Genetic Factors Related to the Development or Progression of Mesoamerican Endemic Nephropathy
Abstract
1. Introduction
2. Genetic Factors Associated with the Development of Mesoamerican Endemic Nephropathy
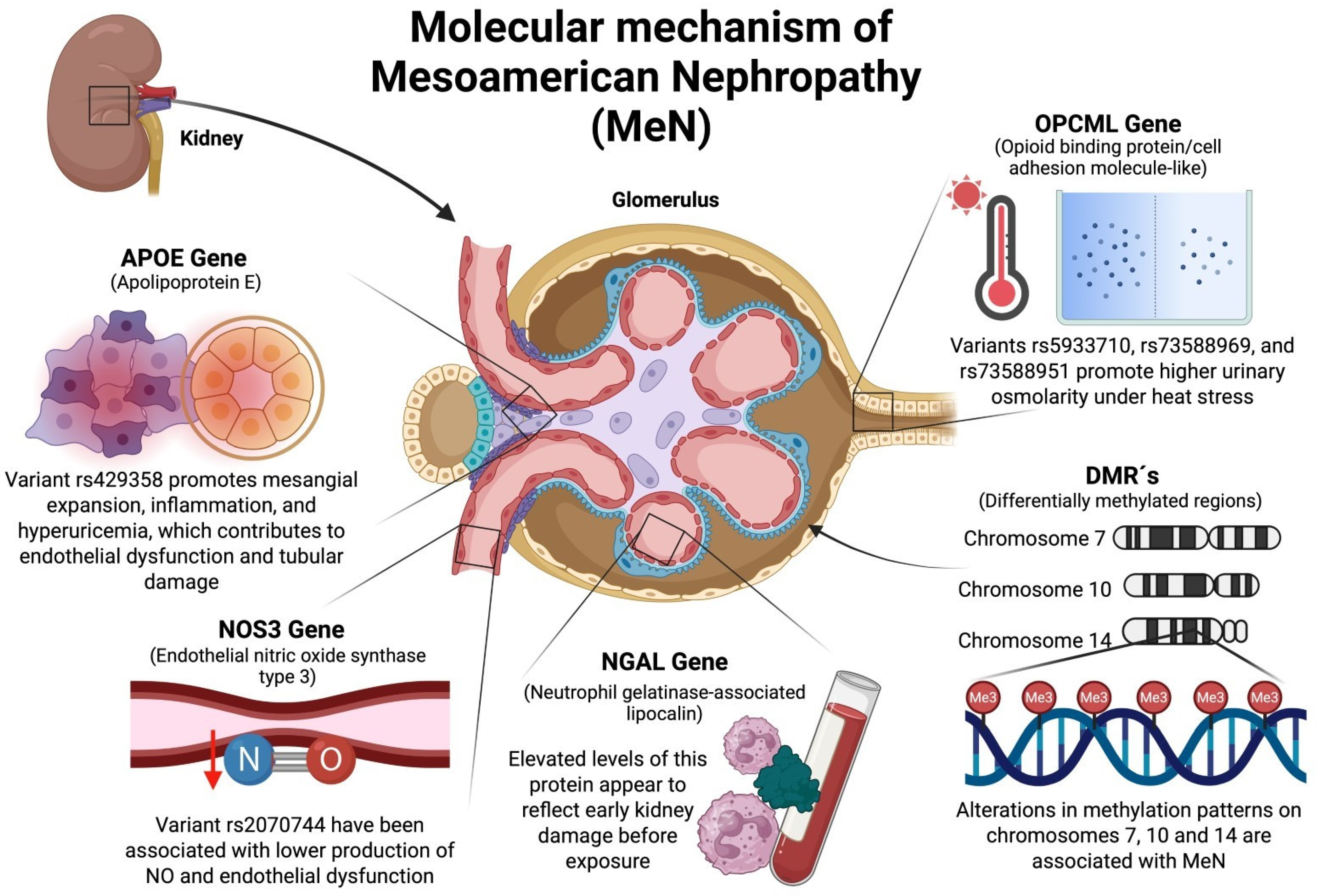
2.1. OPCML (Opioid-Binding Protein/Cell Adhesion Molecule-like) Gene
2.2. NOS3 (Nitric Oxide Synthase Type 3) Gene
2.3. NGAL (Neutrophil Gelatinase-Associated Lipocalin) Gene
2.4. ApoE (Apolipoprotein E) Gene
3. Epigenetic Factors Related to MeN
3.1. Role of Differentially Methylated Regions (DMRs) in the Development of MeN
3.1.1. DMR on Chromosome 7
AMPH (Amphiphysin 1) Gene
3.1.2. DMR on Chromosome 10
SLC29A3 (Solute Carrier Family 29 “Nucleoside Transporters Member 3”) Gene
3.1.3. DMR on Chromosome 14
DIO3 (Iodothyronine Deiodinase 3) Gene
RTL1 (Retrotransposon Gag Like 1) Gene
DLK1 (Delta Like Non-Canonical Notch Ligand 1) Gene
4. Possible Molecular Mechanisms Involving Genetic Factors Associated with MeN
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fischer, R.S.B.; Palma, L.; Murray, K.O.; Vangala, C.; García-Trabanino, R.; Chavarria, D.; Garcia, L.L.; Nolan, M.S.; Garcia, F.; Mandayam, S. Clinical Evidence of Acute Mesoamerican Nephropathy. Am. J. Trop. Med. Hyg. 2017, 97, 1247–1256. [Google Scholar] [CrossRef]
- Fischer, R.S.B.; Vangala, C.; Mandayam, S.; Chavarria, D.; García-Trabanino, R.; Garcia, F.; Garcia, L.L.; Murray, K.O. Clinical Markers to Predict Progression from Acute to Chronic Kidney Disease in Mesoamerican Nephropathy. Kidney Int. 2018, 94, 1205–1216. [Google Scholar] [CrossRef]
- Gunasekara, T.D.K.S.C.; De Silva, P.M.C.S.; Herath, C.; Siribaddana, S.; Siribaddana, N.; Jayasumana, C.; Jayasinghe, S.; Cardenas-Gonzalez, M.; Jayasundara, N. The Utility of Novel Renal Biomarkers in Assessment of Chronic Kidney Disease of Unknown Etiology (CKDu): A Review. Int. J. Environ. Res. Public Health 2020, 17, 9522. [Google Scholar] [CrossRef]
- Pett, J.; Mohamed, F.; Knight, J.; Linhart, C.; Osborne, N.J.; Taylor, R. Two Decades of Chronic Kidney Disease of Unknown Aetiology (CKDu) Research: Existing Evidence and Persistent Gaps from Epidemiological Studies in Sri Lanka. Nephrology 2021, 27, 238–247. [Google Scholar] [CrossRef]
- John, O.; Gummudi, B.; Jha, A.; Gopalakrishnan, N.; Kalra, O.P.; Kaur, P.; Kher, V.; Kumar, V.; Machiraju, R.S.; Osborne, N.; et al. Chronic Kidney Disease of Unknown Etiology in India: What Do We Know and Where We Need to Go. Kidney Int. Rep. 2021, 6, 2743–2751. [Google Scholar] [CrossRef]
- Pavlović, N.M. Balkan endemic nephropathy-current status and future perspectives. Clin. Kidney J. 2013, 6, 257–265. [Google Scholar] [CrossRef]
- Jelakovi, B.; Nikoli, J.; Radovanovi, Z.; Nortier, J.; Cosyns, J.P.; Grollman, A.P.; Bašić-Jukić, N.; Belicza, M.; Bukvić, D.; Čavaljuga, S.; et al. Consensus Statement on Screening, Diagnosis, Classification and Treatment of Endemic (Balkan) Nephropathy. Nephrol. Dial. Transplant. 2013, 29, 2020–2027. [Google Scholar] [CrossRef]
- Stefanovic, V.; Toncheva, D.; Polenakovic, M. Balkan nephropathy. Clin. Nephrol. 2015, 83 (Suppl. S1), 64–69. [Google Scholar] [CrossRef]
- Jayasumana, C. Chronic Interstitial Nephritis in Agricultural Communities (CINAC) in Sri Lanka. Semin. Nephrol. 2019, 39, 278–283. [Google Scholar] [CrossRef]
- Campese, V.M. The Mesoamerican Nephropathy: A Regional Epidemic of Chronic Kidney Disease? Nephrol. Dial. Transplant. 2016, 31, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, Z.E.; Ramirez-Rubio, O.; Scammell, M.K.; Laws, R.L.; Lopez-Pilarte, D.; Amador, J.J.; Ballester, J.; O’Callaghan-Gordo, C.; Brooks, D.R. Climate Trends at a Hotspot of Chronic Kidney Disease of Unknown Causes in Nicaragua, 1973–2014. Int. J. Environ. Res. Public Health 2021, 18, 5418. [Google Scholar] [CrossRef]
- Wijkström, J.; Jayasumana, C.; Dassanayake, R.; Priyawardane, N.; Godakanda, N.; Siribaddana, S.; Ring, A.; Hultenby, K.; Söderberg, M.; Elinder, C.-G.; et al. Morphological and Clinical Findings in Sri Lankan Patients with Chronic Kidney Disease of Unknown Cause (CKDu): Similarities and Differences with Mesoamerican Nephropathy. PLoS ONE 2018, 13, e0193056. [Google Scholar] [CrossRef]
- Wijkström, J.; González-Quiroz, M.; Hernández, M.; Trujillo, Z.; Hultenby, K.; Ring, A.; Söderberg, M.; Aragón, A.; Elinder, C.-G.; Wernerson, A. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. Am. J. Kidney Dis. 2017, 69, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Karanović, S.; Tomić, K.; Dittrich, D.; Borovečki, F.; Zavadil, J.; Vuković-Lela, I.; Karlović, K.; Knežević, M.; Jelaković, B. Endemic (Balkan) nephropathy is aristolochic acid nephropathy. Pril (Makedon Akad Nauk Umet Odd Med. Nauki) 2014, 35, 43–46. [Google Scholar]
- Vukelić, M.; Sostarić, B.; Fuchs, R. Some pathomorphological features of Balkan endemic nephropathy in Croatia. IARC Sci. Publ. 1991, 115, 37–42. [Google Scholar]
- Wesseling, C.; Weiss, I. Enfermedad renal crónica de etiología desconocida o de origen no tradicional: ¿una epidemia global? Arch. Prev. Riesgos. Labor. 2017, 20, 200–202. [Google Scholar] [CrossRef]
- Roncal, J.C.A.; García, T.R.; Wesseling, C.; Johnson, R.J. Mesoamerican Nephropathy or Global Warming Nephropathy? Blood Purif. 2016, 41, 135–138. [Google Scholar] [CrossRef]
- Trivedi, A.; Kumar, S. Chronic Kidney Disease of Unknown Origin: Think Beyond Common Etiologies. Cureus 2023, 15, e38939. [Google Scholar] [CrossRef]
- Sánchez, M.E.S.; Sánchez, B.D.J.; Sequeira, C.D.; Murillo, S.J.A.; Sandoval, L.D. Revisión y Actualización en Nefropatía Mesoamericana. Rev. Clin. Esc. Med. 2019, 9, 8–15. [Google Scholar]
- Aguilar, D.J.; Madero, M. Other Potential CKD Hotspots in the World: The Cases of Mexico and the United States. Semin. Nephrol. 2019, 39, 300–307. [Google Scholar] [CrossRef]
- Strasma, A.; Mejía, F.; Aragón, A.; López, I.; Park, L.P.; Hogan, S.L.; Thielman, N.M.; Wyatt, C.M.; González-Quiroz, M. Kidney Disease Characteristics, Prevalence, and Risk Factors in León, Nicaragua: A Population-Based Study. BMC Nephrol. 2023, 24, 335. [Google Scholar] [CrossRef]
- Fajardo, F.A.; Ramírez-Osorto, L.J.; Pérez, C.M.; Benítez, A.A.; Ordóñez, G.M.; Gómez-Flores, E.O.; Merino, A. Prevalencia Y Caracterización de Nefropatía de Etiología No Determinada En Hospital de Segundo Nivel, Honduras. Rev. Colom. Nefrol. 2024, 11, e76. [Google Scholar]
- Winkler, K. Agrotóxicos En El Cultivo de La Caña de Azúcar Y Sus Impactos En La Salud Humana. Causas Y Orígenes de La Nefropatía Mesoamericana En Guatemala; IDEAR-CONGCOOP. 2018. Available online: https://latin.weeffect.org/app/uploads/2018/07/ESTUDIO-AGROTOX_11-jul-2018_VF.pdf (accessed on 5 April 2025).
- Orantes, C.M.; Herrera, R.; Almaguer, M.; Brizuela, E.G.; Hernández, C.E.; Bayarre, H.; Amaya, J.C.; Calero, D.J.; Orellana, P.; Colindres, R.M.; et al. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev. 2011, 13, 14–22. [Google Scholar]
- Wesseling, C.; van Wendel de Joode, B.; Crowe, J.; Rittner, R.; Sanati, N.A.; Hogstedt, C.; Jakobsson, K. Mesoamerican nephropathy: Geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup. Environ. Med. 2015, 72, 714–721. [Google Scholar] [CrossRef]
- Sellar, G.C.; Watt, K.P.; Rabiasz, G.J.; Stronach, E.A.; Li, L.; Miller, E.P.; Massie, C.E.; Miller, J.; Contreras-Moreira, B.; Scott, D.; et al. OPCML at 11q25 Is Epigenetically Inactivated and Has Tumor-Suppressor Function in Epithelial Ovarian Cancer. Nat. Genet. 2003, 34, 337–343. [Google Scholar] [CrossRef]
- Shark, K.B.; Lee, N.M. Cloning, Sequencing and Localization to Chromosome 11 of a CDNA Encoding a Human Opioid-Binding Cell Adhesion Molecule (OBCAM). Gene 1995, 155, 213–217. [Google Scholar] [CrossRef]
- Shao, Y.; Kong, J.; Xu, H.; Wu, X.; Cao, Y.; Li, W.; Han, J.; Li, D.; Xie, K.; Wu, J. OPCML Methylation and the Risk of Ovarian Cancer: A Meta and Bioinformatics Analysis. Front. Cell. Dev. Biol. 2021, 9, 570898. [Google Scholar] [CrossRef]
- Khamko, R.; Daduang, J.; Settasatian, C.; Limpaiboon, T. OPCML Exerts Antitumor Effects in Cholangiocarcinoma via AXL/STAT3 Inactivation and Rho GTPase Down-Regulation. Cancer Genom. Proteom. 2021, 18, 771–780. [Google Scholar] [CrossRef]
- Li, C.; Tang, L.; Zhao, L.; Li, L.; Xiao, Q.; Peng, X.; Ren, G.; Tao, Q.; Xiang, T. OPCML Is Frequently Methylated in Human Colorectal Cancer and Its Restored Expression Reverses EMT via Downregulation of Smad Signaling. Am. J. Cancer Res. 2015, 5, 1635–1648. [Google Scholar]
- Birtley, J.R.; Alomary, M.; Zanini, E.; Antony, J.; Maben, Z.; Weaver, G.C.; Von Arx, C.; Mura, M.; Marinho, A.T.; Lu, H.; et al. Inactivating Mutations and X-Ray Crystal Structure of the Tumor Suppressor OPCML Reveal Cancer-Associated Functions. Nat. Commun. 2019, 10, 3134. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, T.; Zhang, Z.; Bai, X.; Li, H.; Zhang, J. Luteolin Reverses OPCML Methylation to Inhibit Proliferation of Breast Cancer MDA-MB-231 Cells. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 550–555. [Google Scholar] [PubMed]
- Zhang, Z.; Ye, M.; Li, Q.; You, Y.; Yu, H.; Ma, Y.; Mei, L.; Sun, X.; Wang, L.; Yue, W.; et al. The Schizophrenia Susceptibility Gene OPCML Regulates Spine Maturation and Cognitive Behaviors through Eph-Cofilin Signaling. Cell Rep. 2019, 29, 49–61.e7. [Google Scholar] [CrossRef] [PubMed]
- Habicher, J.; Sanvido, I.; Bühler, A.; Sartori, S.; Piccoli, G.; Carl, M. The Risk Genes for Neuropsychiatric Disorders Negr1 and Opcml Are Expressed throughout Zebrafish Brain Development. Genes 2024, 15, 363. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Leone, D.A.; Amador, J.J.; Kupferman, J.; Francey, L.J.; Lopez-Pilarte, D.; Lau, J.; Delgado, I.; Yih, W.K.; Salinas, A.; et al. Genetic Risk Factors for Mesoamerican Nephropathy. Proc. Natl. Acad. Sci. USA 2024, 121, e2404848121. [Google Scholar] [CrossRef]
- Mercadante, S.; Arcuri, E. Opioids and Renal Function. J. Pain 2004, 5, 2–19. [Google Scholar] [CrossRef]
- Marín-Medina, A.; Esteban-Zubero, E.; Alatorre-Jiménez, M.A.; Alonso-Barragan, S.A.; López-García, C.A.; Gómez-Ramos, J.J.; Santoscoy-Gutiérrez, J.F.; González-Castillo, Z. NOS3 Polymorphisms and Chronic Kidney Disease. J. Bras. Nephrol. 2018, 40, 273–277. [Google Scholar] [CrossRef]
- Marin-Medina, A.; Brambila-Tapia, A.J.; Picos-Cárdenas, V.J.; Gallegos-Arreola, M.P.; Figuera-Villanueva, L.E. eNOS gene Glu298Asp and 4b/a polymorphisms are associated with renal function parameters in Mexican patients with Fabry disease. Genet. Mol. Res. 2016, 15, 1–6. [Google Scholar] [CrossRef]
- Li, R.; Zhao, A.; Diao, X.; Song, J.; Wang, C.; Li, Y.; Qi, X.; Guan, Z.; Zhang, T.; He, Y. Polymorphism of NOS3 Gene and Its Association with Essential Hypertension in Guizhou Populations of China. PLoS ONE 2023, 18, e0278680. [Google Scholar] [CrossRef]
- Yi, K.; Guo, T.; Wang, W.-X.; He, S.-E.; Zhang, X.; Xu, J.-G.; Wang, Z.-Q.; Wang, F.-N.; You, T. The Relationship of Nitric Oxide Synthase 3(NOS3) Gene Polymorphism in the Risk of Pulmonary Arterial Hypertension: A Systematic Review and Meta-Analysis. Nitric. Oxide 2024, 154, 51–76. [Google Scholar] [CrossRef]
- Agúndez, J.A.G.; García-Martín, E.; Rodríguez, C.; Benito-León, J.; Millán-Pascual, J.; Díaz-Sánchez, M.; Calleja, P.; Turpín-Fenoll, L.; Alonso-Navarro, H.; García-Albea, E.; et al. Endothelial Nitric Oxide Synthase (NOS3) Rs2070744 Polymorphism and Risk for Multiple Sclerosis. J. Neural. Transm. 2020, 127, 1167–1175. [Google Scholar] [CrossRef]
- Karimian, M.; Yaqubi, S.; Karimian, Z. The ENOS-G894T Genetic Polymorphism and Risk of Preeclampsia: A Case-Control Study, an Updated Meta-Analysis, and a Bioinformatic Assay. Cytokine 2023, 169, 156283. [Google Scholar] [CrossRef] [PubMed]
- Barbitoff, Y.A.; Tsarev, A.A.; Vashukova, E.S.; Maksiutenko, E.M.; Kovalenko, L.V.; Belotserkovtseva, L.D.; Glotov, A.S. A Data-Driven Review of the Genetic Factors of Pregnancy Complications. Int. J. Mol. Sci. 2020, 21, 3384. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; He, Q.; Shen, J.; Yang, J.; Xue, P.; Ma, M.; Xu, R.; Du, L. The Glu298Asp Polymorphism in the NOS3 Gene and the Risk of Prostate Cancer. Tumour. Biol. 2014, 35, 4735–4739. [Google Scholar] [CrossRef]
- Aytac, H.M.; Pehlivan, M.; Oyaci, Y.; Pehlivan, S. Association of Intron 4 VNTR Polymorphism in the NOS3 Gene with Rapid Cycling and Treatment Resistance in Bipolar Disorder: A Case-Control Study. Neurosciences 2022, 27, 229–236. [Google Scholar] [CrossRef]
- Asensi, V.; Montes, A.H.; Valle, E.; Ocaña, M.G.; Astudillo, A.; Alvarez, V.; López-Anglada, E.; Solís, A.; Coto, E.; Meana, A.; et al. The NOS3 (27-Bp Repeat, Intron 4) Polymorphism Is Associated with Susceptibility to Osteomyelitis. Nitric. Oxide 2006, 16, 44–53. [Google Scholar] [CrossRef]
- Padhi, U.N.; Mulkalwar, M.; Saikrishna, L.; Verma, H.K.; Bhaskar, L. NOS3 Gene Intron 4 A/B Polymorphism Is Associated with ESRD in Autosomal Dominant Polycystic Kidney Disease Patients. J. Bras. Nefrol. 2022, 44, 224–231. [Google Scholar] [CrossRef]
- Marín-Medina, A.; Gómez-Ramos, J.J.; Mendoza-Morales, N.; Figuera-Villanueva, L.E. Association between the Polymorphisms Rs2070744, 4b/a and Rs1799983 of the NOS3 Gene with Chronic Kidney Disease of Uncertain or Non-Traditional Etiology in Mexican Patients. Medicina 2023, 59, 829. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Q.-H.; Zhou, C.-J.; Hu, M.-Z.; Qian, H.-X. Protective Effect of ENOS Overexpression against Ischemia/Reperfusion Injury in Small-For-Size Liver Transplantation. Exp. Ther. Med. 2016, 12, 3181–3188. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Saito, Y.; Nakayama, M.; Shimasaki, Y.; Yoshimura, T.; Yoshimura, M.; Harada, H.; Kajiyama, N.; Kishimoto, I.; Kuwahara, K.; et al. Replication Protein A1 Reduces Transcription of the Endothelial Nitric Oxide Synthase Gene Containing a -786T->c Mutation Associated with Coronary Spastic Angina. Hum. Mol. Genet. 2000, 9, 2629–2637. [Google Scholar] [CrossRef]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-Dependent Metabolism of Fructose Induces Proinflammatory Mediators in Proximal Tubular Cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef]
- Campese, V.M. Con: Mesoamerican Nephropathy: Is the Problem Dehydration or Rehydration? Nephrol. Dial. Transplant. 2017, 32, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, M.F.; Muñoz, P.; Serrano, R.; Alonso, M.; Gil, Y.; Quiroga, B. Nefropatía Endémica Mesoamericana: Una Enfermedad Renal Crónica de Origen No Tan Desconocido. Nefrología (Engl. Ed.) 2021, 41, 612–619. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, J. Endothelial Nitric Oxide Synthase Gene Sequence Variations and Vascular Disease. Mol. Genet. Metab. 2000, 70, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Seijas, M.; Baccino, C.; Nin, N.; Lorente, J.A. Definition and Biomarkers of Acute Renal Damage: New Perspectives. Med. Intensiva 2014, 38, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Lombi, F.; Muryan, A.; Canzonieri, R.; Trimarchi, H.; Lombi, F.; Muryan, A.; Canzonieri, R.; Trimarchi, H. Biomarcadores En La Lesión Renal Aguda: ¿Paradigma O Evidencia? Nefrología 2016, 36, 339–346. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef]
- Ramírez-Rubio, O.; Amador, J.J.; Kaufman, J.S.; Weiner, D.E.; Parikh, C.R.; Khan, U.; McClean, M.D.; Laws, R.L.; López-Pilarte, D.; Friedman, D.J.; et al. Urine Biomarkers of Kidney Injury among Adolescents in Nicaragua, a Region Affected by an Epidemic of Chronic Kidney Disease of Unknown Aetiology. Nephrol. Dial. Transplant. 2015, 31, 424–432. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Li, L.; Xia, H.; Lin, Z.; Zhong, T. AMPH-1 Is Critical for Breast Cancer Progression. J. Cancer 2018, 9, 2175–2182. [Google Scholar] [CrossRef]
- Hsu, C.C.; Kao, W.H.; Coresh, J.; Pankow, J.S.; Marsh-Manzi, J.; Boerwinkle, E.; Bray, M.S. Apolipoprotein E and Progression of Chronic Kidney Disease. JAMA 2005, 293, 2892–2899. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Genis-Mendoza, A.D.; León-Escalante, D.I.; Hernández-Díaz, Y.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; López-Narváez, M.L.; Marín-Medina, A.; Nicolini, H.; Castillo-Avila, R.G.; et al. Possible Association of Cholesterol as a Biomarker in Suicide Behavior. Biomedicines 2021, 9, 1559. [Google Scholar] [CrossRef]
- Araki, S.I.; Koya, D.; Makiishi, T.; Sugimoto, T.; Isono, M.; Kikkawa, R.; Kashiwagi, A.; Haneda, M. APOE Polymorphism and the Progression of Diabetic Nephropathy in Japanese Subjects with Type 2 Diabetes: Results of a Prospective Observational Follow-up Study. Diabetes Care 2003, 26, 2416–2420. [Google Scholar] [CrossRef]
- Landires, I.; Courville, K.; Pimentel-Peralta, G.; Cumbrera, R.; Bustamante, N.; Arcos-Burgos, M.; Núñez-Samudio, V. Exome Analysis Points APOE4 Haplotype as Major Risk to Develop Mesoamerican Nephropathy. Kidney Int. Rep. 2024, 10, S365–S366. [Google Scholar] [CrossRef]
- Courville, K.; Bustamante, N.; Hurtado, B.; Pecchio, M.; Rodríguez, C.; Núñez-Samudio, V.; Landires, I. Chronic Kidney Disease of Nontraditional Causes in Central Panama. BMC Nephrol. 2022, 23, 275. [Google Scholar] [CrossRef]
- Waring, W.S.; Conery, A.; Mishra, V.; Shenkin, A.; Webb, D.J.; Maxwell, S.R.J. Uric Acid Reduces Exercise-Induced Oxidative Stress in Healthy Adults. Clin. Sci. 2003, 105, 425–430. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, M.-L.; Chen, M.; Li, K.; Zhu, X.; Gao, Y. Effect of ApoE ε4 Gene Polymorphism on the Correlation between Serum Uric Acid and Left Ventricular Hypertrophy Remodeling in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2022, 9, 1055790. [Google Scholar] [CrossRef]
- Haryono, A.; Nugrahaningsih, D.A.A.; Sari, D.C.R.; Romi, M.M.; Arfian, N. Reduction of Serum Uric Acid Associated with Attenuation of Renal Injury, Inflammation and Macrophages M1/M2 Ratio in Hyperuricemic Mice Model. Kobe J. Med. Sci. 2018, 64, E107–E114. [Google Scholar]
- Saul, D.; Kosinsky, R.L. Epigenetics of Aging and Aging-Associated Diseases. Int. J. Mol. Sci. 2021, 22, 401. [Google Scholar] [CrossRef]
- Pant, S.; Sharma, M.; Patel, K.; Caplan, S.; Carr, C.M.; Grant, B.D. AMPH-1/Amphiphysin/Bin1 Functions with RME-1/Ehd1 in Endocytic Recycling. Nat. Cell Biol. 2009, 11, 1399–1410. [Google Scholar] [CrossRef]
- Yamamoto, R.; LI, X.; Winter, S.; Francke, U.; Kilimann, M.W. Primary Structure of Human Amphiphysin, the Dominant Autoantigen of Paraneoplastic Stiff—Man Syndrome, and Mapping of Its Gene (AMPH) to Chromosome 7p13–P14. Hum. Mol. Genet 1995, 4, 265–268. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Lin, M.-I.; Weng, W.-C.; Lee, W.-T. Neuropsychiatric Disorders due to Limbic Encephalitis: Immunologic Aspect. Int. J. Mol. Sci. 2020, 22, 389. [Google Scholar] [CrossRef]
- Oomatia, A.; Chervova, O.; Al-Rashed, A.M.; Smpokou, E.-T.; Ecker, S.; Pearce, N.; Heggeseth, B.; Nitsch, D.; Cardenas, A.; Beck, S.; et al. Longitudinal Leucocyte DNA Methylation Changes in Mesoamerican Nephropathy. Environ. Epigenet. 2025, 11, dvaf001. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wan, Z.; Huang, C.; Yin, H.; Song, D. AMPH-1 is a tumor suppressor of lung cancer by inhibiting Ras-Raf-MEK-ERK signal pathway. Lasers Med. Sci. 2019, 34, 473–478. [Google Scholar] [CrossRef]
- Liu, O.; Grant, B.D. Basolateral Endocytic Recycling Requires RAB-10 and AMPH-1 Mediated Recruitment of RAB-5 GAP TBC-2 to Endosomes. PLoS Genet. 2015, 11, e1005514. [Google Scholar] [CrossRef]
- Ma, H.; Qu, J.; Liao, Y.; Liu, L.; Yan, M.; Wei, Y.; Xu, W.; Luo, J.; Dai, Y.; Pang, Z.; et al. Equilibrative Nucleotide Transporter ENT3 (SLC29A3): A Unique Transporter for Inherited Disorders and Cancers. Exp. Cell. Res. 2024, 434, 113892. [Google Scholar] [CrossRef]
- Bakhchane, A.; Kindil, Z.; Charoute, H.; Benchikhi, K.H.; Khadir, K.; Nadifi, S.; Baline, K.; Roky, R.; Barakat, A. Compound Heterozygous SLC29A3 Mutation Causes H Syndrome in a Moroccan Patient: A Case Report. Curr. Res. Transl. Med. 2016, 64, 65–68. [Google Scholar] [CrossRef]
- Raines, N.; Leone, D.; O’Callaghan-Gordo, C.; Ramírez-Rubio, O.; Amador, J.; López, D.; Delgado, I.; Leibler, J.; Embade, N.; Gil-Redondo, R.; et al. Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy. Metabolites 2023, 13, 325. [Google Scholar] [CrossRef]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine Pathway, NAD+ Synthesis, and Mitochondrial Function: Targeting Tryptophan Metabolism to Promote Longevity and Healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Zheng, M.; Cai, J.; Liu, Z.; Shu, S.; Wang, Y.; Tang, C.; Dong, Z. Nicotinamide Reduces Renal Interstitial Fibrosis by Suppressing Tubular Injury and Inflammation. J. Cell. Mol. Med. 2019, 23, 3995–4004. [Google Scholar] [CrossRef]
- Hernandez, A.; Fiering, S.; Martinez, E.; Galton, V.A.; Germain, D.S. The Gene Locus Encoding Iodothyronine Deiodinase Type 3 (Dio3) Is Imprinted in the Fetus and Expresses Antisense Transcripts. Endocrinology 2002, 143, 4483–4486. [Google Scholar] [CrossRef]
- Enterina, J.R.; Enfield, S.; Anderson, C.; Marshall, E.A.; Ng, K.W.; Lam, W.L. DLK1-DIO3 Imprinted Locus Deregulation in Development, Respiratory Disease, and Cancer. Expert. Rev. Respir. Med. 2017, 11, 749–761. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Morelli, M.B.; Gambardella, J.; Lombardi, A.; Santulli, G. Thyroid Hormones Regulate Both Cardiovascular and Renal Mechanisms Underlying Hypertension. J. Clin. Hyperten. 2020, 23, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Wirth, E.K.; Wohlgemuth, F.; Reix, N.; Klein, M.O.; Grüters, A.; Köhrle, J.; Schweizer, U. Aminoaciduria, but Normal Thyroid Hormone Levels and Signalling, in Mice Lacking the Amino Acid and Thyroid Hormone Transporter Slc7a8. Biochem. J. 2011, 439, 249–255. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.A.; Brown, T.A.; Horowitz, B.; Chiu, R.; Schlotterbeck, J.; Bowen, J.; Schmitt, C.A. Thyroid Hormone Coordinately Regulates Na+-K+-ATPase Alpha- and Beta-Subunit MRNA Levels in Kidney. Am. J. Physiol. 1988, 254, C323–C329. [Google Scholar] [CrossRef]
- Trajkovic-Arsic, M.; Visser, T.J.; Darras, V.M.; Frieseman, E.C.H.; Schlott, B.; Mittag, J.; Bauer, K.; Heuer, H. Consequences of Monocarboxylate Transporter 8 Deficiency for Renal Transport and Metabolism of Thyroid Hormones in Mice. Endocrinology 2009, 151, 802–809. [Google Scholar] [CrossRef]
- Lo, J.C.; Chertow, G.M.; Go, A.S.; Hsu, C.-Y. Increased Prevalence of Subclinical and Clinical Hypothyroidism in Persons with Chronic Kidney Disease. Kidney Int. 2005, 67, 1047–1052. [Google Scholar] [CrossRef]
- Hataya, Y.; Igarashi, S.; Yamashita, T.; Komatsu, Y. Thyroid Hormone Replacement Therapy for Primary Hypothyroidism Leads to Significant Improvement of Renal Function in Chronic Kidney Disease Patients. Clin. Exp. Nephrol. 2012, 17, 525–531. [Google Scholar] [CrossRef]
- Kitazawa, M.; Sutani, A.; Kaneko-Ishino, T.; Ishino, F. The Role of Eutherian-Specific RTL1 in the Nervous System and Its Implications for the Kagami-Ogata and Temple Syndromes. Genes Cells. 2021, 26, 165–179. [Google Scholar] [CrossRef]
- Dini, P.; Carossino, M.; Balasuriya, U.B.R.; El-Sheikh Ali, H.; Loux, S.C.; Esteller-Vico, A.; Scoggin, K.E.; Loynachan, A.T.; Kalbfleisch, T.; De Spiegelaere, W.; et al. Paternally Expressed Retrotransposon Gag-like 1 Gene, RTL1, Is One of the Crucial Elements for Placental Angiogenesis in Horses. Biol. Reprod. 2021, 104, 1386–1399. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Hu, M.-C.; Chen, P.-Y.; Hsu, C.-L.; Lin, T.-Y.; Tan, M.-J.; Lee, C.-Y.; Kuo, M.-F.; Huang, P.-H.; Wu, V.-C.; et al. RTL1/PEG11 Imprinted in Human and Mouse Brain Mediates Anxiety-like and Social Behaviors and Regulates Neuronal Excitability in the Locus Coeruleus. Hum. Mol. Gen. 2022, 31, 3161–3180. [Google Scholar] [CrossRef]
- Shiura, H.; Kitazawa, M.; Ishino, F.; Kaneko-Ishino, T. Roles of retrovirus-derived PEG10 and PEG11/RTL1 in mammalian development and evolution and their involvement in human disease. Front. Cell Dev. Biol. 2023, 11, 1273638. [Google Scholar] [CrossRef]
- Traustadóttir, G.A.; Lagoni, L.V.; Ankerstjerne, L.B.; Bisgaard, H.C.; Jensen, C.H.; Andersen, D.C. The Imprinted Gene Delta like Non-Canonical Notch Ligand 1 (Dlk1) Is Conserved in Mammals, and Serves a Growth Modulatory Role during Tissue Development and Regeneration through Notch Dependent and Independent Mechanisms. Cytokine Growth Factor Rev. 2019, 46, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Exposito, L.; Cantero-Navarro, E.; Lavoz, C.; Fierro-Fernández, M.; Poveda, J.; Rayego-Mateos, S.; Rodrigues-Diez, R.R.; Morgado-Pascual, J.L.; Orejudo, M.; Mezzano, S.; et al. Análisis de La Vía Notch Como Una Posible Diana Terapéutica En La Patología Renal. Nefrología 2018, 38, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Bonegio, R.; Susztak, K. Notch signaling in diabetic nephropathy. Exp. Cell Res. 2012, 318, 986–992. [Google Scholar] [CrossRef]
- Horimoto, A.R.V.R.; Xue, D.; Cai, J.; Lash, J.P.; Daviglus, M.L.; Franceschini, N.; Thornton, T.A. Genome-Wide Admixture Mapping of Estimated Glomerular Filtration Rate and Chronic Kidney Disease Identifies European and African Ancestry-of-Origin Loci in Hispanic and Latino Individuals in the United States. J. Am. Soc. Nephrol. 2021, 33, 77–87. [Google Scholar] [CrossRef]
| Type of CKDnT | Geographical Area of High Prevalence | Main Etiological Agents | Main Histopathological Characteristics |
|---|---|---|---|
| BeN | Serbia, Croatia, Bosnia and Herzegovina, Romania, and Bulgaria | Exposure to aristolochic acid, genetic predisposition identified [14]. | Tubulointerstitial nephropathy with fibrosis. Secondary glomerular damage, no chronic vascular alterations are observed [15]. |
| Uddanam nephropathy | India and Sri Lanka | Exposure to pesticides, heavy metals, and other chemical and biological agents. Possible genetic predisposition [5]. | Tubulointerstitial nephropathy with fibrosis. No primary glomerular damage is observed; secondary vascular alterations are present [16,17]. |
| MeN | Mexico, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, and Panama | Occupational exposure to high temperatures, contamination with silica particles. Possible genetic predisposition [10]. | Tubulointerstitial nephropathy with fibrosis, which may be accompanied by primary glomerular damage and secondary vascular alteration [17]. |
| Gene | Chromosomal Location | Possible Relationship with MeN |
|---|---|---|
| OPCML | 11q25 | Variants in this gene may be associated with a lower risk of MeN. This gene appears to play a role in regulating temperature. |
| NOS3 | 7q36 | The rs2070744 variant of this gene is associated with decreased NO production, so this variant appears to be related to endothelial dysfunction and possibly glomerular damage, which could contribute to the rapid progression of the disease. |
| NGAL | 9q34 | It appears to be associated with early kidney damage prior to occupational exposure in areas of high MeN prevalence. |
| APOE | 19q13 | The rs429358 variant appears to be associated with hyperuricemia (a previously described risk factor for the development of this disease); therefore, this variant appears to be linked to accelerated disease progression. |
| Chromosomal Location of DMRs | Regulated Genes | Role in the Development or Progression of MeN |
|---|---|---|
| 7p14 | AMPH | This DMR has not been previosuly associated with kidney disease, so it may be unique to MeN and could play an important role in the genesis of disease. The genes regulated by this region perform functions related to vesicular trafficking and receptor recycling. |
| 10q22 | SLC29A3 | This DMR has not been previosuly associated with kidney disease, so it may be unique to MeN and could play an important role in the genesis of disease. The genes it regulates play a central role in the transport of high-energy molecules (NAD+ and analogues) in tubular cells. |
| 14q32 | DIO3 RTL1 DLK1 | The DMR that controls the expression of these genes appears to be associated with established and rapidly progressing kidney disease, since the genes controlled by this region have functions related to angiogenesis, endothelial function, cell growth and proliferation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Medina, A.; Dávalos-Rodríguez, I.P.; Peña-Durán, E.; Torre-Castellanos, L.E.d.l.; González-Vargas, L.F.; Gómez-Ramos, J.J. Genetic Factors Related to the Development or Progression of Mesoamerican Endemic Nephropathy. Int. J. Mol. Sci. 2025, 26, 4486. https://doi.org/10.3390/ijms26104486
Marín-Medina A, Dávalos-Rodríguez IP, Peña-Durán E, Torre-Castellanos LEdl, González-Vargas LF, Gómez-Ramos JJ. Genetic Factors Related to the Development or Progression of Mesoamerican Endemic Nephropathy. International Journal of Molecular Sciences. 2025; 26(10):4486. https://doi.org/10.3390/ijms26104486
Chicago/Turabian StyleMarín-Medina, Alejandro, Ingrid Patricia Dávalos-Rodríguez, Emiliano Peña-Durán, Luis Eduardo de la Torre-Castellanos, Luis Felipe González-Vargas, and José Juan Gómez-Ramos. 2025. "Genetic Factors Related to the Development or Progression of Mesoamerican Endemic Nephropathy" International Journal of Molecular Sciences 26, no. 10: 4486. https://doi.org/10.3390/ijms26104486
APA StyleMarín-Medina, A., Dávalos-Rodríguez, I. P., Peña-Durán, E., Torre-Castellanos, L. E. d. l., González-Vargas, L. F., & Gómez-Ramos, J. J. (2025). Genetic Factors Related to the Development or Progression of Mesoamerican Endemic Nephropathy. International Journal of Molecular Sciences, 26(10), 4486. https://doi.org/10.3390/ijms26104486







