In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses
Abstract
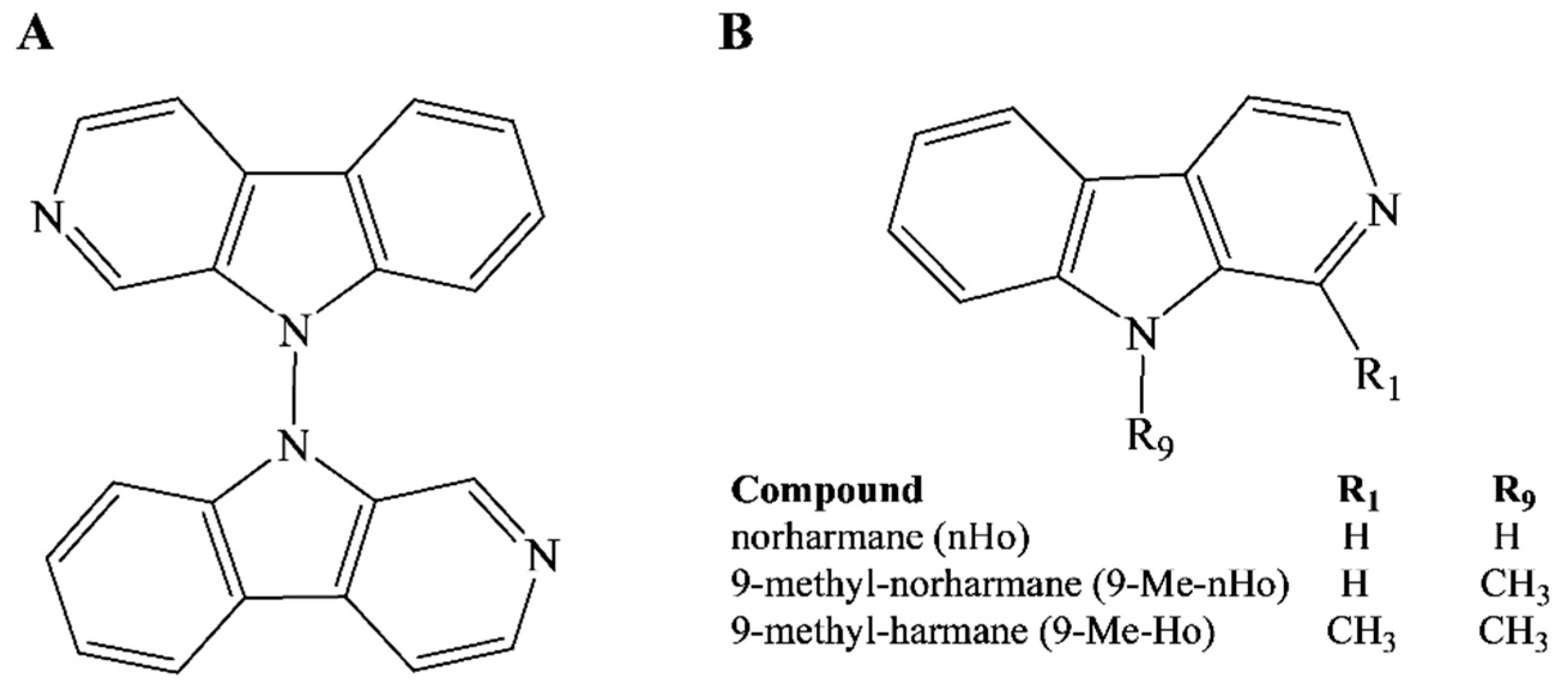
1. Introduction
2. Results
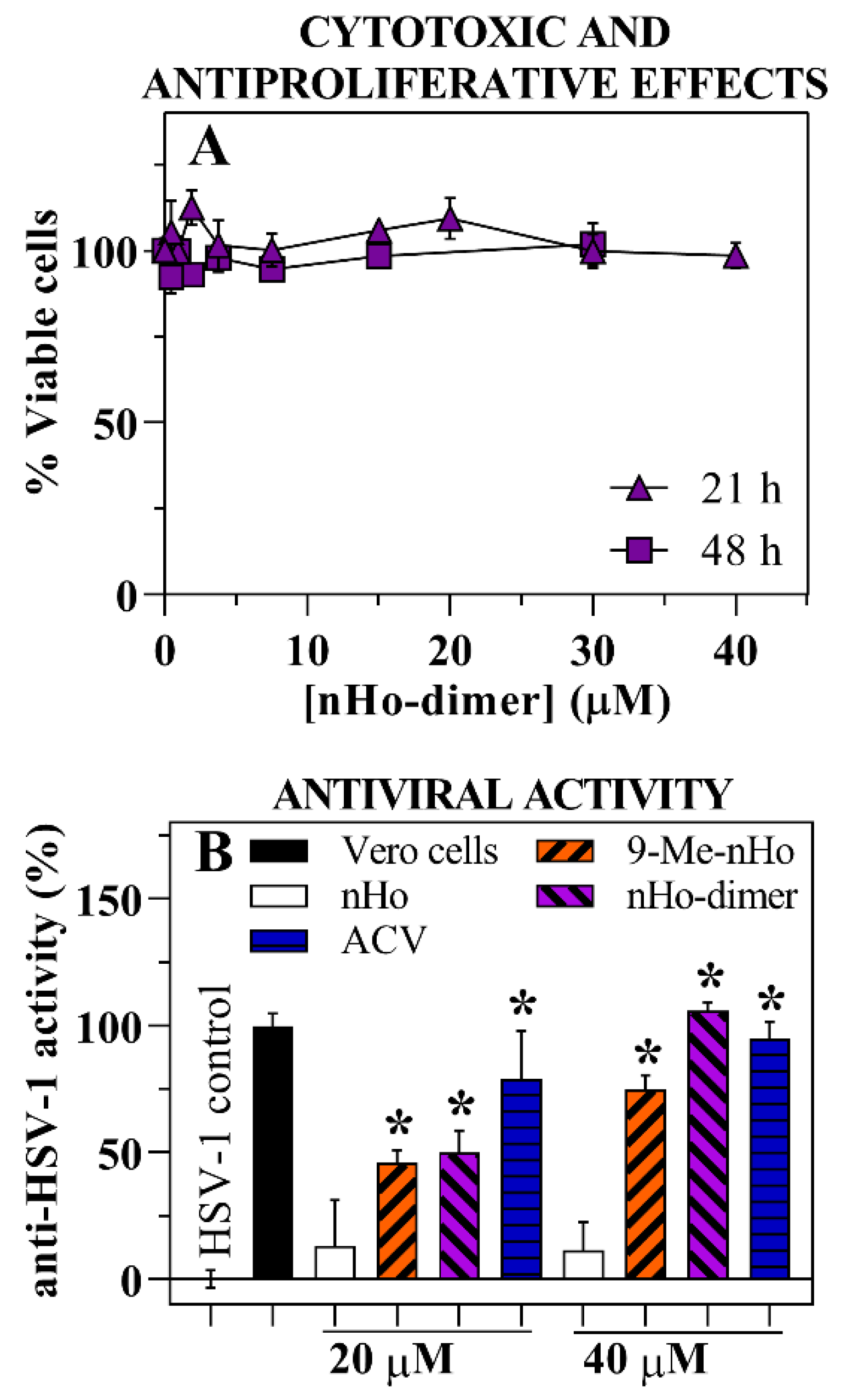
2.1. Cytotoxic and Antiproliferative Effect
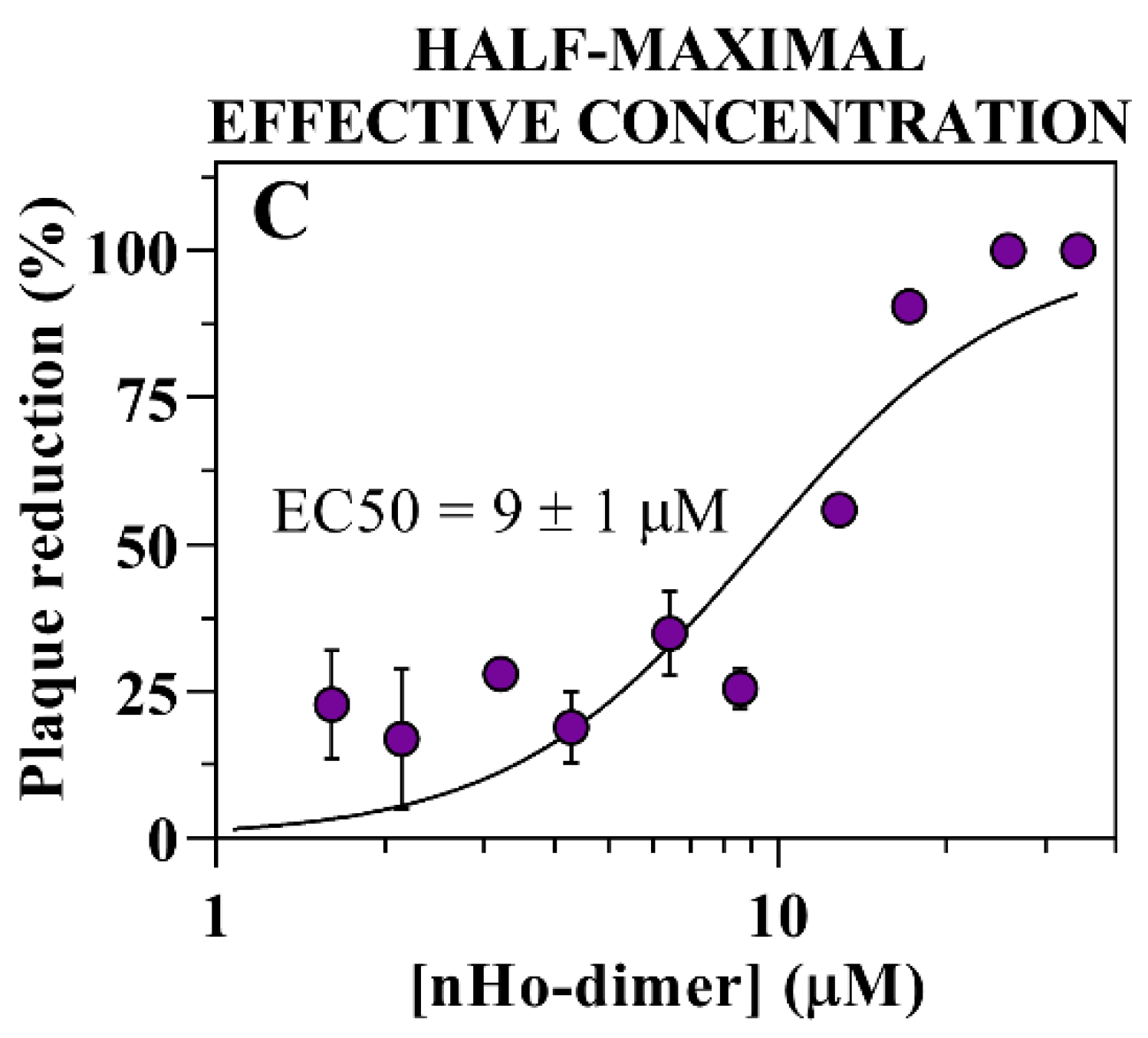
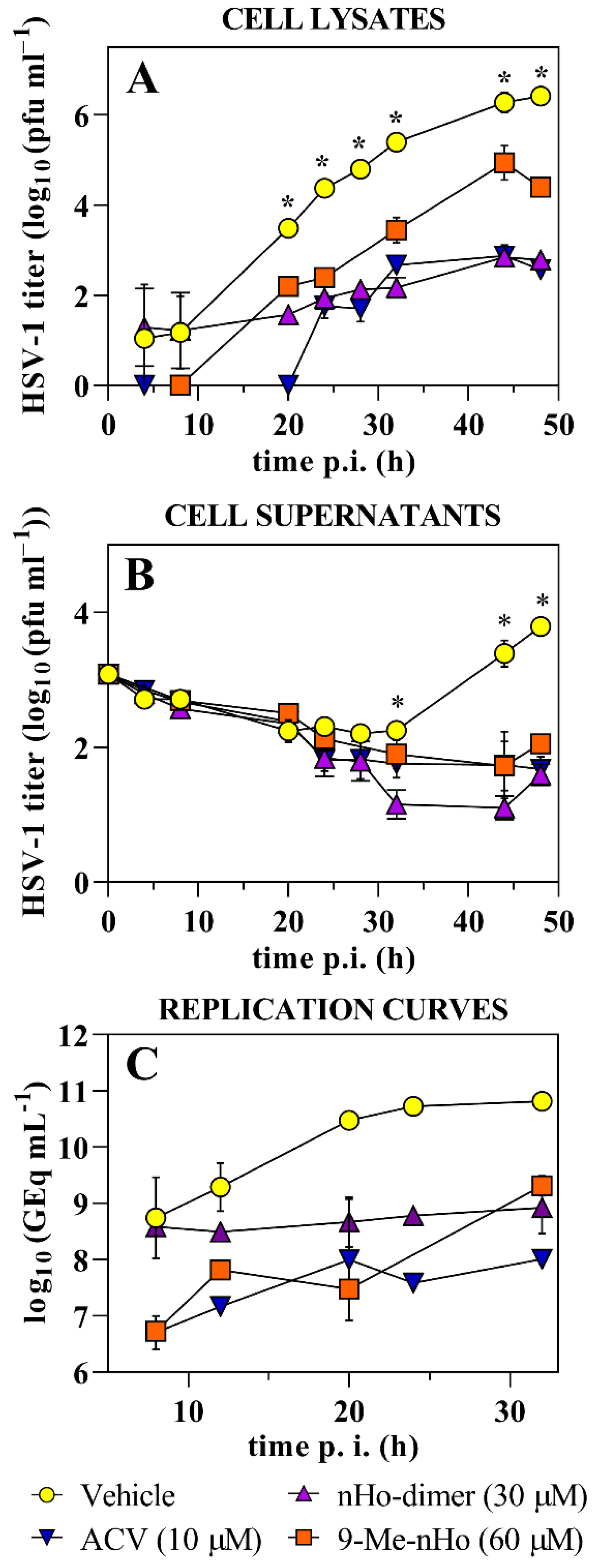
2.2. Antiviral Effect
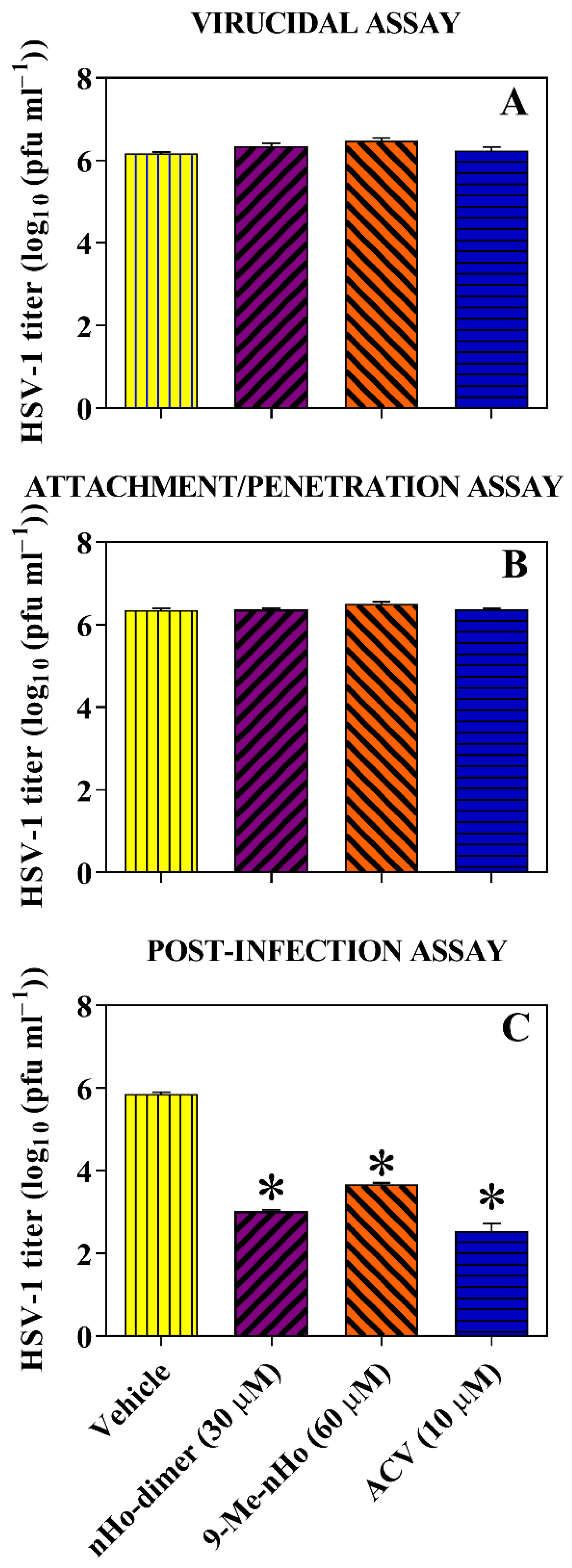
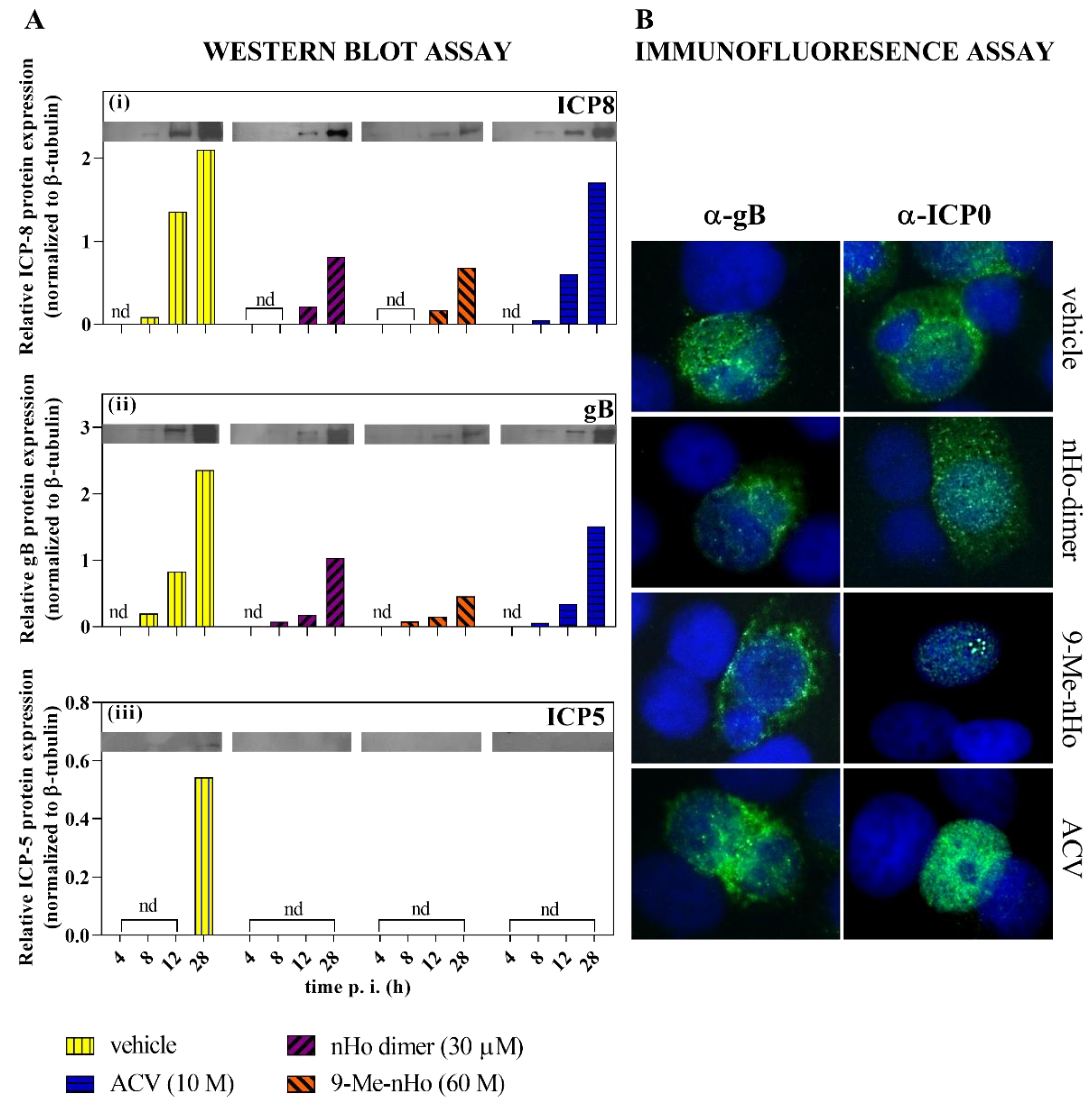
2.3. Effect on the Virus Life Cycle and Its Potential Mechanisms of Action
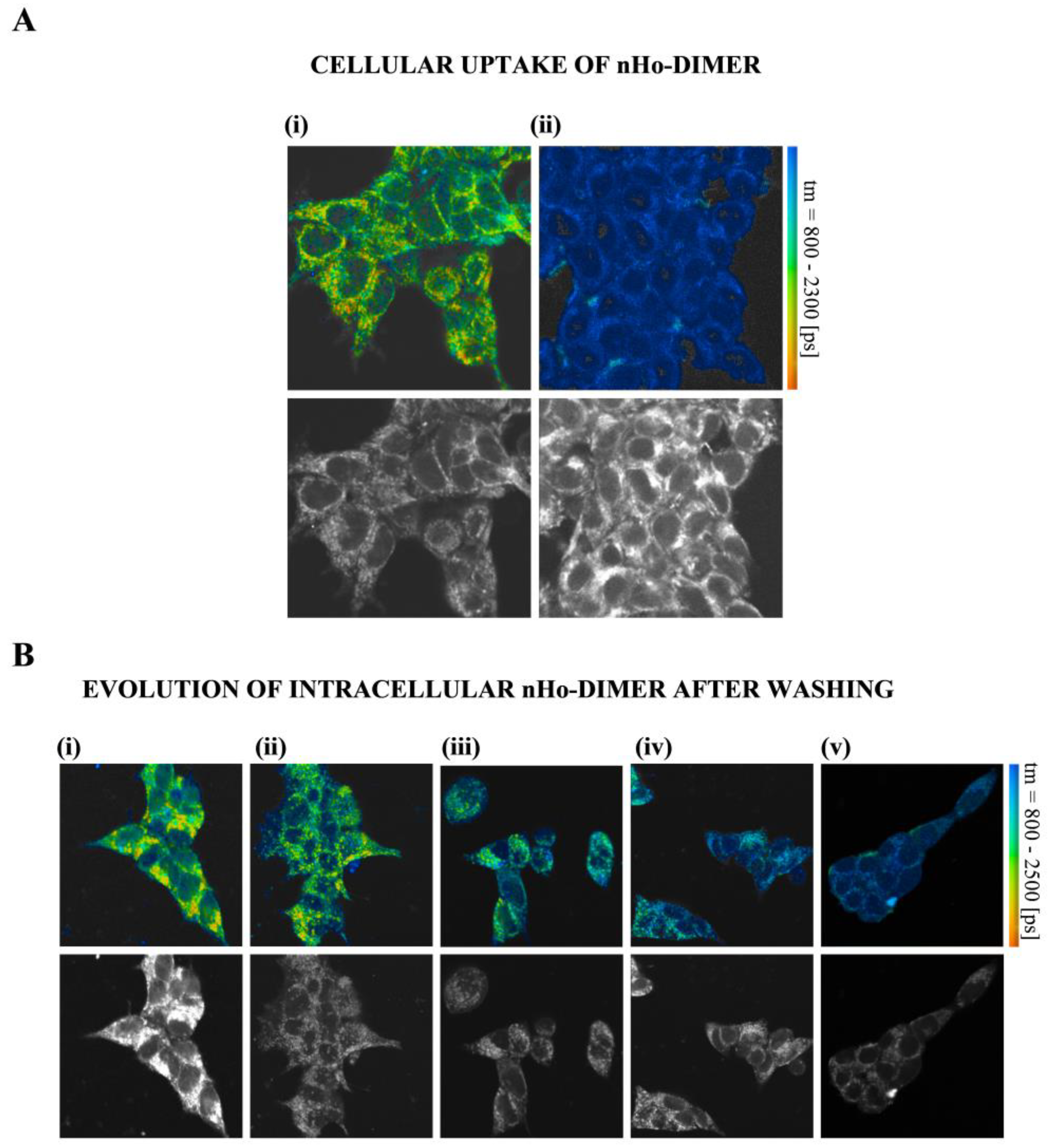
2.4. Cellular Uptake
3. Discussion
4. Materials and Methods
4.1. Chemical and Reactants
Preparation of Stock Solutions
4.2. Virus and Cell Cultures
4.2.1. Cell Culture
4.2.2. Virus Propagation
4.3. Cytotoxicity Assays
4.3.1. MTS Assay
4.3.2. Calculation of Cell Viability
4.4. Evaluation of Antiviral Activity
4.5. Plaque Reduction Assays and EC50 Determination
4.5.1. Plaque Counting and Plaque Reduction Analysis
4.5.2. EC50 Determination
4.6. Experiments to Assess the Effect of nHo-Dimer during Different Stages of Infection. Time-of-Addition Experiments
4.6.1. Virucidal Activity Assay
4.6.2. Assessment of Viral Attachment
4.6.3. Post-Infection Treatment
4.7. Effect of nHo-Dimer on Viral Replication
4.8. Quantitative PCR (qPCR)
4.9. Immunoblotting
4.10. Immunofluorescence Staining and Microscopy
4.11. Other Relevant Information
4.12. Fluorescence Lifetime Imaging Microscopy (FLIM) in Living Cells
4.12.1. Fluorescence Lifetime Images
4.12.2. Fluorescence Lifetime Determination
4.12.3. Incubation of HEK293 Cells with βCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Álvarez, D.M.; Castillo, E.; Duarte, L.F.; Arriagada, J.; Corrales, N.; Farías, M.A.; Henríquez, A.; Agurto-Muñoz, C.; González, P.A. Current antivirals and novel botanical molecules interfering with herpes simplex virus infection. Front. Microbiol. 2020, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A. Optimal management of genital herpes: Current perspectives. Infect. Drug Resist. 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Immunomodulatory strategies in herpes simplex virus encephalitis. Clin. Microbiol. Rev. 2020, 33, e00105-19. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Orroth, K.K.; White, R.G.; Glynn, J.R.; Bakker, R.; Boily, M.-C.; Habbema, D.; Buvé, A.; Hayes, R. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: Simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex. Transm. Infect. 2007, 83 (Suppl. S1), i17–i24. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Resistance of herpes simplex viruses to nucleoside analogues: Mechanisms, prevalence, and management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef] [PubMed]
- Banoth, K.K.; ChandraSekhar KV, G.; Adinarayana, N.; Murugesan, S. Recent evolution on synthesis strategies and anti-leishmanial activity of β-carboline derivatives—An update. Heliyon 2020, 6, e04916. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bai, Z.; Ma, Q.; Fan, W.; Guo, L.; Zhang, G.; Qiu, L.; Yu, H.; Shao, G.; Cao, R. Synthesis and biological evaluation of novel bivalent β-carbolines as potential antitumor agents. MedChemComm 2014, 5, 953–957. [Google Scholar] [CrossRef]
- Ahmad, I.; Fakhri, S.; Khan, H.; Jeandet, P.; Aschner, M.; Yu, Z.-L. Targeting cell cycle by β-carboline alkaloids in vitro: Novel therapeutic prospects for the treatment of cancer. Chem. Biol. Interact. 2020, 330, 109229. [Google Scholar] [CrossRef]
- Alomar, M.L.; Yañuk, J.G.; Angel, S.O.; Gonzalez, M.M.; Cabrerizo, F.M. In vitro Effect of Harmine Alkaloid and Its N-Methyl Derivatives Against Toxoplasma gondii. Front. Microbiol. 2021, 21, 716534. [Google Scholar] [CrossRef]
- Alomar, M.L.; Rasse-Suriani, F.A.; Ganuza, A.; Cóceres, V.M.; Cabrerizo, F.M.; Angel, S.O. In vitro evaluation of β-carboline alkaloids as potential anti-Toxoplasma agents. BMC Res. Notes 2013, 6, 193. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Cerioni, L.; González, M.M.; Cabrerizo, F.M.; Rapisarda, V.A.; Volentini, S.I. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis cinerea. Food Microbiol. 2017, 62, 9–14. [Google Scholar] [CrossRef]
- Wang, N.; An, J.; Zhang, Z.; Liu, Y.; Fang, J.; Yang, Z. The Antimicrobial Activity and Characterization of Bioactive Compounds in Peganum harmala L. Based on HPLC and HS-SPME-GC-MS. Front. Microbiol. 2022, 13, 916371. [Google Scholar] [CrossRef]
- Gonzalez, M.M.; Cabrerizo, F.M.; Baiker, A.; Erra-Balsells, R.; Osterman, A.; Nitschko, H.; Vizoso-Pinto, M.G. β-Carboline derivatives as novel antivirals for herpes simplex virus. Int. J. Antimicrob. Agents 2018, 52, 459–468. [Google Scholar] [CrossRef]
- Bag, P.; Ojha, D.; Mukherjee, H.; Halder, U.C.; Mondal, S.; Biswas, A.; Sharon, A.; Van Kaer, L.; Chakrabarty, S.; Das, G. A dihydro-pyrido-indole potently inhibits HSV-1 infection by interfering the viral immediate early transcriptional events. Antivir. Res. 2014, 105, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Su, A.; Fu, Y.; Wang, X.; Lv, X.; Xu, W.; Xu, S.; Wang, H.; Wu, Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antivir. Res. 2015, 123, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, K.G.; Ahmed, N.; Sabde, S.; Mitra, D.; Singh, I.P.; Bhutani, K.K. Synthesis and evaluation of β-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010, 20, 4416–4419. [Google Scholar] [CrossRef] [PubMed]
- Formagio AS, N.; Santos, P.R.; Zanoli, K.; Ueda-Nakamura, T.; Tonin LT, D.; Nakamura, C.V.; Sarragiotto, M.H. Synthesis and antiviral activity of β-carboline derivatives bearing a substituted carbohydrazide at C-3 against poliovirus and herpes simplex virus (HSV-1). Eur. J. Med. Chem. 2009, 44, 4695–4701. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Liu, Y.; Wang, L.; Wang, Q. Synthesis and antiviral and fungicidal activity evaluation of β-carboline, dihydro-β-carboline, tetrahydro-β-carboline alkaloids, and their derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef]
- Quintana, V.M.; Piccini, L.E.; Zénere, J.D.P.; Damonte, E.B.; Ponce, M.A.; Castilla, V. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antivir. Res. 2016, 134, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.; Mahmoud, S.H.; Elshaier, Y.A.M.M.; Shama, N.M.A.; Nasr, N.F.; Ali, M.A.; El-Shazly, A.M.; Mostafa, I.; Mostafa, A. Antiviral activities of plant-derived indole and β-carboline alkaloids against human and avian influenza viruses. Sci. Rep. 2023, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Harbour, G.C.; Gilmore, J.; Rinehart, K.L., Jr. Eudistomins A, D, G, H, I, J, M, N, O, P, and Q, bromo, hydroxy, pyrrolyl and iminoazepino. beta.-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984, 106, 1526–1528. [Google Scholar] [CrossRef]
- Wu, Z.-N.; Chen, N.-H.; Tang, Q.; Chen, S.; Zhan, Z.-C.; Zhang, Y.-B.; Wang, G.-C.; Li, Y.-L.; Ye, W.-C. β-Carboline alkaloids from the seeds of Peganum harmala and their anti-HSV-2 virus activities. Org. Lett. 2020, 22, 7310–7314. [Google Scholar] [CrossRef] [PubMed]
- Nahapetian, A.T.; Thomas, J.N.; Thilly, W. Optimization of environment for high density Vero cell culture: Effect of dissolved oxygen and nutrient supply on cell growth and changes in metabolites. J. Cell Sci. 1986, 81, 65–103. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Cao, R.; Fan, W.; Guo, L.; Ma, Q.; Chen, X.; Zhang, G.; Qiu, L.; Song, H. Design, synthesis and in vitro and in vivo antitumor activities of novel bivalent β-carbolines. Eur. J. Med. Chem. 2013, 60, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Soh, T.K.; Davies, C.T.; Muenzner, J.; Hunter, L.M.; Barrow, H.G.; Connor, V.; Bouton, C.R.; Smith, C.; Emmott, E.; Antrobus, R. Temporal proteomic analysis of herpes simplex virus 1 infection reveals cell-surface remodeling via pUL56-mediated GOPC degradation. Cell Rep. 2020, 33, 108235. [Google Scholar] [CrossRef] [PubMed]
- Denofrio, M.P.; Rasse-Suriani, F.A.; Paredes, J.M.; Fassetta, F.; Crovetto, L.; Giron, M.D.; Salto, R.; Epe, B.; Cabrerizo, F.M. N-Methyl-β-carboline alkaloids: Structure-dependent photosensitizing properties and localization in subcellular domains. Org. Biomol. Chem. 2020, 18, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Rasse-Suriani, F.A.; García-Einschlag, F.S.; Rafti, M.; Schmidt De Leon, T.; David Gara, P.M.; Erra-Balsells, R.; Cabrerizo, F.M. Photophysical and Photochemical Properties of Naturally Occurring normelinonine F and Melinonine F Alkaloids and Structurally Related N (2)-and/or N (9)-methyl-β-carboline Derivatives. Photochem. Photobiol. 2018, 94, 36–51. [Google Scholar] [CrossRef]
- Jiang, Y.-C.; Feng, H.; Lin, Y.-C.; Guo, X.-R. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Wang, C.; Hu, R.; Wang, T.; Duan, L.; Hou, Q.; Wang, J.; Yang, Z. A bivalent β-carboline derivative inhibits macropinocytosis-dependent entry of pseudorabies virus by targeting the kinase DYRK1A. J. Biol. Chem. 2023, 299, 104605. [Google Scholar] [CrossRef] [PubMed]
- Weerasooriya, S.; DiScipio, K.A.; Darwish, A.S.; Bai, P.; Weller, S.K. Herpes simplex virus 1 ICP8 mutant lacking annealing activity is deficient for viral DNA replication. Proc. Natl. Acad. Sci. USA 2019, 116, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Jin, F.; Wang, Q.; Ren, Z.; Zheng, K.; Wang, Y. siRNAs Targeting viral protein 5: The major capsid protein of herpes simplex virus-1 affects its propagation and cytoskeleton. Trop. J. Pharm. Res. 2015, 14, 391–397. [Google Scholar] [CrossRef][Green Version]
- Connolly, S.A.; Jackson, J.O.; Jardetzky, T.S.; Longnecker, R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.A.; Yao, X.-D.; Gill, N.; Sajic, D.; Patrick, A.J.; Rosenthal, K.L. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J. Infect. Dis. 2004, 190, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Harandi, A.M.; Eriksson, K.; Holmgren, J. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 2003, 77, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, K.; Williams, B.R.; Silverman, R.H.; Halford, W.; Carr, D.J. Distinctive roles for 2′,5′-oligoadenylate synthetases and double-stranded RNA-dependent protein kinase R in the in vivo antiviral effect of an adenoviral vector expressing murine IFN-β. J. Immunol. 2004, 172, 5638–5647. [Google Scholar] [CrossRef]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Gu, H. Infected cell protein 0 functional domains and their coordination in herpes simplex virus replication. World J. Virol. 2016, 5, 1. [Google Scholar] [CrossRef]
- Smith, M.C.; Boutell, C.; Davido, D.J. HSV-1 ICP0: Paving the way for viral replication. Future Virol. 2011, 6, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.C.; Dybas, J.M.; Hughes, J.; Weitzman, M.D.; Boutell, C. The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection. Virus Res. 2020, 285, 198015. [Google Scholar] [CrossRef] [PubMed]
- Kalamvoki, M.; Roizman, B. Nuclear retention of ICP0 in cells exposed to HDAC inhibitor or transfected with DNA before infection with herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 2008, 105, 20488–20493. [Google Scholar] [CrossRef] [PubMed]
- Kalamvoki, M.; Roizman, B. Role of herpes simplex virus ICP0 in the transactivation of genes introduced by infection or transfection: A reappraisal. J. Virol. 2010, 84, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Gu, H. Temporal Analysis of the Nuclear-to-cytoplasmic Translocation of a Herpes Simplex Virus 1 Protein by Immunofluorescent Confocal Microscopy. J. Vis. Exp. 2018, e58504. [Google Scholar] [CrossRef] [PubMed]
- Delboy, M.G.; Siekavizza-Robles, C.R.; Nicola, A.V. Herpes simplex virus tegument ICP0 is capsid associated, and its E3 ubiquitin ligase domain is important for incorporation into virions. J. Virol. 2010, 84, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Van Sant, C.; Roizman, B. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J. Virol. 2001, 75, 3832–3840. [Google Scholar] [CrossRef] [PubMed]
- Samrat, S.K.; Ha, B.L.; Zheng, Y.; Gu, H. Characterization of elements regulating the nuclear-to-cytoplasmic translocation of ICP0 in late herpes simplex virus 1 infection. J. Virol. 2018, 92, e01673-17. [Google Scholar] [CrossRef]
- Kalamvoki, M.; Roizman, B. ICP0 enables and monitors the function of D cyclins in herpes simplex virus 1 infected cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14576–14580. [Google Scholar] [CrossRef]
- Li, D.; Liu, W.; Huang, Y.; Liu, M.; Tian, C.; Lu, H.; Jia, H.; Xu, Z.; Ding, H.; Zhao, Q. Facile synthesis of C1-substituted β-carbolines as CDK4 inhibitors for the treatment of cancer. Bioorg. Chem. 2022, 121, 105659. [Google Scholar] [CrossRef]
- Lu, D.; Qu, L.; Wang, C.; Luo, H.; Li, S.; Yin, F.; Liu, X.; Chen, X.; Luo, Z.; Cui, N. Harmine-based dual inhibitors targeting histone deacetylase (HDAC) and DNA as a promising strategy for cancer therapy. Bioorg. Chem. 2022, 120, 105604. [Google Scholar] [CrossRef] [PubMed]
- Balsells, R.E.; Frasca, A. Photochemical dimerization of β-carboline alkaloids. Tetrahedron 1983, 39, 33–39. [Google Scholar] [CrossRef]
- Gonzalez, M.M.; Arnbjerg, J.; Denofrio, M.P.; Erra-Balsells, R.; Ogilby, P.R.; Cabrerizo, F.M. One-and two-photon excitation of β-carbolines in aqueous solution: pH-dependent spectroscopy, photochemistry, and photophysics. J. Phys. Chem. A. 2009, 113, 6648–6656. [Google Scholar] [CrossRef] [PubMed]
- Burleson, F.G.; Chambers, T.M.; Wiedbrauk, D.L. Virology: A Laboratory Manual; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Kaneko, H.; Putzier, I.; Frings, S.; Gensch, T. Determination of intracellular chloride concentration in dorsal root ganglion neurons by fluorescence lifetime imaging. Curr. Top. Membr. 2002, 53, 167–189. [Google Scholar]
- Gensch, T.; Untiet, V.; Franzen, A.; Kovermann, P.; Fahlke, C. Determination of intracellular chloride concentrations by fluorescence lifetime imaging. In Advanced Time-Correlated Single Photon Counting Applications; Springer Series in Chemical Physics; Becker, W., Ed.; Springer: Cham, Switzerland, 2015; Volume 111, pp. 189–211. [Google Scholar]
- Meyer, J.; Untiet, V.; Fahlke, C.; Gensch, T.; Rose, C.R. Quantitative determination of cellular [Na+] by fluorescence lifetime imaging with CoroNaGreen. J. Gen. Physiol. 2019, 151, 1319–1331. [Google Scholar] [CrossRef]
- Engels, M.; Kalia, M.; Rahmati, S.; Petersilie, L.; Kovermann, P.; van Putten, M.J.; Rose, C.R.; Meijer, H.G.; Gensch, T.; Fahlke, C. Glial chloride homeostasis under transient ischemic stress. Front. Cell. Neurosci. 2021, 15, 735300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, M.M.; Vizoso-Pinto, M.G.; Erra-Balsells, R.; Gensch, T.; Cabrerizo, F.M. In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses. Int. J. Mol. Sci. 2024, 25, 4966. https://doi.org/10.3390/ijms25094966
Gonzalez MM, Vizoso-Pinto MG, Erra-Balsells R, Gensch T, Cabrerizo FM. In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses. International Journal of Molecular Sciences. 2024; 25(9):4966. https://doi.org/10.3390/ijms25094966
Chicago/Turabian StyleGonzalez, María Micaela, Maria Guadalupe Vizoso-Pinto, Rosa Erra-Balsells, Thomas Gensch, and Franco M. Cabrerizo. 2024. "In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses" International Journal of Molecular Sciences 25, no. 9: 4966. https://doi.org/10.3390/ijms25094966
APA StyleGonzalez, M. M., Vizoso-Pinto, M. G., Erra-Balsells, R., Gensch, T., & Cabrerizo, F. M. (2024). In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses. International Journal of Molecular Sciences, 25(9), 4966. https://doi.org/10.3390/ijms25094966






