Distinctive Nucleic Acid Recognition by Lysine-Embedded Phenanthridine Peptides
Abstract
1. Introduction
2. Results and Discussion
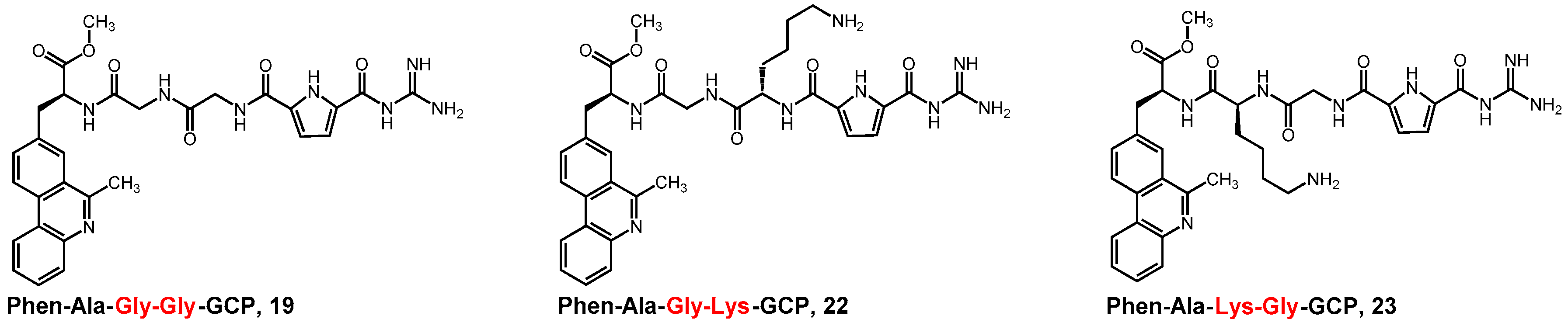
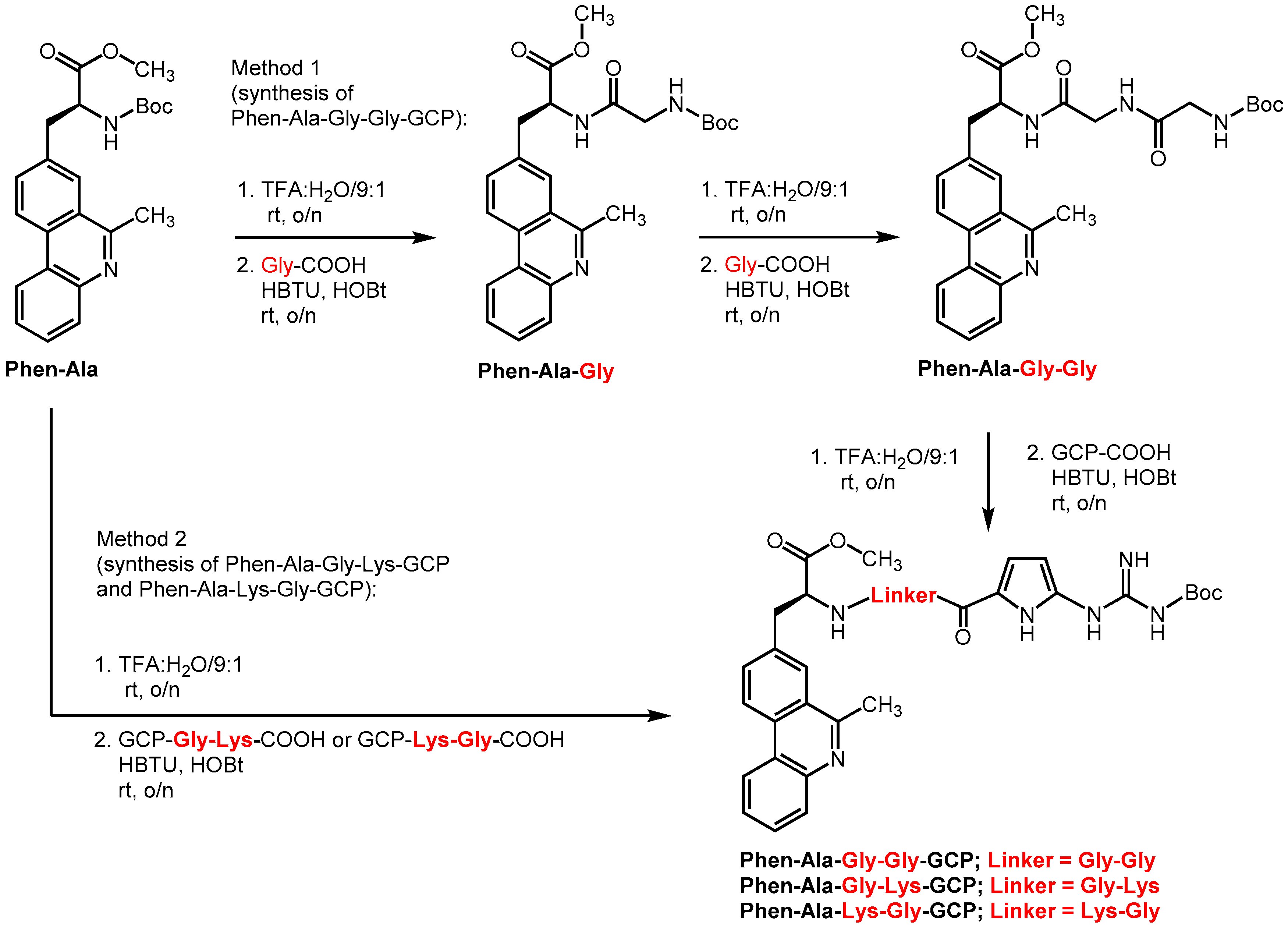
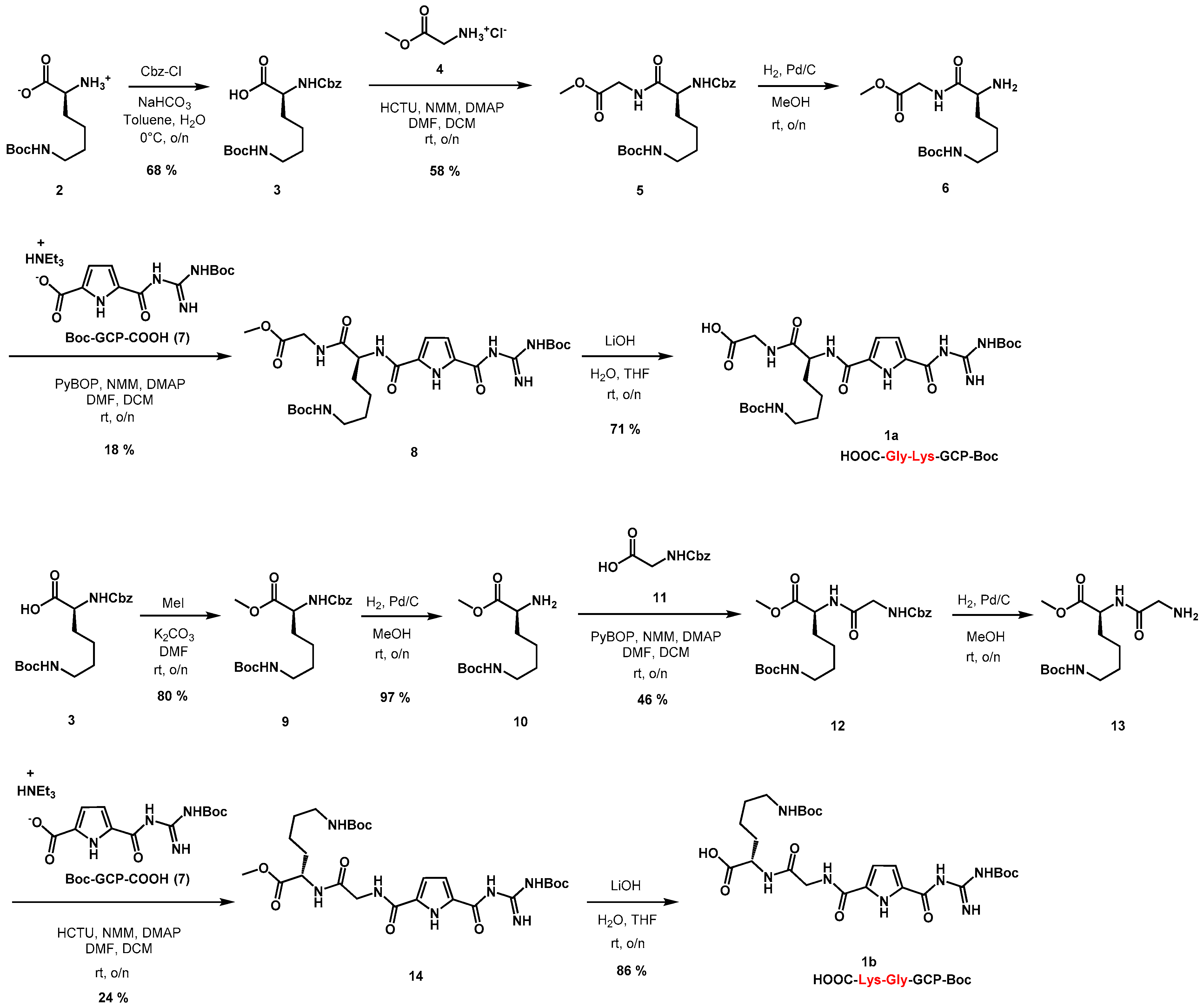
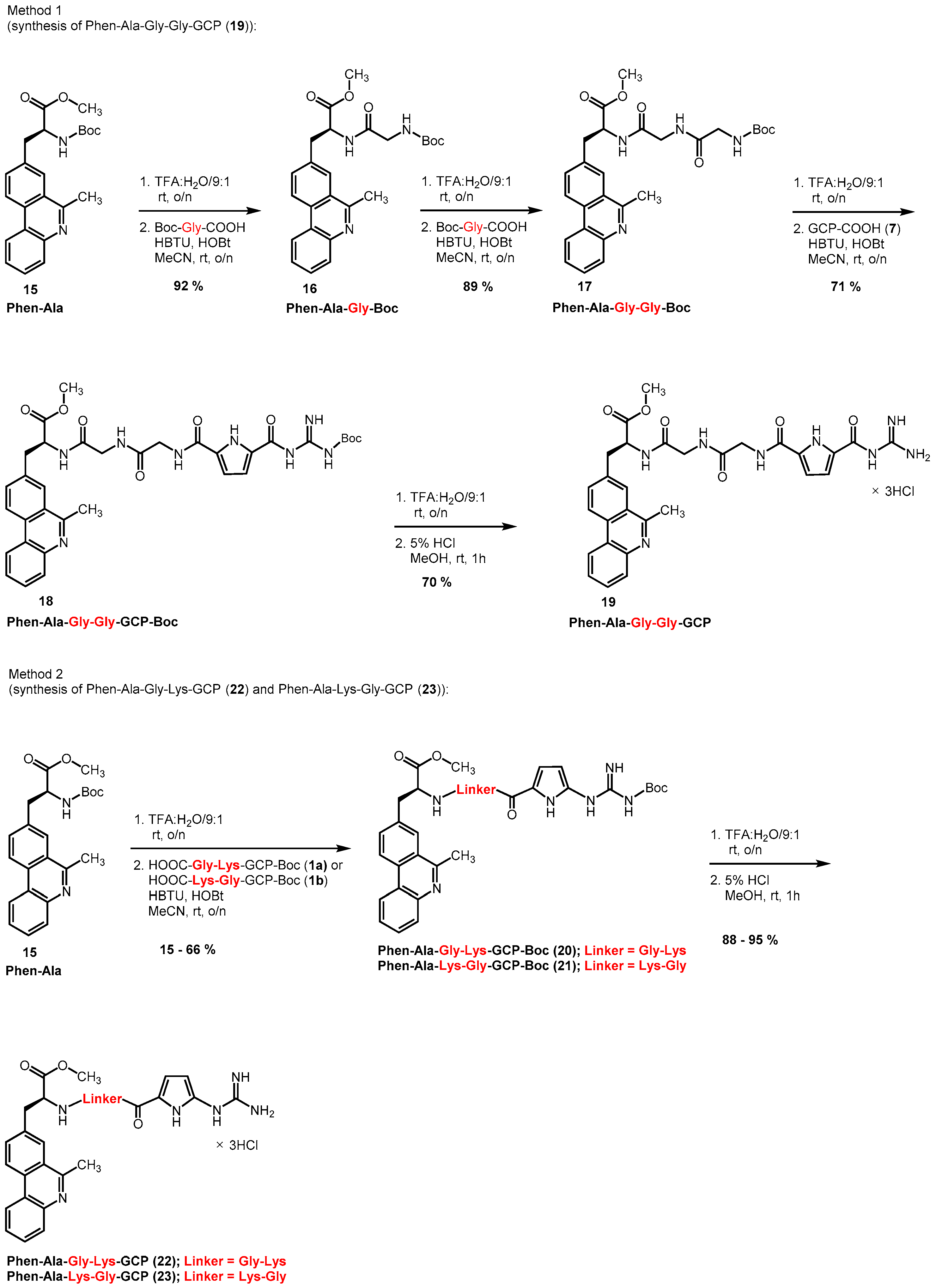
2.1. Synthesis of Phenanthridine–Guanidinocarbonylpyrrole Tetrapeptides
2.2. Spectroscopic Characterization of 19, 22, and 23 in Aqueous Solutions
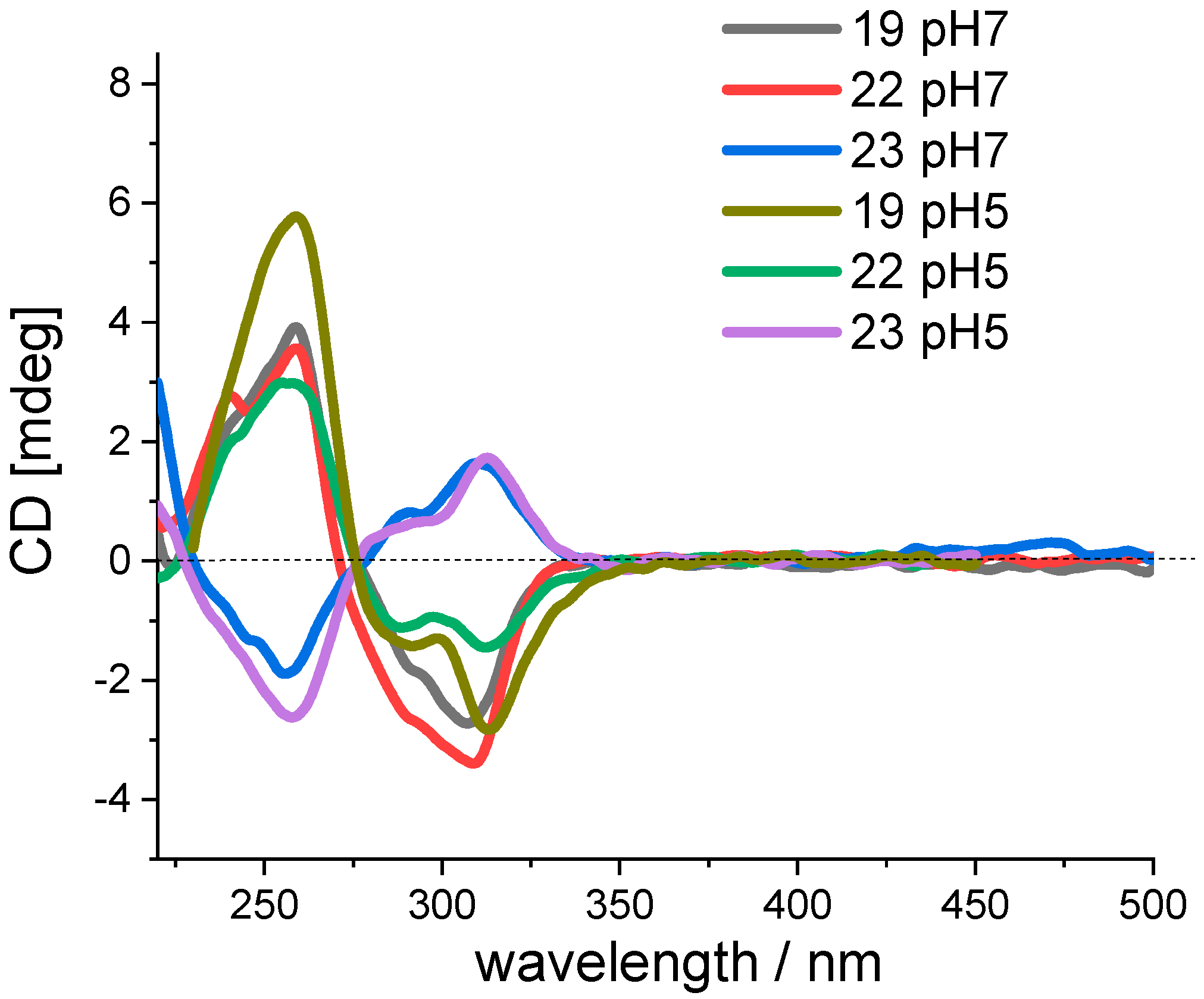
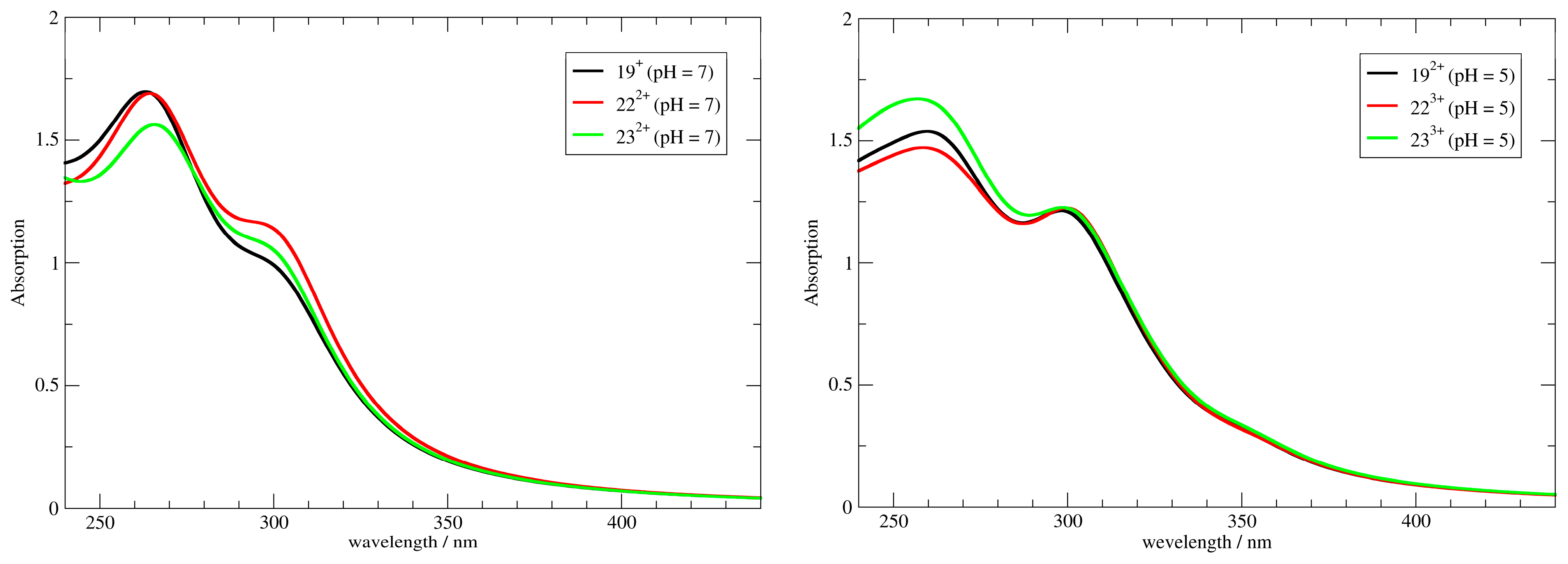
2.2.1. UV/Vis, Fluorescence, and Circular Dichroism (CD) Spectra
2.2.2. Mass Spectra (MS)
2.3. Study of Interactions of 19, 22, and 23 with ds-DNA and ds-RNA in Aqueous Media
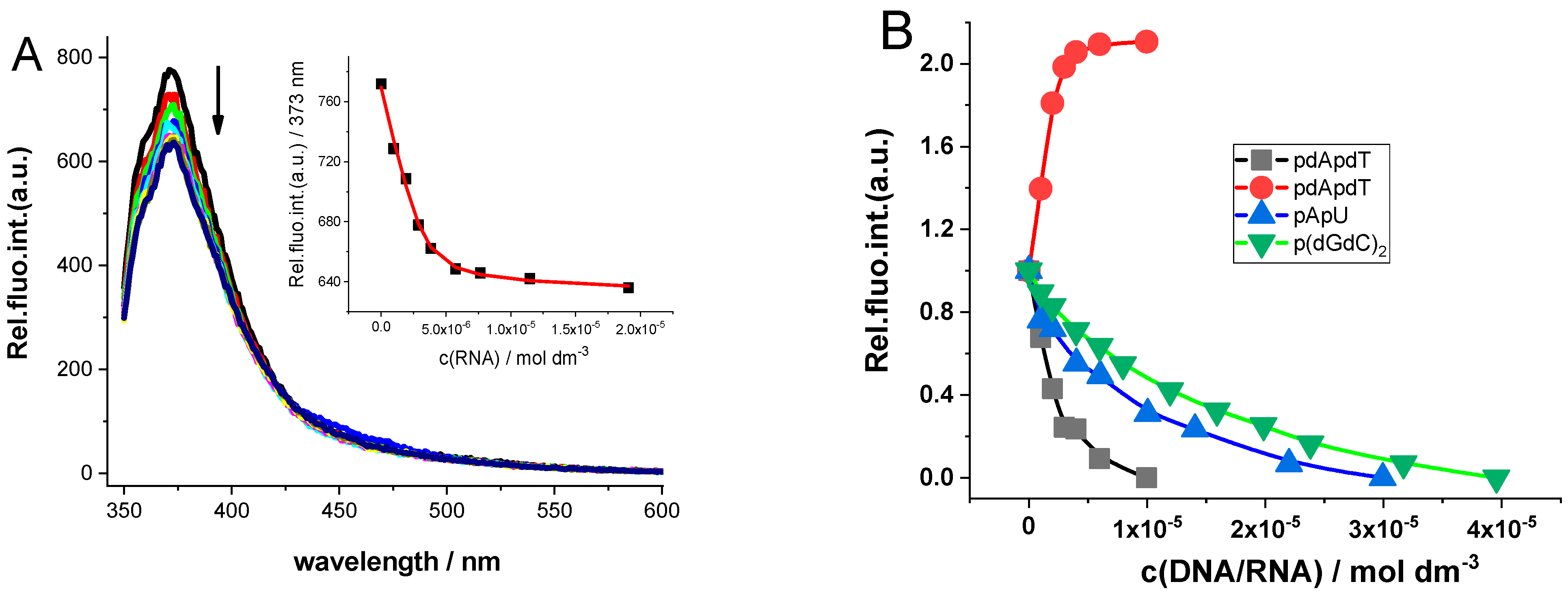
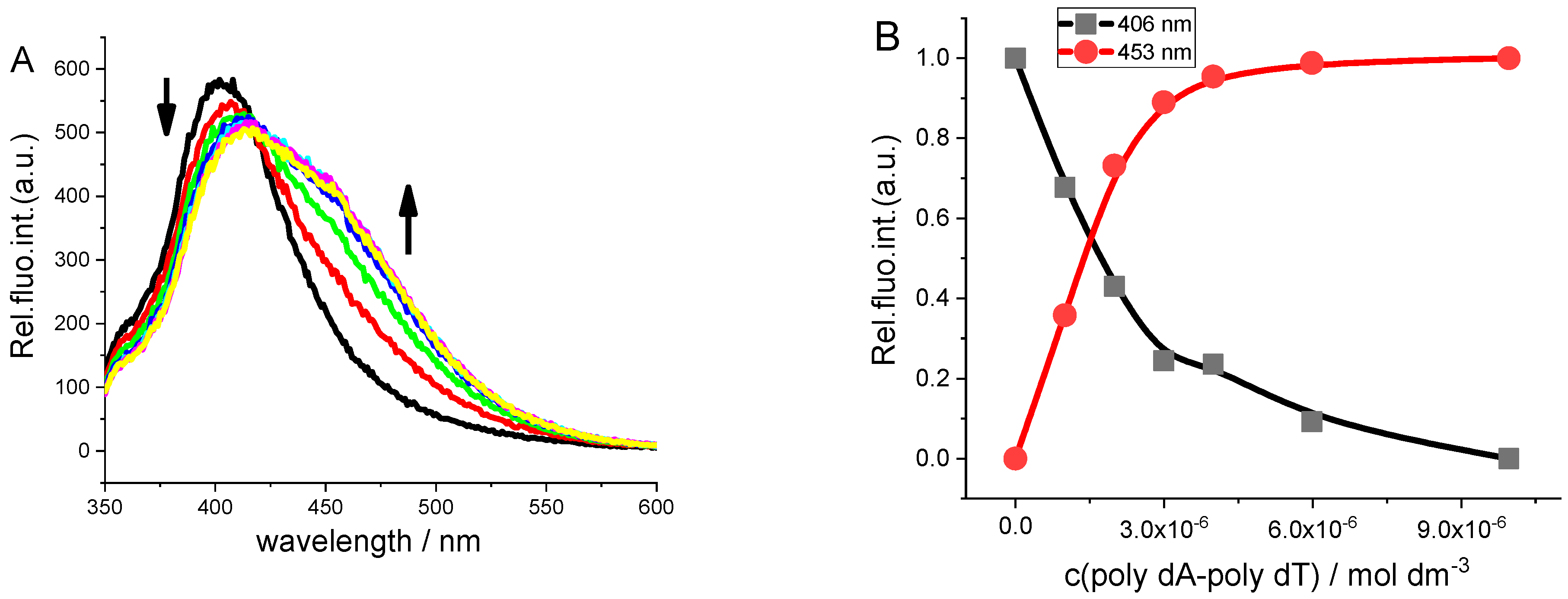
2.3.1. Spectrophotometric Titrations
2.3.2. Thermal Melting Experiments
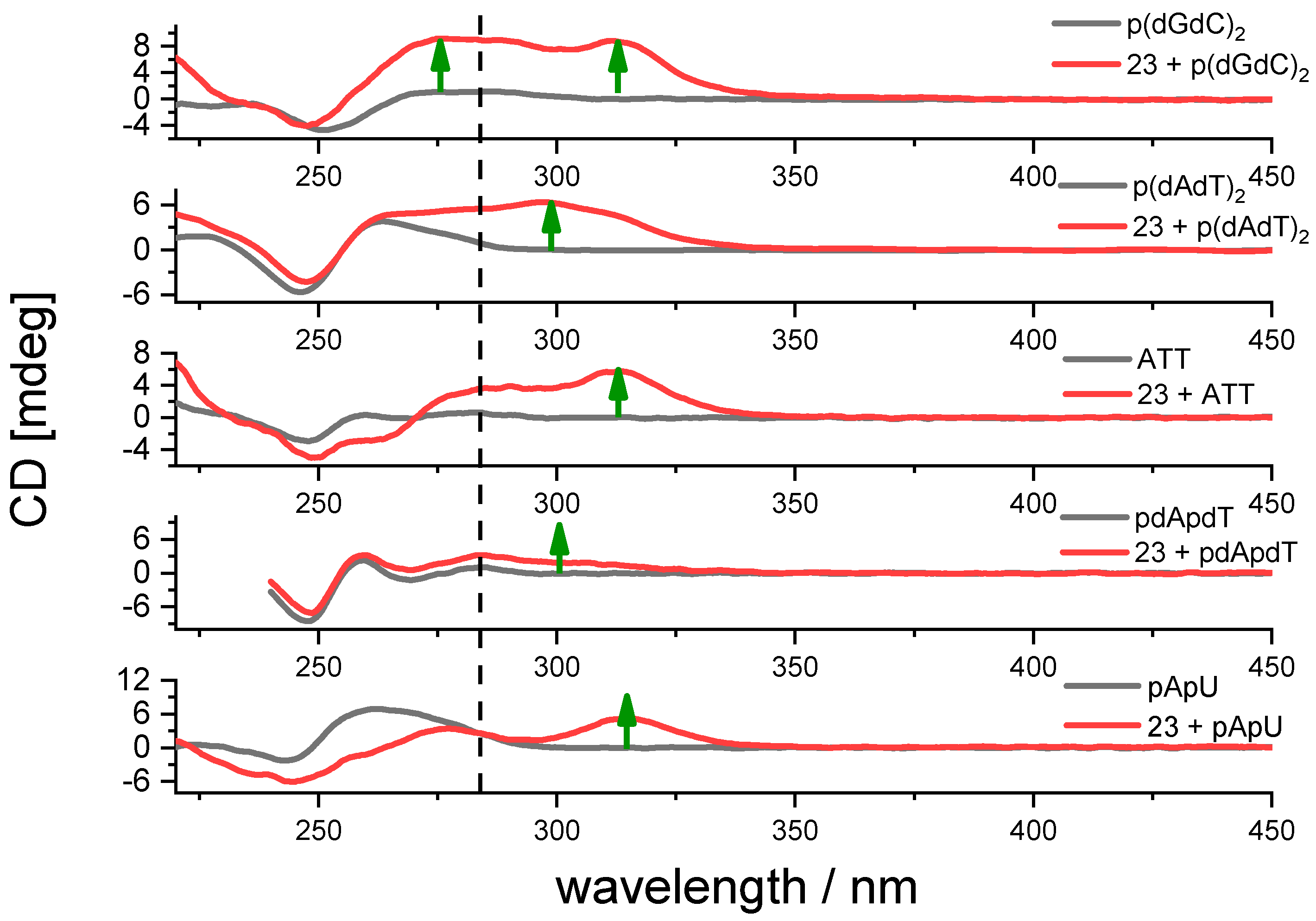
2.3.3. Circular Dichroism (CD) Experiments
2.4. Computational Analysis of Systems 19, 22, and 23 in Aqueous Solution
2.5. Biological Activity of 19, 22, and 23
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. Synthesis of HOOC-Gly-Lys-GCP-Boc (1a)
- Cbz-protection of 2 to obtain acid 3
- 2.
- Coupling of 3 with 4 to obtain peptide 5
- 3.
- Cbz-deprotection of 5 to obtain amine 6; coupling of 6 with 7 to obtain 8
- 4.
- Methyl ester deprotection of 8 to obtain acid 1a
3.1.2. Synthesis of HOOC-Lys-Gly-GCP-Boc (1b)
- Protection of 3 to obtain methyl ester 9
- 2.
- Cbz-deprotection of 9 to obtain amine 10
- 3.
- Coupling of 10 with 11 to obtain peptide 12
- 4.
- Cbz-deprotection of 12 to obtain amine 13; coupling of 13 with 7 to obtain 14
- 5.
- Methyl ester deprotection of 14 to obtain acid 1b
3.1.3. General Procedure for Peptide Coupling—Synthesis of 16, 17, 18, 20, and 21
- Synthesis of Phen-Ala-Gly-Boc (16):
- 2.
- Synthesis of Phen-Ala-Gly-Gly-Boc (17):
- 3.
- Synthesis of Phen-Ala-Gly-Gly-GCP-Boc (18):
- 4.
- Synthesis of Phen-Ala-Gly-Lys-GCP-Boc (20):
- 5.
- Synthesis of Phen-Ala-Lys-Gly-GCP-Boc (21):
3.1.4. General Procedure for –Boc Deprotection and Synthesis of Hydrochloride Salts 19, 22, and 23 (Figure 12)
- Synthesis of Phen-Ala-Gly-Gly-GCP (19):
- 2.
- Synthesis of Phen-Ala-Gly-Lys-GCP (22):
- 3.
- Synthesis of Phen-Ala-Gly-Gly-GCP (23):
3.2. Spectrophotometric Measurements
Mass Spectrometry Experimental for Section 2.2.2
3.3. MTT Assay
3.3.1. Cell Line
3.3.2. Cytotoxicity Assay
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutorine, A.S.; Novopashina, D.S.; Krasheninina, O.A.; Nozeret, K.; Venyaminova, A.G. Fluorescent Probes for Nucleic Acid Visualization in Fixed and Live Cells. Molecules 2013, 18, 15357–15397. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Rhee, W.J.; Tsourkas, A. Fluorescent Probes for Live-Cell RNA Detection. Annu. Rev. Biomed. Eng. 2009, 11, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Khan, A.; Qureshi, R.; Ansari, F.; Nazar, M.; Shah, S. Redox Behavior of Anticancer Chalcone on a Glassy Carbon Electrode and Evaluation of its Interaction Parameters with DNA. Int. J. Mol. Sci. 2008, 9, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Dembska, A.; Juskowiak, B. Pyrene functionalized molecular beacon with pH-sensitive i-motif in a loop. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 150, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.X.; Adhikari, R.; Bi, J.H.; Mazi, W.; Dorh, N.; Wang, J.B.; Conner, N.; Ainsley, J.; Karabencheva-Christova, T.G.; Luo, F.T.; et al. Fluorescent probes for sensitive and selective detection of pH changes in live cells in visible and near-infrared channels. J. Mater. Chem. B 2017, 5, 9579–9590. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.Y.; Dziuba, D.; Benhida, R.; Demchenko, A.P.; Burger, A. Probing of Nucleic Acid Structures, Dynamics, and Interactions With Environment-Sensitive Fluorescent Labels. Front. Chem. 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Gao, F.C.; Wang, Y.D.; Li, H.; Zhang, J.; Sun, Z.W.; Jiang, Y.Y. Fluorescent Organic Small Molecule Probes for Bioimaging and Detection Applications. Molecules 2022, 27, 8421. [Google Scholar] [CrossRef] [PubMed]
- Tumir, L.M.; Stojkovic, M.R.; Piantanida, I. Come-back of phenanthridine and phenanthridinium derivatives in the 21st century. Beilstein J. Org. Chem. 2014, 10, 2930–2954. [Google Scholar] [CrossRef] [PubMed]
- Jothi, D.; Manickam, S.; Sawminathan, S.; Munusamy, S.; R, S.K.; Ashok Kumar, S.K.; Kulathu Iyer, S. Phenanthridine-based fluorescence sensor for the “off-on” determination of thorium ion and its bio-imaging applications. Dye. Pigment. 2022, 197, 109826. [Google Scholar] [CrossRef]
- Sawminathan, S.; Munusamy, S.; Jothi, D.; Iyer, S.K. Phenanthridine-Based Donor/Acceptor Fluorescent Dyes: Synthesis, Photophysical Properties and Fluorometric Sensing of Biogenic Primary Amines. ChemistrySelect 2021, 6, 858–864. [Google Scholar] [CrossRef]
- Dragan, A.I.; Bishop, E.S.; Strouse, R.J.; Casas-Finet, J.R.; Schenerman, M.A.; Geddes, C.D. Metal-enhanced ethidium bromide emission: Application to dsDNA detection. Chem. Phys. Lett. 2009, 480, 296–299. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Liu, Q.; Tor, Y. Synthesis, photophysical properties, and nucleic acid binding of phenanthridinium derivatives based on ethidium. Bioorg. Med. Chem. 2003, 11, 5235–5247. [Google Scholar] [CrossRef]
- Luedtke, N.W.; Liu, Q.; Tor, Y. On the electronic structure of ethidium. Chemistry 2005, 11, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Kubař, T.; Hanus, M.; Ryjáček, F.; Hobza, P. Binding of Cationic and Neutral Phenanthridine Intercalators to a DNA Oligomer Is Controlled by Dispersion Energy: Quantum Chemical Calculations and Molecular Mechanics Simulations. Chem. Eur. J. 2005, 12, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Parenty, A.D.C.; Smith, L.V.; Guthrie, K.M.; Long, D.-L.; Plumb, J.; Brown, R.; Cronin, L. Highly Stable Phenanthridinium Frameworks as a New Class of Tunable DNA Binding Agents with Cytotoxic Properties. J. Med. Chem. 2005, 48, 4504–4506. [Google Scholar] [CrossRef]
- Rangarajan, S.; Friedman, S.H. Design, synthesis, and evaluation of phenanthridine derivatives targeting the telomerase RNA/DNA heteroduplex. Bioorg. Med. Chem. Lett. 2007, 17, 2267–2273. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.-A.; Rájecký, M.; Šebrlová, K.; Mravec, F.; Táborský, P. Influence of Solvent Polarity and DNA-Binding on Spectral Properties of Quaternary Benzo[c]phenanthridine Alkaloids. PLoS ONE 2015, 10, e0129925. [Google Scholar] [CrossRef] [PubMed]
- Matic, J.; Supljika, F.; Tandaric, T.; Duksi, M.; Piotrowski, P.; Vianello, R.; Brozovic, A.; Piantanida, I.; Schmuck, C.; Stojkovic, M.R. DNA/RNA recognition controlled by the glycine linker and the guanidine moiety of phenanthridine peptides. Int. J. Biol. Macromol. 2019, 134, 422–434. [Google Scholar] [CrossRef]
- Scholl, M.T. Synthesis, Structural Characterization and Applications of Hyperbranched Polymers Based on L-Lysine; EPFL: Lausanne, Switzerland, 2007. [Google Scholar]
- Arnon, Z.A.; Kreiser, T.; Yakimov, B.; Brown, N.; Aizen, R.; Shaham-Niv, S.; Makam, P.; Qaisrani, M.N.; Poli, E.; Ruggiero, A.; et al. On-off transition and ultrafast decay of amino acid luminescence driven by modulation of supramolecular packing. iScience 2021, 24, 102695. [Google Scholar] [CrossRef]
- Handula, M.; Chapeau, D.; Seimbille, Y. Orthogonal synthesis of a versatile building block for dual functionalization of targeting vectors. Open Chem. 2023, 21, 20220361. [Google Scholar] [CrossRef]
- Albert, A.; Goldacre, R.; Phillips, J. 455. The strength of heterocyclic bases. J. Chem. Soc. (Resumed) 1948, 2240–2249. [Google Scholar] [CrossRef]
- Brandão-Lima, L.C.; Silva, F.C.; Costa, P.V.C.G.; Alves-Júnior, E.A.; Viseras, C.; Osajima, J.A.; Bezerra, L.R.; de Moura, J.F.P.; de A. Silva, A.G.; Fonseca, M.G.; et al. Clay Mineral Minerals as a Strategy for Biomolecule Incorporation: Amino Acids Approach. Materials 2021, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, C.; Bickert, V.; Merschky, M.; Geiger, L.; Rupprecht, D.; Dudaczek, J.; Wich, P.; Rehm, T.; Machon, U. A Facile and Efficient Multi-Gram Synthesis of N-Protected 5-(Guanidinocarbonyl)-1H-pyrrole-2-carboxylic Acids. Eur. J. Org. Chem. 2007, 2008, 324–329. [Google Scholar] [CrossRef]
- Berova, N. Koji Nakanishi’s enchanting journey in the world of chirality. Chirality 1997, 9, 395–406. [Google Scholar] [CrossRef]
- Berova, N.; Bari, L.D.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, D.; Derguini, F.; Berova, N.; Nakanishi, K.; Harada, N. Unique ultraviolet-visible and circular dichroism behavior due to exciton coupling in a biscyanine dye. J. Am. Chem. Soc. 2002, 113, 7046–7047. [Google Scholar] [CrossRef]
- Baytekin, B.; Baytekin, H.T.; Schalley, C.A. Mass spectrometric studies of non-covalent compounds: Why supramolecular chemistry in the gas phase? Org. Biomol. Chem. 2006, 4, 2825–2841. [Google Scholar] [CrossRef] [PubMed]
- Schalley, C.A. Supramolecular chemistry goes gas phase: The mass spectrometric examination of noncovalent interactions in host-guest chemistry and molecular recognition. Int. J. Mass Spectrom. 2000, 194, 11–39. [Google Scholar] [CrossRef]
- Schalley, C.A. Molecular recognition and supramolecular chemistry in the gas phase. Mass Spectrom. Rev. 2001, 20, 253–309. [Google Scholar] [CrossRef]
- Springer, A.; Schalley, C.A. Mass Spectrometry and Gas-Phase Chemistry of Non-Covalent Complexes; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984; pp. 1–556. [Google Scholar]
- Wilson, W.D.; Wang, Y.H.; Krishnamoorthy, C.R.; Smith, J.C. Poly(dA).cntdot.poly(dT) exists in an unusual conformation under physiological conditions: Propidium binding to poly(dA).cntdot.poly(dT) and poly[d(A-T)].cntdot.poly[d(A-T)]. Biochemistry 2002, 24, 3991–3999. [Google Scholar] [CrossRef]
- McGhee, J.D.; von Hippel, P.H. Theoretical aspects of DNA-protein interactions: Co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J. Mol. Biol. 1974, 86, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Scatchard, G. The Attractions of Proteins for Small Molecules and Ions. Ann. N. Y. Acad. Sci. 2006, 51, 660–672. [Google Scholar] [CrossRef]
- Mergny, J.-L.; Lacroix, L. Analysis of Thermal Melting Curves. Oligonucleotides 2003, 13, 515–537. [Google Scholar] [CrossRef] [PubMed]
- Cantor, C.R.; Schimmel, P.R. Biophysical Chemistry; W. H. Freeman and Company: New York, NY, USA, 1980; p. 365. [Google Scholar]
- Arya, D.P.; Coffee, R.L.; Willis, B.; Abramovitch, A.I. Aminoglycoside–Nucleic Acid Interactions: Remarkable Stabilization of DNA and RNA Triple Helices by Neomycin. J. Am. Chem. Soc. 2001, 123, 5385–5395. [Google Scholar] [CrossRef] [PubMed]
- Arya, D.P. New Approaches Toward Recognition of Nucleic Acid Triple Helices. Acc. Chem. Res. 2010, 44, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Zonjić, I.; Kurutos, A.; Mihovilović, P.; Crnolatac, I.; Tumir, L.-M.; Paić, A.T.; Kralj, J.; Horvat, L.; Brozovic, A.; Stojković, R.; et al. Formation of triplex rA/dA-containing nucleic acid helices induced by new thiazole orange analogues and their biological evaluation. H-aggregate formation conditioned by rA sequence. Dye. Pigment. 2022, 207, 110715. [Google Scholar] [CrossRef]
- Pilch, D.S.; Kirolos, M.A.; Breslauer, K.J. Berenil binding to higher ordered nucleic acid structures: Complexation with a DNA and RNA triple helix. Biochemistry 2002, 34, 16107–16124. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, G.S. Interaction of Isoquinoline Alkaloids with an RNA Triplex: Structural and Thermodynamic Studies of Berberine, Palmatine, and Coralyne Binding to Poly(U).Poly(A)*Poly(U). J. Phys. Chem. B 2009, 113, 13410–13420. [Google Scholar] [CrossRef]
- Dalla Pozza, M.; Abdullrahman, A.; Cardin, C.J.; Gasser, G.; Hall, J.P. Three’s a crowd—Stabilisation, structure, and applications of DNA triplexes. Chem. Sci. 2022, 13, 10193–10215. [Google Scholar] [CrossRef]
- Jain, A.; Wang, G.; Vasquez, K.M. DNA triple helices: Biological consequences and therapeutic potential. Biochimie 2008, 90, 1117–1130. [Google Scholar] [CrossRef]
- Watson, J.D.; Baker, T.A.; Bell, S.P.; Gann, A.A.F.; Levine, M.; Losick, R.M. Molecular Biology of the Gene, 7th ed.; Pearson: London, UK, 2013; p. 912. [Google Scholar]
- Rodger, A.; Nordén, B. Circular Dichroism and Linear Dichroism; Oxford University Press: Oxford, UK, 1997; p. 150. [Google Scholar]
- Šmidlehner, T.; Piantanida, I.; Pescitelli, G. Polarization spectroscopy methods in the determination of interactions of small molecules with nucleic acids—Tutorial. Beilstein J. Org. Chem. 2018, 14, 84–105. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.N.; Perveen, F.; Nazar, M.F.; Mughal, E.U.; Rafique, H.; Tahir, M.N.; Akbar, M.S.; Zahra, S. Synthesis, characterization, DNA-Binding, enzyme inhibition and antioxidant studies of new N -methylated derivatives of pyridinium amine. J. Mol. Struct. 2017, 1137, 84–96. [Google Scholar] [CrossRef]
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular dichroism Principles and Applications, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Eriksson, M.; Nordén, B. Linear and circular dichroism of drug-nucleic acid complexes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; pp. 68–98. [Google Scholar] [CrossRef]
- Bernardi, G. Isochores and the evolutionary genomics of vertebrates. Gene 2000, 241, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Valin, F.; Lamolle, G.; Bernardi, G. Isochores, GC 3 and mutation biases in the human genome. Gene 2002, 300, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ban, Ž.; Žinić, B.; Vianello, R.; Schmuck, C.; Piantanida, I. Nucleobase–Guanidiniocarbonyl-Pyrrole Conjugates as Novel Fluorimetric Sensors for Single Stranded RNA. Molecules 2017, 22, 2213. [Google Scholar] [CrossRef] [PubMed]
- Matić, J.; Tandarić, T.; Radić Stojković, M.; Šupljika, F.; Karačić, Z.; Tomašić Paić, A.; Horvat, L.; Vianello, R.; Tumir, L.-M. Phenanthridine–pyrene conjugates as fluorescent probes for DNA/RNA and an inactive mutant of dipeptidyl peptidase enzyme. Beilstein J. Org. Chem. 2023, 19, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B.; Dattagupta, N.; Crothers, D.M. Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: Equilibrium binding studies on the interaction of daunomycin with deoxyribonucleic acid. Biochemistry 2002, 21, 3933–3940. [Google Scholar] [CrossRef]
- Bresloff, J.L.; Crothers, D.M. Equilibrium studies of ethidium-polynucleotide interactions. Biochemistry 2002, 20, 3547–3553. [Google Scholar] [CrossRef]
- Chalikian, T.V.; Völker, J.; Plum, G.E.; Breslauer, K.J. A more unified picture for the thermodynamics of nucleic acid duplex melting: A characterization by calorimetric and volumetric techniques. Proc. Natl. Acad. Sci. USA 1999, 96, 7853–7858. [Google Scholar] [CrossRef]
- Mickisch, G.; Fajta, S.; Keilhauer, G.; Schlick, E.; Tschada, R.; Alken, P. Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT). Urol. Res. 1990, 18, 131–136. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bauer, P.; Hess, B.; Lindahl, E. GROMACS 2022.5 Manual (2022.5); Zenodo: Geneva, Switzerland, 2023. [Google Scholar]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Jeddi, I.; Saiz, L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci. Rep. 2017, 7, 1178. [Google Scholar] [CrossRef] [PubMed]
- Beč, A.; Vianello, R.; Hranjec, M. Synthesis and spectroscopic characterization of multifunctional D-π—A benzimidazole derivatives as potential pH sensors. J. Mol. Liq. 2023, 386, 122493. [Google Scholar] [CrossRef]
- Boček, I.; Hranjec, M.; Vianello, R. Imidazo[4,5-b]pyridine derived iminocoumarins as potential pH probes: Synthesis, spectroscopic and computational studies of their protonation equilibria. J. Mol. Liq. 2022, 355, 118982. [Google Scholar] [CrossRef]




| UV/Vis | Fluorescence | ||
|---|---|---|---|
| λmax (nm)/ε × 103 (dm3 mol−1 cm−1) | Emission | ||
| pH 5.0 | pH 7.0 | λmax (nm) | |
| 22 | 252/43.6 298/21.4 | 251/48.3 300/23.3 | 397 a/372 b |
| 23 | 252/46.7 297/24.1 | 251/44.1 297/22.5 | 397 a/372 b |
| Phen-AA | 251/49.5 300/4.8 | 250/50.6 300/4.5 | 402 a/370 b |
| Phen-Gly | 251/51.9 300/5.1 | 250/53.7 300/4.8 | 403 a/370 b |
| GCP | 250/4.0 297/27.3 | 250/6.1 297/28.4 | - |
| pH | ctDNA | p(dAdT)2 | p(dGdC)2 | pApU | pdApdT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log Ka | n | I/I0 b | log Ka | n | I/I0 b | log Ka | n | I/I0 b | log Ka | n | I/I0 b | log Ka | n | I/I0 b | ||
| 7.0 | 22 | - c | - c | - c | 6.7 | 0.3 | 0.9 | 6.1 | 0.8 | 0.9 | - c | - c | - c | - c | - c | - c |
| 23 | 7.0 | 0.5 | 0.8 | 6.7 | 0.2 | 0.9 | 5.8 | 0.4 | 0.6 | 7.1 | 0.3 | 0.8 | 6.6 | 0.3 | 0.9 | |
| 5.0 | 22 | 5.7 | 0.3 | 0.7 | 5.3 | 0.5 | 0.6 | 5.3 | 0.4 | 0.3 | 5.5 | 0.5 | 0.8 | - c | - c | - c |
| 23 | 5.5 | 0.5 | 0.8 | 6.0 | 0.2 | 0.6 | 5.3 | 0.3 | 0.3 | 5.8 | 0.2 | 0.8 | 6.3 d 7.4 e | 0.5 0.4 | 0.8 2.1 | |
| b r = 0.3 | ctDNA | pApU | pdApdT | p(dAdT)2 | |
|---|---|---|---|---|---|
| pH 7.0 | 19 | 0.6 | 0 | 0.7 | 1.2 |
| 22 | 0.9 | 0 | 1.5 | 2.6 | |
| 23 | 0.7 | 2.5 | -/2.1 c | 1.0 | |
| pH 5.0 | 19 | 1.3 | 1.1/1.0 d | 0.5 | 1.3 |
| 22 | 1.2 | 0/0.8 d | 1.3 | 3.9 | |
| 23 | 1.3 | 0/0.8 d | -/0.7 c | 3.0 |
| b r = 0.3 | pdApdT DUPLEX | ATT TRIPLEX | ||
|---|---|---|---|---|
| Tm | ΔTm | Tm | ΔTm | |
| pH 5.0 | 70.1 | - | 23.8/70.3 c | - |
| 27.3/70.2 | -/0.1 | 29.3/70.0 | 5.5/0.3 | |
| pH 7.0 | 70.8 | - | 25.1/71.3 c | - |
| 71.3 | 0.5 | 26.2/71.1 | 1.1/−0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matić, J.; Piotrowski, P.; Vrban, L.; Kobetić, R.; Vianello, R.; Jurić, I.; Fabijanić, I.; Pernar Kovač, M.; Brozovic, A.; Piantanida, I.; et al. Distinctive Nucleic Acid Recognition by Lysine-Embedded Phenanthridine Peptides. Int. J. Mol. Sci. 2024, 25, 4866. https://doi.org/10.3390/ijms25094866
Matić J, Piotrowski P, Vrban L, Kobetić R, Vianello R, Jurić I, Fabijanić I, Pernar Kovač M, Brozovic A, Piantanida I, et al. Distinctive Nucleic Acid Recognition by Lysine-Embedded Phenanthridine Peptides. International Journal of Molecular Sciences. 2024; 25(9):4866. https://doi.org/10.3390/ijms25094866
Chicago/Turabian StyleMatić, Josipa, Patryciusz Piotrowski, Lucija Vrban, Renata Kobetić, Robert Vianello, Ivona Jurić, Ivana Fabijanić, Margareta Pernar Kovač, Anamaria Brozovic, Ivo Piantanida, and et al. 2024. "Distinctive Nucleic Acid Recognition by Lysine-Embedded Phenanthridine Peptides" International Journal of Molecular Sciences 25, no. 9: 4866. https://doi.org/10.3390/ijms25094866
APA StyleMatić, J., Piotrowski, P., Vrban, L., Kobetić, R., Vianello, R., Jurić, I., Fabijanić, I., Pernar Kovač, M., Brozovic, A., Piantanida, I., Schmuck, C., & Radić Stojković, M. (2024). Distinctive Nucleic Acid Recognition by Lysine-Embedded Phenanthridine Peptides. International Journal of Molecular Sciences, 25(9), 4866. https://doi.org/10.3390/ijms25094866









