Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen
Abstract
1. Introduction
2. Results
2.1. Plant Production of the RBDw-Fc Recombinant Antigen
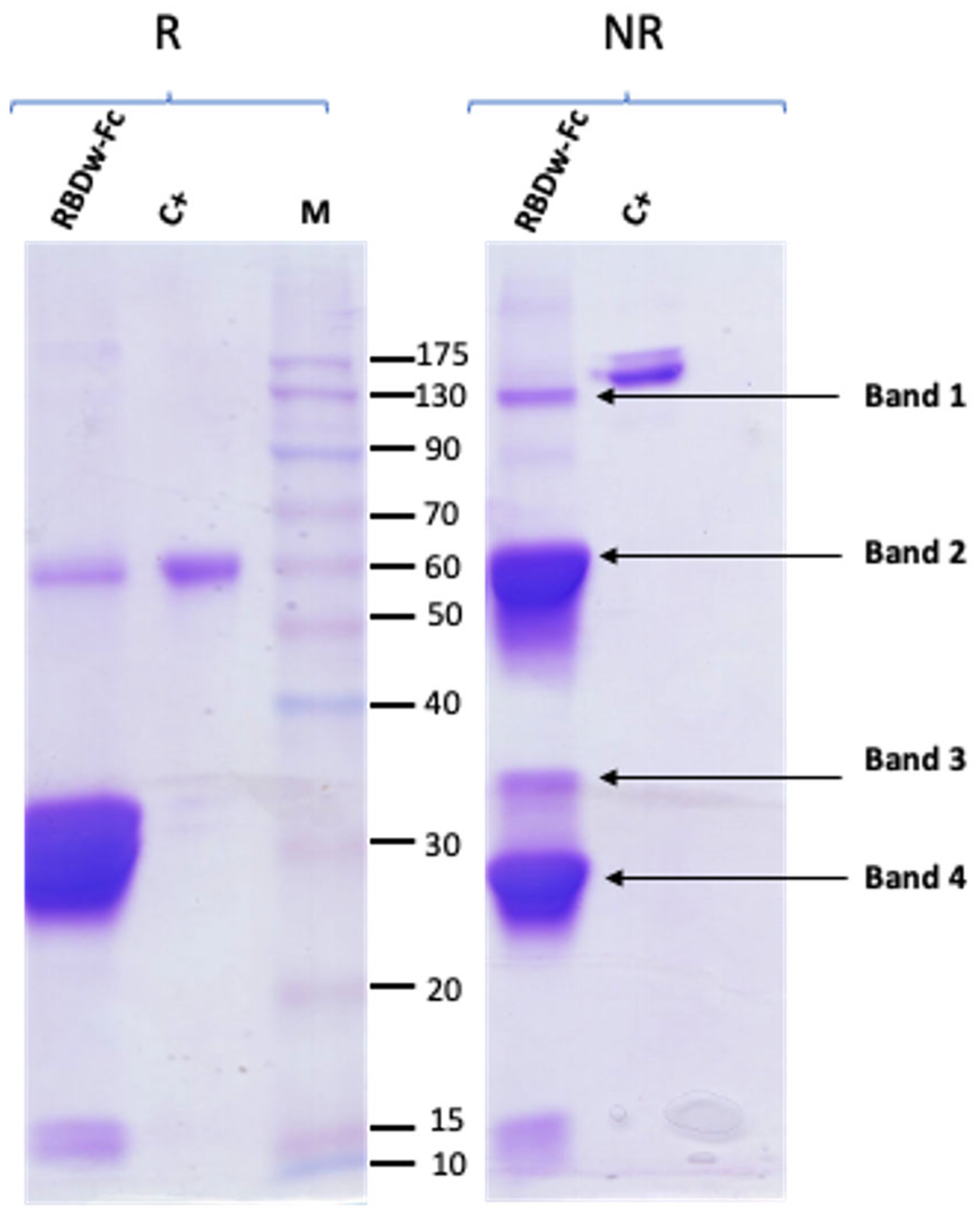
2.2. RBDw-Fc Purification and Characterization
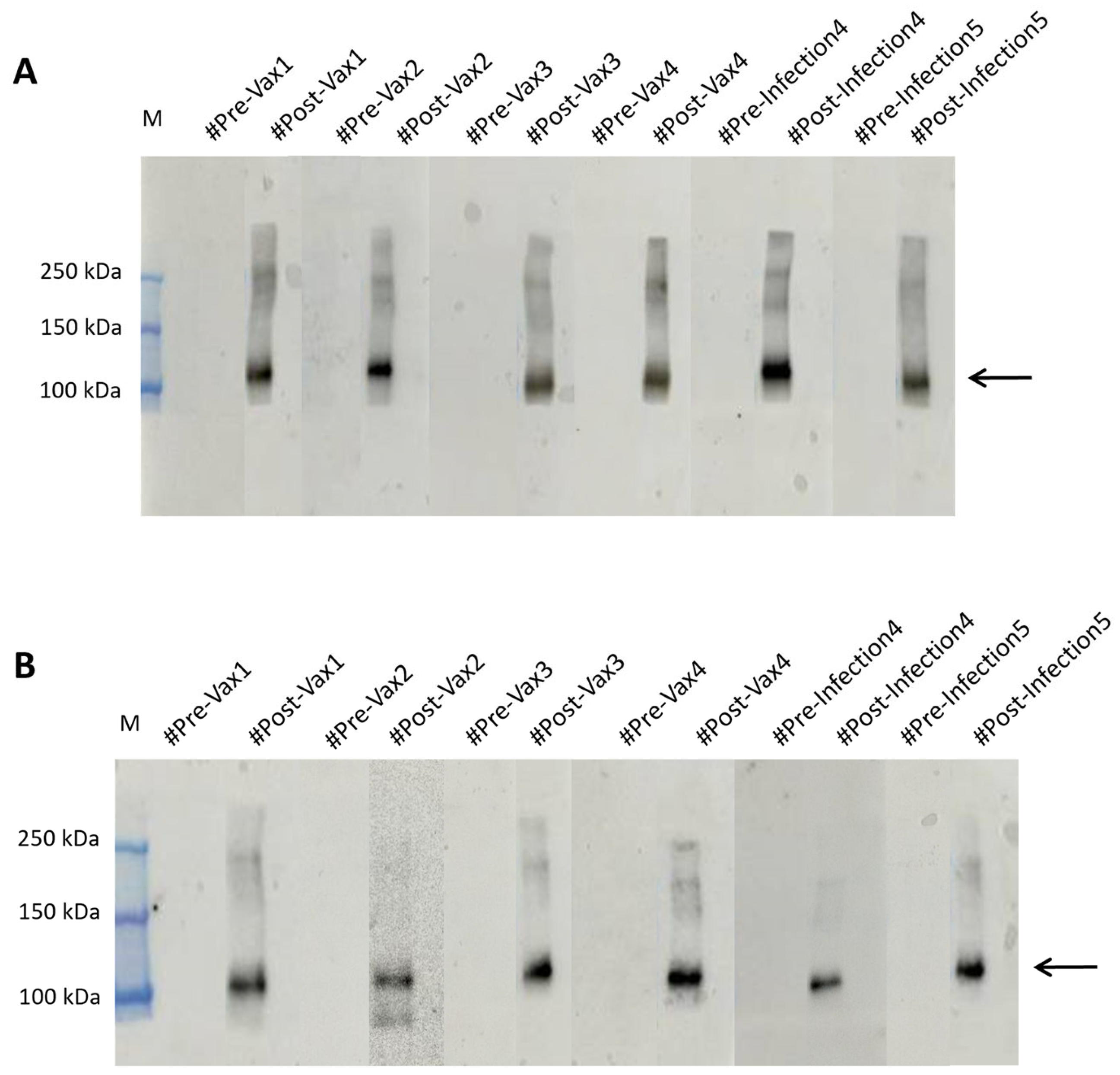
2.3. The RBDw-Fc Antigen-Based Test Development and Validation
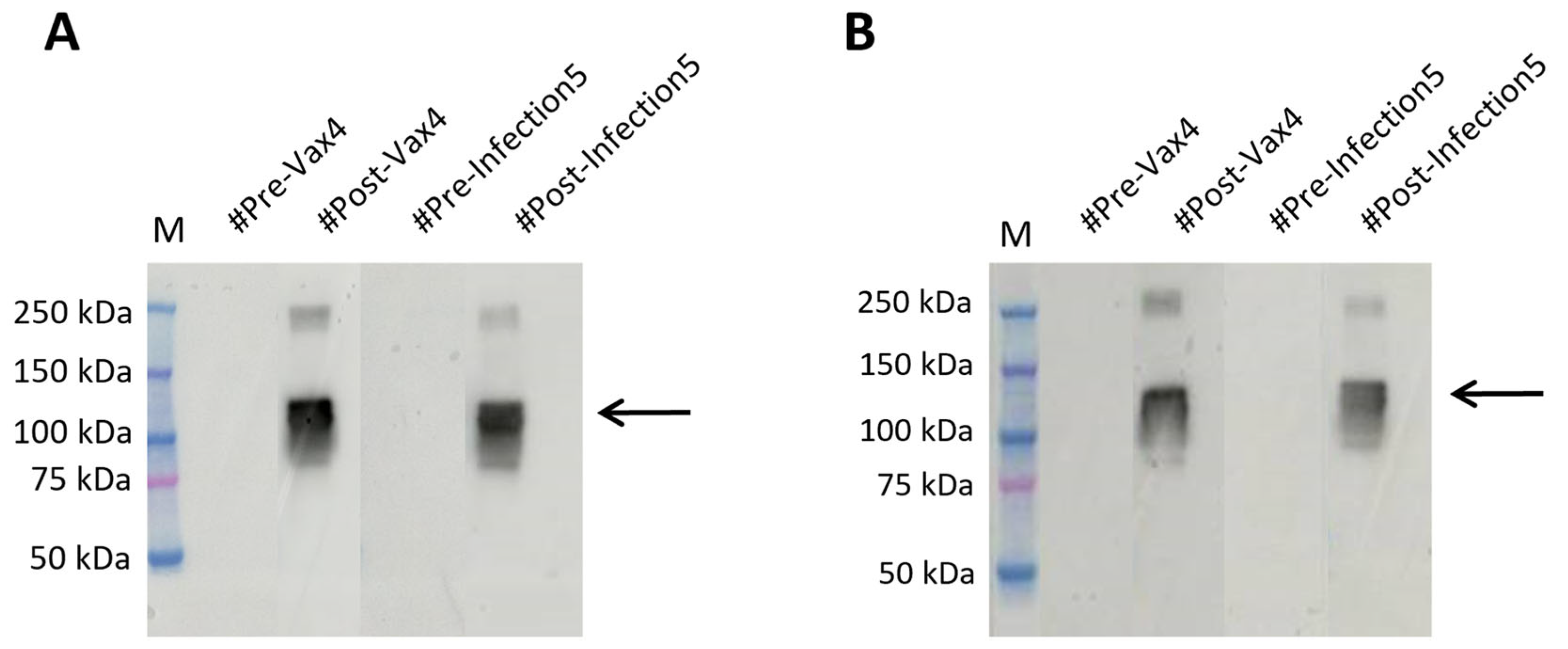
2.4. RBDw-Fc Antigen-Based Test for SARS-CoV-2 Infection Rapid Screening
2.5. RBDw-Fc Antigen in Plants: A Cheap and Innovative Test
3. Discussion
4. Materials and Methods
4.1. Plant Production of RBDw-Fc
4.2. Analysis of Plant-Produced RBDw-Fc by Western Blotting
4.3. RBDw-Fc Extraction and Protein-A Affinity Chromatography
4.4. Biological Samples Analyzed
4.5. Western Blot Analysis of Blood Samples
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obembe, O.O.; Popoola, J.O.; Leelavathi, S.; Reddy, S.V. Advances in plant molecular farming. Biotechnol. Adv. 2011, 29, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.E.; Woodard, S.L.; Howard, J.A. Plant molecular farming: Systems and products. Plant Cell Rep. 2004, 22, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Siriwattananon, K.; Wangkanont, K.; Phoolcharoen, W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 2020, 38, 10–18. [Google Scholar]
- Shanmugaraj, B.; Jirarojwattana, P.; Phoolcharoen, W. Molecular Farming Strategy for the Rapid Production of Protein-Based Reagents for Use in Infectious Disease Diagnostics. Planta Med. 2023, 89, 1010–1020. [Google Scholar] [CrossRef]
- Bharathi, J.K.; Suresh, P.; Prakash, M.A.S.; Muneer, S. Exploring recent progress of molecular farming for therapeutic and recombinant molecules in plant systems. Heliyon 2024, 10, e37634. [Google Scholar] [CrossRef]
- Lico, C.; Santi, L.; Baschieri, S.; Noris, E.; Marusic, C.; Donini, M.; Pedrazzini, E.; Maga, G.; Franconi, R.; Di Bonito, P.; et al. Plant Molecular Farming as a Strategy Against COVID-19—The Italian Perspective. Front. Plant Sci. 2020, 11, 609910. [Google Scholar] [CrossRef]
- Demurtas, O.C.; Massa, S.; Illiano, E.; De Martinis, D.; Chan, P.K.S.; Di Bonito, P.; Franconi, R. Antigen Production in Plant to Tackle Infectious Diseases Flare Up: The Case of SARS. Front. Plant Sci. 2016, 7, 54. [Google Scholar] [CrossRef]
- Tiwari, S.; Verma, P.C.; Singh, P.K.; Tuli, R. Plants as bioreactors for the production of vaccine antigens. Biotechnol. Adv. 2009, 27, 449–467. [Google Scholar] [CrossRef]
- Zahmanova, G.; Takova, K.; Valkova, R.; Toneva, V.; Minkov, I.; Andonov, A.; Lukov, G.L. Plant-Derived Recombinant Vaccines against Zoonotic Viruses. Life 2022, 12, 156. [Google Scholar] [CrossRef]
- Jung, J.W.; Park, P.G.; Lee, W.K.; Shin, J.H.; Jang, M.H.; Seo, E.H.; An, T.; Kim, Y.B.; Moon, M.H.; Choi, S.K.; et al. Production of Plant-Derived Japanese Encephalitis Virus Multi-Epitope Peptide in Nicotiana benthamiana and Immunological Response in Mice. Int. J. Mol. Sci. 2023, 24, 11643. [Google Scholar] [CrossRef]
- Vo, D.K.; Trinh, K.T.L. Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines. Vaccines 2025, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis -A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Frigerio, R.; Salzano, A.M.; Scaloni, A.; Marusic, C.; Donini, M. Plant production of recombinant antigens containing the receptor binding domain (RBD) of two SARS-CoV-2 variants. Biotechnol. Lett. 2024, 46, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S. Plant Molecular Pharming and Plant-Derived Compounds towards Generation of Vaccines and Therapeutics against Coronaviruses. Vaccines 2022, 10, 1805. [Google Scholar] [CrossRef]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef]
- Song, S.; Kim, H.; Jang, E.Y.; Jeon, H.; Diao, H.; Khan, R.I.; Lee, M.; Lee, Y.J.; Nam, J.; Kim, S.; et al. SARS-CoV-2 spike trimer vaccine expressed in Nicotiana benthamiana adjuvanted with Alum elicits protective immune responses in mice. Plant Biotechnol. J. 2022, 20, 2298–2312. [Google Scholar] [CrossRef]
- Zahmanova, G.; Aljabali, A.A.A.; Takova, K.; Minkov, G.; Tambuwala, M.M.; Minkov, I.; Lomonossoff, G.P. Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17575. [Google Scholar] [CrossRef]
- Moussavou, G.; Ko, K.; Lee, J.H.; Choo, Y.K. Production of monoclonal antibodies in plants for cancer immunotherapy. Biomed. Res. Int. 2015, 2015, 306164. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 153359, 258–259. [Google Scholar] [CrossRef]
- Freeman, K.G.; Wetzel, K.S.; Zhang, Y.; Zack, K.M.; Jacobs-Sera, D.; Walters, S.M.; Barbeau, D.J.; McElroy, A.K.; Williams, J.V.; Hatfull, G.F. A Mycobacteriophage-Based Vaccine Platform: SARS-CoV-2 Antigen Expression and Display. Microorganisms 2021, 9, 2414. [Google Scholar] [CrossRef]
- Klumpp-Thomas, C.; Kalish, H.; Drew, M.; Hunsberger, S.; Snead, K.; Fay, M.P.; Mehalko, J.; Shunmugavel, A.; Wall, V.; Frank, P.; et al. Standardization of enzyme-linked immunosorbent assays for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. medRxiv 2020. [Google Scholar]
- Diego-Martin, B.; González, B.; Vazquez-Vilar, M.; Selma, S.; Mateos-Fernández, R.; Gianoglio, S.; Fernández-Del-Carmen, A.; Orzáez, D. Pilot Production of SARS-CoV-2 Related Proteins in Plants: A Proof of Concept for Rapid Repurposing of Indoor Farms Into Biomanufacturing Facilities. Front. Plant Sci. 2020, 11, 612781. [Google Scholar] [CrossRef] [PubMed]
- Rattanapisit, K.; Bulaon, C.J.I.; Khorattanakulchai, N.; Shanmugaraj, B.; Wangkanont, K.; Phoolcharoen, W. Plant-produced SARS-CoV-2 receptor binding domain (RBD) variants showed differential binding efficiency with anti-spike specific monoclonal antibodies. PLoS ONE 2021, 16, e0253574. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-J.; König-Beihammer, J.; Vavra, U.; Schwestka, J.; Kienzl, N.F.; Klausberger, M.; Laurent, E.; Grünwald-Gruber, C.; Vierlinger, K.; Hofner, M.; et al. N-Glycosylation of the SARS-CoV-2 Receptor Binding Domain Is Important for Functional Expression in Plants. Front. Plant Sci. 2021, 12, 689104. [Google Scholar] [CrossRef]
- Siriwattananon, K.; Manopwisedjaroen, S.; Shanmugaraj, B.; Rattanapisit, K.; Phumiamorn, S.; Sapsutthipas, S.; Trisiriwanich, S.; Prompetchara, E.; Ketloy, C.; Buranapraditkun, S.; et al. Plant-Produced Receptor-Binding Domain of SARS-CoV-2 Elicits Potent Neutralizing Responses in Mice and Non-human Primates. Front. Plant Sci. 2021, 12, 682953. [Google Scholar] [CrossRef]
- Jutras, P.V.; Goulet, M.C.; Lavoie, P.O.; D’Aoust, M.A.; Sainsbury, F.; Michaud, D. Recombinant protein susceptibility to proteolysis in the plant cell secretory pathway is pH-dependent. Plant Biotechnol. J. 2018, 16, 1928–1938. [Google Scholar] [CrossRef]
- Donini, M.; Lombardi, R.; Lonoce, C.; Di Carli, M.; Marusic, C.; Morea, V.; Di Micco, P. Antibody proteolysis: A common picture emerging from plants. Bioengineered 2015, 6, 299–302. [Google Scholar] [CrossRef]
- Jutras, P.V.; Marusic, C.; Lonoce, C.; Deflers, C.; Goulet, M.C.; Benvenuto, E.; Michaud, D.; Donini, M. An Accessory Protease Inhibitor to Increase the Yield and Quality of a Tumour-Targeting mAb in Nicotiana benthamiana Leaves. PLoS ONE 2016, 11, e0167086. [Google Scholar] [CrossRef]
- Hehle, V.K.; Paul, M.J.; Roberts, V.A.; van Dolleweerd, C.J.; Ma, J.K. Site-targeted mutagenesis for stabilization of recombinant monoclonal antibody expressed in tobacco (Nicotiana tabacum) plants. FASEB J. 2016, 30, 590–598. [Google Scholar] [CrossRef]
- Tusé, D.; Nandi, S.; McDonald, K.A.; Buyel, J.F. The Emergency Response Capacity of Plant-Based Biopharmaceutical Manufacturing-What It Is and What It Could Be. Front. Plant Sci. 2020, 11, 594019. [Google Scholar] [CrossRef]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef]
- Lombardi, R.; Circelli, P.; Villani, M.E.; Buriani, G.; Nardi, L.; Coppola, V.; Bianco, L.; Benvenuto, E.; Donini, M.; Marusic, C. High-level HIV-1 Nef transient expression in Nicotiana benthamiana using the P19 gene silencing suppressor protein of Artichoke Mottled Crinckle Virus. BMC Biotechnol. 2009, 9, 96. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miele, C.; Ramadan, D.; Lupacchini, L.; Marusic, C.; D’Argenio, V.; Valente, M.G.; Spila, A.; Gessoni, G.; Alfano, V.; Ferroni, P.; et al. Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen. Int. J. Mol. Sci. 2025, 26, 4500. https://doi.org/10.3390/ijms26104500
Miele C, Ramadan D, Lupacchini L, Marusic C, D’Argenio V, Valente MG, Spila A, Gessoni G, Alfano V, Ferroni P, et al. Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen. International Journal of Molecular Sciences. 2025; 26(10):4500. https://doi.org/10.3390/ijms26104500
Chicago/Turabian StyleMiele, Chiara, Dania Ramadan, Leonardo Lupacchini, Carla Marusic, Valeria D’Argenio, Maria Giovanna Valente, Antonella Spila, Gianluca Gessoni, Veronica Alfano, Patrizia Ferroni, and et al. 2025. "Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen" International Journal of Molecular Sciences 26, no. 10: 4500. https://doi.org/10.3390/ijms26104500
APA StyleMiele, C., Ramadan, D., Lupacchini, L., Marusic, C., D’Argenio, V., Valente, M. G., Spila, A., Gessoni, G., Alfano, V., Ferroni, P., Donini, M., & Guadagni, F. (2025). Rapid and Cost-Effective Diagnostic Blot Assays Based on the Use of Plant-Produced Recombinant Antigens: Lessons Learned from the SARS-CoV-2 RBD Antigen. International Journal of Molecular Sciences, 26(10), 4500. https://doi.org/10.3390/ijms26104500






