Biomarkers of Extracellular Matrix Fragments in Patients with Psoriasis
Abstract
1. Introduction
2. Results
2.1. ECM Fragment Levels in Patients with PSO Compared with HC
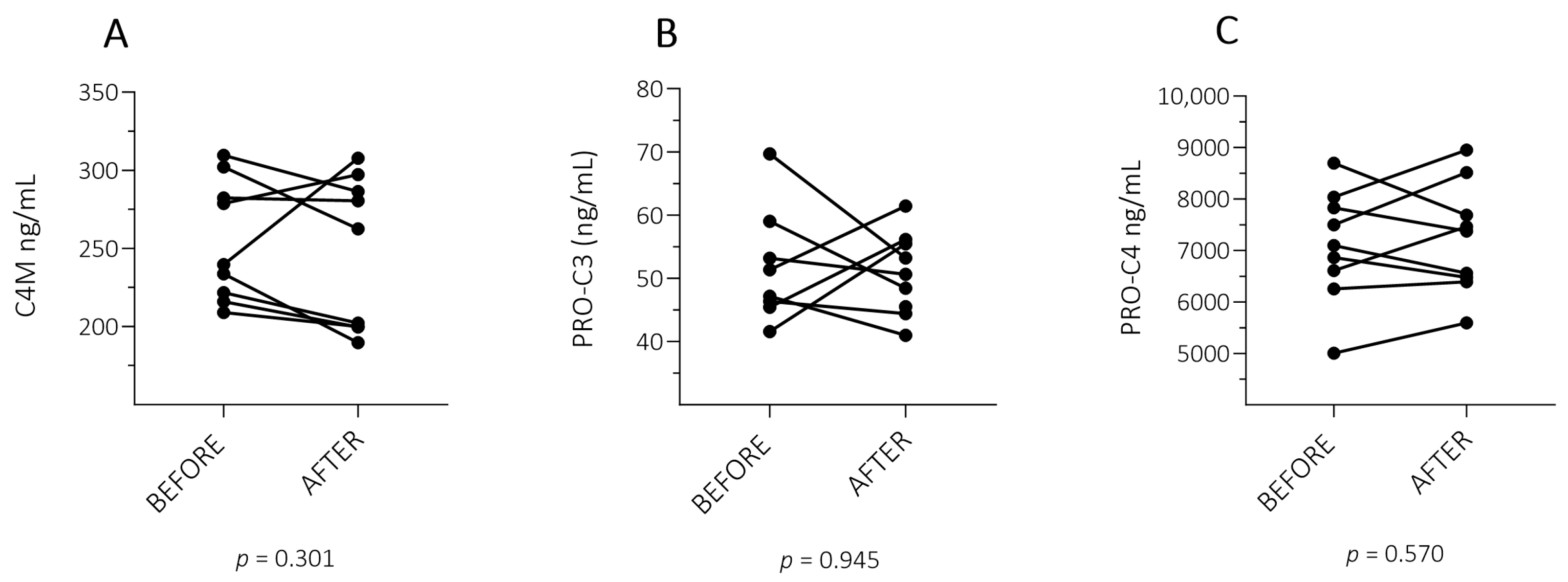
2.2. ECM Fragment Levels in Patients with PSO with a Successful Treatment Response
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Study Population
4.2. ECM Fragments Assessment in EDTA Plasma
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of Psoriatic Arthritis in Patients with Psoriasis: A Systematic Review and Meta-Analysis of Observational and Clinical Studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef]
- Corbett, M.; Ramessur, R.; Marshall, D.; Acencio, M.L.; Ostaszewski, M.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Haddad, S.; Jensen, A.H.M.; et al. Biomarkers of Systemic Treatment Response in People with Psoriasis: A Scoping Review. Br. J. Dermatol. 2022, 187, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Ramessur, R.; Corbett, M.; Marshall, D.; Acencio, M.L.; Barbosa, I.A.; Dand, N.; Di Meglio, P.; Haddad, S.; Jensen, A.H.M.; Koopmann, W.; et al. Biomarkers of Disease Progression in People with Psoriasis: A Scoping Review. Br. J. Dermatol. 2022, 187, 481. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular Matrix Remodeling: The Common Denominator in Connective Tissue Diseases Possibilities for Evaluation and Current Understanding of the Matrix as More than a Passive Architecture, but a Key Player in Tissue Failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef]
- Pfisterer, K.; Weninger, W.; Shaw, L.E.; Symmank, D. The Extracellular Matrix in Skin Inflammation and Infection. Cell Dev. Biol 2021, 9, 682414. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastases and Elastokines: Elastin Degradation and Its Significance in Health and Disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Mezentsev, A.; Nikolaev, A.; Bruskin, S. Matrix Metalloproteinases and Their Role in Psoriasis. Gene 2014, 540, 1–10. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Matrix Molecules and Skin Biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Magee, C.; Groen, S.S.; Sinkevičiūtė, D.; Bay-Jensen, A.C.; Karsdal, M.A.; Pennington, S.R.; FitzGerald, O. Differentiating Patients with Psoriasis from Psoriatic Arthritis Using Collagen Biomarkers. Clin. Exp. Rheumatol. 2023, 41, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, Function, and Biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Sinkeviciute, D.; Madsen, D.H.; Önnerfjord, P.; Hansen, M.; Schmidt, H.; Karsdal, M.A.; Svane, I.M.; Willumsen, N. Granzyme B Degraded Type IV Collagen Products in Serum Identify Melanoma Patients Responding to Immune Checkpoint Blockade. Cancers 2020, 12, 2786. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Hambro, C.A.; Johnston, A.; Stuart, P.E.; Tsoi, L.C.; Nair, R.P.; Elder, J.T. Neutrophil Extracellular Traps Induce Human Th17 Cells: Effect of Psoriasis-Associated TRAF3IP2 Genotype. J. Investig. Dermatol. 2019, 139, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.P.; Kimber, I. A Review on the Potential Role of Basement Membrane Laminin in the Pathogenesis of Psoriasis. Scand. J. Immunol. 2016, 83, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.F.M.G.; Theodoro, T.R.; Filho, C.D.A.S.M.; Oyafuso, L.K.M.; Pinhal, M.A.S. Extracellular Matrix Alterations in the Skin of Patients Affected by Psoriasis. BMC Mol. Cell Biol. 2021, 22, 55. [Google Scholar] [CrossRef]
- Karas, A.; Holmannova, D.; Borsky, P.; Fiala, Z.; Andrys, C.; Hamakova, K.; Svadlakova, T.; Palicka, V.; Krejsek, J.; Rehacek, V.; et al. Significantly Altered Serum Levels of NAD, AGE, RAGE, CRP, and Elastin as Potential Biomarkers of Psoriasis and Aging—A Case-Control Study. Biomedicines 2022, 10, 1133. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, O.; Ayyangar, U.; Kurbet, A.S.; Ashok, D.; Raghavan, S. Unraveling the ECM-Immune Cell Crosstalk in Skin Diseases. Front. Cell Dev. Biol. 2019, 7, 447867. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Strafella, C.; Termine, A.; Campione, E.; Bianchi, L.; Novelli, G.; Giardina, E.; Cascella, R. RNAseq-Based Prioritization Revealed COL6A5, COL8A1, COL10A1 and MIR146A as Common and Differential Susceptibility Biomarkers for Psoriasis and Psoriatic Arthritis: Confirmation from Genotyping Analysis of 1417 Italian Subjects. Int. J. Mol. Sci. 2020, 21, 2740. [Google Scholar] [CrossRef]
- Starodubtseva, N.L.; Sobolev, V.V.; Soboleva, A.G.; Nikolaev, A.A.; Bruskin, S.A. Expression of Genes for Metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) Associated with Psoriasis. Genetika 2011, 47, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Sun, S.; Bay-Jensen, A.C.; Karsdal, M.; Sørensen, I.J.; Weber, U.; Loft, A.G.; Kollerup, G.; Thamsborg, G.; Madsen, O.R.; et al. Levels of Extracellular Matrix Metabolites Are Associated with Changes in Ankylosing Spondylitis Disease Activity Score and MRI Inflammation Scores in Patients with Axial Spondyloarthritis during TNF Inhibitor Therapy. Arthritis Res. Ther. 2022, 24, 279. [Google Scholar] [CrossRef] [PubMed]
- Port, H.; Holm Nielsen, S.; Frederiksen, P.; Madsen, S.F.; Bay-Jensen, A.C.; Sørensen, I.J.; Jensen, B.; Loft, A.G.; Madsen, O.R.; Østergaard, M.; et al. Extracellular Matrix Turnover Biomarkers Reflect Pharmacodynamic Effects and Treatment Response of Adalimumab in Patients with Axial Spondyloarthritis—Results from Two Randomized Controlled Trials. Arthritis Res. Ther. 2023, 25, 157. [Google Scholar] [CrossRef] [PubMed]
- Gudmann, N.S.; Manon-Jensen, T.; Sand, J.M.B.; Diefenbach, C.; Sun, S.; Danielsen, A.; Karsdal, M.A.; Leeming, D.J. Lung Tissue Destruction by Proteinase 3 and Cathepsin G Mediated Elastin Degradation Is Elevated in Chronic Obstructive Pulmonary Disease. Biochem. Biophys. Res. Commun. 2018, 503, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; Sinkeviciute, D.; Manon-Jensen, T.; Domislović, V.; Mccall, K.; Thudium, C.S.; Brinar, M.; Önnerfjord, P.; Goodyear, C.S.; Krznarić, Z.; et al. A Specific Calprotectin Neo-Epitope [CPa9-HNE] in Serum from Inflammatory Bowel Disease Patients Is Associated with Neutrophil Activity and Endoscopic Severity. J. Crohn’s Colitis 2022, 16, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Barascuk, N.; Veidal, S.S.; Larsen, L.; Larsen, D.V.; Larsen, M.R.; Wang, J.; Zheng, Q.; Xing, R.; Cao, Y.; Rasmussen, L.M.; et al. A Novel Assay for Extracellular Matrix Remodeling Associated with Liver Fibrosis: An Enzyme-Linked Immunosorbent Assay (ELISA) for a MMP-9 Proteolytically Revealed Neo-Epitope of Type III Collagen. Clin. Biochem. 2010, 43, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Veidal, S.S.; Karsdal, M.A.; Nawrocki, A.; Larsen, M.R.; Dai, Y.; Zheng, Q.; Hägglund, P.; Vainer, B.; Skjøt-Arkil, H.; Leeming, D.J. Assessment of Proteolytic Degradation of the Basement Membrane: A Fragment of Type IV Collagen as a Biochemical Marker for Liver Fibrosis. Fibrogenesis Tissue Repair 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Veidal, S.S.; Karsdal, M.A.; Vassiliadis, E.; Nawrocki, A.; Larsen, M.R.; Nguyen, Q.H.T.; Hägglund, P.; Luo, Y.; Zheng, Q.; Vainer, B.; et al. MMP Mediated Degradation of Type VI Collagen Is Highly Associated with Liver Fibrosis—Identification and Validation of a Novel Biochemical Marker Assay. PLoS ONE 2011, 6, e24753. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The Neo-Epitope Specific PRO-C3 ELISA Measures True Formation of Type III Collagen Associated with Liver and Muscle Parameters. Am. J. Transl. Res. 2013, 5, 303. [Google Scholar]
- Frimodt-Møller, M.; Hansen, T.W.; Rasmussen, D.G.K.; Theilade, S.; Nielsen, S.H.; Karsdal, M.A.; Genovese, F.; Rossing, P. A Marker of Type VI Collagen Formation (PRO-C6) Is Associated with Higher Arterial Stiffness in Type 1 Diabetes. Acta Diabetol. 2019, 56, 711–712. [Google Scholar] [CrossRef]
- Holtsche, M.M.; Van Beek, N.; Hashimoto, T.; Di Zenzo, G.; Zillikens, D.; Prost-Squarcioni, C.; Titeux, M.; Hovnanian, A.; Schmidt, E.; Goletz, S. Diagnosis of Epidermolysis Bullosa Acquisita: Multicentre Comparison of Different Assays for Serum Anti-Type VII Collagen Reactivity. Acta Derm.-Venereol. 2021, 101, adv00420. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Nielsen, M.J.; Dai, Y.; Veidal, S.S.; Vassiliadis, E.; Zhang, C.; He, Y.; Vainer, B.; Zheng, Q.; Karsdal, M.A. Enzyme-Linked Immunosorbent Serum Assay Specific for the 7S Domain of Collagen Type IV (P4NP 7S): A Marker Related to the Extracellular Matrix Remodeling during Liver Fibrogenesis. Hepatol. Res. 2012, 42, 482–493. [Google Scholar] [CrossRef]
- Crespo-Bravo, M.; Thorlacius-Ussing, J.; Nissen, N.I.; Pedersen, R.S.; Boisen, M.K.; Liljefors, M.; Johansen, A.Z.; Johansen, J.S.; Karsdal, M.A.; Willumsen, N. Levels of Type XVII Collagen (BP180) Ectodomain Are Elevated in Circulation from Patients with Multiple Cancer Types and Is Prognostic for Patients with Metastatic Colorectal Cancer. BMC Cancer 2023, 23, 949. [Google Scholar] [CrossRef]
- Madsen, E.A.; Thorlacius-Ussing, J.; Nissen, N.I.; Jensen, C.; Chen, I.M.; Johansen, J.S.; Diab, H.M.H.; Jørgensen, L.N.; Hansen, C.P.; Karsdal, M.A.; et al. Type XXII Collagen Complements Fibrillar Collagens in the Serological Assessment of Tumor Fibrosis and the Outcome in Pancreatic Cancer. Cells 2022, 11, 3763. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Sardar, S.; Siebuhr, A.S.; Schlemmer, A.; Schmidt, E.B.; Bay-Jensen, A.C.; Karsdal, M.A.; Christensen, J.H.; Kristensen, S. Effect of N-3 PUFA on Extracellular Matrix Protein Turnover in Patients with Psoriatic Arthritis: A Randomized, Double-Blind, Placebo-Controlled Trial. Rheumatol. Int. 2021, 41, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Raaby, L.; Zachariae, C.; Østensen, M.; Heickendorff, L.; Thielsen, P.; Grønbæk, H.; Skov, L.; Kyvsgaard, N.; Madsen, J.T.; Heidenheim, M.; et al. Methotrexate Use and Monitoring in Patients with Psoriasis: A Consensus Report Based on a Danish Expert Meeting. Acta Derm.-Venereol. 2017, 97, 426–432. [Google Scholar] [CrossRef]
- Lee, J.H.M.; Loo, C.H.; Tan, W.C.; Lee, C.K.; Jamil, A.; Khor, Y.H. Comparison of Noninvasive Screening Tools for Hepatic Fibrosis, Association with Methotrexate Cumulative Dose, and Risk Factors in Psoriasis Patients. Dermatol. Ther. 2022, 35, e15203. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, H.; Muhammad Aslam, H.; Bjerring, P.; Søgaard, H.; Zachariae, E.; Heickendorff, L. Serum Aminoterminal Propeptide of Type III Procollagen in Psoriasis and Psoriatic Arthritis: Relation to Liver Fibrosis and Arthritis. J. Am. Acad. Dermatol. 1991, 25, 50–53. [Google Scholar] [CrossRef]
- Juhl, P.; Vinderslev Iversen, L.; Karlsmark, T.; Asser Karsdal, M.; Bay-Jensen, A.C.; Mogensen, M.; Siebuhr, A.S. Association of Metabolites Reflecting Type III and VI Collagen Formation with Modified Rodnan Skin Score in Systemic Sclerosis—A Cross-Sectional Study. Biomarkers 2019, 24, 373–378. [Google Scholar] [CrossRef]
- Dobrota, R.; Jordan, S.; Juhl, P.; Maurer, B.; Wildi, L.; Bay-Jensen, A.C.; Karsdal, M.A.; Herrick, A.L.; Distler, J.H.W.; Allanore, Y.; et al. Circulating Collagen Neo-Epitopes and Their Role in the Prediction of Fibrosis in Patients with Systemic Sclerosis: A Multicentre Cohort Study. Lancet Rheumatol. 2021, 3, e175–e184. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Z.; Wei, Y.; Zhang, R.; Deng, Y.; Li, D. The Role of Dermal Fibroblasts in Autoimmune Skin Diseases. Front. Immunol. 2024, 15, 1379490. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shao, X.; Liu, W.; Wang, M.; Li, Q.; Wang, M.; Xiao, Y.; Li, K.; Liang, H.; Wang, N.; et al. The Mechano-Chemical Circuit in Fibroblasts and Dendritic Cells Drives Basal Cell Proliferation in Psoriasis. Cell Rep. 2024, 43, 114513. [Google Scholar] [CrossRef] [PubMed]
- van Haaften, W.T.; Mortensen, J.H.; Dige, A.K.; Grønbæk, H.; Hvas, C.L.; Bay-Jensen, A.C.; Karsdal, M.A.; Olinga, P.; Manon-Jensen, T.; Dijkstra, G. Serological Biomarkers of Tissue Turnover Identify Responders to Anti-TNF Therapy in Crohn’s Disease: A Pilot Study. Clin. Transl. Gastroenterol. 2020, 11, e00217. [Google Scholar] [CrossRef] [PubMed]
- Domislovic, V.; Høg Mortensen, J.; Lindholm, M.; Kaarsdal, M.A.; Brinar, M.; Barisic, A.; Manon-Jensen, T.; Krznaric, Z. Inflammatory Biomarkers of Extracellular Matrix Remodeling and Disease Activity in Crohn’s Disease and Ulcerative Colitis. J. Clin. Med. 2022, 11, 5907. [Google Scholar] [CrossRef] [PubMed]
- Lønsmann, I.; Grove, J.I.; Haider, A.; Kaye, P.; Karsdal, M.A.; Leeming, D.J.; Aithal, G.P. Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL). Biology 2023, 12, 1087. [Google Scholar] [CrossRef]
- Nedergaard, A.; Karsdal, M.A.; Sun, S.; Henriksen, K. Serological Muscle Loss Biomarkers: An Overview of Current Concepts and Future Possibilities. J. Cachexia Sarcopenia Muscle 2013, 4, 1–17. [Google Scholar] [CrossRef]
- Torp, N.; Israelsen, M.; Nielsen, M.J.; Åstrand, C.P.; Juhl, P.; Johansen, S.; Hansen, C.D.; Madsen, B.; Villesen, I.F.; Leeming, D.J.; et al. Binge Drinking Induces an Acute Burst of Markers of Hepatic Fibrogenesis (PRO-C3). Liver Int. 2022, 42, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Erhardtsen, E.; Rasmussen, D.G.K.; Frederiksen, P.; Leeming, D.J.; Shevell, D.; Gluud, L.L.; Karsdal, M.A.; Aithal, G.P.; Schattenberg, J.M. Determining a Healthy Reference Range and Factors Potentially Influencing PRO-C3—A Biomarker of Liver Fibrosis. JHEP Rep. 2021, 3, 100317. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.G.; Simpson, J.K.; Saini, G.; Bentley, J.H.; Russell, A.M.; Braybrooke, R.; Molyneaux, P.L.; McKeever, T.M.; Wells, A.U.; Flynn, A.; et al. Longitudinal Change in Collagen Degradation Biomarkers in Idiopathic Pulmonary Fibrosis: An Analysis from the Prospective, Multicentre PROFILE Study. Lancet Respir. Med. 2015, 3, 462–472. [Google Scholar] [CrossRef]
- Breisnes, H.W.; Leeming, D.J.; Karsdal, M.A.; Burke, H.; Freeman, A.; Wilkinson, T.; Fazleen, A.; Bülow Sand, J.M. Biomarkers of Tissue Remodelling Are Elevated in Serum of COVID-19 Patients Who Develop Interstitial Lung Disease—An Exploratory Biomarker Study. BMC Pulm. Med. 2024, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Groen, S.S.; Yao, Y.; Jørgensen, A.H.R.; Nielsen, V.W.; Karsdal, M.; Gehring, K.; Bay-Jensen, A.C.; Thomsen, S.F. Biomarkers of Tissue Turnover and Systemic Inflammation Are Associated with Disease Severity and Activity in Patients with Hidradenitis Suppurativa. J. Investig. Dermatol. 2023, 143, 328–331.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Jin, H.Z. Role of Neutrophils in Psoriasis. J. Immunol. Res. 2020, 2020, 3709749. [Google Scholar] [CrossRef] [PubMed]
- Todberg, T.; Egeberg, A.; Zachariae, C.; Sørensen, N.; Pedersen, O.; Skov, L. Patients with Psoriasis Have a Dysbiotic Taxonomic and Functional Gut Microbiota. Br. J. Dermatol. 2022, 187, 89–98. [Google Scholar] [CrossRef]
- Gineyts, E.; Millet, M.; Borel, O.; Coutant, F.; Rousseau, J.C.; Chapurlat, R.; Marotte, H.; Garnero, P. Serum Col3-4: A New Type III and IV Collagen Biochemical Marker of Synovial Tissue Turnover in Patients with Rheumatoid Arthritis. PLoS ONE 2023, 18, e0282954. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Henriksen, K.; Karsdal, M.A.; Byrjalsen, I.; Rittweger, J.; Armbrecht, G.; Belavy, D.L.; Felsenberg, D.; Nedergaard, A.F. Collagen Type III and VI Turnover in Response to Long-Term Immobilization. PLoS ONE 2015, 10, e0144525. [Google Scholar] [CrossRef]
- Todberg, T.; Egeberg, A.; Zachariae, C.; Sørensen, N.; Pedersen, O.; Skov, L. Successful Treatment of Psoriasis with Adalimumab Induced No Changes in the Gut Microbiota. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e464–e467. [Google Scholar] [CrossRef]

| Variables | Patients with PSO (n = 59) | PSO After Treatment (n = 9) | Healthy Controls (n = 52) |
|---|---|---|---|
| Age, years, mean (SD) | 47 (±16) | 36 (±12) | 46 (±14) |
| Men, n (%) | 33 (56%) | 5 (56%) | 28 (54%) |
| Age at diagnosis, years, mean (SD) | 24 (±16) | 17 (±10) | - |
| Family history, n (%) | |||
| Yes | 33 (56%) | 5 (56%) | - |
| No | 23 (39%) | 2 (22%) | - |
| Unknown | 4 (7%) | 2 (22%) | - |
| PASI all patients, median (min-max) | 10.0 (8.0–36.8) | - | - |
| PASI before treatment, median (min-max) | - | 10.2 (8.1–21.4) | - |
| PASI after treatment, median (min-max) | - | 0.0 (0.0–2.0) | - |
| Psoriatic arthritis (PsA), n (%) | 9 (15%) | 1 (11%) | - |
| Age at diagnosis, years, median (min-max) | 31 (16–42) | - | - |
| Abbreviation | Description | Function | Reference |
|---|---|---|---|
| ELP-3 | Neo-epitope of proteinase-3 mediated degradation of elastin | Degradation of elastin | [24] |
| C4G | Fragment of type IV collagen released by granzyme-B | T-cell migration through the basement membrane by the degradation of type IV collagen | [14] |
| CPa9-HNE | Fragment of S100A9 (calprotectin) released by neutrophil elastase | Neutrophil activity and neutrophil extracellular trap formation (NETosis) | [25] |
| C3M | Fragment of type III collagen released by MMP-9 | ECM degradation | [26] |
| C4M | Fragment of type IV collagen released by MMP (multiple) | Basement membrane degradation and ECM degradation | [27] |
| C6M | Fragment of type VIa1 collagen released by MMP-2/9 | Interface matrix degradation and fibrogenesis | [28] |
| PRO-C3 | Fragment of N-terminal type III collagen | Fibrogenesis and ECM formation | [29] |
| PRO-C4 | Fragment of the internal 7S domain of type IV collagen | Basement membrane synthesis and ECM formation | [32] |
| PRO-C6 | Fragment of C-terminal type VIa3 collagen (Endotrophin) | Pro-fibrotic signalling and ECM formation | [30] |
| PRO-C7 | Fragment of C-terminal type VII collagen | ECM formation | [31] |
| PRO-C17 | Fragment of C-terminal type XVII collagen | ECM formation and epithelial damage | [33] |
| PRO-C22 | C-terminal of type XXII collagen, neoepitope-specific | Connective tissue formation | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansen, M.B.; Nielsen, S.H.; Port, H.; Todberg, T.; Løvendorf, M.B.; Skov, L. Biomarkers of Extracellular Matrix Fragments in Patients with Psoriasis. Int. J. Mol. Sci. 2025, 26, 261. https://doi.org/10.3390/ijms26010261
Johansen MB, Nielsen SH, Port H, Todberg T, Løvendorf MB, Skov L. Biomarkers of Extracellular Matrix Fragments in Patients with Psoriasis. International Journal of Molecular Sciences. 2025; 26(1):261. https://doi.org/10.3390/ijms26010261
Chicago/Turabian StyleJohansen, Mila Broby, Signe Holm Nielsen, Helena Port, Tanja Todberg, Marianne Bengtson Løvendorf, and Lone Skov. 2025. "Biomarkers of Extracellular Matrix Fragments in Patients with Psoriasis" International Journal of Molecular Sciences 26, no. 1: 261. https://doi.org/10.3390/ijms26010261
APA StyleJohansen, M. B., Nielsen, S. H., Port, H., Todberg, T., Løvendorf, M. B., & Skov, L. (2025). Biomarkers of Extracellular Matrix Fragments in Patients with Psoriasis. International Journal of Molecular Sciences, 26(1), 261. https://doi.org/10.3390/ijms26010261







