Adropin/Tirzepatide Combination Mitigates Cardiac Metabolic Aberrations in a Rat Model of Polycystic Ovarian Syndrome, Implicating the Role of the AKT/GSK3β/NF-κB/NLRP3 Pathway
Abstract
1. Introduction
2. Results
2.1. Adropin and/or Tirze Ameliorated the PCOS-Induced Alterations in Blood Glucose, Serum Insulin, HOMA/IR, and Both Hormonal and Lipid Profiles
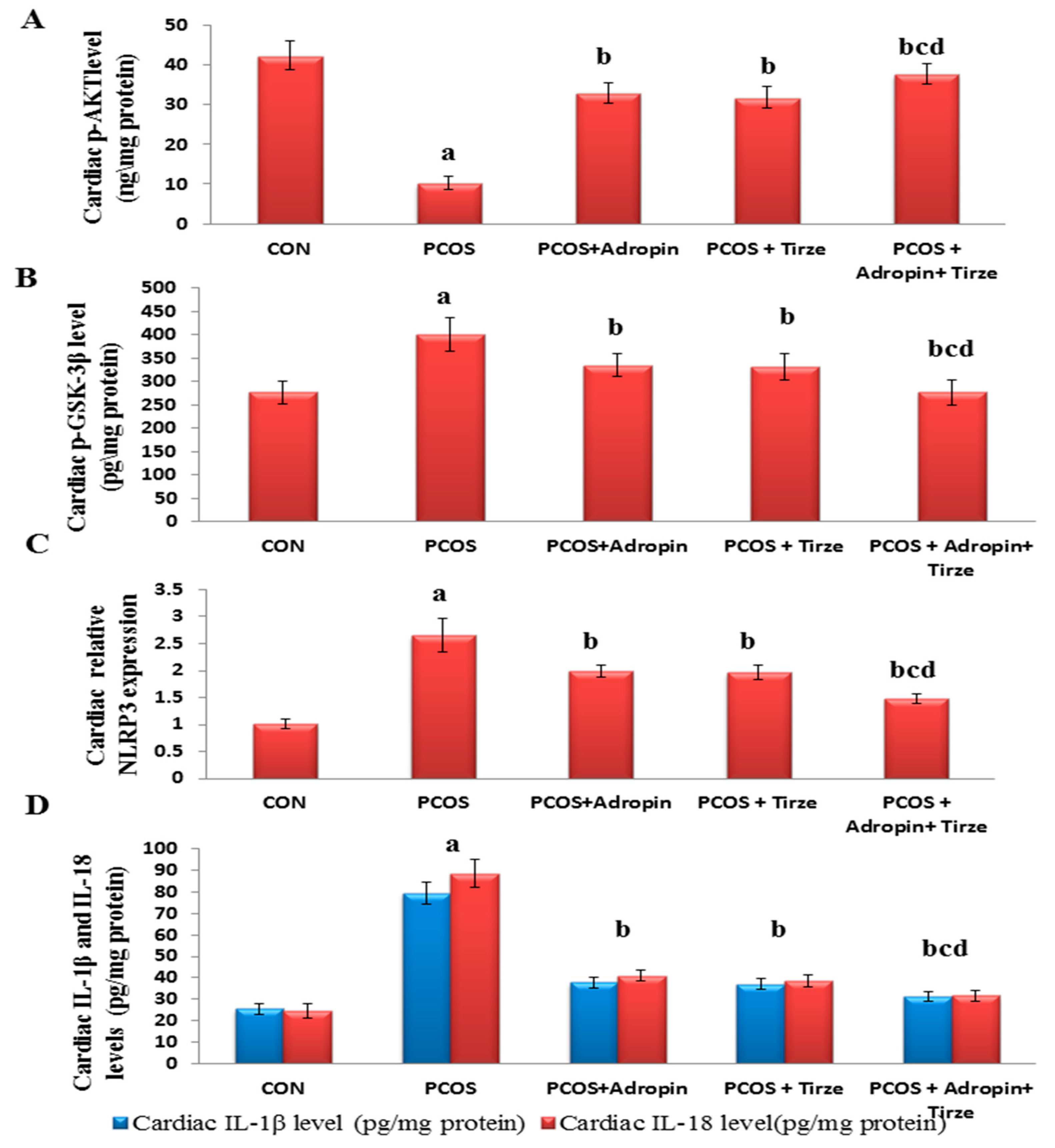
2.2. Adropin and/or Tirze Intervention Reversed the PCOS-Induced Disturbance in the Cardiac AKT/GSK-3β Signaling Pathway
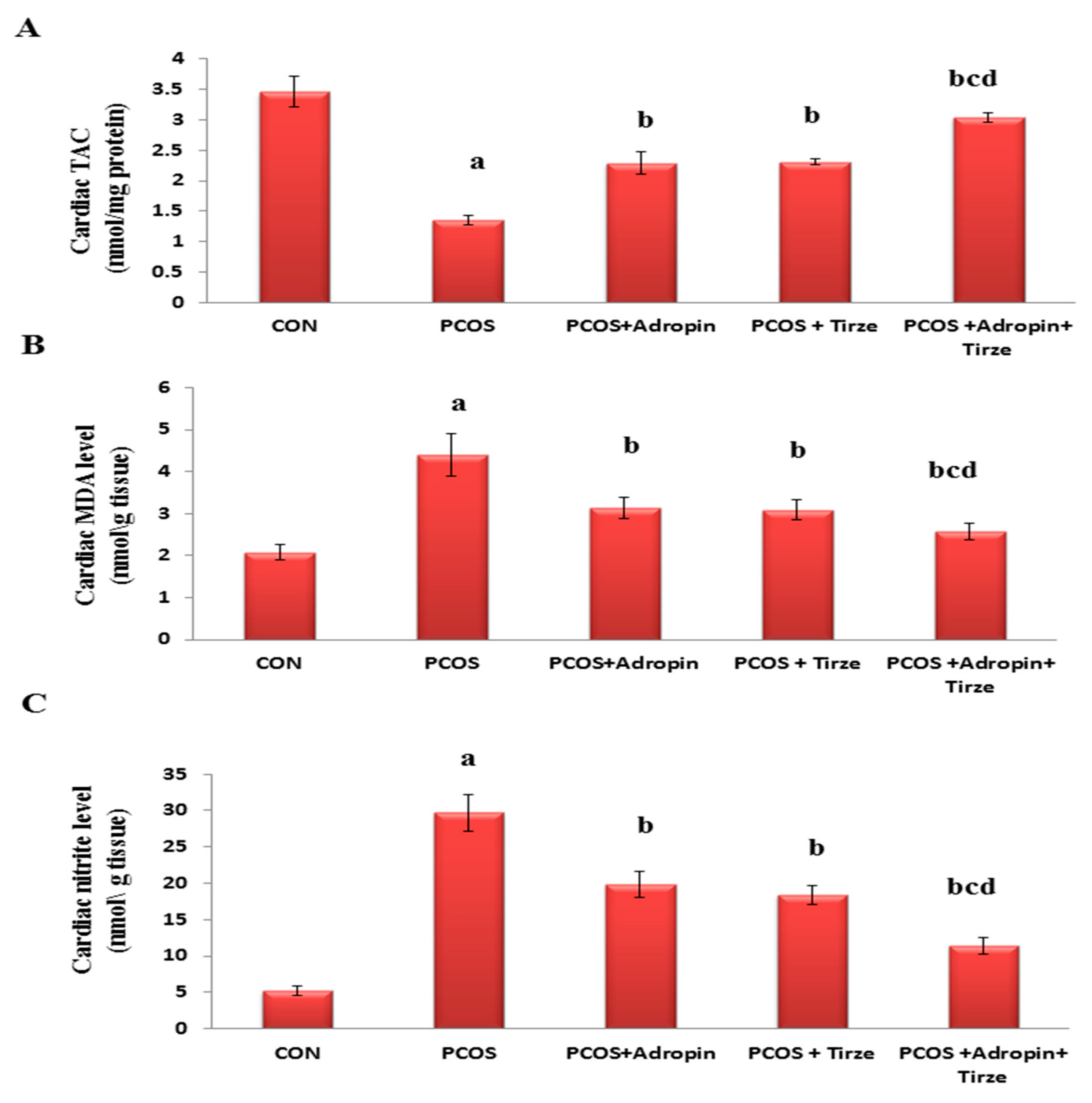
2.3. Impact of Adropin and/or Tirze on the Cardiac Redox State
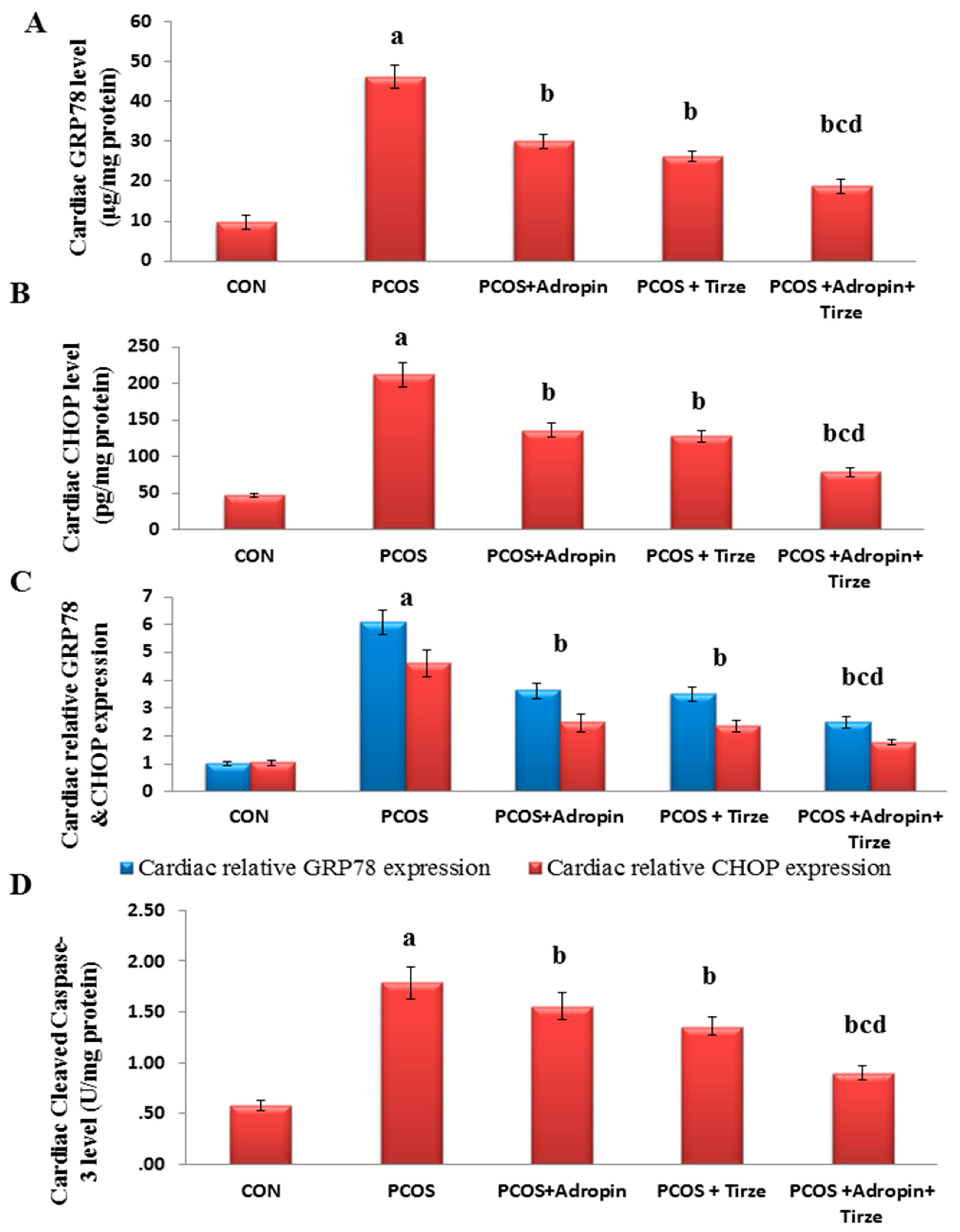
2.4. Impact of Adropin and/or Tirze on the Cardiac ER Stress Markers; GRP78 and CHOP and Apoptotic Marker Cleaved Caspase-3
2.5. Impact of Adropin and/or Tirze on Histological, Immunohistochemical, and Electron Microscopic Results
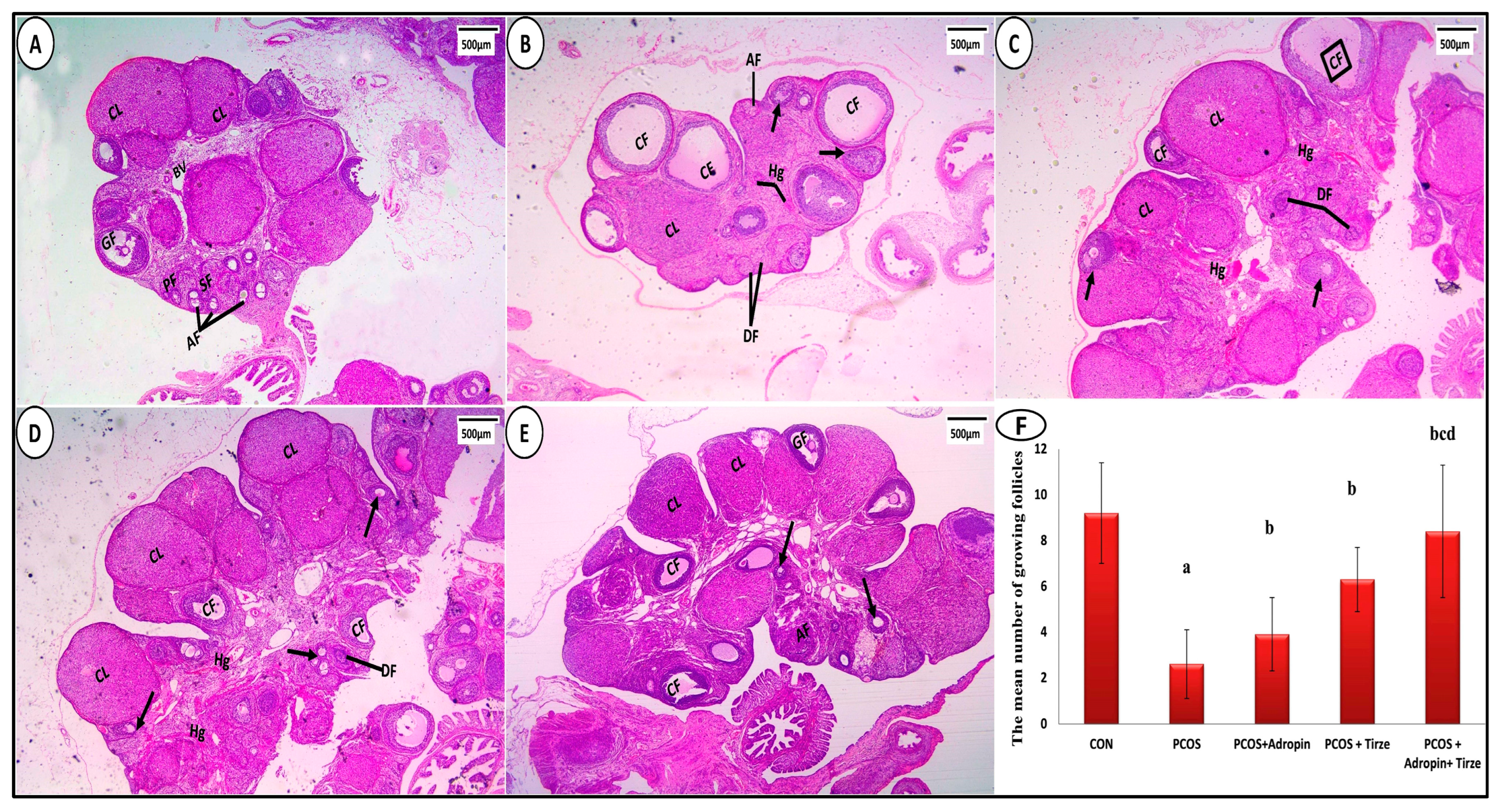
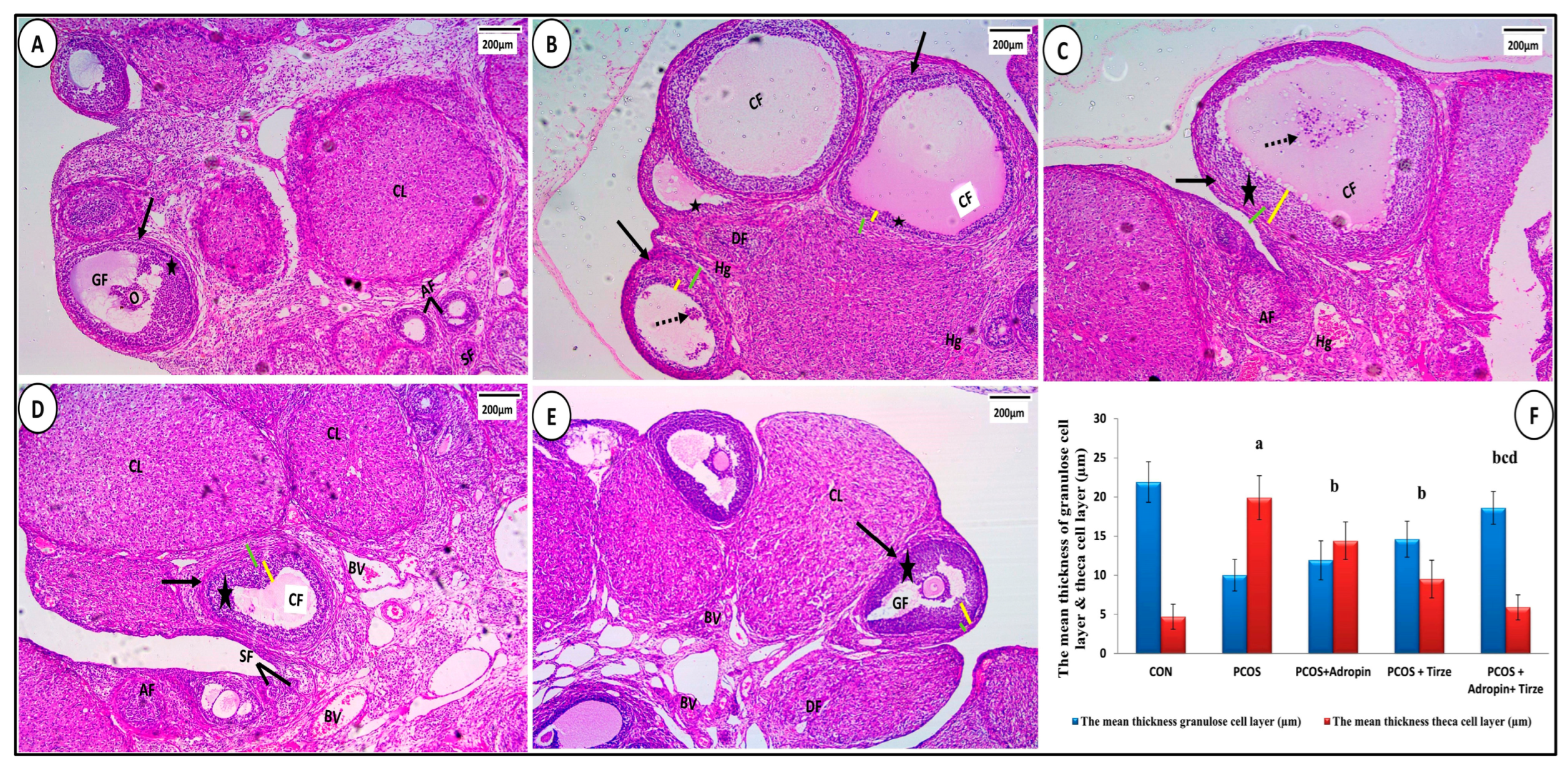
2.5.1. Hematoxylin and Eosin Staining
2.5.2. Statistical Evaluation of Morphometric Measures
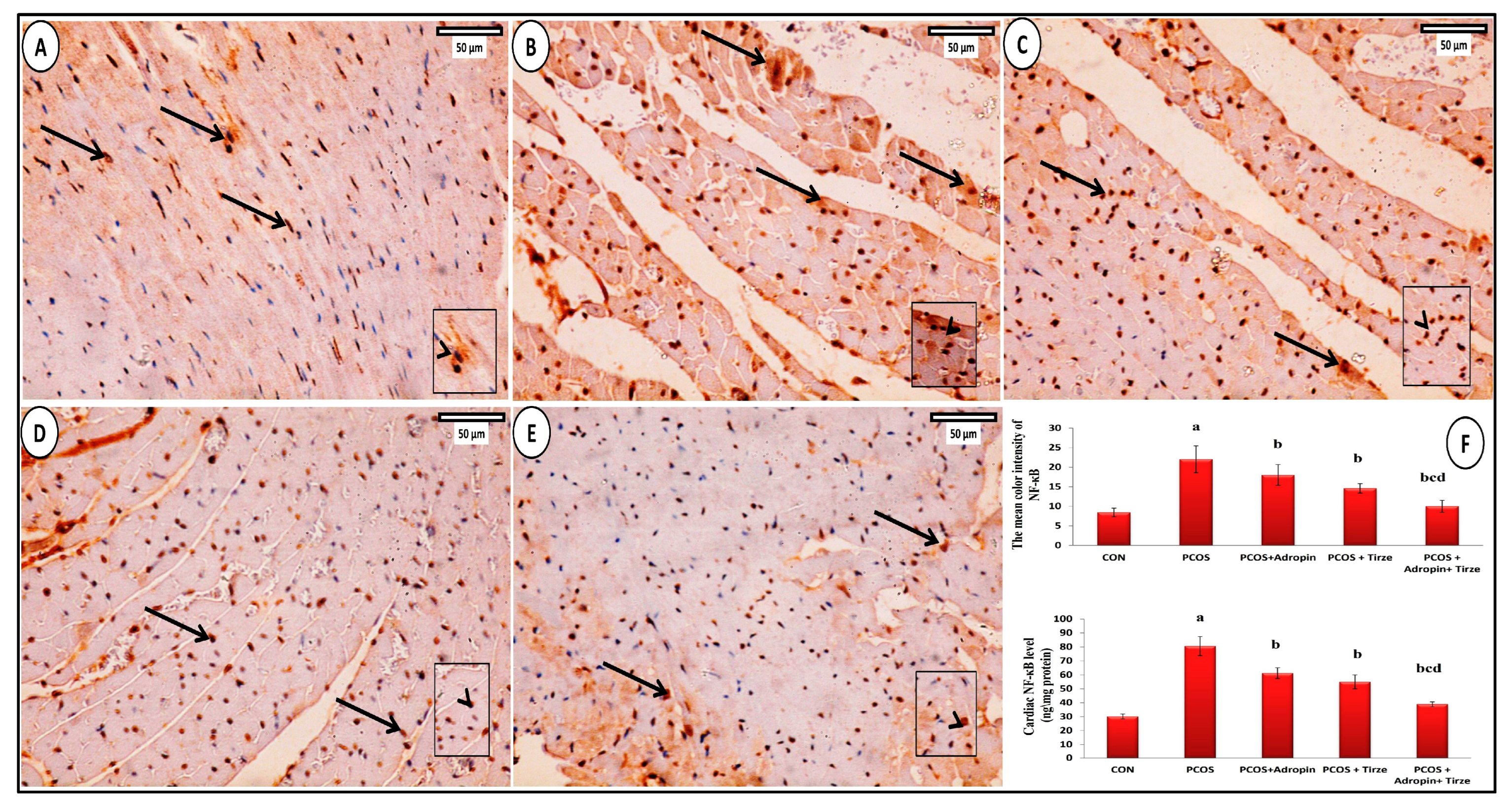
2.6. Immunohistochemical Staining Study
NF-κB Immunohistochemical Staining
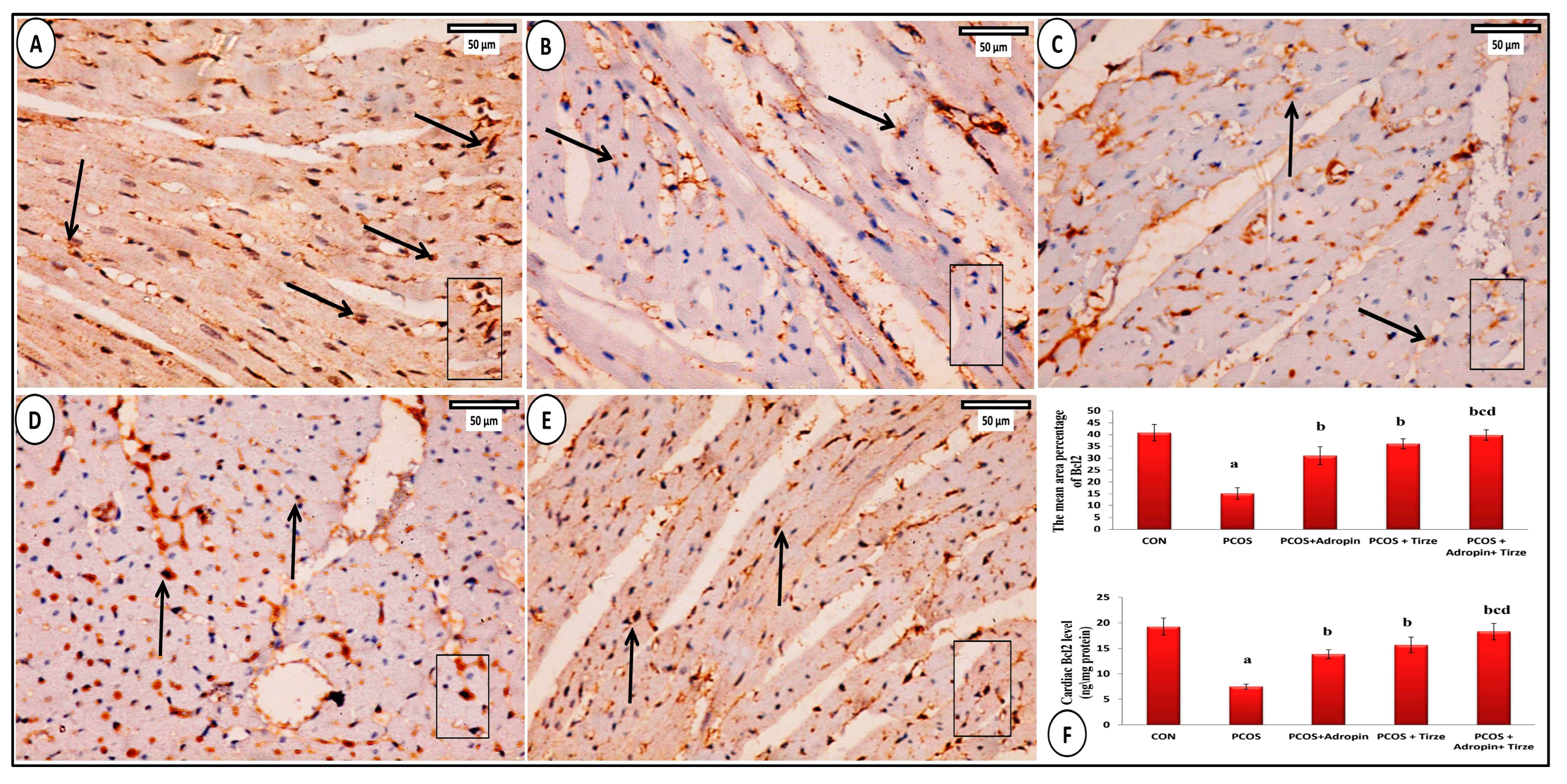
2.7. Bcl2 Immunohistochemical Staining
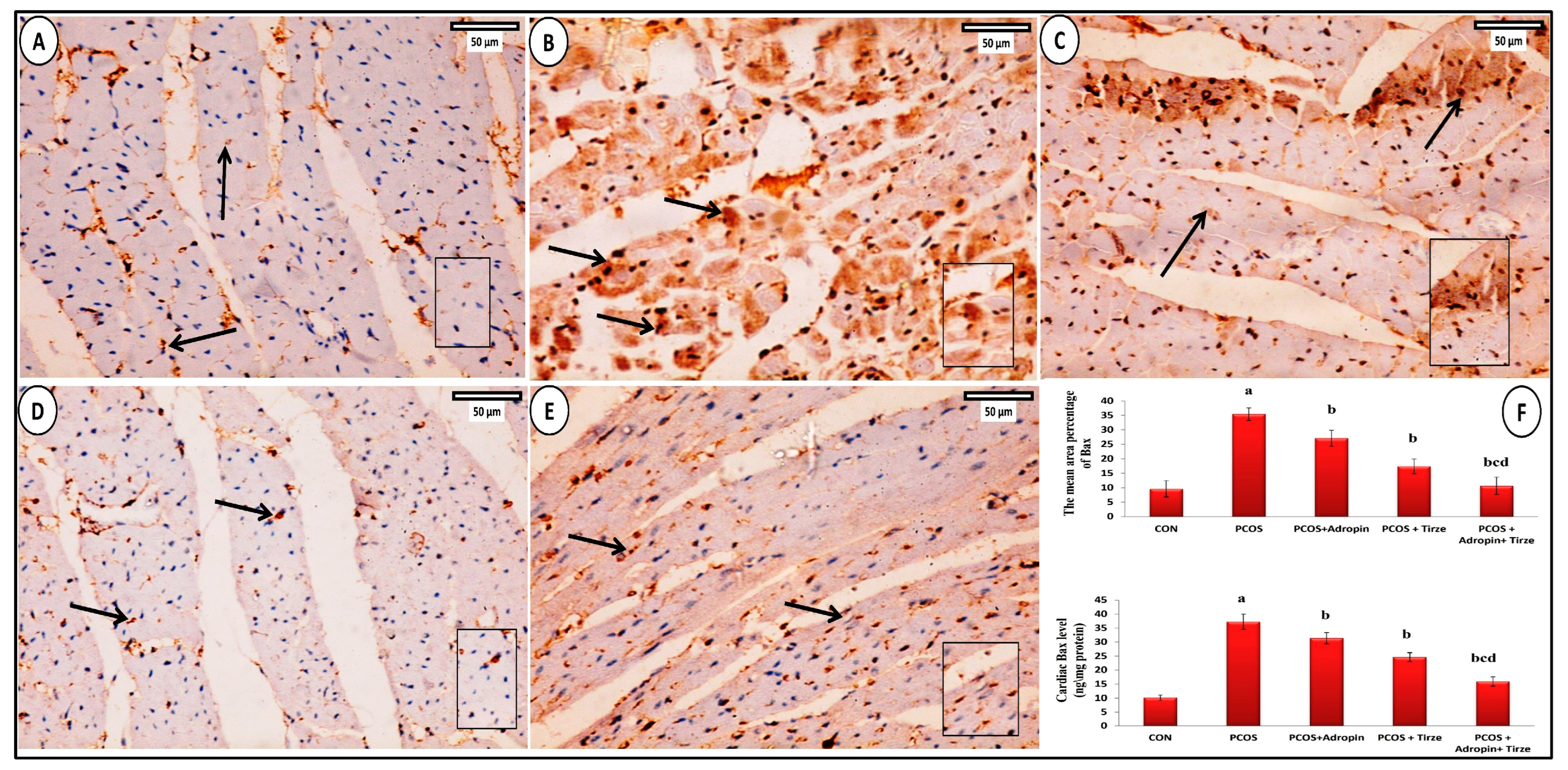
2.8. Bax Immunohistochemical Staining
2.9. Electron Microscope Examination
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Animals and Experimental Design
- CON group: (1% CMC; 2 mL/kg/day, p.o.);
- PCOS + Adropin group (Adropin; 2.1 μg/kg/day, i.p.) [55];
- PCOS + Adropin+ Tirze (received both adropin and Tirze concomitantly as previously mentioned).
4.3. Blood and Tissue Collection
4.4. Biochemical and Immunoassay Investigations
4.5. Relative NLRP3, GRP78, and CHOP Gene Expression Assay by Quantitative Real-Time PCR
4.6. Histological, Immunohistochemical, and Electron Microscopic Studies
4.7. Morphometric Study
- Mean number of ovarian growing follicles (the primary, secondary, and antral follicles numbers were counted);
- Mean thickness of the granulosa cell layer;
- Mean thickness of the theca cell layer;
- The mean diameter of cardiomyocytes;
- Mean color intensity of NF-κB in immunostained sections;
- Mean area %of Bcl2 in immunostained sections;
- Mean area % of Bax in immunostained sections.
4.8. Statistical Analysis
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gao, L.; Zhao, Y.; Wu, H.; Lin, X.; Guo, F.; Li, J.; Long, Y.; Zhou, B.; She, J.; Zhang, C.; et al. Polycystic Ovary Syndrome Fuels Cardiovascular Inflammation and Aggravates Ischemic Cardiac Injury. Circulation 2023, 148, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Al-Jefout, M.; Alsafar, H.; Kirtley, S.; Lindgren, C.M.; Missmer, S.A.; Becker, C.M.; Zondervan, K.T.; Rahmioglu, N. Prevalence of common gynecological conditions in the middle east: Systematic review and meta-analysis. Front. Reprod. Health 2021, 3, 661360. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A.S.; Thackray, V.G.; Ryan, G.E.; Tolson, K.P.; Glidewell-Kenney, C.A.; Semaan, S.J.; Poling, M.C.; Iwata, N.; Breen, K.M.; Duleba, A.J.; et al. A Novel Letrozole Model Recapitulates Both the Reproductive and Metabolic Phenotypes of Polycystic Ovary Syndrome in Female Mice. Biol. Reprod. 2015, 93, 69. [Google Scholar] [CrossRef] [PubMed]
- Ollila, M.-M.; Arffman, R.K.; Korhonen, E.; Morin-Papunen, L.; Franks, S.; Junttila, J.; Piltonen, T.T. Women with PCOS have an increased risk for cardiovascular disease regardless of diagnostic criteria—A prospective population-based cohort study. Eur. J. Endocrinol. 2023, 189, 96–105. [Google Scholar] [CrossRef]
- Zore, T.; Joshi, N.V.; Lizneva, D.; Azziz, R. Polycystic Ovarian Syndrome: Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 271–281. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Zhu, L.; Tan, F.; Qin, Y.; Huang, H.; Yu, Y. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 2018, 17, 185. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, W.; Xue, W.; Tan, Y.; Hein, D.W.; Li, X.-K.; Cai, L. Inactivation of GSK-3β by Metallothionein Prevents Diabetes-Related Changes in Cardiac Energy Metabolism, Inflammation, Nitrosative Damage, and Remodeling. Diabetes 2009, 58, 1391–1402. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, X.D.; Hao, Z.H.; Xu, D. Insulin-like growth factor-1 improves diabetic cardiomyopathy through antioxidative and anti-inflammatory processes along with modulation of Akt/GSK-3β signaling in rats. Korean J. Physiol. Pharmacol. 2016, 20, 613–619. [Google Scholar] [CrossRef]
- Kumar, K.G.; Trevaskis, J.L.; Lam, D.D.; Sutton, G.M.; Koza, R.A.; Chouljenko, V.N.; Kousoulas, K.G.; Rogers, P.M.; Kesterson, R.A.; Thearle, M.; et al. Identification of Adropin as a Secreted Factor Linking Dietary Macronutrient Intake with Energy Homeostasis and Lipid Metabolism. Cell Metab. 2008, 8, 468–481. [Google Scholar] [CrossRef]
- Gao, S.; McMillan, R.P.; Zhu, Q.; Lopaschuk, G.D.; Hulver, M.W.; Butler, A.A. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 2015, 4, 310–324. [Google Scholar] [CrossRef]
- Thapa, D.; Xie, B.; Zhang, M.; Stoner, M.W.; Manning, J.R.; Huckestein, B.R.; Edmunds, L.R.; Mullett, S.J.; McTiernan, C.F.; Wendell, S.G.; et al. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J. Mol. Cell. Cardiol. 2019, 129, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, T.R.; Gao, S.; Karwi, Q.G.; Fukushima, A.; Rawat, S.; Wagg, C.S.; Zhang, L.; Lopaschuk, G.D. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism 2019, 98, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Xie, B.; Mushala, B.A.; Zhang, M.; Manning, J.R.; Bugga, P.; Stoner, M.W.; Jurczak, M.J.; Scott, I. Diet-induced obese mice are resistant to improvements in cardiac function resulting from short-term adropin treatment. Curr. Res. Physiol. 2022, 5, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fang, J.; Chen, L.; Zhao, Z.; Luo, Y.; Lin, C.; Fan, L. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 751–758. [Google Scholar] [CrossRef]
- Aydin, S.; Eren, M.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Alatas, O.; Kuloglu, T.; Balaban, H.; Cakmak, T.; Kobalt, M.; et al. Adropin as a potential marker of enzyme-positive acute coronary syndrome. Cardiovasc. J. Afr. 2017, 28, 40–47. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lin, Y.; Luo, M.J.; Considine, G.; Cox, A.L.; Bowsman, L.M.; Robins, D.A.; Haupt, A.; Duffin, K.L.; Ruotolo, G. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes. Metab. 2021, 24, 148–153. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Bhatt, D.L.; Buse, J.B.; Del Prato, S.; E Kahn, S.; Lincoff, A.M.; McGuire, D.K.; A Nauck, M.; E Nissen, S.; Sattar, N.; et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am. Heart J. 2023, 267, 1–11. [Google Scholar] [CrossRef]
- Jahan, S.; Munir, F.; Razak, S.; Mehboob, A.; Ain, Q.U.; Ullah, H.; Afsar, T.; Shaheen, G.; Almajwal, A. Ameliorative effects of rutin against metabolic, biochemical and hormonal disturbances in polycystic ovary syndrome in rats. J. Ovarian Res. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Ke, Y.; Hu, J.; Zhu, Y.; Wang, Y.; Chen, S.; Liu, S. Correlation Between Circulating Adropin Levels and Patients with PCOS: An Updated Systematic Review and Meta-analysis. Reprod. Sci. 2022, 29, 3295–3310. [Google Scholar] [CrossRef]
- Ali, I.I.; D’souza, C.; Singh, J.; Adeghate, E. Adropin’s Role in Energy Homeostasis and Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 8318. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. 2013, 125, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Rizk, F.H.; El Saadany, A.A.; Elshamy, A.M.; Ellatif, R.A.A.; El-Guindy, D.M.; Helal, D.S.; Hamama, M.G.; El-Sharnoby, J.A.E.; Ghafar, M.T.A.; Faheem, H. Ameliorating effects of adropin on letrozole-induced polycystic ovary syndrome via regulating steroidogenesis and the microbiota inflammatory axis in rats. J. Physiol. 2024, 602, 3621–3639. [Google Scholar] [CrossRef] [PubMed]
- Anala, A.D.; Saifudeen, I.S.H.; Ibrahim, M.; Nanda, M.; Naaz, N.; Atkin, S.L. The Potential Utility of Tirzepatide for the Management of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 4575. [Google Scholar] [CrossRef]
- Lee, C.J.; Mao, H.; Thieu, V.T.; Landó, L.F.; Thomas, M.K. Tirzepatide as monotherapy improved markers of beta-cell function and insulin sensitivity in type 2 diabetes (SURPASS-1). J. Endocr. Soc. 2023, 7, bvad056. [Google Scholar] [CrossRef]
- Min, T.; Bain, S.C. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2020, 12, 143–157. [Google Scholar] [CrossRef]
- Lv, X.; Wang, H.; Chen, C.; Zhao, Y.; Li, K.; Wang, Y.; Wang, L.; Fu, S.; Liu, J. The Effect of Tirzepatide on Weight, Lipid Metabolism and Blood Pressure in Overweight/Obese Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 701–714. [Google Scholar] [CrossRef]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, J.; Kong, B.; Shuai, W.; Huang, H. Tirzepatide attenuates lipopolysaccharide-induced left ventricular remodeling and dysfunction by inhibiting the TLR4/NF-kB/NLRP3 pathway. Int. Immunopharmacol. 2023, 120, 110311. [Google Scholar] [CrossRef]
- Hankosky, E.R.; Wang, H.; Neff, L.M.; Kan, H.; Wang, F.; Ahmad, N.N.; Griffin, R.; Stefanski, A.; Garvey, W.T. Tirzepatide reduces the predicted risk of atherosclerotic cardiovascular disease and improves cardiometabolic risk factors in adults with obesity or overweight: SURMOUNT-1 post hoc analysis. Diabetes Obes. Metab. 2023, 26, 319–328. [Google Scholar] [CrossRef]
- Yu, W.; Zha, W.; Ke, Z.; Min, Q.; Li, C.; Sun, H.; Liu, C. Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway. J. Diabetes Res. 2016, 2016, 4158591. [Google Scholar] [CrossRef] [PubMed]
- Landa-Galvan, H.V.; Rios-Castro, E.; Romero-Garcia, T.; Rueda, A.; Olivares-Reyes, J.A. Metabolic syndrome diminishes insulin-induced Akt activation and causes a redistribution of Akt-interacting proteins in cardiomyocytes. PLoS ONE 2020, 15, e0228115. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-N.; Jiang, Y.; Yan, L.-Y.; Yin, S.-Y.; Wang, Y.-H.; Wang, S.-B.; Fang, L.-H.; Du, G.-H. Aesculin suppresses the NLRP3 inflammasome-mediated pyroptosis via the Akt/GSK3β/NF-κB pathway to mitigate myocardial ischemia/reperfusion injury. Phytomedicine 2021, 92, 153687. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Sasi, A.K.; Hussen, B.M.; Shoorei, H.; Siddiq, A.; Taheri, M.; Ayatollahi, S.A. Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 2022, 49, 9767–9781. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lei, M.; Zhao, J.; Wu, M.; Ren, Z.; Yang, X.; Ouyang, C.; Liu, X.; Liu, C.; Chen, Q. Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation response in diabetic rats. Front. Pharmacol. 2023, 14, 1146960. [Google Scholar] [CrossRef]
- Hassan, N.F.; Ragab, D.; Ibrahim, S.G.; El-Galil, M.M.A.; Abd-El-Hamid, A.H.; Hamed, D.M.; William, M.M.; Salem, M.A. The potential role of Tirzepatide as adjuvant therapy in countering colistin-induced nephro and neurotoxicity in rats via modulation of PI3K/p-Akt/GSK3-β/NF-kB p65 hub, shielding against oxidative and endoplasmic reticulum stress, and activation of p-CREB/BDNF/TrkB cascade. Int. Immunopharmacol. 2024, 135, 112308. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Ruan, B.; Yang, H.; Yu, Y. Tirzepatide protects against doxorubicin-induced cardiotoxicity by inhibiting oxidative stress and inflammation via PI3K/Akt signaling. Peptides 2024, 178, 171245. [Google Scholar] [CrossRef]
- Xue, H.; Fang, W.; Chen, K.; Chen, S.; Yang, W.; Shen, T.; Chen, X.; Zhang, P.; Ling, W. Adropin protects against liver injury in nonalcoholic steatohepatitis via the Nrf2 mediated antioxidant capacity. Redox Biol. 2018, 21, 101068. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Li, Y.; Zhao, G.; Guo, Z.; Luo, Y.; et al. Exercise suppresses NLRP3 inflammasome activation in mice with diet-induced NASH: A plausible role of adropin. Mod. Pathol. 2020, 101, 369–380. [Google Scholar] [CrossRef]
- Kandar, C.C.; Sen, D.; Maity, A. Anti-inflammatory Potential of GSK-3 Inhibitors. Curr. Drug Targets 2021, 22, 1464–1476. [Google Scholar] [CrossRef]
- Hyderali, B.N.; Mala, K. Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 191, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, A.S.; Bülbül, M.; Çeker, T.; Özak, A.; Tanrıöver, G.; Gürer, I.E.; Balaban, H.T.; Göksu, E.; Aslan, M. Protective Effects of Adropin in Experimental Subarachnoid Hemorrhage. Neuroscience 2024, 551, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fang, J.; Yuan, X.; Xiong, C.; Chen, L. Adropin reduces hypoxia/reoxygenation-induced myocardial injury via the reperfusion injury salvage kinase pathway. Exp. Ther. Med. 2019, 18, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, W.; Zheng, X.-L.; Yin, W.-D.; Tang, C.-K. A novel peptide adropin in cardiovascular diseases. Clin. Chim. Acta 2016, 453, 107–113. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Zhou, Y.; Deng, J.; Wu, L. Tirzepatide alleviates oxidative stress and inflammation in diabetic nephropathy via IL-17 signaling pathway. Mol. Cell. Biochem. 2024. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, A.; Yan, J.; Liu, B.; Liu, Q.; Zhang, J.; Zhang, X.; Ou, C.; Chen, M. Resveratrol Ameliorates Cardiac Dysfunction by Inhibiting Apoptosis via the PI3K/Akt/FoxO3a Pathway in a Rat Model of Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Helmstädter, J.; Keppeler, K.; Küster, L.; Münzel, T.; Daiber, A.; Steven, S. Glucagon-like peptide-1 (GLP-1) receptor agonists and their cardiovascular benefits—The role of the GLP-1 receptor. Br. J. Pharmacol. 2021, 179, 659–676. [Google Scholar] [CrossRef]
- Rani, M.P.; Raj, P.S.; Nair, A.; Ranjith, S.; Rajankutty, K.; Raghu, K. In vitro and in vivo studies reveal the beneficial effects of chlorogenic acid against ER stress mediated ER-phagy and associated apoptosis in the heart of diabetic rat. Chem.-Biol. Interact. 2022, 351, 109755. [Google Scholar]
- Yang, D.; Li, S.; Gao, L.; Lv, Z.; Bing, Q.; Lv, Q.; Zheng, X.; Li, R.; Zhang, Z. Dietary grape seed procyanidin extract protects against lead-induced heart injury in rats involving endoplasmic reticulum stress inhibition and AKT activation. J. Nutr. Biochem. 2018, 62, 43–49. [Google Scholar] [CrossRef]
- Taktaz, F.; Scisciola, L.; Fontanella, R.A.; Pesapane, A.; Ghosh, P.; Franzese, M.; Tortorella, G.; Puocci, A.; Sommella, E.; Signoriello, G.; et al. Evidence that tirzepatide protects against diabetes-related cardiac damages. Cardiovasc. Diabetol. 2024, 23, 112. [Google Scholar] [CrossRef]
- Bai, B.; Li, D.; Xue, G.; Feng, P.; Wang, M.; Han, Y.; Wang, Y.; Hölscher, C. The novel GLP-1/GIP dual agonist DA3-CH is more effective than liraglutide in reducing endoplasmic reticulum stress in diabetic rats with cerebral ischemia-reperfusion injury. Nutr. Metab. Cardiovasc. Dis. 2020, 31, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, J.; Liu, S.; Huang, W.; Gong, Y.; Gu, X. Glucagon-like peptide-1 attenuates endoplasmic reticulum stress–induced apoptosis in H9c2 cardiomyocytes during hypoxia/reoxygenation through the GLP-1R/PI3K/Akt pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Kafali, H.; Iriadam, M.; Ozardalı, I.; Demir, N. Letrozole-induced polycystic ovaries in the rat: A new model for cystic ovarian disease. Arch. Med. Res. 2004, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Olaniyi, K.S.; Areloegbe, S.E. Suppression of PCSK9/NF-kB-dependent pathways by acetate ameliorates cardiac inflammation in a rat model of polycystic ovarian syndrome. Life Sci. 2022, 300, 120560. [Google Scholar] [CrossRef]
- El Gheit, R.E.A.; Atef, M.M.; El Deeb, O.S.; Badawi, G.A.; Alshenawy, H.A.; Elwan, W.M.; Arakeep, H.M.; Emam, M.N. Unique Novel Role of Adropin in a Gastric Ulcer in a Rotenone-Induced Rat Model of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 3077–3088. [Google Scholar] [CrossRef]
- Samms, R.J.; Christe, M.E.; Collins, K.A.; Pirro, V.; Droz, B.A.; Holland, A.K.; Friedrich, J.L.; Wojnicki, S.; Konkol, D.L.; Cosgrove, R.; et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J. Clin. Investig. 2021, 131, e146353. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.; Treacher, D.F.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M. 9—The Hematoxylins and Eosin. In Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone: Edinburgh, UK, 2008; pp. 121–134. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Kiupel, M.; Baszler, T.; Bliven, L.; Brodersen, B.; Chelack, B.; West, K.; Czub, S.; Del Piero, F.; Dial, S.; et al. Suggested Guidelines for Immunohistochemical Techniques in Veterinary Diagnostic Laboratories. J. Veter- Diagn. Investig. 2008, 20, 393–413. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.E.; Stirling, J.W. 30—Electron Microscopy. In Theory and Practice of Histological Techniques, 6th ed.; Bancroft, J.D., Gamble, M., Eds.; Churchill Livingstone: Edinburgh, UK, 2008; pp. 601–640. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegab, I.I.; El-Horany, H.E.-s.; Abd-Ellatif, R.N.; Nasef, N.A.; Okasha, A.H.; Emam, M.N.; Hassan, S.; Elseady, W.S.; Radwan, D.A.; ElEsawy, R.O.; et al. Adropin/Tirzepatide Combination Mitigates Cardiac Metabolic Aberrations in a Rat Model of Polycystic Ovarian Syndrome, Implicating the Role of the AKT/GSK3β/NF-κB/NLRP3 Pathway. Int. J. Mol. Sci. 2025, 26, 1. https://doi.org/10.3390/ijms26010001
Hegab II, El-Horany HE-s, Abd-Ellatif RN, Nasef NA, Okasha AH, Emam MN, Hassan S, Elseady WS, Radwan DA, ElEsawy RO, et al. Adropin/Tirzepatide Combination Mitigates Cardiac Metabolic Aberrations in a Rat Model of Polycystic Ovarian Syndrome, Implicating the Role of the AKT/GSK3β/NF-κB/NLRP3 Pathway. International Journal of Molecular Sciences. 2025; 26(1):1. https://doi.org/10.3390/ijms26010001
Chicago/Turabian StyleHegab, Islam Ibrahim, Hemat El-sayed El-Horany, Rania Nagi Abd-Ellatif, Nahla Anas Nasef, Asmaa H. Okasha, Marwa Nagy Emam, Shereen Hassan, Walaa S. Elseady, Doaa A. Radwan, Rasha Osama ElEsawy, and et al. 2025. "Adropin/Tirzepatide Combination Mitigates Cardiac Metabolic Aberrations in a Rat Model of Polycystic Ovarian Syndrome, Implicating the Role of the AKT/GSK3β/NF-κB/NLRP3 Pathway" International Journal of Molecular Sciences 26, no. 1: 1. https://doi.org/10.3390/ijms26010001
APA StyleHegab, I. I., El-Horany, H. E.-s., Abd-Ellatif, R. N., Nasef, N. A., Okasha, A. H., Emam, M. N., Hassan, S., Elseady, W. S., Radwan, D. A., ElEsawy, R. O., Hafez, Y. M., Hassan, M. E., Mansour, N. M., Abdelkader, G. E., Fouda, M. H., Abd El Maged, A. M., & Abdallah, H. M. (2025). Adropin/Tirzepatide Combination Mitigates Cardiac Metabolic Aberrations in a Rat Model of Polycystic Ovarian Syndrome, Implicating the Role of the AKT/GSK3β/NF-κB/NLRP3 Pathway. International Journal of Molecular Sciences, 26(1), 1. https://doi.org/10.3390/ijms26010001





