Molecular Characterization and Functional Analysis of Odorant-Binding Proteins in Ectropis grisescens
Abstract
1. Introduction
2. Results
2.1. Identification of OBP in E. grisescens
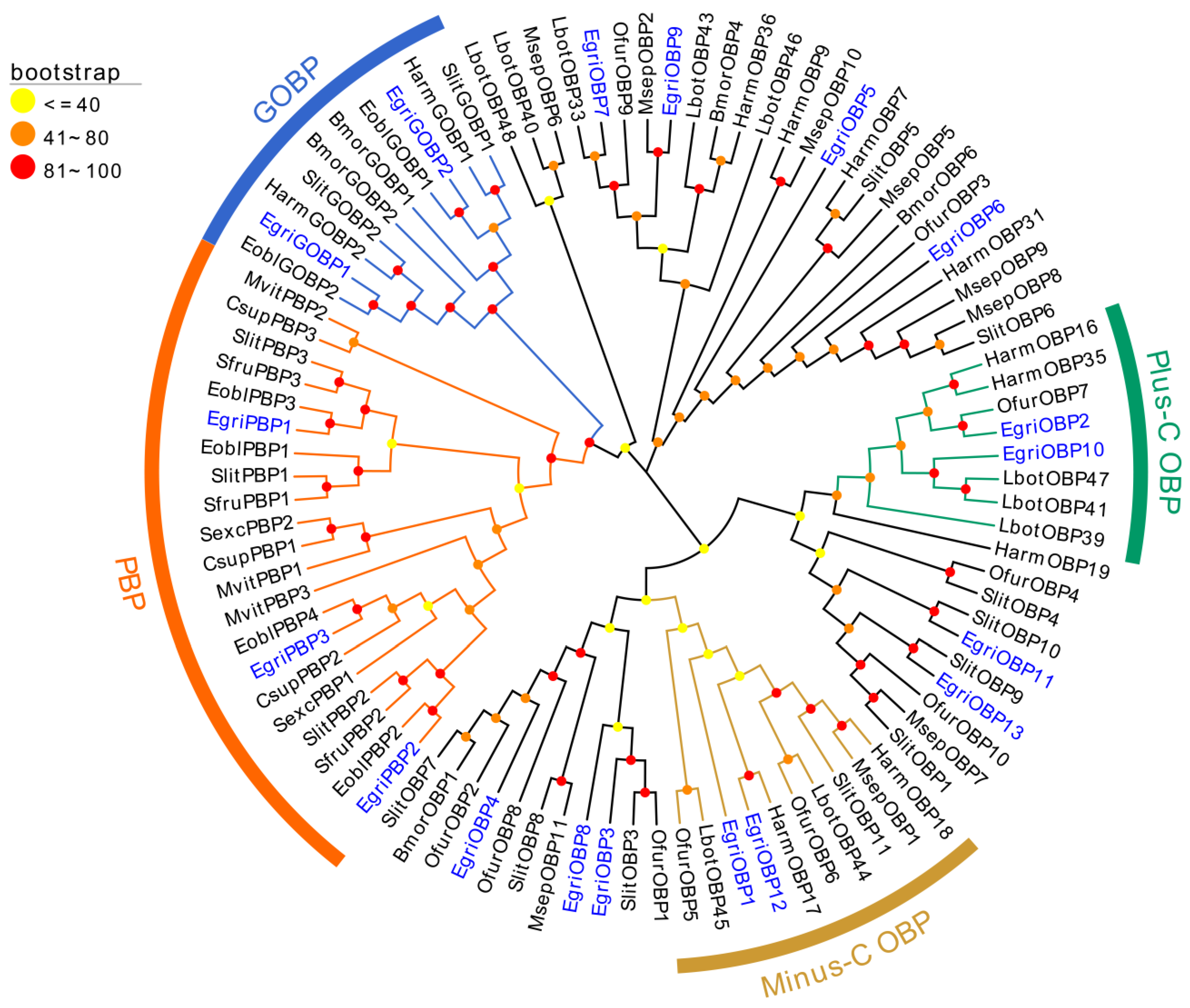
2.2. Phylogenetic Analysis
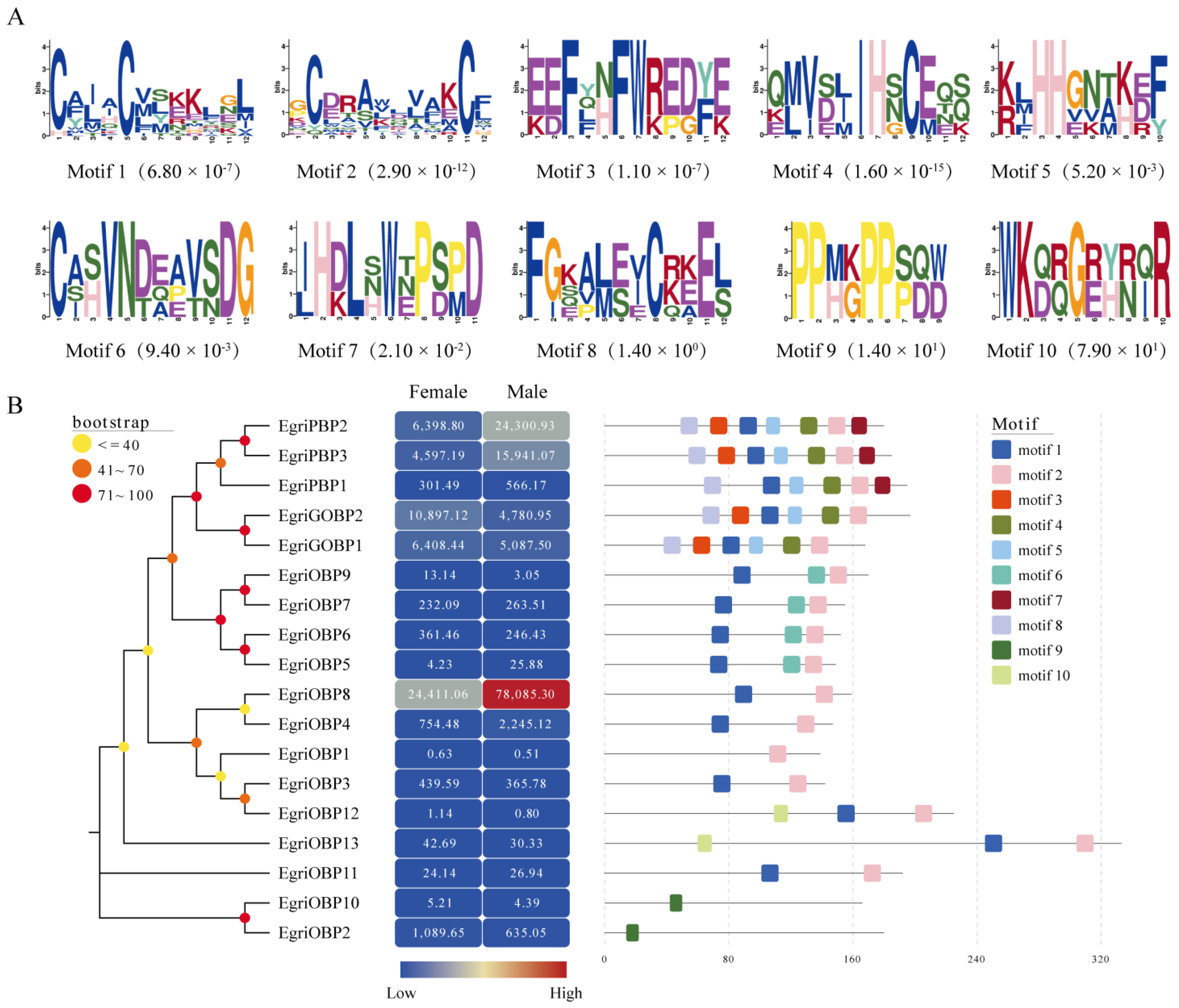
2.3. Motif Pattern Analysis
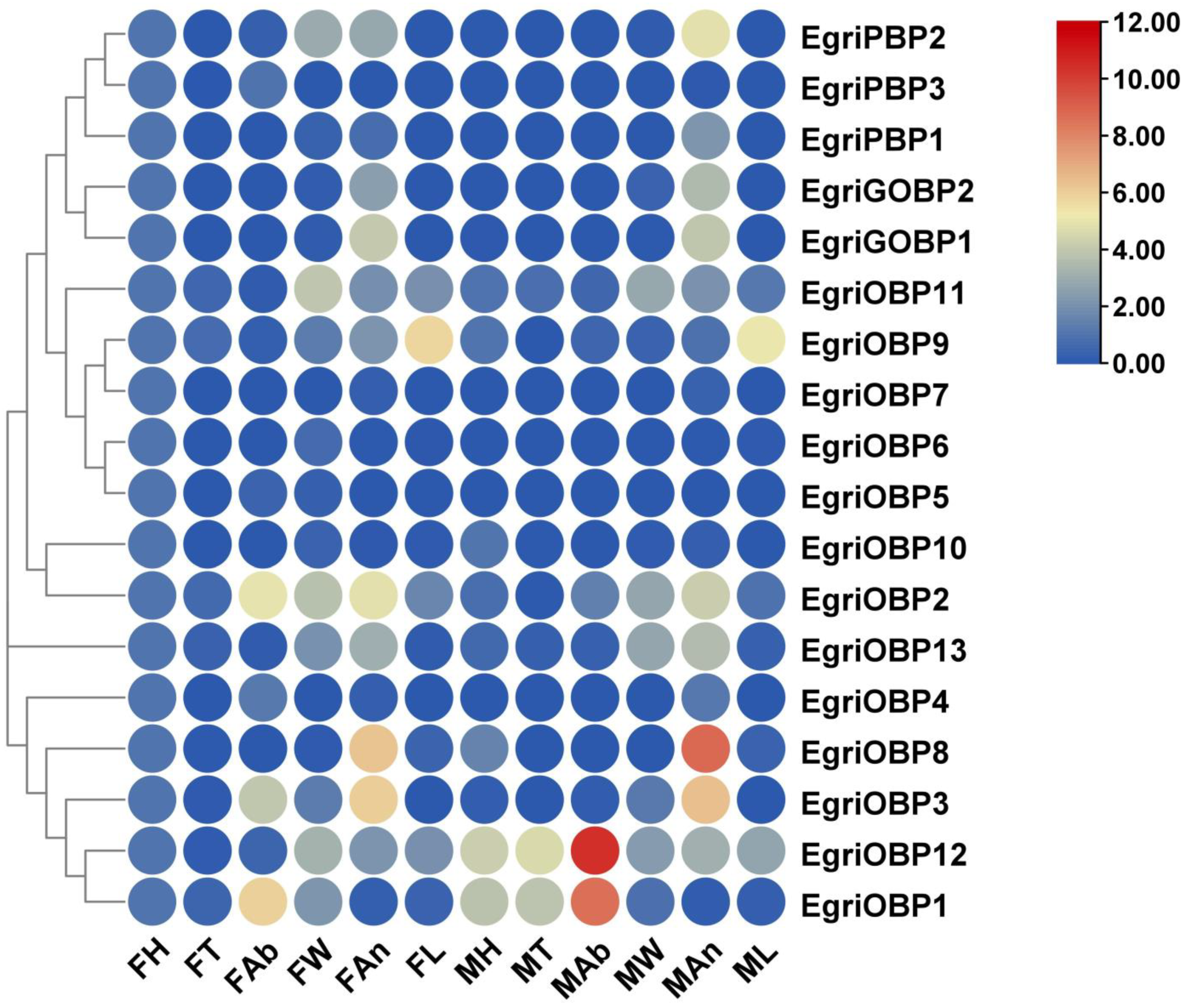
2.4. Expression Profiles of EgriOBPs
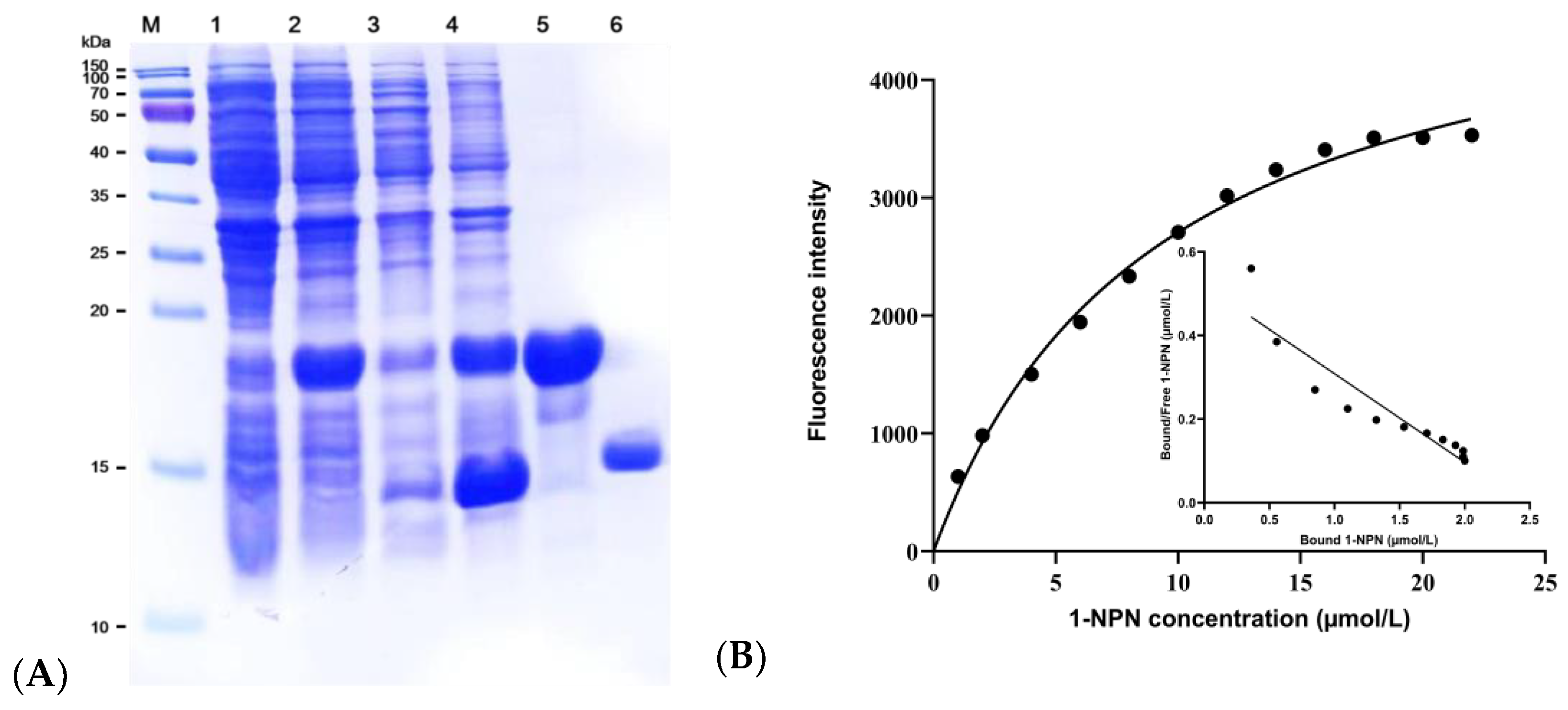
2.5. Expression and Purification of EgriGOBP
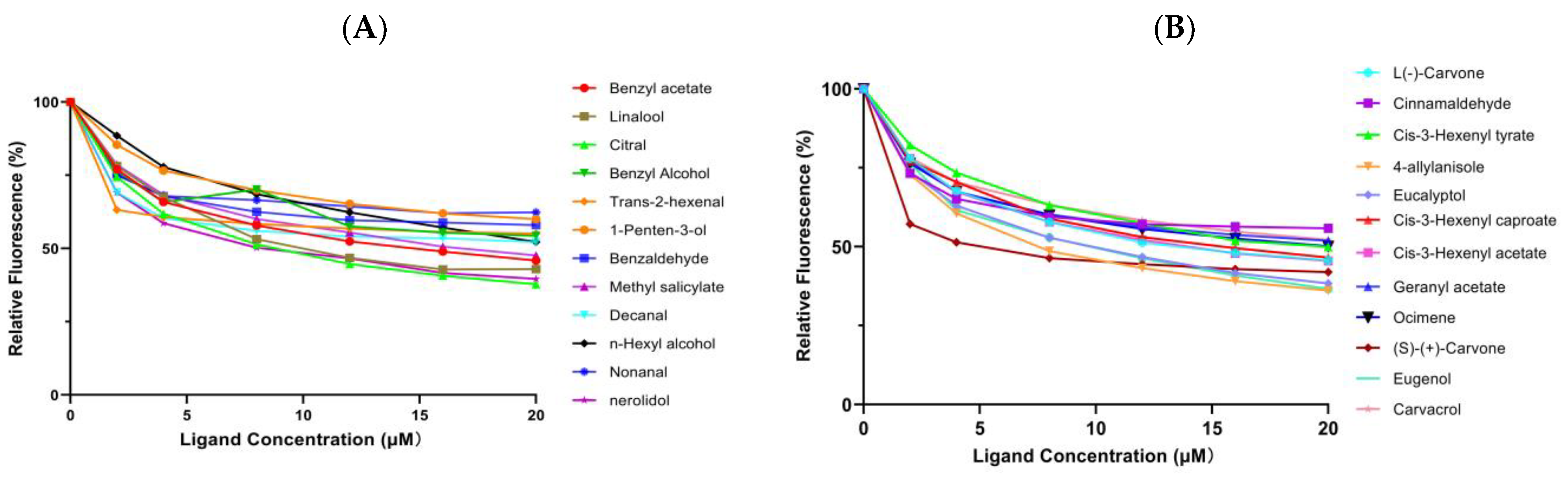
2.6. Fluorescence Competitive Binding Analyses of EgriGOBP2
3. Discussion
4. Materials and Methods
4.1. Insect Rearing, Sample Collection, and RNA Extraction
4.2. Identificantion and Analysis of EgriOBPs
4.3. Quantitative Real-Time PCR (qRT-PCR) Assay and Data Analysis
4.4. Expression and Purification of Recombinant EgriOBPs
4.5. Fluorescence Competitive Binding Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the Odor World: Olfactory Receptors and Their Role for Signal Transduction in Insects. Cell Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Benton, R. Molecular Mechanisms of Olfactory Detection in Insects: Beyond Receptors. Open Biol. 2020, 10, 200252. [Google Scholar] [CrossRef] [PubMed]
- Rihani, K.; Ferveur, J.F.; Briand, L. The 40-year Mystery of Insect Odorant-binding Proteins. Biomolecules 2021, 11, 509. [Google Scholar] [CrossRef]
- Tian, J.H.; Zhan, H.X.; Dewer, Y.; Zhang, B.Y.; Qu, C.; Luo, C.; Li, F.; Yang, S. Whitefly Network Analysis Reveals Gene Modules Involved in Host Plant Selection, Development and Evolution. Front. Physiol. 2021, 12, 656649. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Xiao, S.; Carlson, J.R. The Diverse Small Proteins Called Odorant-binding Proteins. Open Biol. 2018, 8, 180208. [Google Scholar] [CrossRef]
- Zhan, Y.D.; Zhang, J.H.; Xu, M.X.; Francis, F.; Liu, Y. Pheromone-Binding Protein 1 Performs a Dual Function for Intra- and Intersexual Signaling in a Moth. Int. J. Mol. Sci. 2024, 25, 13125. [Google Scholar] [CrossRef]
- Guo, J.L.; Liu, P.J.; Zhang, X.F.; An, J.J.; Li, Y.F.; Zhang, T.; Gao, Z.L. Characterization of the Ligand Binding Properties of Odorant-binding Protein 38 from Riptortus pedestris when Interacting with Soybean Volatiles. Front. Physiol. 2025, 15, 1475489. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Q.; Zhang, Y.X.; Tu, X.H.; Yan, Y.T.; Wang, Q.; Dong, K.; Zhang, Y.J.; Xiao, Q. The Sensilla Trichodea-biased EoblPBP1 Binds Sex Pheromones and Green Leaf Volatiles in Ectropis obliqua Prout, a Geometrid Moth Pest That Uses Type-II Sex Pheromones. J. Insect Physiol. 2019, 116, 17–24. [Google Scholar] [CrossRef]
- Khuhro, S.A.; Liao, H.; Dong, X.T.; Yu, Q.; Yan, Q.; Dong, S.L. Two General Odorant Binding Proteins Display High Bindings to Both Host Plant Volatiles and Sex Pheromones in a Pyralid Moth Chilo suppressalis (Lepidoptera: Pyralidae). J. Asia Pac. Entomol. 2017, 20, 521–528. [Google Scholar] [CrossRef]
- Vogt, R.G.; Riddiford, L.M. Pheromone Binding and Inactivation by Moth Antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef]
- Yao, R.X.; Zhao, M.M.; Zhong, L.; Li, Y.; Li, D.Z.; Deng, Z.N.; Ma, X.F. Characterization of the Binding Ability of the Odorant Binding Protein BminOBP9 of Bactrocera minax to Citrus Volatiles. Pest Manag. Sci. 2020, 77, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.D.; Liu, J.M.; Liu, L.H.; Yu, Y.H.; Zhao, H.Y.; Lu, W. De Novo Transcriptome Identifies Olfactory Genes in Diachasmimorpha longicaudata (Ashmead). Genes 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Q.; Song, Z.Y.; Dong, J.F.; Lv, Q.H.; Chen, Q.X.; Sun, H.Z. Identifcation and Comparative Expression Analysis of Odorant-binding Proteins in the Reproductive System and Antennae of Athetis dissimilis. Sci. Rep. 2021, 11, 13941. [Google Scholar]
- Gao, Y.Q.; Chen, Z.Z.; Liu, M.Y.; Song, C.Y.; Jia, Z.F.; Liu, F.H.; Qu, C.; Dewer, Y.; Zhao, H.P.; Xu, Y.Y.; et al. Characterization of Antennal Chemosensilla and Associated Chemosensory Genes in the Orange Spiny Whitefly, Aleurocanthus spiniferus (Quaintanca). Front. Physiol. 2022, 13, 847895. [Google Scholar] [CrossRef]
- Cui, X.N.; Liu, D.G.; Sun, K.K.; He, Y.; Shi, X.Q. Expression Profiles and Functional Characterization of Two Odorant-binding Proteins from the Apple Buprestid Beetle Agrilus mali (Coleoptera: Buprestidae). J. Econ. Entomol. 2018, 111, 1420–1432. [Google Scholar] [CrossRef]
- Chen, Z.M.; Zhou, L.; Yang, M.; Luo, F.J.; Lou, Z.Y.; Zhang, X.Z.; Sun, H.Z.; Wang, X.R. Index Design and Safety Evaluation of Pesticides Application based on a Fuzzy AHP Model for Beverage Crops: Tea as a Casestudy. Pest Manag. Sci. 2020, 75, 520–526. [Google Scholar] [CrossRef]
- Li, X.; Li, J.W.; Sun, W.X.; Li, W.; Gao, H.Y.; Liu, T.X.; Qu, M.J. Candidate Chemosensory Genes Identified in the Adult Antennae of Sympiezomias velatus and Binding Property of Odorant-binding Protein 15. Front. Physiol. 2022, 13, 907667. [Google Scholar] [CrossRef]
- Diallo, S.; Shahbaaz, M.; Makwatta, J.O.; Muema, J.M.; Masiga, D.; Christofells, A.; Getahun, M.N. Antennal Enriched Odorant Binding Proteins are Required for Odor Communication in Glossina f. fuscipes. Biomolecules 2021, 11, 541. [Google Scholar] [CrossRef]
- Scheuermann, E.A.; Smith, D.P. Odor-specific Deactivation Defects in a Drosophila odorant-binding Protein Mutant. Genetics 2019, 213, 897–909. [Google Scholar] [CrossRef]
- Cranham, J.E. Tea Pests and Their Control. Annu. Rev. Entomol. 1966, 11, 491–514. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhou, H.; Zhao, J. Potential Climate-suitable Distribution of Ectropis grisescens in China based on the CLIMEX and ArcGIS Prediction. Chin. J. Tea Sci. 2020, 40, 817–829. [Google Scholar]
- Pan, Y.J.; Fang, G.Q.; Wang, Z.B.; Cao, Y.H.; Liu, Y.J.; Li, G.Y.; Liu, X.J.; Xiao, Q.; Zhan, S. Chromosome-level Genome Reference and Genome Editing of the Tea Geometrid. Mol. Ecol. Resour. 2021, 21, 2034–2049. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, S.X.; Xue, D.Y.; Tang, M.J.; Xiao, Q.; Han, H.X. External Morphology and Molecular Identification of Two Tea Geometrid Moth From Southern China. Chin. J. Appl. Entomol. 2014, 51, 987–1002. [Google Scholar]
- Gao, T.; Zhang, Y.; Sun, W.P.; Li, Q.K.; Huang, X.Y.; Zhi, D.; Zi, H.B.; Ji, R.J.; Long, Y.H.; Gong, C.M.; et al. The Symbiont Wolbachia Increases Resistance to Bifenthrin in Ectropis grisescens by Regulating the Host Detoxification Function. Ecotoxicol. Environ. Saf. 2025, 289, 117666. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, Y.; Wei, P.L.; Qu, C.; Luo, C. Molecular and Functional Characterization of One Odorant-binding Protein Gene OBP3 in Bemisia tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2020, 113, 299–305. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, G.W.; Xu, X.L.; Wu, J.X. Molecular and Functional Characterization of Odorant Binding Protein 7 from the Oriental Fruit Moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Front. Physiol. 2018, 9, 1762. [Google Scholar] [CrossRef]
- Dong, Y.H.; Li, T.; Liu, J.; Sun, M.X.; Chen, X.Y.; Liu, Y.J.; Xu, P.J. Sex- and Stage-dependent Expression Patterns of Odorant Binding and Chemosensory Protein Genes in Spodoptera exempta. PeerJ 2021, 9, e12132. [Google Scholar] [CrossRef]
- Ma, T.; Xiao, Q.; Yu, Y.G.; Wang, C.; Zhu, C.Q.; Sun, Z.H.; Wen, X.J. Analysis of Tea Geometrid (Ectropis grisescens) Pheromone Gland Extracts using GC-EAD and GC×GC/TOFMS. J. Agr. Food Chem. 2016, 64, 3161–3166. [Google Scholar] [CrossRef]
- Zhang, F.M.; Chen, Y.J.; Zhao, X.C.; Guo, S.B.; Hong, F.; Zhi, Y.N.; Zhang, L.; Zhou, Z.; Zhang, Y.H.; Zhou, X.G.; et al. Antennal Transcriptomic Analysis of Carboxylesterases and Glutathione S-transferases Associated with Odorant Degradation in the Tea Gray Geometrid, Ectropis grisescens (Lepidoptera, Geometridae). Front. Physiol. 2023, 14, 1183610. [Google Scholar] [CrossRef]
- Gu, S.H.; Zhou, J.J.; Gao, S.; Wang, D.H.; Li, X.C.; Guo, Y.Y.; Zhang, Y.J. Identification and Comparative Expression Analysis of Odorant Binding Protein Genes in the Tobacco Cutworm Spodoptera litura. Sci. Rep. 2015, 5, 13800. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.D.; Liu, M.; Li, W.; Liu, M.; Di, S.F.; Yang, S.; Fan, C.; Bai, L.; Lai, Y.C. Identification and Expression Profiles Analysis of Odorant-binding Proteins in Soybean Aphid, Aphis glycines (Hemiptera: Aphididae). Insect Sci. 2020, 27, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, F.Q.; Zhang, W.; Zhang, X.M.; Qu, C.; Tetreau, G.; Sun, L.J.; Luo, C.; Zhou, J. Identification and Expression Profile Analysis of Odorant Binding Protein and Chemosensory Protein Genes in Bemisia tabaci MED by Head Transcriptome. PLoS ONE 2017, 12, e0171739. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wilde, E.; Kuebler, L.S.; Bucks, S.; Vogel, H.; Wicher, D.; Hansson, B.S. Antennal Transcriptome of Manduca sexta. Proc. Natl. Acad. Sci. USA 2011, 108, 7449–7454. [Google Scholar] [CrossRef]
- Li, M.Y.; Jiang, X.Y.; Qi, Y.Z.; Huang, Y.J.; Li, S.G.; Liu, S. Identification and Expression Profiles of 14 Odorant-binding Protein Genes from Pieris rapae (Lepidoptera: Pieridae). J. Insect Sci. 2020, 20, 2. [Google Scholar] [CrossRef]
- Campanini, E.B.; De Brito, R.A. Molecular Evolution of Odorant-binding Proteins Gene Family in Two Closely Related Anastrepha Fruit Flies. BMC Evol. Biol. 2016, 16, 198. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, X.; He, D.F.; Wu, Q.; Tang, R.; Xing, L.S.; Liu, W.X.; Wang, W.K.; Liu, B.; Xi, Y.; et al. Comparative Genomics Provide Insights into Function and Evolution of Odorant Binding Proteins in Cydia pomonella. Front. Physiol. 2021, 12, 690185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Li, W.B.; Tao, J.; Zong, S.X. Antennal Transcriptome Analyses and Olfactory Protein Identification in an Important Wood-boring Moth Pest, Streltzoviella insularis (Lepidoptera: Cossidae). Sci. Rep. 2019, 9, 17951. [Google Scholar] [CrossRef]
- Liu, N.Y.; Liu, C.C.; Dong, S.L. Functional Differentiation of Pheromone-binding Proteins in the Common Cutworm Spodoptera litura. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 254–262. [Google Scholar] [CrossRef]
- Bruno, D.; Grossi, G.; Salvia, R.; Scala, A.; Farina, D.; Grimaldi, A.; Zhou, J.J.; Bufo, S.A.; Vogel, H.; Grosse-Wilde, E.; et al. Sensilla Morphology and Complex Expression Pattern of Odorant Binding Proteins in the Vetch Aphid Megoura viciae (Hemiptera: Aphididae). Front. Physiol. 2018, 9, 777. [Google Scholar] [CrossRef]
- Gao, K.X.; Zhang, S.; Luo, J.Y.; Wang, C.Y.; Lu, L.M.; Zhang, L.J.; Zhu, X.Z.; Wang, L.; Cui, J.J. Molecular Characterization and Ligand Binding Properties of Six Odorant Binding Proteins (OBPs) from Aphis gossypii. J. Asia-Pac. Entomol. 2018, 21, 914–925. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hu, Y.W.; Bi, J.; Kong, X.T.; Long, G.Y.; Zheng, Y.; Liu, K.Y.; Wang, Y.F.; Xu, H.L.; Guan, C.X.; et al. Odorant-binding Proteins Involved in Sex Pheromone and Host-plant Recognition of the Sugarcane Borer Chilo infuscatellus (Lepidoptera: Crambidae). Pest Manag. Sci. 2020, 76, 4064–4076. [Google Scholar] [CrossRef]
- Sun, M.; Liu, Y.; Wang, G. Expression Patterns and Binding Properties of Three Pheromone Binding Proteins in the Diamondback Moth, Plutella xyllotella. J. Insect Physiol. 2013, 59, 46–55. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Q.; Wang, Q.; Dong, K.; Xiao, Y.; Zhang, Y.J. Identification and Characterization of Odorant Binding Proteins in the Forelegs of Adelphocoris lineolatus (Goeze). Front. Physiol. 2017, 8, 735. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Xu, K.; Ma, W.H.; Su, W.T.; Tai, M.M.; Zhao, H.T.; Jiang, Y.S.; Li, X.C. Contact Chemosensory Genes Identified in Leg Transcriptome of Apis cerana Cerana (Hymenoptera: Apidae). J. Econ. Entomol. 2019, 112, 2015–2029. [Google Scholar] [CrossRef] [PubMed]
- Frederick, L.; Anupama, D.; Linnea, A.W.; Jae Young, K.; John, R.C. The Molecular and Cellular Basis of Taste Coding in the Legs of Drosophila. J. Neurosci. 2014, 34, 7148. [Google Scholar]
- Wang, S.; Minter, M.; Homem, R.A.; Michaelson, L.V.; Venthur, H.; Lim, K.S.; Withers, A.; Xi, J.; Jones, C.M.; Zhou, J.J. Odorant Binding Proteins Promote Flight Activity in the Migratory Insect, Helicoverpa armigera. Mol. Ecol. 2020, 29, 3795–3808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, C.; Fu, G.; Tang, R.; Yang, N.; Liu, W.; Qian, W.; Wan, F. Molecular and Functional Characterization of Three General Odorant-Binding Protein 2 Genes in Cydia pomonella (Lepidoptera: Tortricidae). Int. J. Mol. Sci. 2024, 25, 1746. [Google Scholar] [CrossRef]
- Tu, J.J.; Wang, Z.H.; Yang, F.; Liu, H.; Qiao, G.H.; Zhang, A.H.; Wang, S.N. The Female-biased General Odorant Binding Protein 2 of Semiothisa cinerearia Displays Binding Affinity for Biologically Active Host Plant Volatiles. Biology 2024, 13, 274. [Google Scholar] [CrossRef]
- Huang, X.D.; Xiao, T.X.; Deng, M.Q.; Zhao, X.Y.; Wang, W.X.; Li, J.; Xu, X.Y.; Yang, Z.M.; Sun, Z.X.; Lu, K. Binding properties of the general odorant-binding protein GOBP2 to herbicides and insecticides in Spodoptera litura. J. Agric. Food Chem. 2025, 73, 3977–3989. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Fu, X.B.; Cui, H.C.; Zhao, L.; Yu, J.Z.; Li, H.L. Functional Characteristics, Electrophysiological and Antennal Immunolocalization of General Odorant-binding Protein 2 in Tea Geometrid, Ectropis obliqua. Int. J. Mol. Sci. 2018, 19, 875. [Google Scholar] [CrossRef]
- Liu, X.L.; Wu, Z.R.; Liao, W.; Zhang, X.Q.; Pei, Y.W.; Lu, M. The Binding Affinity of Two General Odorant Binding Proteins in Spodoptera frugiperda to General Volatiles and Insecticides. Int. J. Biol. Macromol. 2023, 252, 126338. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.F.; Wang, K.; Sun, Y.L.; Tian, C.H.; Wang, S.L. Antennal Transcriptome Analysis of Odorant-binding Proteins and Characterization of GOBP2 in the Variegated Cutworm Peridroma saucia. Front Physiol. 2023, 14, 1241324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Zhang, Z.J.; Hafeez, M.; Huang, J.; Zhang, J.M.; Wang, L.K.; Lu, Y.B. Rosmarinus officinialis L. (Lamiales: Lamiaceae), a Promising Repellent Plant for Thrips Management. J. Econ. Entomol. 2021, 114, 131–141. [Google Scholar] [CrossRef]
- Rosa, J.S.; Oliveira, L.; Sousa, R.M.O.F.; Escobar, C.B.; Fernandes-Ferreira, M. Bioactivity of some Apiaceae Essential Oils and Their Constituents against Sitophilus zeamais (Coleoptera: Curculionidae). Bull. Entomol. Res. 2020, 110, 406–416. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.R.M.; Ricci-Júnior, E. An Approach to Natural Insect Repellent Formulations: From Basic Research to Technological Development. Acta Trop. 2020, 212, 105419. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, X.K.; Zhang, L.; Chen, W.Y.; Lyu, Q.J.; Du, S.S. The Essential Oil of Kochia scoparia (L.) Schrad. as a Potential Repellent Against Stored-Product Insects. Environ. Sci. Pollut. Res. Int. 2023, 30, 24416–124424. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, S.; Luo, J.Y.; Wang, S.B.; Dong, S.L.; Cui, J.J. Odorant-binding Proteins Display High Affinities for Behavioral Attractants and Repellents in the Natural Predator Chrysopa pallens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 185, 51–57. [Google Scholar] [CrossRef]
- Liggri, P.; Tsitsanou, K.; Stamati, E.C.V.; Saitta, F.; Drakou, C.E.; Leonidas, D.D.; Fessas, D.; Zographos, S.E. The Structure of AgamOBP5 in Complex with the Natural Insect Repellents Carvacrol and Thymol: Crystallographic, Fuorescence and Thermodynamic Binding Studies. Int. J. Biol. Macromol. 2023, 237, 124009. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Schneidmiller, R.G.; Hoover, D.R.; Zhou, G.J.; Margaryan, A.; Bryant, P. Essential Oils as Spatial Repellents for the Brown Marmorated Stinkbug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). J. Appl. Entomol. 2014, 138, 490–499. [Google Scholar] [CrossRef]
- Baley, T.L.; Elkan, C. Fitting a Mixture Model by Expectation Maximization to Discover Motifs in Biopolymers. In Proceedings of the 2nd International Conference on Intelligent Systems for Molecular Biology, Stanford, CA, USA, 14–17 August 1994; Volume 2, pp. 28–36. [Google Scholar]
- Zhai, Y.Y.; Zhang, F.; Tian, T.Q.; Yang, Y.W.; Li, Y.; Ren, B.W.; Hong, B. The Sequence Characteristics and Binding Properties of the Odorant-binding Protein SvelOBP1 from Sympiezomias velatus (Coleoptera: Curculionidae) to Jujube Volatiles. Life 2024, 14, 192. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, G.C.; Gao, Y.; Zhang, X.Z.; Xin, Z.J.; Chen, Z.M. Volatiles Emitted from Tea Plants Infested by Ectropis obliqua Larvae are Attractive to Conspecific Moths. J. Chem. Ecol. 2014, 40, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Schneidmiller, R.; Hoover, D. Essential Oils and Their Compositions as Spatial Repellents for Pestiferous Social Wasps. Pest Manag. Sci. 2013, 69, 542–552. [Google Scholar] [CrossRef] [PubMed]
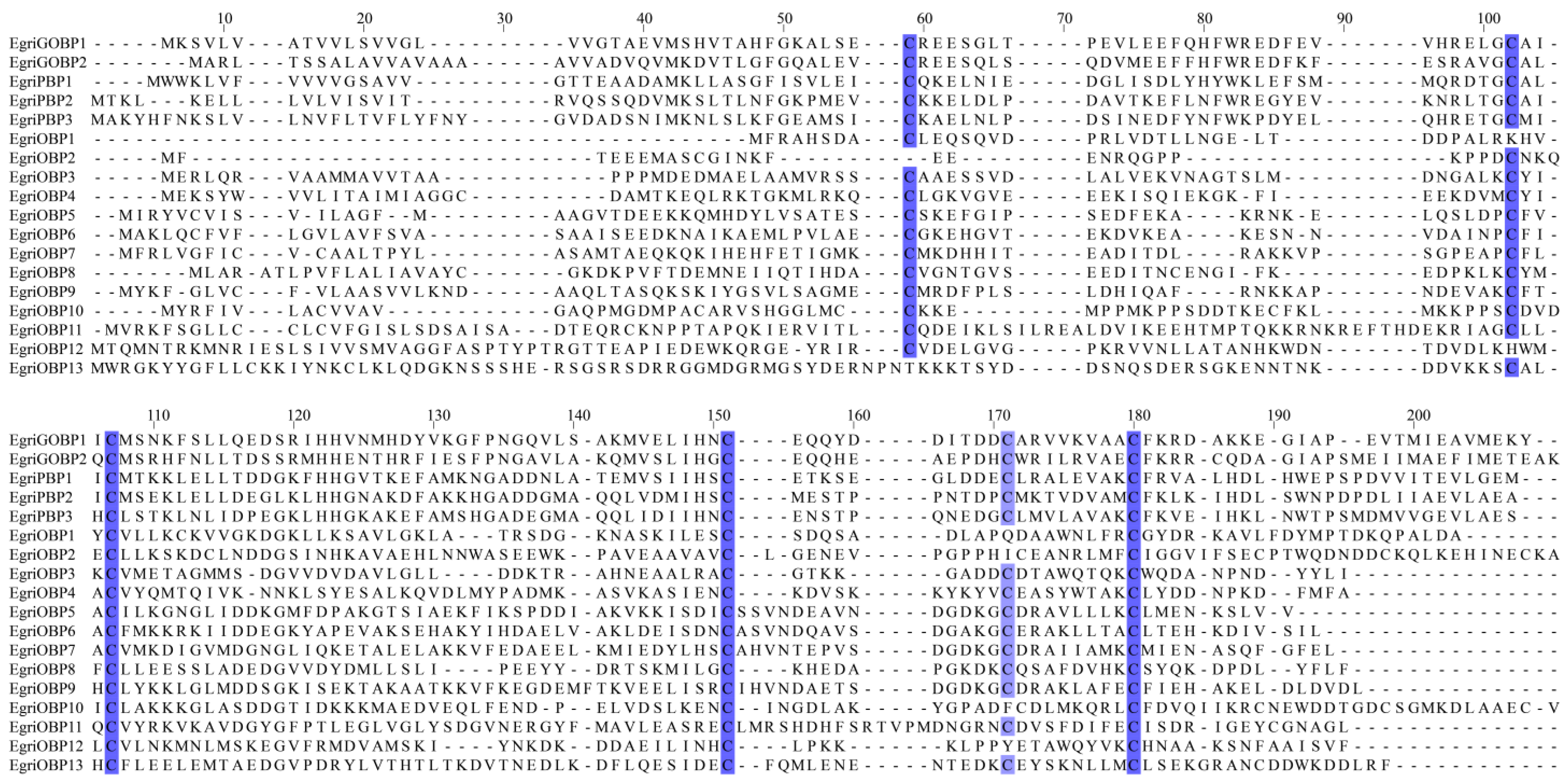
| Gene Name | Accession Number | Length (bp) | ORF (aa) | Blastx Best Hit (Name and Species) | Accession Number | E Value | Identity (%) | Signal Peptide | pI | Mw (kDa) |
|---|---|---|---|---|---|---|---|---|---|---|
| EgriGOBP1 | ON380526 | 504 | 160 | general odorant-binding protein 2 [Ectropis obliqua] | ACN29681.1 | 2 × 10−93 | 100.00 | NO | 5.8 | 19.0 |
| EgriGOBP2 | ON380510 | 591 | 214 | general odorant-binding protein 1 [Ectropis obliqua] | ACN29680.1 | 4 × 10−92 | 98.58 | YES | 5.9 | 22.2 |
| EgriPBP1 | ON380520 | 585 | 162 | pheromone-binding protein 3 [Ectropis obliqua] | ALS03849.1 | 1 × 10−91 | 100.00 | NO | 4.9 | 21.7 |
| EgriPBP2 | ON380512 | 540 | 163 | pheromone-binding protein 2 [Ectropis obliqua] | ALS03848.1 | 2 × 10−89 | 100.00 | NO | 5.3 | 19.9 |
| EgriPBP3 | ON380514 | 555 | 170 | pheromone-binding protein 4 [Ectropis obliqua] | ALS03850.1 | 9 × 10−119 | 100.00 | NO | 6.1 | 20.8 |
| EgriOBP1 | ON380521 | 417 | 120 | odorant-binding protein [Semiothisa cinerearia] | QRF70927.1 | 2 × 10−72 | 79.41 | NO | 8.3 | 15.0 |
| EgriOBP2 | ON380524 | 540 | 134 | odorant-binding protein 10 [Ectropis obliqua] | ALS03858.1 | 5 × 10−106 | 100.00 | YES | 5.1 | 20.0 |
| EgriOBP3 | ON380515 | 426 | 138 | odorant-binding protein [Semiothisa cinerearia] | QRF70921.1 | 3 × 10−43 | 81.58 | NO | 4.4 | 15.1 |
| EgriOBP4 | ON380517 | 441 | 142 | odorant-binding protein 11 [Ectropis obliqua] | ALS03859.1 | 5 × 10−92 | 99.29 | YES | 8.6 | 16.8 |
| EgriOBP5 | ON380527 | 447 | 145 | odorant-binding protein 18 [Ectropis obliqua] | ALS03866.1 | 3 × 10−90 | 98.62 | NO | 5.5 | 16.2 |
| EgriOBP6 | ON380523 | 456 | 150 | odorant-binding protein 6 [Ectropis obliqua] | ALS03854.1 | 8 × 10−92 | 98.65 | NO | 5.3 | 16.4 |
| EgriOBP7 | ON380525 | 465 | 151 | odorant-binding protein 14 [Ectropis obliqua] | ALS03862.1 | 2 × 10−105 | 100.00 | NO | 5.3 | 17.0 |
| EgriOBP8 | ON380513 | 477 | 156 | odorant-binding protein 9 [Ectropis obliqua] | ALS03857.1 | 3 × 10−96 | 99.29 | NO | 4.4 | 17.5 |
| EgriOBP9 | ON380518 | 510 | 160 | odorant-binding protein 17 [Ectropis obliqua] | ALS03865.1 | 7 × 10−93 | 100.00 | NO | 7.0 | 18.9 |
| EgriOBP10 | ON380511 | 498 | 163 | odorant-binding protein OBP47 [Lobesia botrana] | AXF48744.1 | 6 × 10−32 | 52.17 | NO | 5.1 | 18.3 |
| EgriOBP11 | ON380516 | 576 | 184 | odorant-binding protein 4 [Ectropis obliqua] | ALS03852.1 | 2 × 10−125 | 100.00 | YES | 6.5 | 21.5 |
| EgriOBP12 | ON380519 | 675 | 210 | odorant-binding protein 18 [Dendrolimus punctatus] | ARO70177.1 | 7 × 10−20 | 40.50 | NO | 8.4 | 25.9 |
| EgriOBP13 | ON380522 | 999 | 331 | odorant-binding protein [Semiothisa cinerearia] | QRF70922.1 | 1 × 10−104 | 75.74 | NO | 5.5 | 38.7 |
| Ligands | Formula | CAS No# | Purity (%) | EgriGOBP2 | ||
|---|---|---|---|---|---|---|
| IC50 (µM) | Ki (µM) | |||||
| Host volatiles | Benzyl acetate | C9H10O2 | 140-11-4 | 99.00 | 14.35 | 12.95 |
| Linalool | C10H18O | 78-70-6 | 97.00 | 11.92 | 10.76 | |
| Citral | C10H16O | 5392-40-5 | 95.00 | 9.12 | 8.23 | |
| Benzyl alcohol | C7H8O | 100-51-6 | 99.00 | 29.97 | 27.05 | |
| E-2-hexenal | C10H16O | 6728-26-3 | 98.00 | - | - | |
| 1-penten-3-ol | C5H10O | 616-25-1 | 99.00 | 44.84 | 40.57 | |
| Benzaldehyde | C7H6O | 100-52-7 | 99.50 | - | - | |
| Methyl salicylate | C8H8O3 | 119-36-8 | 98.00 | 17.27 | 15.59 | |
| Decanal | C10H20O | 112-31-2 | 98.00 | - | - | |
| n-hexyl alcohol | C6H14O | 111-27-3 | 98.00 | 23.48 | 21.20 | |
| Nonanal | C9H18O | 124-19-6 | 95.00 | - | - | |
| Nerolidol | C15H26O | 7212-44-4 | 98.00 | 8.79 | 7.93 | |
| Plant essential oil-derived volatiles | Cinnamaldehyde | C9H8O | 104-55-2 | 95.00 | - | - |
| Cis-3-hexenyl tyrate | C10H18O2 | 16491-36-4 | 95.00 | 20.63 | 18.62 | |
| 4-allylanisole | C10H12O | 140-67-0 | 98.00 | 8.10 | 7.31 | |
| Eucalyptol | C10H18O | 406-67-7 | 95.00 | 9.36 | 8.61 | |
| Cis-3-hexenyl caproate | C12H22O2 | 31501-11-8 | 99.00 | 15.50 | 13.99 | |
| Cis-3-hexenyl acetate | C8H14O2 | 3681-71-8 | 98.00 | 14.48 | 13.07 | |
| Geranyl acetate | C12H20O2 | 105-87-3 | 97.00 | 24.72 | 22.31 | |
| Ocimene | C10H16 | 13877-91-3 | 90.00 | 19.92 | 17.98 | |
| (S)-(+)-carvone | C10H14O | 2244-16-8 | 98.00 | 5.36 | 4.84 | |
| Eugenol | C10H12O2 | 97-53-0 | 99.00 | 9.28 | 8.38 | |
| Carvacrol | C10H14O | 499-75-2 | 98.00 | 23.58 | 21.29 | |
| L(-)-carvone | C10H14O | 6485-40-1 | 99.00 | 12.87 | 11.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Sun, H.; Geng, S.; Guo, S.; Zhou, Z.; Shi, H.; Zhou, X.; Li, X. Molecular Characterization and Functional Analysis of Odorant-Binding Proteins in Ectropis grisescens. Int. J. Mol. Sci. 2025, 26, 4568. https://doi.org/10.3390/ijms26104568
Zhang F, Sun H, Geng S, Guo S, Zhou Z, Shi H, Zhou X, Li X. Molecular Characterization and Functional Analysis of Odorant-Binding Proteins in Ectropis grisescens. International Journal of Molecular Sciences. 2025; 26(10):4568. https://doi.org/10.3390/ijms26104568
Chicago/Turabian StyleZhang, Fangmei, Haohan Sun, Shubao Geng, Shibao Guo, Zhou Zhou, Hongzhong Shi, Xuguo Zhou, and Xiangrui Li. 2025. "Molecular Characterization and Functional Analysis of Odorant-Binding Proteins in Ectropis grisescens" International Journal of Molecular Sciences 26, no. 10: 4568. https://doi.org/10.3390/ijms26104568
APA StyleZhang, F., Sun, H., Geng, S., Guo, S., Zhou, Z., Shi, H., Zhou, X., & Li, X. (2025). Molecular Characterization and Functional Analysis of Odorant-Binding Proteins in Ectropis grisescens. International Journal of Molecular Sciences, 26(10), 4568. https://doi.org/10.3390/ijms26104568








