Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading
Abstract
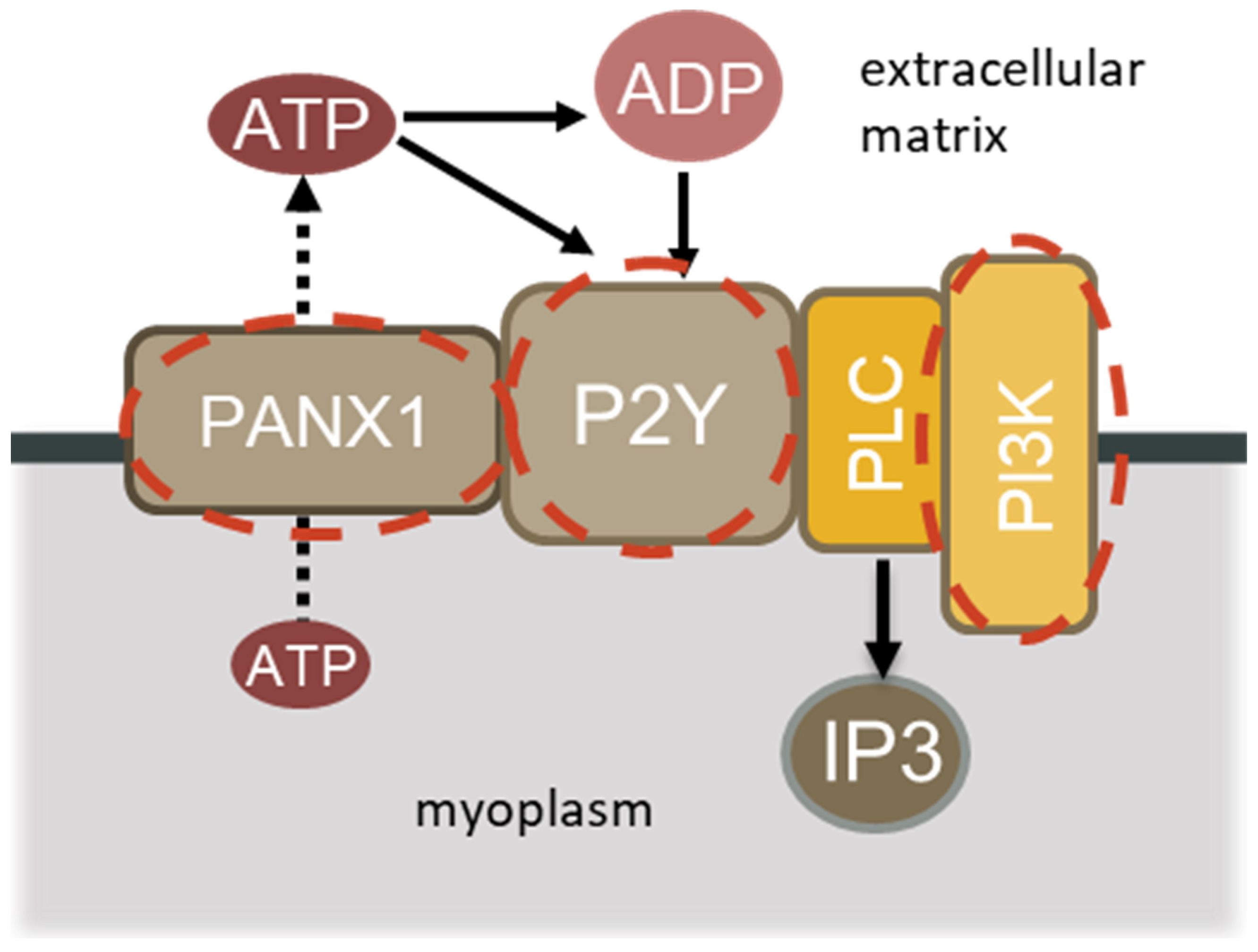
1. Introduction
2. Results
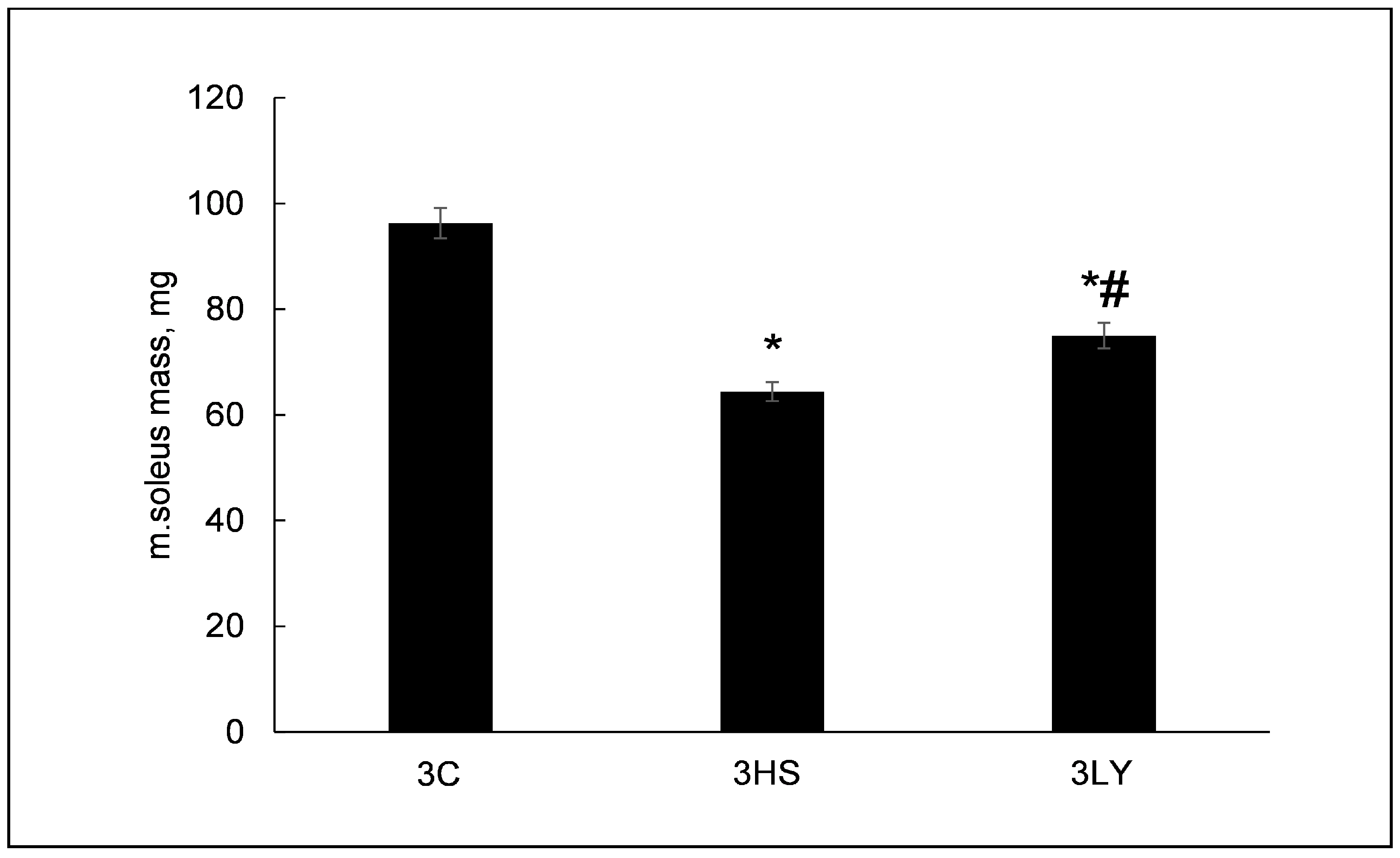
2.1. The Effect of PI3K Inhibition with LY294002 on Unloaded Muscle Mass
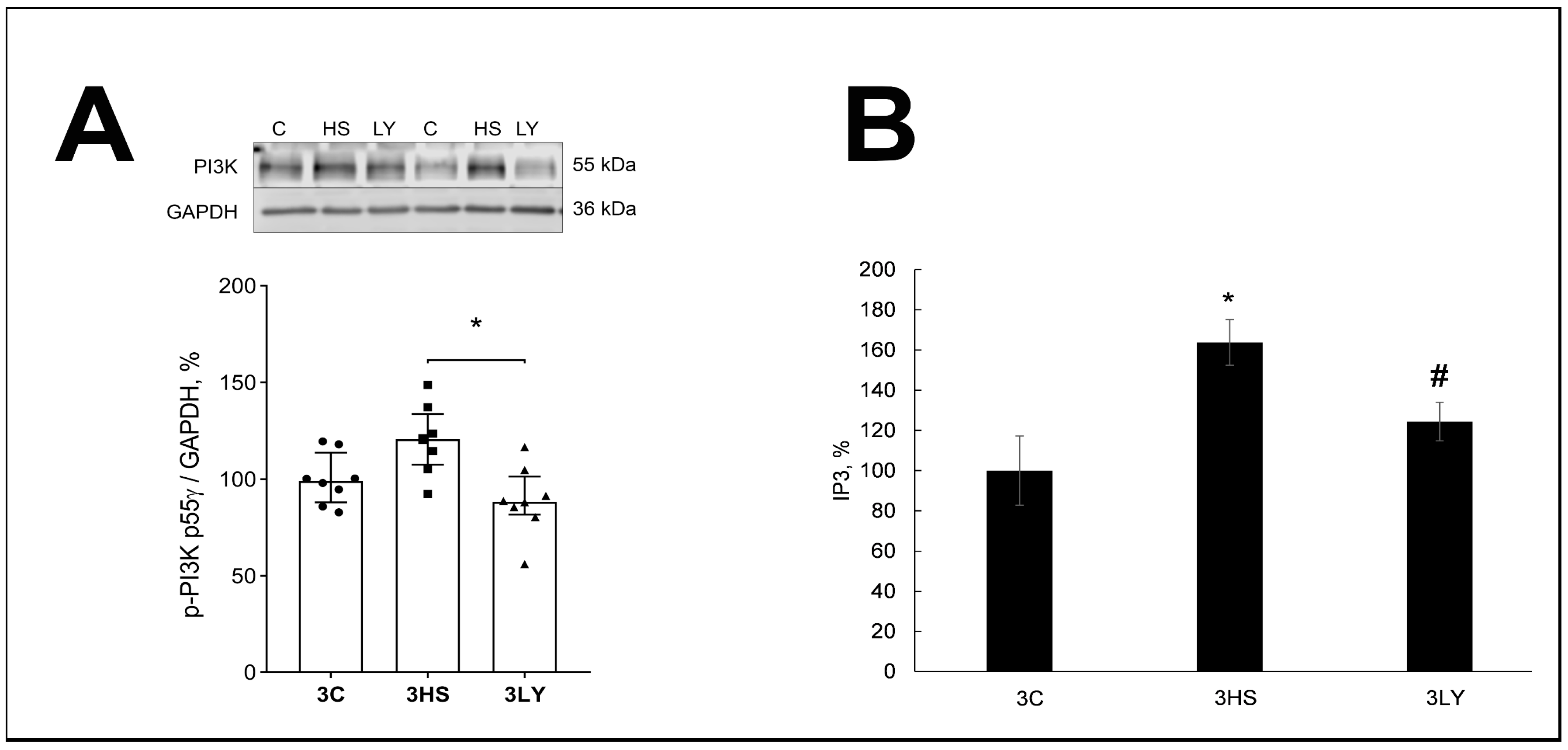
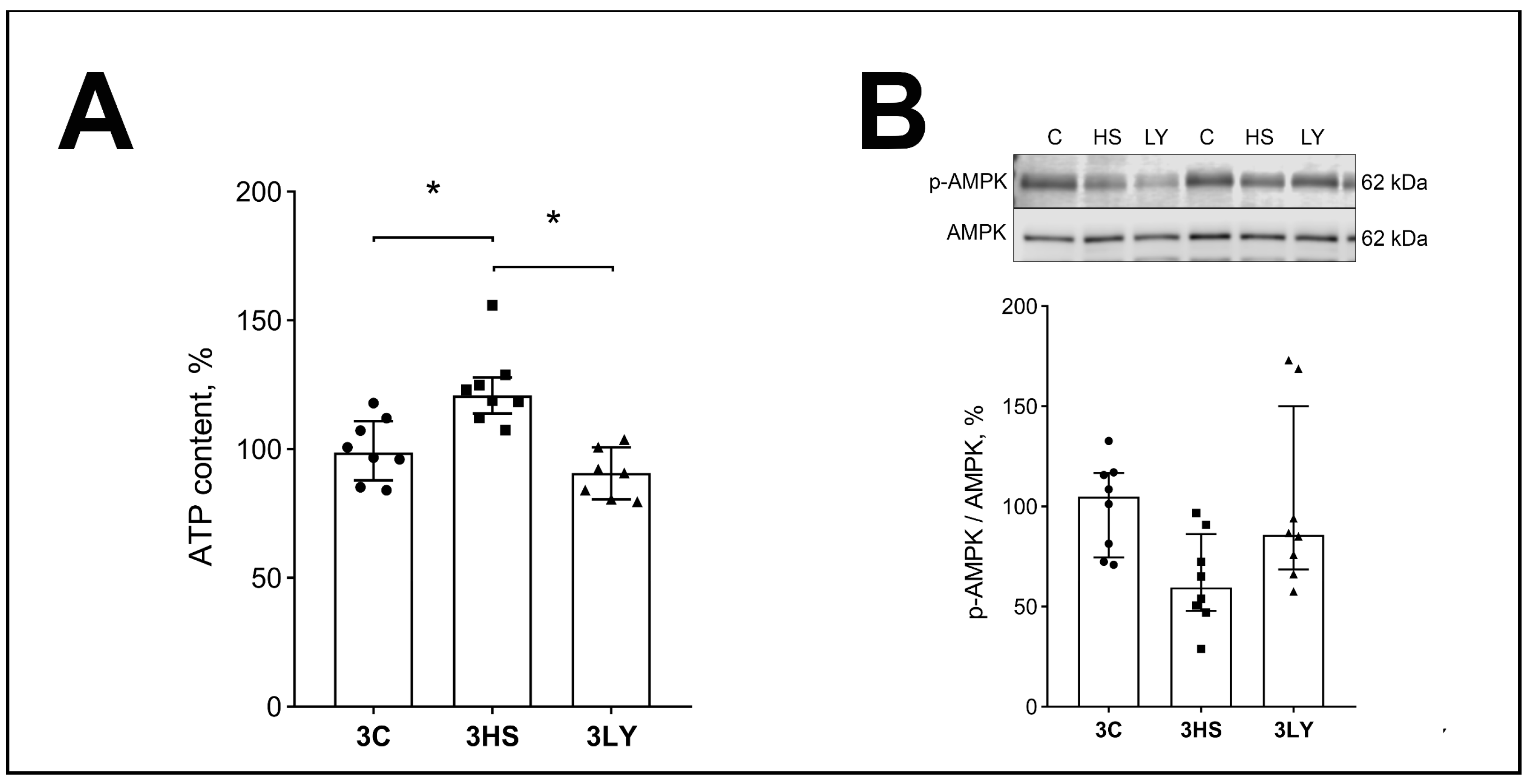
2.2. The Effect of PI3K Inhibition with LY294002 on Phospho-PI3K, IP3, ATP, and Phospho-AMPK Content in Unloaded Muscle
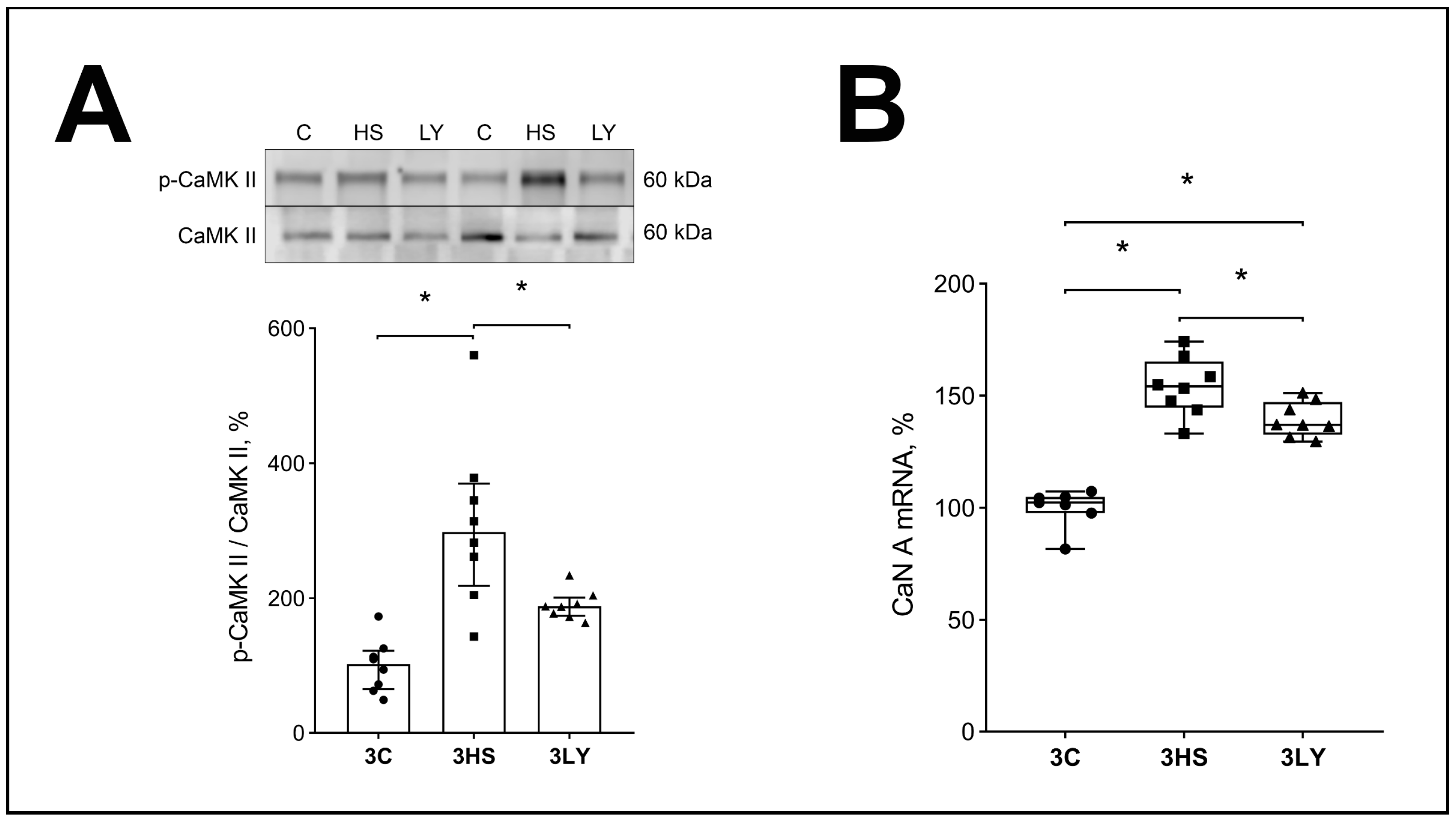
2.3. The Effect of PI3K Inhibition with LY294002 on Ca2+ Signaling and IP3R Content in Unloaded Muscle
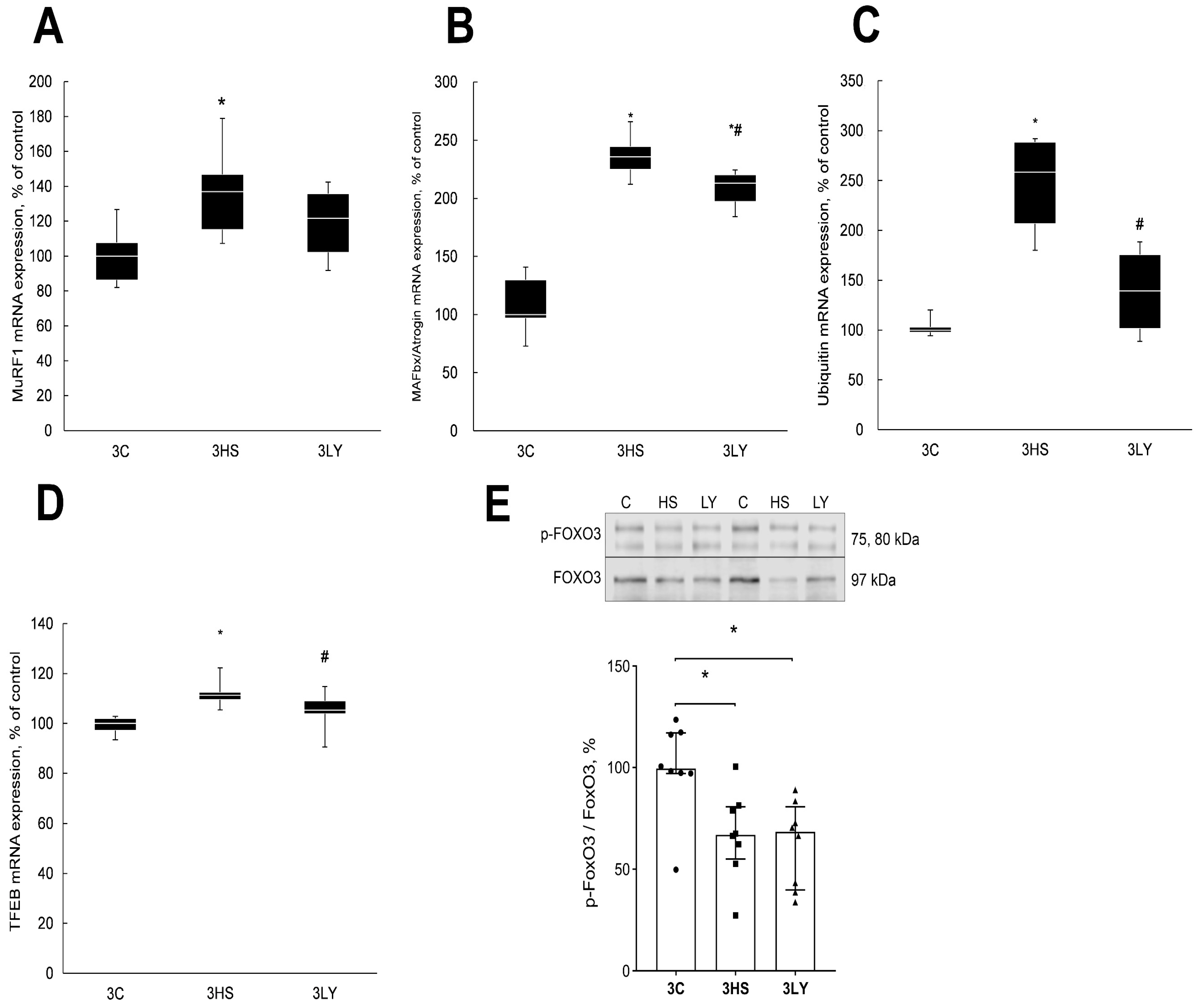
2.4. The Effect of PI3K Inhibition with LY294002 on Markers of Catabolic Signaling in Unloaded Muscle
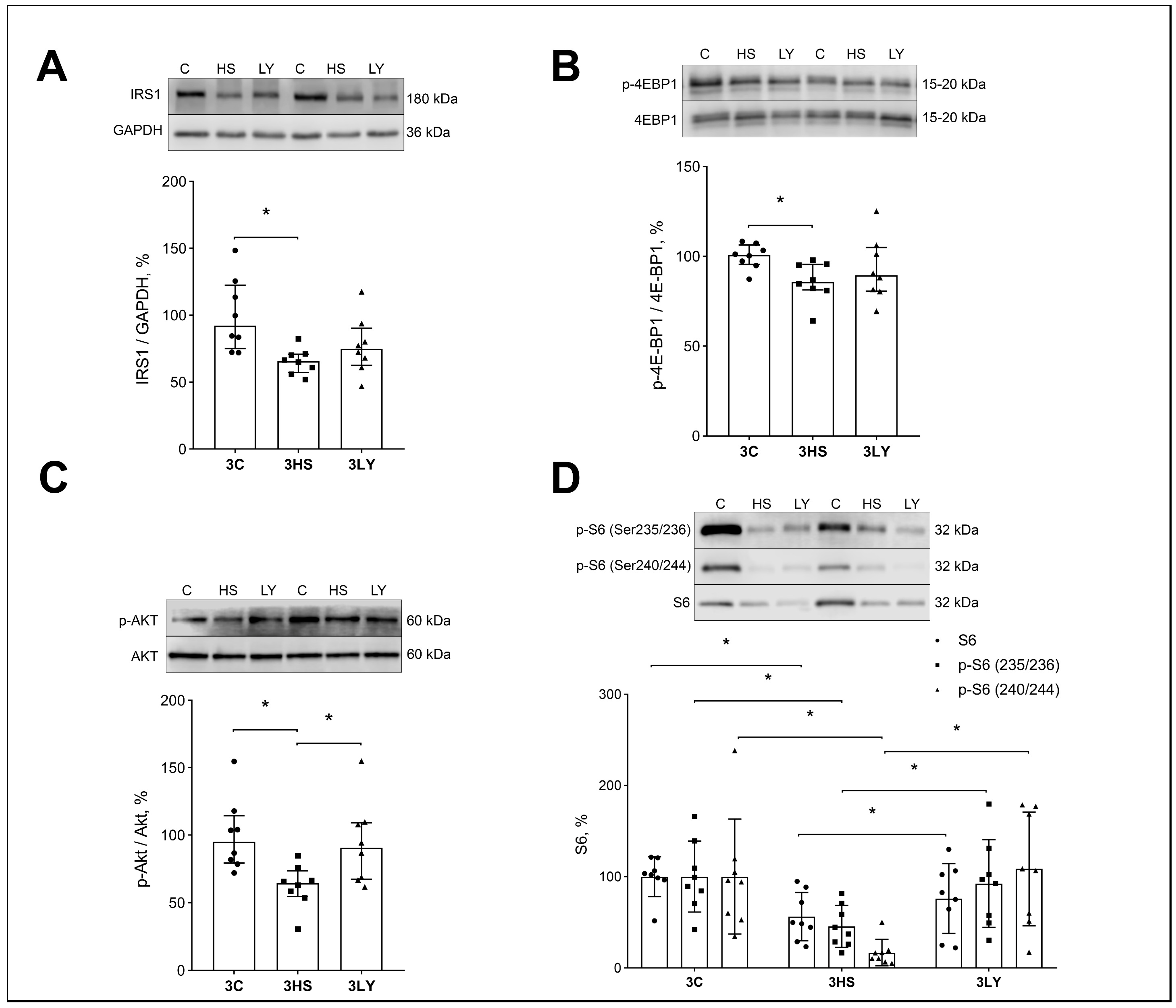
2.5. The Effect of PI3K Inhibition with LY294002 on Markers of Anabolic Signaling in Unloaded Muscle
3. Discussion
3.1. Effect of LY294002 Administration on the Content of ATP and AMPK in the Soleus Muscle of Rats
3.2. Effect of LY294002 Administration on Ca2+ Signaling
3.3. Effect of LY294002 Administration on the Level of Muscle Catabolic Signaling Markers During Unloading
3.4. Effect of LY294002 Administration on the Level of Anabolic Signaling Markers
4. Materials and Methods
4.1. Animal Protocol Approval
4.2. Animal Procedures
4.3. Western Blotting
4.4. Muscle ATP Content Evaluation
4.5. Muscle IP3 Content Evaluation
4.6. RNA Isolation and Reverse Transcription
4.7. Quantitative PCR Analysis
4.8. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirzoev, T.M.; Shenkman, B.S. Regulation of Protein Synthesis in Inactivated Skeletal Muscle: Signal Inputs, Protein Kinase Cascades, and Ribosome Biogenesis. Biochemistry 2018, 83, 1299–1317. [Google Scholar] [CrossRef] [PubMed]
- Ingalls, C.P.; Warren, G.L.; Armstrong, R.B. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J. Appl. Physiol. 1999, 87, 386–390. [Google Scholar] [CrossRef]
- Zaripova, K.A.; Kalashnikova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Role of Pannexin 1 ATP-Permeable Channels in the Regulation of Signaling Pathways during Skeletal Muscle Unloading. Int. J. Mol. Sci. 2021, 22, 10444. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Nemirovskaya, T.L. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle Res. Cell Motil. 2008, 29, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Razgovorova, I.A.; Heiny, J.A.; Krivoi, I.I. Isoform-specific Na,K-ATPase alterations precede disuse-induced atrophy of rat soleus muscle. BioMed Res. Int. 2015, 2015, 720172. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct alpha2 Na,K-ATPase membrane pools are differently involved in early skeletal muscle remodeling during disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP signaling in skeletal muscle: From fiber plasticity to regulation of metabolism. Exerc. Sport. Sci. Rev. 2014, 42, 110–116. [Google Scholar] [CrossRef]
- Nemirovskaya, T.L.; Sharlo, K.A. Roles of ATP and SERCA in the Regulation of Calcium Turnover in Unloaded Skeletal Muscles: Current View and Future Directions. Int. J. Mol. Sci. 2022, 23, 6937. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, W.; Li, J.; Liu, J. Lysosomal exocytosis of ATP is coupled to P2Y2 receptor in marginal cells in the stria vascular in neonatal rats. Cell Calcium 2018, 76, 62–71. [Google Scholar] [CrossRef]
- Vilchinskaya, N.A.; Mochalova, E.P.; Paramonova, I.; Belova, S.P.; Mirzoev, T.; Shenkman, B. The Effect of β-GPA on the Markers of Anabolic and Catabolic Signaling Pathways in Rat Soleus Muscle at the Initial Stage of Hindlimb Unloading. Biochemistry 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Eisner, V.; Jaimovich, E. Skeletal muscle excitation-metabolism coupling. Arch. Biochem. Biophys. 2019, 664, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Buvinic, S.; Almarza, G.; Bustamante, M.; Casas, M.; Lopez, J.; Riquelme, M.; Saez, J.C.; Huidobro-Toro, J.P.; Jaimovich, E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J. Biol. Chem. 2009, 284, 34490–34505. [Google Scholar] [CrossRef] [PubMed]
- Zaripova, K.A.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. P2Y1 and P2Y2 receptors differ in their role in the regulation of signaling pathways during unloading-induced rat soleus muscle atrophy. Arch. Biochem. Biophys. 2024, 751, 109844. [Google Scholar] [CrossRef] [PubMed]
- May, C.; Weigl, L.; Karel, A.; Hohenegger, M. Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem. Pharmacol. 2006, 71, 1497–1509. [Google Scholar] [CrossRef]
- Bony, C.; Roche, S.; Shuichi, U.; Sasaki, T.; Crackower, M.A.; Penninger, J.; Mano, H.; Puceat, M. A specific role of phosphatidylinositol 3-kinase gamma. A regulation of autonomic Ca2+ oscillations in cardiac cells. J. Cell Biol. 2001, 152, 717–728. [Google Scholar] [CrossRef]
- Jaconi, M.; Bony, C.; Richards, S.M.; Terzic, A.; Arnaudeau, S.; Vassort, G.; Puceat, M. Inositol 1,4,5-trisphosphate directs Ca2+ flow between mitochondria and the Endoplasmic/Sarcoplasmic reticulum: A role in regulating cardiac autonomic Ca2+ spiking. Mol. Biol. Cell 2000, 11, 1845–1858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossi, A.M.; Taylor, C.W. IP(3) receptors—lessons from analyses ex cellula. J. Cell Sci. 2018, 132, jcs222463. [Google Scholar] [CrossRef]
- Jaimovich, E.; Reyes, R.; Liberona, J.L.; Powell, J.A. IP(3) receptors, IP(3) transients, and nucleus-associated Ca2+ signals in cultured skeletal muscle. Am. J. Physiol. Cell Physiol. 2000, 278, C998–C1010. [Google Scholar] [CrossRef] [PubMed]
- Eltit, J.M.; Garcia, A.A.; Hidalgo, J.; Liberona, J.L.; Chiong, M.; Lavandero, S.; Maldonado, E.; Jaimovich, E. Membrane electrical activity elicits inositol 1,4,5-trisphosphate-dependent slow Ca2+ signals through a Gbetagamma/phosphatidylinositol 3-kinase gamma pathway in skeletal myotubes. J. Biol. Chem. 2006, 281, 12143–12154. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, C.; Liberona, J.L.; Molgo, J.; Colasante, C.; Mignery, G.A.; Jaimovich, E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J. Cell Sci. 2005, 118, 3131–3140. [Google Scholar] [CrossRef]
- Casas, M.; Altamirano, F.; Jaimovich, E. Measurement of calcium release due to inositol trisphosphate receptors in skeletal muscle. Methods Mol. Biol. 2012, 798, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Arias-Calderon, M.; Almarza, G.; Diaz-Vegas, A.; Contreras-Ferrat, A.; Valladares, D.; Casas, M.; Toledo, H.; Jaimovich, E.; Buvinic, S. Characterization of a multiprotein complex involved in excitation-transcription coupling of skeletal muscle. Skelet. Muscle 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.A.; Carrasco, M.A.; Adams, D.S.; Drouet, B.; Rios, J.; Muller, M.; Estrada, M.; Jaimovich, E. IP(3) receptor function and localization in myotubes: An unexplored Ca2+ signaling pathway in skeletal muscle. J. Cell Sci. 2001, 114, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Araya, R.; Liberona, J.L.; Cardenas, J.C.; Riveros, N.; Estrada, M.; Powell, J.A.; Carrasco, M.A.; Jaimovich, E. Dihydropyridine receptors as voltage sensors for a depolarization-evoked, IP3R-mediated, slow calcium signal in skeletal muscle cells. J. Gen. Physiol. 2003, 121, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Ali, K.; Bilancio, A.; Geering, B.; Foukas, L.C. Signalling by PI3K isoforms: Insights from gene-targeted mice. Trends Biochem. Sci. 2005, 30, 194–204. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Vogt, P.K.; Rommel, C. PI3K: From the bench to the clinic and back. Curr. Top. Microbiol. Immunol. 2010, 347, 1–19. [Google Scholar] [CrossRef]
- Gulluni, F.; Martini, M.; De Santis, M.C.; Campa, C.C.; Ghigo, A.; Margaria, J.P.; Ciraolo, E.; Franco, I.; Ala, U.; Annaratone, L.; et al. Mitotic Spindle Assembly and Genomic Stability in Breast Cancer Require PI3K-C2alpha Scaffolding Function. Cancer Cell 2017, 32, 444–459.e7. [Google Scholar] [CrossRef] [PubMed]
- Devereaux, K.; Dall’Armi, C.; Alcazar-Roman, A.; Ogasawara, Y.; Zhou, X.; Wang, F.; Yamamoto, A.; De Camilli, P.; Di Paolo, G. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS ONE 2013, 8, e76405. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Braccini, L.; Ciraolo, E.; Morello, F.; Perino, A. Twice upon a time: PI3K’s secret double life exposed. Trends Biochem. Sci. 2009, 34, 244–248. [Google Scholar] [CrossRef]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K isoforms in cell signalling and vesicle trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kgamma-Akt-AS160 in skeletal muscle cells. Diabetes 2013, 62, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Pan, F.; Guo, Y.; Cao, L.; Yan, W.; Gao, Y. New Findings: Hindlimb Unloading Causes Nucleocytoplasmic Ca2+ Overload and DNA Damage in Skeletal Muscle. Cells 2023, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S. How Postural Muscle Senses Disuse? Early Signs and Signals. Int. J. Mol. Sci. 2020, 21, 5037. [Google Scholar] [CrossRef] [PubMed]
- Morello, F.; Perino, A.; Hirsch, E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc. Res. 2009, 82, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Sharlo, K.A.; Lvova, I.D.; Tyganov, S.A.; Zaripova, K.A.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. The Effect of SERCA Activation on Functional Characteristics and Signaling of Rat Soleus Muscle upon 7 Days of Unloading. Biomolecules 2023, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; Hughes, D.C.; Waddell, D.S.; Bodine, S.C. SnapShot: Skeletal muscle atrophy. Cell 2022, 185, 1618–1618.e11. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z. Role of PI3 kinase gamma in excitation-contraction coupling and heart disease. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 295–304. [Google Scholar] [CrossRef]
- Kawano, F.; Ishihara, A.; Ohira, Y. Air-righting responses to chronic hindlimb suspension and ambulation recovery in adult rats. Biol. Sci. Space 2002, 16, 149–150. [Google Scholar]
- Ito, N.; Ruegg, U.T.; Takeda, S. ATP-Induced Increase in Intracellular Calcium Levels and Subsequent Activation of mTOR as Regulators of Skeletal Muscle Hypertrophy. Int. J. Mol. Sci. 2018, 19, 2804. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.A.; Leite, M.F.; Bennett, A.M.; Nathanson, M.H. Calcium signaling in the nucleus. Can. J. Physiol. Pharmacol. 2006, 84, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Scheffler, T.L.; Gerrard, D.E. Chronic high cytosolic calcium decreases AICAR-induced AMPK activity via calcium/calmodulin activated protein kinase II signaling cascade. Cell Calcium 2011, 50, 73–83. [Google Scholar] [CrossRef]
- Witczak, C.A.; Sharoff, C.G.; Goodyear, L.J. AMP-activated protein kinase in skeletal muscle: From structure and localization to its role as a master regulator of cellular metabolism. Cell. Mol. Life Sci. 2008, 65, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Raney, M.A.; Turcotte, L.P. Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J. Appl. Physiol. 2008, 104, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Hatano, N.; Fujiwara, Y.; Sha’ri, A.; Takabatake, S.; Akano, H.; Kanayama, N.; Magari, M.; Nozaki, N.; Tokumitsu, H. AMP-activated protein kinase-mediated feedback phosphorylation controls the Ca2+/calmodulin (CaM) dependence of Ca2+/CaM-dependent protein kinase kinase. J. Biol. Chem. 2017, 292, 19804–19813. [Google Scholar] [CrossRef]
- Chin, E.R. The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc. Nutr. Soc. 2004, 63, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; Rietze, B.A.; Chambers, P.J.; Bellissimo, C.; Bombardier, E.; Quadrilatero, J.; Tupling, A.R. Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload. Am. J. Physiol. Cell Physiol. 2017, 313, C154–C161. [Google Scholar] [CrossRef]
- Sharlo, K.; Paramonova, I.; Turtikova, O.; Tyganov, S.; Shenkman, B. Plantar mechanical stimulation prevents calcineurin-NFATc1 inactivation and slow-to-fast fiber type shift in rat soleus muscle under hindlimb unloading. J. Appl. Physiol. 2019, 126, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Mochalova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Differences in the Role of HDACs 4 and 5 in the Modulation of Processes Regulating MAFbx and MuRF1 Expression during Muscle Unloading. Int. J. Mol. Sci. 2020, 21, 4815. [Google Scholar] [CrossRef]
- Kubokawa, M.; Nakamura, K.; Komagiri, Y. Interaction between Calcineurin and Ca/Calmodulin Kinase-II in Modulating Cellular Functions. Enzym. Res. 2011, 2011, 587359. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, S.J.; Liu, L. Phosphorylation and acetylation modifications of FOXO3a: Independently or synergistically? Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Kalashnikova, E.P.; Tyganov, S.A.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Effect of enhanced muscle tone on the expression of atrogenes and cytoskeletal proteins during postural muscle unloading. Arch. Biochem. Biophys. 2022, 725, 109291. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Dooley, M.S.; Kim, C.R.; Kim, E.J.; Xu, W.; Goodman, C.A.; Hornberger, T.A. A DGK zeta-FoxO-ubiquitin proteolytic axis controls fiber size during skeletal muscle remodeling. Sci. Signal. 2018, 11, eaao6847. [Google Scholar] [CrossRef]
- Yudushkin, I. Getting the Akt Together: Guiding Intracellular Akt Activity by PI3K. Biomolecules 2019, 9, 67. [Google Scholar] [CrossRef]
- Belova, S.P.; Vilchinskaya, N.A.; Mochalova, E.P.; Mirzoev, T.M.; Nemirovskaya, T.L.; Shenkman, B.S. Elevated p70S6K phosphorylation in rat soleus muscle during the early stage of unloading: Causes and consequences. Arch. Biochem. Biophys. 2019, 674, 108105. [Google Scholar] [CrossRef]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, B.S.; Belova, S.P.; Lomonosova, Y.N.; Kostrominova, T.Y.; Nemirovskaya, T.L. Calpain-dependent regulation of the skeletal muscle atrophy following unloading. Arch. Biochem. Biophys. 2015, 584, 36–41. [Google Scholar] [CrossRef]
- Glass, D.J. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 2003, 5, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.O.; Ahn, C.B.; Cho, H.J.; Choi, J.Y.; Son, K.H.; Yoon, M.S. Simulated microgravity inhibits C2C12 myogenesis via phospholipase D2-induced Akt/FOXO1 regulation. Sci. Rep. 2019, 9, 14910. [Google Scholar] [CrossRef] [PubMed]
- Amar-Schwartz, A.; Ben Hur, V.; Jbara, A.; Cohen, Y.; Barnabas, G.D.; Arbib, E.; Siegfried, Z.; Mashahreh, B.; Hassouna, F.; Shilo, A.; et al. S6K1 phosphorylates Cdk1 and MSH6 to regulate DNA repair. Elife 2022, 11, e79128. [Google Scholar] [CrossRef]
- Han, B.; Zhu, M.J.; Ma, C.; Du, M. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Pattan, G.; Rafeequi, T. Eukaryotic elongation factor-2 (eEF2): Its regulation and peptide chain elongation. Cell Biochem. Funct. 2011, 29, 227–234. [Google Scholar] [CrossRef]
- Sharlo, K.; Tyganov, S.A.; Tomilovskaya, E.; Popov, D.V.; Saveko, A.A.; Shenkman, B.S. Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling. Int. J. Mol. Sci. 2021, 23, 468. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Grundy, D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Exp. Physiol. 2015, 100, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.; Globus, R.K.; Kaplansky, A.; Durnova, G. The hindlimb unloading rat model: Literature overview, technique update and comparison with space flight data. Adv. Space Biol. Med. 2005, 10, 7–40. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef] [PubMed]

| Gene Description | Primer Sequence |
|---|---|
| CaN | 5′-GCACACATAGATGGTCGGC-3′ 5′-CAGGTGCATGCTTTGATCGC-3′ |
| MAFbx | 5′-CTACGATGTTGCAGCCAAGA-3′ 5′-GGCAGTCGAGAAGTCCAGTC-3′ |
| MuRF-1 | 5′-GCCAATTTGGTGCTTTTTGT-3′ 5′-AAATTCAGTCCTCTCCCCGT-3′ |
| RPL19 | 5′-GTACCCTTCCTCTTCCCTATGC-3′ 5′-CAATGCCAACTCTCGTCAACAG-3′ |
| TFEB | 5′-CCTTCCCCATCATAGGACTGC-3′ 5′-TTTGGACTTAGTGCCTGCCTGG-3′ |
| Ubiquitin | 5′-CACCAAGAAGGTCAAACAGGA-3′ 5′-GCAAGAACTTTATTCAAAGTGCAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaripova, K.A.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading. Int. J. Mol. Sci. 2025, 26, 414. https://doi.org/10.3390/ijms26010414
Zaripova KA, Belova SP, Kostrominova TY, Shenkman BS, Nemirovskaya TL. Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading. International Journal of Molecular Sciences. 2025; 26(1):414. https://doi.org/10.3390/ijms26010414
Chicago/Turabian StyleZaripova, Ksenia A., Svetlana P. Belova, Tatiana Y. Kostrominova, Boris S. Shenkman, and Tatiana L. Nemirovskaya. 2025. "Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading" International Journal of Molecular Sciences 26, no. 1: 414. https://doi.org/10.3390/ijms26010414
APA StyleZaripova, K. A., Belova, S. P., Kostrominova, T. Y., Shenkman, B. S., & Nemirovskaya, T. L. (2025). Role of PI3 Kinases in Cell Signaling and Soleus Muscle Atrophy During Three Days of Unloading. International Journal of Molecular Sciences, 26(1), 414. https://doi.org/10.3390/ijms26010414










