The α1- and β1-Subunits of Nitric Oxide-Sensitive Guanylyl Cyclase in Pericytes of Healthy Human Dental Pulp
Abstract
1. Introduction
2. Results
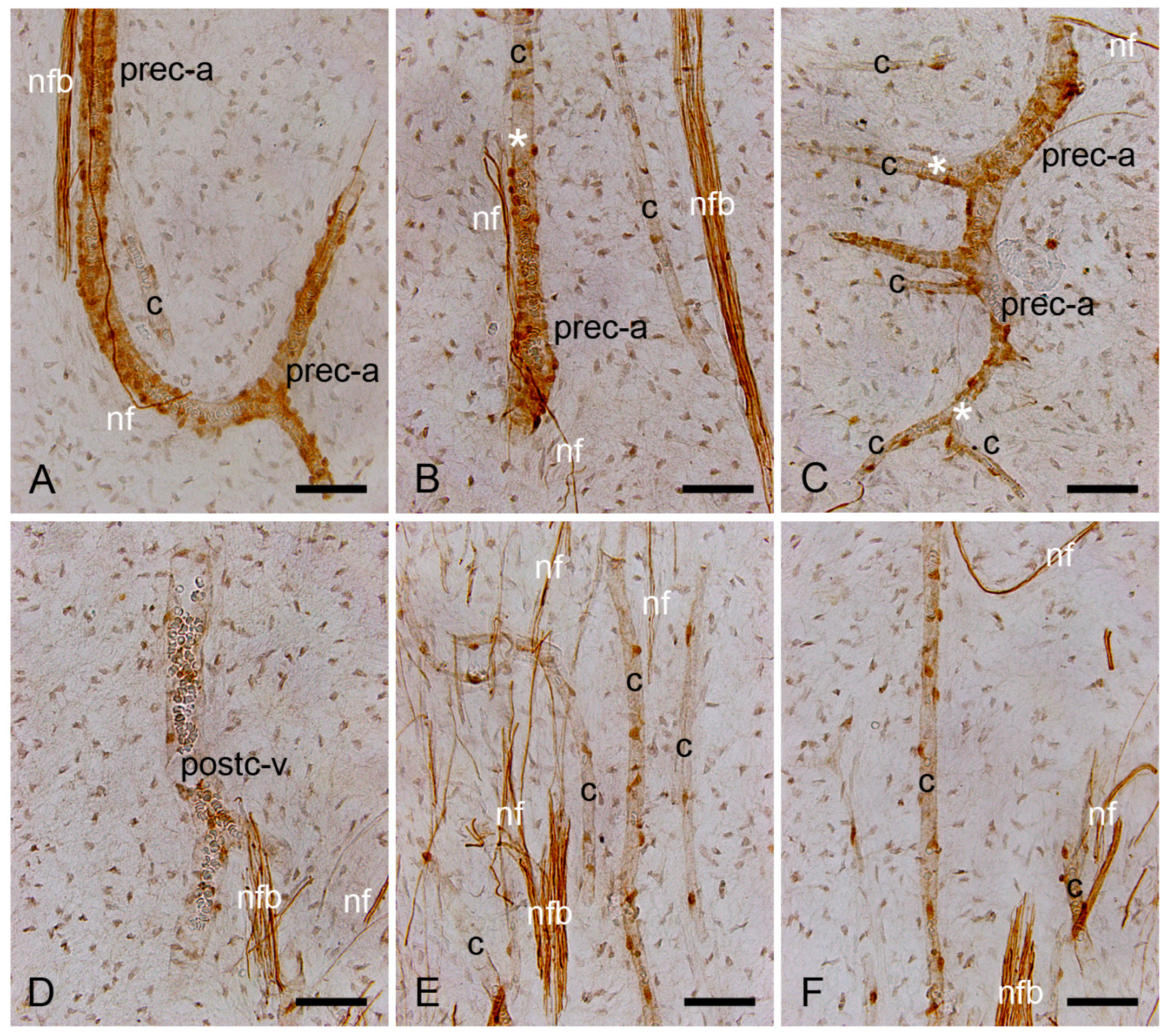
2.1. Characterization of the Healthy Human Dentin–Pulp Complex
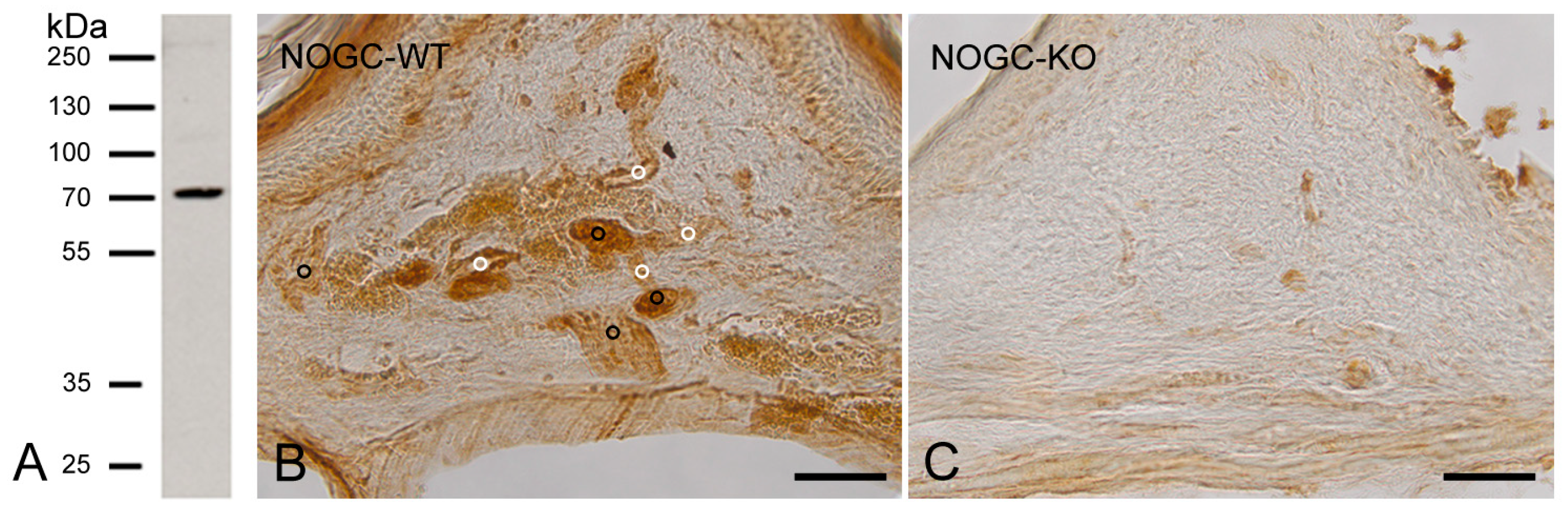
2.2. Specificity of NO-GC α1 and β1 Antibodies
2.2.1. Specificity of NO-GCα1 Antibody
2.2.2. Specificity of NO-GCβ1 Antibody
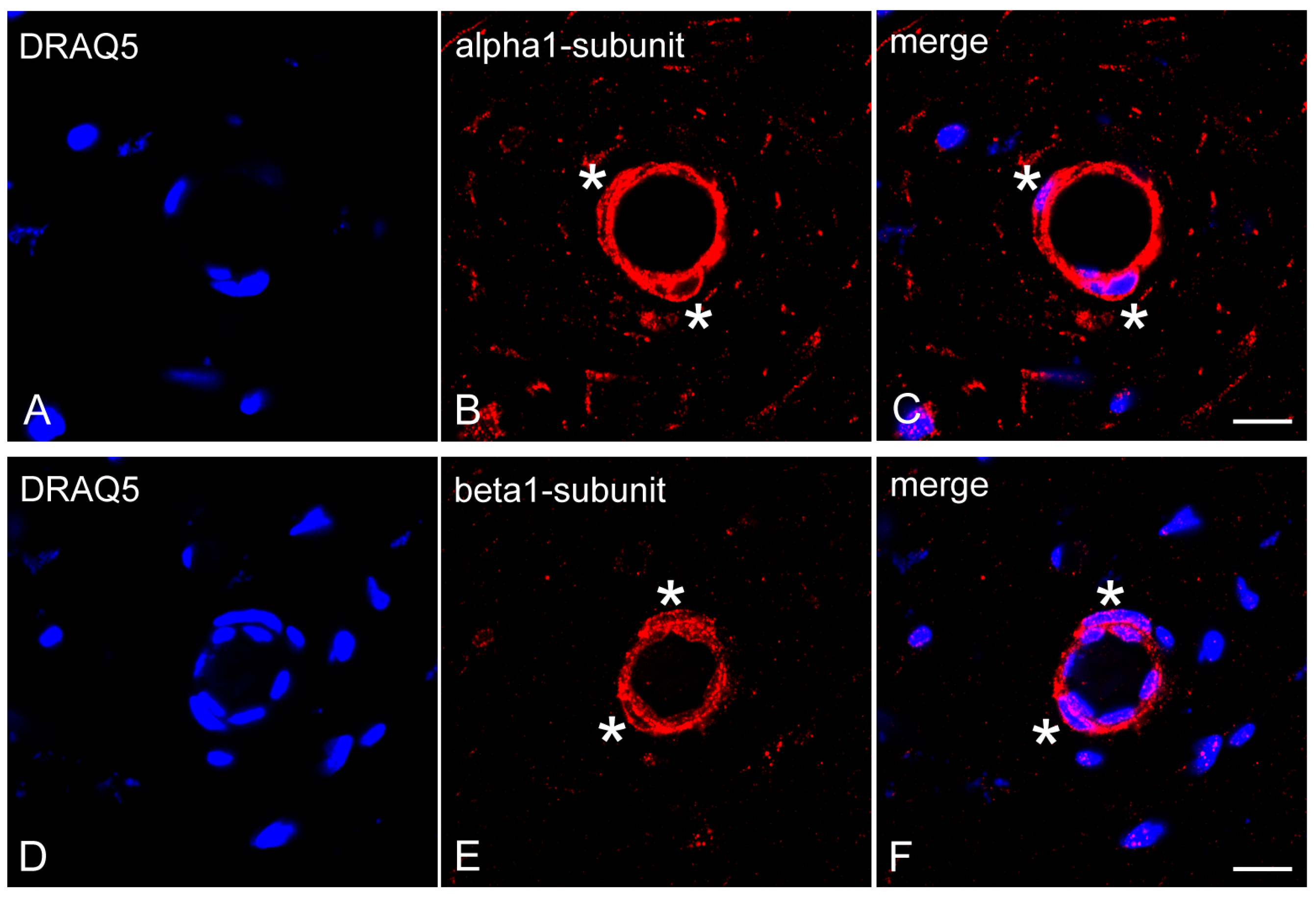
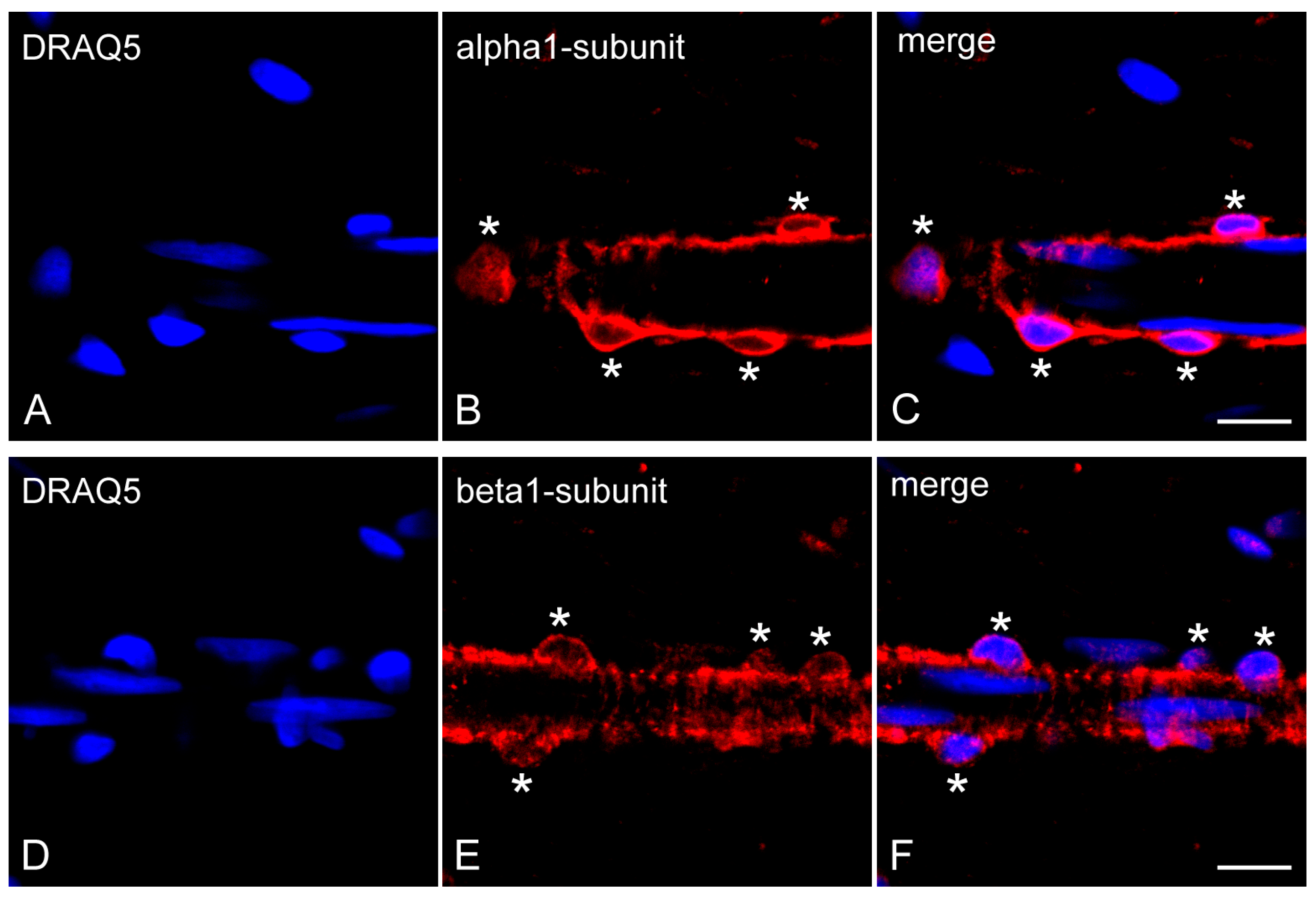
2.3. Expression of NO-GCα1 and β1 Subunits in Pericytes of Decalcified Dental Pulp
2.4. Expression of NO-GCα1 and β1 Subunits in Pericytes of Non-Decalcified Dental Pulp
2.5. Immunohistochemical Controls
2.6. Statistical Analysis of the Fluorescence Intensity of NO-GCα1 and NO-GCβ1 in Pericytes of Decalcified and Non-Decalcified Dental Pulp
3. Discussion
4. Materials and Methods
4.1. Clinical Evaluation and Collection of Human Molars
4.2. Tissue Preparation
4.3. Histopathological Evaluation of Healthy and Inflamed Human Dental Pulp
4.4. Specificity of NO-GC α1 and β1 Subunit Antibodies
4.4.1. Characterization of NO-GCα1 Antibody
4.4.2. Development and Characterization of NO-GCβ1 Antibody
GCKO-Mice and Characterization of NO-GCβ1 Antibody in GCKO Mice
Western Blots with NO-GCβ1 Antibody Using Human Placenta Tissue Samples
Mass Spectrometry Analysis
4.5. Immunohistochemical Methods
4.5.1. Avidin–Biotin–Peroxidase Complex Method
4.5.2. Immunofluorescence
4.5.3. Double Immunofluorescence
4.6. Fluorescence Intensities of the NO-GCα1 and NO-GCβ1 in Pericytes and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; H S, A.K.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Investig. 2006, 116, 2552–2561. [Google Scholar] [CrossRef]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Haskó, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Foster, P.; Scotland, R.S.; McLean, P.G.; Mathur, A.; Perretti, M.; Moncada, S.; Hobbs, A.J. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of Pselectin expression and leukocyte recruitment. Proc. Natl. Acad. Sci. USA 2004, 101, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Koesling, D.; Mergia, E.; Russwurm, M. Physiological Functions of NO-Sensitive Guanylyl Cyclase Isoforms. Curr. Med. Chem. 2016, 23, 2653–2665. [Google Scholar] [CrossRef]
- Herbert, S.P.; Stainier, D.Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat. Rev. Mol. Cell. Biol. 2011, 12, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Maharjan, S.; Kuang, X.; Wang, Z.; Mille, L.S.; Tao, M.; Yu, P.; Cao, X.; Lian, L.; Lv, L.; et al. Microfluidic bioprinting of tough hydrogel-based vascular conduits for functional blood vessels. Sci. Adv. 2022, 8, eabq6900. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 5–60. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Haefliger, I.O.; Zschauer, A.; Anderson, D.R. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Investig. Ophthalmol. Vis. Sci. 1994, 35, 991–997. [Google Scholar]
- Sloan, A.J.; Smith, A.J. Stem cells and the dental pulp: Potential roles in dentine regeneration and repair. Oral. Dis. 2007, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Feki, A.; Papaccio, G.; Catón, J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011, 23, 275–279. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Mantesso, A.; Sharpe, P.T. Perivascular cells as mesenchymal stem cells. Expert. Opin. Biol. Ther. 2010, 10, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis--a common pathway to organ injury and failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.J.; Bentley, J.P.; Marshall, F.J. Age-related changes in reducible crosslinks of human dental pulp collagen. Arch. Oral. Biol. 1983, 28, 759–764. [Google Scholar] [CrossRef]
- Morse, D.R. Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg. Oral. Med. Oral. Pathol. 1991, 72, 721–745. [Google Scholar] [CrossRef]
- Bettaga, N.; Jager, R.; Dunnes, S.; Groneberg, D.; Friebe, A. Cellspecific impact of nitric oxide-dependent guanylyl cyclase on arteriogenesis and angiogenesis in mice. Angiogenesis 2015, 18, 245–254. [Google Scholar] [CrossRef]
- Fukutani, T.; Iino, S.; Nojyo, Y. The expression of soluble guanylate cyclase in the vasculature of rat skeletal muscle. Arch. Histol. Cytol. 2009, 72, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Theilig, F.; Bostanjoglo, M.; Pavenstädt, H.; Grupp, C.; Holland, G.; Slosarek, I.; Gressner, A.M.; Russwurm, M.; Koesling, D.; Bachmann, S. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J. Am. Soc. Nephrol. 2001, 12, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, H.; Wang, T.; Su, N.; Zhang, F.; Jiang, K.; Zhu, J.; Zhang, C.; Niu, K.; Wang, L.; et al. Single-Cell Transcriptomic Analysis Reveals a Hepatic Stellate Cell-Activation Roadmap and Myofibroblast Origin During Liver Fibrosis in Mice. Hepatology 2021, 74, 2774–2790. [Google Scholar] [CrossRef] [PubMed]
- Aue, A.; Englert, N.; Harrer, L.; Schwiering, F.; Gaab, A.; König, P.; Adams, R.; Schmidtko, A.; Friebe, A.; Groneberg, D. NO-sensitive guanylyl cyclase discriminates pericyte-derived interstitial from intra-alveolar myofibroblasts in murine pulmonary fibrosis. Respir. Res. 2023, 24, 167. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yang, W.; Su, N.; Zhang, C.; Dai, J.; Han, F.; Singhal, M.; Bai, W.; Zhu, X.; Zhu, J.; et al. Activating NO-sGC crosstalk in the mouse vascular niche promotes vascular integrity and mitigates acute lung injury. J. Exp. Med. 2023, 220, e20211422. [Google Scholar] [CrossRef]
- Englert, N.; Burkard, P.; Aue, A.; Rosenwald, A.; Nieswandt, B.; Friebe, A. Anti-Fibrotic and Anti-Inflammatory Role of NO-Sensitive Guanylyl Cyclase in Murine Lung. Int. J. Mol. Sci. 2023, 24, 11661. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Roggendorf, H.C.; Siefer, O.G.; Seehawer, J.; Imhof, T.; Plomann, M.; Bloch, W.; Friebe, A.; Huebbers, C.U. Downregulation of the α1- and β1-subunit of sGC in Arterial Smooth Muscle Cells of OPSCC Is HPV-Independent. J. Dent. Res. 2018, 97, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Plomann, M.; Puladi, B.; Demirbas, A.; Bloch, W.; Deschner, J. Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase. Int. J. Mol. Sci. 2023, 24, 901. [Google Scholar] [CrossRef]
- Kim, S. Neurovascular interactions in the dental pulp in health and inflammation. J. Endod. 1990, 16, 48–53. [Google Scholar] [CrossRef]
- Berggreen, E.; Bletsa, A.; Heyeraas, K.J. Circulation in normal and inflamed dental pulp. Endod. Top. 2010, 17, 2–11. [Google Scholar] [CrossRef]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dong, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hu, J.; Lønstrup, M.; Brodin, B.; et al. Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proc. Natl. Acad. Sci. USA 2021, 118, e2023749118. [Google Scholar] [CrossRef]
- Shah, R.C.; Sanker, S.; Wood, K.C.; Durgin, B.G.; Straub, A.C. Redox regulation of soluble guanylyl cyclase. Nitric Oxide 2018, 76, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Lang, H.; Beikler, T.; Cho, B.; Behrends, S.; Bloch, W.; Addicks, K.; Raab, W.H. Irreversible inflammation is associated with decreased levels of the α1-, β1- and α2-subunit of sGC in human odontoblasts. J. Dent. Res. 2011, 90, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Yianni, V.; Sharpe, P.T. Perivascular-Derived Mesenchymal Stem Cells. J. Dent. Res. 2019, 98, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fu, L.L.; Nada, O.A.; Chen, L.M.; Gosau, M.; Smeets, R.; Feng, H.C.; Friedrich, R.E. Evaluation of the Effects of Human Dental Pulp Stem Cells on the Biological Phenotype of Hypertrophic Keloid Fibroblasts. Cells 2021, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Di Tinco, R.; Bertani, G.; Orlandi, G.; Bertoni, L.; Pignatti, E.; Orciani, M.; Sena, P.; Bertacchini, J.; Salvarani, C.; et al. Human dental pulp stem cells (hDPSCs) promote the lipofibroblast transition in the early stage of a fibro-inflammatory process. Front. Cell Dev. Biol. 2023, 11, 1196023. [Google Scholar] [CrossRef]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, Y.; Pryymachuk, G.; Schroeter, M.M.; Puladi, B.; Piekarek, N.; Appel, S.; Bloch, W.; Lackmann, J.-W.; Deschner, J.; Friebe, A. The α1- and β1-Subunits of Nitric Oxide-Sensitive Guanylyl Cyclase in Pericytes of Healthy Human Dental Pulp. Int. J. Mol. Sci. 2025, 26, 30. https://doi.org/10.3390/ijms26010030
Korkmaz Y, Pryymachuk G, Schroeter MM, Puladi B, Piekarek N, Appel S, Bloch W, Lackmann J-W, Deschner J, Friebe A. The α1- and β1-Subunits of Nitric Oxide-Sensitive Guanylyl Cyclase in Pericytes of Healthy Human Dental Pulp. International Journal of Molecular Sciences. 2025; 26(1):30. https://doi.org/10.3390/ijms26010030
Chicago/Turabian StyleKorkmaz, Yüksel, Galyna Pryymachuk, Mechthild M. Schroeter, Behrus Puladi, Nadin Piekarek, Sarah Appel, Wilhelm Bloch, Jan-Wilm Lackmann, James Deschner, and Andreas Friebe. 2025. "The α1- and β1-Subunits of Nitric Oxide-Sensitive Guanylyl Cyclase in Pericytes of Healthy Human Dental Pulp" International Journal of Molecular Sciences 26, no. 1: 30. https://doi.org/10.3390/ijms26010030
APA StyleKorkmaz, Y., Pryymachuk, G., Schroeter, M. M., Puladi, B., Piekarek, N., Appel, S., Bloch, W., Lackmann, J.-W., Deschner, J., & Friebe, A. (2025). The α1- and β1-Subunits of Nitric Oxide-Sensitive Guanylyl Cyclase in Pericytes of Healthy Human Dental Pulp. International Journal of Molecular Sciences, 26(1), 30. https://doi.org/10.3390/ijms26010030






