Long-Term 5-HT1A Receptor Agonist NLX-112 Treatment Improves Functional Recovery After Spinal Cord Injury
Abstract
1. Introduction
2. Results
2.1. Two-Week Delayed NLX-112 Treatment Significantly Improves Locomotion After SCI in a Dose-Dependent Manner
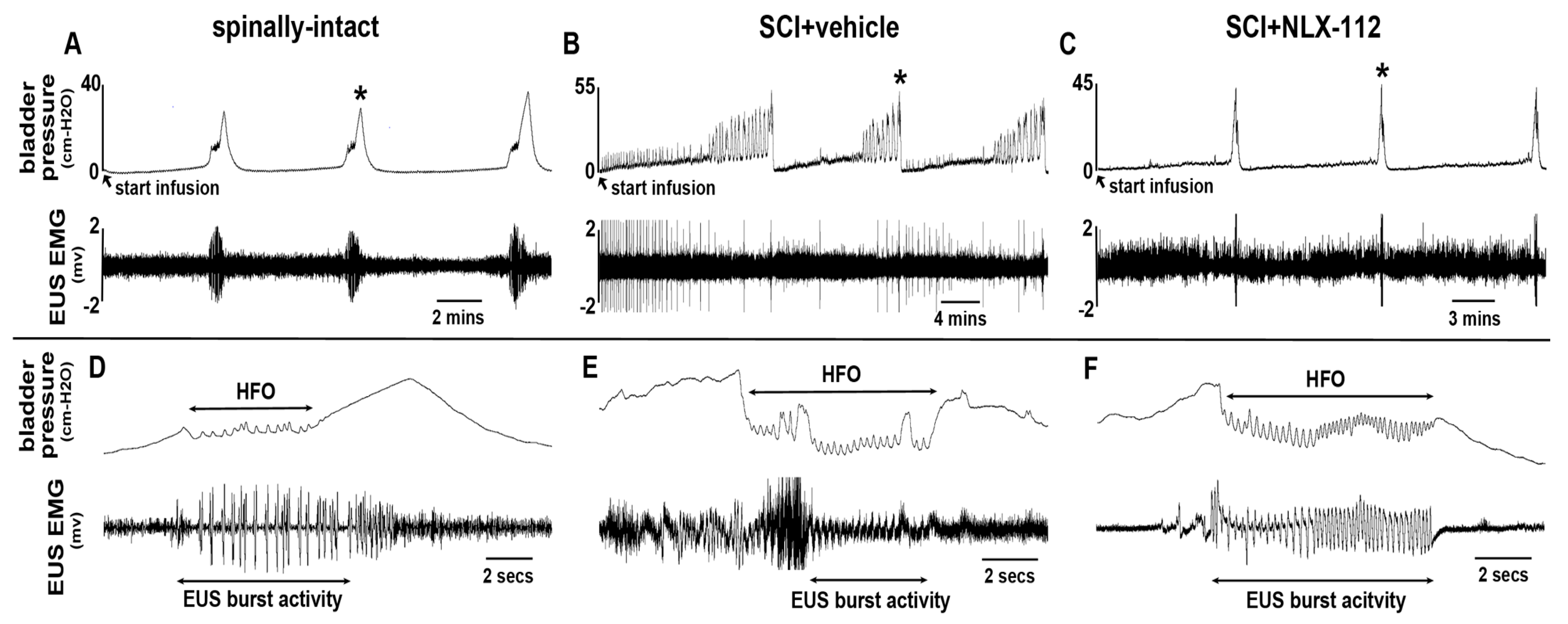
2.2. NLX-112 Treatment Significantly Improves LUT Function at 8 Weeks After SCI
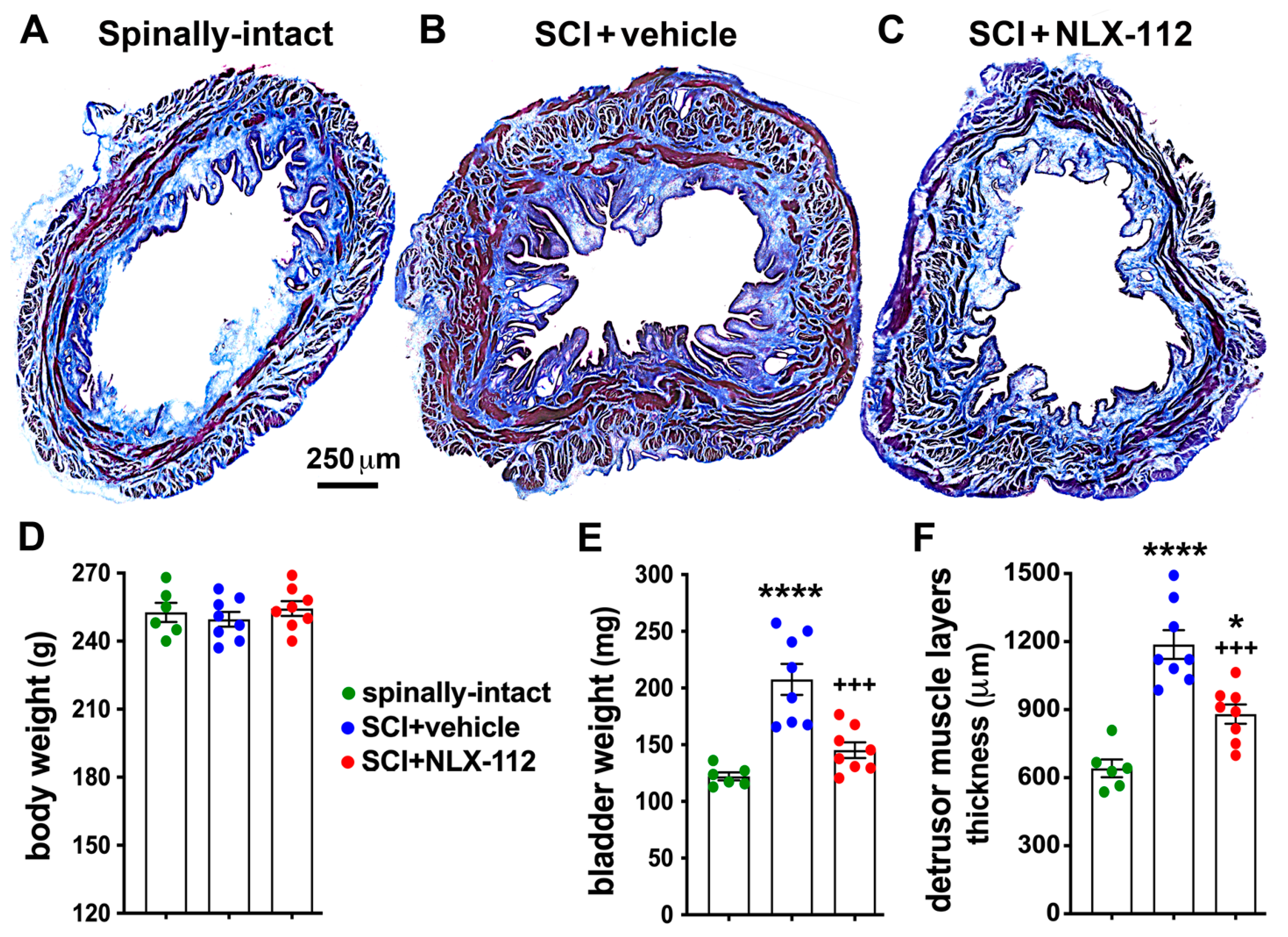
2.3. NLX-112 Treatment Significantly Improves Bladder Morphology After SCI
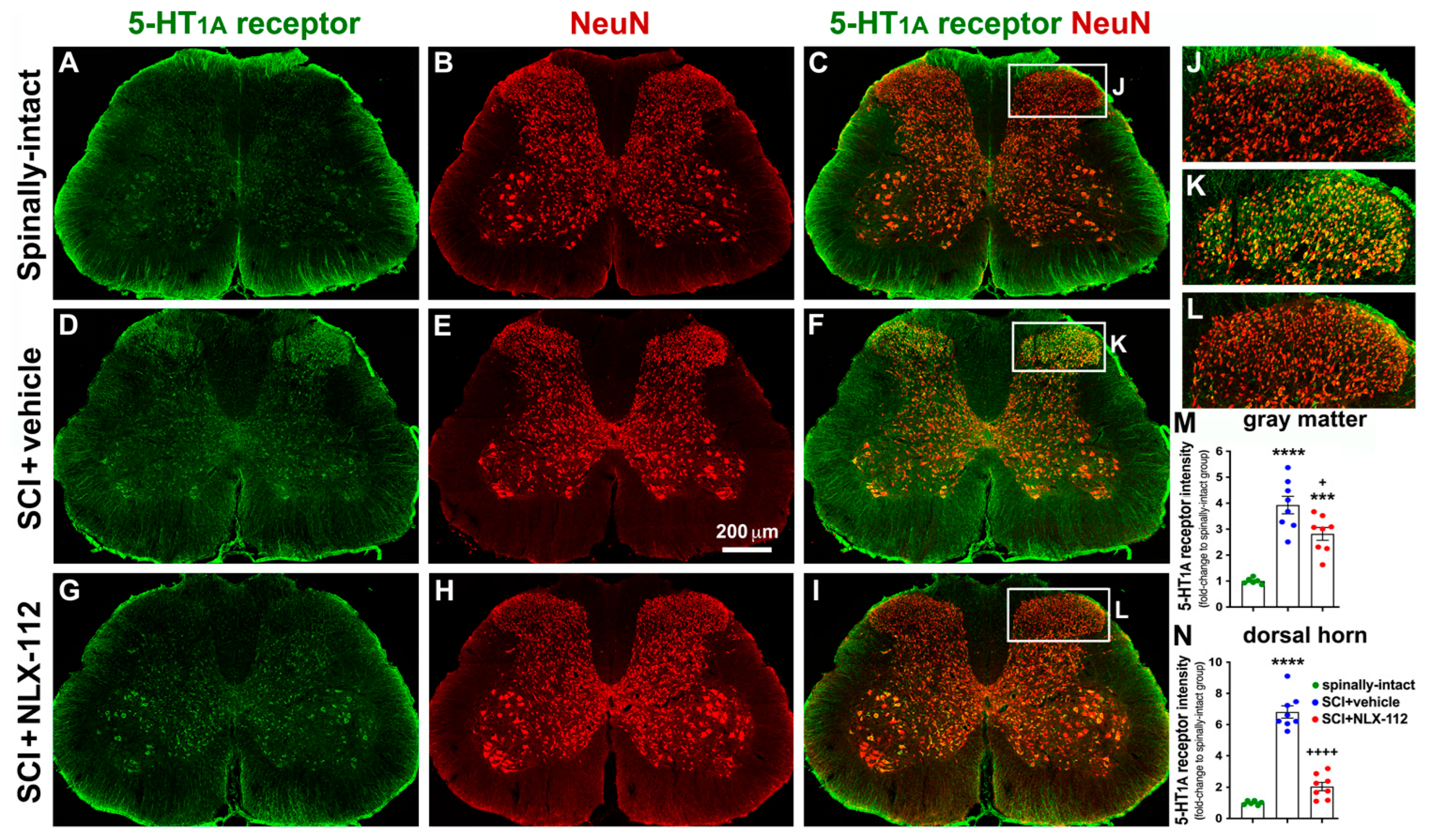
2.4. NLX-112 Treatment Significantly Decreases SCI-Upregulated Spinal 5-HT1A Receptors
3. Discussion
4. Materials and Methods
4.1. Animal Groups and Experimental Design
4.2. T8 Contusive SCI Surgery
4.3. The BBB Open Field Locomotion Test to Assess Hindlimb Locomotion
4.4. Awake Urodynamics and EUS EMG Recording
4.5. Quantification of CMG and EUS EMG Patterns
4.6. Analyses of Bladder Morphology
4.7. Immunohistochemistry for 5-HT1A Receptors and Analyses of Immunoreactivity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Adriaansen, J.J.; van Asbeck, F.W.A.; Tepper, M.; Faber, W.X.; Visser-Meily, J.M.A.; de Kort, L.M.O.; Post, M.W.M. Bladder-emptying methods, neurogenic lower urinary tract dysfunction and impact on quality of life in people with long-term spinal cord injury. J. Spinal Cord. Med. 2017, 40, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Elliott, S.; Noonan, V.K.; Thorogood, N.P.; Fallah, N.; Aludino, A.; Dvorak, M.F. Impact of bladder, bowel and sexual dysfunction on health status of people with thoracolumbar spinal cord injuries living in the community. J. Spinal Cord. Med. 2017, 40, 548–559. [Google Scholar] [CrossRef]
- Dolber, P.C.; Gu, B.; Zhang, X.; Fraser, M.O.; Thor, K.B.; Reiter, J.P. Activation of the external urethral sphincter central pattern generator by a 5-HT(1A) receptor agonist in rats with chronic spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1699–R1706. [Google Scholar] [CrossRef]
- Chang, H.Y.; Cheng, C.L.; Chen, J.J.; de Groat, W.C. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F1044–F1053. [Google Scholar] [CrossRef]
- Cheng, C.L.; de Groat, W.C. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. Am. J. Physiol. Ren. Physiol. 2010, 298, F771–F778. [Google Scholar] [CrossRef]
- Tai, C.; Miscik, C.L.; Ungerer, T.D.; Roppolo, J.R.; de Groat, W.C. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp. Neurol. 2006, 199, 427–437. [Google Scholar] [CrossRef]
- Peroutka, S.J. Selective interaction of novel anxiolytics with 5-hydroxytryptamine1A receptors. Biol. Psychiatry 1985, 20, 971–979. [Google Scholar] [CrossRef]
- Wu, W.-P.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; Koek, W.; Colpaert, F.C. The very-high-efficacy 5-HT1A receptor agonist, F 13640, preempts the development of allodynia-like behaviors in rats with spinal cord injury. Eur. J. Pharmacol. 2003, 478, 131–137. [Google Scholar] [CrossRef]
- Colpaert, F.C.; Wu, W.-P.; Hao, J.-X.; Royer, I.; Sautel, F.; Wiesenfeld-Hallin, Z.; Xu, X.-J. High-efficacy 5-HT1A receptor activation causes a curative-like action on allodynia in rats with spinal cord injury. Eur. J. Pharmacol. 2004, 497, 29–33. [Google Scholar] [CrossRef]
- Hains, B.C.; Willis, W.D.; Hulsebosch, C.E. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp. Brain Res. 2003, 149, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Antri, M.; Orsal, D.; Barthe, J.Y. Locomotor recovery in the chronic spinal rat: Effects of long-term treatment with a 5-HT2 agonist. Eur. J. Neurosci. 2002, 16, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Antri, M.; Mouffle, C.; Orsal, D.; Barthe, J.Y. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 2003, 18, 1963–1972. [Google Scholar] [CrossRef]
- Ganzer, P.D.; Beringer, C.R.; Shumsky, J.S.; Nwaobasi, C.; Moxon, K.A. Serotonin receptor and dendritic plasticity in the spinal cord mediated by chronic serotonergic pharmacotherapy combined with exercise following complete SCI in the adult rat. Exp. Neurol. 2018, 304, 132–142. [Google Scholar] [CrossRef]
- Lin, C.Y.; Sparks, A.; Lee, Y.S. Improvement of lower urinary tract function by a selective serotonin 5-HT1A receptor agonist, NLX-112, after chronic spinal cord injury. Exp. Neurol. 2020, 332, 113395. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front. Neural Circuits 2014, 8, 151. [Google Scholar] [CrossRef]
- Slawinska, U.; Miazga, K.; Jordan, L.M. The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol. Exp. 2014, 74, 172–187. [Google Scholar] [CrossRef]
- Antri, M.; Barthe, J.Y.; Mouffle, C.; Orsal, D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci. Lett. 2005, 384, 162–167. [Google Scholar] [CrossRef]
- Freyvert, Y.; Yong, N.A.; Morikawa, E.; Zdunowski, S.; Sarino, M.E.; Gerasimenko, Y.; Edgerton, V.R.; Lu, D.C. Engaging cervical spinal circuitry with non-invasive spinal stimulation and buspirone to restore hand function in chronic motor complete patients. Sci. Rep. 2018, 8, 15546. [Google Scholar] [CrossRef]
- Guertin, P.A.; Ung, R.V.; Rouleau, P. Oral administration of a tri-therapy for central pattern generator activation in paraplegic mice: Proof-of-concept of efficacy. Biotechnol. J. 2010, 5, 421–426. [Google Scholar] [CrossRef]
- Ramage, A.G. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res. Bull. 2001, 56, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.N.; Belton, A.L.; de Groat, W.C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993, 264, R1157–R1163. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C. Anatomy and physiology of the lower urinary tract. Urol. Clin. N. Am. 1993, 20, 383–401. [Google Scholar] [CrossRef]
- De Groat, W.C. Anatomy of the central neural pathways controlling the lower urinary tract. Eur. Urol. 1998, 34 (Suppl. S1), 2–5. [Google Scholar] [CrossRef]
- Ramage, A.G. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br. J. Pharmacol. 2006, 147 (Suppl. S2), S120–S131. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, K.; Thalluri, R.; Lee, Y.S. Upregulated 5-HT(1A) Receptors Regulate Lower Urinary Tract Function in Rats after Complete Spinal Cord Injury. J. Neurotrauma 2023, 40, 845–861. [Google Scholar] [CrossRef]
- Laporte, A.M.; Fattaccini, C.M.; Lombard, M.C.; Chauveau, J.; Hamon, M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J. Neural Transm. Gen. Sect. 1995, 100, 207–223. [Google Scholar] [CrossRef]
- Giroux, N.; Rossignol, S.; Reader, T.A. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J. Comp. Neurol. 1999, 406, 402–414. [Google Scholar] [CrossRef]
- Otoshi, C.K.; Walwyn, W.M.; Tillakaratne, N.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. J. Neurotrauma 2009, 26, 575–584. [Google Scholar] [CrossRef]
- Golder, F.J.; Mitchell, G.S. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J. Neurosci. 2005, 25, 2925–2932. [Google Scholar] [CrossRef]
- Hains, B.C.; Willis, W.D.; Hulsebosch, C.E. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003, 970, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Newman-Tancredi, A.; Bardin, L.; Auclair, A.; Colpaert, F.; Depoortère, R.; Varney, M. NLX-112, a highly selective 5-HT1A receptor agonist, mediates analgesia and antidepressant-like activity in rats via spinal cord and prefrontal cortex 5-HT1A receptors, respectively. Brain Res. 2018, 1688, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Buritova, J.; Tarayre, J.P.; Besson, J.M.; Colpaert, F. The novel analgesic and high-efficacy 5-HT1A receptor agonist, F 13640 induces c-Fos protein expression in spinal cord dorsal horn neurons. Brain Res. 2003, 974, 212–221. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Yoshimura, N. Changes in afferent activity after spinal cord injury. Neurourol. Urodyn. 2010, 29, 63–76. [Google Scholar] [CrossRef]
- Perrin, F.E.; Gerber, Y.N.; Teigell, M.; Lonjon, N.; Boniface, G.; Bauchet, L.; Rodriguez, J.J.; Hugnot, J.P. Anatomical study of serotonergic innervation and 5-HT(1A) receptor in the human spinal cord. Cell Death Dis. 2011, 2, e218. [Google Scholar] [CrossRef]
- Yu, H.; Nagi, S.S.; Usoskin, D.; Hu, Y.; Kupari, J.; Bouchatta, O.; Yan, H.; Cranfill, S.L.; Gautam, M.; Su, Y.; et al. Leveraging deep single-soma RNA sequencing to explore the neural basis of human somatosensation. Nat. Neurosci. 2024, 27, 2326–2340. [Google Scholar] [CrossRef]
- Buritova, J.; Larrue, S.; Aliaga, M.; Besson, J.M.; Colpaert, F. Effects of the high-efficacy 5-HT1A receptor agonist, F 13640 in the formalin pain model: A c-Fos study. Eur. J. Pharmacol. 2005, 514, 121–130. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lin, C.-Y.; Jiang, H.-H.; DePaul, M.; Lin, V.W.; Silver, J. Nerve regeneration restores supraspinal control of bladder function after complete spinal cord injury. J. Neurosci. 2013, 33, 10591–10606. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Li, K.; Gitchell, T.; Lee, Y.-S. Long-Term 5-HT1A Receptor Agonist NLX-112 Treatment Improves Functional Recovery After Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 239. https://doi.org/10.3390/ijms26010239
Lin C-Y, Li K, Gitchell T, Lee Y-S. Long-Term 5-HT1A Receptor Agonist NLX-112 Treatment Improves Functional Recovery After Spinal Cord Injury. International Journal of Molecular Sciences. 2025; 26(1):239. https://doi.org/10.3390/ijms26010239
Chicago/Turabian StyleLin, Ching-Yi, Kevin Li, Thomas Gitchell, and Yu-Shang Lee. 2025. "Long-Term 5-HT1A Receptor Agonist NLX-112 Treatment Improves Functional Recovery After Spinal Cord Injury" International Journal of Molecular Sciences 26, no. 1: 239. https://doi.org/10.3390/ijms26010239
APA StyleLin, C.-Y., Li, K., Gitchell, T., & Lee, Y.-S. (2025). Long-Term 5-HT1A Receptor Agonist NLX-112 Treatment Improves Functional Recovery After Spinal Cord Injury. International Journal of Molecular Sciences, 26(1), 239. https://doi.org/10.3390/ijms26010239




