The Potential of Edible Bird’s Nests in Reducing Cardiovascular Disease Risk Factors: A Narrative Review
Abstract
1. Introduction
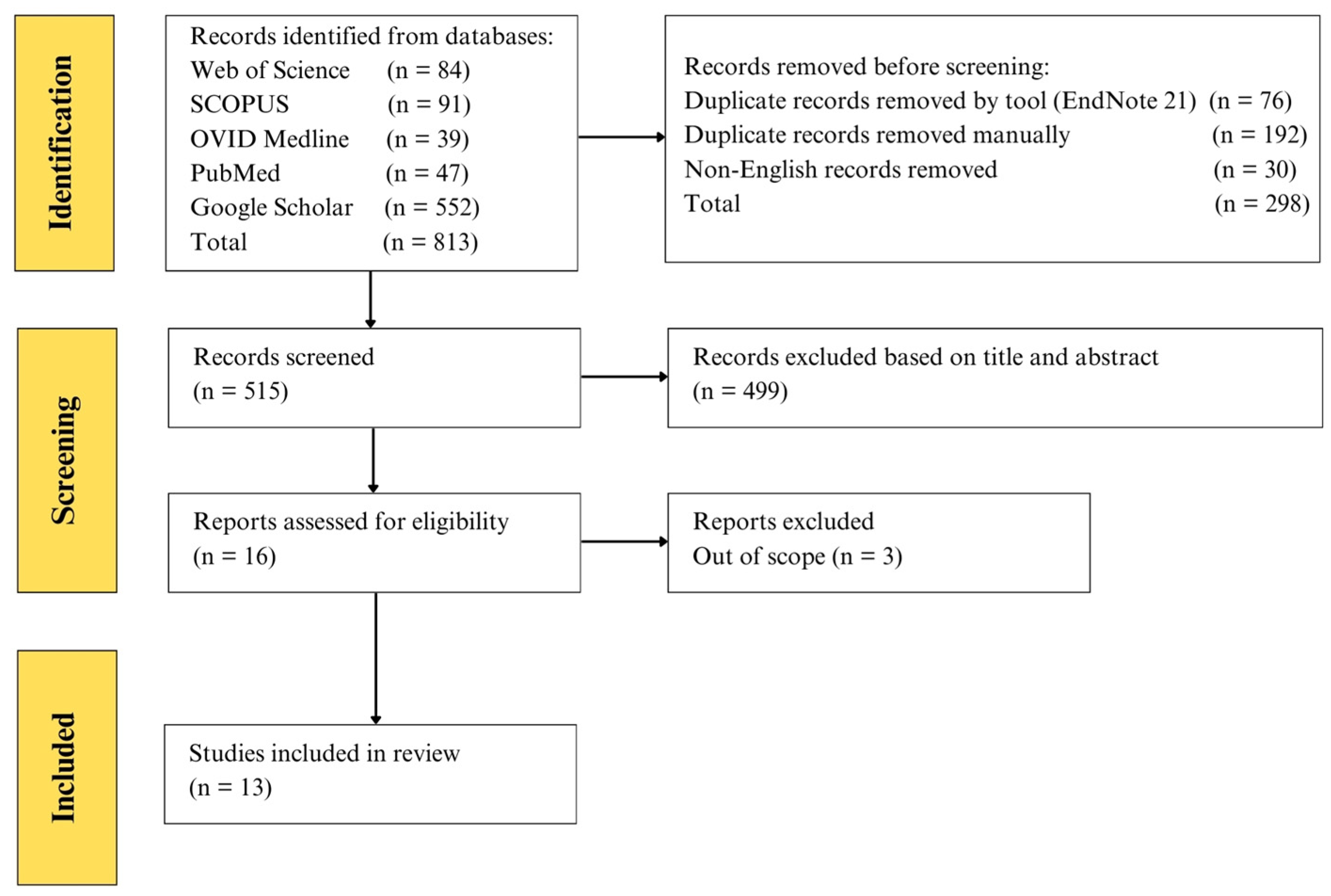
2. Literature Search Strategy for Narrative Review
3. Effects of EBN on Dyslipidemia and Lipid Metabolism
4. Effects of EBN on Obesity
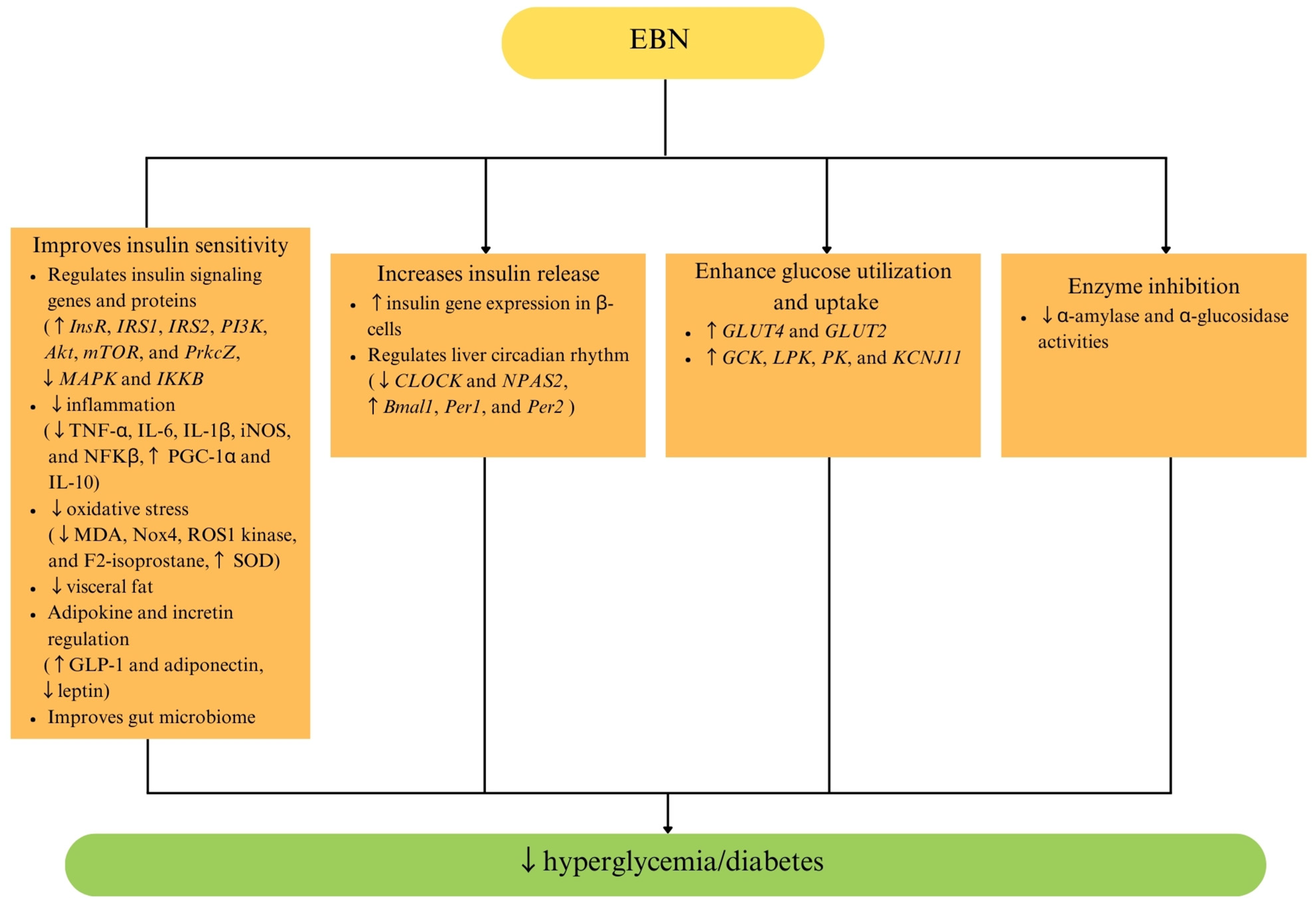
5. Effects of EBN on Diabetes Mellitus and Glucose Metabolism
6. Variability in EBN Composition
7. Safety of EBN
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023.
- Tada, H.; Melander, O.; Louie, J.Z.; Catanese, J.J.; Rowland, C.M.; Devlin, J.J.; Kathiresan, S.; Shiffman, D. Risk prediction by genetic risk scores for coronary heart disease is independent of self-reported family history. Eur. Heart J. 2015, 37, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Essa, M.M.; Bishir, M.; Bhat, A.; Chidambaram, S.B.; Al-Balushi, B.; Hamdan, H.; Govindarajan, N.; Freidland, R.P.; Qoronfleh, M.W. Functional foods and their impact on health. J. Food Sci. Technol. 2023, 60, 820–834. [Google Scholar] [CrossRef]
- Mashau, M.E.; Ramashia, S.E. Role of Functional Food in Treating and Preventing Cardiovascular Diseases. In Functional Foods—Phytochemicals and Health Promoting Potential; Arshad, M.S., Ahmad, M.H., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Chok, K.C.; Ng, M.G.; Ng, K.Y.; Koh, R.Y.; Tiong, Y.L.; Chye, S.M. Edible Bird’s Nest: Recent Updates and Industry Insights Based On Laboratory Findings. Front. Pharmacol. 2021, 12, 746656. [Google Scholar] [CrossRef]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef]
- Bai, W.; Liu, X.; Fan, Q.; Lian, J.; Guo, B. Study of the antiaging effects of bird’s nest peptide based on biochemical, cellular, and animal models. J. Funct. Foods 2023, 103, 105479. [Google Scholar] [CrossRef]
- Choong, M.J.; Dewadas, H.D.; Cheng Lim, L.; Sukuru, S.D.; Tan, C.H.; Cheong, S.K.; Lim, Y.M. Effects of house-cultivated edible bird’s nest on immunoglobulin and cytokine release in vitro. Vet. World 2024, 17, 1370–1384. [Google Scholar] [CrossRef]
- Daud, N.A.; Salma, M.Y.; Salam, B.A.; Joe, L.S.; Razid, S.S.; Hui Yan, T. Edible Bird’s Nest: Physicochemical Properties, Production, and Application of Bioactive Extracts and Glycopeptides. Food Rev. Int. 2021, 37, 177–196. [Google Scholar] [CrossRef]
- Chinese Academy of Inspection and Quarantine (CAIQ). Development Report of Imported Bird’s Nest in 2021; Chinese Academy of Inspection and Quarantine (CAIQ): Beijing, China, 2021. [Google Scholar]
- Aswir Abd Rashed, A.A.R.; Wan Nazaimoon, W. Effect of edible bird’s nest on Caco-2 cell proliferation. J. Food Technol. 2010, 8, 126–130. [Google Scholar] [CrossRef]
- Ma, F.; Liu, D. Sketch of the edible bird’s nest and its important bioactivities. Food Res. Int. 2012, 48, 559–567. [Google Scholar] [CrossRef]
- Sims, R. The identification of Malaysian species of swiftlets Collocalia. Ibis 2008, 103a, 205–210. [Google Scholar] [CrossRef]
- Zulkifli, D.A.; Mansor, R.; Md Ajat, M.M.; Abas, F.; Ideris, A.; Abu, J. Differentiation of Malaysian Farmed and Commercialised Edible Bird’s Nests through Nutritional Composition Analysis. Pertanika J. Trop. Agric. Sci. 2019, 42, 871–881. [Google Scholar]
- But, P.P.; Jiang, R.W.; Shaw, P.C. Edible bird’s nests--how do the red ones get red? J. Ethnopharmacol. 2013, 145, 378–380. [Google Scholar] [CrossRef]
- Quek, M.C.; Chin, N.L.; Yusof, Y.A.; Law, C.L.; Tan, S.W. Characterization of edible bird’s nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res. Int. 2018, 109, 35–43. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, J.; Wang, Y.; Chen, Y.; Jiang, L. A comprehensive review of edible bird’s nest. Food Res. Int. 2021, 140, 109875. [Google Scholar] [CrossRef]
- Wong, Z.C.; Chan, G.K.; Wu, L.; Lam, H.H.; Yao, P.; Dong, T.T.; Tsim, K.W. A comprehensive proteomics study on edible bird’s nest using new monoclonal antibody approach and application in quality control. J. Food Compos. Anal. 2018, 66, 145–151. [Google Scholar] [CrossRef]
- Babji, A.; Syarmila, E.I.; D’Aliah, N.; Nadia, N.M.; Akbar, H.D.; Norrakiah, A.; Ghassem, M.; Najafian, L.; Salma, M. Assessment on bioactive components of hydrolysed edible bird nest. Int. Food Res. J. 2018, 25, 1936–1941. [Google Scholar]
- Lee, C.H.; Hamdan, N.; Nyakuma, B.B.; Wong, S.L.; Wong, K.Y.; Tan, H.; Jamaluddin, H.; Lee, T.H. Purification, identification and molecular docking studies of antioxidant and anti-inflammatory peptides from Edible Bird’s Nest. Food Chem. 2024, 454, 139797. [Google Scholar] [CrossRef]
- Chua, K.H.; Mohamed, I.N.; Mohd Yunus, M.H.; Shafinaz Md Nor, N.; Kamil, K.; Ugusman, A.; Kumar, J. The anti-viral and anti-inflammatory properties of edible bird’s nest in influenza and coronavirus infections: From pre-clinical to potential clinical application. Front. Pharmacol. 2021, 12, 633292. [Google Scholar] [CrossRef] [PubMed]
- Haghani, A.; Mehrbod, P.; Safi, N.; Aminuddin, N.A.; Bahadoran, A.; Omar, A.R.; Ideris, A. In vitro and in vivo mechanism of immunomodulatory and antiviral activity of Edible Bird’s Nest (EBN) against influenza A virus (IAV) infection. J. Ethnopharmacol. 2016, 185, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Vimala, B.; Hussain, H.; Nazaimoon, W.W. Effects of edible bird’s nest on tumour necrosis factor-alpha secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Agric. Immunol. 2012, 23, 303–314. [Google Scholar] [CrossRef]
- Wulandari, E.; Hapsari, R.A.F.; Wulanuri, I.; Nasution, A.R. The Role Cardioprotective of Edible Bird’s nest (Collacalia fuciphaga) Extract Through Suppression of Oxidative Stress. J. Cardiovasc. Dis. Res. 2021, 12, 22–28. [Google Scholar]
- Hu, Q.; Li, G.; Yao, H.; He, S.; Li, H.; Liu, S.; Wu, Y.; Lai, X. Edible bird’s nest enhances antioxidant capacity and increases lifespan in Drosophila Melanogaster. Cell Mol. Biol. (Noisy-Le-Grand) 2016, 62, 116–122. [Google Scholar]
- Akmal, M.N.; Intan-Shameha, A.R.; Mansor, R.; Ideris, A.; Rahman, A. High-dose edible bird’s nest extract (ebn) upregulates ldl-r via suppression of hmgcr gene expression in hepg2 cell lines. Sains Malays. 2020, 49, 2433–2442. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Hou, Z.P. Edible bird’s nest attenuates procoagulation effects of high-fat diet in rats. Drug Des. Dev. Ther. 2015, 9, 3951–3959. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest attenuates high fat diet-induced oxidative stress and inflammation via regulation of hepatic antioxidant and inflammatory genes. BMC Complement. Altern. Med. 2015, 15, 310. [Google Scholar] [CrossRef]
- Verschuren, W.M.M.; Boer, J.M.A.; Temme, E.H.M. Optimal diet for cardiovascular and planetary health. Heart 2022, 108, 1234–1239. [Google Scholar] [CrossRef]
- Al-Rubaye, H.; Perfetti, G.; Kaski, J.C. The Role of Microbiota in Cardiovascular Risk: Focus on Trimethylamine Oxide. Curr. Probl. Cardiol. 2019, 44, 182–196. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, Y.W.; Hazeena, S.H.; Tain, Y.L.; Hsieh, C.W.; Chen, D.Q.; Liu, R.Y.; Shih, M.K. Cardiovascular risk of dietary trimethylamine oxide precursors and the therapeutic potential of resveratrol and its derivatives. FEBS Open Bio 2024, 14, 358–379. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Boarescu, P.-M.; Bocsan, C.I.; Gherman, M.L.; Chedea, V.S.; Jianu, E.-M.; Roșian, Ș.H.; Boarescu, I.; Ranga, F.; Tomoiagă, L.L.; et al. Anti-Inflammatory and Antioxidant Effects of White Grape Pomace Polyphenols on Isoproterenol-Induced Myocardial Infarction. Int. J. Mol. Sci. 2025, 26, 2035. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alharbi, H.O.A.; Khan, A.A.; Babiker, A.Y.; Rizvi, M.M.A. Therapeutic potential of resveratrol, a polyphenol in the prevention of liver injury induced by diethylnitrosamine (DEN) through the regulation of inflammation and oxidative stress. Funct. Foods Health Dis. 2024, 14, 898–920. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed. Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef]
- Ramachandran, R.; Babji, A.S.; Sani, N.A. Antihypertensive potential of bioactive hydrolysate from edible bird’s nest. Proc. AIP Conf. Proc. 2018, 1940, 020099. [Google Scholar]
- Lee, C.H.; Hamdan, N.; Nyakuma, B.B.; Wong, S.L.; Wong, K.Y.; Tan, H.Y.; Jamaluddin, H.; Lee, T.H. Production and characterisation of dual-function antioxidant and anti-inflammatory peptides from edible bird’s nest (EBN) protein hydrolysates. J. Food Meas. Charact. 2025, 19, 952–973. [Google Scholar] [CrossRef]
- Addisu, B.; Bekele, S.; Wube, T.B.; Hirigo, A.T.; Cheneke, W. Dyslipidemia and its associated factors among adult cardiac patients at Ambo university referral hospital, Oromia region, west Ethiopia. BMC Cardiovasc. Disord. 2023, 23, 321. [Google Scholar] [CrossRef]
- Dybiec, J.; Baran, W.; Dąbek, B.; Fularski, P.; Młynarska, E.; Radzioch, E.; Rysz, J.; Franczyk, B. Advances in Treatment of Dyslipidemia. Int. J. Mol. Sci. 2023, 24, 13288. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, M.; Liu, X.; Que, M.; Dekyi, K.; Zheng, L.; Zhang, Y.; Lv, Y.; Fan, Q.; Wang, X.; et al. Edible bird’s nest regulates glucose and lipid metabolic disorders via the gut-liver axis in obese mice. Food Funct. 2024, 15, 7577–7591. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ooi, D.J.; Sarega, N.; Azmi, N.H.; Ismail, N.; Chan, K.W.; Hou, Z.; Yusuf, N.B. Edible Bird’s Nest Prevents High Fat Diet-Induced Insulin Resistance in Rats. J. Diabetes Res. 2015, 2015, 760535. [Google Scholar] [CrossRef]
- Yida, Z.; Al-Shuwayah, H.; Ismail, M.; Imam, M.U. Edible Bird’s Nest Regulates Hepatic Cholesterol Metabolism through Transcriptional Regulation of Cholesterol Related Genes. Evid. Based Complement. Altern. Med. 2022, 2022, 8882993. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.N.; Intan-Shameha, A.R.; Ajat, M.; Abu Bakar, M.Z.; Mansor, R.; Ideris, A. Edible Bird’s Nest Soup (EBNS) Serves as Anti-Obesity and Antilipemic After 6-Weeks of Supplementation in High-Fat Diet (HFD)-Fed Rats. Sains Malays. 2024, 53, 3545–3553. [Google Scholar] [CrossRef]
- Mohamad Nasir, N.N.; Mohamad Ibrahim, R.; Mahmud, R.; Ab Razak, N.A.; Ismail, N.; Chan, K.W.; Abu Bakar, M.Z. Edible Bird’s Nest Effectively Attenuates Atherosclerosis through Modulation of Cholesterol Metabolism via Activation of PPARγ/LXRα Signaling Pathway In Vivo. J. Food Biochem. 2023, 2023, 7861265. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Permatasari, Q.I.; Taslim, N.A.; Subali, D.; Kurniawan, R.; Surya, R.; Qhabibi, F.R.; Tanner, M.J.; Batubara, S.C.; Mayulu, N.; et al. Revealing Edible Bird Nest as Novel Functional Foods in Combating Metabolic Syndrome: Comprehensive In Silico, In Vitro, and In Vivo Studies. Nutrients 2023, 15, 3886. [Google Scholar] [CrossRef]
- Hou, Z.; Imam, M.U.; Ismail, M.; Ooi, D.J.; Ideris, A.; Mahmud, R. Nutrigenomic effects of edible bird’s nest on insulin signaling in ovariectomized rats. Drug Des. Devel Ther. 2015, 9, 4115–4125. [Google Scholar] [CrossRef]
- Fan, Q.; Lian, J.; Liu, X.; Zou, F.; Wang, X.; Chen, M. A Study on the Skin Whitening Activity of Digesta from Edible Bird’s Nest: A Mucin Glycoprotein. Gels 2021, 8, 24. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Liu, Y.; Xu, D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019, 18, 173. [Google Scholar] [CrossRef]
- Payazdan, M.; Khatami, S.; Galehdari, H.; Delfan, N.; Shafiei, M.; Heydaran, S. The anti-inflammatory effects of sialic acid on the human glia cells by the upregulation of IL-4 and IL-10 genes’ expressions. Gene Rep. 2021, 24, 101218. [Google Scholar] [CrossRef]
- Hou, Z.P.; Imam, M.U.; Ismail, M.; Azmi, N.H.; Ismail, N.; Ideris, A.; Mahmud, R. Lactoferrin and ovotransferrin contribute toward antioxidative effects of Edible Bird’s Nest against hydrogen peroxide-induced oxidative stress in human SH-SY5Y cells. Biosci. Biotechnol. Biochem. 2015, 79, 1570–1578. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Barham, G.S.; Marghazani, I.B. Lactoferrin’s role in modulating NF-κB pathway to alleviate diabetes-associated inflammation: A novel in-silico study. Heliyon 2024, 10, e34051. [Google Scholar] [CrossRef]
- Ranjan, S.; Trivedi, S.; Sharma, S.; Khan, S.; Pandey, R. Bakuchiol modulates acetylcholine synthesis and alleviates Aβ proteotoxicity. Nat. Prod. Res. 2024, 38, 3876–3880. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.N.; Mahmud, S.; Ark, S.M.A.; Kamruzzaman, M.; Hasan, M.K. Bakuchiol, a natural constituent and its pharmacological benefits. F1000Res 2023, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Panuganti, K.K.; Nguyen, M.; Kshirsagar, R.K. Obesity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.J.W.; Chang, L.S.; Babji, A.S.; Latip, J.; Koketsu, M.; Lim, S.J. Review of sialic acid’s biochemistry, sources, extraction and functions with special reference to edible bird’s nest. Food Chem. 2022, 367, 130755. [Google Scholar] [CrossRef]
- Li, D.; Lin, Q.; Luo, F.; Wang, H. Insights into the Structure, Metabolism, Biological Functions and Molecular Mechanisms of Sialic Acid: A Review. Foods 2023, 13, 145. [Google Scholar] [CrossRef]
- Đức, N.M.; Nguyen, M.T.; Can, M.V.; Trinh, H.N.T.; Ngo, L.; Vo, T.T.B.; Nghiem, M.N. Nucleobindin-2 (NUCB2) Gene Transcription Activity and Nesfatin-1 Levels in Correlation with Anthropometric and Biochemical Parameters in Type 2 Diabetes Mellitus Patient Groups in Vietnam. medRxiv 2021. [Google Scholar] [CrossRef]
- Gharanei, S.; Ramanjaneya, M.; Patel, A.H.; Patel, V.; Shabir, K.; Auld, C.; Karteris, E.; Kyrou, I.; Randeva, H.S. NUCB2/Nesfatin-1 Reduces Obesogenic Diet Induced Inflammation in Mice Subcutaneous White Adipose Tissue. Nutrients 2022, 14, 1409. [Google Scholar] [CrossRef]
- Luo, J.-j.; Wen, F.-j.; Qiu, D.; Wang, S.-z. Nesfatin-1 in lipid metabolism and lipid-related diseases. Clin. Chim. Acta 2021, 522, 23–30. [Google Scholar] [CrossRef]
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes mellitus: From molecular mechanism to pathophysiology and pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Petersmann, A.; Nauck, M.; Muller-Wieland, D.; Kerner, W.; Muller, U.A.; Landgraf, R.; Freckmann, G.; Heinemann, L. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Alexandra, F.D.; Satriyandi, M.; Frethernety, A.; Triawanti, T. Influence of Extract Swiftlet Nest (Collocalia Fuciphago) as Antihyperglycemia to Circulating Endothelial Cells in Rat (Rattus novergicus) Induced by Streptozotocin. Berk. Kedokt. 2018, 14, 1. [Google Scholar] [CrossRef]
- Alexandra, F.D.; Frethernety, A.; Trinovita, E.; Triawanti, T. Effect of Swiftlet’s (Collocalia Fuciphago) Nest Extract on the Malondialdehyde (MDA) and Superoxyde Dismutase (SOD) Activity on Hyperglycemic Rattus Norvegicius. Maj. Obat Tradis. 2020, 25, 161–166. [Google Scholar] [CrossRef]
- Komolkriengkrai, M.; Matsathit, U.; Sirinupong, N.; Khimmaktong, W. The effectiveness of edible bird’s nest in lowering VEGF, CD31, and PDGFR-β levels in diabetic retinopathy in rats with type 1 diabetes. Histol. Histopathol. 2025, 40, 669–678. [Google Scholar] [CrossRef]
- Choy, K.W.; Zain, Z.M.; Murugan, D.D.; Giribabu, N.; Zamakshshari, N.H.; Lim, Y.M.; Mustafa, M.R. Effect of Hydrolyzed Bird’s Nest on β-Cell Function and Insulin Signaling in Type 2 Diabetic Mice. Front. Pharmacol. 2021, 12, 632169. [Google Scholar] [CrossRef]
- Tan, Y.; Miao, L.; Xiao, J.; Cheang, W.S. 3,3′,4,5′-Tetramethoxy-trans-stilbene Improves Insulin Resistance by Activating the IRS/PI3K/Akt Pathway and Inhibiting Oxidative Stress. Curr. Issues Mol. Biol. 2022, 44, 2175–2185. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, X.; Huang, X.; Wang, C.; Li, Y. The IRS/PI3K/Akt signaling pathway mediates olanzapine-induced hepatic insulin resistance in male rats. Life Sci. 2019, 217, 229–236. [Google Scholar] [CrossRef]
- Taheri, R.; Mokhtari, Y.; Yousefi, A.-M.; Bashash, D. The PI3K/Akt signaling axis and type 2 diabetes mellitus (T2DM): From mechanistic insights into possible therapeutic targets. Cell Biol. Int. 2024, 48, 1049–1068. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the regulation of body weigh. Keio J. Med. 2011, 60, 1–9. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.R.; Lee, T.H.; Cheong, S.K.; Lim, Y.M. Untargeted metabolite profiling on the water-soluble metabolites of edible bird’s nest through liquid chromatography-mass spectrometry. Vet. World 2020, 13, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Babji, A.S.; Sajak, A.A.B.; Daud, N.A.; Rahman, H.A.; Sermwittayawong, D.; Patninan, K. Potential Anti-diabetic Activities from Edible Bird Nest and Its Hydrolysates. Curr. Adv. Chem. Biochem. 2021, 3, 77–86. [Google Scholar]
- Chan, K.W.; Ismail, M.; Esa, N.M.; Khong, N.M. Research Article Defatted Kenaf (Hibiscus cannabinus L.) Seed Meal and Its Phenolic-Saponin-Rich Extract Protect Hypercholesterolemic Rats Against Oxidative Stress and Systemic Inflammation via Transcriptional Modulation of Hepatic Antioxidant Genes; Hindawi: London, UK, 2018. [Google Scholar]
- Liu, Z.; Xu, X.; Liang, Z.; Chen, Y.; Wang, Q.; Kang, W.; Zhang, Y.; Cong, B. Bakuchiol ameliorates glycolipid homeostasis by reducing inflammation. Food Sci. Hum. Wellness 2024, 13, 3159–3170. [Google Scholar] [CrossRef]
- Poon, P.M.-Y.; Sze, E.T.-P. Development of Method for Evaluation of Edible Bird’s Nest Content in Ready-to-Eat Beverages. J. AOAC Int. 2023, 106, 1003–1009. [Google Scholar] [CrossRef]
- Elfita, L.; Ietje, W.; Dondin, S.; Indra, B.; Huda Shalahudin, D. The diversity in nutritional profile of farmed edible bird’s nests from several regions in Indonesia. Biodiversitas J. Biol. Divers. 2020, 21. [Google Scholar] [CrossRef]
- Hamzah, Z.; Ibrahim, N.H.; Hussin, K.; Hashim, O.; Lee, B.-B. Nutritional properties of edible bird nest. J. Asian Sci. Res. 2013, 3, 600. [Google Scholar]
- Saengkrajang, W.; Matan, N.; Matan, N. Nutritional composition of the farmed edible bird’s nest (Collocalia fuciphaga) in Thailand. J. Food Compos. Anal. 2013, 31, 41–45. [Google Scholar] [CrossRef]
- Wurster, C.M.; Munksgaard, N.; Zwart, C.; Bird, M. The biogeochemistry of insectivorous cave guano: A case study from insular Southeast Asia. Biogeochemistry 2015, 124, 163–175. [Google Scholar] [CrossRef]
- Yeo, B.-H.; Tang, T.-K.; Wong, S.-F.; Tan, C.-P.; Wang, Y.; Cheong, L.-Z.; Lai, O.-M. Potential Residual Contaminants in Edible Bird’s Nest. Front. Pharmacol. 2021, 12, 631136. [Google Scholar] [CrossRef]
- Rahayu, S.; Suhartono, M.T.; Suryapratama, W.; Bata, M. Keratinolytic Enzymes For Cleaning Edible Bird’s Nest. Biosci. Biotech. Res. Asia 2017, 14, 989–996. [Google Scholar] [CrossRef]
- Zulkefle, N.N.; Ibrahim, M.A.; Azuan, N.F.; Ch’ng, S.E.; Ismail, N.; Abu Bakar, M.Z.; Chan, K.W. A Review on the Edible Bird’s Nest Quality and Manufacturing Standards of the Three Largest Exporting Countries in the World. J. Food Qual. 2024, 2024, 5608357. [Google Scholar] [CrossRef]
- Xing, Y.N.; Ni, H.G.; Chen, Z.Y. Semicarbazide in selected bird’s nest products. J. Food Prot. 2012, 75, 1654–1659. [Google Scholar] [CrossRef]
- Delhalle, L.; Taminiau, B.; Fastrez, S.; Fall, A.; Ballesteros, M.; Burteau, S.; Daube, G. Evaluation of Enzymatic Cleaning on Food Processing Installations and Food Products Bacterial Microflora. Front. Microbiol. 2020, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Haraharap, M.A.; Sjofjan, O.; Radiati, L.E.; Natsir, M.H.; Abdi Syahputra, R.; Nurkolis, F. A current insight and future perspective of edible bird nest as caviar of the east. Pharmacia 2023, 70, 1135–1155. [Google Scholar] [CrossRef]
- Kew, P.E.; Wong, S.F.; Ling, S.J.; Lim, P.K.C.; Mak, J.W. Isolation and identification of mites associated with raw and commercial farm edible bird nests. Trop. Biomed. 2015, 32, 761–775. [Google Scholar]
- Tan, S.N.; Sani, D.; Lim, C.W.; Ideris, A.; Stanslas, J.; Lim, C.T.S. Proximate Analysis and Safety Profile of Farmed Edible Bird’s Nest in Malaysia and Its Effect on Cancer Cells. Evid. Based Complement. Altern. Med. 2020, 2020, 8068797. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for its Application. In Food Hygiene—Basic Texts, 3rd ed.; FAO/WHO: Rome, Italy, 2003; Available online: http://www.fao.org/3/y1579e/y1579e03.htm (accessed on 5 May 2025).
- World Health Organization (WHO). Good Manufacturing Practices and Inspection; WHO Technical Report Series, No. 961, Annex 3; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- ISO 22000:2018; Food Safety Management Systems—Requirements for Any Organization in the Food Chain. International Organization for Standardization (ISO): Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/65464.html (accessed on 5 May 2025).
- CAIQ-RZ-2015001; Bird’s Nest Product Processing Enterprise Hygienic Technical Specifications. Chinese Academy of Inspection and Quarantine (CAIQ): Beijing, China, 2015.
- CAIQ-RZ-2015002; Bird’s Nest Product Certification Implementation Rules. Chinese Academy of Inspection and Quarantine (CAIQ): Beijing, China, 2015.
- Li, G.; Lee, T.H.; Chan, G.; Tsim, K.W.K. Editorial: Edible Bird’s Nest—Chemical Composition and Potential Health Efficacy and Risks. Front. Pharmacol. 2022, 12, 819461. [Google Scholar] [CrossRef]
- Lee, T.H.; Wani, W.A.; Lee, C.H.; Cheng, K.K.; Shreaz, S.; Wong, S.; Hamdan, N.; Azmi, N.A. Edible Bird’s Nest: The Functional Values of the Prized Animal-Based Bioproduct from Southeast Asia—A Review. Front. Pharmacol. 2021, 12, 626233. [Google Scholar] [CrossRef]
- Guo, C.T.; Takahashi, T.; Bukawa, W.; Takahashi, N.; Yagi, H.; Kato, K.; Hidari, K.I.; Miyamoto, D.; Suzuki, T.; Suzuki, Y. Edible bird’s nest extract inhibits influenza virus infection. Antivir. Res. 2006, 70, 140–146. [Google Scholar] [CrossRef]


| EBN Preparation and Source | EBN Dose | Experimental Model | Findings | Proposed Mechanism and Suggested Active Compound | Reference |
|---|---|---|---|---|---|
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | ↓ TC, TG, LDLc ↑ HDLc ↓ oxLDL | EBN improves lipid profile by increasing adiponectin and reducing leptin levels. Suggested active compound: not stated. | [30] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | ↓ TC, TG, LDLc ↑ HDLc ↓ oxLDL ↓ serum lipase | EBN improves hypercholesterolemia by regulating the expression of hepatic cholesterol metabolism genes (↑ LDLR and CYP7A1, and ↓ PCSK9, APOB and HMGCR). Suggested active compounds: sialic acid, lactoferrin, and ovotransferrin. | [44] |
| Full stew EBN and EBN stew extract Terengganu, Malaysia | 500 mg/kg/day for 12 weeks | High-fat, high-cholesterol diet-induced atherosclerotic New Zealand white rabbits | ↓ TC, TG, LDLc ↑ HDLc ↓ atherogenic indices | EBN ameliorates hypercholesterolemia by:
| [46] |
| Fresh stewed EBN Xiamen, China | 2777, 5555 and 11,111 mg/kg/day for 10 weeks | High-fat diet-induced obese C57BL/6J mice | ↓ TC, TG, LDLc ↑ HDLc ↑ sialic acid content in LDL ↓ oxLDL | EBN improves lipid profile by:
| [42] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | ↓ TC, TG, LDLc ↑ HDLc | The lipid-lowering mechanisms of EBN were not elucidated in the study. Suggested active compounds: sialic acid, lactoferrin, and ovotransferrin. | [43] |
| EBN powder Sarawak, Malaysia | 1.5% and 3% EBN for 12 weeks | Ovariectomy-induced menopausal Sprague Dawley rats | ↓ TC, TG, LDLc ↑ HDLc | EBN improves lipid profile by enhancing estrogen levels and through its estrogen-mimetic effects. Suggested active compound: not stated. | [48] |
| EBN powder East Java, Indonesia | In vitro: 50–250 µg/mL In vivo: 22.5 and 45 mg/kg/day for 6 weeks | High-cholesterol, high-fat diet-induced obese Rattus norwegicus | ↓ TC, TG, LDLc ↑ HDLc | EBN improves lipid profile by reducing HMGCR and lipase activities. Suggested active compounds: bakuchiol and dehydrolinestrenolide. | [47] |
| EBN soup and extract Perak, Malaysia | 843.2 mg/kg/day EBN soup or 6.5 mg/kg/day EBN extract for 6 weeks | High-fat diet-induced obese Sprague Dawley rats | ↓ TC, TG, LDLc, VLDLc ↑ HDLc ↓ atherogenic indices | The lipid-lowering mechanisms of EBN were not elucidated in the study. Suggested active compound: sialic acid. | [45] |
| EBN Preparation and Source | EBN Dose | Experimental Model | Findings | Proposed Mechanism and Suggested Active Compound | Reference |
|---|---|---|---|---|---|
| Fresh stewed EBN Xiamen, China | 2777, 5555 and 11,111 mg/kg/day for 8 weeks | High-fat diet-induced obese C57BL/6J mice | ↓ body weight gain ↓ body fat mass ↓ liver weight and fat accumulation ↑ energy expenditure | EBN attenuates obesity by:
| [42] |
| EBN powder Sarawak, Malaysia | 1.5% and 3% for 12 weeks | Ovariectomy-induced menopausal Sprague Dawley rats | ↓ body weight gain | EBN reduces weight gain during menopause by enhancing estrogen levels and through its estrogen-mimetic effects. Suggested active compound: not stated. | [48] |
| EBN powder East Java, Indonesia | In vitro: 50–250 µg/mL In vivo: 22.5 and 45 mg/kg/day for 6 weeks | In vitro enzyme inhibitory assays High-cholesterol, high-fat diet-induced obese Rattus norwegicus | ↓ lipase activity in vitro ↓ body weight ↓ fat mass and obesity-associated proteins | EBN demonstrates anti-obesity effect by reducing serum lipase activity. Suggested active compounds: bakuchiol and dehydrolindestrenolide. | [47] |
| Full stew EBN and EBN stew extract Terengganu, Malaysia | 500 mg/kg/day for 12 weeks | High-fat, high-cholesterol diet-induced New Zealand white rabbits | ↓ body weight gain | The weight-lowering mechanisms of EBN are not elucidated in the study. Suggested active compounds: sialic acid and protein nucelobindin-2. | [46] |
| EBN soup and extract Perak, Malaysia | 843.2 mg/kg/day EBN soup or 6.5 mg/kg/day EBN extract for 6 weeks | High-fat diet-induced obese Sprague Dawley rats | ↓ body weight gain ↓ subcutaneous and visceral fat mass | The weight-lowering mechanisms of EBN are not elucidated in the study. Suggested active compound: sialic acid. | [45] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | No significant change in body weight with EBN supplementation. | - | [43] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | No significant change in body weight with EBN supplementation. | - | [44] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% EBN for 12 weeks | High-fat diet-induced obese Sprague Dawley rats | No significant change in body weight with EBN supplementation. | - | [30] |
| EBN Preparation and Source | EBN Dose | Experimental Model | Findings | Proposed Mechanism and Suggested Active Compound | Reference |
|---|---|---|---|---|---|
| Fresh stewed EBN Xiamen, China | 2777, 5555, and 11,111 mg/kg/day for 8 weeks | High-fat diet-induced obese C57BL/6J mice | ↓ FBG Improve glucose tolerance ↓ serum insulin ↓ HOMA-IR | EBN reduces insulin resistance by:
| [42] |
| EBN powder Terengganu, Malaysia | 2.5% and 20% for 12 weeks | High-fat diet-induced insulin-resistant Sprague Dawley rats | Improve glucose tolerance ↓ insulin levels ↓ HOMA-IR | EBN reduces insulin resistance by:
| [43] |
| Hydrolyzed EBN Selangor, Malaysia | 75 and 150 mg/kg/day for 28 days | Type 2 diabetic db/db mice | ↓ FBG Improve glucose tolerance ↓ serum insulin | The antidiabetic effects of EBN are mediated by:
| [72] |
| EBN powder Sarawak, Malaysia | 1.5% and 3% for 12 weeks | Ovariectomy-induced menopausal Sprague Dawley rats | Improve glucose tolerance ↓ HOMA-IR | EBN prevents insulin resistance by:
| [48] |
| EBN powder East Java, Indonesia | In vitro: 50–250 µg/mL In vivo: 22.5 mg/kg/day and 45 mg/kg/day for 6 weeks | In vitro enzyme inhibitory assays High-cholesterol, high-fat diet-induced obese Rattus norwegicus | ↓ α-glucosidase and α-amylase activities ↓ blood glucose | The antidiabetic effect of EBN is contributed by:
| [47] |
| EBN aqueous extract Kalimantan, Indonesia | 1, 10 and 100 mg/kg/day for 4 weeks | Streptozotocin-induced type 1 diabetic Rattus norwegicus | ↓ blood glucose | EBN improves blood glucose of diabetic rats by reducing oxidative stress (↑ SOD). Suggested active compound: not stated. | [70] |
| EBN aqueous extract Kalimantan, Indonesia | 1, 10 and 100 mg/kg/day for 4 weeks | Streptozotocin-induced type 1 diabetic Rattus norwegicus | ↓ blood glucose | The glucose-lowering mechanisms of EBN are not elucidated in the study. Suggested active compound: not stated. | [69] |
| EBN aqueous extract Nakhon Si Thammarat, Thailand | 75 and 150 mg/kg/day for 8 weeks | Streptozotocin-induced type 1 diabetic Wistar rats | ↓ blood glucose | The glucose-lowering mechanisms of EBN are not elucidated in the study. Suggested active compound: not stated. | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanuar, N.D.; Aminuddin, A.; Hamid, A.A.; Kumar, J.; Hui, C.K.; Mahadi, M.K.; Ugusman, A. The Potential of Edible Bird’s Nests in Reducing Cardiovascular Disease Risk Factors: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 4619. https://doi.org/10.3390/ijms26104619
Rusanuar ND, Aminuddin A, Hamid AA, Kumar J, Hui CK, Mahadi MK, Ugusman A. The Potential of Edible Bird’s Nests in Reducing Cardiovascular Disease Risk Factors: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(10):4619. https://doi.org/10.3390/ijms26104619
Chicago/Turabian StyleRusanuar, Nina Diyana, Amilia Aminuddin, Adila A. Hamid, Jaya Kumar, Chua Kien Hui, Mohd Kaisan Mahadi, and Azizah Ugusman. 2025. "The Potential of Edible Bird’s Nests in Reducing Cardiovascular Disease Risk Factors: A Narrative Review" International Journal of Molecular Sciences 26, no. 10: 4619. https://doi.org/10.3390/ijms26104619
APA StyleRusanuar, N. D., Aminuddin, A., Hamid, A. A., Kumar, J., Hui, C. K., Mahadi, M. K., & Ugusman, A. (2025). The Potential of Edible Bird’s Nests in Reducing Cardiovascular Disease Risk Factors: A Narrative Review. International Journal of Molecular Sciences, 26(10), 4619. https://doi.org/10.3390/ijms26104619






