Aldose Reductase: A Promising Therapeutic Target for High-Altitude Pulmonary Edema
Abstract
1. Introduction
2. Results
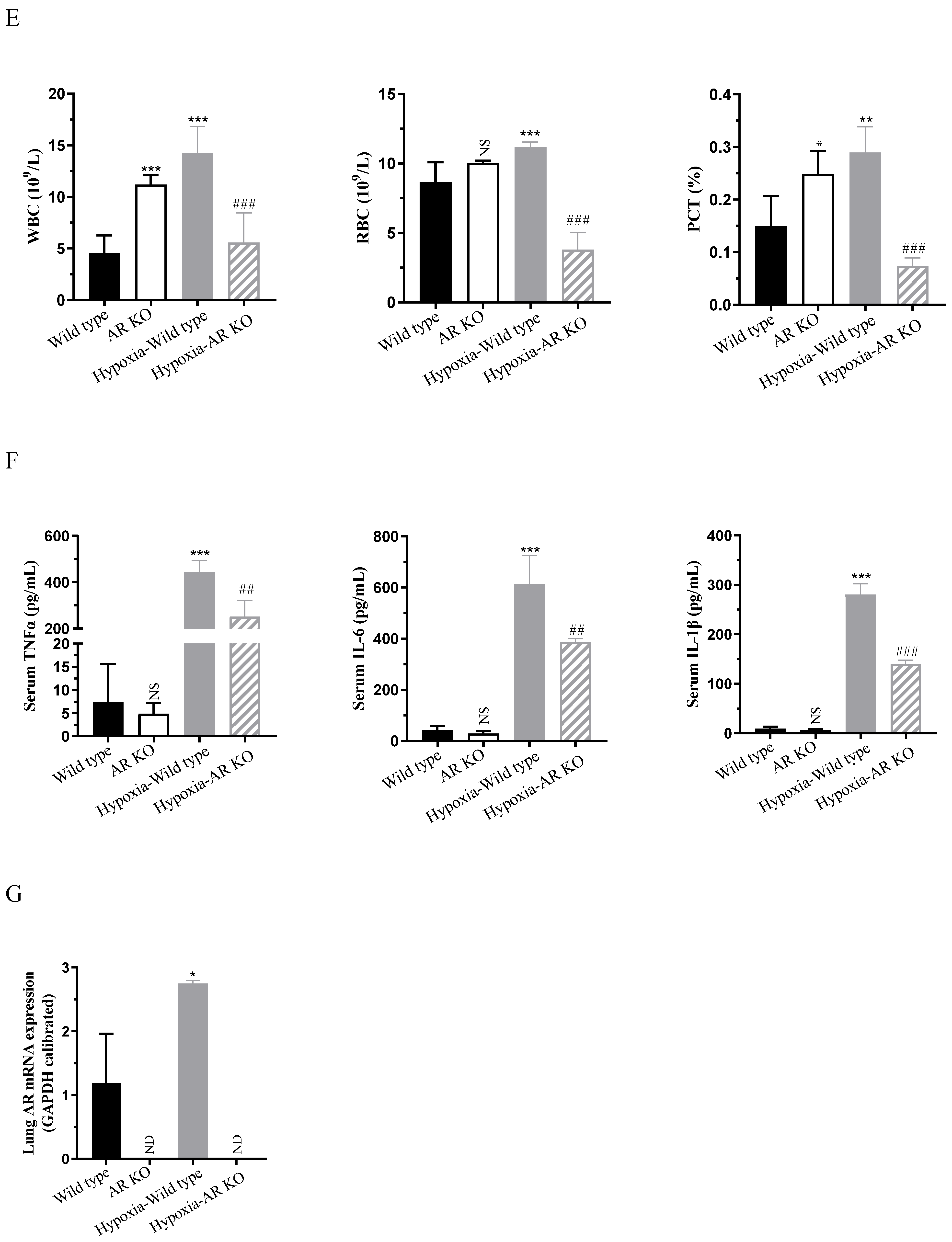
2.1. Knockout of AR Alleviates Hypoxia-Induced Pulmonary Edema in Mice
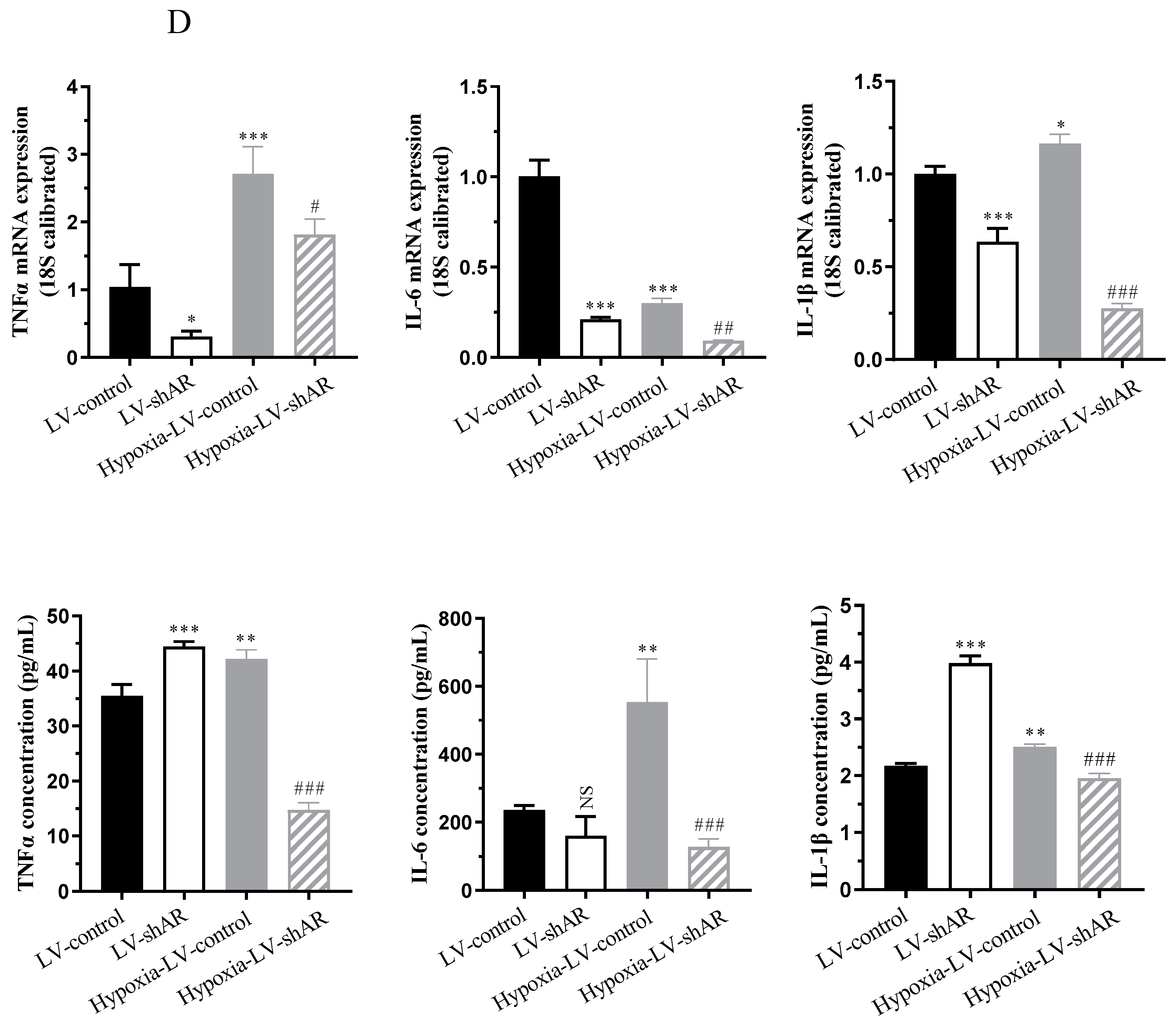
2.2. AR Knockdown Ameliorates Hypoxia-Induced Expression of Vascular Pressure and Inflammatory Factors In Vitro
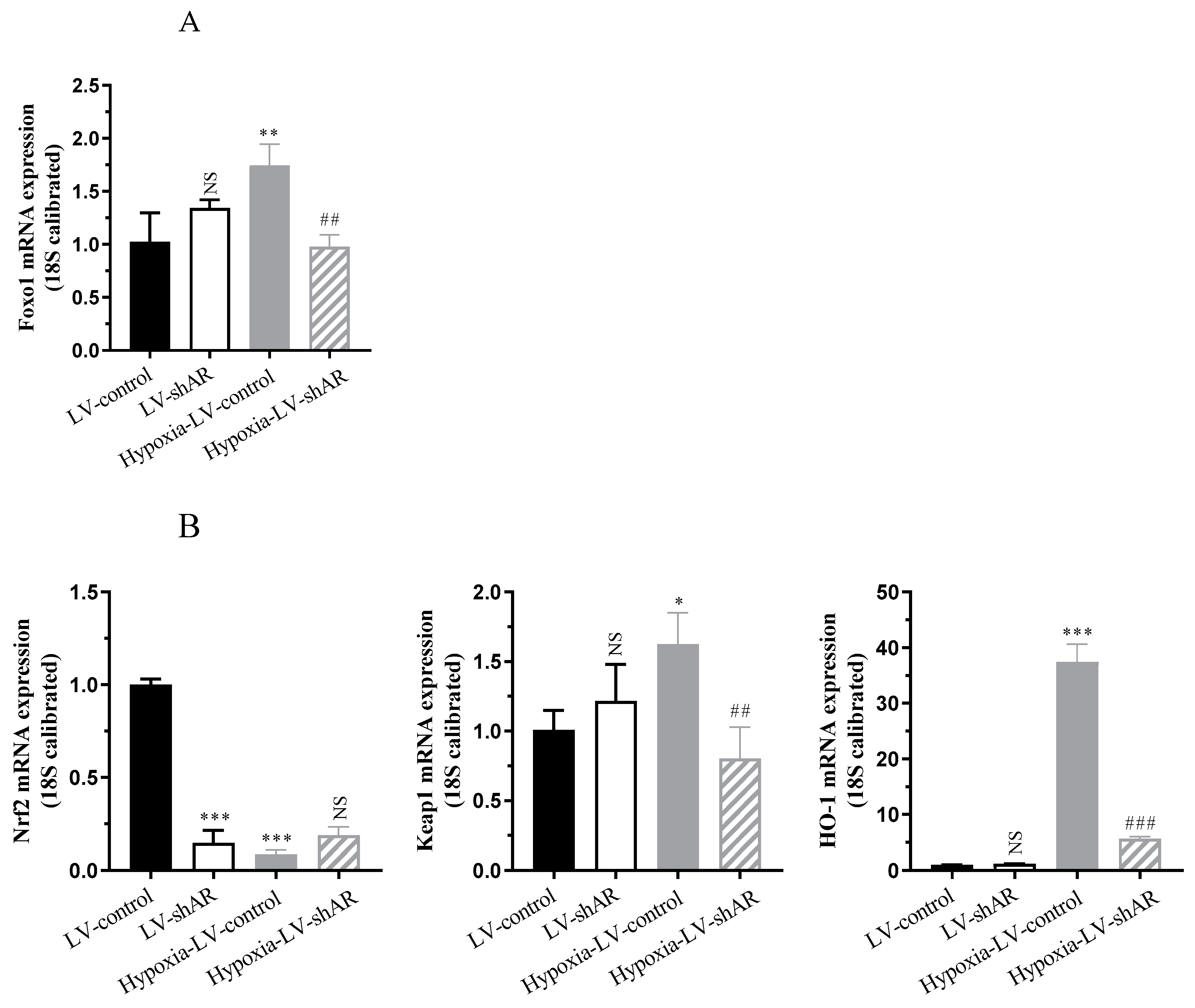
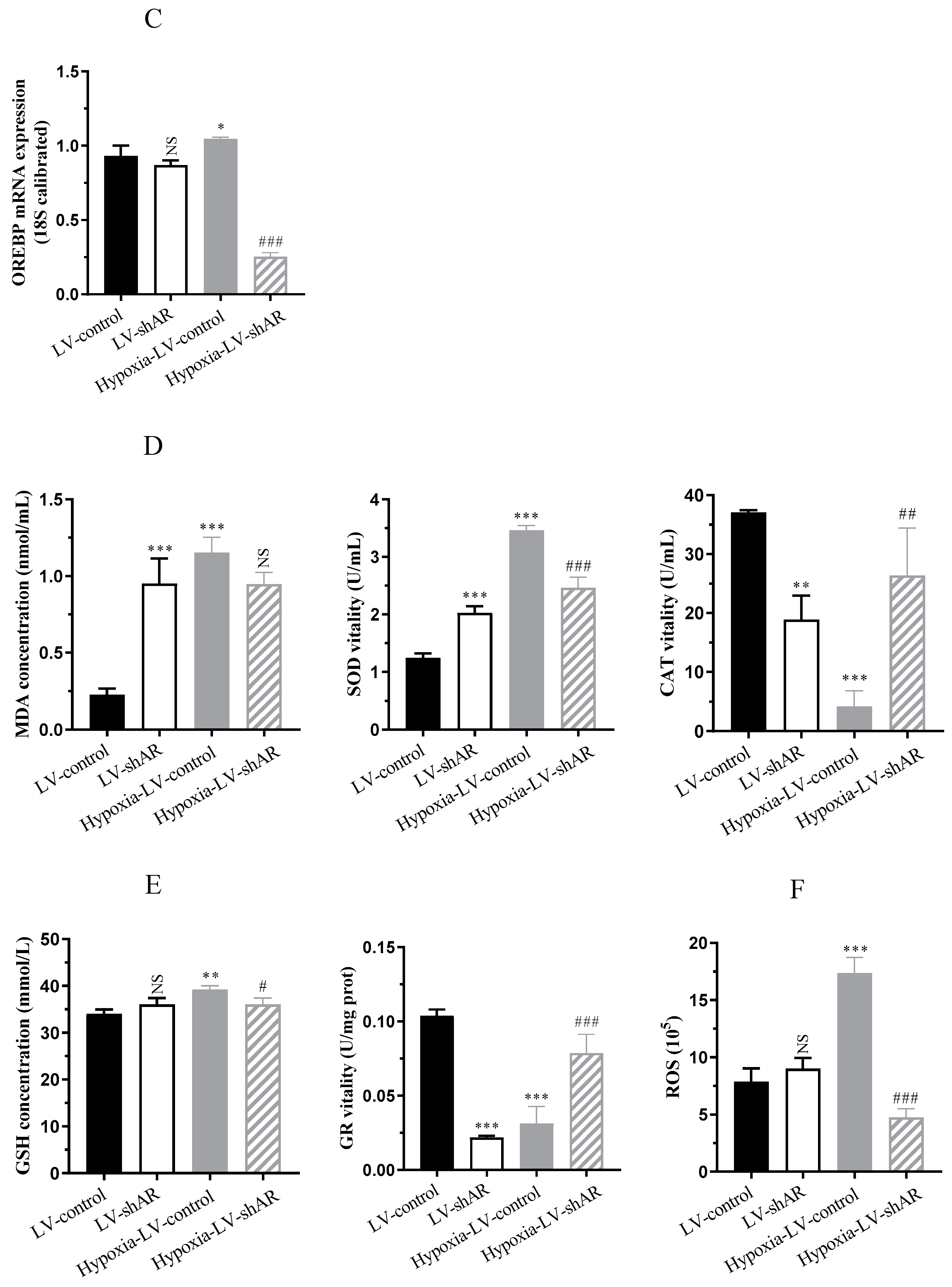
2.3. Knocking Down AR Improves the Expression of Oxidative Stress Induced by Hypoxia
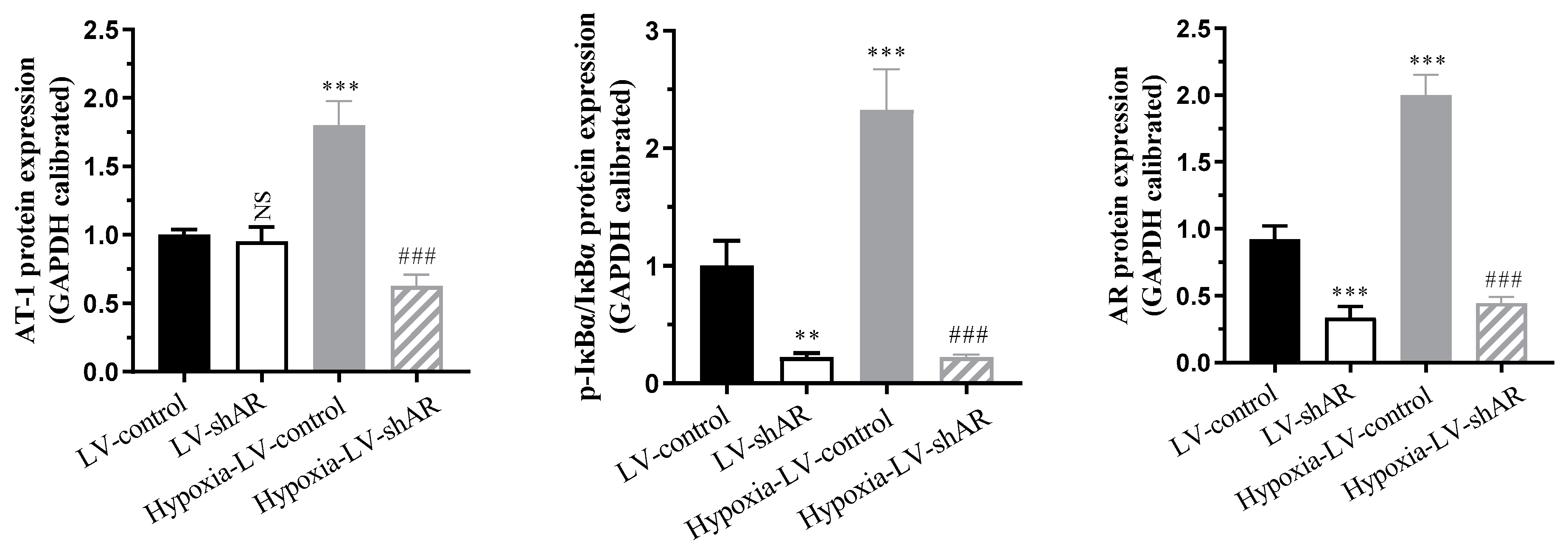
2.4. Verify the Regulatory Role of AR on Various Physiological Indicators Under Hypoxic Stimulation at the Protein Expression Level
2.5. AR Regulation of Blood Glucose and Lactate Accumulation Under Hypoxic Conditions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animal Treatment
4.3. Lung Water Content
4.4. Bronchoalveolar Lavage Fluid
4.5. Hematoxylin and Eosin Staining
4.6. Cell Culture
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Real-Time PCR
4.9. Western Blot
4.10. Statistical Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhang, Y.; Zhang, Y. Research advances in pathogenesis and prophylactic measures of acute high altitude illness. Respir. Med. 2018, 145, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Woods, P.; Alcock, J. High-altitude pulmonary edema. Evol. Med. Public Health 2021, 9, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Richalet, J.P.; Jeny, F.; Callard, P.; Bernaudin, J.F. High-altitude pulmonary edema: The intercellular network hypothesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2023, 325, L155–L173. [Google Scholar] [CrossRef] [PubMed]
- Gatterer, H.; Villafuerte, F.C.; Ulrich, S.; Bhandari, S.S.; Keyes, L.E.; Burtscher, M. Altitude illnesses. Nat. Rev. Dis. Primers 2024, 10, 43. [Google Scholar] [CrossRef]
- Bian, S.Z.; Zhang, C.; Rao, R.S.; Ding, X.H.; Huang, L. Systemic Blood Predictors of Elevated Pulmonary Artery Pressure Assessed by Non-invasive Echocardiography After Acute Exposure to High Altitude: A Prospective Cohort Study. Front. Cardiovasc. Med. 2022, 9, 866093. [Google Scholar] [CrossRef] [PubMed]
- Mounier, R.; Amonchot, A.; Caillot, N.; Gladine, C.; Citron, B.; Bedu, M.; Chirico, E.; Coudert, J.; Pialoux, V. Pulmonary arterial systolic pressure and susceptibility to high altitude pulmonary edema. Respir. Physiol. Neurobiol. 2011, 179, 294–299. [Google Scholar] [CrossRef]
- Lee, S.Y.; Li, M.H.; Shi, L.S.; Chu, H.; Ho, C.W.; Chang, T.C. Rhodiola crenulata Extract Alleviates Hypoxic Pulmonary Edema in Rats. Evid. Based Complement. Alternat Med. 2013, 2013, 718739. [Google Scholar] [CrossRef] [PubMed]
- Sarada, S.K.; Titto, M.; Himadri, P.; Saumya, S.; Vijayalakshmi, V. Curcumin prophylaxis mitigates the incidence of hypobaric hypoxia-induced altered ion channels expression and impaired tight junction proteins integrity in rat brain. J. Neuroinflamm. 2015, 12, 113. [Google Scholar] [CrossRef]
- Miao, Q.; Shi, X.P.; Ye, M.X.; Zhang, J.; Miao, S.; Wang, S.W.; Li, B.; Jiang, X.X.; Zhang, S.; Hu, N.; et al. Polydatin attenuates hypoxic pulmonary hypertension and reverses remodeling through protein kinase C mechanisms. Int. J. Mol. Sci. 2012, 13, 7776–7787. [Google Scholar] [CrossRef]
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; Guan, X.; Chen, J.; Wang, C. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-kappaB signaling pathway. Int. Immunopharmacol. 2016, 40, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Arya, A.; Gangwar, A.; Bhargava, K.; Ahmad, Y. Size restricted silymarin suspension evokes integrated adaptive response against acute hypoxia exposure in rat lung. Free Radic. Biol. Med. 2016, 96, 139–151. [Google Scholar] [CrossRef]
- Penning, T.M.; Drury, J.E. Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch. Biochem. Biophys. 2007, 464, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef]
- Dschietzig, T.; Alexiou, K.; Richter, C.; Bobzin, M.; Baumann, G.; Stangl, K.; Brunner, F. Endotoxin causes pulmonary hypertension by upregulating smooth muscle endothelin type-B receptors: Role of aldose reductase. Shock 2008, 30, 189–196. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Eum, W.S.; Shin, M.J.; Yeo, H.J.; Yeo, E.J.; Choi, Y.J.; Kwon, H.J.; Cho, S.W.; Park, J.; Han, K.H.; et al. Tat-aldose reductase prevents dopaminergic neuronal cell death by inhibiting oxidative stress and MAPK activation. Int. J. Mol. Med. 2021, 47, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Yadav, U.C.; Reddy, A.B.; Saxena, A.; Tammali, R.; Shoeb, M.; Ansari, N.H.; Bhatnagar, A.; Petrash, M.J.; Srivastava, S.; et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem. Biol. Interact. 2011, 191, 330–338. [Google Scholar] [CrossRef]
- Tammali, R.; Saxena, A.; Srivastava, S.K.; Ramana, K.V. Aldose reductase inhibition prevents hypoxia-induced increase in hypoxia-inducible factor-1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) by regulating 26 S proteasome-mediated protein degradation in human colon cancer cells. J. Biol. Chem. 2011, 286, 24089–24100. [Google Scholar] [CrossRef]
- Huang, W.; Liu, H.; Wang, T.; Zhang, T.; Kuang, J.; Luo, Y.; Chung, S.S.; Yuan, L.; Yang, J.Y. Tonicity-responsive microRNAs contribute to the maximal induction of osmoregulatory transcription factor OREBP in response to high-NaCl hypertonicity. Nucleic Acids Res. 2011, 39, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, M.; Zhang, Y.; Zhao, X.; Zhang, Y.; Yue, H.; Zhang, L. The Anti-Hypoxic Mechanism of Sesamoside Determined Using Network Pharmacology. Dose Response 2024, 22, 15593258241282574. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Xiao, H.; Li, Y. Hypoxia dissociates HDAC6/FOXO1 complex and aggregates them into nucleus to regulate autophagy and osteogenic differentiation. J. Periodontal Res. 2023, 58, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wen, H.; Li, X.; Yang, J.; Li, G.; Zhang, M.; Li, J.; He, F. Acute hypoxia effects on Keap1/Nrf2 (Mafs)-GST pathway related oxidative metabolism in muscle of Japanese flounder (Paralichthys olivaceus). Sci. Total Environ. 2021, 795, 148646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Irarrazabal, C.E.; Williams, C.K.; Ely, M.A.; Birrer, M.J.; Garcia-Perez, A.; Burg, M.B.; Ferraris, J.D. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J. Biol. Chem. 2008, 283, 2554–2563. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, H.; Li, F.; Yuan, Y.; Zheng, X.; Zhang, X.; Sun, J. Ammidin ameliorates myocardial hypoxia/reoxygenation injury by inhibiting the ACSL4/AMPK/mTOR-mediated ferroptosis pathway. BMC Complement. Med. Ther. 2023, 23, 459. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Keykhaee, M.; Khorramdelazad, H.; Karimi, M.Y.; Nejatbakhsh Samimi, L.; Aghamohamadi, N.; Karimi, M.; Falak, R.; Khoobi, M. Catalase application in cancer therapy: Simultaneous focusing on hypoxia attenuation and macrophage reprogramming. Biomed. Pharmacother. 2022, 153, 113483. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, A.; Heyder, L.; Daude, M.; Plessner, M.; Krippner, S.; Grosse, R.; Diederich, W.E.; Culmsee, C. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic. Biol. Med. 2018, 117, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.E.; de Souza, L.F.; Dos Santos, B.; Maher, P.; Lopes, F.M.; Londero, G.F.; Klamt, F.; Dafre, A.L. Methylglyoxal-Induced Protection Response and Toxicity: Role of Glutathione Reductase and Thioredoxin Systems. Neurotox. Res. 2017, 32, 340–350. [Google Scholar] [CrossRef]
- Serrano-Duenas, M. Acute mountain sickness: The clinical characteristics of a cohort of 615 patients. Med. Clin. 2000, 115, 441–445. [Google Scholar] [CrossRef]
- Oltmanns, K.M.; Gehring, H.; Rudolf, S.; Schultes, B.; Rook, S.; Schweiger, U.; Born, J.; Fehm, H.L.; Peters, A. Hypoxia causes glucose intolerance in humans. Am. J. Respir. Crit. Care Med. 2004, 169, 1231–1237. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, Y.Q.; Liu, C.; Yang, J.; Zhang, J.H.; Jin, J.; Tan, H.; Yuan, F.Z.; Ke, J.B.; He, C.Y.; et al. Association between physiological responses after exercise at low altitude and acute mountain sickness upon ascent is sex-dependent. Mil. Med. Res. 2020, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Zubieta-Calleja, G.R.; Zubieta-DeUrioste, N. High Altitude Pulmonary Edema, High Altitude Cerebral Edema, and Acute Mountain Sickness: An enhanced opinion from the High Andes—La Paz, Bolivia 3500 m. Rev. Environ. Health 2023, 38, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Sydykov, A.; Mamazhakypov, A.; Maripov, A.; Kosanovic, D.; Weissmann, N.; Ghofrani, H.A.; Sarybaev, A.S.; Schermuly, R.T. Pulmonary Hypertension in Acute and Chronic High Altitude Maladaptation Disorders. Int. J. Environ. Res. Public Health 2021, 18, 1692. [Google Scholar] [CrossRef] [PubMed]
- El Alam, S.; Pena, E.; Aguilera, D.; Siques, P.; Brito, J. Inflammation in Pulmonary Hypertension and Edema Induced by Hypobaric Hypoxia Exposure. Int. J. Mol. Sci. 2022, 23, 12656. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Shihan, M.; Seeger, W.; Schermuly, R.T.; Novoyatleva, T. Genetic Delivery and Gene Therapy in Pulmonary Hypertension. Int. J. Mol. Sci. 2021, 22, 1179. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, U.; Rexhaj, E.; Jayet, P.Y.; Allemann, Y.; Sartori, C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog. Cardiovasc. Dis. 2010, 52, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.A.; Thompson, A.A.; Baillie, J.K.; MacDougall, M.; Hirani, N. Change in plasma vascular endothelial growth factor during onset and recovery from acute mountain sickness. Respir. Med. 2007, 101, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Tschop, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschop, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kador, P.F. The contributions of Jin H. Kinoshita to aldose reductase research. Exp. Eye Res. 1990, 50, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Kawashima, K.; Suzuki, S.; Asakura, Y.; Asano, M.; Tsurumaru, K.; Dai, H.; Tachi, Y.; Bannai, C.; Saitoh, M.; et al. Restoration of nitric oxide production by aldose reductase inhibitor in human endothelial cells cultured in high-glucose medium. Life Sci. 1997, 60, PL53–PL56. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; King, G.L. The role of protein kinase C activation in diabetic nephropathy. Kidney Int. Suppl. 2007, 72, S49–S53. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Wang, M.; Zhao, X.; Zhang, Y.; Zhang, Y.; Hao, X.; Yuan, J.; Tang, H. Aldose Reductase: A Promising Therapeutic Target for High-Altitude Pulmonary Edema. Int. J. Mol. Sci. 2025, 26, 341. https://doi.org/10.3390/ijms26010341
Song D, Wang M, Zhao X, Zhang Y, Zhang Y, Hao X, Yuan J, Tang H. Aldose Reductase: A Promising Therapeutic Target for High-Altitude Pulmonary Edema. International Journal of Molecular Sciences. 2025; 26(1):341. https://doi.org/10.3390/ijms26010341
Chicago/Turabian StyleSong, Dan, Mengjie Wang, Xinjie Zhao, Yanru Zhang, Yiyi Zhang, Xiaohua Hao, Jialu Yuan, and Haojie Tang. 2025. "Aldose Reductase: A Promising Therapeutic Target for High-Altitude Pulmonary Edema" International Journal of Molecular Sciences 26, no. 1: 341. https://doi.org/10.3390/ijms26010341
APA StyleSong, D., Wang, M., Zhao, X., Zhang, Y., Zhang, Y., Hao, X., Yuan, J., & Tang, H. (2025). Aldose Reductase: A Promising Therapeutic Target for High-Altitude Pulmonary Edema. International Journal of Molecular Sciences, 26(1), 341. https://doi.org/10.3390/ijms26010341





