Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy
Abstract
1. Introduction
2. Immune Checkpoint Inhibitors (ICIs)
3. Vitamin D
3.1. The Synthesis Process of Vitamin D
3.2. The Catabolic Process and Its Regulatory Mechanisms
3.3. Physiological Function
4. The Interrelation Between Vitamin D and Lung Cancer
4.1. Impairment of Vitamin D Metabolism in Individuals with Lung Cancer
4.2. The Anti-Tumor Mechanism of Vitamin D
4.3. Vitamin D Status and Prognosis in Lung Carcinoma: Clinical Correlation Studies
4.4. The Influence of Vitamin D on the Efficacy and Adverse Effects of ICIs
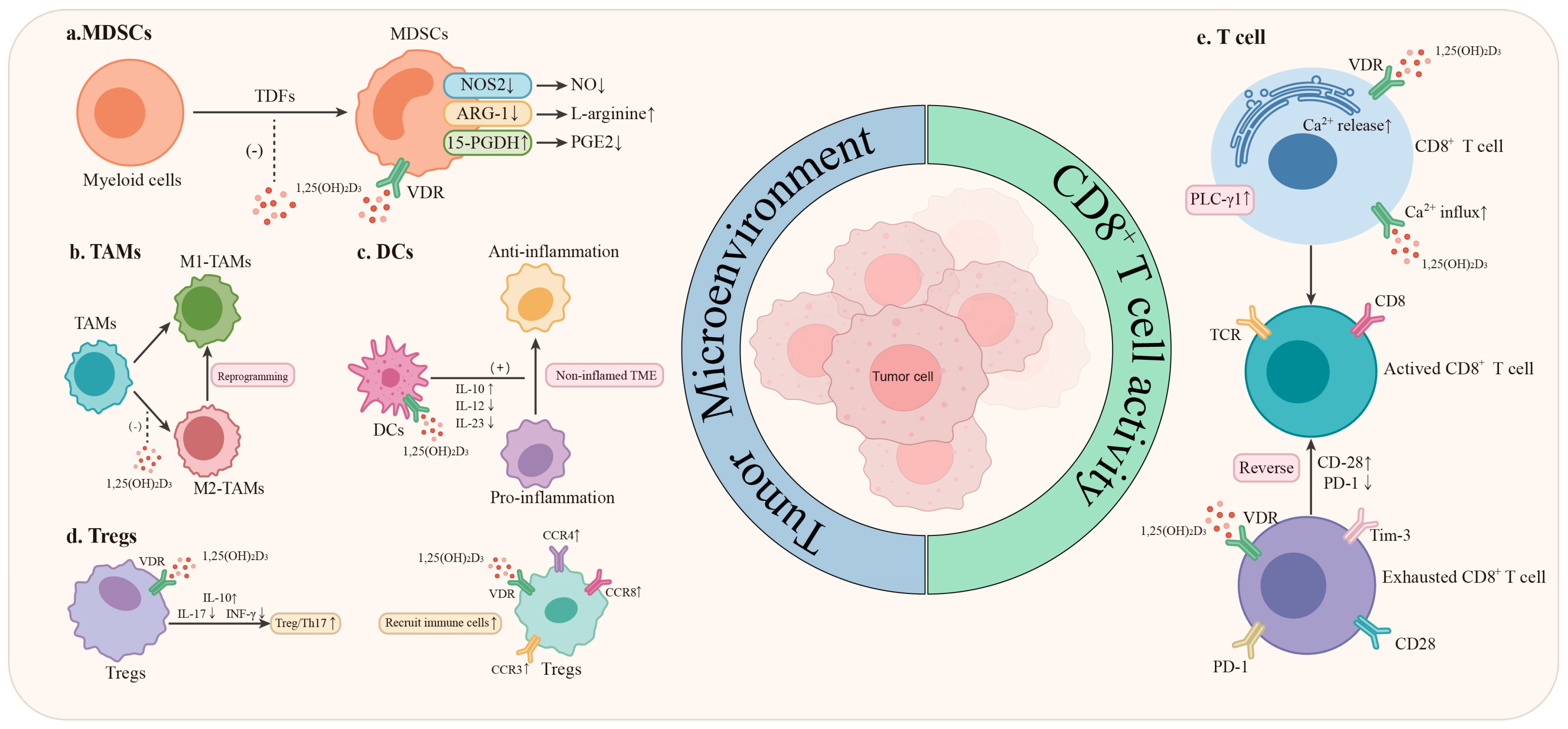
5. The Mechanism Through Which Vitamin D Enhances the Effectiveness of ICIs in Lung Cancer Patients
5.1. Innate Immunity System
5.1.1. Myeloid-Derived Suppressor Cells (MDSCs)
5.1.2. Tumor-Associated Macrophages (TAMs)
5.1.3. Dendritic Cells (DCs)
5.1.4. Natural Killer Cells (NK Cells)
5.1.5. Modulation of the Microbiome
5.2. Adaptive Immunity System
5.2.1. The Activation of CD8+ T Lymphocytes
5.2.2. Mitigate the Depletion of CD8+ T Cells Within the TME
5.2.3. CD4+ T Cell
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Han, B.F.; Zheng, R.S.; Zeng, H.M.; Wang, S.M.; Sun, K.X.; Chen, R.; Li, L.; Wei, W.Q.; He, J. Cancer Incidence and Mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Batra, A.; Boldt, G.; Shah, P.S.; Blanchette, P.; Rodrigues, G.; Vincent, M.D. Predictors of Survival Benefit from Immune Checkpoint Inhibitors in Patients with Advanced Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin. Lung Cancer 2020, 21, 106–113.e5. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, Y.S.; Liu, Z.Q.; Tian, W.T.; Zeng, Y.; Liu, J.Q.; Zhang, S.J.; Peng, Y.R.; Wu, F. Efficacy and Safety of First-Line Immune Checkpoint Inhibitors Combined with Chemotherapy for Extensive-Stage Small Cell Lung Cancer: A Network Meta-Analysis. Lung Cancer 2023, 178, 47–56. [Google Scholar] [CrossRef]
- Desai, A.P.; Adashek, J.J.; Reuss, J.E.; West, H.J.; Mansfield, A.S. Perioperative Immune Checkpoint Inhibition in Early-Stage Non-Small Cell Lung Cancer: A Review. JAMA Oncol. 2023, 9, 135–142. [Google Scholar] [CrossRef]
- Mariniello, A.; Borgeaud, M.; Weiner, M.; Frisone, D.; Kim, F.; Addeo, A. Primary and Acquired Resistance to Immunotherapy with Checkpoint Inhibitors in NSCLC: From Bedside to Bench and Back. BioDrugs 2025, 39, 215–235. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kawaura, A.; Kato, S.; Takeda, E.; Okano, T. 1 Alpha,25-Dihydroxyvitamin D3 Is a Preventive Factor in the Metastasis of Lung Cancer. Carcinogenesis 2005, 26, 429–440. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An Update on Vitamin D Signaling and Cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, X.H.; Cao, G.C.; Wu, R.; Li, K.; Yuan, W.H.; Chen, B.Y.; Sun, G.D.; Xia, X.C.; Zhang, H.; et al. 1α,25(OH)2D3 Reverses Exhaustion and Enhances Antitumor Immunity of Human Cytotoxic T Cells. J. Immunother. Cancer 2022, 10, e003477. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Burcham, G.N.; Calvert, R.D.; Elzey, B.D.; Ratliff, T.L. 1α, 25 Dihydroxyvitamin D (1,25(OH)2D) Inhibits the T Cell Suppressive Function of Myeloid Derived Suppressor Cells (MDSC). J. Steroid. Biochem. Mol. Biol. 2020, 198, 105557. [Google Scholar] [CrossRef]
- Thumkeo, D.; Punyawatthananukool, S.; Prasongtanakij, S.; Matsuura, R.; Arima, K.; Nie, H.; Yamamoto, R.; Aoyama, N.; Hamaguchi, H.; Sugahara, S.; et al. PGE2-EP2/EP4 Signaling Elicits Immunosuppression by Driving the mregDC-Treg Axis in Inflammatory Tumor Microenvironment. Cell Rep. 2022, 39, 110914. [Google Scholar] [CrossRef]
- Galus, Ł.; Michalak, M.; Lorenz, M.; Stoińska-Swiniarek, R.; Tusień Małecka, D.; Galus, A.; Kolenda, T.; Leporowska, E.; Mackiewicz, J. Vitamin D Supplementation Increases Objective Response Rate and Prolongs Progression-Free Time in Patients with Advanced Melanoma Undergoing Anti–PD-1 Therapy. Cancer 2023, 129, 2047–2055. [Google Scholar] [CrossRef]
- Bersanelli, M.; Cortellini, A.; Leonetti, A.; Parisi, A.; Tiseo, M.; Bordi, P.; Michiara, M.; Bui, S.; Cosenza, A.; Ferri, L.; et al. Systematic Vitamin D Supplementation Is Associated with Improved Outcomes and Reduced Thyroid Adverse Events in Patients with Cancer Treated with Immune Checkpoint Inhibitors: Results from the Prospective PROVIDENCE Study. Cancer Immunol. Immunother. 2023, 72, 3707–3716. [Google Scholar] [CrossRef]
- Jeon, H.; Wang, S.; Song, J.M.; Gill, H.; Cheng, H.Y. Update 2025: Management of Non-small-Cell Lung Cancer. Lung 2025, 203, 53. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Novello, S.; Kowalski, D.M.; Luft, A.; Gümüş, M.; Vicente, D.; Mazières, J.; Rodríguez-Cid, J.; Tafreshi, A.; Cheng, Y.; Lee, K.H.; et al. Pembrolizumab plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without Tremelimumab, plus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer: 3-Year Overall Survival Update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh-Gavgani, E.; Majidazar, R.; Lotfinejad, P.; Kazemi, T.; Shamekh, A. Immune Checkpoint Molecules: A Review on Pathways and Immunotherapy Implications. Immun. Inflamm. Dis. 2025, 13, e70196. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Guryev, O.; Carvalho, R.A.; Usanov, S.; Gilep, A.; Estabrook, R.W. A Pathway for the Metabolism of Vitamin D3: Unique Hydroxylated Metabolites Formed during Catalysis with Cytochrome P450scc (CYP11A1). Proc. Natl. Acad. Sci. USA 2003, 100, 14754–14759. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Li, W.; Szczesniewski, A.; Tuckey, R.C. The Cytochrome P450scc System Opens an Alternate Pathway of Vitamin D3 Metabolism. FEBSJ 2005, 272, 4080–4090. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-Mediated Metabolism of Vitamin D. J. Lipid. Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D Receptor (VDR)-Mediated Actions of 1α,25(OH)2 vitamin D3: Genomic and Non-Genomic Mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Hobrath, J.V.; Oak, A.S.W.; Tang, E.K.Y.; Tieu, E.W.; Li, W.; Tuckey, R.C.; Jetten, A.M. Endogenously Produced Nonclassical Vitamin D Hydroxy-Metabolites Act as “Biased” Agonists on VDR and Inverse Agonists on RORα and RORγ. J. Steroid. Biochem. Mol. Biol. 2017, 173, 42–56. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-Related Orphan Receptors (RORs): Critical Roles in Development, Immunity, Circadian Rhythm, and Cellular Metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef] [PubMed]
- Matsunawa, M.; Amano, Y.; Endo, K.; Uno, S.; Sakaki, T.; Yamada, S.; Makishima, M. The Aryl Hydrocarbon Receptor Activator Benzo[a]Pyrene Enhances Vitamin D3 Catabolism in Macrophages. Toxicol. Sci. 2009, 109, 50–58. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Wang, Z.; Chen, G.; Ray, P.; Lin, J.; Zhang, Z.; Zhao, L.; Beer, D.; Ray, D.; Ramnath, N. Oncogenic Potential of CYP24A1 in Lung Adenocarcinoma. J. Thorac. Oncol. 2017, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Naseeb, M.A.; Basaqr, R.O.; Albajri, E.A.; Khan, M.I.; Dev, K.; Beg, M.M.A. Cell-Free SLC30A10 Messenger Ribonucleic Acid (mRNA) Expression and Their Association with Vitamin-D Level among Non-Small Cell Lung Cancer (NSCLC) Patients. J. Cancer Res. Ther. 2023, 19, S764–S769. [Google Scholar] [CrossRef]
- De Groot, P.; Munden, R.F. Lung Cancer Epidemiology, Risk Factors, and Prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef]
- Hackshaw, A.K. Lung Cancer and Passive Smoking. Stat. Methods Med. Res. 1998, 7, 119–136. [Google Scholar] [CrossRef]
- Liang, Y.G.; Zhang, X.Q.; Peng, J.H.; Liu, J.; Chen, H.; Guo, S.X. Vitamin D-Mediated tsRNA-07804 Triggers Mitochondrial Dysfunction and Suppresses Non-Small Cell Lung Cancer Progression by Targeting CRKL. J. Cancer Res. Clin. Oncol. 2024, 150, 51. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.S.; Zhou, Y.J.; Xiao, Z.G.; Zhang, L.; Zhang, H.Y.; Li, X.B.; Li, W.P.; et al. Repurposing Vitamin D for Treatment of Human Malignancies via Targeting Tumor Microenvironment. Acta. Pharm. Sin. B 2019, 9, 203–219. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.F.; Fu, Y.; Li, Y.; Lu, W.Y.; Pan, Y.M.; Yang, J.X.; Kong, J. Inhibition of Lung Cancer by Vitamin D Depends on Downregulation of Histidine-Rich Calcium-Binding Protein. J. Adv. Res. 2021, 29, 13–22. [Google Scholar] [CrossRef]
- Yiyan, S.Y.; Yang, S.Y.S.; Li, D.J.; Li, W. Vitamin D Affects the Warburg Effect and Stemness Maintenance of Non-Small-Cell Lung Cancer Cells by Regulating the PI3K/AKT/mTOR Signaling Pathway. Curr. Cancer Drug Targets 2022, 22, 86–95. [Google Scholar] [CrossRef]
- Huang, J.D.; Dong, C.H.; Shao, S.W.; Gu, T.J.; Hu, Z.L.; Ying, J.; Zhou, D.F.; Xie, Y.P. Circulating 25-Hydroxyvitamin D Level and Prognosis of Lung Cancer Patients: A Systematic Review and Meta-Analysis. Bull. Cancer 2017, 104, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Zhang, Y.; Liu, Z.R.; Pei, Y.R.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.Y.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3717. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.Q.; Zhang, H.; Dong, Z.Q.; Zhou, Y.; Ma, J.P. Circulating 25-Hydroxyvitamin D and Lung Cancer Risk and Survival: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Medicine 2017, 96, e8613. [Google Scholar] [CrossRef]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D Supplementation and Survival of Patients with Non-Small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef]
- Zhou, W.; Heist, R.S.; Liu, G.; Asomaning, K.; Neuberg, D.S.; Hollis, B.W.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Su, L.; et al. Circulating 25-Hydroxyvitamin D Levels Predict Survival in Early-Stage Non–Small-Cell Lung Cancer Patients. JCO 2007, 25, 479–485. [Google Scholar] [CrossRef]
- Heist, R.S.; Zhou, W.; Wang, Z.; Liu, G.; Neuberg, D.; Su, L.; Asomaning, K.; Hollis, B.W.; Lynch, T.J.; Wain, J.C.; et al. Circulating 25-Hydroxyvitamin D, VDR Polymorphisms, and Survival in Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 5596–5602. [Google Scholar] [CrossRef]
- Vashi, P.G.; Edwin, P.; Popiel, B.; Gupta, D. The Relationship between Circulating 25-Hydroxyvitamin D and Survival in Newly Diagnosed Advanced Non-Small-Cell Lung Cancer. BMC Cancer 2015, 15, 1012. [Google Scholar] [CrossRef]
- You, W.; Liu, X.Y.; Tang, H.; Lu, B.; Zhou, Q.Y.; Li, Y.; Chen, M.J.; Zhao, J.; Xu, Y.; Wang, M.Z.; et al. Vitamin D Status Is Associated with Immune Checkpoint Inhibitor Efficacy and Immune-Related Adverse Event Severity in Lung Cancer Patients: A Prospective Cohort Study. J. Immunother. 2023, 46, 236. [Google Scholar] [CrossRef]
- Cusato, J.; Genova, C.; Tomasello, C.; Carrega, P.; Ottonello, S.; Pietra, G.; Mingari, M.C.; Cossu, I.; Rijavec, E.; Leggieri, A.; et al. Influence of Vitamin D in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab. Cancers 2019, 11, 125. [Google Scholar] [CrossRef]
- Grover, S.; Dougan, M.; Tyan, K.; Giobbie-Hurder, A.; Blum, S.M.; Ishizuka, J.; Qazi, T.; Elias, R.; Vora, K.B.; Ruan, A.B.; et al. Vitamin D Intake Is Associated with Decreased Risk of Immune Checkpoint Inhibitor-Induced Colitis. Cancer 2020, 126, 3758–3767. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The Hallmarks of Cancer Immune Evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef] [PubMed]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory Role of Vitamin D: A Review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Peláez, B.; Jiménez-Cortegana, C.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Role of Nutrients Regulating Myeloid Derived Suppressor Cells in Cancer: A Scoping Review. Curr. Issues Mol. Biol. 2024, 46, 9286–9297. [Google Scholar] [CrossRef]
- Najjar, Y.G.; Finke, J.H. Clinical Perspectives on Targeting of Myeloid Derived Suppressor Cells in the Treatment of Cancer. Front. Oncol. 2013, 3, 49. [Google Scholar] [CrossRef]
- Hughes, D.; Otani, T.; Yang, P.; Newman, R.A.; Yantiss, R.K.; Altorki, N.K.; Port, J.L.; Yan, M.; Markowitz, S.D.; Mazumdar, M.; et al. NAD+-Dependent 15-Hydroxyprostaglandin Dehydrogenase Regulates Levels of Bioactive Lipids in Non–Small Cell Lung Cancer. Cancer Prev. Res. 2008, 1, 241–249. [Google Scholar] [CrossRef]
- Tulimilli, S.V.; Karnik, M.; Bettadapura, A.D.S.; Sukocheva, O.A.; Tse, E.; Kuppusamy, G.; Natraj, S.M.; Madhunapantula, S.V. The Tumor Suppressor Role and Epigenetic Regulation of 15-Hydroxyprostaglandin Dehydrogenase (15-PGDH) in Cancer and Tumor Microenvironment (TME). Pharmacol. Ther. 2025, 268, 108826. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends. Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Y.Z.; Wang, L. Shaping Polarization of Tumor-Associated Macrophages in Cancer Immunotherapy. Front. Immunol. 2022, 13, 888713. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Q.; Peng, H.L. Tumor-Associated Macrophages: New Insights on Their Metabolic Regulation and Their Influence in Cancer Immunotherapy. Front. Immunol. 2023, 14, 1157291. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.H.; Li, Y.N.; Geng, X.; Xia, X.D.; Zhang, L.S.; Yang, H. Arachidonic Acid Metabolism Controls Macrophage Alternative Activation through Regulating Oxidative Phosphorylation in PPARG Dependent Manner. Front. Immunol. 2021, 12, 618501. [Google Scholar] [CrossRef]
- LI, Y.; Zhang, C.G.; Jiang, A.M.; Lin, A.Q.; Liu, Z.Q.; Cheng, X.S.; Wang, W.T.; Cheng, Q.; Zhang, J.; Wei, T.; et al. Potential Anti-Tumor Effects of Regulatory T Cells in the Tumor Microenvironment: A Review. J. Transl. Med. 2024, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, F.; Yang, W.Q.; Shi, W.Q.; Wan, J.M.; Li, J.; Pan, J.J.; Wang, P.; Qiu, J.L.; Zhang, Z.L.; et al. Effect of 1α,25(OH)2 D3 -Treated M1 and M2 Macrophages on Cell Proliferation and Migration Ability in Ovarian Cancer. Nutr. Cancer 2022, 74, 2632–2643. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Christofyllakis, K.; Neumann, F.; Bewarder, M.; Thurner, L.; Kaddu-Mulindwa, D.; Kos, I.A.; Lesan, V.; Bittenbring, J.T. Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies. Nutrients 2023, 15, 3498. [Google Scholar] [CrossRef] [PubMed]
- Giampazolias, E.; da Costa, M.P.; Lam, K.C.; Lim, K.H.J.; Cardoso, A.; Piot, C.; Chakravarty, P.; Blasche, S.; Patel, S.; Biram, A.; et al. Vitamin D Regulates Microbiome-Dependent Cancer Immunity. Science 2024, 384, 428–437. [Google Scholar] [CrossRef]
- Von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Ødum, N.; Geisler, C. Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T Cell Exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell. 2018, 33, 547–562. [Google Scholar] [CrossRef]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Tsuji, A.; Yoshikawa, S.; Morikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Asai, T.; Matsuda, S. Potential Tactics with Vitamin D and Certain Phytochemicals for Enhancing the Effectiveness of Immune-Checkpoint Blockade Therapies. Explor. Target. Antitumor. Ther. 2023, 4, 460–473. [Google Scholar] [CrossRef]
- Xia, X.D.; Xu, F.; Dai, D.X.; Xiong, A.; Sun, R.M.; Ling, Y.L.; Qiu, L.; Wang, R.; Ding, Y.; Lin, M.Y.; et al. VDR Is a Potential Prognostic Biomarker and Positively Correlated with Immune Infiltration: A Comprehensive Pan-Cancer Analysis with Experimental Verification. Biosci. Rep. 2024, 44, BSR20231845. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Lill, J.K.; Altenburger, L.M. How Chemokines Organize the Tumour Microenvironment. Nat. Rev. Cancer 2024, 24, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. Perioperative Nivolumab in Resectable Lung Cancer. N. Engl. J. Med. 2024, 390, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Spicer, J.D.; Garassino, M.C.; Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Chen, K.-N.; Dooms, C.; Majem, M.; et al. Neoadjuvant Pembrolizumab plus Chemotherapy Followed by Adjuvant Pembrolizumab Compared with Neoadjuvant Chemotherapy Alone in Patients with Early-Stage Non-Small-Cell Lung Cancer (KEYNOTE-671): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2024, 404, 1240–1252. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.-W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.-Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update from KEYNOTE-010: Pembrolizumab versus Docetaxel for Previously Treated, Programmed Death-Ligand 1–Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef]
- Lu, S.; Wu, L.; Jian, H.; Cheng, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Han, L.; Sun, M.; et al. Sintilimab plus Chemotherapy for Patients with EGFR-Mutated Non-Squamous Non-Small-Cell Lung Cancer with Disease Progression after EGFR Tyrosine-Kinase Inhibitor Therapy (ORIENT-31): Second Interim Analysis from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2023, 11, 624–636. [Google Scholar] [CrossRef]
- Xie, J.; Wu, X.; Wu, J.; Huang, F.; Xu, L. Meta-Analysis of the Efficacy and Safety of Sintilimab for Treating Advanced Non-Small Cell Lung Cancer. Oncol. Lett. 2022, 24, 425. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, G.; Niu, Y.; Zhang, G.; Ji, Y.; Yan, X.; Zhang, X.; Wang, Q.; Jing, X.; Wang, J.; et al. Sintilimab with Two Cycles of Chemotherapy for the Treatment of Advanced Squamous Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. Nat. Commun. 2024, 15, 1512. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Xu, X.; Jiang, T.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; et al. Camrelizumab plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; Hu, Y.; et al. Camrelizumab plus Carboplatin and Pemetrexed as First-Line Therapy for Advanced Non-Squamous Non-Small-Cell Lung Cancer: 5-Year Outcomes of the CameL Randomized Phase 3 Study. J. ImmunoTher. Cancer 2024, 12, e009240. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-R.; Chen, Q.; Zhou, C.; Wu, L.; Li, W.; Zhang, H.; Li, Y.; Xu, F.; Xiong, J.; Wang, Q.; et al. Effectiveness and Safety of Camrelizumab in Inoperable or Advanced Non-Small Cell Lung Cancer Patients: A Multicenter Real-World Retrospective Observational Study (CTONG2004-ADV). Transl. Lung Cancer Res. 2023, 12, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Wang, W.; Liu, H.; Chen, Q.; Chen, C.; Liu, L.; Zhang, P.; Zhao, G.; Yang, F.; Han, G.; et al. Perioperative Tislelizumab plus Neoadjuvant Chemotherapy for Patients with Resectable Non-Small-Cell Lung Cancer (RATIONALE-315): An Interim Analysis of a Randomised Clinical Trial. Lancet Respir. Med. 2025, 13, 119–129. [Google Scholar] [CrossRef]
- Daei Sorkhabi, A.; ZareDini, M.; Fazlollahi, A.; Sarkesh, A.; Naseri, A.; Mousavi, S.E.; Nejadghaderi, S.A.; Sullman, M.J.M.; Kolahi, A.-A.; Safiri, S. The Safety and Efficacy of Tislelizumab, Alone or in Combination with Chemotherapy, for the Treatment of Non-Small Cell Lung Cancer: A Systematic Review of Clinical Trials. BMC Pulm. Med. 2023, 23, 495. [Google Scholar] [CrossRef]
- Ul Bassar, W.; Ogedegbe, O.J.; Qammar, A.; Sumia, F.; Ul Islam, M.; Chaudhari, S.S.; Ntukidem, O.L.; Khan, A. Efficacy of Tislelizumab in Lung Cancer Treatment: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus 2025, 17, e80609. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Duan, Z.; Cheng, Q.; Liu, M.; Zhang, H.; Zhao, H. Efficacy and Safety of Toliparibumab for the Treatment of Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2024, 14, 1444312. [Google Scholar] [CrossRef]
- Zhong, J.; Fei, K.; Wu, L.; Li, B.; Wang, Z.; Cheng, Y.; Li, X.; Wang, X.; Han, L.; Wu, X.; et al. Toripalimab plus Chemotherapy for First Line Treatment of Advanced Non-Small Cell Lung Cancer (CHOICE-01): Final OS and Biomarker Exploration of a Randomized, Double-Blind, Phase 3 Trial. Signal Transduct. Target. Ther. 2024, 9, 369. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, W.; Wu, L.; Zhou, C.; Wang, D.; Xia, B.; Bi, M.; Fu, X.; Li, C.; Lv, D.; et al. Toripalimab plus Chemotherapy as a First-Line Therapy for Extensive-Stage Small Cell Lung Cancer: The Phase 3 EXTENTORCH Randomized Clinical Trial. JAMA Oncol. 2025, 11, 16. [Google Scholar] [CrossRef]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients with Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA. 2022, 328, 1223. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, Y.; Arkania, E.; Kilickap, S.; Ying, K.; Xu, F.; Wu, L.; Wang, X.; Viguro, M.; Makharadze, T.; et al. A Global Phase 3 Study of Serplulimab plus Chemotherapy as First-Line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer (ASTRUM-004). Cancer Cell 2024, 42, 198–208.e3. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in Combination with Carboplatin plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-Squamous Non-Small-Cell Lung Cancer (IMpower130): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus Docetaxel for Patients with Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Barlesi, F.; Cho, B.C.; Goldberg, S.B.; Yoh, K.; Zimmer Gelatti, A.C.; Mann, H.; Gopinathan, A.; Bielecka, Z.F.; Newton, M.; Aggarwal, C. PACIFIC-9: Phase III Trial of Durvalumab + Oleclumab or Monalizumab in Unresectable Stage III Non-Small-Cell Lung Cancer. Future Oncol. 2024, 20, 2137–2147. [Google Scholar] [CrossRef]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus Platinum–Etoposide versus Platinum–Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-human Phase I Study of Envafolimab, a Novel Subcutaneous Single-domain anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, M.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Sun, S.; Chen, J.; et al. Interim Survival Analysis of the Randomized Phase III GEMSTONE-302 Trial: Sugemalimab or Placebo plus Chemotherapy as First-Line Treatment for Metastatic NSCLC. Nat. Cancer 2023, 4, 860–871. [Google Scholar] [CrossRef]
- Gan, Y.; Shi, F.; Zhu, H.; Han, S.; Li, D. Adebrelimab plus Chemotherapy vs. Chemotherapy for Treatment of Extensive-Stage Small-Cell Lung Cancer from the US and Chinese Healthcare Sector Perspectives: A Cost-Effectiveness Analysis to Inform Drug Pricing. Front. Pharmacol. 2023, 14, 1241130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, J.; Zhang, W.; Xie, C.; Hu, Q.; Zhou, N.; Huang, C.; Wei, S.; Sun, H.; Li, X.; et al. Benmelstobart, Anlotinib and Chemotherapy in Extensive-Stage Small-Cell Lung Cancer: A Randomized Phase 3 Trial. Nat. Med. 2024, 30, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.-W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab with or without Tremelimumab in Combination with Chemotherapy as First-Line Therapy for Metastatic Non–Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2023, 41, 1213–1227. [Google Scholar] [CrossRef]
- Gao, X.; Xu, N.; Li, Z.; Shen, L.; Ji, K.; Zheng, Z.; Liu, D.; Lou, H.; Bai, L.; Liu, T.; et al. Safety and Antitumour Activity of Cadonilimab, an Anti-PD-1/CTLA-4 Bispecific Antibody, for Patients with Advanced Solid Tumours (COMPASSION-03): A Multicentre, Open-Label, Phase 1b/2 Trial. Lancet Oncol. 2023, 24, 1134–1146. [Google Scholar] [CrossRef]
- Ma, L.X.; Zhang, Y.; Fang, Y.; Hao, C.; Fan, Q.; Jiang, D.; Lu, L.; Su, F.; Yang, C.; Liu, Z.; et al. Anti-PD-L1 Envafolimab Combined with Anti-VEGF Suvemcitug in Pretreated Solid Tumors and Hepatocellular Carcinoma: An Open-Label Phase II Study with Safety Run-in Stage. Investig. New Drugs 2025, 43, 181–190. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Zhao, Y.; Zhao, H.; Zhou, N.; Zhang, Y.; Chen, L.; Zhou, T.; Chen, G.; Wu, T.; et al. QL1706 (Anti-PD-1 IgG4/CTLA-4 Antibody) plus Chemotherapy with or without Bevacizumab in Advanced Non-Small Cell Lung Cancer: A Multi-Cohort, Phase II Study. Signal Transduct. Target. Ther. 2024, 9, 23. [Google Scholar] [CrossRef]

| Participants | Country | Design | Follow-up | Outcomes |
|---|---|---|---|---|
| 8 studies [41] | 4 countries | Meta-analysis | 10.8 months–20 years | OS |
| 17 studies [43] | More than 5 countries | Meta-analysis | NA | OS |
| 155 patients [44] | Japan | A randomized, double-blind trial | 1 year | OS |
| 77 patients [48] | China | A prospective study | More than 1 year | OS, PFS |
| 45 patients [49] | Italy | A clinical trial | 60 days | The level of 25(OH)D and nivolumab |
| References | Design | Participants | Cohorts | Main Findings | Conclusions |
|---|---|---|---|---|---|
| [15] | A prospective controlled study | 200 patients with advanced melanoma | ∙ Group A*: Reduced VitD levels ∙ Group B*: Normal VitD levels | ∙ ORR 36.2%; mPFS 5.6 months ∙ ORR 56%; mPFS 11.25 months | Maintaining normal VitD levels may improve ICI efficacy |
| [16] | A prospective controlled study | 164 patients with advanced cancer | ∙ Cohort 1: 101 patients (anti-PD-1 + early VitD repletion) ∙ Cohort 2: 63 patients (anti-PD-1 + delayed VitD repletion) ∙ Control cohort: 238 patients (without systematic VitD repletion) | ∙ mOS 15.9 months; mTTF 5.2 months; ORR 33.3% ∙ mOS not achieved ; mTTF 23.2 months; ORR 33.3% ∙ mOS 7.1 months; mTTF 3.1 months; ORR 25% | Early VitD repletion may improve outcomes in advanced cancer patients on ICIs |
| [48] | A prospective controlled study | 77 patients with advanced lung cancers | Baseline VitD level ∙ Sufficiency (<10 ng/mL) ∙ Insufficiency (10–20 ng/mL) ∙ Deficiency (≥20 ng/mL) | ∙ mPFS 606 days VS 326 days VS 308 days ∙ PR patients’ VitD baseline level higher than non-PR patients ∙ irAE 37% | ∙ Baseline VitD levels may correlate with ICI efficacy and prognosis ∙ Supplementing VitD might enhance ICI efficacy and reduce moderate-to-severe irAEs |
| [49] | An observational study | 45 patients with advanced lung cancers | The level of Nivolumab and VitD ∙ Day 0 ∙ Day 15 ∙ Day 30 ∙ Day 45 | ∙ 25(OH)D3 12.8 ng/mL (D0) 13.6 ng/mL (D15) 11.8 ng/mL (D30) 12.9 ng /mL(D45) ∙ Nivolumab 12.5 μg/mL (D15) 22.3 μg/mL (D30) 27.1 μg/mL (D45) | The concentration of nivolumab is correlated with the VitD level |
| [50] | A retrospective study | 213 melanoma patients who received ICIs and developed irAE | ∙ The discovery cohort (213 patients) ∙ The confirmatory cohort (169 patients) | Compared with patients not taking vitamin D ∙ ICI-related colitis decreased by 65% ∙ ICI-related colitis decreased by 54% | Patients using VitD had significantly decreased likelihood of developing ICI-related colitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, Y.; Zhong, W.; Zhao, J.; Liu, X.; Gao, X.; Chen, M.; Wang, M. Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy. Int. J. Mol. Sci. 2025, 26, 4511. https://doi.org/10.3390/ijms26104511
Zhang Y, Xu Y, Zhong W, Zhao J, Liu X, Gao X, Chen M, Wang M. Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy. International Journal of Molecular Sciences. 2025; 26(10):4511. https://doi.org/10.3390/ijms26104511
Chicago/Turabian StyleZhang, Yu, Yan Xu, Wei Zhong, Jing Zhao, Xiaoyan Liu, Xiaoxing Gao, Minjiang Chen, and Mengzhao Wang. 2025. "Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy" International Journal of Molecular Sciences 26, no. 10: 4511. https://doi.org/10.3390/ijms26104511
APA StyleZhang, Y., Xu, Y., Zhong, W., Zhao, J., Liu, X., Gao, X., Chen, M., & Wang, M. (2025). Vitamin D and Immune Checkpoint Inhibitors in Lung Cancer: A Synergistic Approach to Enhancing Treatment Efficacy. International Journal of Molecular Sciences, 26(10), 4511. https://doi.org/10.3390/ijms26104511





