Bioavailability for the Improved Therapeutic Profile of trans-Dehydrocrotonin Incorporated into a Copaiba Oil Self-Nanoemulsifying Drug Delivery System: Formulation, Physicochemical Characterizations, and Antioxidant In Vitro Effect
Abstract
1. Introduction
Colloidal Delivery Systems in Improving Drug Bioavailability
2. Results and Discussion
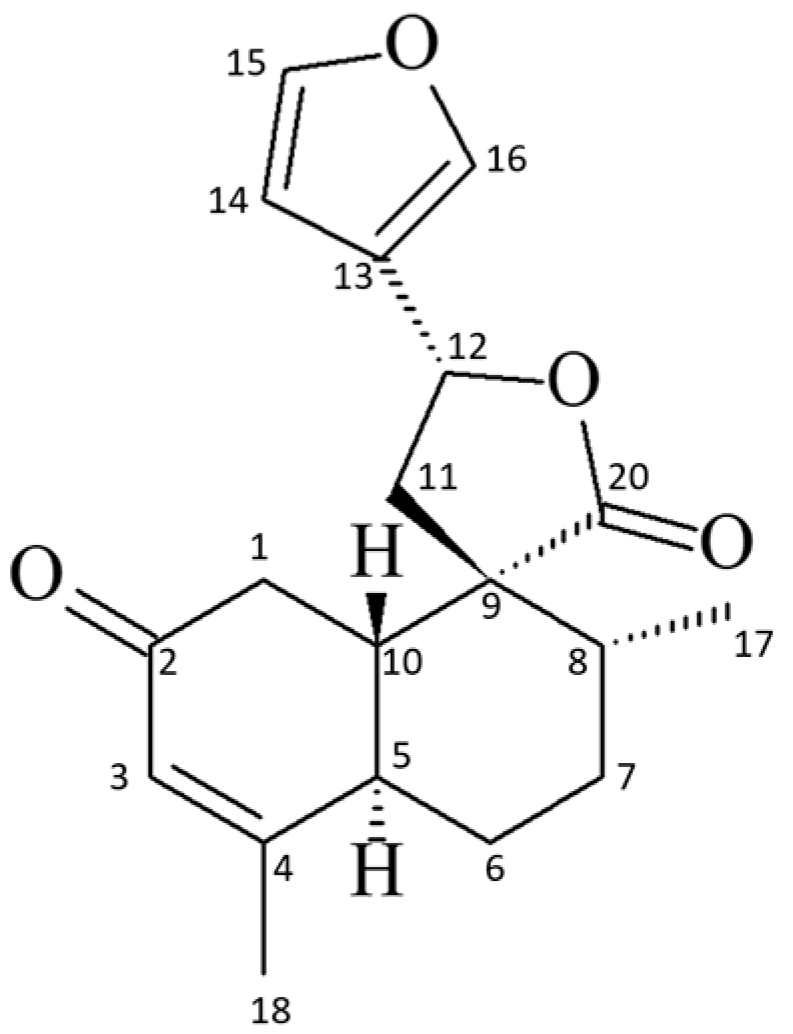
2.1. Phytochemistry Analysis of Croton cajucara Benth
Spectral Data of the 19-nor-Clerodane trans-Dehydrocrotonin
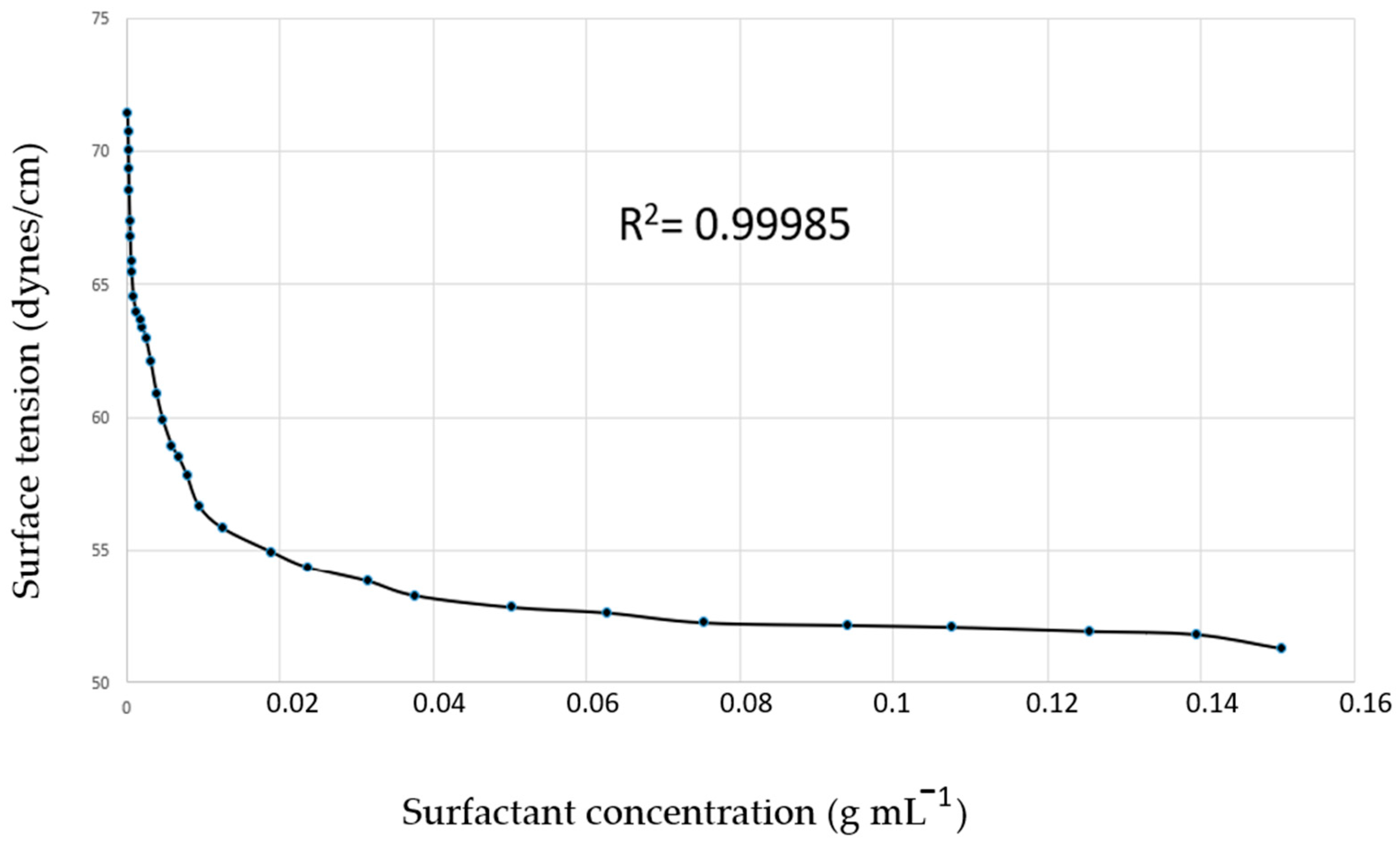
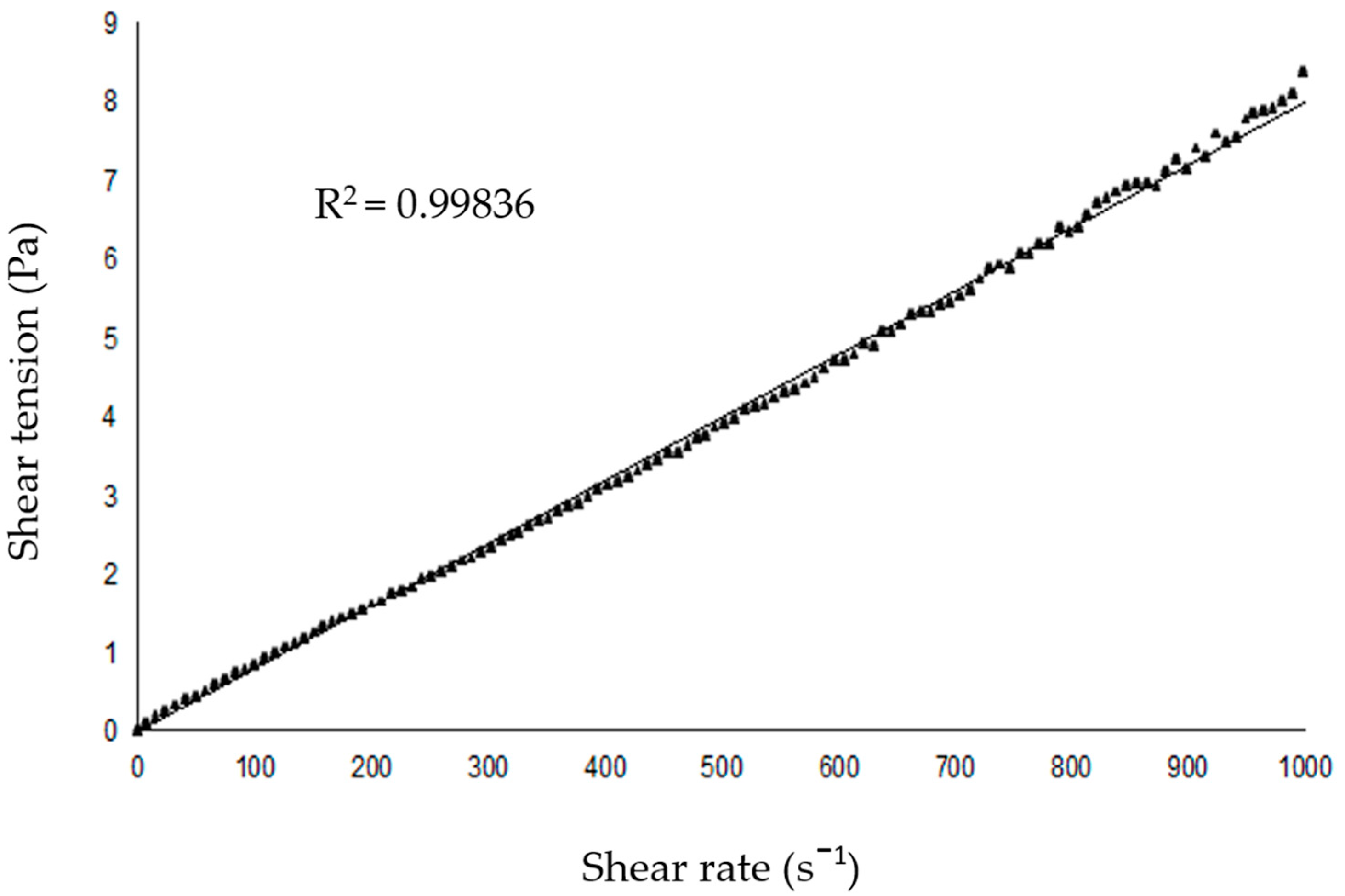
2.2. SNEDDS-Copaiba Oil Colloidal Formulation, Characterization, and Stability Analysis
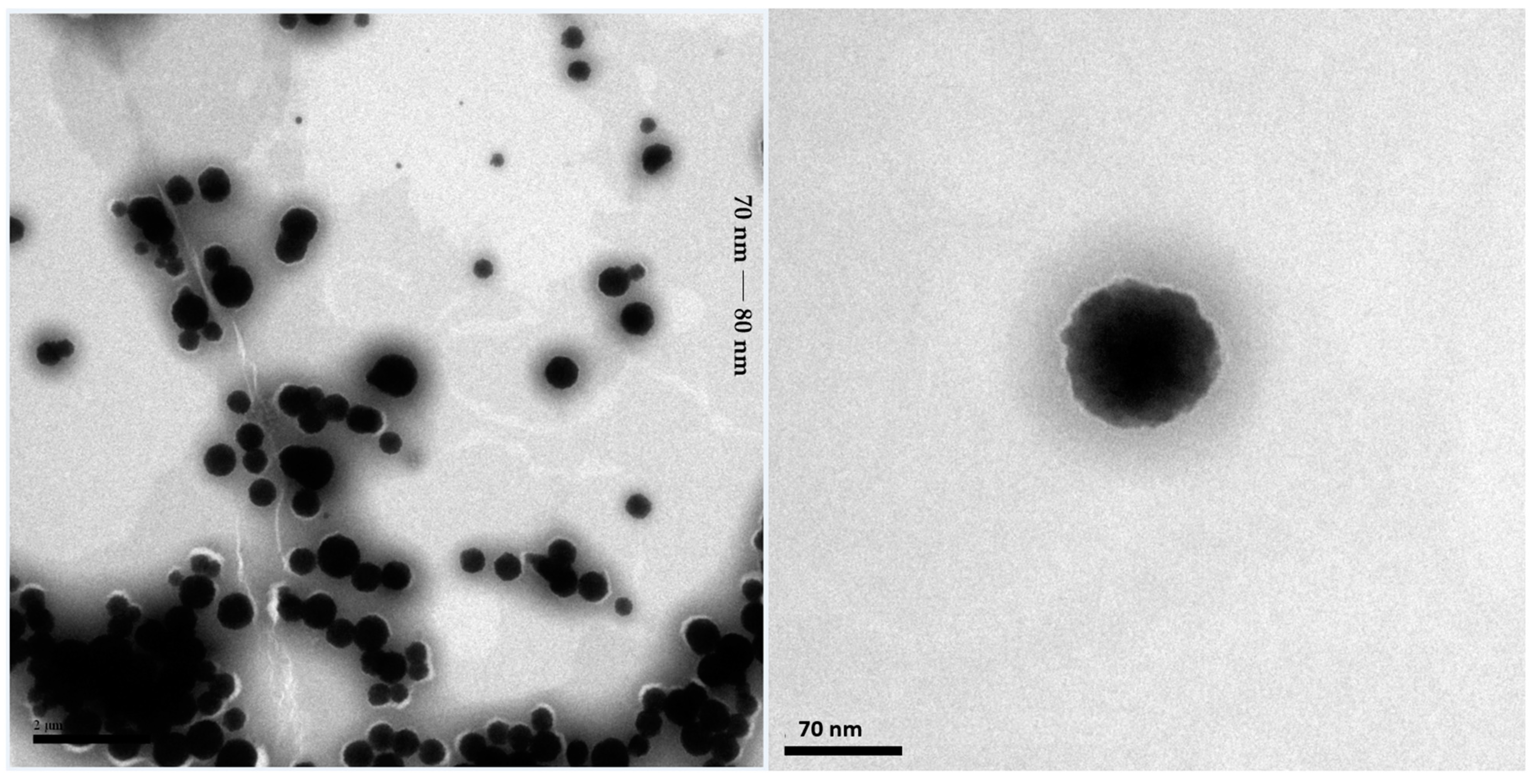
2.2.1. Transmission Electron Microscopy Analysis of the SNEDDS-CO System
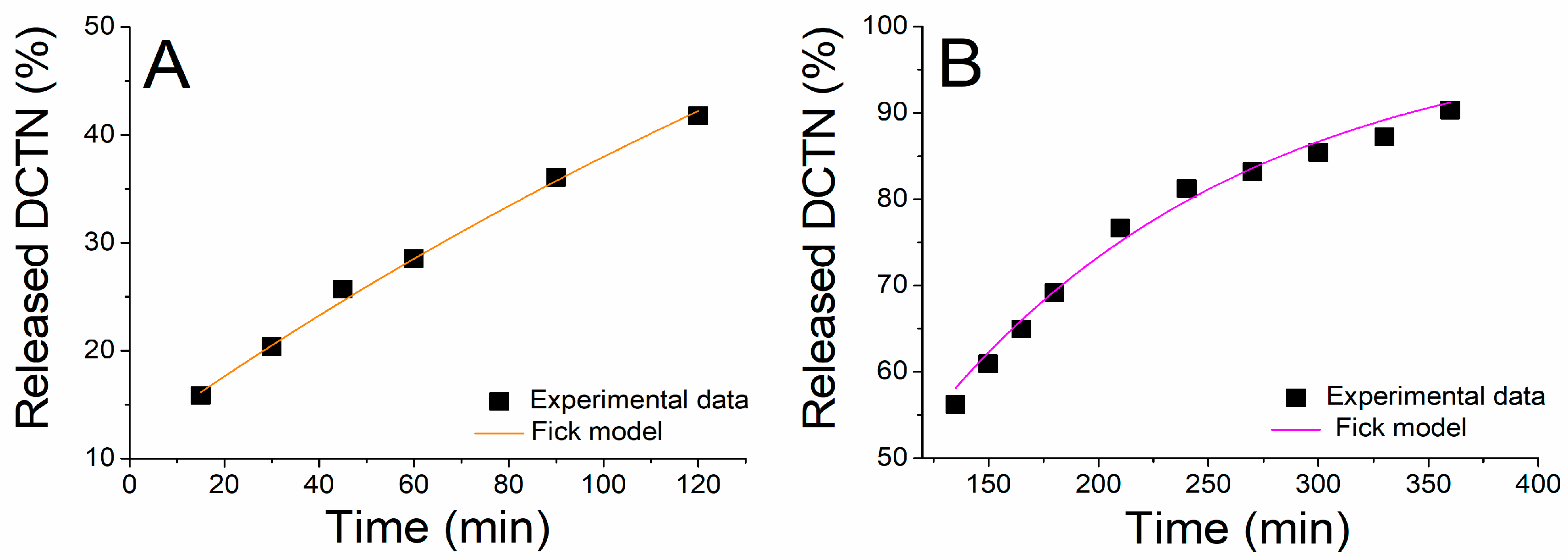
2.2.2. In Vitro Release Kinetic of trans-Dehydrocrotonin Loaded into the SNEDDS-CO System
2.3. In Vitro Antioxidant Analysis of trans-Dehydrocrotonin Loaded into the SNEDDS-CO System
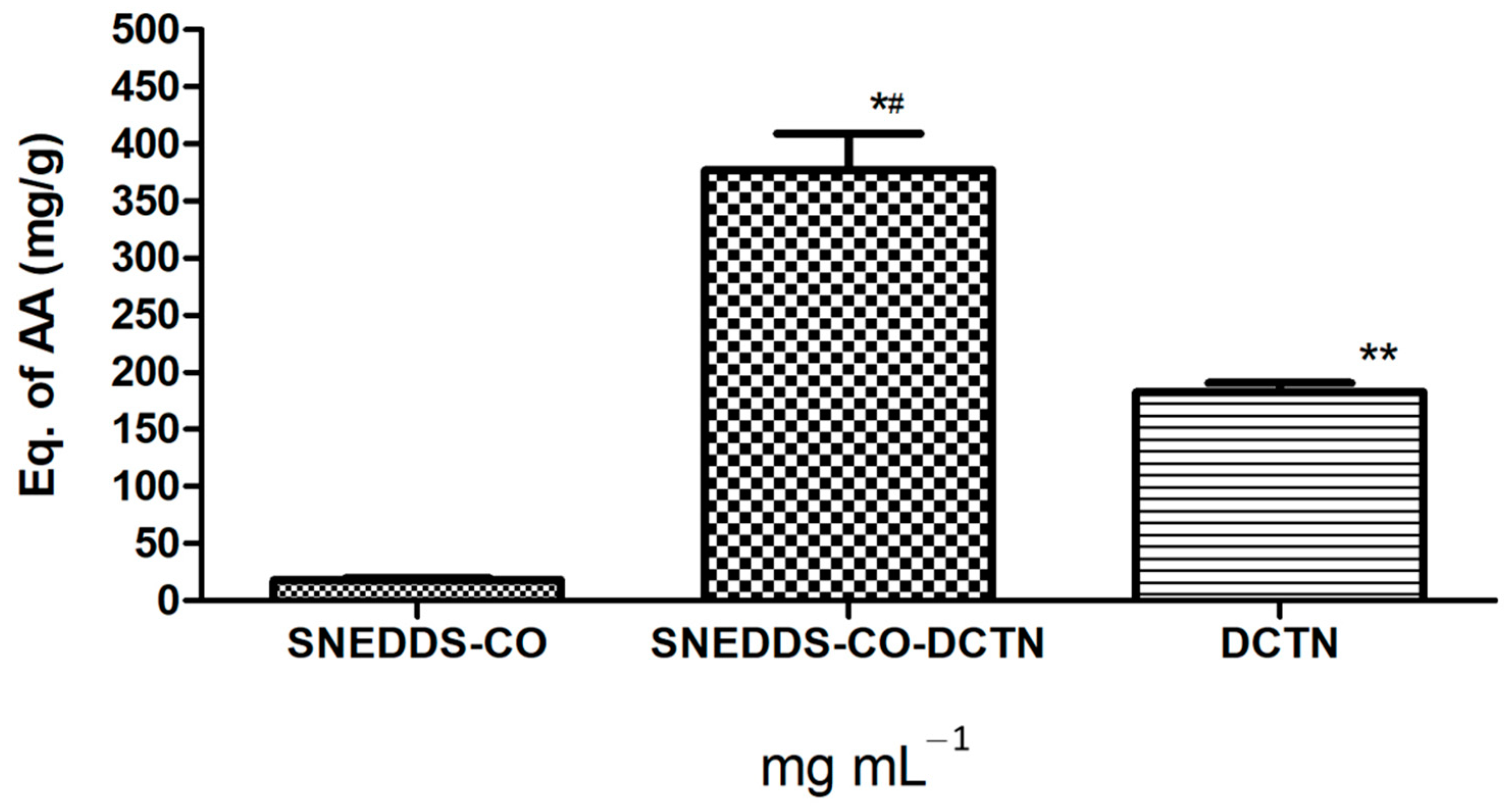
2.3.1. Determination of Total Antioxidant Capacity
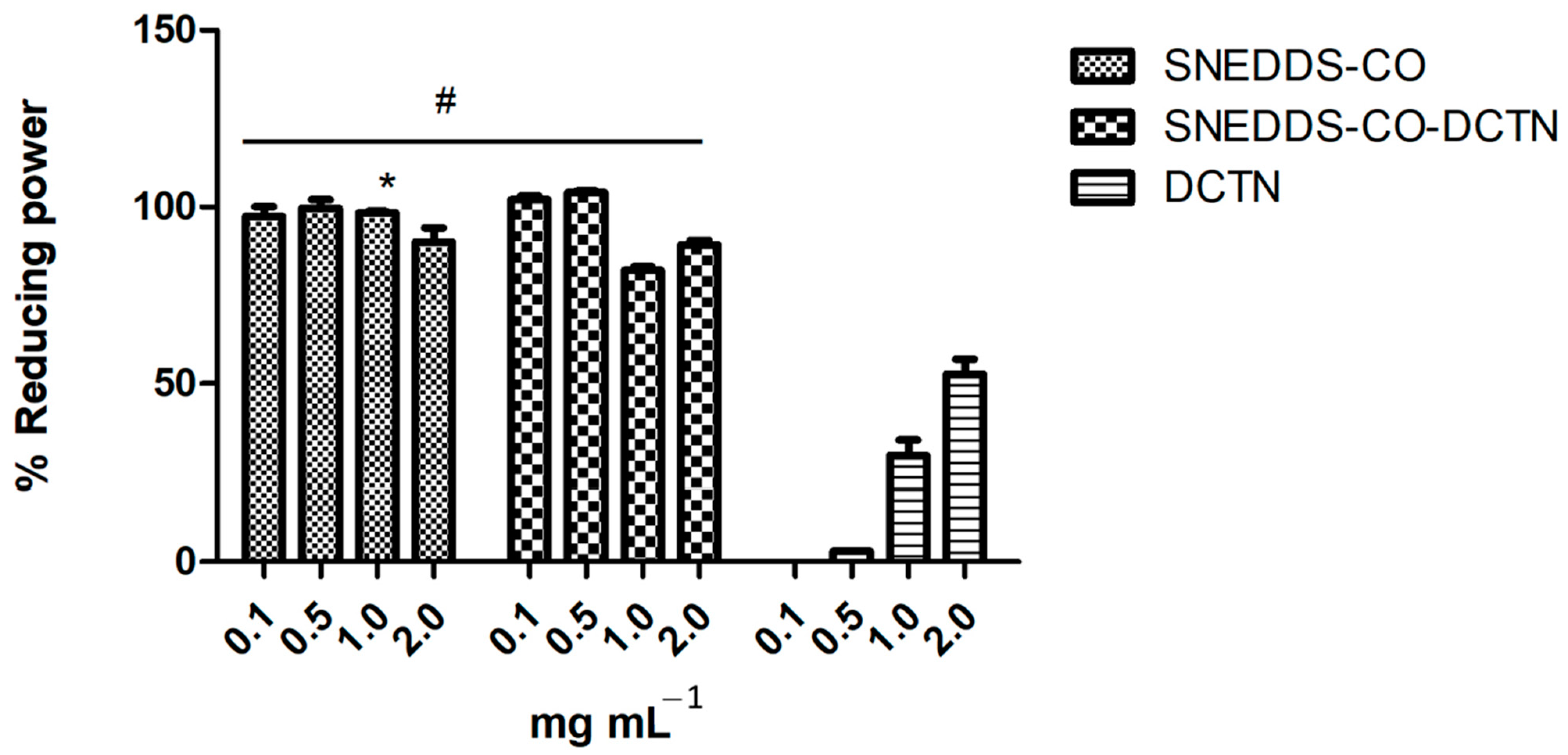
2.3.2. Antioxidant Capacity via Reducing Power
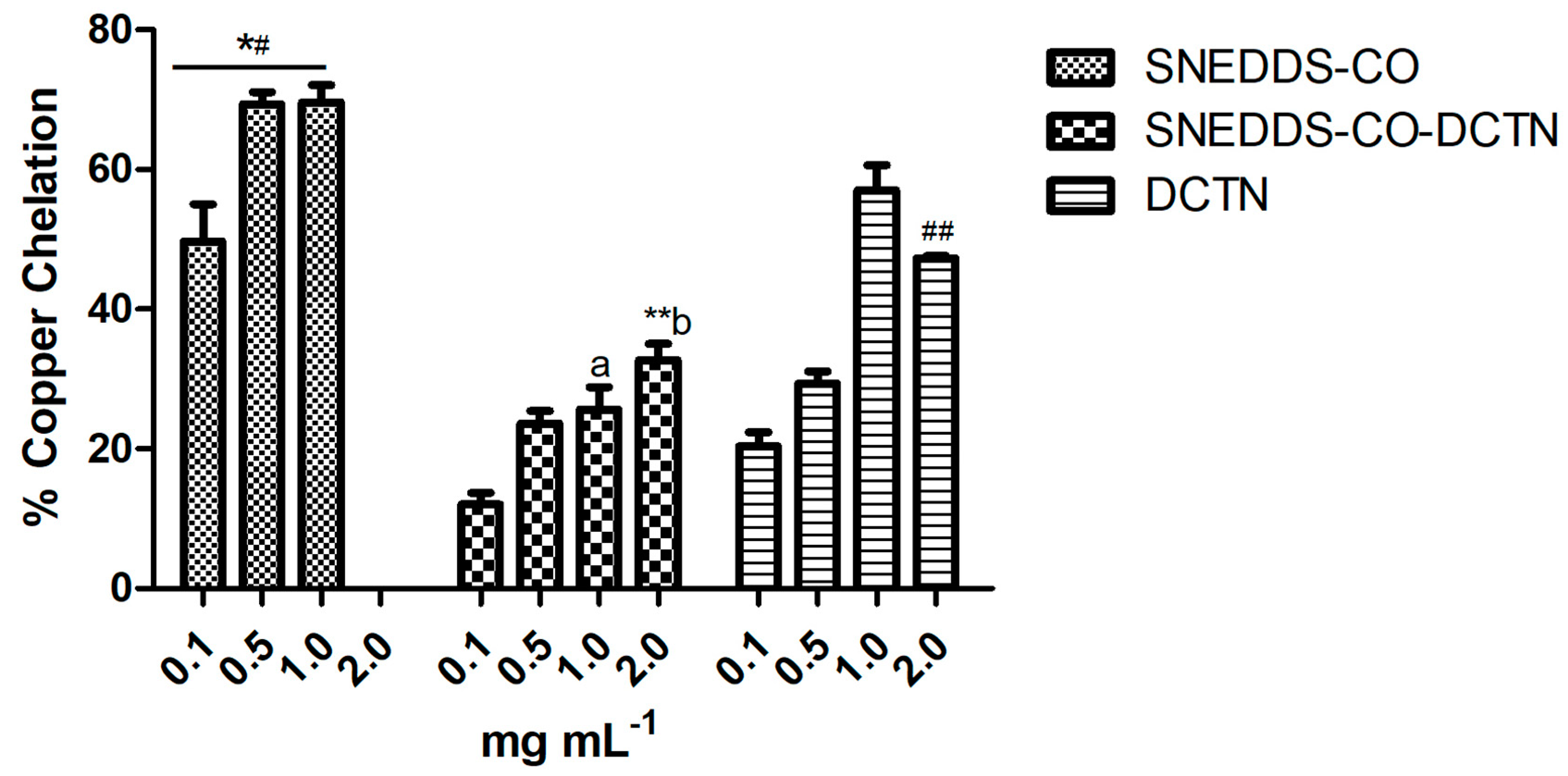
2.3.3. Copper Ion Chelation Method
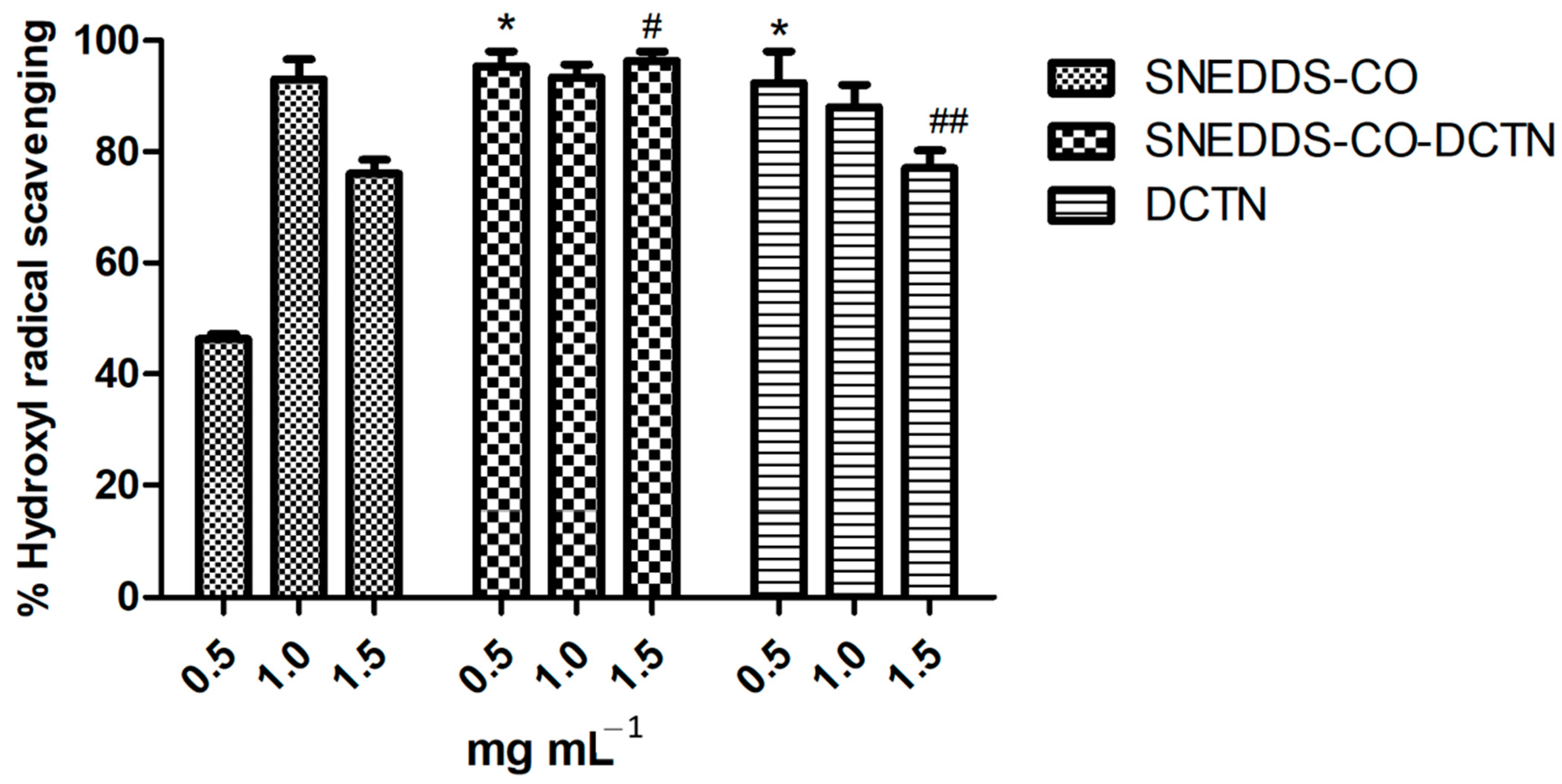
2.3.4. Hydroxyl Radical Sequestration Capability
3. Materials and Methods
3.1. Chromatography Analysis of Copaifera reticulata Ducke
3.2. Chromatography Analysis of Croton cajucara Benth
3.3. SNEDDS-Copaiba Oil Colloidal Formulation
3.4. Loading of trans-Dehydrocrotonin and Effectiveness of the Solubility in the SNEDDS-CO System
3.5. Particle Size, Polydispersity Index, and Zeta Potential Analysis
3.6. In Vitro Release Kinetics
3.7. Antioxidant Activity Assays
3.7.1. Determination of Total Antioxidant Capacity (TAC)
3.7.2. Reducing Power
3.7.3. Copper Chelation
3.7.4. Hydroxyl Radical Scavenging Assay
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maciel, M.A.M.; Pinto, A.C.; Arruda, A.C.; Pamplona, S.G.S.R.; Vanderline, F.A.; Lapa, A.J.; Echevarria, A.; Grynberg, N.F.; Côlus, I.M.S.; Farias, R.A.F.; et al. Ethnopharmacology, phytochemistry and pharmacology: A successful combination in the study of Croton cajucara. J. Ethnopharmacol. 2000, 70, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Maciel, M.A.M.; Pinto, A.C.; Veiga Junior, V.F.; Grynberg, N.F.; Echevarria, A. Medicinal plants: The need for multidisciplinary scientific studies. Quim. Nova 2002, 25, 429–438. [Google Scholar] [CrossRef]
- Lima, L.R.; Da Silva Júnior, F.L.; Rufino Arcanjo, D.D.; Maciel, M.A.M. Croton cajucara: Patents and nanotechnological advances. Recent. Pat. Nanotechnol. 2024, 18, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.T.; Silva, M.F.C.; Maciel, M.A.M.; Pinto, A.C.; Nunes, D.S.; Lima, R.M.; Bastos, J.K.; Sarti, S.J. Investigation of anti-inflammatory and antinociceptive activities of trans-dehydrocrotonin, a 19-nor-clerodane diterpene from Croton cajucara. Part 1. Planta Med. 1996, 62, 402–404. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Ather, A.; Pinto, A.C.; Maciel, M.A.M. Potential benefits of the 19-nor-clerodane trans-dehydrocrotonin on the central nervous system. Rev. Bras. Farmacogn. 2009, 9, 7–13. [Google Scholar] [CrossRef]
- Perazzo, F.F.; Carvalho, J.C.T.; Rodrigues, M.; Morais, E.K.L.; Maciel, M.A.M. Comparative anti-inflammatory and antinociceptive effects of terpenoids and an aqueous extract obtained from Croton cajucara Benth. Rev. Bras. Farmacogn. 2007, 17, 521–528. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Spadari-Bratfisch, R.C.; Grassi-Kassisse, D.M.; Souza-Brito, A.R.M. Antiulcerogenic mechanisms of dehydrocrotonin, a diterpene lactone obtained from Croton cajucara. Planta Med. 1999, 65, 325–330. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Hiruma-Lima, C.A.; Souza-Brito, A.R.M. Antiulcer activity and subacute toxicity of trans-dehydrocrotonin from Croton cajucara. Hum. Exp. Toxicol. 2004, 23, 455–461. [Google Scholar] [CrossRef]
- Luna Costa, A.M.; Silva, J.C.; Campos, A.R.; Rao, V.S.; Maciel, M.A.; Pinto, A.C. Antioestrogenic effect of trans-dehydrocrotonin, a nor-clerodane diterpene from Croton cajucara Benth. in rats. Phytother. Res. 1999, 13, 689–691. [Google Scholar] [CrossRef]
- Farias, R.A.F.; Rao, V.S.N.; Viana, C.S.B.; Silveira, E.R.; Maciel, M.A.M.; Pinto, A.C. Hypoglycemic effect of trans-dehydrocrotonin, a nor-clerodane diterpene from Croton cajucara. Planta Med. 1997, 63, 558–560. [Google Scholar] [CrossRef]
- Silva, R.M.; Santos, F.A.; Rao, V.S.N.; Maciel, M.A.M.; Pinto, A.C. The lipid-lowering effect of trans-dehydrocrotonin, a clerodane diterpene from Croton cajucara Benth. in mice fed on high-fat diet. J. Pharm. Pharmacol. 2001, 53, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Santos, F.A.; Rao, V.S.N.; Maciel, M.A.M.; Pinto, A.C. Blood glucose- and triglyceride-lowering effect of trans-dehydrocrotonin, a diterpene from Croton cajucara Benth, in rats. Diabetes Obes. Metab. 2001, 3, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Santos, F.A.; Maciel, M.A.M.; Pinto, A.C.; Rao, V.S.N. Effect of trans-dehydrocrotonin, a 19-nor-clerodane diterpene from Croton cajucara on experimental hypertriglyceridaemia and hypercholesterolaemia induced by triton WR 1339 (tyloxapol) in mice. Planta Med. 2001, 67, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Agner, A.R.; Maciel, M.A.M.; Pinto, A.C.; Cólus, I.M.S. Antigenotoxicity of trans-dehydrocrotonin, a clerodane diterpene from Croton cajucara. Planta Med. 2001, 67, 815–819. [Google Scholar] [CrossRef]
- Agner, A.R.; Maciel, M.A.M.; Pinto, A.C.; Pamplona, S.G.S.R.; Cólus, I.M.S. Investigation of genotoxicity activity of trans-dehydrocrotonin, a clerodane diterpene from Croton cajucara. Teratog. Carcinog. Mutagen. 1999, 19, 377–384. [Google Scholar] [CrossRef]
- Santos, F.V.; Mesquita, S.F.P.; Faria, M.J.S.S.; Poersh, A.; Maciel, M.A.M.; Pinto, A.C.; Morimoto, H.K.; Cólus, I.M.S. Absence of mutagenicity in somatic and germ cells of mice submitted to subchronic treatment with an extract of Croton cajucara Benth (Euphorbiaceae). Genet. Mol. Biol. 2011, 29, 159–165. [Google Scholar] [CrossRef]
- Lima, G.; Machado, G.; Maciel, M.M.; Echevarria, A. Antitrypanosomal and antileishmanial effects of the hydroalcoholic extract of Croton cajucara benth and its 19-nor-clerodane chromatographic fractions. Pharmacogn. Mag. 2021, 17, 302–306. [Google Scholar] [CrossRef]
- Lima, G.S.; Castro-Pinto, D.B.; Machado, G.C.; Maciel, M.A.M.; Echevarria, A. Antileishmanial activity and trypanothione reductase effects of terpenes from the Amazonian species Croton cajucara Benth (Euphorbiaceae). Phytomedicine 2015, 22, 1133–1137. [Google Scholar] [CrossRef]
- Campos, M.C.O.; Salomão, K.; Castro-Pinto, D.B.; Leon, L.L.; Barbosa, H.S.; Maciel, M.A.M.; De Castro, S. Croton cajucara crude extract and isolated terpenes: Activity on Trypanosoma cruzi. Parasitol. Res. 2010, 107, 1193–1204. [Google Scholar] [CrossRef]
- Silva, R.M.; Oliveira, F.M.; Cunha, K.M.A.; Maia, J.L.; Maciel, M.A.M.; Pinto, A.C.; Nascimento, N.R.F.; Santos, F.A.; Rao, V.S.N. Cardiovascular effects of trans-dehydrocrotonin, a diterpene from Croton cajucara in rats. Vascul. Pharmacol. 2005, 43, 11–18. [Google Scholar] [CrossRef]
- Zeng, N.; Zhang, Q.; Yao, Q.; Fu, G.; Su, W.; Wang, W.; Li, B. A Comprehensive Review of the Classification, Sources, Phytochemistry, and Pharmacology of Norditerpenes. Molecules 2024, 29, 60. [Google Scholar] [CrossRef] [PubMed]
- Esteves-Souza, A.; Pissinate, K.; Maciel, M.A.M.; Echevarria, A. Synthesis of new trans-dehydrocrotonin nitrogenated derivatives and their cytotoxic and DNA-Topoisomerase I Inhibition Activities. J. Braz. Chem. Soc. 2018, 29, 133–139. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Cortez, J.K.P.C.; Gomes, F.E.S. The genus Croton and relevant aspects of clerodane diterpenes. Rev. Fitos 2006, 2, 54–73. [Google Scholar] [CrossRef]
- Pinto, E.P.; Menezes, R.P.; Tavares, W.S.; Ferreira, A.M.; De Sousa, F.F.O.; Da Silva, G.A.; Zamora, R.R.M.; Araújo, R.S.; De Souza, T.M. Copaiba essential oil loaded-nanocapsule film as potential candidate for treating skin disorders: Preparations, characterization, and antibacterial properties. Int. J. Pharm. 2023, 633, 122608. [Google Scholar] [CrossRef]
- De Medeiros, M.L.; Araújo-Filho, I.; Soares, C.D.; Rossi, C.G.F.T.; De Mello Salgueiro, C.C.; Ramalho, H.M.M.; Xavier Júnior, F.H.; Veiga Júnior, V.F.; Maciel, M.A.M. Copaiba oil loaded into self-nanoemulsifying drug delivery system enriched with powdered coconut water as a strategy for therapeutic enhancement of skin wound healing. In Applications and Industrialisation of Nanotechnology; Ahmed, W., Maciel, M.A.M., Eds.; One Central Press: Manchester, UK, 2022; pp. 1–25. [Google Scholar]
- De Medeiros, M.L.; De Oliveira Netto, J.R.; Xavier Júnior, F.H.; Veiga Júnior, V.F.; Maciel, M.A.M. Copaiba oil as a natural product challenge in the chemistry, pharmacological and biotecnological fields. In Applications and Industrialisation of Nanotechnology; Ahmed, W., Maciel, M.A.M., Eds.; One Central Press: Manchester, UK, 2022; pp. 220–262. [Google Scholar]
- Emerenciano, D.P.; Baracho, B.B.D.; Medeiros, M.L.; Rocha, H.A.O.; Xavier-Júnior, F.H.; Veiga-Júnior, V.F.; Maciel, M.A.M. Physicochemical characterizations and antioxidant property of copaiba oil loaded into SNEDDS systems. J. Braz. Chem. Soc. 2019, 30, 234–246. [Google Scholar] [CrossRef]
- Lucca, L.G.; De Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga-Júnior, V.F.; De Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory effect from a hydrogel containing nanoemulsified copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech. 2018, 19, 522–530. [Google Scholar] [CrossRef]
- Oliveira Neves, J.K.; Apolinário, A.C.; Saraiva, K.L.A.; Da Silva, D.T.C.; Araújo Reis, M.Y.F.; Damasceno, B.P.G.L.; Pessoa Junior, A.; Galvão, M.A.M.; Soares, L.A.L.; Veiga-Júnior, V.F.; et al. Microemulsions containing Copaifera multijuga Hayne oil-resin: Challengs to acieve na efficient system for β-caryophyllene delivery. Ind. Crop. Prod. 2018, 111, 185–192. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; Ramos, A.S.; Falcão, D.Q.; Ferreira, J.L.P.; Basso, S.L.; Silva, J.R.A.; Amaral, A.C.F. Development of nanoemulsions to enhance the antileishmanial activity of Copaifera paupera oleoresins. Biomed. Res. Int. 2018, 9, 9781724. [Google Scholar] [CrossRef]
- Dias, D.O.; Colombo, M.; Kelmann, R.G.; Kaiser, S.; Lucca, L.G.; Teixeira, H.F.; Limberger, R.P.; Veiga Junior, V.F.; Koester, L.S. Optimization of copaiba oil-based nanoemulsions obtained by different preparation methods. Ind. Crops. Prod. 2014, 59, 154–162. [Google Scholar] [CrossRef]
- Xavier Junior, F.H.; Huang, N.; Vachon, J.J.; Rehder, V.L.; Egito, E.S.; Vauthier, C. Match of solubility parameters between oil and surfactants as a rational approach for the formulation of microemulsion with a high dispersed volume of copaiba oil and low surfactant content. Pharm. Res. 2016, 33, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Ames-Sibin, A.P.; Chagas, A.C.; Ferreira, S.B.S.; Mandim, F.; Finimundy, T.C.; Calhelha, R.C.; Peralta, R.M.; Sá-Nakanishi, A.B.; Bracht, L.; Bruschi, M.L.; et al. Characterization and bioactivity of copaiba essential oil carried in a self-nanoemulsifying drug delivery system. J. Drug Deliv. Sci. Technol. 2024, 91, 105206. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, J.-G.; Yun, T.-H.; Cho, J.-H.; Kim, K.-S. Enhanced stability and improved oral absorption of enzalutamide with self-nanoemulsifying drug delivery system. Int. J. Mol. Sci. 2024, 25, 1197. [Google Scholar] [CrossRef]
- Mohite, P.; Sule, S.; Pawar, A.; Alharbi, H.M.; Maitra, S.; Subramaniyan, V.; Kumarasamy, V.; Uti, D.E.; Ogbu, C.O.; Oodo, S.I.; et al. Development and characterization of a self-nano emulsifying drug delivery system (SNEDDS) for Ornidazole to improve solubility and oral bioavailability of BCS class II drugs. Sci. Rep. 2024, 14, 27724. [Google Scholar] [CrossRef]
- Morakul, B.; Teeranachaideekul, V.; Limwikrant, W.; Junyaprasert, V.B. Dissolution and antioxidant potential of apigenin self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery. Sci. Rep. 2024, 14, 8851. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Calado, P.F.; Pereira, J.D.S.; Medeiros, M.I.T. Sistemas Carreadores Coloidais e Aplicações na Odontologia. In Contribuições Científicas em Odontologia: Pesquisas, Práticas e Novos Paradigmas; Casais, P.M., Lins, L.S.S., Eds.; AMPLLA: Campina Grande, Brasil, 2022; Volume 1, pp. 477–517. [Google Scholar]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Préat, V. Self-nano-emulsifying drug-delivery systems: From the development to the current applications and challenges in oral drug delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Rani, S.; Rana, R.; Saraogi, G.K.; Kumar, V.; Gupta, U. Self-emulsifying oral lipid drug delivery systems: Advances and challenges. AAPS PharmSciTech. 2019, 20, 129. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing drug solubility, bioavailability, and targeted therapeutic applications through magnetic nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Haripriyaa, M.; Suthindhiran, K. Pharmacokinetics of nanoparticles: Current knowledge, future directions and its implications in drug delivery. Futur. J. Pharm. Sci. 2023, 9, 113. [Google Scholar] [CrossRef]
- Saikia, P.; Das, A.; Chutia, A. Nano tech for better drugs: Exploring the advantages of self-nano emulsifying drug delivery systems. Int. J. Pharm. Sci. Nanotechnol. 2024, 2, 1734–1752. [Google Scholar] [CrossRef]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, in vitro and in vivo Assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS): Challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Sinde, A.; Shinde, P.; Deshmukh, A. A complete review on self-nanoemulsifying drug delivery system. Int. J. Anal. Exp. Modal Anal. 2021, 13, 1459–1473. [Google Scholar]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Ther. Drug. Carrier. Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and microemulsions in biomedicine: From theory to practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef]
- Almeida, A.R.; Morais, W.A.; Oliveira, N.D.; Silva, W.C.G.; Gomes, A.P.B.; Espindola, L.S.; Araujo, M.O.; Araujo, R.M.; Albernaz, L.C.; De Sousa, D.P.; et al. Nanoemulsions and solid microparticles containing pentyl cinnamate to control Aedes aegypti. Int. J. Mol. Sci. 2023, 24, 12141. [Google Scholar] [CrossRef]
- Xavier Júnior, F.H.; Silva, K.G.H.; Farias, I.E.G.; Morais, A.R.V.; Alencar, E.N.; Araujo, I.B.; Oliveira, A.G.; Egito, E.S.T. Prospective study for the development of emulsion systems containing natural oil products. J. Drug. Deliv. Sci. Technol. 2012, 22, 367–372. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.D.L. Nanoemulsions: Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Arab, D.; Kantzas, A.; Bryant, S.L. Nanoparticle stabilized oil in water emulsions: A critical review. J. Pet. Sci. Eng. 2018, 163, 217–242. [Google Scholar] [CrossRef]
- Salager, J.-L.; Marquez, R.; Rodón, M.; Bullón, J.; Graciaa, A. Review on some confusion produced by the bicontinuous microemulsion terminology and its domains microcurvature: A simple spatiotemporal model at optimum formulation of surfactant-oil-water systems. ACS Omega 2023, 8, 9040–9057. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Celma, M.J.G. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Kupikowska-Stobba, B.; Kasprzak, M. Fabrication of nanoparticles for bone regeneration: New insight into applications of nanoemulsion technology. J. Mater. Chem. B. 2021, 9, 5221–5244. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C. Nanoemulsion for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Friberg, S.; Corkery, R.; Blute, I. Phase inversion temperature (PIT) emulsification process. J. Chem. Eng. Data 2011, 56, 4282–4290. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Zhang, M.; Pang, Y.; Li, Z.; Zhao, A.; Feng, J. Self-emulsifying drug delivery system and the applications in herbal drugs. Drug Deliv. 2015, 22, 475–486. [Google Scholar] [CrossRef]
- Yan, B.; Ma, Y.; Guo, J.; Wang, Y. Self-microemulsifying delivery system for improving bioavailability of water insoluble drugs. J. Nanopart. Res. 2020, 22, 18. [Google Scholar] [CrossRef]
- Telles, L.O.; Silva, B.S.; Paulino, A.M.B.; Mendonça, S.T.; Sinhorin, V.D.G.; Lima, M.C.F.; Junior, V.F.V.; Andrighetti, C.R.; Nascimento, A.F.; Bomfim, G.F.; et al. Copaiba oleoresin presents anti-obesogenic effect and mitigates inflammation and redox imbalance in adipose tissue. Acta Amaz. 2022, 52, 331–338. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne-a comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Zunino, L.; Patitucci, M.L.; Pinto, A.C.; Calixto, J.B. The inhibition of paw oedema formation caused by the oil of Copaifera multijuga Hayne and its fractions. J. Pharm. Pharmacol. 2006, 58, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Pinto, A.C.; De Lima, H.C. The essential oil composition of Copaifera trapezifolia Hayne leaves. J. Essent. Oil Res. 2006, 18, 430–431. [Google Scholar] [CrossRef]
- Frazão, D.R.; Cruz, J.N.; Santana de Oliveira, M.; Baia-da-Silva, D.C.; Nazário, R.M.F.; Rodrigues, M.F.L.; Saito, M.T.; Souza-Rodrigues, R.D.; Lima, R.R. Evaluation of the biological activities of Copaiba (Copaifera spp): A comprehensive review based on scientometric analysis. Front. Pharmacol. 2023, 14, 1215437. [Google Scholar] [CrossRef]
- Mauro, M.; Da Silva, R.M.; De Campos, M.L.; Bauermeister, A.; Lopes, N.P.; Moraes, N.V. In vitro metabolism of copalic and kaurenoic acids in rat and human liver microsomes. Quim Nova 2021, 44, 700–708. [Google Scholar] [CrossRef]
- Símaro, G.V.; Lemos, M.; Mangabeira da Silva, J.J.; Ribeiro, V.P.; Arruda, C.; Schneider, A.H.; Wanderley, C.W.S.; Carneiro, L.J.; Mariano, R.L.; Ambrósio, S.R.; et al. Antinociceptive and anti-inflammatory activities of Copaifera pubiflora Benth oleoresin and its major metabolite ent-hardwickiic acid. J. Ethnopharmacol. 2021, 271, 113883. [Google Scholar] [CrossRef]
- Couto, R.S.D.; Rodrigues, M.F.S.D.; Ferreira, L.S.; Diniz, I.M.A.; Silva, F.S.; Lopez, T.C.C.; Lima, R.R.; Marques, M.M. Evaluation of resin-based material containing copaiba oleoresin (Copaifera reticulata Ducke): Biological effects on the human dental pulp stem cells. Biomolecules 2020, 10, 972. [Google Scholar] [CrossRef]
- Moraes, T.S.; Leandro, L.F.; Santiago, M.B.; De Oliveira Silva, L.; Bianchi, T.C.; Veneziani, R.C.S.; Ambrósio, S.R.; Ramos, S.B.; Bastos, J.K.; Martins, C.H.G. Assessment of the antibacterial, antivirulence, and action mechanism of Copaifera pubiflora oleoresin and isolated compounds against oral bacteria. Biomed. Pharmacother. 2020, 129, 110467. [Google Scholar] [CrossRef]
- Arruda, C.; Aldana Mejía, J.A.; Ribeiro, V.P.; Gambeta Borges, C.H.; Gomes Martins, C.H.; Sola Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus: A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef]
- Senedese, J.M.; Rinaldi-Neto, F.; Furtado, R.A.; Nicollela, H.D.; De Souza, L.D.R.; Ribeiro, A.B.; Ferreira, L.S.; Magalhães, G.M.; Carlos, I.Z.; Da Silva, J.J.M.; et al. Chemopreventive role of Copaifera reticulata Ducke oleoresin in colon carcinogenesis. Biomed. Pharmacother. 2019, 111, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Abrão, F.; Alves, J.A.; Andrade, G.; De Oliveira, P.F.; Ambrósio, S.R.; Veneziani, R.C.S.; Tavares, D.C.; Bastos, J.K.; Martins, C.H.G. Antibacterial effect of Copaifera duckei Dwyer oleoresin and its main diterpenes against oral pathogens and their cytotoxic effect. Front. Microbiol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Da Trindade, R.; Da Silva, J.K.; Setzer, W.N. Copaifera of the neotropics: A review of the phytochemistry and pharmacology. Int. J. Mol. Sci. 2018, 19, 1511. [Google Scholar] [CrossRef]
- Souza, M.G.M.; Leandro, L.F.; Moraes, T.S.; Abrão, F.; Veneziani, R.C.S.; Ambrosio, S.R.; Martins, C.H.G. ent-Copalic acid antibacterial and anti-biofilm properties against Actinomyces naeslundii and Peptostreptococcus anaerobius. Anaerobe 2018, 52, 43–49. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Pinto, A.C. The Copaifera L. genus. Quim. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Yin, J.L.; Wong, W.S. Production of santalenes and bergamotene in nicotiana tabacum plants. PLoS ONE 2019, 14, e0203249. [Google Scholar] [CrossRef]
- Li, H.; Ge, Y.; Zhou, Y.; Zhang, X.; Zhang, J.; Fu, Q. Evaluation of the chemical composition, antioxidant and anti-inflammatory activities of distillate and residue fractions of sweet basil essential oil. J. Food. Sci. Technol. 2017, 54, 1882–1890. [Google Scholar] [CrossRef]
- Bardají, D.K.; Da Silva, J.J.; Bianchi, T.C.; De Souza Eugênio, D.; De Oliveira, P.F.; Leandro, L.F.; Rogez, H.L.; Venezianni, R.C.; Ambrosio, S.R.; Tavares, D.C.; et al. Copaifera reticulata oleoresin: Chemical characterization and antibacterial properties against oral pathogens. Anaerobe 2016, 40, 18–27. [Google Scholar] [CrossRef]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef]
- Singh, A.K.; Chanotiya, C.S.; Yadav, A.; Kalra, A. Volatile of Callicarpa macropylla: A rich source of selineneisomer. Nat. Prod. Commun. 2010, 5, 269–272. [Google Scholar]
- Borghi, S.M.; Mizokami, S.S.; Carvalho, T.T.; Rasquel-Oliveira, F.S.; Ferraz, C.R.; Fattori, V.; Hayashida, T.H.; Peron, J.P.S.; Camilios-Neto, D.; Ambrosio, S.R.; et al. The diterpene from Sphagneticola trilobata (L.) Pruski, kaurenoic acid, reduces lipopolysaccharide-induced peritonitis and pain in mice. J. Ethnopharmacol. 2021, 273, 113980. [Google Scholar] [CrossRef] [PubMed]
- Moreti, D.L.C.; Leandro, L.F.; Da Silva Moraes, T.; Moreira, M.R.; Sola Veneziani, R.C.; Ambrosio, S.R.; Gomes, B.P.; Martins, C.H.G. Mikania glomerata sprengel extract and its major compound ent-kaurenoic acid display activity against bacteria present in endodontic infections. Anaerobe 2017, 47, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Cunha, K.M.A.; Silveira, E.R.; Santos, F.A.; Rao, V.S. Effects of kaurenoic acid, a bioactive diterpene on embryo implantation and pregnancy outcome in mice. Curr. Top. Toxicol. 2011, 7, 89–94. [Google Scholar]
- Maciel, M.A.M.; Pinto, A.C.; Kaiser, C.R. NMR and structure review of some natural furoclerodanes. Magn. Reson. Chem. 2003, 41, 278–282. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Pinto, A.C.; Brabo, S.N.; Silva, M.N. Terpenoids from Croton cajucara. Phytochemistry 1998, 49, 823–828. [Google Scholar] [CrossRef]
- Soares, B.A.; Firme, C.L.; Maciel, M.A.M.; Kaiser, C.R.; Schilling, E.; Bortoluzzi, A.J. Experimental and NMR theoretical methodology applied to geometric analysis of the bioactive clerodane trans-dehydrocrotonin. J. Braz. Chem. Soc. 2014, 25, 629–638. [Google Scholar] [CrossRef]
- Eisenreich, W.; Rohdich, F.; Bacher, A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001, 6, 78–84. [Google Scholar] [CrossRef]
- Meerts, P.; Hano, C. Book Review: Teucrium Species: Biology and Applications; Stanković, M., Ed.; Springer Nature: Cham, Switzerland, 2020; ISBN 978-3-030-52158-5. [Google Scholar] [CrossRef]
- Ahmed, W.; Maciel, M.A.M. (Eds.) Applications and Industrialisation of Nanotechnology; One Central Press: Manchester, UK, 2022. [Google Scholar]
- Guerrero Pabón, M.F.; Ortiz, S.A.P.; Puebla Ibáñez, D.P. Vascular interactions of Croton Schiedeanus major flavonoids in isolated aortic rings from wistar rats. Vitae 2021, 28, 343923. [Google Scholar] [CrossRef]
- Vieira, G.I.A.; Bittencourt, C.B.; Andrade, I.M. Prospecção científica e tecnológica da espécie Croton sonderianus Muell. Arg. Cad. Prospecç. 2024, 17, 225–240. [Google Scholar] [CrossRef]
- Maciel, M.A.M.; Dantas, T.N.C.; Câmara, J.K.P.; Pinto, A.C.; Veiga Jr, V.F.; Kaiser, C.R.; Pereira, N.A.; Carneiro, C.M.T.S.; Vanderline, F.A.; Lapa, A.J.; et al. Pharmacological and biochemical profiling of lead compounds from traditional remedies: The case of Croton cajucara. In Advances in Phytomedicine; Khan, M.T.H., Ather, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 225–253. [Google Scholar]
- Maciel, M.A.M.; Gomes, F.E.S.; Soares, B.A.; Grynberg, N.F.; Echevarria, A.; Cólus, I.M.S.; Kaiser, C.; Morais, W.A.; Magalhães, N.S.S. Biological Effectiveness and Recent Advancing of Natural Products on the Discovery of Anticancer Agents, In Bioactive Phytochemicals: Perspectives for Modern Medicine; Khan, M.T., Ather, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 239–293. [Google Scholar]
- Varghese, N.; Sykes, T.C.; Quetzeri-Santiago, M.A.; Castrejón-Pita, A.A.; Castrejón-Pita, J.R. Effect of Surfactants on the Splashing Dynamics of Drops Impacting Smooth Substrates. Langmuir 2024, 40, 8781–8790. [Google Scholar] [CrossRef]
- Dos Santos, R.G. Surface Tension and Its Derivative Properties. In Fundamentals of Surface Thermodynamics; Dos Santos, R.G., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 73–87. [Google Scholar] [CrossRef]
- Rossi, C.G.F.T.; Dantas, T.N.C.; Dantas Neto, A.A.; Maciel, M.A.M. Microemulsões: Uma abordagem básica e perspectivas para aplicabilidade industrial. Rev. Univ. Rural Sér. Ciênc. Exatas Terra 2007, 26, 45. [Google Scholar]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.G.F.T.; Dantas, T.N.C.; Dantas Neto, A.A.; Maciel, M.A.M. Tensoativos: Uma abordagem básica e perspectivas Para aplicabilidade industrial. Rev. Univ. Rural Sér. Ciênc. Exatas Terra 2006, 25, 73–85. [Google Scholar]
- Lindman, B.; Medronho, B.; Karlström, G. Clouding of nonionic surfactants. Curr. Opin. Colloid Interface Sci. 2016, 22, 23–29. [Google Scholar] [CrossRef]
- Mudwadkar, A.; Sonawane, G.; Patil, T. Thermodynamic of clouding behavior of non-ionic surfactant, polyoxyethylene (10) cetyl ether (Brij-56) with and without non-polar additives. Chem. Sci. Rev. Lett. 2014, 3, 962–969. [Google Scholar]
- Kupikowska-Stobba, B.; Domagała, J.; Kasprzak, M.M. Critical review of techniques for food emulsion characterization. Appl. Sci. 2024, 14, 1069. [Google Scholar] [CrossRef]
- Fischer, P.; Windhab, E.J. Rheology of food materials. Curr. Opin. Colloid Interface Sci. 2011, 16, 36–40. [Google Scholar] [CrossRef]
- Zhou, B.; Drusch, S.; Hogan, S.A. Confined flow behavior under high shear rates and stability of oil/water high internal phase emulsions (HIPEs) stabilized by whey protein isolate: Role of protein concentration and pH. Food Res. Int. 2022, 160, 111674. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Koller, T.M.; Fröba, A.P. Effective thermal conductivity, effective viscosity, and particle diffusion coefficient of microemulsions consisting of water, n-decane, and a non-ionic surfactant in different regions of the phase diagram. Int. J. Heat Mass Transf. 2024, 232, 125901. [Google Scholar] [CrossRef]
- Izham, M.N.M.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of self-nanoemulsifying drug delivery system loaded with citraland its antiproliferative effect on colorectal cells in vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef]
- Moraes, A.R.D.P.; Tavares, G.D.; Rocha, F.J.S.; Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, B.; Lassoued, M.A.; Seguin, J.; Lai-Kuen, R.; Dhotel, H.; Sfar, S.; Mignet, N. Self-emulsifying drug delivery system developed by the HLB-RSM approach: Characterization by transmission electron microscopy and pharmacokinetic study. Int. J. Pharm. 2015, 487, 56–63. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Thymoquinone-Loaded Chitosan Nanoparticles as Natural Preservative Agent in Cosmetic Products. Int. J. Mol. Sci. 2022, 23, 898. [Google Scholar] [CrossRef]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; De Almeida, M.P.; Pereira, E.; West, P. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef]
- Zhao, T.; Maniglio, D.; Wang, K.; Chen, B.; Motta, A.; Migliaresi, C. Design and optimization of self-nanoemulsifying formulations for lipophilic drugs. Nanotechnology 2015, 26, 125102. [Google Scholar] [CrossRef]
- Niknam, S.M.; Escudero, I.; Benito, J.M. Formulation and preparation of water-in-oil-in-water emulsions loaded with a phenolic-rich inner aqueous phase by application of high energy emulsification methods. Foods 2020, 9, 1411. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Adv. Colloid Interface Sci. 2021, 288, 102344. [Google Scholar] [CrossRef]
- Nogueira Barradas, T.; Araujo Cardoso, S.; De Castro Grimaldi, P.; Lohan-Codeço, M.; Escorsim Machado, D.; Medina de Mattos, R.; Eurico Nasciutti, L.; Palumbo Jr, A. Development, characterization and evidence of anti-endometriotic activity of phytocannabinoid-rich nanoemulsions. Int. J. Pharm. 2023, 643, 123049. [Google Scholar] [CrossRef]
- Venturini, C.G.; Bruinsmann, F.A.; Contri, R.V.; Fonseca, F.N.; Frank, L.A.; D’Amore, C.M.; Raffin, R.P.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Co-encapsulation of imiquimod and copaiba oil in novel nanostructured systems: Promising formulations against skin carcinoma. Eur. J. Pharm. Sci. 2015, 79, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.V.D.; Ribeiro, L.N.D.M.; Moura, L.D.D.; Rodrigues Da Silva, G.H.; Mitsutake, H.; Mendonça, T.C.; Geronimo, G.; Breitkreitz, M.C.; De Paula, E. Docetaxel loaded in copaiba oil-nanostructured lipid carriers as a promising DDS for breast cancer treatment. Molecules 2022, 27, 8838. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Urru, M.; Chiarugi, C.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 583, 119361. [Google Scholar] [CrossRef]
- Arriagada, F.; Günter, G.; Morales, J. Nanoantioxidant–based silica particles as flavonoid carrier for drug delivery applications. Pharmaceutics 2020, 2, 302. [Google Scholar] [CrossRef]
- Estime, N.; Teychené, S.; Autret, J.-M.; Biscans, B. Influence of pH, temperature and impurities on the solubility of an active pharmaceutical ingredient (API). Int. J. Chem. React. Eng. 2010, 8, 1–14. [Google Scholar] [CrossRef][Green Version]
- Lapenda, T.L.S.; Morais, W.A.; Almeida, F.J.F.; Ferraz, M.S.; Lira, M.C.B.; Santos, N.P.S.; Maciel, M.A.M.; Santos-Magalhães, N.S. Encapsulation of trans-dehydrocrotonin in liposomes: An enhancement of the antitumor activity. J. Biomed. Nanotechnol. 2013, 9, 499–510. [Google Scholar] [CrossRef]
- Morais, W.A.; Barros Neto, B.; Cavalcanti, I.M.F.; Xavier Júnior, F.H.; Santos-Magalhães, N.S.; Maciel, M.A.M. Coencapsulation of trans-dehydrocrotonin and trans-dehydrocrotonin:hydroxypropyl-β-cyclodextrin into microparticles. J. Braz. Chem. Soc. 2017, 28, 1494–1505. [Google Scholar] [CrossRef]
- Tran, M.T.; Gomez, S.V.; Alenicheva, V.; Remcho, V.T. A paper-based assay for the determination of total antioxidant capacity in human serum samples. Biosensors 2024, 14, 559. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative stress in liver pathophysiology and disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Wang, J.-H.; Lee, S.-B.; Lee, D.-S.; Son, C.-G. Total antioxidant capacity in HBV carriers, a promising biomarker for evaluating hepatic fibrosis: A pilot study. Antioxidants 2021, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative stress markers in inflammatory bowel diseases: Systematic review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef]
- Souza-Barbosa, P.C.; Medeiros, R.S.; Sampaio, P.T.B.; Vieira, G.; Wiedemann, L.S.M.; Veiga Junior, V.F. Influence of abiotic factors on the chemical composition of copaiba oil (Copaifera multijuga Hayne): Soil composition, seasonality and diameter at breast height. J. Braz. Chem. Soc. 2012, 23, 1823–1833. [Google Scholar] [CrossRef]
- Barylski, M.; Kowalczyk, E.; Banach, M.; Ciećwierz, J.; Pawlicki, L.; Kowalski, J. Plasma total antioxidant activity in comparison with plasma NO and VEGF levels in patients with metabolic syndrome. Angiology 2009, 60, 87–92. [Google Scholar] [CrossRef]
- Barreto Júnior, A.G.; Biscaia Junior, E.C.; Veiga Junior, V.F.; Pinto, A.C.; Carvalhaes, S.F.; Maciel, M.A.M. Cromatografia de troca-iônica aplicada ao isolamento da fração ácida do óleo de copaíba (Copaifera multijuga) e da sacaca (Croton cajucara). Quim. Nova 2005, 28, 719–722. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of reactive oxygen species (ros) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Fidelis, G.P.; Costa, M.S.; Telles, C.B.; Dantas-Santos, N.; Oliveira Elias, S.; Ribeiro, V.B.; Barth, A.L.; Macedo, A.J.; Leite, E.L.; et al. In vitro antioxidant, anticoagulant and antimicrobial activity and in inhibition of cancer cell proliferation by xylan extracted from corn cobs. Int. J. Mol. Sci. 2012, 13, 409–426. [Google Scholar] [CrossRef]
- Melo, K.R.; Camara, R.B.G.; Queiroz, M.F.; Vidal, A.A.J.; Lima, C.R.; Melo-Silveira, R.F.; Almeida-Lima, J.; Rocha, H.A.O. Evaluation of sulfated polysaccharides from the brown seaweed Dictyopteris justii as antioxidant agents and as inhibitors of the formation of calcium oxalate crystals. Molecules 2013, 18, 14543–14563. [Google Scholar] [CrossRef]



| Triplicate Analysis | Ø (nm) | PDI | ζ (mV) | |
|---|---|---|---|---|
| A | 11.15 | 0.083 | −3.60 | |
| SNEDDS-CO-DCTN | B | 11.31 | 0.107 | −3.63 |
| C | 11.43 | 0.130 | −3.11 | |
| D | 11.46 | 0.135 | −4.13 | |
| SNEDDS-CO | E | 11.71 | 0.188 | −3.39 |
| F | 11.81 | 0.200 | −4.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Netto, J.R.; Corrêa, N.P.; Aragão de Araujo, L.B.; Paiva, W.d.S.; Oliveira Rocha, H.A.; Morais Lima, W.d.A.; Oliveira do Nascimento, J.H.; dos Santos Macedo, D.C.; Santos-Magalhães, N.S.; da Veiga Júnior, V.F.; et al. Bioavailability for the Improved Therapeutic Profile of trans-Dehydrocrotonin Incorporated into a Copaiba Oil Self-Nanoemulsifying Drug Delivery System: Formulation, Physicochemical Characterizations, and Antioxidant In Vitro Effect. Int. J. Mol. Sci. 2025, 26, 4469. https://doi.org/10.3390/ijms26104469
de Oliveira Netto JR, Corrêa NP, Aragão de Araujo LB, Paiva WdS, Oliveira Rocha HA, Morais Lima WdA, Oliveira do Nascimento JH, dos Santos Macedo DC, Santos-Magalhães NS, da Veiga Júnior VF, et al. Bioavailability for the Improved Therapeutic Profile of trans-Dehydrocrotonin Incorporated into a Copaiba Oil Self-Nanoemulsifying Drug Delivery System: Formulation, Physicochemical Characterizations, and Antioxidant In Vitro Effect. International Journal of Molecular Sciences. 2025; 26(10):4469. https://doi.org/10.3390/ijms26104469
Chicago/Turabian Stylede Oliveira Netto, José Robério, Natália Pignataro Corrêa, Leonardo Bruno Aragão de Araujo, Weslley de Souza Paiva, Hugo Alexandre Oliveira Rocha, Waldenice de Alencar Morais Lima, José Heriberto Oliveira do Nascimento, Daniel Charles dos Santos Macedo, Nereide Stela Santos-Magalhães, Valdir Florêncio da Veiga Júnior, and et al. 2025. "Bioavailability for the Improved Therapeutic Profile of trans-Dehydrocrotonin Incorporated into a Copaiba Oil Self-Nanoemulsifying Drug Delivery System: Formulation, Physicochemical Characterizations, and Antioxidant In Vitro Effect" International Journal of Molecular Sciences 26, no. 10: 4469. https://doi.org/10.3390/ijms26104469
APA Stylede Oliveira Netto, J. R., Corrêa, N. P., Aragão de Araujo, L. B., Paiva, W. d. S., Oliveira Rocha, H. A., Morais Lima, W. d. A., Oliveira do Nascimento, J. H., dos Santos Macedo, D. C., Santos-Magalhães, N. S., da Veiga Júnior, V. F., & Maciel, M. A. M. (2025). Bioavailability for the Improved Therapeutic Profile of trans-Dehydrocrotonin Incorporated into a Copaiba Oil Self-Nanoemulsifying Drug Delivery System: Formulation, Physicochemical Characterizations, and Antioxidant In Vitro Effect. International Journal of Molecular Sciences, 26(10), 4469. https://doi.org/10.3390/ijms26104469









