Therapeutic Applications of Poly-miRNAs and miRNA Sponges
Abstract
1. Introduction
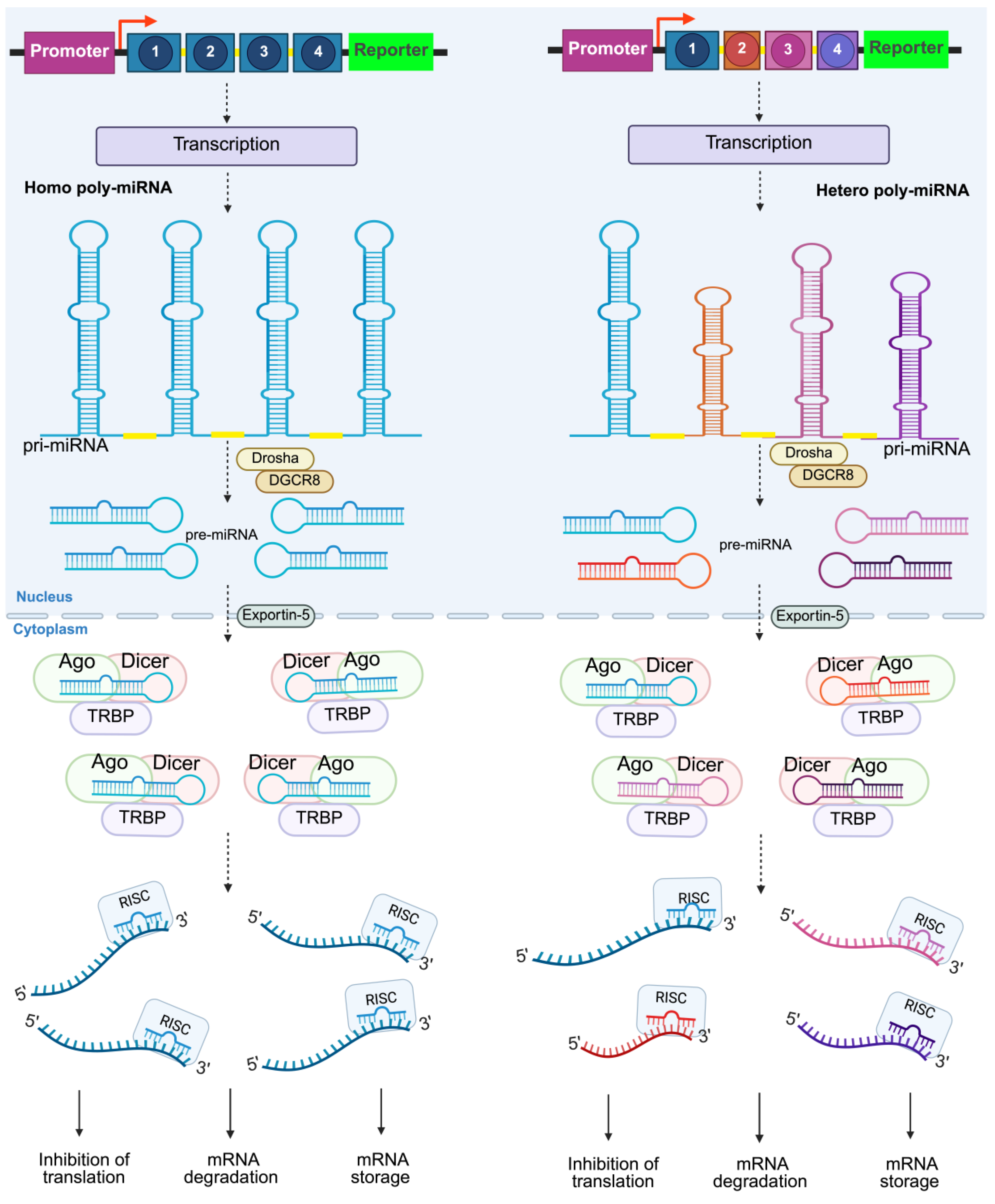
2. Overview of Synthetic Poly-miRNAs
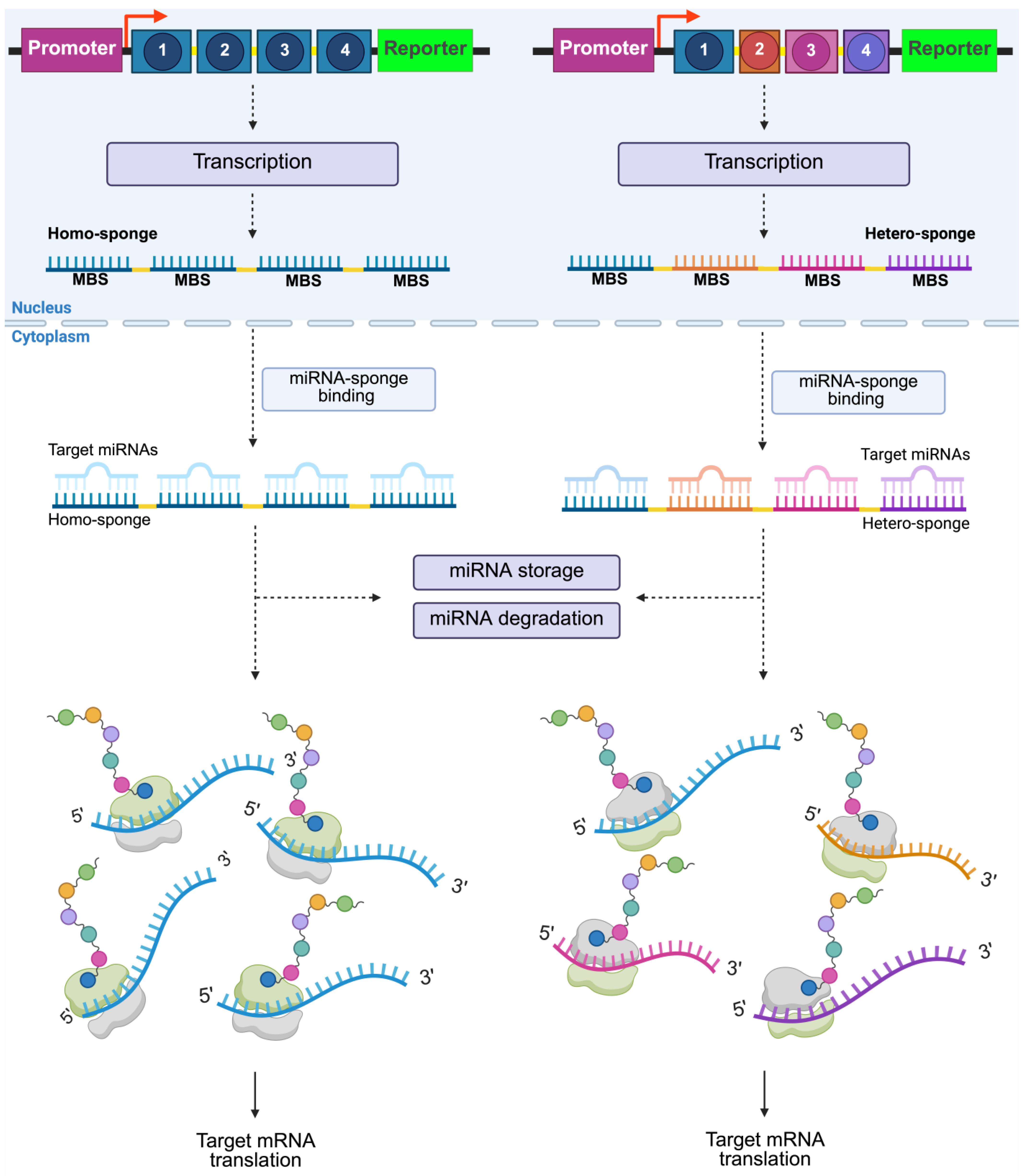
3. Overview of Synthetic miRNA Sponges
4. Applications in Cardiovascular Diseases
5. Role in Metabolic Disorders
5.1. Role of miRNAs and Applications in Metabolic Disorders
5.2. Role in Obesity
5.3. Role in Diabetes and Insulin Resistance
5.4. Role in Dyslipidemia
6. Applications in Inflammatory and Autoimmune Diseases
7. Cancer Therapeutics
8. Targeted Delivery Approaches
9. Challenges and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RNA | Ribo nucleic acid |
| mRNA | Messenger RNA |
| miRNA | MicroRNA |
| Pol | Polymerase |
| Pri-miRNA | Primary micro-RNA |
| Pre-miRNA | Precursor micro-RNA |
| NCBI | National Center for Biotechnology Information |
| siRNA | Small interfering RNA |
| shRNA | Short hairpin RNA |
| ncRNA | Non-coding RNA |
| ceRNA | Competing endogenous RNA |
| lncRNAs | Long non-coding RNA |
| circRNAs | Circular RNA |
| AGO | Argonaute |
| XPO5 | Exportin 5 |
| RISC | RNA-induced silencing complex |
| TNRC6 | Trinucleotide repeat-containing gene 6A protein |
| Poly-miR | Polycistronic miRNA |
| MBS | Multi-binding site |
| CVD | Cardiovascular disease |
| DGCR8 | DiGeorge syndrome critical region 8 protein |
| SRSF | Serine/arginine-rich splicing factor |
| ERH | Enhancer of rudimentary homolog |
| KO | Knock out |
| HFD | High-fat diet |
| AAV | Adeno-associated virus |
| BIC | B-cell integration cluster |
| MASLD | Metabolic dysfunction-associated liver disease |
| AgRP | Agouti-related peptide |
| IR | Insulin-resistant |
| T2DM | Type 2 diabetes |
| GLUT4 | Glucose transporter type 4 |
| LDL-Ch | Low-density lipoprotein cholesterol |
| HDL-Ch | High-density lipoprotein cholesterol |
| NF-κβ | Nuclear factor kappa B |
| SLE | Systemic lupus erythematosus |
| IBD | Inflammatory bowel disease |
| HCV | Hepatitis C virus |
| EMT | Epithelial–mesenchymal transition |
| HCC | Hepatocellular carcinoma |
| LNP | Lipid nanoparticle |
References
- Bernardo, B.C.; Gregorevic, P.; Ritchie, R.H.; McMullen, J.R. Generation of MicroRNA-34 Sponges and Tough Decoys for the Heart: Developments and Challenges. Front. Pharmacol. 2018, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Huang, Z. Recent Progress in microRNA-Based Delivery Systems for the Treatment of Human Disease. ExRNA 2019, 1, 24. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. miRNA Biogenesis and Regulation of Diseases: An Updated Overview. In MicroRNA Profiling: Methods and Protocols; Rani, S., Ed.; Springer: New York, NY, USA, 2023; pp. 1–12. ISBN 978-1-0716-2823-2. [Google Scholar]
- Ambros, V. The Functions of Animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bofill-De Ros, X.; Vang Ørom, U.A. Recent Progress in miRNA Biogenesis and Decay. RNA Biol. 2024, 21, 36–43. [Google Scholar] [CrossRef]
- Ambros, V. microRNAs: Tiny Regulators with Great Potential. Cell 2001, 107, 823–826. [Google Scholar] [CrossRef]
- Castanotto, D.; Rossi, J.J. The Promises and Pitfalls of RNA-Interference-Based Therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef]
- Kotowska-Zimmer, A.; Pewinska, M.; Olejniczak, M. Artificial miRNAs as Therapeutic Tools: Challenges and Opportunities. WIREs RNA 2021, 12, e1640. [Google Scholar] [CrossRef]
- McDonald, M.F.; Hossain, A.; Momin, E.N.; Hasan, I.; Singh, S.; Adachi, S.; Gumin, J.; Ledbetter, D.; Yang, J.; Long, L.; et al. Tumor-Specific Polycistronic miRNA Delivered by Engineered Exosomes for the Treatment of Glioblastoma. Neuro Oncol. 2023, 26, 236–250. [Google Scholar] [CrossRef]
- Altuvia, Y.; Landgraf, P.; Lithwick, G.; Elefant, N.; Pfeffer, S.; Aravin, A.; Brownstein, M.J.; Tuschl, T.; Margalit, H. Clustering and Conservation Patterns of Human microRNAs. Nucleic Acids Res. 2005, 33, 2697–2706. [Google Scholar] [CrossRef]
- Baskerville, S.; Bartel, D.P. Microarray Profiling of microRNAs Reveals Frequent Coexpression with Neighboring miRNAs and Host Genes. RNA 2005, 11, 241–247. [Google Scholar] [CrossRef]
- Wang, T.; Xie, Y.; Tan, A.; Li, S.; Xie, Z. Construction and Characterization of a Synthetic MicroRNA Cluster for Multiplex RNA Interference in Mammalian Cells. ACS Synth. Biol. 2016, 5, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Kluiver, J.; Gibcus, J.H.; Hettinga, C.; Adema, A.; Richter, M.K.S.; Halsema, N.; Slezak-Prochazka, I.; Ding, Y.; Kroesen, B.-J.; Berg, A. van den Rapid Generation of MicroRNA Sponges for MicroRNA Inhibition. PLoS ONE 2012, 7, e29275. [Google Scholar] [CrossRef]
- Rama, A.R.; Perazzoli, G.; Cabeza, L.; Mesas, C.; Quiñonero, F.; García-Pinel, B.; Vélez, C. Novel MicroRNA Sponges to Specifically Modulate Gene Expression in Colon Cancer Cells. Nucleic Acid Ther. 2020, 30, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Montaño-Samaniego, M.; Sánchez-Cedillo, J.; Lucas-González, A.; Bravo-Estupiñan, D.M.; Alarcón-Hernández, E.; Rivera-Gutiérrez, S.; Balderas-López, J.A.; Ibáñez-Hernández, M. Targeted Expression to Liver of an antimiR-33 Sponge as a Gene Therapy Strategy against Hypercholesterolemia: In Vitro Study. Curr. Issues Mol. Biol. 2023, 45, 7043–7057. [Google Scholar] [CrossRef]
- Rad, S.M.A.H.; Halpin, J.C.; Tawinwung, S.; Suppipat, K.; Hirankarn, N.; McLellan, A.D. MicroRNA-Mediated Metabolic Reprogramming of Chimeric Antigen Receptor T Cells. Immunol. Cell Biol. 2022, 100, 424–439. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Comeau, D.C.; Connor, R.; DiCuccio, M.; Farrell, C.M.; Feldgarden, M.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2023, 52, D33–D43. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Melegari, M.; Sridhar, S.; Rogler, C.E.; Zhu, L. Multi-miRNA Hairpin Method That Improves Gene Knockdown Efficiency and Provides Linked Multi-Gene Knockdown. BioTechniques 2006, 41, 59–63. [Google Scholar] [CrossRef]
- Bhaskaran, V.; Nowicki, M.O.; Idriss, M.; Jimenez, M.A.; Lugli, G.; Hayes, J.L.; Mahmoud, A.B.; Zane, R.E.; Passaro, C.; Ligon, K.L.; et al. The Functional Synergism of microRNA Clustering Provides Therapeutically Relevant Epigenetic Interference in Glioblastoma. Nat. Commun. 2019, 10, 442. [Google Scholar] [CrossRef]
- Pushparaj, P.N.; Aarthi, J.J.; Manikandan, J.; Kumar, S.D. siRNA, miRNA, and shRNA: In Vivo Applications. J. Dent. Res. 2008, 87, 992–1003. [Google Scholar] [CrossRef]
- Cui, S.; Yu, S.; Huang, H.-Y.; Lin, Y.-C.-D.; Huang, Y.; Zhang, B.; Xiao, J.; Zuo, H.; Wang, J.; Li, Z.; et al. miRTarBase 2025: Updates to the Collection of Experimentally Validated microRNA–Target Interactions. Nucleic Acids Res. 2025, 53, D147–D156. [Google Scholar] [CrossRef] [PubMed]
- What Will It Take to Get miRNA Therapies to Market? [editorial]. Nat. Biotechnol. 2024, 42, 1623–1624. [CrossRef] [PubMed]
- Bhaskaran, V.; Yao, Y.; Bei, F.; Peruzzi, P. Engineering, Delivery, and Biological Validation of Artificial microRNA Clusters for Gene Therapy Applications. Nat. Protoc. 2019, 14, 3538–3553. [Google Scholar] [CrossRef]
- Auyeung, V.C.; Ulitsky, I.; McGeary, S.E.; Bartel, D.P. Beyond Secondary Structure: Primary-Sequence Determinants License Pri-miRNA Hairpins for Processing. Cell 2013, 152, 844–858. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I Study of MRX34, a Liposomal miR-34a Mimic, Administered Twice Weekly in Patients with Advanced Solid Tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of microRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Askou, A.L.; Aagaard, L.; Kostic, C.; Arsenijevic, Y.; Hollensen, A.K.; Bek, T.; Jensen, T.G.; Mikkelsen, J.G.; Corydon, T.J. Multigenic Lentiviral Vectors for Combined and Tissue-Specific Expression of miRNA- and Protein-Based Antiangiogenic Factors. Mol. Ther.-Methods Clin. Dev. 2015, 2, 14064. [Google Scholar] [CrossRef]
- Yang, X.; Marcucci, K.; Anguela, X.; Couto, L.B. Preclinical Evaluation of An Anti-HCV miRNA Cluster for Treatment of HCV Infection. Mol. Ther. 2013, 21, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.-Y.; Stern, P.; Guo, Z.; Chen, J. Expression of Multiple Artificial MicroRNAs from a Chicken miRNA126-Based Lentiviral Vector. PLoS ONE 2011, 6, e22437. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.P.; Haasnoot, J.; ter Brake, O.; Berkhout, B.; Konstantinova, P. Inhibition of HIV-1 by Multiple siRNAs Expressed from a Single microRNA Polycistron. Nucleic Acids Res. 2008, 36, 2811–2824. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kasina, V.; Wahane, A.; Liu, C.-H.; Yang, L.; Nieh, M.-P.; Slack, F.J.; Bahal, R. Next-Generation Poly-L-Histidine Formulations for miRNA Mimic Delivery. Mol. Ther.-Methods Clin. Dev. 2023, 29, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Diener, C.; Keller, A.; Meese, E. The miRNA–Target Interactions: An Underestimated Intricacy. Nucleic Acids Res. 2024, 52, 1544–1557. [Google Scholar] [CrossRef]
- Jie, J.; Liu, D.; Wang, Y.; Wu, Q.; Wu, T.; Fang, R. Generation of MiRNA Sponge Constructs Targeting Multiple MiRNAs. J. Clin. Lab. Anal. 2022, 36, e24527. [Google Scholar] [CrossRef]
- Bak, R.O.; Mikkelsen, J.G. miRNA Sponges: Soaking up miRNAs for Regulation of Gene Expression. Wiley Interdiscip. Rev. RNA 2014, 5, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.C.; Lim, J.K.; Zhu, H.; Hin, L.C.; Wang, S. Using Artificial microRNA Sponges to Achieve microRNA Loss-of-Function in Cancer Cells. Adv. Drug Deliv. Rev. 2015, 81, 117–127. [Google Scholar] [CrossRef]
- Barta, T.; Peskova, L.; Hampl, A. miRNAsong: A Web-Based Tool for Generation and Testing of miRNA Sponge Constructs in Silico. Sci. Rep. 2016, 6, 36625. [Google Scholar] [CrossRef]
- Ortega, M.M.; Bouamar, H. Guidelines on Designing MicroRNA Sponges: From Construction to Stable Cell Line. In MicroRNA Profiling: Methods and Protocols; Rani, S., Ed.; Springer: New York, NY, USA, 2017; pp. 221–233. ISBN 978-1-4939-6524-3. [Google Scholar]
- Ebert, M.S.; Sharp, P.A. MicroRNA Sponges: Progress and Possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef]
- Tang, L.; Chen, H.-Y.; Hao, N.-B.; Tang, B.; Guo, H.; Yong, X.; Dong, H.; Yang, S.-M. microRNA Inhibitors: Natural and Artificial Sequestration of microRNA. Cancer Lett. 2017, 407, 139–147. [Google Scholar] [CrossRef]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA Sponges: Competitive Inhibitors of Small RNAs in Mammalian Cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef]
- Alkan, A.H.; Akgül, B. Endogenous miRNA Sponges. In miRNomics: MicroRNA Biology and Computational Analysis; Allmer, J., Yousef, M., Eds.; Springer: New York, NY, USA, 2022; pp. 91–104. ISBN 978-1-0716-1170-8. [Google Scholar]
- Le, T.D.; Zhang, J.; Liu, L.; Li, J. Computational Methods for Identifying miRNA Sponge Interactions. Brief. Bioinform. 2017, 18, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Mafi, A.; Rahmati, A.; Babaei Aghdam, Z.; Salami, R.; Salami, M.; Vakili, O.; Aghadavod, E. Recent Insights into the microRNA-Dependent Modulation of Gliomas from Pathogenesis to Diagnosis and Treatment. Cell. Mol. Biol. Lett. 2022, 27, 65. [Google Scholar] [CrossRef] [PubMed]
- Witten, L.; Slack, F.J. miR-155 as a Novel Clinical Target for Hematological Malignancies. Carcinogenesis 2020, 41, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Bhattacharjee, S.; Sharma, A.R.; Sharma, G.; Lee, S.-S.; Chakraborty, C. miRNAs in Alzheimer Disease—A Therapeutic Perspective. Curr. Alzheimer Res. 2017, 14, 1198–1206. [Google Scholar] [CrossRef]
- Hinkel, R.; Penzkofer, D.; Zühlke, S.; Fischer, A.; Husada, W.; Xu, Q.-F.; Baloch, E.; van Rooij, E.; Zeiher, A.M.; Kupatt, C.; et al. Inhibition of microRNA-92a Protects against Ischemia/Reperfusion Injury in a Large-Animal Model. Circulation 2013, 128, 1066–1075. [Google Scholar] [CrossRef]
- Cakmak, H.A.; Coskunpinar, E.; Ikitimur, B.; Barman, H.A.; Karadag, B.; Tiryakioglu, N.O.; Kahraman, K.; Vural, V.A. The Prognostic Value of Circulating microRNAs in Heart Failure: Preliminary Results from a Genome-Wide Expression Study. J. Cardiovasc. Med. 2015, 16, 431–437. [Google Scholar] [CrossRef]
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/es/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 August 2024).
- WHO La OMS Revela Las Principales Causas de Muerte y Discapacidad En El Mundo: 2000–2019. Available online: https://www.who.int/es/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 20 July 2024).
- Aghaei, S.M.; Hosseini, S.M. Inflammation-Related miRNAs in Obesity, CVD, and NAFLD. Cytokine 2024, 182, 156724. [Google Scholar] [CrossRef]
- Hata, A. Functions of MicroRNAs in Cardiovascular Biology and Disease. Annu. Rev. Physiol. 2013, 75, 69–93. [Google Scholar] [CrossRef]
- Sayed, D.; Abdellatif, M. MicroRNAs in Development and Disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef]
- Wronska, A. The Role of microRNA in the Development, Diagnosis, and Treatment of Cardiovascular Disease: Recent Developments. J. Pharmacol. Exp. Ther. 2023, 384, 123–132. [Google Scholar] [CrossRef]
- Dong, S.; Cheng, Y.; Yang, J.; Li, J.; Liu, X.; Wang, X.; Wang, D.; Krall, T.J.; Delphin, E.S.; Zhang, C. MicroRNA Expression Signature and the Role of MicroRNA-21 in the Early Phase of Acute Myocardial Infarction. J. Biol. Chem. 2009, 284, 29514–29525. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, L.; Zhang, Y.; Xu, S.; Liu, S.; Ye, J.; Liu, P. Inhibition of miR-214-3p Attenuates Ferroptosis in Myocardial Infarction via Regulating ME2. Biochem. Biophys. Res. Commun. 2023, 661, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Izawa, H.; Hirashiki, A.; Cheng, X.W.; Inden, Y.; Nomura, M.; Murohara, T. Altered microRNA Expression Associated with Reduced Catecholamine Sensitivity in Patients with Chronic Heart Failure. J. Cardiol. 2011, 57, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Z.; Yin, Q.; Fu, C.; Barszczyk, A.; Zhang, X.; Wang, J.; Yang, D. Cardiac-specific Overexpression of miR-122 Induces Mitochondria-dependent Cardiomyocyte Apoptosis and Promotes Heart Failure by Inhibiting Hand2. J. Cell. Mol. Med. 2021, 25, 5326–5334. [Google Scholar] [CrossRef] [PubMed]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.-L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 Controls Cardiac Hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Price, N.L.; Goedeke, L.; Suárez, Y.; Fernández-Hernando, C. miR-33 in Cardiometabolic Diseases: Lessons Learned from Novel Animal Models and Approaches. EMBO Mol. Med. 2021, 13, e12606. [Google Scholar] [CrossRef]
- Rotllan, N.; Ramírez, C.M.; Aryal, B.; Esau, C.C.; Fernández-Hernando, C. Therapeutic Silencing of MicroRNA-33 Inhibits the Progression of Atherosclerosis in Ldlr−/− Mice—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1973–1977. [Google Scholar] [CrossRef]
- Goedeke, L.; Salerno, A.; Ramírez, C.M.; Guo, L.; Allen, R.M.; Yin, X.; Langley, S.R.; Esau, C.; Wanschel, A.; Fisher, E.A.; et al. Long-term Therapeutic Silencing of miR-33 Increases Circulating Triglyceride Levels and Hepatic Lipid Accumulation in Mice. EMBO Mol. Med. 2014, 6, 1133–1141. [Google Scholar] [CrossRef]
- Price, N.L.; Singh, A.K.; Rotllan, N.; Goedeke, L.; Wing, A.; Canfrán-Duque, A.; Diaz-Ruiz, A.; Araldi, E.; Baldán, Á.; Camporez, J.-P.; et al. Genetic Ablation of miR-33 Increases Food Intake, Enhances Adipose Tissue Expansion, and Promotes Obesity and Insulin Resistance. Cell Rep. 2018, 22, 2133–2145. [Google Scholar] [CrossRef]
- Price, N.L.; Zhang, X.; Fernández-Tussy, P.; Singh, A.K.; Burnap, S.A.; Rotllan, N.; Goedeke, L.; Sun, J.; Canfrán-Duque, A.; Aryal, B.; et al. Loss of Hepatic miR-33 Improves Metabolic Homeostasis and Liver Function without Altering Body Weight or Atherosclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2006478118. [Google Scholar] [CrossRef]
- Ortega, R.; Liu, B.; Persaud, S.J. Effects of miR-33 Deficiency on Metabolic and Cardiovascular Diseases: Implications for Therapeutic Intervention. Int. J. Mol. Sci. 2023, 24, 10777. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, J.-W.; Lin, J.-Y.; Miao, R.; Zhong, J.-C. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc. Toxicol. 2020, 20, 463–473. [Google Scholar] [CrossRef]
- Šatrauskienė, A.; Navickas, R.; Laucevičius, A.; Krilavičius, T.; Užupytė, R.; Zdanytė, M.; Ryliškytė, L.; Jucevičienė, A.; Holvoet, P. Mir-1, miR-122, miR-132, and miR-133 Are Related to Subclinical Aortic Atherosclerosis Associated with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 1483. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, T.; Yang, N.; Zhang, T.; Wen, R.; Liu, C. Inhibition of Micro RNA miR-122-5p Prevents Lipopolysaccharide-Induced Myocardial Injury by Inhibiting Oxidative Stress, Inflammation and Apoptosis via Targeting GIT1. Bioengineered 2021, 12, 1902–1915. [Google Scholar] [CrossRef]
- Hsu, S.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential Metabolic, Anti-Inflammatory, and Anti-Tumorigenic Functions of miR-122 in Liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Ghoshal, K. miR-122 Is a Unique Molecule with Great Potential in Diagnosis, Prognosis of Liver Disease, and Therapy Both as miRNA Mimic and Antimir. Curr. Gene Ther. 2015, 15, 142–150. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Luo, L.; Nong, S.; Li, T. circFOXO3 Induced by KLF16 Modulates Clear Cell Renal Cell Carcinoma Growth and Natural Killer Cell Cytotoxic Activity through Sponging miR-29a-3p and miR-122-5p. Dis. Markers 2022, 2022, 6062236. [Google Scholar] [CrossRef]
- Eshraghi, R.; Rafiei, M.; Hadian Jazi, Z.; Shafie, D.; Raisi, A.; Mirzaei, H. MicroRNA-155 and Exosomal microRNA-155: Small Pieces in the Cardiovascular Diseases Puzzle. Pathol. Res. Pract. 2024, 257, 155274. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Rajagopalan, S.; Natarajan, R.; Deiuliis, J.A. Noncoding RNAs in Cardiovascular Disease: Pathological Relevance and Emerging Role as Biomarkers and Therapeutics. Am. J. Hypertens. 2018, 31, 150–165. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.-P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. miR-21, miR-210, miR-34a, and miR-146a/b Are up-Regulated in Human Atherosclerotic Plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Holland, A.; Enrick, M.; Diaz, A.; Yin, L. Is miR-21 A Therapeutic Target in Cardiovascular Disease? Int. J. Drug Discov. Pharmacol. 2023, 2, 26–36. [Google Scholar] [CrossRef]
- Huang, C.-K.; Bär, C.; Thum, T. miR-21, Mediator, and Potential Therapeutic Target in the Cardiorenal Syndrome. Front. Pharmacol. 2020, 11, 726. [Google Scholar] [CrossRef]
- Laggerbauer, B.; Engelhardt, S. MicroRNAs as Therapeutic Targets in Cardiovascular Disease. J. Clin. Investig. 2022, 132, e159179. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, D.; Sassi, Y.; Laggerbauer, B.; Engelhardt, S. Viral Vector-Based Targeting of miR-21 in Cardiac Nonmyocyte Cells Reduces Pathologic Remodeling of the Heart. Mol. Ther. 2016, 24, 1939–1948. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 Contributes to Myocardial Disease by Stimulating MAP Kinase Signalling in Fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ponnusamy, M.; Zhang, L.; Zhang, Y.; Liu, C.; Yu, W.; Wang, K.; Li, P. The Role of miR-214 in Cardiovascular Diseases. Eur. J. Pharmacol. 2017, 816, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Yang, L.; Gong, W.; Chaugai, S.; Wang, F.; Chen, C.; Wang, P.; Zou, M.-H.; Wang, D.W. MicroRNA-214 Is Upregulated in Heart Failure Patients and Suppresses XBP1-Mediated Endothelial Cells Angiogenesis. J. Cell. Physiol. 2015, 230, 1964–1973. [Google Scholar] [CrossRef]
- Feng, Y.; Wan, P.; Yin, L. Long Noncoding RNA X-Inactive Specific Transcript (XIST) Promotes Osteogenic Differentiation of Periodontal Ligament Stem Cells by Sponging MicroRNA-214-3p. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e918932-1–e918932-8. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Huang, J.; Li, W.-Z.; Wang, J.-J.; Song, D.-Y.; Ni, J.-D. LncRNA-MALAT1 Promotes Osteogenic Differentiation through Regulating ATF4 by Sponging miR-214: Implication of Steroid-Induced Avascular Necrosis of the Femoral Head. Steroids 2020, 154, 108533. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Ooi, J.Y.Y.; Matsumoto, A.; Tham, Y.K.; Singla, S.; Kiriazis, H.; Patterson, N.L.; Sadoshima, J.; Obad, S.; Lin, R.C.Y.; et al. Sex Differences in Response to miRNA-34a Therapy in Mouse Models of Cardiac Disease: Identification of Sex-, Disease- and Treatment-Regulated miRNAs. J. Physiol. 2016, 594, 5959–5974. [Google Scholar] [CrossRef]
- Hua, C.-C.; Liu, X.-M.; Liang, L.-R.; Wang, L.-F.; Zhong, J.-C. Targeting the microRNA-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 8, 784044. [Google Scholar] [CrossRef]
- Huang, Y.; Qi, Y.; Du, J.-Q.; Zhang, D. MicroRNA-34a Regulates Cardiac Fibrosis after Myocardial Infarction by Targeting Smad4. Expert Opin. Ther. Targets 2014, 18, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.-W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.-D.; Fisch, S.; Unno, K.; Sereti, K.-I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Marchetti, M.; Garner, K.; Littlejohns, B.; Sala-Newby, G.; Xenophontos, N.; Floris, I.; Suleiman, M.-S.; Madeddu, P.; Caporali, A.; et al. Local Inhibition of MicroRNA-24 Improves Reparative Angiogenesis and Left Ventricle Remodeling and Function in Mice With Myocardial Infarction. Mol. Ther. 2013, 21, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Di Martino, M.T.; De Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gullà, A.; Tagliaferri, P.; Tassone, P.; et al. Mir-34: A New Weapon Against Cancer? Mol. Ther.-Nucleic Acids 2014, 3, e195. [Google Scholar] [CrossRef]
- Bruen, R.; Fitzsimons, S.; Belton, O. miR-155 in the Resolution of Atherosclerosis. Front. Pharmacol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Heymans, S.; Corsten, M.F.; Verhesen, W.; Carai, P.; van Leeuwen, R.E.W.; Custers, K.; Peters, T.; Hazebroek, M.; Stöger, L.; Wijnands, E.; et al. Macrophage microRNA-155 Promotes Cardiac Hypertrophy and Failure. Circulation 2013, 128, 1420–1432. [Google Scholar] [CrossRef]
- Li, X.; Kong, D.; Chen, H.; Liu, S.; Hu, H.; Wu, T.; Wang, J.; Chen, W.; Ning, Y.; Li, Y.; et al. miR-155 Acts as an Anti-Inflammatory Factor in Atherosclerosis-Associated Foam Cell Formation by Repressing Calcium-Regulated Heat Stable Protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, L.; Liang, X.; Zhu, G. MicroRNA-155 Promotes Atherosclerosis Inflammation via Targeting SOCS1. Cell. Physiol. Biochem. 2015, 36, 1371–1381. [Google Scholar] [CrossRef]
- Chung, K.-H.; Hart, C.C.; Al-Bassam, S.; Avery, A.; Taylor, J.; Patel, P.D.; Vojtek, A.B.; Turner, D.L. Polycistronic RNA Polymerase II Expression Vectors for RNA Interference Based on BIC/miR-155. Nucleic Acids Res. 2006, 34, e53. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 August 2024).
- Rahman, M.A.; Islam, M.M.; Ripon, M.A.R.; Islam, M.M.; Hossain, M.S. Regulatory Roles of MicroRNAs in the Pathogenesis of Metabolic Syndrome. Mol. Biotechnol. 2024, 66, 1599–1620. [Google Scholar] [CrossRef] [PubMed]
- Masoumi-Ardakani, Y.; Eghbalian, M.; Fallah, H.; Jafari, A.; Shahouzehi, B. Exploring Serum miR-33b as a Novel Diagnostic Marker for Hypercholesterolemia and Obesity: Insights from a Pilot Case-Control Study. BMC Endocr. Disord. 2025, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Elkhawaga, S.Y.; Ismail, A.; Elsakka, E.G.E.; Doghish, A.S.; Elkady, M.A.; El-Mahdy, H.A. miRNAs as Cornerstones in Adipogenesis and Obesity. Life Sci. 2023, 315, 121382. [Google Scholar] [CrossRef]
- Ibarra, P.E.; García-Solís, P.; Solís-Sáinz, J.C.; Cruz-Hernández, A. Expression of miRNA in Obesity and Insulin Resistance: A Review. Endokrynol. Pol. 2021, 72, 73–80. [Google Scholar] [CrossRef]
- Kilic, I.D.; Dodurga, Y.; Uludag, B.; Alihanoglu, Y.I.; Yildiz, B.S.; Enli, Y.; Secme, M.; Bostancı, H.E. microRNA -143 and -223 in Obesity. Gene 2015, 560, 140–142. [Google Scholar] [CrossRef]
- Silveira, A.; Gomes, J.; Roque, F.; Fernandes, T.; de Oliveira, E.M. MicroRNAs in Obesity-Associated Disorders: The Role of Exercise Training. Obes. Facts 2022, 15, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tussy, P.; Cardelo, M.P.; Zhang, H.; Sun, J.; Price, N.L.; Boutagy, N.E.; Goedeke, L.; Cadena-Sandoval, M.; Xirouchaki, C.E.; Brown, W.; et al. miR-33 Deletion in Hepatocytes Attenuates MASLD-MASH-HCC Progression. JCI Insight 2024, 9, e168476. [Google Scholar] [CrossRef]
- Price, N.L.; Fernández-Tussy, P.; Varela, L.; Cardelo, M.P.; Shanabrough, M.; Aryal, B.; de Cabo, R.; Suárez, Y.; Horvath, T.L.; Fernández-Hernando, C. microRNA-33 Controls Hunger Signaling in Hypothalamic AgRP Neurons. Nat. Commun. 2024, 15, 2131. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zeng, D.; Xiong, J.; Luo, J.; Chen, X.; Chen, T.; Xi, Q.; Sun, J.; Ren, X.; et al. The Novel Importance of miR-143 in Obesity Regulation. Int. J. Obes. 2023, 47, 100–108. [Google Scholar] [CrossRef]
- Yi, C.; Xie, W.; Li, F.; Lv, Q.; He, J.; Wu, J.; Gu, D.; Xu, N.; Zhang, Y. MiR-143 Enhances Adipogenic Differentiation of 3T3-L1 Cells through Targeting the Coding Region of Mouse Pleiotrophin. FEBS Lett. 2011, 585, 3303–3309. [Google Scholar] [CrossRef]
- Zarkesh, M.; Tabaei, K.; Akbarzadeh, M.; Daneshafrooz, A.; Zadeh-Vakili, A. Association of miR-34a and miR-143 Levels with PPARγ Gene Expression in Adipose Tissues of Non-Diabetic Adults. J. Physiol. Anthropol. 2022, 41, 13. [Google Scholar] [CrossRef] [PubMed]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 Regulates Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zeng, D.; Wang, H.; Wang, Y.; Xiong, J.; Chen, X.; Luo, J.; Chen, T.; Xi, Q.; et al. miR-143-Null Is against Diet-Induced Obesity by Promoting BAT Thermogenesis and Inhibiting WAT Adipogenesis. Int. J. Mol. Sci. 2022, 23, 13058. [Google Scholar] [CrossRef]
- Lin, X.; Du, Y.; Lu, W.; Gui, W.; Sun, S.; Zhu, Y.; Wang, G.; Eserberg, D.T.; Zheng, F.; Zhou, J.; et al. CircRNF111 Protects Against Insulin Resistance and Lipid Deposition via Regulating miR-143-3p/IGF2R Axis in Metabolic Syndrome. Front. Cell Dev. Biol. 2021, 9, 663148. [Google Scholar] [CrossRef] [PubMed]
- Lhamyani, S.; Gentile, A.-M.; Giráldez-Pérez, R.M.; Feijóo-Cuaresma, M.; Romero-Zerbo, S.Y.; Clemente-Postigo, M.; Zayed, H.; Oliva-Olivera, W.; Bermúdez-Silva, F.J.; Salas, J.; et al. miR-21 Mimic Blocks Obesity in Mice: A Novel Therapeutic Option. Mol. Ther.-Nucleic Acids 2021, 26, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Sekar, D.; Venugopal, B.; Sekar, P.; Ramalingam, K. Role of microRNA 21 in Diabetes and Associated/Related Diseases. Gene 2016, 582, 14–18. [Google Scholar] [CrossRef]
- Seeger, T.; Fischer, A.; Muhly-Reinholz, M.; Zeiher, A.M.; Dimmeler, S. Long-Term Inhibition of miR-21 Leads to Reduction of Obesity in Db/Db Mice. Obesity 2014, 22, 2352–2360. [Google Scholar] [CrossRef]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential Therapeutic Effects of Exosomes Packed with a miR-21-Sponge Construct in a Rat Model of Glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef]
- Ma, F.; Cao, D.; Liu, Z.; Li, Y.; Ouyang, S.; Wu, J. Identification of Novel Circulating miRNAs Biomarkers for Healthy Obese and Lean Children. BMC Endocr. Disord. 2023, 23, 238. [Google Scholar] [CrossRef]
- de Almeida-Faria, J.; Duque-Guimarães, D.E.; Ong, T.P.; Pantaleão, L.C.; Carpenter, A.A.; Loche, E.; Kusinski, L.C.; Ashmore, T.J.; Antrobus, R.; Bushell, M.; et al. Maternal Obesity during Pregnancy Leads to Adipose Tissue ER Stress in Mice via miR-126-Mediated Reduction in Lunapark. Diabetologia 2021, 64, 890–902. [Google Scholar] [CrossRef]
- Shen, L.; He, J.; Zhao, Y.; Niu, L.; Chen, L.; Tang, G.; Jiang, Y.; Hao, X.; Bai, L.; Li, X.; et al. MicroRNA-126b-5p Exacerbates Development of Adipose Tissue and Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 10261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.-Z.; Yang, D.-G.; Zhang, Q.-Y.; Deng, Z.-N.; Wang, K.; Mao, X.-J. MiR-21 Antagomir Improves Insulin Resistance and Lipid Metabolism Disorder in Streptozotocin-Induced Type 2 Diabetes Mellitus Rats. Ann. Palliat. Med. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Liu, R.; Liu, C.; He, X.; Sun, P.; Zhang, B.; Yang, H.; Shi, W.; Ruan, Q. MicroRNA-21 Promotes Pancreatic β Cell Function through Modulating Glucose Uptake. Nat. Commun. 2022, 13, 3545. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Das, F.; Mariappan, M.M.; Mandal, C.C.; Ghosh-Choudhury, N.; Kasinath, B.S.; Choudhury, G.G. MicroRNA-21 Orchestrates High Glucose-Induced Signals to TOR Complex 1, Resulting in Renal Cell Pathology in Diabetes. J. Biol. Chem. 2011, 286, 25586–25603. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.K.; Chen, H.Y.; Dong, Y.; Meng, X.M.; Li, R.; Yang, W.; Hou, F.F.; Lan, H.Y. miR-21 Is a Key Therapeutic Target for Renal Injury in a Mouse Model of Type 2 Diabetes. Diabetologia 2013, 56, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Abusree Ahmed, A.; Hassanien, R.T.M.; Mahfouz, H.; Salah, M.; Amr, H.M.; Fahim, S.A. Emerging Role of Hsa_circ_0000652, Hsa-miR-21, SMAD2, and Foxo1 in Type 2 Diabetes Mellitus Pathogenesis. Hum. Gene 2025, 43, 201386. [Google Scholar] [CrossRef]
- Ghaffari, M.; Razi, S.; Zalpoor, H.; Nabi-Afjadi, M.; Mohebichamkhorami, F.; Zali, H. Association of MicroRNA-146a with Type 1 and 2 Diabetes and Their Related Complications. J. Diabetes Res. 2023, 2023, 2587104. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Nelson, M.C.; Lee, S.-H.; Voth, W.; Alexander, M.; Hu, R.; Wallace, J.; Petersen, C.; Panic, V.; Villanueva, C.J.; et al. Anti-Inflammatory microRNA-146a Protects Mice from Diet-Induced Metabolic Disease. PLoS Genet. 2019, 15, e1007970. [Google Scholar] [CrossRef]
- Peng, X.; He, F.; Mao, Y.; Lin, Y.; Fang, J.; Chen, Y.; Sun, Z.; Zhuo, Y.; Jiang, J. miR-146a Promotes M2 Macrophage Polarization and Accelerates Diabetic Wound Healing by Inhibiting the TLR4/NF-κB Axis. J. Mol. Endocrinol. 2022, 69, 315–327. [Google Scholar] [CrossRef]
- Roos, J.; Dahlhaus, M.; Funcke, J.-B.; Kustermann, M.; Strauss, G.; Halbgebauer, D.; Boldrin, E.; Holzmann, K.; Möller, P.; Trojanowski, B.M.; et al. miR-146a Regulates Insulin Sensitivity via NPR3. Cell. Mol. Life Sci. CMLS 2021, 78, 2987–3003. [Google Scholar] [CrossRef]
- Rohm, T.V.; Castellani Gomes Dos Reis, F.; Isaac, R.; Murphy, C.; Cunha e Rocha, K.; Bandyopadhyay, G.; Gao, H.; Libster, A.M.; Zapata, R.C.; Lee, Y.S.; et al. Adipose Tissue Macrophages Secrete Small Extracellular Vesicles That Mediate Rosiglitazone-Induced Insulin Sensitization. Nat. Metab. 2024, 6, 880–898. [Google Scholar] [CrossRef]
- Ying, W.; Gao, H.; Dos Reis, F.C.G.; Bandyopadhyay, G.; Ofrecio, J.M.; Luo, Z.; Ji, Y.; Jin, Z.; Ly, C.; Olefsky, J.M. MiR-690, an Exosomal-Derived miRNA from M2-Polarized Macrophages, Improves Insulin Sensitivity in Obese Mice. Cell Metab. 2021, 33, 781–790.e5. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhao, C.; Guo, X.; Ding, H.; Cui, Y.; Shen, R.; Liu, J. Differential Expression of MicroRNAs in Omental Adipose Tissue From Gestational Diabetes Mellitus Subjects Reveals miR-222 as a Regulator of ERα Expression in Estrogen-Induced Insulin Resistance. Endocrinology 2014, 155, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, G.; Gea-Sorlí, S.; Otero-Mateo, M.; Fillat, C. Inhibition of miR-222 by Oncolytic Adenovirus-Encoded miRNA Sponges Promotes Viral Oncolysis and Elicits Antitumor Effects in Pancreatic Cancer Models. Cancers 2021, 13, 3233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, F.; Chen, X.; Liu, Z.; Ouyang, Y.; Zhao, W.; Yu, D. Downregulation of miR-221/222 by a microRNA Sponge Promotes Apoptosis in Oral Squamous Cell Carcinoma Cells through Upregulation of PTEN. Oncol. Lett. 2016, 12, 4419–4426. [Google Scholar] [CrossRef]
- Chuang, T.-Y.; Wu, H.-L.; Chen, C.-C.; Gamboa, G.M.; Layman, L.C.; Diamond, M.P.; Azziz, R.; Chen, Y.-H. MicroRNA-223 Expression Is Upregulated in Insulin Resistant Human Adipose Tissue. J. Diabetes Res. 2015, 2015, 943659. [Google Scholar] [CrossRef]
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962. [Google Scholar] [CrossRef]
- Sánchez-Ceinos, J.; Rangel-Zuñiga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcalá-Diaz, J.F.; Guzmán-Ruiz, R.; López-Miranda, J.; Malagón, M.M. miR-223-3p as a Potential Biomarker and Player for Adipose Tissue Dysfunction Preceding Type 2 Diabetes Onset. Mol. Ther.-Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef]
- Huerta-Zavala, M.L.; Lopez-Castillejos, E.S.; Requenez-Contreras, J.L.; Granados-Riveron, J.T.; Aquino-Jarquin, G. A Single miRNA and miRNA Sponge Expression System for Efficient Modulation of miR-223 Availability in Mammalian Cells. J. Gene Med. 2019, 21, e3100. [Google Scholar] [CrossRef]
- M’baya-Moutoula, E.; Marchand, A.; Six, I.; Bahrar, N.; Celic, T.; Mougenot, N.; Maitrias, P.; Massy, Z.A.; Lompré, A.-M.; Metzinger, L.; et al. Inhibition of miR-223 Expression Using a Sponge Strategy Decreases Restenosis in Rat Injured Carotids. Curr. Vasc. Pharmacol. 2020, 18, 507–516. [Google Scholar] [CrossRef]
- Gao, F.; Wei, L.; Li, J.; Zeng, L.; Wang, M.; Lan, P.; Liang, S.; Huang, X.; Chen, L.; Jiang, H. Circular RNA CircVmn2r1 Acts as a miR-223-3p Sponge to Promote Kidney Aging by Regulating NLRP3 Expression in Mice. J. Gerontol. Ser. A 2024, 79, glae067. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Sun, J.; Huang, J.; Yao, F.; Chen, X.; Zhu, B.; Zhao, D. Circular RNA circRNA_0000094 Sponges microRNA-223-3p and up-Regulate F-Box and WD Repeat Domain Containing 7 to Restrain T Cell Acute Lymphoblastic Leukemia Progression. Hum. Cell 2021, 34, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, G.-F.; Wang, J.-C.; Ji, S.-H.; Wu, X.-W.; Lu, X.-J.; Chen, J.-L.; Li, J.-T. Hsa_circ_0070963 Inhibits Liver Fibrosis via Regulation of miR-223-3p and LEMD3. Aging 2020, 12, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dai, Y.; Zhang, D.; Zhang, X.; He, Z.; Xie, X.; Cai, C. LINC00961 Inhibits the Migration and Invasion of Colon Cancer Cells by Sponging miR-223-3p and Targeting SOX11. Cancer Med. 2020, 9, 2514–2523. [Google Scholar] [CrossRef]
- Chen, W.; Xu, J.; Wu, Y.; Liang, B.; Yan, M.; Sun, C.; Wang, D.; Hu, X.; Liu, L.; Hu, W.; et al. The Potential Role and Mechanism of circRNA/miRNA Axis in Cholesterol Synthesis. Int. J. Biol. Sci. 2023, 19, 2879–2896. [Google Scholar] [CrossRef]
- Mirzaei, R.; Karampoor, S.; Korotkova, N.L. The Emerging Role of miRNA-122 in Infectious Diseases: Mechanisms and Potential Biomarkers. Pathol. Res. Pract. 2023, 249, 154725. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, H.; Bai, M.; Ning, T.; Deng, T.; Liu, R.; Fan, Q.; Zhu, K.; Li, J.; et al. Exosome-delivered circRNA Promotes Glycolysis to Induce Chemoresistance through the miR-122-PKM2 Axis in Colorectal Cancer. Mol. Oncol. 2020, 14, 539–555. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, S.; Qin, S.; Shen, X.; Ju, S. Exosomal circRNAs: Sorting Mechanisms, Roles and Clinical Applications in Tumors. Front. Cell Dev. Biol. 2020, 8, 581558. [Google Scholar] [CrossRef]
- Fernández-Tussy, P.; Ruz-Maldonado, I.; Fernández-Hernando, C. MicroRNAs and Circular RNAs in Lipoprotein Metabolism. Curr. Atheroscler. Rep. 2021, 23, 33. [Google Scholar] [CrossRef]
- Goedeke, L.; Rotllan, N.; Ramírez, C.M.; Aranda, J.F.; Canfrán-Duque, A.; Araldi, E.; Fernández-Hernando, A.; Langhi, C.; de Cabo, R.; Baldán, Á.; et al. miR-27b Inhibits LDLR and ABCA1 Expression but Does Not Influence Plasma and Hepatic Lipid Levels in Mice. Atherosclerosis 2015, 243, 499–509. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b Is a Regulatory Hub in Lipid Metabolism and Is Altered in Dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Zhu, L.; Zhang, H.; Feng, L. Inhibition of miR-27b Regulates Lipid Metabolism in Skeletal Muscle of Obese Rats During Hypoxic Exercise by Increasing PPARγ Expression. Front. Physiol. 2020, 11, 1090. [Google Scholar] [CrossRef]
- Galicia, U.; Benito-Vicente, A.; Jebari-Benslaiman, S.; Belloso-Uribe, K.; Larrea, A.; José, A.S.; Plagaro, C.M. Statin-Induced miR-33a and miR-27b up-Regulation Contributes to the Development of New-Onset Type 2 Diabetes. Atherosclerosis 2023, 379, S30. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G.; Labbaye, C. miR-146 and miR-155: Two Key Modulators of Immune Response and Tumor Development. Non-Coding RNA 2017, 3, 22. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, L.; Wang, K.; Qi, Q.; Wang, M.; Yang, L.; Sun, P.; Mu, H. MicroRNA-146a Inhibits NF-κB Activation and pro-Inflammatory Cytokine Production by Regulating IRAK1 Expression in THP-1 Cells. Exp. Ther. Med. 2019, 18, 3078–3084. [Google Scholar] [CrossRef]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef]
- Garchow, B.; Kiriakidou, M. MicroRNA-21 Deficiency Protects from Lupus-like Autoimmunity in the Chronic Graft-Versus-Host Disease Model of Systemic Lupus Erythematosus. Clin. Immunol. 2016, 162, 100–106. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-Derived miR-223 Regulates Intestinal Inflammation via Repression of the NLRP3 Inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Kourti, M.; Sokratous, M.; Katsiari, C.G. Regulation of microRNA in Systemic Lupus Erythematosus: The Role of miR-21 and miR-210. Mediterr. J. Rheumatol. 2020, 31, 71–74. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. The Neuroscientist 2018, 24, 221–245. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Okarski, M.; Wawak, M.; Przewłocki, T.; Podolec, J. Cardiac microRNAs: Diagnostic and Therapeutic Potential. Arch. Med. Sci. AMS 2023, 19, 1360–1381. [Google Scholar] [CrossRef]
- Panigrahi, M.; Palmer, M.A.; Wilson, J.A. MicroRNA-122 Regulation of HCV Infections: Insights from Studies of miR-122-Independent Replication. Pathogens 2022, 11, 1005. [Google Scholar] [CrossRef]
- Abidin, S.Z.; Mat Pauzi, N.A.; Mansor, N.I.; Mohd Isa, N.I.; Hamid, A.A. A New Perspective on Alzheimer’s Disease: microRNAs and Circular RNAs. Front. Genet. 2023, 14, 1231486. [Google Scholar] [CrossRef]
- Li, H.; Liu, T.; Yang, Y.; Cho, W.C.; Flynn, R.J.; Harandi, M.F.; Song, H.; Luo, X.; Zheng, Y. Interplays of Liver Fibrosis-Associated microRNAs: Molecular Mechanisms and Implications in Diagnosis and Therapy. Genes Dis. 2023, 10, 1457–1469. [Google Scholar] [CrossRef]
- Felekkis, K.; Pieri, M.; Papaneophytou, C. Exploring the Feasibility of Circulating miRNAs as Diagnostic and Prognostic Biomarkers in Osteoarthritis: Challenges and Opportunities. Int. J. Mol. Sci. 2023, 24, 13144. [Google Scholar] [CrossRef]
- Bhatia, A.; Upadhyay, A.K.; Sharma, S. miRNAs Are Now Starring in “No Time to Die: Overcoming the Chemoresistance in Cancer”. IUBMB Life 2023, 75, 238–256. [Google Scholar] [CrossRef]
- Mehterov, N. Role of MicroRNAs in Cancer Development and Treatment. Int. J. Mol. Sci. 2023, 24, 11058. [Google Scholar] [CrossRef]
- Romano, G.; Acunzo, M.; Nana-Sinkam, P. microRNAs as Novel Therapeutics in Cancer. Cancers 2021, 13, 1526. [Google Scholar] [CrossRef]
- Sell, M.C.; Ramlogan-Steel, C.A.; Steel, J.C.; Dhungel, B.P. MicroRNAs in Cancer Metastasis: Biological and Therapeutic Implications. Expert Rev. Mol. Med. 2023, 25, e14. [Google Scholar] [CrossRef]
- Arghiani, N.; Shah, K. Modulating microRNAs in Cancer: Next-Generation Therapies. Cancer Biol. Med. 2022, 19, 289–304. [Google Scholar] [CrossRef]
- Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. COVID-19: Fighting the Invisible Enemy with microRNA. Expert Rev. Anti Infect. Ther. 2021, 19, 137–145. [Google Scholar] [CrossRef]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-Based Biomarkers, Therapies, and Resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, E.; Bayraktar, R.; Oztatlici, H.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Targeting miRNAs and Other Non-Coding RNAs as a Therapeutic Approach: An Update. Non-Coding RNA 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Milán Rois, P.; Latorre, A.; Rodriguez Diaz, C.; Del Moral, Á.; Somoza, Á. Reprogramming Cells for Synergistic Combination Therapy with Nanotherapeutics against Uveal Melanoma. Biomimetics 2018, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. miRNA Oligonucleotide and Sponge for miRNA-21 Inhibition Mediated by PEI-PLL in Breast Cancer Therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef]
- Davenport, M.L.; Echols, J.B.; Silva, A.D.; Anderson, J.C.; Owens, P.; Yates, C.; Wei, Q.; Harada, S.; Hurst, D.R.; Edmonds, M.D. miR-31 Displays Subtype Specificity in Lung Cancer. Cancer Res. 2021, 81, 1942–1953. [Google Scholar] [CrossRef]
- Van Roosbroeck, K.; Fanini, F.; Setoyama, T.; Ivan, C.; Rodriguez-Aguayo, C.; Fuentes-Mattei, E.; Xiao, L.; Vannini, I.; Redis, R.S.; D’Abundo, L.; et al. Combining Anti-Mir-155 with Chemotherapy for the Treatment of Lung Cancers. Clin. Cancer Res. 2017, 23, 2891–2904. [Google Scholar] [CrossRef]
- Garofalo, M.; Quintavalle, C.; Romano, G.; Croce, C.M.; Condorelli, G. miR221/222 in Cancer: Their Role in Tumor Progression and Response to Therapy. Curr. Mol. Med. 2012, 12, 27–33. [Google Scholar] [CrossRef]
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Moshiri, F.; Callegari, E.; D’Abundo, L.; Corrà, F.; Lupini, L.; Sabbioni, S.; Negrini, M. Inhibiting the Oncogenic Mir-221 by microRNA Sponge: Toward microRNA-Based Therapeutics for Hepatocellular Carcinoma. Gastroenterol. Hepatol. Bed Bench 2014, 7, 43–54. [Google Scholar]
- Miller, T.E.; Ghoshal, K.; Ramaswamy, B.; Roy, S.; Datta, J.; Shapiro, C.L.; Jacob, S.; Majumder, S. MicroRNA-221/222 Confers Tamoxifen Resistance in Breast Cancer by Targeting p27Kip1. J. Biol. Chem. 2008, 283, 29897–29903. [Google Scholar] [CrossRef]
- Ouyang, Y.X.; Feng, J.; Wang, Z.; Zhang, G.J.; Chen, M. miR-221/222 Sponge Abrogates Tamoxifen Resistance in ER-Positive Breast Cancer Cells through Restoring the Expression of ERα. Mol. Biomed. 2021, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, X.; Yin, S.; Liang, B.; Zhang, Y.; Fan, M.; Fu, Z.; Shen, C.; Han, Y.; et al. Function of microRNA-124 in the Pathogenesis of Cancer (Review). Int. J. Oncol. 2024, 64, 6. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Yang, L. miR-124 Inhibits the Growth of Glioblastoma through the Downregulation of SOS1. Mol. Med. Rep. 2013, 8, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA. Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Cao, Z.; Qiu, J.; Yang, G.; Liu, Y.; Luo, W.; You, L.; Zheng, L.; Zhang, T. MiR-135a Biogenesis and Regulation in Malignancy: A New Hope for Cancer Research and Therapy. Cancer Biol. Med. 2020, 17, 569–582. [Google Scholar] [CrossRef]
- Sun, X.; Xue, H.; Xiong, Y.; Yu, R.; Gao, X.; Qian, M.; Wang, S.; Wang, H.; Xu, J.; Chen, Z.; et al. GALE Promotes the Proliferation and Migration of Glioblastoma Cells and Is Regulated by miR-Let-7i-5p. Cancer Manag. Res. 2019, 11, 10539–10554. [Google Scholar] [CrossRef]
- Nair, R.; Westin, J. CAR T-Cells. In Immunotherapy; Naing, A., Hajjar, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 215–233. ISBN 978-3-030-41008-7. [Google Scholar]
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcón-Hernández, E.; Ibáñez-Hernández, M. Strategies for Targeting Gene Therapy in Cancer Cells With Tumor-Specific Promoters. Front. Oncol. 2020, 10, 605380. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-Associated Virus as a Delivery Vector for Gene Therapy of Human Diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef]
- Eid, F.-E.; Chen, A.T.; Chan, K.Y.; Huang, Q.; Zheng, Q.; Tobey, I.G.; Pacouret, S.; Brauer, P.P.; Keyes, C.; Powell, M.; et al. Systematic Multi-Trait AAV Capsid Engineering for Efficient Gene Delivery. Nat. Commun. 2024, 15, 6602. [Google Scholar] [CrossRef]
- Puzzo, F.; Zhang, C.; Powell Gray, B.; Zhang, F.; Sullenger, B.A.; Kay, M.A. Aptamer-Programmable Adeno-Associated Viral Vectors as a Novel Platform for Cell-Specific Gene Transfer. Mol. Ther. Nucleic Acids 2023, 31, 383–397. [Google Scholar] [CrossRef]
- Santiago-Ortiz, J.L.; Schaffer, D.V. Adeno-Associated Virus (AAV) Vectors in Cancer Gene Therapy. J. Control. Release 2016, 240, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Vyas, K.; Patel, M.M. Insights on Drug and Gene Delivery Systems in Liver Fibrosis. Asian J. Pharm. Sci. 2023, 18, 100779. [Google Scholar] [CrossRef]
- Bravo-Estupiñan, D.M.; Montaño-Samaniego, M.; Mora-Rodríguez, R.A.; Ibáñez-Hernández, M. Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis. Appl. Sci. 2024, 14, 10892. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging Non-Viral Vectors for Gene Delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Wei, P.-S.; Thota, N.; John, G.; Chang, E.; Lee, S.; Wang, Y.; Ma, Z.; Tsai, Y.-H.; Mei, K.-C. Enhancing RNA-Lipid Nanoparticle Delivery: Organ- and Cell-Specificity and Barcoding Strategies. J. Control. Release 2024, 375, 366–388. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Wang, S.; Zhang, L.; Jiang, X. Microfluidics-Assembled Nanovesicles for Nucleic Acid Delivery. Acc. Chem. Res. 2025, 58, 570–582. [Google Scholar] [CrossRef]
- Zheng, C.; Baum, B.J. Evaluation of Promoters for Use in Tissue-Specific Gene Delivery. Methods Mol. Biol. 2008, 434, 205–219. [Google Scholar] [CrossRef][Green Version]
- Meuwissen, R.; Berns, A. Mouse Models for Human Lung Cancer. Genes Dev. 2005, 19, 643–664. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Watanabe, S.; Ohtsuka, M.; Maehara, T.; Ishihara, M.; Yokomine, T.; Sato, M. Cre-loxP System as a Versatile Tool for Conferring Increased Levels of Tissue-Specific Gene Expression from a Weak Promoter. Mol. Reprod. Dev. 2008, 75, 1085–1093. [Google Scholar] [CrossRef]
- Shepelev, M.V.; Kopantzev, E.P.; Vinogradova, T.V.; Sverdlov, E.D.; Korobko, I.V. hTERT and BIRC5 Gene Promoters for Cancer Gene Therapy: A Comparative Study. Oncol. Lett. 2016, 12, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Liu, X.; Wu, S.; Wang, M.; Turowski, S.G.; Spernyak, J.A.; Tracz, A.; Abdelaal, A.M.; Sudarshan, K.; et al. Developing Folate-Conjugated miR-34a Therapeutic for Prostate Cancer: Challenges and Promises. Int. J. Mol. Sci. 2024, 25, 2123. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Sohal, I.S.; Iyer, S.G.; Sudarshan, K.; Orellana, E.A.; Ozcan, K.E.; dos Santos, A.P.; Low, P.S.; Kasinski, A.L. Selective Targeting of Chemically Modified miR-34a to Prostate Cancer Using a Small Molecule Ligand and an Endosomal Escape Agent. Mol. Ther. Nucleic Acids 2024, 35, 102193. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Therapeutic Advances of miRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. [Google Scholar] [CrossRef]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Brillante, S.; Volpe, M.; Indrieri, A. Advances in MicroRNA Therapeutics: From Preclinical to Clinical Studies. Hum. Gene Ther. 2024, 35, 628–648. [Google Scholar] [CrossRef]
- Estevez-Fraga, C.; Tabrizi, S.J.; Wild, E.J. Huntington’s Disease Clinical Trials Corner: March 2024. J. Huntingt. Dis. 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Kotecki, N.; Opdam, F.; Robbrecht, D.; Strijbos, M.; Kroon, K.; Janicot, M.; Yahyanejad, S.; Telford, B.; van den Bosch, M.; Alemdehy, F.; et al. Phase I/Ib Study with INT-1B3, a Novel LNP-Formulated Micro-RNA (miR-193a-3p Mimic) Therapeutic for Patients with Advanced Solid Cancer. J. Clin. Oncol. 2021, 39, TPS2666. [Google Scholar] [CrossRef]
- Plotnikova, O.; Baranova, A.; Skoblov, M. Comprehensive Analysis of Human microRNA-mRNA Interactome. Front. Genet. 2019, 10, 933. [Google Scholar] [CrossRef]
- Toden, S.; Zumwalt, T.J.; Goel, A. Non-Coding RNAs and Potential Therapeutic Targeting in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188491. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer (Review). Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, Y.; Li, J. MicroRNA-21: A Central Regulator of Fibrotic Diseases via Various Targets. Curr. Pharm. Des. 2015, 21, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of miRNA Therapeutics: In a Drug Target Perspective. Drug Des. Dev. Ther. 2021, 15, 721–733. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, D.-Y.; Huang, L. In Vivo Delivery of miRNAs for Cancer Therapy: Challenges and Strategies. Adv. Drug Deliv. Rev. 2015, 81, 128–141. [Google Scholar] [CrossRef]
- Parveen, A.; Mustafa, S.H.; Yadav, P.; Kumar, A. Applications of Machine Learning in miRNA Discovery and Target Prediction. Curr. Genom. 2019, 20, 537–544. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, J.; Chen, Z.; Liu, Y.; Dura, B.; Kwak, M.; Xavier-Ferrucio, J.; Lu, Y.-C.; Zhang, M.; Roden, C.; et al. Single-Cell microRNA-mRNA Co-Sequencing Reveals Non-Genetic Heterogeneity and Mechanisms of microRNA Regulation. Nat. Commun. 2019, 10, 95. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Kamaroddin, M.F. Innovative Approaches in Transforming microRNAs into Therapeutic Tools. Wiley Interdiscip. Rev. RNA 2023, 14, e1768. [Google Scholar] [CrossRef]
- Orellana, E.A.; Abdelaal, A.M.; Rangasamy, L.; Tenneti, S.; Myoung, S.; Low, P.S.; Kasinski, A.L. Enhancing MicroRNA Activity through Increased Endosomal Release Mediated by Nigericin. Mol. Ther. Nucleic Acids 2019, 16, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Fromm, B.; Zhong, X.; Tarbier, M.; Friedländer, M.R.; Hackenberg, M. The Limits of Human microRNA Annotation Have Been Met. RNA 2022, 28, 781–785. [Google Scholar] [CrossRef]
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723. [Google Scholar] [CrossRef]
- Bofill-De Ros, X.; Kasprzak, W.K.; Bhandari, Y.; Fan, L.; Cavanaugh, Q.; Jiang, M.; Dai, L.; Yang, A.; Shao, T.-J.; Shapiro, B.A.; et al. Structural Differences between Pri-miRNA Paralogs Promote Alternative Drosha Cleavage and Expand Target Repertoires. Cell Rep. 2019, 26, 447–459.e4. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, A.S.; Parchem, R.J. KRAS Hijacks the miRNA Regulatory Pathway in Cancer. Cancer Res. 2023, 83, 1563–1572. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, L.; Li, S.; Chen, F.; Au-Yeung, K.K.-W.; Shi, C. MicroRNA as an Important Target for Anticancer Drug Development. Front. Pharmacol. 2021, 12, 736323. [Google Scholar] [CrossRef] [PubMed]
- Ricarte-Filho, J.C.; Casado-Medrano, V.; Reichenberger, E.; Spangler, Z.; Scheerer, M.; Isaza, A.; Baran, J.; Patel, T.; MacFarland, S.P.; Brodeur, G.M.; et al. DICER1 RNase IIIb Domain Mutations Trigger Widespread miRNA Dysregulation and MAPK Activation in Pediatric Thyroid Cancer. Front. Endocrinol. 2023, 14, 1083382. [Google Scholar] [CrossRef]
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-miRNA Oligonucleotides: A Comprehensive Guide for Design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef]
- Hussen, B.M.; Rasul, M.F.; Abdullah, S.R.; Hidayat, H.J.; Faraj, G.S.H.; Ali, F.A.; Salihi, A.; Baniahmad, A.; Ghafouri-Fard, S.; Rahman, M.; et al. Targeting miRNA by CRISPR/Cas in Cancer: Advantages and Challenges. Mil. Med. Res. 2023, 10, 32. [Google Scholar] [CrossRef]
- Vervaeke, P.; Borgos, S.E.; Sanders, N.N.; Combes, F. Regulatory Guidelines and Preclinical Tools to Study the Biodistribution of RNA Therapeutics. Adv. Drug Deliv. Rev. 2022, 184, 114236. [Google Scholar] [CrossRef]
- Banoun, H. mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues. Int. J. Mol. Sci. 2023, 24, 10514. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Portugal, C.; Montaño-Samaniego, M.; Guttman-Bazbaz, R.; Bravo-Estupiñan, D.M.; Ibáñez-Hernández, M. Therapeutic Applications of Poly-miRNAs and miRNA Sponges. Int. J. Mol. Sci. 2025, 26, 4535. https://doi.org/10.3390/ijms26104535
Avendaño-Portugal C, Montaño-Samaniego M, Guttman-Bazbaz R, Bravo-Estupiñan DM, Ibáñez-Hernández M. Therapeutic Applications of Poly-miRNAs and miRNA Sponges. International Journal of Molecular Sciences. 2025; 26(10):4535. https://doi.org/10.3390/ijms26104535
Chicago/Turabian StyleAvendaño-Portugal, Cynthia, Mariela Montaño-Samaniego, Raquel Guttman-Bazbaz, Diana M. Bravo-Estupiñan, and Miguel Ibáñez-Hernández. 2025. "Therapeutic Applications of Poly-miRNAs and miRNA Sponges" International Journal of Molecular Sciences 26, no. 10: 4535. https://doi.org/10.3390/ijms26104535
APA StyleAvendaño-Portugal, C., Montaño-Samaniego, M., Guttman-Bazbaz, R., Bravo-Estupiñan, D. M., & Ibáñez-Hernández, M. (2025). Therapeutic Applications of Poly-miRNAs and miRNA Sponges. International Journal of Molecular Sciences, 26(10), 4535. https://doi.org/10.3390/ijms26104535





