Maximal Intensity Exercise Induces Adipokine Secretion and Disrupts Prooxidant–Antioxidant Balance in Young Men with Different Body Composition
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Plasma Volume Changes Following Maximal Intensity Exercise
2.3. Effect of Maximal Intensity Exercise on Biochemical Markers
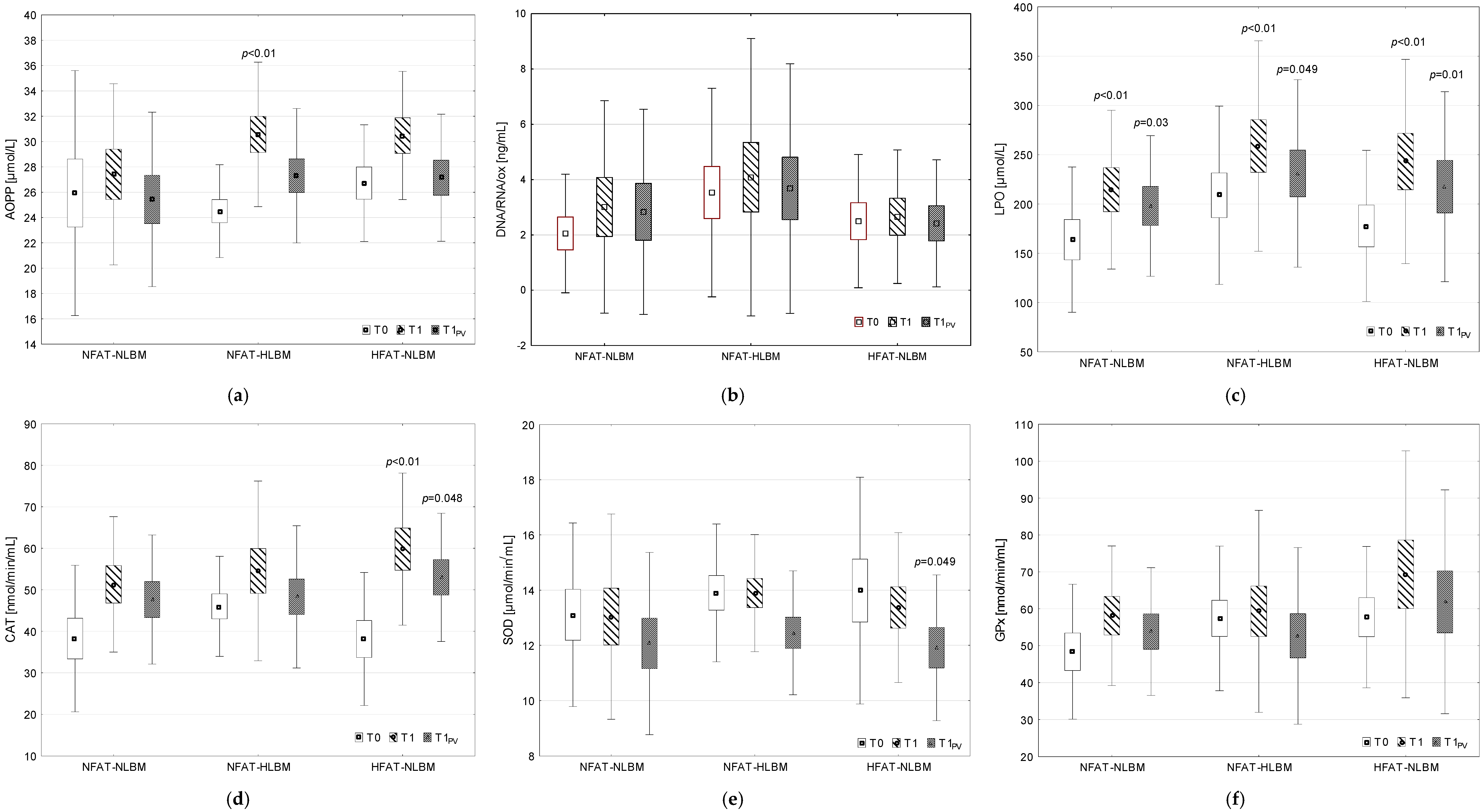
2.3.1. Prooxidant–Antioxidant Balance
2.3.2. Adipokine Concentration
2.4. Correlations
3. Discussion
3.1. Effect of Exercise on Prooxidant–Antioxidant Balance Indices
3.2. Influence of Exercise on Adipokine Levels
3.3. Limitations of the Study
4. Materials and Methods
4.1. Participant Qualification
- NFAT-NLBM group—men with average %FAT and average LBM;
- NFAT-HLBM group—men with average %FAT and high LBM;
- HFAT-NLBM group—men with high %FAT and average LBM.
4.2. Diet Control
4.3. Exercise Protocol
4.4. Blood Sampling and Biochemical Analyses
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, M.; Maciejczyk, M.; Szymura, J.; Szygula, Z. Effect of maximal-intensity exercise on systemic nitro-oxidative stress in men and women. Redox Rep. 2017, 22, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, M.; Maciejczyk, M.; Szymura, J.; Szygula, Z.; Kantorowicz, M. Changes in Non-Enzymatic Antioxidants in the Blood Following Anaerobic Exercise in Men and Women. PLoS ONE 2015, 10, e0143499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wiecek, M.; Maciejczyk, M.; Szymura, J.; Szygula, Z. Sex differences in oxidative stress after eccentric and concentric exercise. Redox Rep. 2017, 22, 478–485. [Google Scholar] [CrossRef]
- Wiecek, M.; Maciejczyk, M.; Szymura, J.; Szygula, Z. Changes in oxidative stress and acid-base balance in men and women following maximal-intensity physical exercise. Physiol. Res. 2015, 64, 93–102. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Anaerobic Exercise-Induced Activation of Antioxidant Enzymes in the Blood of Women and Men. Front. Physiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Radak, Z.; Pan, L.; Zhou, L.; Mozaffaritabar, S.; Gu, Y.; A Pinho, R.; Zheng, X.; Ba, X.; Boldogh, I. Epigenetic and “redoxogenetic” adaptation to physical exercise. Free Radic. Biol. Med. 2024, 210, 65–74. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sports Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Sakurai, T.; Ogasawara, J.; Shirato, K.; Izawa, T.; Oh-Ishi, S.; Ishibashi, Y.; Radák, Z.; Ohno, H.; Kizaki, T. Exercise Training Attenuates the Dysregulated Expression of Adipokines and Oxidative Stress in White Adipose Tissue. Oxid. Med. Cell Longev. 2017, 2017, 9410954. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Cánovas-Cervera, I.; Pallardó, F.V. Oxidative stress and metabolism meet epigenetic modulation in physical exercise. Free Radic. Biol. Med. 2024, 213, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Ziemann, E.E.; Kasperska, A.; Żurek, P.; Rynkiewicz, M.; Rynkiewicz, T.; Laskowski, R.; Hubner-Woźniak, E. Association between cytokine activity and body composition in highly trained athletes. Med. Sports 2013, 66, 199–209. [Google Scholar]
- Görgens, S.W.; Eckardt, K.; Jensen, J.; Drevon, C.A.; Eckel, J. Exercise and regulation of adipokine and myokine production. Prog. Mol. Biol. Transl. Sci. 2015, 135, 313–336. [Google Scholar]
- Lu, Y.; Wiltshire, H.D.; Baker, J.S.; Wang, Q. Effects of High Intensity Exercise on Oxidative Stress and Antioxidant Status in Untrained Humans: A Systematic Review. Biology 2021, 10, 1272. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- El Abed, K.; Rebai, H.; Bloomer, R.J.; Trabelsi, K.; Masmoudi, L.; Zbidi, A.; Sahnoun, Z.; Hakim, A.; Tabka, Z. Antioxidant status and oxidative stress at rest and in response to acute exercise in judokas and sedentary men. J. Strength. Cond. Res. 2011, 25, 2400–2409. [Google Scholar] [CrossRef]
- Ji, L.L.; Radak, Z.; Goto, S. Hormesis and exercise: How the cell copes with oxidative stress. Am. J. Pharmacol. Toxicol. 2008, 3, 41–55. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear Factor Erythroid-Derived 2-like 2 (NFE2L2, Nrf2) Mediates Exercise-Induced Mitochondrial Biogenesis and the Anti-Oxidant Response in Mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glenn, J.M.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; et al. Effects of Aerobic-, Anaerobic- and Combined-Based Exercises on Plasma Oxidative Stress Biomarkers in Healthy Untrained Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 2601. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; McGuckin, T.A.; Leicht, A.S. Influence of exercise intensity on systemic oxidative stress and antioxidant capacity. Clin. Physiol. Funct. Imaging. 2014, 34, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef]
- Wiecek, M.; Maciejczyk, M.; Szymura, J.; Wiecha, S.; Kantorowicz, M.; Szygula, Z. Effect of body composition, aerobic performance and physical activity on exercise-induced oxidative stress in healthy subjects. J. Sports Med. Phys. Fit. 2017, 57, 942–952. [Google Scholar] [CrossRef]
- Alessio, H.M.; Goldfarb, A.H. Lipid peroxidation and scavenger enzymes during exercise: Adaptive response to training. J. Appl. Physiol. 1988, 64, 1333–1336. [Google Scholar] [CrossRef]
- Berzosa, C.; Cebrián, I.; Fuentes-Broto, L.; Gómez-Trullén, E.; Piedrafita, E.; Martínez-Ballarín, E.; López-Pingarrón, L.; Reiter, R.J.; García, J.J. Acute Exercise Increases Plasma Total Antioxidant Status and Antioxidant Enzyme Activities in Untrained Men. J. Biomed. Biotechnol. 2011, 2011, 540458. [Google Scholar] [CrossRef]
- Finkler, M.; Hochman, A.; Pinchuk, I.; Lichtenberg, D. In Healthy Young Men, a Short Exhaustive Exercise Alters the Oxidative Stress Only Slightly, Independent of the Actual Fitness. Oxidative Med. Cell. Longev. 2016, 2016, 9107210. [Google Scholar] [CrossRef]
- Otocka-Kmiecik, A.; Lewandowski, M.; Stolarek, R.; Szkudlarek, U.; Nowak, D.; Orlowska-Majdak, M. Effect of single bout of maximal excercise on plasma antioxidant status and paraoxonase activity in young sportsmen. Redox Rep. 2010, 15, 275–281. [Google Scholar] [CrossRef]
- Leaf, D.A.; Kleinman, M.T.; Hamilton, M.; Barstow, T.J. The effect of exercise intensity on lipid peroxidation. Med. Sci. Sports Exerc. 1997, 29, 1036–1039. [Google Scholar] [CrossRef]
- Ajmani, R.S.; Fleg, J.L.; Demehin, A.A.; Wright, J.G.; O'Connor, F.; Heim, J.M.; Tarien, E.; Rifkind, J.M. Oxidative stress and hemorheological changes induced by acute treadmill exercise. Clin. Hemorheol. Microcirc. 2003, 28, 29–40. [Google Scholar] [PubMed]
- Hessel, E.; Haberland, A.; Müller, M.; Lerche, D.; Schimke, I. Oxygen radical generation of neutrophils: A reason for oxidative stress during marathon running? Clin. Chim. Acta 2000, 298, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Jammes, Y.; Steinberg, J.G.; Brégeon, F.; Delliaux, S. The oxidative stress in response to routine incremental cycling exercise in healthy sedentary subjects. Respir. Physiol. Neurobiol. 2004, 144, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fry, A.C.; Falvo, M.J.; Moore, C.A. Protein carbonyls are acutely elevated following single set anaerobic exercise in resistance trained men. J. Sci. Med. Sports 2007, 10, 411–417. [Google Scholar] [CrossRef]
- Park, S.Y.; Kwak, Y.S. Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes. J. Exerc. Rehabil. 2016, 12, 113–117. [Google Scholar] [CrossRef]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef]
- Terblanche, S.E. The effects of exhaustive exercise on the activity levels of catalase in various tissues of male and female rats. Cell Biol. Int. 2000, 23, 749–753. [Google Scholar] [CrossRef]
- Djordjevic, D.Z.; Cubrilo, D.G.; Barudzic, N.S.; Vuletic, M.S.; Zivkovic, V.I.; Nesic, M.; Radovanovic, D.; Djuric, D.M.; Jakovljevic, V.L. Comparison of blood pro/antioxidant levels before and after acute exercise in athletes and non-athletes. Gen. Physiol. Biophys. 2012, 31, 211–219. [Google Scholar] [CrossRef]
- Bouassida, A.I.; Chatard, J.C.; Chamari, K.; Zaouali, M.; Feki, Y.; Gharbi, N.; Zbidi, A.; Tabka, Z. Effect of energy expenditure and training status on leptin response to sub-maximal cycling. J. Sports Sci. Med. 2009, 8, 190–196. [Google Scholar]
- Pérusse, L.; Collier, G.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Nadeau, A.; Zimmet, P.Z.; Bouchard, C. Acute and chronic effects of exercise on leptin levels in humans. J. Appl. Physiol. 1997, 83, 5–10. [Google Scholar] [CrossRef]
- Ferguson, M.A.; White, L.J.; McCoy, S.; Kim, H.W.; Petty, T.; Wilsey, J. Plasma adiponectin response to acute exercise in healthy subjects. Eur. J. Appl. Physiol. 2004, 91, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Phillips, S.A. Adipokine responses to acute resistance exercise in trained and untrained men. Med. Sci. Sports Exerc. 2010, 42, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Huminska-Lisowska, K.; Mieszkowski, J.; Kochanowicz, A.; Bojarczuk, A.; Niespodziński, B.; Brzezińska, P.; Stankiewicz, B.; Michałowska-Sawczyn, M.; Grzywacz, A.; Petr, M.; et al. Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 8782. [Google Scholar] [CrossRef] [PubMed]
- Landt, M.; Lawson, G.M.; Helgeson, J.M.; Davila-Roman, V.G.; Ladenson, J.H.; Jaffe, A.S.; Hickner, R.C. Prolonged exercise decreases serum leptin concentrations. Metabolism 1997, 46, 1109–1112. [Google Scholar] [CrossRef]
- Christensen, R.A.; Malinowski, K.; Massenzio, A.M.; Hafs, H.D.; Scanes, C.G. Acute effects of short-term feed deprivation and refeeding on circulating concentrations of metabolites, insulin-like growth factor, I.; insulin-like growth factor binding proteins, somatotropin, and thyroid hormones in adult geldings. J. Anim. Sci. 1997, 75, 1351–1358. [Google Scholar] [CrossRef]
- Horner, K.; Hopkins, M.; Finlayson, G.; Gibbons, C.; Brennan, L. Biomarkers of appetite: Is there a potential role for metabolomics? Nutr. Res. Rev. 2020, 33, 271–286. [Google Scholar] [CrossRef]
- Bobbert, T.; Wegewitz, U.; Brechtel, L.; Freudenberg, M.; Mai, K.; Möhlig, M.; Diederich, S.; Ristow, M.; Rochlitz, H.; Pfeiffer, A.F.; et al. Adiponectin oligomers in human serum during acute and chronic exercise: Relation to lipid metabolism and insulin sensitivity. Int. J. Sports Med. 2007, 28, 1–8. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Jürimäe, T. Adiponectin is altered after maximal exercise in highly trained male rowers. Eur. J. Appl. Physiol. 2005, 93, 502–505. [Google Scholar] [CrossRef]
- Numao, S.; Katayama, Y.; Hayashi, Y.; Matsuo, T.; Tanaka, K. Influence of acute aerobic exercise on adiponectin oligomer concentrations in middle-aged abdominally obese men. Metabolism 2011, 60, 186–194. [Google Scholar] [CrossRef]
- Jamurtas, A.Z.; Theocharis, V.; Koukoulis, G.; Stakias, N.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur. J. Appl. Physiol. 2006, 97, 122–126. [Google Scholar] [CrossRef]
- Jürimäe, J.; Rämson, R.; Mäestu, J.; Purge, P.; Jürimäe, T.; Arciero, P.J.; von Duvillard, S.P. Plasma visfatin and ghrelin response to prolonged sculling in competitive male rowers. Med. Sci. Sports Exerc. 2009, 41, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Frydelund-Larsen, L.; Akerstrom, T.; Nielsen, S.; Keller, P.; Keller, C.; Pedersen, B.K. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercice. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E24–E31. [Google Scholar] [CrossRef] [PubMed]
- AL-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Role of adiponectin and, P.BEF/visfatin as regulators of inflammation: Involvement in obesity-associated diseases. Clin. Sci. 2008, 114, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jin, S.H.; Park, J.J.; Park, J.W.; Namgoong, I.S.; Kim, Y.I.; Lee, B.J.; Kim, J.G. Visfatin induces sickness responses in the brain. PLoS ONE 2011, 6, e15981. [Google Scholar] [CrossRef]
- Fortes, Y.M.; Souza-Gomes, A.F.; Moreira, A.R.S.; Campos, L.N.; de Moura, S.S.; Barroso, L.S.S.; de Faria, M.H.S.; de Barros Fernandes, H.; de Miranda, A.S.; Martins-Costa, H.C.; et al. A single session of strength training changed plasma levels of resistin, but not leptin in overweight and obese men. Sports Med. Health Sci. 2023, 6, 324–330. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine/Adipokine Response to “Aerobic” Exercise: Is It Just a Matter of Exercise Load? Front. Physiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Czajkowska, A.; Ambroszkiewicz, J.; Mróz, A.; Witek, K.; Nowicki, D.; Małek, Ł. The Effect of the Ultra-Marathon Run. at a Distance of 100 Kilometers on the Concentration of Selected Adipokines in Adult Men. Int. J. Environ. Res. Public Health 2020, 17, 4289. [Google Scholar] [CrossRef]
- Roupas, N.D.; Mamali, I.; Maragkos, S.; Leonidou, L.; Armeni, A.K.; Markantes, G.K.; Tsekouras, A.; Sakellaropoulos, G.C.; Markou, K.B.; Georgopoulos, N.A. The effect of prolonged aerobic exercise on serum adipokine levels during an ultra-marathon endurance race. Hormones 2013, 12, 275–282. [Google Scholar] [CrossRef]
- Ceylan, H.İ.; Saygın, Ö.; Özel Türkcü, Ü. Assessment of acute aerobic exercise in the morning versus evening on asprosin, spexin, lipocalin-2, and insulin level in overweight/obese versus normal weight adult men. Chronobiol. Int. 2020, 37, 1252–1268. [Google Scholar] [CrossRef]
- Ceylan, H.İ.; Öztürk, M.E.; Öztürk, D.; Silva, A.F.; Albayrak, M.; Saygın, Ö.; Eken, Ö.; Clemente, F.M.; Nobari, H. Acute effect of moderate and high-intensity interval exercises on asprosin and, B.DNF levels in inactive normal weight and obese individuals. Sci. Rep. 2023, 13, 7040. [Google Scholar] [CrossRef] [PubMed]
- Schumann, U.; Qiu, S.; Enders, K.; Bosnyák, E.; Laszlo, R.; Machus, K.; Trájer, E.; Jaganathan, S.; Zügel, M.; Steinacker, J.M. Asprosin, a newly identified fasting-induced hormone is not elevated in obesity and is insensitive to acute exercise. Med. Sci. Sports Exerc. 2017, 49, 1023. [Google Scholar] [CrossRef]
- Wiecek, M.; Szymura, J.; Maciejczyk, M.; Kantorowicz, M.; Szygula, Z. Acute Anaerobic Exercise Affects the Secretion of Asprosin, Irisin, and Other Cytokines—A Comparison Between Sexes. Front. Physiol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Huang, M.; Zhang, M.; Zhu, C.; Chen, X.; Bennett, S.; Xu, J.; Zou, J. The Effects of Asprosin on Exercise-Intervention in Metabolic Diseases. Front. Physiol. 2022, 13, 907358. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Sénéchal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef]
- Daskalopoulou, S.S.; Cooke, A.B.; Gomez, Y.H.; Mutter, A.F.; Filippaios, A.; Mesfum, E.T.; Mantzoros, C.S. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur. J. Endocrinol. 2014, 171, 343–352. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw = Album of Photographs of Food Products and Dishes; Institute of Food and Nutrition: Warszawa, Poland, 2008. [Google Scholar]
- Dill, D.; Costill, D. Calculation of percentage changes in volumes of blood, plasma and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.H.; Graveney, M.J.; Cochrane, L.A. Some sources of error in the calculation of relative change in plasma volume. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 50, 13–21. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Brown, B.S. Alterations in plasma-volume-corrected blood components of marathon runners and concomitant relationship to performance. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Krzych, L. Interpretation of the statistical analysis of data. Kardiochirurgia Torakochirurgia Pol. 2007, 4, 315–321. [Google Scholar]
- Prajapati, B.; Dunne, M.; Armstrong, R. Sample size estimation and statistical power analyses. Optom. Today 2010, 16, 10–18. [Google Scholar]

| GROUP | One-Way ANOVA | Kruskal–Wallis Test | |||
|---|---|---|---|---|---|
| VARIABLE | NFAT-NLBM Mean ± SD (N = 13) | NFAT-HLBM Mean ± SD (N = 16) | HFAT-NLBM Mean ± SD (N = 13) | p-Value (F) | p-Value (H) |
| Age (years) | 21.23 ± 1.42 | 21.50 ± 2.10 | 21.62 ± 2.90 | 0.89 (0.23) | |
| BM (kg) | 73.35 ± 2.34 | 85.06 ± 5.28 * | 80.19 ± 4.40 * | <0.01 (25.46) | |
| BMI (kg × m−2) | 22.94 ± 1.33 | 24.76 ± 1.54 * | 25.36 ± 1.12 * | <0.01 (11.35) | |
| %FAT (%) | 16.27 ± 1.60 | 16.86 ± 2.45 | 23.09 ± 1.89 *# | <0.01 (45.40) | |
| LBM (kg) | 61.38 ± 1.29 | 70.74 ± 4.97 * | 61.62 ± 2.68 # | <0.01 (34.56) | |
| VO2max × BM−1 (mL × kg−1 × min−1) | 58.23 ± 5.84 | 52.94 ± 5.13 * | 50.25 ± 4.57 * | <0.01 (7.97) | |
| GROUP | One-Way ANOVA | Kruskal–Wallis Test | |||
|---|---|---|---|---|---|
| VARIABLE | NFAT-NLBM Mean ± SD (N = 13) | NFAT-HLBM Mean ± SD (N = 16) | HFAT-NLBM Mean ± SD (N = 13) | p-Value (F) | p-Value (H) |
| Erythrocytes (106 × μL−1) | 4.97 ± 0.34 | 5.06 ± 0.23 | 5.16 ± 0.22 | 0.19 (1.72) | |
| Hemoglobin (g × dL−1) | 14.97 ± 1.17 | 15.12 ± 0.74 | 15.50 ± 0.66 | 0.29 (1.28) | |
| Hematocrit (%) | 43.77 ± 2.79 | 44.31 ± 1.65 | 45.34 ± 1.88 | 0.17 (1.83) | |
| Leukocytes (103 × μL−1) | 5.43 ± 0.95 | 5.87 ± 0.98 | 5.74 ± 1.26 | 0.53 (0.64) | |
| Neutrophils (%) | 56.62 ± 11.90 | 53.79 ± 6.95 | 53.38 ± 6.89 | 0.59 (0.54) | |
| Lymphocytes (%) | 35.12 ± 8.46 | 36.28 ± 7.94 | 36.13 ± 6.63 | 0.91 (0.09) | |
| Monocytes (%) | 7.42 ± 1.49 | 7.19 ± 2.23 | 7.77 ± 1.51 | 0.70 (0.36) | |
| Eosinophils (%) | 2.88 ± 1.98 | 2.33 ± 1.32 | 2.37 ± 1.58 | 0.79 (0.47) | |
| Basophils (%) | 0.23 ± 0.10 | 0.28 ± 0.20 | 0.22 ± 0.10 | 0.51 (1.35) | |
| Platelets (103 × μL−1) | 216.0 ± 46.8 | 236.9 ± 48.3 | 232.6 ± 34.5 | 0.43 (0.87) | |
| Glucose (mmol × L−1) | 4.10 ± 0.30 | 4.12 ± 0.45 | 4.40 ± 0.38 | 0.13 (4.02) | |
| Total cholesterol (mmol × L−1) | 4.21 ± 0.67 | 4.28 ± 0.56 | 4.47 ± 0.95 | 0.65 (0.43) | |
| HDL-cholesterol (mmol × L−1) | 1.37 ± 0.20 | 1.34 ± 0.18 | 1.40 ± 0.30 | 0.77 (0.26) | |
| LDL-cholesterol (mmol × L−1) | 2.43 ± 0.73 | 2.54 ± 0.49 | 2.61 ± 0.82 | 0.79 (0.24) | |
| Triglycerides (mmol × L−1) | 0.73 ± 0.27 | 0.90 ± 0.26 | 1.00 ± 0.50 | 0.20 (3.25) | |
| Glycated hemoglobin (%) | 5.14 ± 0.33 | 5.12 ± 0.74 | 5.12 ± 0.27 | 0.95 (0.09) | |
| ESR (mm × h−1) | 2.08 ± 0.28 | 3.31 ± 1.58 | 3.23 ± 2.20 | 0.03 (7.07) | |
| CRP (mg × L−1) | 0.36 ± 0.20 | 0.84 ± 0.80 | 0.79 ± 0.62 | 0.16 (3.64) | |
| Results of Two-Way RM ANOVA | ||||
|---|---|---|---|---|
| BODY COMPOSITION | EXERCISE | BODY COMPOSITION × EXERCISE | ||
| VARIABLE | Measuring Points | F (p) η2 | F (p) η2 | F (p) η2 |
| AOPP | (1) %dPV uncorrected | 0.37 (0.69) 0.02 | 26.79 (<0.01) 0.41 | 3.44 (0.04) 0.15 |
| (μmol × L−1) | (2) %dPV corrected | 0.18 (0.83) 0.01 | 1.73 (0.20) 0.04 | 2.17 (0.13) 0.10 |
| DNA/RNA/ox | (1) %dPV uncorrected | 0.69 (0.51) 0.03 | 3.31 (0.08) 0.08 | 0.53 (0.59) 0.03 |
| (ng × mL−1) | (2) %dPV corrected | 0.64 (0.53) 0.03 | 1.01 (0.32) 0.03 | 0.80 (0.46) 0.04 |
| LPO | (1) %dPV uncorrected | 093 (0.40) 0.05 | 76.60 (<0.01) 0.66 | 0.62 (0.54) 0.03 |
| (μmol × L−1) | (2) %dPV corrected | 0.81 (0.45) 0.04 | 28.72 (<0.01) 0.42 | 0.81 (0.45) 0.04 |
| CAT | (1) %dPV uncorrected | 0.56 (0.58) 0.03 | 23.38 (<0.01) 0.37 | 1.69 (0.20) 0.08 |
| (nmol × min−1 × mL−1) | (2) %dPV corrected | 0.38 (0.69) 0.02 | 10.28 (<0.01) 0.21 | 1.82 (0.18) 0.09 |
| SOD | (1) %dPV uncorrected | 0.31 (0.74) 0.02 | 0.38 (0.54) 0.01 | 0.26 (0.77) 0.01 |
| (μmol × min−1 × mL−1) | (2) %dPV corrected | 0.16 (0.85) 0.01 | 15.46 (<0.01) 0.28 | 0.57 (0.57) 0.03 |
| GPx | (1) %dPV uncorrected | 0.87 (0.43) 0.04 | 3.99 (0.05) 0.09 | 0.63 (0.54) 0.03 |
| (nmol × min−1 × mL−1) | (2) %dPV corrected | 0.72 (0.49) 0.04 | 0.21 (0.65) 0.01 | 0.83 (0.44) 0.04 |
| Visfatin | (1) %dPV uncorrected | 0.67 (0.52) 0.03 | 31.32 (<0.01) 0.45 | 2.33 (0.11) 0.11 |
| (ng × mL−1) | (2) %dPV corrected | 0.71 (0.50) 0.04 | 17.15 (<0.01) 0.31 | 3.36 (0.04) 0.15 |
| Leptin | (1) %dPV uncorrected | 3.06 (0.05) 1.14 | 14.36 (<0.01) 0.27 | 1.74 (0.19) 0.08 |
| (ng × mL−1) | (2) %dPV corrected | 2.94 (0.06) 0.13 | 0.73 (0.40) 0.02 | 0.48 (0.62) 0.02 |
| Resistin | (1) %dPV uncorrected | 1.03 (0.37) 0.05 | 226.07 (<0.01) 0.85 | 0.24 (0.79) 0.01 |
| (ng × mL−1) | (2) %dPV corrected | 1.34 (0.27) 0.16 | 102.11 (<0.01) 0.72 | 0.02 (0.98) <0.01 |
| Adiponectin | (1) %dPV uncorrected | 1.78 (0.32) 0.06 | 38.70 (<0.01) 0.50 | 0.09 (0.91) <0.01 |
| (µg × mL−1) | (2) %dPV corrected | 1.51 (0.23) 0.07 | 1.98 (0.17) 0.05 | 0.90 (0.42) 0.04 |
| Asprosin | (1) %dPV uncorrected | 0.20 (0.82) 0.01 | 0.89 (0.35) 0.02 | 1.09 (0.35) 0.05 |
| (ng × mL−1) | (2) %dPV corrected | 0.22 (0.80) 0.01 | 6.34 (0.02) 0.14 | 0.75 (0.48) 0.04 |
| Irisin | (1) %dPV uncorrected | 0.09 (0.91) <0.01 | 11.15 (<0.01) 0.22 | 0.48 (0.62) 0.02 |
| (µg × mL−1) | (2) %dPV corrected | 0.13 (0.88) 0.01 | 1.44 (0.24) 0.04 | 0.86 (0.43) 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiecek, M.; Mardyla, M.; Szymura, J.; Kantorowicz, M.; Kusmierczyk, J.; Maciejczyk, M.; Szygula, Z. Maximal Intensity Exercise Induces Adipokine Secretion and Disrupts Prooxidant–Antioxidant Balance in Young Men with Different Body Composition. Int. J. Mol. Sci. 2025, 26, 350. https://doi.org/10.3390/ijms26010350
Wiecek M, Mardyla M, Szymura J, Kantorowicz M, Kusmierczyk J, Maciejczyk M, Szygula Z. Maximal Intensity Exercise Induces Adipokine Secretion and Disrupts Prooxidant–Antioxidant Balance in Young Men with Different Body Composition. International Journal of Molecular Sciences. 2025; 26(1):350. https://doi.org/10.3390/ijms26010350
Chicago/Turabian StyleWiecek, Magdalena, Mateusz Mardyla, Jadwiga Szymura, Malgorzata Kantorowicz, Justyna Kusmierczyk, Marcin Maciejczyk, and Zbigniew Szygula. 2025. "Maximal Intensity Exercise Induces Adipokine Secretion and Disrupts Prooxidant–Antioxidant Balance in Young Men with Different Body Composition" International Journal of Molecular Sciences 26, no. 1: 350. https://doi.org/10.3390/ijms26010350
APA StyleWiecek, M., Mardyla, M., Szymura, J., Kantorowicz, M., Kusmierczyk, J., Maciejczyk, M., & Szygula, Z. (2025). Maximal Intensity Exercise Induces Adipokine Secretion and Disrupts Prooxidant–Antioxidant Balance in Young Men with Different Body Composition. International Journal of Molecular Sciences, 26(1), 350. https://doi.org/10.3390/ijms26010350






