Unveiling the Chemical Composition, Antioxidant, and Antimicrobial Potentials of Foeniculum vulgare Mill: A Combined In Vitro and In Silico Approach
Abstract
1. Introduction
2. Results
2.1. Quality Control of Plant Material
2.2. Phytochemical Screening of Plant Material
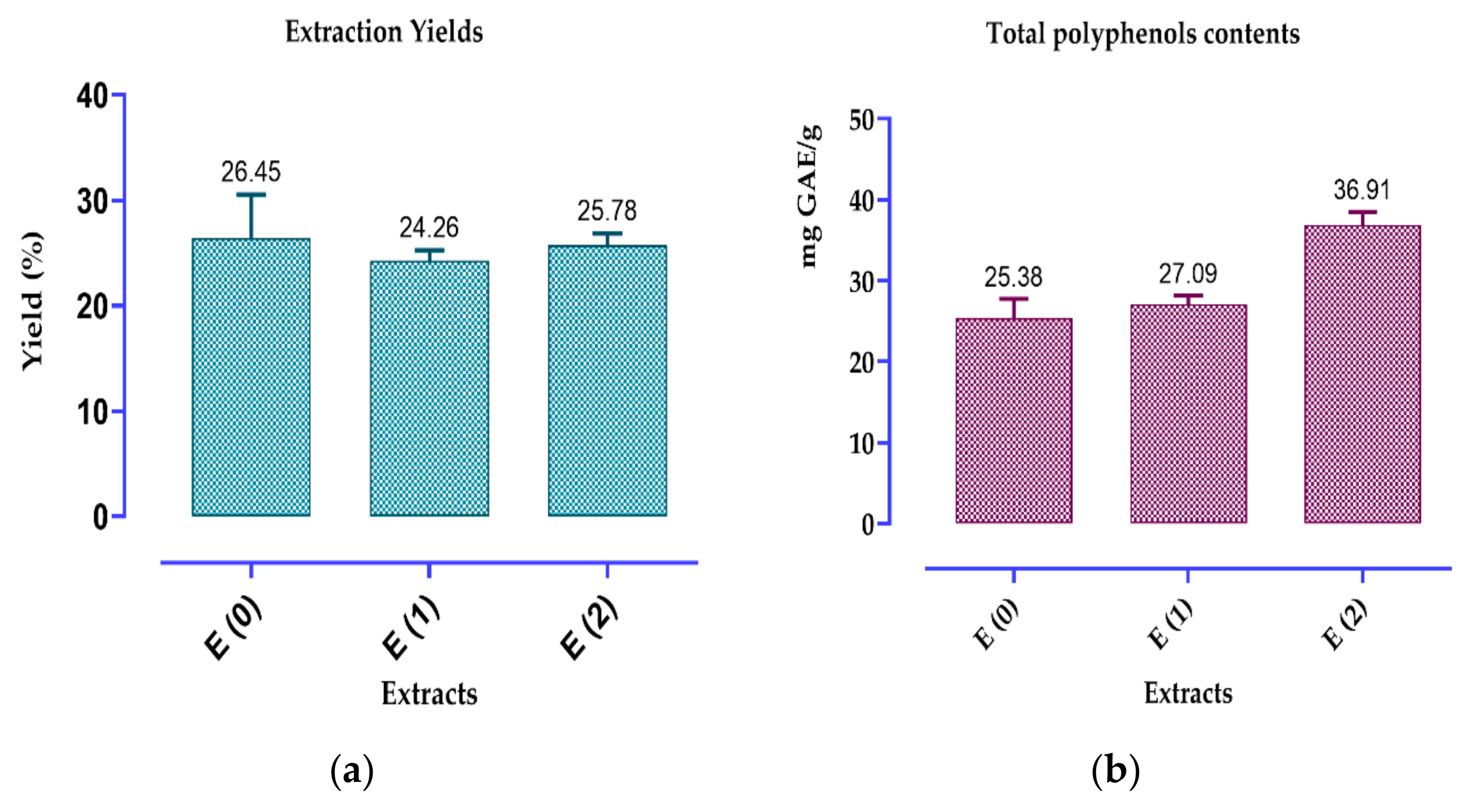
2.3. Contents of Polyphenols, Flavonoids and Condensed Tannins
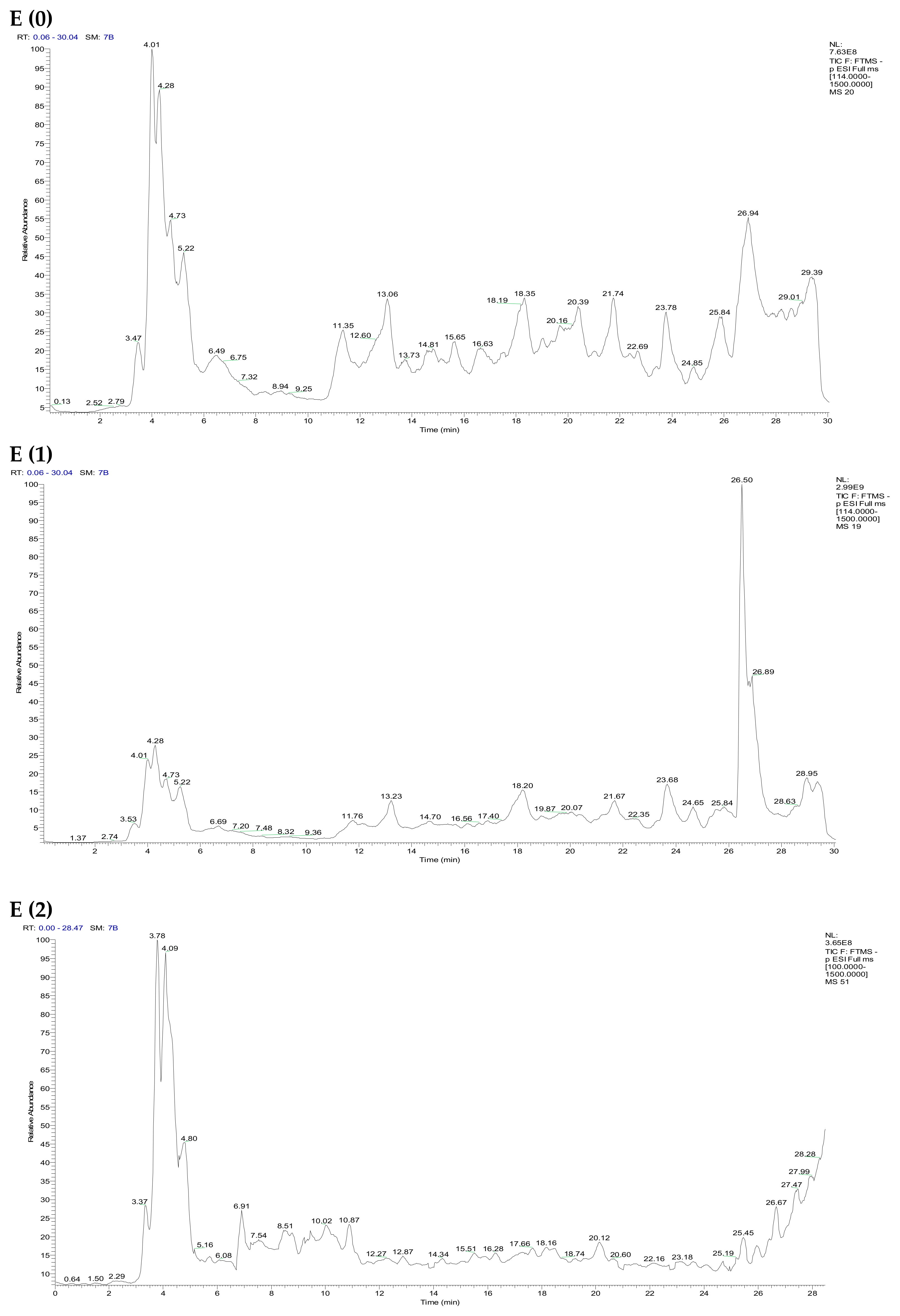
2.4. Identification of the Chemical Composition of Phenolic Compounds from the Extracts of F. vulgare by LC/UV
2.5. Yields and Quality Control of EO
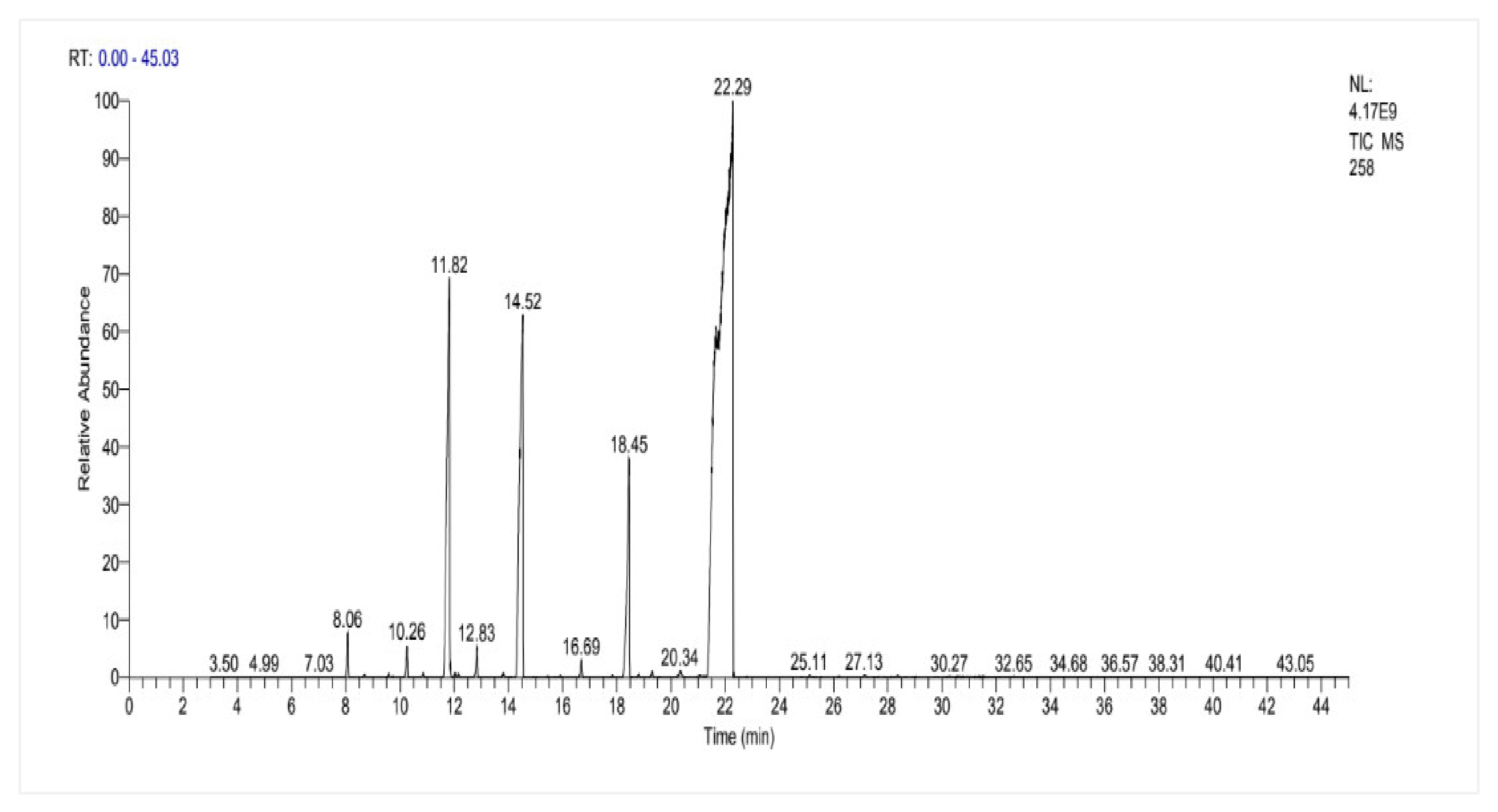
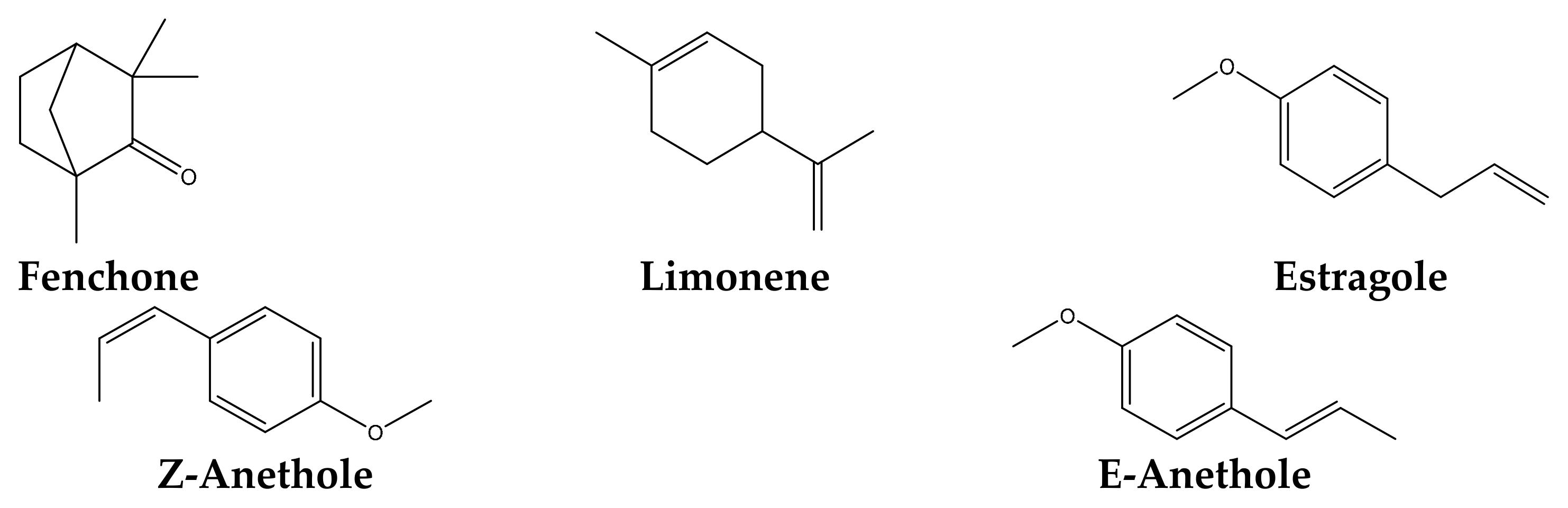
2.6. Chemical Composition of F. vulgare Seed EO
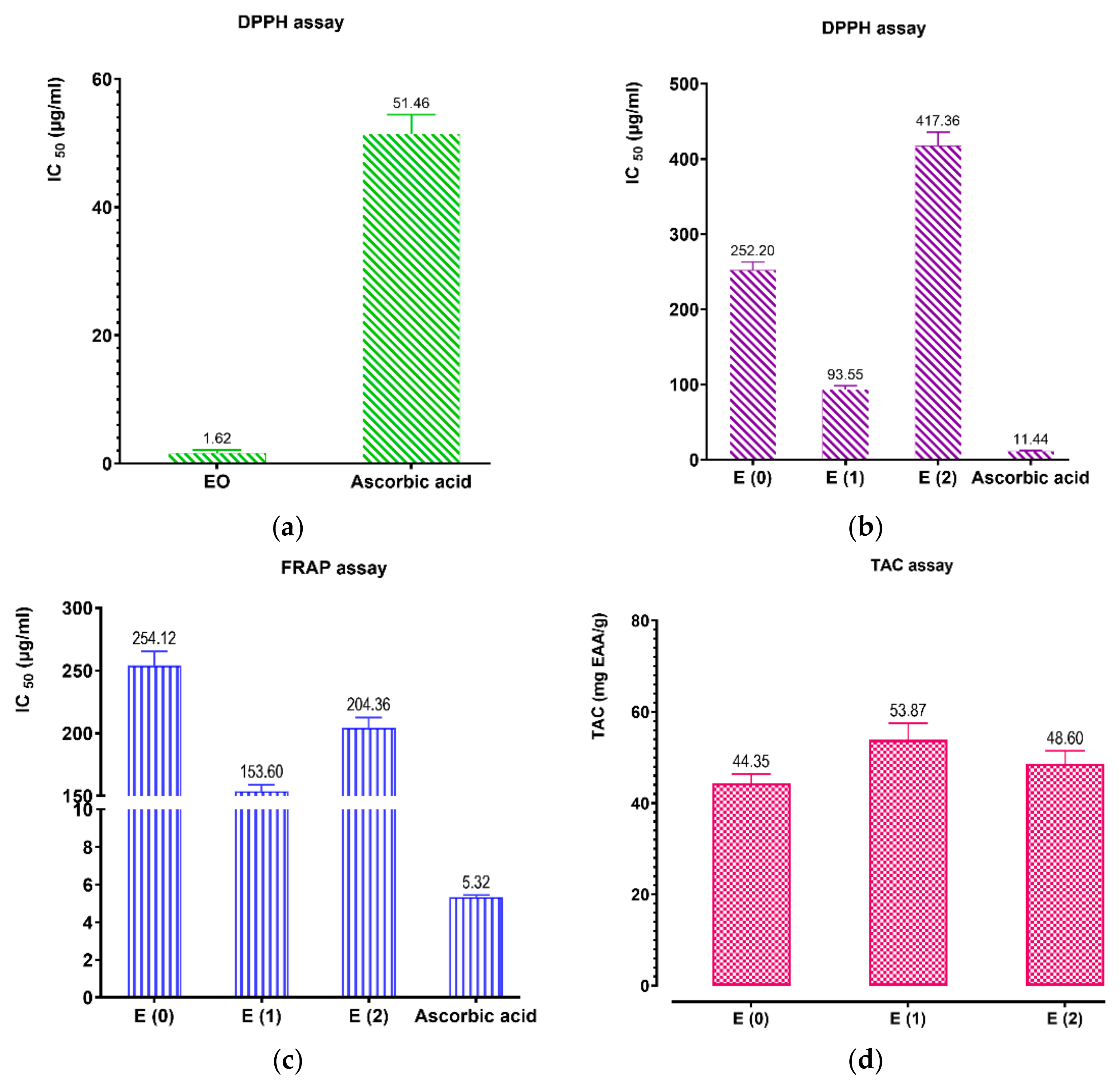
2.7. Antioxidant Activities
2.8. Antimicrobial Activity of Essential Oils and Extracts of F. vulgare
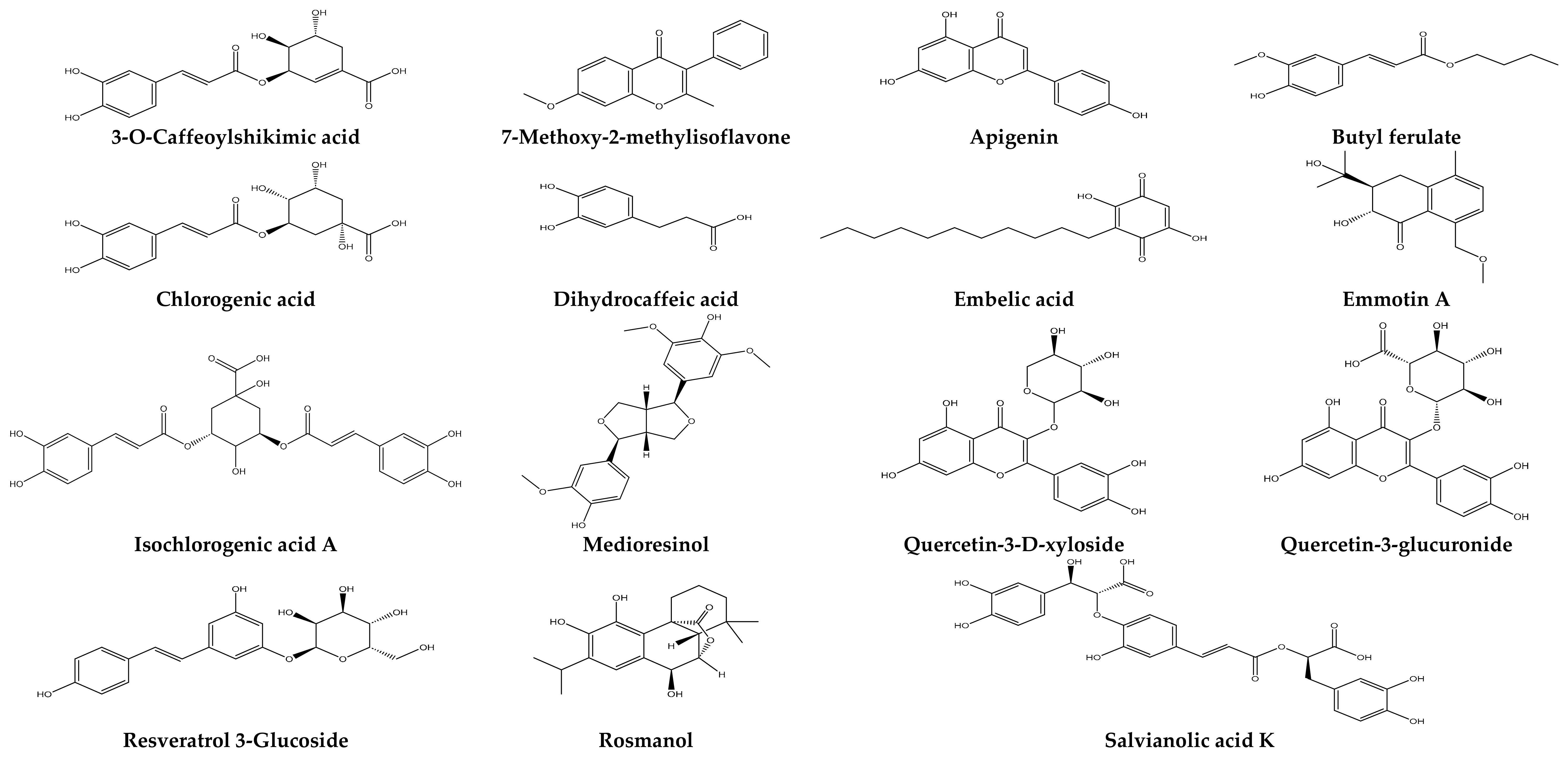
2.9. Molecular Docking
2.9.1. Interaction with Antibacterial Proteins
2.9.2. Interaction with Antioxidant Proteins
| Molecules\Proteins | 3RAE | 3KP5 | ||
|---|---|---|---|---|
| 2D | 3D | 2D | 3D | |
| Chlorogenic acid |  |  |  |  |
| Isochlorogenic acid A |  | 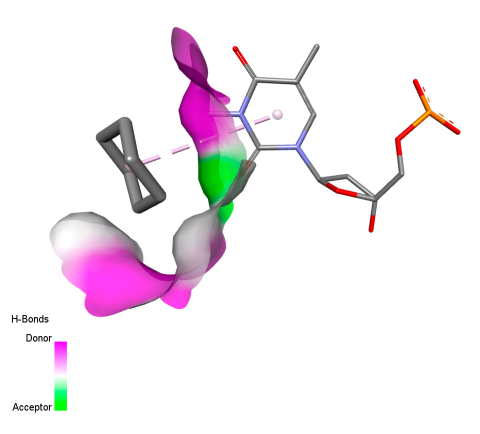 |  |  |
| Quercetin-3-D-xyloside |  |  | 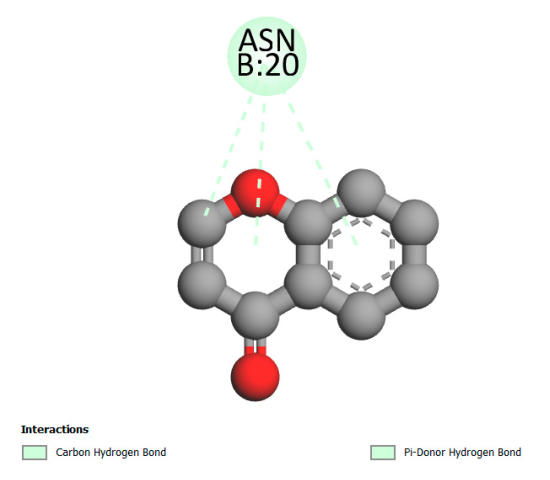 | 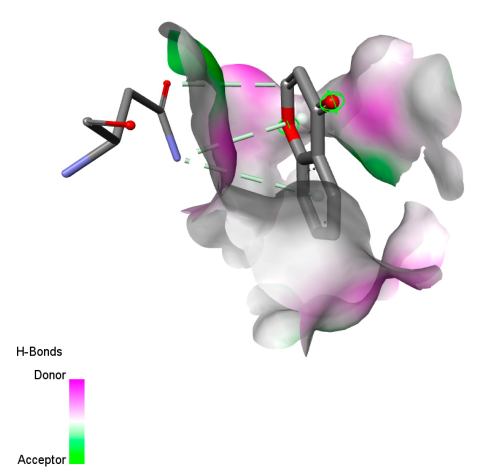 |
| Quercetin-3-glucuronide | 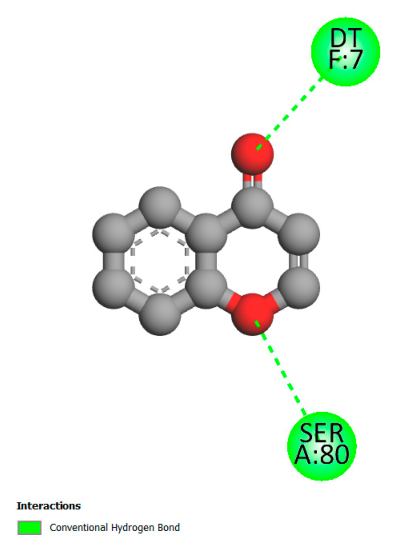 |  | 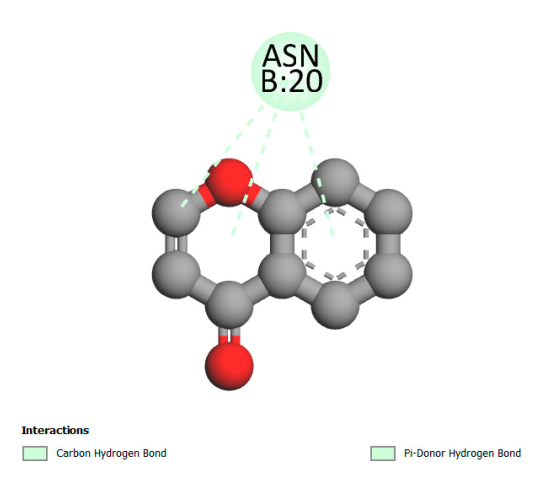 | 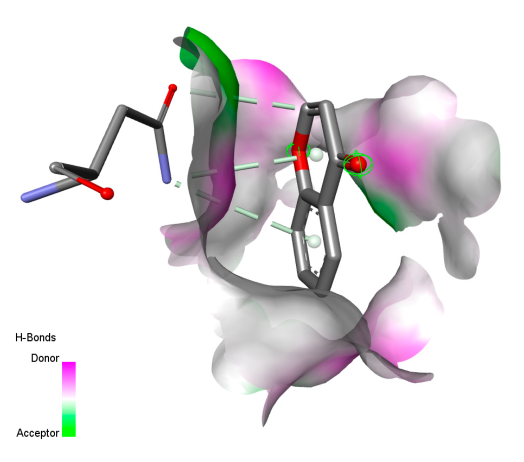 |
| Rosmanol | 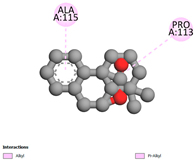 |  |  | 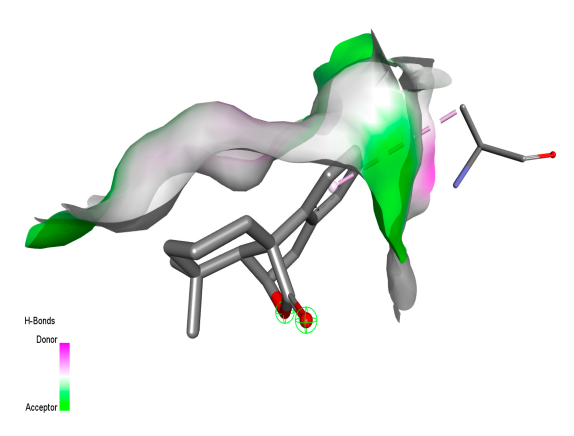 |
| Salvianolic acid K | 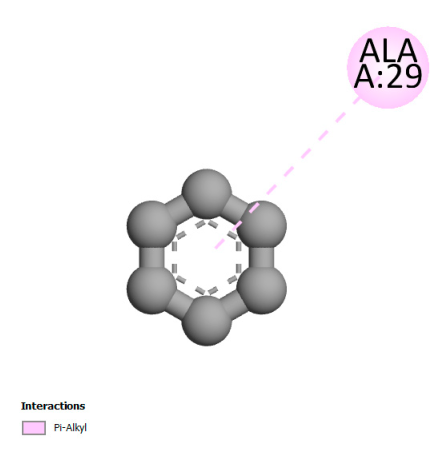 |  |  |  |
3. Materials and Methods
3.1. Plant Material
3.2. Quality Control of Plant Material
3.2.1. Moisture Content (MC)
3.2.2. Determination of pH
3.2.3. Determination of Titratable Acidity
3.2.4. Ash Content
3.2.5. Dosage of Metallic Trace Elements (MTE) by ICP-AES
3.3. Phytochemical Screening
3.3.1. Primary Metabolites
3.3.2. Secondary Metabolites
3.4. Preparation of Seed Extracts of F. vulgare
3.4.1. Extraction by Soxhlet
3.4.2. Extraction by Decoction
3.5. Dosage of Phenolic Compounds
3.5.1. Determination of Total Polyphenols
3.5.2. Dosage of Flavonoids
3.5.3. Dosage of Condensed Tannins
3.6. Identification of Chemical Composition by HPLC/UV-DAD
3.7. Extraction and Determination of Essential Oil Yield
3.7.1. Density
3.7.2. Analysis of Essential Oil by Gas Chromatography Mass Spectrometry
3.8. Antimicrobial Activity
3.8.1. Microbial Material
3.8.2. Determination of Minimum Inhibitory Concentration, Minimum Bactericidal Concentration, and Minimum Fungicidal Concentration
3.9. Antioxidant Activity
3.9.1. DPPH* Trapping Free Radicals
3.9.2. FRAP Iron Reduction Power Test
3.9.3. Total Antioxidant Capacity (TAC)
3.10. Molecular Docking
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Tufail, T.; Badar Ul Ain, H.; Awuchi, C.G. Pharmacological, Nutraceutical, Functional and Therapeutic Properties of Fennel (Foeniculum vulgare). Int. J. Food Prop. 2023, 26, 915–927. [Google Scholar] [CrossRef]
- Rahimi, R.; Ardekani, M.R.S. Medicinal Properties of Foeniculum vulgare Mill. in Traditional Iranian Medicine and Modern Phytotherapy. Chin. J. Integr. Med. 2013, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Saddiqi, H.A.; Iqbal, Z. Usage and Significance of Fennel (Foeniculum vulgare Mill.) Seeds in Eastern Medicine. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 461–467. ISBN 978-0-12-375688-6. [Google Scholar]
- Grover, S.; Malik, C.; Hora, A.; Kushwaha, H. Botany, Cultivation, Chemical Constituents and Genetic Diversity in Fennel (Foeniculum vulgare Mill): A Review. LS Int. J. Life Sci. 2013, 2, 128. [Google Scholar] [CrossRef]
- Rani, S.N.; Aslam, Z.N.; Babar, S.N. Foeniculum vulgare (Fennel): A Versatile Herb With Culinary, Medicinal, and Environmental Significance. In Therapeutic and Pharmacological Applications of Ethnobotany; IGI Global Scientific Publishing: Hershey, PA, USA, 2024; pp. 155–188. ISBN 979-8-3693-1986-4. [Google Scholar]
- Magagula, T.I.P. Assessment of the Invasive Potential of Foeniculum vulgare (Fennel) in the Sarah Baartman District and Nelson Mandela Bay Municipalities in South Africas Eastern Cape Province; University of the Witwatersrand: Johannesburg, South Africa, 2018. [Google Scholar]
- Debnath, S.; Kumar, H.; Sharma, A. Foeniculum vulgare from Spice to Pharma: Recent Advances in Its Medicinal Value, Bioactivities and Perspectives. Tradit. Integr. Med. 2023, 217–229. [Google Scholar] [CrossRef]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef]
- Authority (EFSA), E.F.S. Protocol for the Scientific Opinion on the Evaluation of the Safety in Use of Preparations from the Fruits of Sweet and Bitter Fennel (Foeniculum vulgare Mill. and Foeniculum Piperitum (Ucria) C.Presl). EFSA Support. Publ. 2023, 20, 8245E. [Google Scholar] [CrossRef]
- Guidi, L.; Landi, M. Aromatic Plants: Use and Nutraceutical Properties. In Novel Plant Bioresources; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 303–345. ISBN 978-1-118-46056-6. [Google Scholar]
- Zafar, S.; Khan, M.K.; Perveen, S.; Iqbal, M.; AL-Huqail, A.A. Fennel. In Essentials of Medicinal and Aromatic Crops; Zia-Ul-Haq, M., Abdulkreem AL-Huqail, A., Riaz, M., Farooq Gohar, U., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 483–514. ISBN 978-3-031-35403-8. [Google Scholar]
- Sayed Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Chokr, A.; Merah, O. Fennel Oil and By-Products Seed Characterization and Their Potential Applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Rasul, A.; Akhtar, N.; Khan, B.A.; Mahmood, T.; Zaman, S.U.; Khan, H.M.S. Formulation Development of a Cream Containing Fennel Extract: In Vivo Evaluation for Anti-Aging Effects. Pharm. Int. J. Pharm. Sci. 2012, 67, 54–58. [Google Scholar] [CrossRef]
- Milenković, A.; Ilić, Z.; Stanojević, L.; Milenković, L.; Šunić, L.; Lalević, D.; Stanojević, J.; Danilović, B.; Cvetković, D. Essential Oil Yield, Composition, Antioxidant and Microbial Activity of Wild Fennel (Foeniculum vulgare Mill.) from Monte Negro Coast. Horticulturae 2022, 8, 1015. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, X.; Liang, J. In Vitro Antifungal Activity and Mechanism of Essential Oil from Fennel (Foeniculum vulgare L.) on Dermatophyte Species. J. Med. Microbiol. 2015, 64, 93–103. [Google Scholar] [CrossRef]
- Syed, F.Q.; Mirza, M.B.; Elkady, A.I.; Hakeem, K.R.; Alkarim, S. An Insight of Multitudinous and Inveterate Pharmacological Applications of Foeniculum vulgare (Fennel). In Plant and Human Health, Volume 3: Pharmacology and Therapeutic Uses; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 231–254. ISBN 978-3-030-04408-4. [Google Scholar]
- Lim, T.K. Foeniculum vulgare. In Edible Medicinal And Non-Medicinal Plants: Volume 5, Fruits; Lim, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 36–59. ISBN 978-94-007-5653-3. [Google Scholar]
- Singh, S.; Datta, R.; Johri, P.; Trivedi, M. Phytopharmaceuticals and Biotechnology of Herbal Plants; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-000-81129-2. [Google Scholar]
- Choi, E.-M.; Hwang, J.-K. Antiinflammatory, Analgesic and Antioxidant Activities of the Fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.; Saleh, A.; Mohammed, A.-D.; Mansour, A.-S.; Mohammed, A.-Y.; Ibrahim, A.-M. Gastroprotective Effect of Fennel “Foeniculum vulgare” a Commonly Used Spice in Arab Traditional Medicine. Обзoры пo клиническoй фармакoлoгии и лекарственнoй терапии 2012, 10, 91. [Google Scholar]
- Cioanca, O.; Hancianu, M.; Mircea, C.; Trifan, A.; Hritcu, L. Essential Oils from Apiaceae as Valuable Resources in Neurological Disorders: Foeniculi Vulgare Aetheroleum. Ind. Crops Prod. 2016, 88, 51–57. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical Composition, Antibacterial Activity and Mechanism of Action of Essential Oil from Seeds of Fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and Vapour-Phase Antifungal Activities of Essential Oils against Candida Albicans and Non-Albicans Candida. BMC Complement. Altern. Med. 2016, 16, 330. [Google Scholar] [CrossRef]
- Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. [Google Scholar] [CrossRef]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and Anticarcinogenic Effects of Methanolic Extract and Volatile Oil of Fennel Seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef]
- Abou, N.; Abou El-Soud, N.; El-Laithy, N.; El-Saeed, G.; Wahby, M.S.; Khalil, M.; Morsy, F.; Shaffie, N.; Abou, C.; Wahby, S. Antidiabetic Activities of Foeniculum vulgare Mill. Essential Oil in Streptozotocin-Induced Diabetic Rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Koppula, S.; Kumar, H. Foeniculum vulgare Mill (Umbelliferae) Attenuates Stress and Improves Memory in Wister Rats. Trop. J. Pharm. Res. 2013, 12, 553–558. [Google Scholar] [CrossRef]
- Kooti, W.; Moradi, M.; Ali-Akbari, S.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Therapeutic and Pharmacological Potential of Foeniculum vulgare Mill: A Review. J. Herb. Med. Pharmacol. 2015, 4, 1–9. [Google Scholar]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare Mill.) Seeds from Pakistan. Flavour. Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare L.) and Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Vella, F.M.; Pignone, D.; Laratta, B. The Mediterranean Species Calendula Officinalis and Foeniculum vulgare as Valuable Source of Bioactive Compounds. Molecules 2024, 29, 3594. [Google Scholar] [CrossRef]
- He, W.; Huang, B. A Review of Chemistry and Bioactivities of a Medicinal Spice: Foeniculum vulgare. J. Med. Plants Res. 2011, 5, 3595–3600. [Google Scholar]
- Anka, Z.M.; Gimba, S.; Nanda, A.; Salisu, L. Phytochemistry and Pharmacological Activities of Foeniculum vulgare. IOSR J. Pharm. 2020, 10, 1–10. [Google Scholar]
- Tomou, E.-M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef]
- Jamal, K.; Al-Taweel, A.; Bukhari, S.I.; Orfali, R.; Moubayed, N.M.S.; Al-Qahtani, J.; Aati, H.; Taglialatela-Scafati, O.; Peng, J.; Perveen, S. Isochlorogenic Acid Glucosides from the Arabian Medicinal Plant Artemisia Sieberi and Their Antimicrobial Activities. Molecules 2023, 28, 7460. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Chapter Three-Pharmacological Action and Potential Targets of Chlorogenic Acid. In Advances in Pharmacology; Du, G., Ed.; Pharmacological Advances in Natural Product Drug Discovery; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 71–88. [Google Scholar]
- Raal, A.; Orav, A.; Arak, E. Essential Oil Composition of Foeniculum vulgare Mill. Fruits from Pharmacies in Different Countries. Nat. Prod. Res. 2012, 26, 1173–1178. [Google Scholar] [CrossRef]
- Božović, M.; Garzoli, S.; Vujović, S.; Sapienza, F.; Ragno, R. Foeniculum vulgare Miller, a New Chemotype from Montenegro. Plants 2022, 11, 42. [Google Scholar] [CrossRef]
- Akarca, G.; Şevik, R.; Denzikara, A.; Kilinç, M.; Aşçioğlu, Ç. Bioactive Properties and Volatile Compounds of the Essential Oil of the Leaves of Giant Fennel (Çaksır) Growing in Türkiye. J. Essent. Oil Bear. Plants 2023, 26, 1338–1349. [Google Scholar] [CrossRef]
- Afifi, S.M.; El-Mahis, A.; Heiss, A.G.; Farag, M.A. Gas Chromatography–Mass Spectrometry-Based Classification of 12 Fennel (Foeniculum vulgare Miller) Varieties Based on Their Aroma Profiles and Estragole Levels as Analyzed Using Chemometric Tools. ACS Omega 2021, 6, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Garga, C.; Khan, S.; Ansari, S.; Suman, A.; Garg, M. Chemical Composition, Therapeutic Potential and Perspectives of Foeniculum vulgare. Pharmacogn. Rev. 2009, 3, 346. [Google Scholar]
- Ramteke, A.M. Naturally Isolated Compounds from Spices and Herbs and Their Medicinal Uses. In The Chemistry Inside Spices & Herbs: Research and Development; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; pp. 138–153. [Google Scholar]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical Composition, Antimicrobial and Antioxidant Activities of Anethole-Rich Oil from Leaves of Selected Varieties of Fennel [Foeniculum vulgare Mill. Ssp. Vulgare Var. Azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef]
- Lei, J.-D.; Li, Q.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Wei, S.; Ma, P.-A.; Hu, Y.-S. Transcriptomic and Biochemical Analyses Revealed Antifungal Mechanism of Trans-Anethole on Aspergillus Flavus Growth. Appl. Microbiol. Biotechnol. 2023, 107, 7213–7230. [Google Scholar] [CrossRef]
- Šunić, L.; Ilić, Z.S.; Stanojević, L.; Milenković, L.; Stanojević, J.; Kovač, R.; Milenković, A.; Cvetković, D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.). Horticulturae 2023, 9, 364. [Google Scholar] [CrossRef]
- Cvetkovic, D.; Stanojevic, J.; Djordjevic, N.; Karabegović, I.; Stanojevic, L.; Pavlic, B.; Danilović, B. Effect of Different Extraction Techniques on the Composition of Essential Oil Isolated from Fennel (Foeniculum vulgare Mill.) Rhizome. J. Essent. Oil Res. 2023, 35, 24–34. [Google Scholar] [CrossRef]
- Pessoa, M.L.d.S.; Silva, L.M.O.; Araruna, M.E.C.; Serafim, C.A.d.L.; Alves Júnior, E.B.; Silva, A.O.; Pessoa, M.M.B.; Diniz Neto, H.; de Oliveira Lima, E.; Batista, L.M. Antifungal Activity and Antidiarrheal Activity via Antimotility Mechanisms of (-)-Fenchone in Experimental Models. World J. Gastroenterol. 2020, 26, 6795–6809. [Google Scholar] [CrossRef]
- Araruna, M.E.C.; Alves Júnior, E.B.; de Lima Serafim, C.A.; Pessoa, M.M.B.; de Souza Pessôa, M.L.; Alves, V.P.; Sobral, M.V.; da Silva, M.S.; Alves, A.F.; de Paiva Sousa, M.C.; et al. (-)-Fenchone Ameliorates TNBS-Induced Colitis in Rats via Antioxidant, Immunomodulatory, and Cytoprotective Mechanisms. Pharmaceuticals 2025, 18, 18. [Google Scholar] [CrossRef]
- Aćimović, M.; Jeremić, J.S.; Cvetković, M.; Lončar, B.; Pezo, L. Comparative Analysis of Essential Oils and Hydrolates from Aniseed, Coriander, and Fennel Fruits. Chem. Biodivers. 2025, n/a, e202500022. [Google Scholar] [CrossRef]
- Akbar, S. Foeniculum vulgare Mill. (Apiaceae/Umbelliferae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Akbar, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 931–945. ISBN 978-3-030-16807-0. [Google Scholar]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Chatterjee, S. Assessment of Free Radical Scavenging Potential and Oxidative DNA Damage Preventive Activity of Trachyspermum ammi L. (Carom) and Foeniculum vulgare Mill. (Fennel) Seed Extracts. BioMed Res. Int. 2014, 2014, 582767. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Antioxidant Activities of Polyphenols from Sage (Salvia Officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant Properties of Hydroxycinnamic Acids: A Review of Structure- Activity Relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Akinsemolu, A.A.; Onyeaka, H.N. Microorganisms Associated with Food Spoilage and Foodborne Diseases. In Food Safety and Quality in the Global South; Ogwu, M.C., Izah, S.C., Ntuli, N.R., Eds.; Springer Nature: Singapore, 2024; pp. 489–531. ISBN 978-981-97-2428-4. [Google Scholar]
- Hashemi, S.M.B.; Khaneghah, A.M.; Sant’Ana, A.d.S. Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-14934-7. [Google Scholar]
- Khammassi, M.; Habiba, K.; Mighri, H.; Mouna, S.; Oumayma, K.; Seçer, E.; Ismail, A.; Jamoussi, B.; Yassine, M. Phytochemical Screening of Essential Oils and Methanol Extract Constituents of Wild Foeniculum vulgare Mill.: A Potential Natural Source for Bioactive Molecules. Chem. Afr. 2023, 6, 1227–1240. [Google Scholar] [CrossRef]
- Santoro, V.; Rosa, E.; Donadio, G.; Polito, F.; De Feo, V.; De Tommasi, N. Foeniculum vulgare Miller Bracts, Revalorization of a Local Food Waste. Sci. Rep. 2024, 14, 31287. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological Properties of Essential Oils: An Updated Review. Flavour. Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Qureshi, M.I.; Hashmi, N.; Akhtar, M.S.; A. Khan, M.M. Antimicrobial and Antioxidant Activities of Fennel Oil. Bioinformation 2022, 18, 795–800. [Google Scholar] [CrossRef]
- Jadid, N.; Widodo, A.F.; Ermavitalini, D.; Sa’adah, N.N.; Gunawan, S.; Nisa, C. The Medicinal Umbelliferae Plant Fennel (Foeniculum vulgare Mill.): Cultivation, Traditional Uses, Phytopharmacological Properties, and Application in Animal Husbandry. Arab. J. Chem. 2023, 16, 104541. [Google Scholar] [CrossRef]
- Rafieian, F.; Amani, R.; Rezaei, A.; Karaça, A.C.; Jafari, S.M. Exploring Fennel (Foeniculum vulgare): Composition, Functional Properties, Potential Health Benefits, and Safety. Crit. Rev. Food Sci. Nutr. 2024, 64, 6924–6941. [Google Scholar] [CrossRef]
- Elbaz, M.; Abdesslem, S.B.; St-Gelais, A.; Boulares, M.; Moussa, O.B.; Timoumi, M.; Hassouna, M.; Aider, M. Essential Oils Profile, Antioxidant and Antibacterial Potency of Tunisian Fennel (Foeniculum vulgare Mill.) Leaves Grown under Conventional and Organic Conditions. Food Chem. Adv. 2024, 4, 100734. [Google Scholar] [CrossRef]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Foeniculum vulgare Essential Oils: Chemical Composition, Antioxidant and Antimicrobial Activities. Nat. Prod. Commun. 2010, 5, 1934578X1000500231. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive Phenolic and Free Amino Acid Analysis of Rosemary Infusions: Influence on the Antioxidant Potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Chaowuttikul, C.; Palanuvej, C.; Ruangrungsi, N. Quantification of Chlorogenic Acid, Rosmarinic Acid, and Caffeic Acid Contents in Selected Thai Medicinal Plants Using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 56, e17547. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of Different Molecular Weights of Chitosan by P-Coumaric Acid: Preparation, Characterization and Effect of Molecular Weight on Its Water Solubility and Antioxidant Property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly for Particle Engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-Based Antimicrobial Polymers. Molecules 2021, 26, 559. [Google Scholar] [CrossRef]
- Dangles, O. Antioxidant Activity of Plant Phenols: Chemical Mechanisms and Biological Significance. Curr. Org. Chem. 2012, 16, 692–714. [Google Scholar] [CrossRef]
- Hosseini, E.; Majidi, M.M.; Ehtemam, M.H.; Ghanadian, M.; Huyghe, C. Variation in a Worldwide Collection of Fennel (Foeniculum vulgare Var. Vulgare). Crop Pasture Sci. 2021, 72, 1008–1021. [Google Scholar] [CrossRef]
- Alrub, M.A. Unveiling the Chemical Profiling, Antioxidant, Anticancer and Antibacterial Activities of Essential Oils Derived from Fennel (Foeniculum vulgare), M. Fruticose and Alyosia Citriodora. Ph.D. Thesis, Faculty of Graduate Studies, An-Najah National University, Nablus, Palestine, 2023. [Google Scholar]
- Saidi, S.; Remok, F.; Handaq, N.; Drioiche, A.; Gourich, A.A.; Menyiy, N.E.; Amalich, S.; Elouardi, M.; Touijer, H.; Bouhrim, M.; et al. Phytochemical Profile, Antioxidant, Antimicrobial, and Antidiabetic Activities of Ajuga Iva (L.). Life 2023, 13, 1165. [Google Scholar] [CrossRef] [PubMed]
- Remok, F.; Saidi, S.; Gourich, A.A.; Zibouh, K.; Maouloua, M.; Makhoukhi, F.E.; Menyiy, N.E.; Touijer, H.; Bouhrim, M.; Sahpaz, S.; et al. Phenolic Content, Antioxidant, Antibacterial, Antihyperglycemic, and α-Amylase Inhibitory Activities of Aqueous Extract of Salvia Lavandulifolia Vahl. Pharmaceuticals 2023, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, L. Effet de La Teneur En Eau Du Sol Sur Le Rendement et La Qualité Des Fruits Du Bleuet Nain; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 1995. [Google Scholar]
- Skujins, S. Handbook for ICP-AES (Varian-Vista); A short guide to vista series ICP-AES operation. Version 1; Varian Int. AG: Zug, Switzerland, 1998. [Google Scholar]
- Dohou, R.; Yamni, K.; Tahrouch, S.; Hassani, L.I.; Badoc, A.; Gmira, N. Screening Phytochimique d’une Endémique Iberomarocaine, Thymelaea Lythroides. Bull. Bull.-Société De Pharm. De Bordx. 2003, 142, 61–78. [Google Scholar]
- Judith, M.D. Étude Phytochimique et Pharmacologique de Cassia Nigricans Vhal (Caeslpiniaceae) Utilisée Dans Le Traitement Des Dermatoses Au Tchad. Ph.D. Thesis, Université de Bamako, Bamako, Mali, 2005. [Google Scholar]
- Mezzoug, N.; Elhadri, A.; Dallouh, A.; Amkiss, S.; Skali, N.S.; Abrini, J.; Zhiri, A.; Baudoux, D.; Diallo, B.; El Jaziri, M. Investigation of the Mutagenic and Antimutagenic Effects of Origanum Compactum Essential Oil and Some of Its Constituents. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 629, 100–110. [Google Scholar] [CrossRef]
- Bekro, Y.A.; Jana, A.; Mamyrbekova, B.; Bousa, B.; Fézan, H.; Tra, B.; Ehile, E.E. Etude Ethnobotanique et Sceening Phtochimque de Caesalpina Benthamiana (Ball). Herend et Zarucchi (Caesalpiniaceae). Sci. Nat. 2007, 4, 217–225. [Google Scholar]
- Bruneton, J. Pharmacognosie: Phytochimie, Plantes Médicinales, 4th ed.; Tec & Doc Lavoisier: Paris, France, 2009; ISBN 978-2-7430-1188-8. [Google Scholar]
- N’Guessan, K.; Kadja, B.; Zirihi, G.; Traoré, D.; Aké-Assi, L. Screening Phytochimique de Quelques Plantes Médicinales Ivoiriennes Utilisées En Pays Krobou (Agboville, Côte-d’Ivoire). Sci. Nat. 2009, 6, 1–15. [Google Scholar] [CrossRef]
- El Karkouri, J.; Kchibale, A.; Chroho, M.; Eddamsyry, B.; Touijer, H.; El Makhoukhi, F.; Handaq, N.; Eto, B.; Salamatullah, A.M.; Bourhia, M.; et al. Phytochemical Profile, Antioxidant Activity, Anti-Hyperglycemic Effect and Toxicity Assessment of Ridolfia Segetum (L.) Moris Extract. Life 2023, 13, 44. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hung, P.V.; Maeda, T.; Miyatake, K.; Morita, N. Total Phenolic Compounds and Antioxidant Capacity of Wheat Graded Flours by Polishing Method. Food Res. Int. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A Critical Evaluation of the Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Gourich, A.A.; Touijer, H.; Drioiche, A.; Asbabou, A.; Remok, F.; Saidi, S.; Siddique, F.; Ailli, A.; Bourhia, M.; Salamatullah, A.M.; et al. Insight into Biological Activities of Chemically Characterized Extract from Marrubium vulgare L. in Vitro, in Vivo and in Silico Approaches. Front. Chem. 2023, 11, 1238346. [Google Scholar] [CrossRef] [PubMed]
- Tagnaout, I.; Zerkani, H.; Bencheikh, N.; Amalich, S.; Bouhrim, M.; Mothana, R.A.; Alhuzani, M.R.; Bouharroud, R.; Hano, C.; Zair, T. Chemical Composition, Antioxidants, Antibacterial, and Insecticidal Activities of Origanum Elongatum (Bonnet) Emberger & Maire Aerial Part Essential Oil from Morocco. Antibiotics 2023, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Ailli, A.; Handaq, N.; Touijer, H.; Gourich, A.A.; Drioiche, A.; Zibouh, K.; Eddamsyry, B.; El Makhoukhi, F.; Mouradi, A.; Bin Jardan, Y.A. Phytochemistry and Biological Activities of Essential Oils from Six Aromatic Medicinal Plants with Cosmetic Properties. Antibiotics 2023, 12, 721. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Kovats, E.S. Gas Chromatographic Characterization of Organic Substances in the Retention Index System. Adv. Chromatogr. 1965, 1, 229–247. [Google Scholar]
- Drioiche, A.; Baammi, S.; Zibouh, K.; Al Kamaly, O.; Alnakhli, A.M.; Remok, F.; Saidi, S.; Amaiach, R.; El Makhoukhi, F.; Elomri, A. A Study of the Synergistic Effects of Essential Oils from Origanum Compactum and Origanum Elongatum with Commercial Antibiotics against Highly Prioritized Multidrug-Resistant Bacteria for the World Health Organization. Metabolites 2024, 14, 210. [Google Scholar] [CrossRef]
- Liu, M.H.; Otsuka, N.; Noyori, K.; Shiota, S.; Ogawa, W.; Kuroda, T.; Hatano, T.; Tsuchiya, T. Synergistic Effect of Kaempferol Glycosides Purified from Laurus Nobilis and Fluoroquinolones on Methicillin-Resistant Staphylococcus Aureus. Biol. Pharm. Bull. 2009, 32, 489–492. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Khiya, Z.; Oualcadi, Y.; Gamar, A.; Berrekhis, F.; Zair, T.; Hilali, F.E. Correlation of Total Polyphenolic Content with Antioxidant Activity of Hydromethanolic Extract and Their Fractions of the Salvia Officinalis Leaves from Different Regions of Morocco. J. Chem. 2021, 2021, e8585313. [Google Scholar] [CrossRef]



| Species | MC (%) | pH | Acidity | MM (%) | MO (%) |
|---|---|---|---|---|---|
| F. vulgare | 25.12 ± 0.001 | 5.5 ± 0.00 | 0.11 ± 0.00 | 6.4 ± 0.067 | 93.6 |
| Species | Arsenic (As) | Chrome (Cr) | Antimoine (Sb) | Plomb (Pb) | Cadmium (Cd) | Iron (Fe) | Copper (Cu) | Titanium (Ti) |
|---|---|---|---|---|---|---|---|---|
| F. vulgare | 0.0058 | 0.0008 | 0.0023 | Undetectable | Undetectable | 0.271 | 0.003 | Undetectable |
| Maximum Limit (mg/L) | 0.05 | 0.05 | 0.005 | 0.05 | 0.005 | 20 | 1 | - |
| Chemical Group | F. vulgare | ||
|---|---|---|---|
| Secondary Metabolites | Lipids (Lieberman–Burchard reaction) | ++ | |
| Protein | Biuret reaction | + | |
| Xanthoprotein reaction | ++ | ||
| Reducing sugar | + | ||
| Polysaccharide | + | ||
| Secondary Metabolites | Tannins | +++ | |
| Catechic tannins | +++ | ||
| Gallic tannins | + | ||
| Flavonoids | ++ | ||
| Cyanidin reaction | Flavones | ||
| Leucoanthocyanins | ++ | ||
| Saponosides | + | ||
| Alkaloids | + | ||
| Reducing compounds | ++ | ||
| Monosaccharides and holosides | ++ | ||
| Mucilages | ++ | ||
| Sterols and triterpenes | ++ | ||
| Classes | R.A (%) | ||
|---|---|---|---|
| E (0) | E (1) | E (2) | |
| Carboxylic ester | 1.04 | 0 | 0.66 |
| Dipeptide | 0 | 0 | 5.42 |
| Ester | 0 | 0.55 | 0 |
| Lignan | 0 | 1.21 | 7.82 |
| Phenolic compound | 7.12 | 3.54 | 2.36 |
| Fatty acid | 0 | 0 | 1.67 |
| Phenolic acid | 49.96 | 33.39 | 37.3 |
| Flavonoid | 24.4 | 42.91 | 19.72 |
| Phenolic diterpene | 0 | 1.29 | 8.08 |
| Polyphenol | 11.2 | 9.94 | 7.84 |
| Quinone | 0 | 0 | 0.49 |
| Terpenoid | 4.42 | 6.58 | 6.46 |
| Vitamin | 1.82 | 0.56 | 1.84 |
| Species | Yield (%) | Density (g/mL) |
|---|---|---|
| F. vulgare Mill | 2.500 ± 0.067 | 0.964 ± 0.002 |
| Compound | RA % | KI | |||
|---|---|---|---|---|---|
| α–Pinene | 1.1 | 939 | |||
| Camphene | 0.08 | 954 | |||
| Sabinene | 0.1 | 975 | |||
| β–Pinene | 0.03 | 979 | |||
| Myrcene | 0.82 | 990 | |||
| α–Phellandrene | 0.13 | 1002 | |||
| β–Phellandrene | 0.05 | 1029 | |||
| Limonene | 20.48 | 1029 | |||
| 1,8-Cineole | 0.11 | 1031 | |||
| β–cis-Ocimene | 0.11 | 1037 | |||
| γ–Terpinene | 0.85 | 1059 | |||
| Fenchone | 24.72 | 1086 | |||
| Terpinolene | 0.13 | 1088 | |||
| Cis-Thujone | 0.03 | 1102 | |||
| Trans-Pinene hydrate | 0.05 | 1122 | |||
| Camphor | 0.52 | 1146 | |||
| Terpinen-4-ol | 0.06 | 1177 | |||
| Methyl chavicol (estragole) | 8.79 | 1196 | |||
| Fenchyl acetate <endo-> | 0.07 | 1220 | |||
| Fenchyl acetate <exo-> | 0.18 | 1232 | |||
| Cis-anethole | 19.18 | 1252 | |||
| Trans-anethole | 22.22 | 1284 | |||
| Anisyl methyl ketone | 0.06 | 1382 | |||
| Germacrene D | 0.07 | 1481 | |||
| Trans-Methyl isoeugenol | 0.05 | 1492 | |||
| Identified compounds (%) | 99.99 | ||||
| Monoterpenes (%) | 22.90 | ||||
| Oxygenated monoterpenes (%) | 77.02 | ||||
| Sesquiterpenes (%) | 0.07 | ||||
| Oxygenated sesquiterpenes (%) | 0.0 | ||||
| Strains | EO (mg/mL) | Extracts (mg/mL) | Gentamicin | Terbinafin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E (0) | E (1) | E (2) | ||||||||
| MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | MIC (µg/mL) | MIC (µg/mL) | |
| Enterobacter cloacae | 25 | 25 | 50 | 50 | 12.5 | 25 | 50 | 50 | >4 | - |
| Klebsiella pneumoniae | 25 | 50 | >50 | >50 | 25 | 25 | >50 | >50 | <=1 | - |
| Escherichia coli sauvage | 25 | 25 | >50 | >50 | 50 | 50 | >50 | >50 | 2 | - |
| Staphylococcus aureus BLACT | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | <0.5 | - |
| Staphylococcus epidermidis | 50 | 100 | 50 | 50 | 50 | 50 | >50 | >50 | 2 | - |
| Candida albicans | 3.13 | 6.25 | 50 | 50 | 50 | 50 | 50 | 50 | - | 12.500 |
| Candida dubliniensis | 25 | 25 | 50 | 50 | 50 | 50 | >50 | >50 | - | 3.125 |
| Candida tropicalis | 12.5 | 25 | 50 | 50 | 12.5 | 12.5 | >50 | >50 | - | 12.500 |
| Candida parapsilosis | 25 | 50 | 50 | 50 | 0.78 | 0.78 | >50 | >50 | - | 6.250 |
| Aspergillus niger | 6.25 | 6.25 | 50 | 50 | 25 | 25 | >50 | >50 | - | 3.125 |
| Molecules\Proteins | Antimicrobial Activities | Antioxidant Activities | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7TI1 | 3RAE | 4DUH | 2W9S | 1JIJ | 3KP5 | 7RJB | 5V5Z | 4YBF | 4ZA5 | 5qj2 | 3nrz | 1og5 | 1n8q | 2cdu | ||
| Extracts | 3-O-Caffeoylshikimic acid | −7.4 | −8.4 | −6.4 | −7.9 | −7.4 | −8.5 | −8.5 | −7.6 | −7.1 | −7.9 | −8.4 | −8.6 | −8.7 | −8.8 | −8.3 |
| 7-Methoxy-2-methylisoflavone | −6.9 | −7.9 | −6 | −7 | −6.9 | −7.7 | −9.5 | −7.3 | −6.9 | −8.2 | −7.9 | −8.1 | −8.5 | −8.6 | −8.2 | |
| Apigenin | −7 | −8.4 | −6.6 | −8.1 | −7 | −8.2 | −8.6 | −7.2 | −7 | −8.4 | −8.2 | −9.1 | −8.8 | −9.1 | −7.9 | |
| Butyl ferulate | −6.2 | −6.4 | −4.8 | −6.1 | −6.2 | −6.4 | −7.1 | −6.1 | −5.9 | −6.8 | −6.1 | −7.2 | −6.8 | −7.3 | −7.5 | |
| Chlorogenic acid | −7.2 | −9 | −6.5 | −7.9 | −7.2 | −8.5 | −8.5 | −7.6 | −7.2 | −8.4 | −8 | −8.9 | −8.8 | −8.7 | −8.2 | |
| Dihydrocaffeic acid | −5.9 | −6.2 | −5.1 | −6.6 | −5.9 | −6.3 | −6.3 | −5.8 | −5.7 | −6.3 | −6 | −7.2 | −6.3 | −6.6 | −6.4 | |
| Embelic acid | −5.5 | −6.4 | −4.6 | −6.3 | −5.5 | −7 | −6.9 | −6.3 | −5.4 | −6.7 | −6.3 | −7.3 | −6.9 | −7.6 | −7.3 | |
| Isochlorogenic acid A | −8.1 | −9.3 | −8.2 | −8.6 | −8.1 | −10.3 | −9.7 | −8.5 | −8.6 | −9.2 | −8.7 | −9.9 | −9.1 | −9 | −8.3 | |
| Medioresinol | −7.2 | −8.6 | −6.5 | −7.9 | −7.2 | −8.9 | −8.3 | −7.6 | −7.2 | −7.9 | −9 | −7.8 | −7.8 | −8.4 | −8.7 | |
| Quercetin-3-D-xyloside | −7.6 | −9.9 | −7.1 | −8 | −7.6 | −8.5 | −9.1 | −7.6 | −7.7 | −8.5 | −9.2 | −8.1 | −8.8 | −9.8 | −8.1 | |
| Quercetin-3-glucuronide | −7.6 | −9.3 | −7.1 | −8 | −7.6 | −8.5 | −9.1 | −7.6 | −7.7 | −8.6 | −9.1 | −8.1 | −8.8 | −9.1 | −8.1 | |
| Rosmanol | −7.8 | −9.9 | −7.2 | −7.5 | −7.8 | −6.8 | −8.3 | −7.7 | −7.8 | −8.2 | −9.1 | −7.9 | −9.4 | −8.4 | −8.6 | |
| Salvianolic acid K | −8 | −9.4 | −7.3 | −9 | −8 | −9.5 | −8.7 | −8.6 | −8 | −9.5 | −9 | −10.9 | −10 | −9.6 | −7.9 | |
| EO | Fenchone | −5.6 | −5.1 | −4.8 | −5.5 | −5.6 | −6.5 | −5.6 | −5.7 | −5.4 | −6 | −5.4 | −5.7 | −5.9 | −6.1 | −6.3 |
| Trans-anethole | −5.7 | −5.1 | −4.3 | −5.2 | −5.7 | −5.7 | −6.8 | −5.3 | −5 | −5.6 | −5 | −6.5 | −6 | −6.1 | −6.4 | |
| Limonene | −5.8 | −5 | −4.3 | −5 | −5.8 | −6.1 | −6.9 | −5.5 | −5.1 | −5.8 | −5.4 | −6.5 | −5.8 | −6 | −6.5 | |
| Cis-anethole | −5.7 | −5 | −4.2 | −5.2 | −5.7 | −6.1 | −6.6 | −5.7 | −4.9 | −5.5 | −5 | −6.3 | −5.8 | −5.9 | −6.5 | |
| Estragole | −5.8 | −5.2 | −4.2 | −5.2 | −5.8 | −5.9 | −6.6 | −5.4 | −4.8 | −5.6 | −5.2 | −6.2 | −5.7 | −6 | −6.1 | |
| Molecules\Proteins | 3NRZ | 1OG5 | ||
|---|---|---|---|---|
| 2D | 3D | 2D | 3D | |
| Apigenin |  | 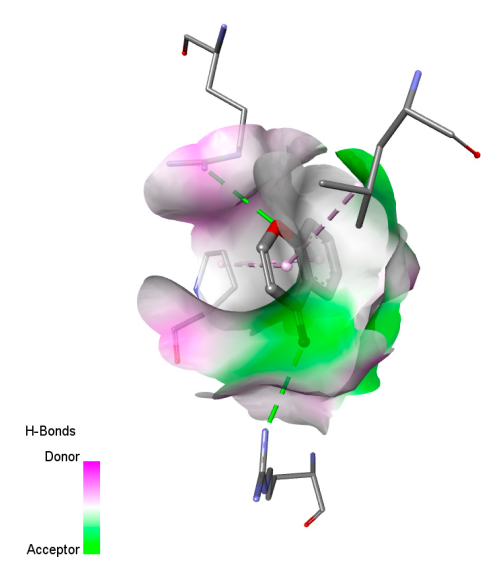 | 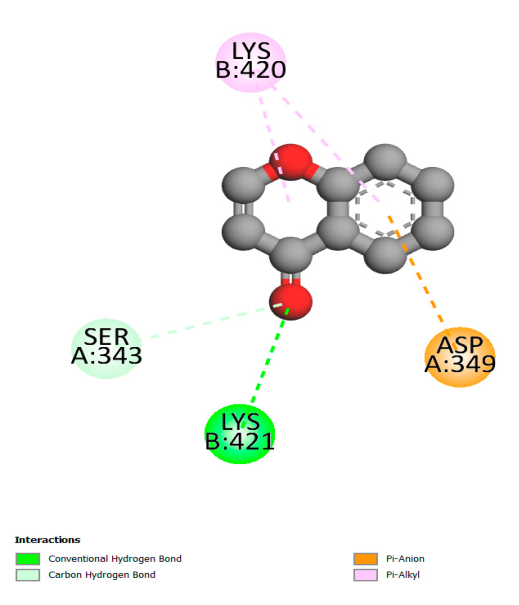 |  |
| Chlorogenic acid |  |  |  |  |
| Isochlorogenic acid A |  |  | 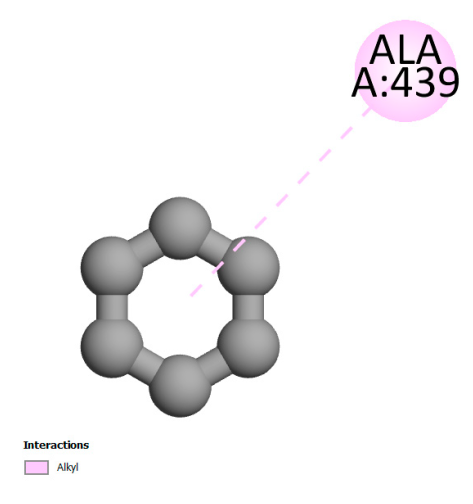 | 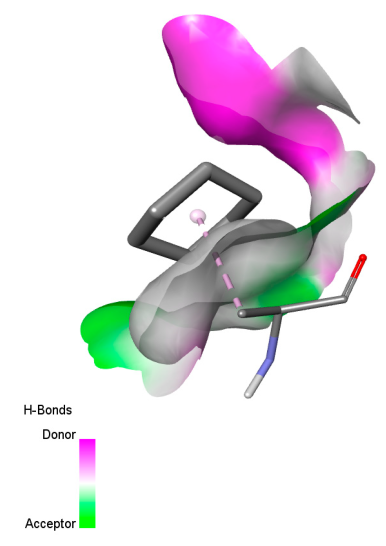 |
| Rosmanol | 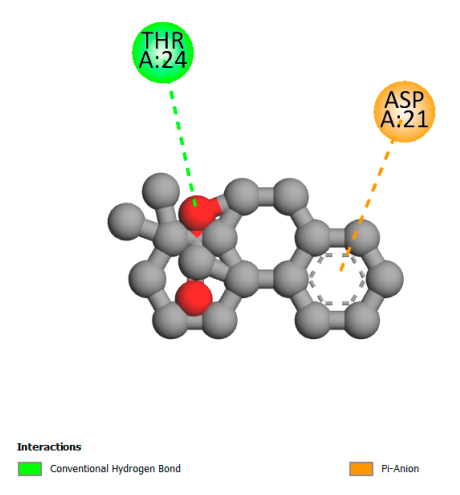 | 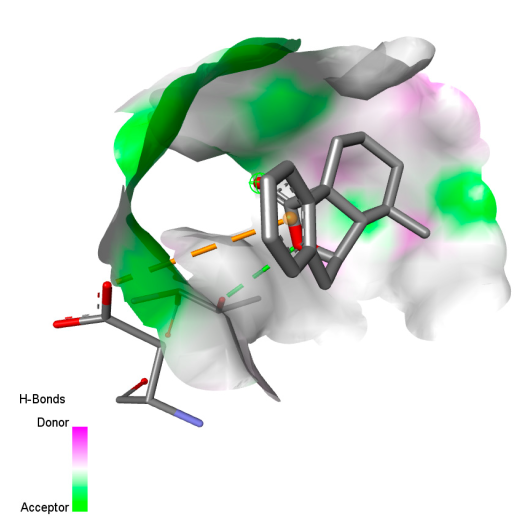 | 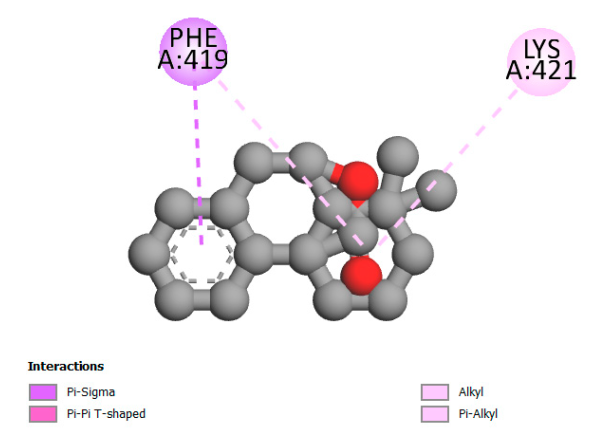 | 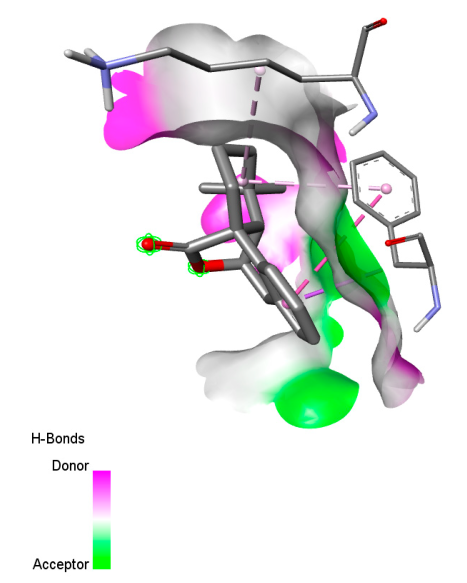 |
| Salvianolic acid K | 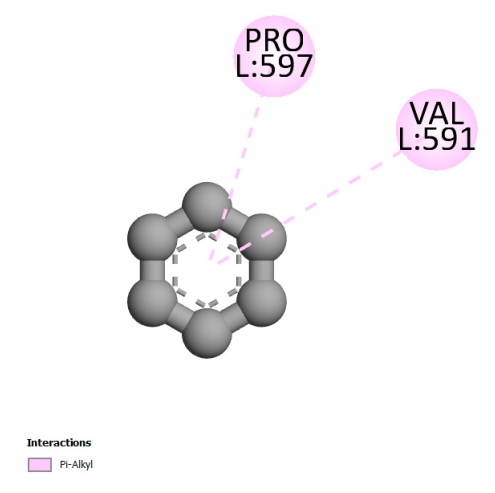 | 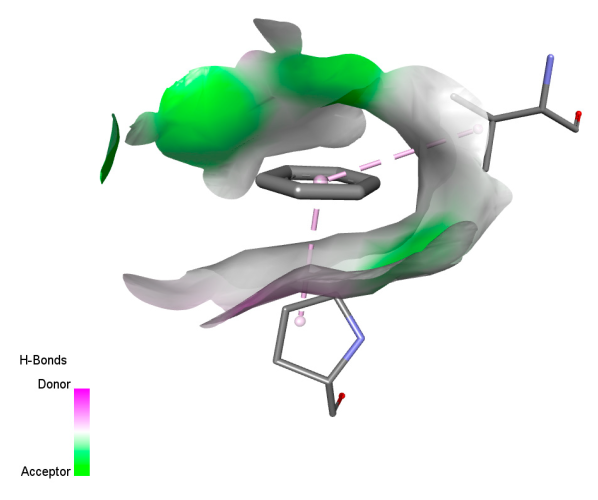 |  |  |
| Plant Species | Vernacular | Harvest Site | Parts Used | Latitude (x) | Longitude (y) | Altitude (m) | Harvest Year | |
|---|---|---|---|---|---|---|---|---|
| Region | Locality | |||||||
| F. vulgare Mill | Fenouil | Meknès | Ain jerry | Seeds | 5°49′55″ O | 33°85′86″ N | 546 | 2024 |
| Reign | Plantae |
| Kingdom | Fenouil |
| Class | Equisetopsida |
| Order | Apiales |
| Family | Apiaceae |
| Genus | Foeniculum |
| Species | Foeniculum vulgare |
| Extraction Method | Solvants | Codification |
|---|---|---|
| Decoction | Water | E (2) |
| Soxhlet | Ethanol/Water (70/30) | E (1) |
| Water | E (0) |
| Bacterial Strains | References | Fungal Strains | References |
|---|---|---|---|
| Enterobacter cloacae | 02EV317 | Candida albicans | Ca |
| Klebsiella pneumoniae | 3DT1823 | Candida dubliniensis | Cd |
| Escherichia coli sauvage | 3DT1938 | Candida tropicalis | Ct |
| Staphylococcus aureus BLACT | 4IH2510 | Candida parapsilosis | Cpa |
| Staphylococcus epidermidis | 5994 | Aspergillus niger | AspN |
| Activities | Targets | PDB ID | Grid Box Center Coordinates | Grid Box Size |
|---|---|---|---|---|
| Antibacterial activity | Beta-lactamase | 7TI1 | center_x = −56.105 center_y = 21.017 center_z = 50.112 | size_x = 30 size_y = 22 size_z = 24 |
| DNA topoisomerase 4 subunit A | 3RAE | center_x = −54.023 center_y = 68.181 center_z = −18.042 | size_x = 22 size_y = 36 size_z = 34 | |
| DNA gyrase subunit B | 4DUH | center_x = 21.304 center_y = 12.134 center_z = 25.205 | size_x = 24 size_y = 24 size_z = 22 | |
| DIHYDROFOLATE REDUCTASE TYPE 1 FROM TN4003 | 2W9S | center_x = 6.027 center_y = −1.060 center_z = 30.037 | size_x = 24 size_y = 28 size_z = 30 | |
| tyrosyl-tRNA synthetase | 1JIJ | center_x = −9.074 center_y = 18.180 center_z = 93.030 | size_x = 24 size_y = 28 size_z = 30 | |
| Transcriptional regulator TcaR | 3KP5 | center_x = −27.301 center_y = −30.531 center_z = −1.040 | size_x = 26 size_y = 28 size_z = 24 | |
| Ubiquinol--cytochrome-c reductase subunit | 7RJB | center_x = 148.091 center_y = 127.219 center_z = 147.014 | size_x = 24 size_y = 24 size_z = 28 | |
| Lanosterol 14-alpha demethylase | 5V5Z | center_x = −44.181 center_y = −14.027 center_z = 22.108 | size_x = 28 size_y = 30 size_z = 36 | |
| Candidapepsin-2 | 4YBF | center_x = 9.025 center_y = 1.007 center_z = 15.741 | size_x = 26 size_y = 32 size_z = 34 | |
| Structure of A. niger Fdc1 | 4ZA5 | center_x = 19.027 center_y = 1.012 center_z = 19.118 | size_x = 24 size_y = 24 size_z = 22 | |
| Antioxidant activity | Myeloperoxidase | 5qj2 | center_x = −49.012 center_y = 9.017 center_z = 29.115 | size_x = 22 size_y = 28 size_z = 32 |
| Xanthine dehydrogenase/oxidase | 3NRZ | center_x = 58.097 center_y = 3.009 center_z = 35.108 | size_x = 22 size_y = 28 size_z = 32 | |
| Cytochrome P450 2C9 | 1OG5 | center_x = −38.207 center_y = 61.001 center_z = 27.024 | size_x = 24 size_y = 22 size_z = 28 | |
| Lipoxygenase-3 | 1N8Q | center_x = 26.027 center_y = 0.050 center_z = 16.130 | size_x = 20 size_y = 28 size_z = 34 | |
| NADPH oxidase | 2CDU | center_x = 11.204 center_y = 1.035 center_z = 24.135 | size_x = 20 size_y = 28 size_z = 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moumen, B.E.; Bouzoubaa, A.; Drioiche, A.; Eddahmouny, M.; Al Kamaly, O.; Shahat, A.A.; Touijer, H.; Hadi, N.; Kharchouf, S.; Cherrat, A.; et al. Unveiling the Chemical Composition, Antioxidant, and Antimicrobial Potentials of Foeniculum vulgare Mill: A Combined In Vitro and In Silico Approach. Int. J. Mol. Sci. 2025, 26, 4499. https://doi.org/10.3390/ijms26104499
Moumen BE, Bouzoubaa A, Drioiche A, Eddahmouny M, Al Kamaly O, Shahat AA, Touijer H, Hadi N, Kharchouf S, Cherrat A, et al. Unveiling the Chemical Composition, Antioxidant, and Antimicrobial Potentials of Foeniculum vulgare Mill: A Combined In Vitro and In Silico Approach. International Journal of Molecular Sciences. 2025; 26(10):4499. https://doi.org/10.3390/ijms26104499
Chicago/Turabian StyleMoumen, Bouchra El, Amal Bouzoubaa, Aziz Drioiche, Mohamed Eddahmouny, Omkulthom Al Kamaly, Abdelaaty Abdelaziz Shahat, Hanane Touijer, Nadia Hadi, Samira Kharchouf, Ali Cherrat, and et al. 2025. "Unveiling the Chemical Composition, Antioxidant, and Antimicrobial Potentials of Foeniculum vulgare Mill: A Combined In Vitro and In Silico Approach" International Journal of Molecular Sciences 26, no. 10: 4499. https://doi.org/10.3390/ijms26104499
APA StyleMoumen, B. E., Bouzoubaa, A., Drioiche, A., Eddahmouny, M., Al Kamaly, O., Shahat, A. A., Touijer, H., Hadi, N., Kharchouf, S., Cherrat, A., Fadili, K., El Ouadni, H., Bari, A., & Zair, T. (2025). Unveiling the Chemical Composition, Antioxidant, and Antimicrobial Potentials of Foeniculum vulgare Mill: A Combined In Vitro and In Silico Approach. International Journal of Molecular Sciences, 26(10), 4499. https://doi.org/10.3390/ijms26104499








