Pharmacotherapy for Keloids and Hypertrophic Scars
Abstract
1. Introduction
2. Comparison of Biological Characteristics between Keloids and Hypertrophic Scars
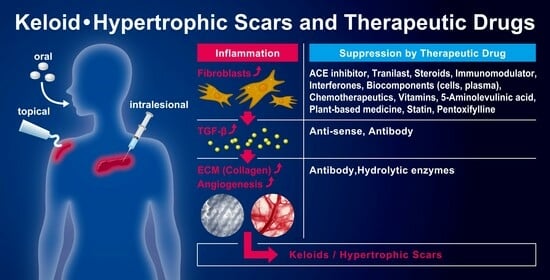
3. Pharmacotherapy for Keloids and Hypertrophic Scars
3.1. ACE Inhibitors (Captopril, Enalapril, Losartan)
3.2. Antiallergic Agent (Tranilast)
3.3. Antisense Drug (Antisense Oligodeoxynucleotides of TGF-β1, SMAD3, and TERT)
3.4. Anti-Viral Cytokines (Interferons)
3.5. Calcium Antagonists (Verapamil)
3.6. Chemotherapeutics (Bleomycin, 5-Fluorouracil, Mitomycin C, Paclitaxel, Tamoxifen)
3.6.1. Bleomycin (BLM)
3.6.2. Hydroxycamptothecin (HCPT)
3.6.3. 5-Fluorouracil (5-FU)
3.6.4. Mitomycin C (MMC)
3.6.5. Paclitaxel (PCT)
3.6.6. Tamoxifen (TAM)
3.7. Enzyme (Collagenase, Hyaluronidase)
3.7.1. Collagenase
3.7.2. Hyaluronidase
3.8. Fat-Soluble Vitamins (Vitamin A, Vitamin D3, Vitamin E)
3.8.1. Vitamin A
3.8.2. Vitamin D
3.8.3. Vitamin E (α-Tocopherol)
3.9. Immunomodulator (Tacrolimus, Imiquimod)
3.9.1. Tacrolimus
3.9.2. Imiquimod
3.10. Monoclonal Antibody (Dupilumab, Anti-TGF-β1 Antibody, Anti-VEGF-A Antibody)
3.10.1. Dupilumub
3.10.2. Anti-TGF-β1 Antibody
3.10.3. Anti-VEGF-A Antibody
3.11. Neurotoxin (Botulinum Toxin, BTX)
3.12. Peripheral Vasodilator (Pentoxifylline)
3.13. Photosensitizer Prodrug (5-Aminolevulinic Acid, Methyl Aminolevulinate)
3.14. Plant-Based Medicine (Aloe Vera, Centella asiatica, Curcuminoids (Curcumin), Green Tea (Catechins), Hyperforin, Loureirin A/B, Onion Extract (Quercetin), Resveratrol, Saireito, Shikonin, Emodin, Glabridin, Kaempferol, Tripterine, Wubeizi)
3.14.1. Aloe Vera
3.14.2. Centella asiatica (Asiaticoside, Asiatic Acid, Madecasosside, Madecassic Acid)
3.14.3. Curcuminoids (Curcumin)
3.14.4. Green Tea Extract (Catechins Especially (-)-Epigallocatechin-3-gallate, EGCG)
3.14.5. Hyperforin
3.14.6. Loureirin A/B
3.14.7. Onion Extract, Contractubex® Gel
3.14.8. Resveratrol
3.14.9. Saireito (or Sairei-to)
3.14.10. Shikonin
3.14.11. Emodin
3.14.12. Other Plant-Based Medicines (Glabridin, Kaempferol, Tripterine, Wubeizi)
3.15. Statins (Simvastatin, Lovastatin, Pravastatin, Atorvastatin)
3.15.1. Simvastatin
3.15.2. Atorvastatin, Lovastatin, Pravastatin
3.16. Steroids (Triamcinolone acetonide, Dexamethasone, Hydrocortisone Acetate, Methylprednisolone)
3.16.1. Triamcinolone Acetonide (TAC)
3.16.2. Dexamethasone (DEX)
3.17. Regenerative Medicine (Fat Grafting, Platelet-Rich Plasma, Adipose-Derived Stroma Vascu-Lar Fraction, Adipose-Derived Stem Cells, Mesenchymal Stem Cells, Hyaluronic Acid)
3.17.1. Fat Grafting
3.17.2. Platelet-Rich Plasma (PRP)
3.17.3. Adipose-Derived Stromal Vascular Fraction (SVF)
3.17.4. Adipose-Derived Stem Cells (ASC)
3.17.5. Mesenchymal Stem Cells (MSC)
3.17.6. Hyaluronic Acid (HyA)
3.18. Comparison of Clinical Efficacy between Triamcinolone Acetonide and Other Drugs
| Drugs | Pharmacological Action |
|---|---|
| ACE inhibitor Captopril Enalapril Losartan | Angiotensin-converting enzyme (ACE) inhibitors reduce fibroblast proliferation, suppress collagen and TGF-β1 expression, and downregulate the phosphorylation of SMAD2/3 and TAK1. ACE inhibitors such as captopril, enalapril, and losartan inhibit the production of angiotensin II, TGF-β1 and ECMs such as collagen [28,29,30]. |
| Antiallergic agent Tranilast | Tranilast, an orally administered drug, suppresses type I allergic reactions by inhibiting the release of chemical mediators such as histamine and leukotrienes from mast cells and various inflammatory cells. It also inhibits the production of collagen, TGF-β, INF-γ, IL-6, IL-10, IL-17, VEGF, MMP-2, MMP-9, TNF-α, some other angiogenic, and inflammatory factors [38,40,42,43]. |
| Antisense drug TGF-β1 antisense SMAD3 antisense hTERT antisense | Topically applied TGF-β1 antisense preparations downregulate TGF-β1 protein levels and improve scar histology as determined by the scar elevation index in vivo [62]. Treatment with SMAD3 antisense inhibits SMAD3, a primary inducer of fibrosis, and suppresses collagen production in KD fibroblasts [63]. Human telomerase reverse transcriptase (hTERT) antisense oligodeoxynucleotide suppresses the growth and proliferation of KD fibroblasts and inhibits telomerase activity in KD fibroblasts [64]. |
| Antiviral cytokines Interferons | Interferon (IFN)-α, β, and γ suppress collagen synthesis by dermal fibroblasts. IFN-γ also suppresses collagen synthesis by myofibroblasts, synovial fibroblast-like cells, and type II collagen synthesis in human articular chondrocytes [68]. |
| Calcium-channel blockers Verapamil | Verapamil inhibits transmembrane calcium influx, the growth and proliferation of vascular smooth muscle cells and fibroblasts, and the synthesis of ECM proteins (collagen, fibronectin, proteoglycans) [76,78]. |
| Chemotherapeutics Bleomycin Camptothecin 5-Fluorouracil Mitomycin C Paclitaxel Tamoxifen | Chemotherapeutics such as bleomycin, camptothecin and 5-fluorouracil can induce apoptosis, autophagy and cell cycle arrest in tumor cells by inhibiting DNA synthesis and interfering with RNA. Metabolites of mitomycin C also interfere with the synthesis of DNA, RNA and proteins [95,96,97,122]. Liposomal paclitaxel can suppress the production of TNF-α, IL-6 and TGF-β and inhibit the expression of α-SMA and collagen I in human KD fibroblast [133]. Tamoxifen decreases the expression of TGF-β1, with the consequent inhibitions of both fibroblast proliferation and collagen production [135]. |
| Enzyme Collagenase Hyaluronidase | The intralesional injection of collagenase can degrade collagen fiber directly and decrease KD volume promptly [145]. Hyaluronidase produces low-molecular-weight fragments during the digestion of high-molecular-weight hyaluronic acid. These fragments are known to stimulate angiogenesis and activate mesenchymal stem cells [144]. |
| Fat-soluble vitamin Vitamin A Vitamin D3 Vitamin E | Vitamins are effective in the treatment of inflammatory dermatoses, acne, pigmentation disorders and wound healing [153]. Vitamin A significantly reduces fibroblast proliferation and collagen synthesis in vitro and in vivo [158]. Vitamin D3 slows the progression of tissue fibrosis by KD fibroblasts and inhibits collagen synthesis in dermal fibrosis [170,173]. Vitamin E supplementation is beneficial for wound repair and immune functions [176]. |
| Immunomodulator Tacrolimus Imiquimod | Tacrolimus inhibits KD fibroblast proliferation, migration and collagen production enhanced by TGF-β1. The increase in TGF-β receptor I and II expression in TGF-β1-treated KD fibroblasts is suppressed by tacrolimus treatment [179]. It also suppresses smooth muscle actin, reduces mucin, and improves the quality of collagen fibers and the density of elastic fibers [183]. Imiquimod and its metabolite, immune-modulators, induce IFN-α in human blood cells, and IL-1, IL-6, IL-8, and TNF-α in human PBMC cultures in vitro [185,186] |
| Monoclonal antibody Dupilumab Anti-TGF-β1 antibody Anti-VEGF-A antibody | Dupilumab, a human monoclonal IgG4 antibody, inhibits IL-4 and IL-13 signaling by binding to the IL-4Rα receptor subunit affecting cellular transcription [195,196,197,198,199]. TGF-β has differential temporal effects in the healing of the wound, and anti-TGF-β1 antibodies can modify the healing process [201,202]. VEGF-A is a key cytokine in developing blood vessels in normal tissues and other tissues undergoing abnormal angiogenesis. Anti-VEGF antibodies exhibit therapeutic utility in blocking VEGF-induced angiogenesis [207]. |
| Neurotoxin Botulinum toxin A | Botulinum toxin A is a potent neurotoxin protein that exerts its effect at the neuromuscular junction by inhibiting the release of acetylcholine, which causes temporary chemical denervation, induces temporary muscular paralysis, and relieves the tension on wound edges [202,203,204,205,206,207,208,209]. |
| Peripheral vasodilator Pentoxifylline | Pentoxifylline regulates TGF-β1-induced fibroblast activation, modifies the expression of collagen types I and III by human fibroblasts, and inhibits the proliferation and rate of collagen synthesis of fibroblasts [230,415,416]. |
| Photosensitizer prodrug 5-Aminolevulinic acid | Photodynamic therapy using topical 5-aminolevulinic acid and red light can reduce cell viability and TGF-β1-mediated signaling by inducing cell apoptosis in human HTS fibroblasts [246]. |
| Plant-based Aloe vera Centella asiatica Contractubex Curcuminoids (curcumin) Green tea (EGCG), Hyperforin Loureirin A/B Onion extract (quercetin) Resveratrol Saireito Shikonin Emodin Glabridin Kaempferol Tripterine Wubeizi | Aloe vera exhibits potential antioxidant, anti-inflammatory, and wound-healing activities [257,263]. Centella asiatica decreases fibroblast proliferation, inhibits type I and type III collagen protein and mRNA expressions, and reduces the expression of both TGF-βRI and TGF-βRII at the transcriptional and translational level [265,268]. Contractubex has a softening and smoothing effect and improves the quality of wound healing by reducing scar formation [295,297]. Curcumin blocks the elevation of ECM and TGF-β1/p-SMAD-2 levels in a dose-dependent manner in KD fibroblasts [273]. EGCG can downregulate the expression levels of collagen by inhibiting the TGF-β/Smad3 signaling pathway [278,280]. Hyperforin reduces the viability of human dermal fibroblasts by reducing the proliferation of human dermal fibroblasts [285]. Loureirin A/B inhibits the proliferation, migration and TGF-β1-induced myofibroblast differentiation of KD fibroblasts [287,289]. Onion extract contains quercetin and kaempferol, which inhibit fibroblast proliferation and collagen production by inhibiting TGF-β1, TGF-β2 and SMAD proteins [291]. Resveratrol inhibits collagen synthesis in KD fibroblasts, by downregulating HIF-1α [305]. Orally administered saireito can reduce postoperative edema after blepharoptosis surgery by suppressing TGF-β1-induced Smad 2/3 phosphorylation [254,314]. Shikonin inhibits the expression of p63, cytokeratin 10, α-SMA, TGF-β1, and collagen I [310]. Emodin exerts an anti-fibrotic effect by suppressing TGF-β1 signaling and subsequently inhibiting inflammation, myofibroblast differentiation and ECM deposition [316,318]. Glabridin can suppress the human KD fibroblast cells’ proliferation by inducing apoptosis and reducing collagen production [320]. Kaempferol inhibits TGF-β1/Smads signaling and inhibits fibroblast collagen synthesis, proliferation and activation in HTSs [322]. Tripterine inhibits proliferation and promotes the apoptosis of KD fibroblasts by inducing ROS generation and activating the JNK signalling pathway [323]. Wubeizi ointment suppressed KD formation by inhibiting fibroblast proliferation and promoting fibroblast apoptosis [326]. |
| Statin Pravastatin Simvastatin | Pravastatin reduces the scar elevation index and decreases type I/III collagen content and myofibroblast persistence in the wound. Simvastatin is an effective inhibitor of the TGF-β1-induced production of type I collagen, connective tissue growth factor, and α-SMA production in KD fibroblasts [330,335,336]. |
| Steroids Triamcinolone acetonide, Dexamethasone, Hydrocortisone acetate, Methylprednisolone | Triamcinolone acetonide is the criterion standard in the nonsurgical management of KDs and HTSs, and these glucocorticoids inhibit the cellular proliferation and production of collagen, glycosaminoglycan, hyaluronic acid, and TGF-β1 by dermal fibroblasts [340,344]. Other glucocorticoids such as dexamethasone, hydrocortisone and methylprednisolone also suppress VFGA expression. |
3.19. Combination Pharmacotherapy of Triamcinolone Acetonide with Other Drug(s)
3.19.1. Combination of Triamcinolone Acetonide with 5-Fluorouracil
3.19.2. Combination of Triamcinolone Acetonide with Verapamil
3.19.3. Combination of Triamcinolone Acetonide with Other Drugs (Bleomycin, Botulinum Toxin A, Interferon, Pentoxifylline, Platelet-Rich-Plasma)
3.20. Combination of Pharmacotherapy with Physical Therapy
3.20.1. Pharmacotherapy Combined with Surgical Excision and Cryotherapy
3.20.2. Pharmacotherapy Combined with Surgical Excision and Laser Therapy
3.20.3. Pharmacotherapy Combined with Surgical Excision and Radiotherapy
3.20.4. Pharmacotherapy Combined with Surgical Excision and Silicone Gel/Sheeting
3.21. Prevention of Recurrence of KD/HTS Scars after Surgical Excision
4. Discussion
4.1. Effect of the Starting Time of Pharmacotherapy
4.2. Effect of the Dose and Dosing Formulation
4.3. Effect of Evaluation Method of Clinical Efficacy
4.4. Effect of the Timing of Evaluation of Clinical Efficacy
4.5. Effect of Treatment Techniques
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogawa, R.; Akaishi, S.; Kuribayashi, S.; Miyashita, T. Keloids and hypertrophic scars can now be cured completely: Recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J. Nippon. Med. Sch. 2016, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jang, Y.J. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef] [PubMed]
- Barone, N.; Safran, T.; Vorstenbosch, J.; Davison, P.G.; Cugno, S.; Murphy, A.M. Current advances in hypertrophic scar and keloid management. Semin. Plast. Surg. 2021, 35, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guarino, M.; Bacci, S.; Pérez González, L.A.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. The role of physical therapies in wound healing and assisted scarring. Int. J. Mol. Sci. 2023, 24, 7487. [Google Scholar] [CrossRef] [PubMed]
- Limmer, E.E.; Glass, D.A., II. A review of current keloid management: Mainstay monotherapies and emerging approaches. Dermatol. Ther. 2020, 10, 931–948. [Google Scholar] [CrossRef] [PubMed]
- Ekstein, S.F.; Wyles, S.P.; Moran, S.L.; Meves, A. Keloids: A review of therapeutic management. Int. J. Dermatol. 2021, 60, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids: A 2020 update of the algorithms published 10 years ago. Plast Reconstr. Surg. 2022, 149, 79e–94e. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Upton, Z.; Leavesley, D.; Fan, C.; Wang, X.Q. Vascular and collagen target: A rational approach to hypertrophic scar management. Adv. Wound Care 2023, 12, 38–55. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Bran, G.M.; Goessler, U.R.; Hormann, K.; Riedel, F.; Sadick, H. Keloids: Current concepts of pathogenesis (review). Int. J. Mol. Med. 2009, 24, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and hypertrophic scars: Pathophysiology, classification, and treatment. Dermatol. Surg. 2017, 43 (Suppl. S1), S3–S18. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R. Wide spread scars, hypertrophic scars, and keloids. Clin. Plast. Surg. 1987, 14, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Sun, X.L.; Liu, D.Z.; Xu, F.; Feng, S.J.; Zhang, S.Y.; Li, L.Z.; Zhou, J.L.; Wang, Y.T.; Zhang, L.; et al. Epidemiological and clinical features of hypertrophic scar and keloid in Chinese college students: A university-based cross-sectional survey. Heliyon 2023, 9, e15345. [Google Scholar] [CrossRef] [PubMed]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front. Cell Dev. Biol. 2020, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C. Epidemiology of skin diseases in people of color. Cutis 2003, 71, 271–275. [Google Scholar] [PubMed]
- Brown, J.J.; Ollier, W.; Arscott, G.; Ke, X.; Lamb, J.; Day, P.; Bayat, A. Genetic susceptibility to keloid scarring: SMAD gene SNP frequencies in Afro-Caribbeans. Exp. Dermatol. 2008, 17, 610–613. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Zargham, R.; Gilardino, M.S.; Sasseville, D.; Jafarian, F. Insights into the pathophysiology of hypertrophic scars and keloids: How do they differ? Adv. Skin Wound Care 2018, 31, 582–595. [Google Scholar] [CrossRef]
- Hellström, M.; Hellström, S.; Engström-Laurent, A.; Bertheim, U. The structure of the basement membrane zone differs between keloids, hypertrophic scars and normal skin: A possible background to an impaired function. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.W.; Boyd, C.D.; Mackenzie, J.W.; Norton, P.; Olson, R.M.; Deak, S.B. Regulation of collagen gene expression in keloids and hypertrophic scars. J. Surg. Res. 1993, 55, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Shin, E.; Kim, H.; Kwak, I.S.; Choi, Y. Interleukin-31, interleukin-31RA, and OSMR expression levels in post-burn hypertrophic scars. J. Pathol. Transl. Med. 2018, 52, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Miyauchi, S.; Miki, Y. Proliferating activity of dermal fibroblasts in keloids and hypertrophic scars. Acta Derm. Venereol. 1995, 75, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Garikaparthi, S.; Smith, E.; Newburger, J. A novel hydrogel scaffold for the prevention or reduction of the recurrence of keloid scars postsurgical excision. J. Am. Acad. Dermatol. 2013, 69, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Akita, S.; Akaishi, S.; Aramaki-Hattori, N.; Dohi, T.; Hayashi, T.; Kishi, K.; Kono, T.; Matsumura, H.; Muneuchi, G.; et al. Diagnosis and treatment of keloids and hypertrophic scars-Japan Scar Workshop Consensus Document 2018. Burns Trauma. 2019, 7, 39. [Google Scholar] [CrossRef]
- Fang, Q.Q.; Wang, X.F.; Zhao, W.Y.; Ding, S.L.; Shi, B.H.; Xia, Y.; Yang, H.; Wu, L.H.; Li, C.Y.; Tan, W.Q. Angiotensin-converting enzyme inhibitor reduces scar formation by inhibiting both canonical and noncanonical TGF-β1 pathways. Sci. Rep. 2018, 8, 3332. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.Y.; Wang, Z.C.; Fang, Q.Q.; Wang, X.F.; Du, Y.Z.; Shi, B.H.; Lou, D.; Xuan, G.D.; Tan, W.Q. The compound losartan cream inhibits scar formation via TGF-β/Smad pathway. Sci. Rep. 2022, 12, 14327. [Google Scholar] [CrossRef]
- Brown, S.; Nores, G.D.G.; Sarker, A.; Ly, C.; Li, C.; Park, H.J.; Hespe, G.E.; Gardenier, J.; Kuonqui, K.; Campbell, A.; et al. Topical captopril: A promising treatment for secondary lymphedema. Transl. Res. 2023, 257, 43–53. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, S.; Liu, Y.; Cen, Y.; Nicolas, C. Effect of captopril on collagen metabolisms in keloid fibroblast cells. ANZ J. Surg. 2016, 86, 1046–1051. [Google Scholar] [CrossRef]
- Ardekani, G.S.; Aghaie, S.; Nemati, M.H.; Handjani, F.; Kasraee, B. Treatment of a postburn keloid scar with topical captopril: Report of the first case. Plast. Reconstr. Surg. 2009, 123, 112e–113e. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Parand, A.; Kardeh, S.; Janati, M.; Mohammadi, S. Efficacy of topical enalapril in treatment of hypertrophic scars. World J. Plast. Surg. 2018, 7, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Bitik, O.; Hekimoğlu, R.; Atilla, P.; Kaykçoğlu, A.U. Angiotensin-converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast. Reconstr. Surg. 2013, 132, 361e–371e. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fang, Q.Q.; Wang, X.F.; Shi, B.H.; Zhao, W.Y.; Chen, C.Y.; Zhang, M.X.; Zhang, L.Y.; Hu, Y.Y.; Shi, P.; et al. The effect of topical ramipril and losartan cream in inhibiting scar formation. Biomed. Pharmacother. 2019, 118, 109394. [Google Scholar] [CrossRef] [PubMed]
- Hedayatyanfard, K.; Ziai, S.A.; Niazi, F.; Habibi, I.; Habibi, B.; Moravvej, H. Losartan ointment relieves hypertrophic scars and keloid: A pilot study. Wound Repair Regen. 2018, 26, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Akershoek, J.J.; Brouwer, K.M.; Vlig, M.; Boekema, B.K.H.L.; Beelen, R.H.J.; Middelkoop, E.; Ulrich, M.M.W. Differential effects of losartan and atorvastatin in partial and full thickness burn wounds. PLoS ONE 2017, 12, e0179350. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef]
- Yamada, H.; Tajima, S.; Nishikawa, T.; Murad, S.; Pinnell, S.R. Tranilast, a selective inhibitor of collagen synthesis in human skin fibroblasts. J. Biochem. 1994, 116, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Tajima, S.; Nishikawa, T. Tranilast inhibits collagen synthesis in normal, scleroderma and keloid fibroblasts at a late passage culture but not at an early passage culture. J. Dermatol. Sci. 1995, 9, 45–47. [Google Scholar] [CrossRef]
- Konneh, M. Tranilast Kissei Pharmaceutical. IDrugs 1998, 1, 141–146. [Google Scholar]
- Chakrabarti, R.; Subramaniam, V.; Abdalla, S.; Jothy, S.; Prud’homme, G.J. Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs 2009, 20, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Norooznezhad, A.H.; Norooznezhad, F.; Ahmadi, K. Next target of tranilast: Inhibition of corneal neovascularization. Med. Hypotheses 2014, 82, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R.; Nashibi, R.; Houshmandfar, S.; Tahmaseby Gandomkari, S.; Khodadadi, A. Tranilast: A potential anti-inflammatory and NLRP3 inflammasome inhibitor drug for COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 247–258. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawatus, T.; Okada, N.; Hata, S.; Higashi, N.; Suzuki, T.; Kawatsu, T.; Yamada, T.; Okumura, M.; Akimoto, N.; et al. Clinical evaluation of tranilast for keloid and hypertrophic scar. Ski Res. 1992, 34, 129–138. [Google Scholar]
- Nanba, K.; Oura, T.; Soeda, S.; Sioya, N.; Tsukada, S.; Hanaoka, K. Clinical evaluation of tranilast for keloid and hypertrophic scarring. Optimal dose finding study in a double blind study. Nessho 1992, 18, 30–45. [Google Scholar]
- Sun, X.; Suzuki, K.; Nagata, M.; Kawauchi, Y.; Yano, M.; Ohkoshi, S.; Matsuda, Y.; Kawachi, H.; Watanabe, K.; Asakura, H.; et al. Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol. Int. 2010, 60, 93–101. [Google Scholar] [CrossRef]
- Shigeki, S.; Murakami, T.; Yata, N.; Ikuta, Y. Treatment of keloid and hypertrophic scars by iontophoretic transdermal delivery of tranilast. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1997, 31, 151–158. [Google Scholar] [CrossRef]
- Murakami, T.; Yoshioka, M.; Yumoto, R.; Higashi, Y.; Shigeki, S.; Ikuta, Y.; Yata, N. Topical delivery of keloid therapeutic drug, tranilast, by combined use of oleic acid and propylene glycol as a penetration enhancer: Evaluation by skin microdialysis in rats. J. Pharm. Pharmacol. 1998, 50, 49–54. [Google Scholar] [CrossRef]
- Hori, N.; Fujii, M.; Yamanouchi, S.; Miyagi, M.; Saito, N.; Matsumoto, M. In vitro release of tranilast from oily gels and penetration of the drug into Yucatan micropig skin. Biol. Pharm. Bull. 1998, 21, 300–303. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y. Therapeutic effects of gel ointments containing tranilast nanoparticles on paw edema in adjuvant-induced arthritis rats. Biol. Pharm. Bull. 2014, 37, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Jung, H.R.; Kim, H.M. Effects of topical tranilast on corneal haze after photorefractive keratectomy. J. Cataract. Refract. Surg. 2005, 31, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Chikama, T.; Takahashi, M.; Nishida, T. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea 2011, 30, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- See, G.L.; Sagesaka, A.; Sugasawa, S.; Todo, H.; Sugibayashi, K. Eyelid skin as a potential site for drug delivery to conjunctiva and ocular tissues. Int. J. Pharm. 2017, 533, 198–205. [Google Scholar] [CrossRef]
- See, G.L.; Arce, F., Jr.; Itakura, S.; Todo, H.; Sugibayashi, K. Prolonged distribution of tranilast in the eyes after topical application onto eyelid skin. Chem. Pharm. Bull. 2020, 68, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Yuan, R.; Wang, W. An experimental study of antisense TGF-beta 1 inhibiting keloid fibroblast proliferation in vitro. Zhonghua Zheng Xing Wai Ke Za Zhi 2001, 17, 325–327. [Google Scholar] [PubMed]
- Naim, R.; Naumann, A.; Barnes, J.; Sauter, A.; Hormann, K.; Merkel, D.; Aust, W.; Braun, T.; Bloching, M. Transforming growth factor-beta1-antisense modulates the expression of hepatocyte growth factor/scatter factor in keloid fibroblast cell culture. Aesthetic Plast. Surg. 2008, 32, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Sadick, H.; Herberger, A.; Riedel, K.; Bran, G.; Goessler, U.; Hoermann, K.; Riedel, F. TGF-beta1 antisense therapy modulates expression of matrix metalloproteinases in keloid-derived fibroblasts. Int. J. Mol. Med. 2008, 22, 55–60. [Google Scholar] [PubMed]
- Bran, G.M.; Goessler, U.R.; Baftiri, A.; Hormann, K.; Riedel, F.; Sadick, H. Effect of transforming growth factor-beta1 antisense oligonucleotides on matrix metalloproteinases and their inhibitors in keloid fibroblasts. Otolaryngol.-Head Neck Surg. 2010, 143, 66–71. [Google Scholar] [CrossRef]
- Bran, G.M.; Sommer, U.J.; Goessler, U.R.; Hörmann, K.; Riedel, F.; Sadick, H. TGF-ß1 antisense impacts the SMAD signalling system in fibroblasts from keloid scars. Anticancer Res. 2010, 30, 3459–3463. [Google Scholar]
- Ponedal, A.; Zhu, S.; Sprangers, A.J.; Wang, X.Q.; Yeo, D.C.; Lio, D.C.S.; Zheng, M.; Capek, M.; Narayan, S.P.; Meckes, B.; et al. Attenuation of abnormal scarring using spherical nucleic acids targeting transforming growth factor beta 1. ACS Appl. Bio Mater. 2020, 3, 8603–8610. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Kim, C.K.; Kim, Y.B. Cellular delivery of cationic lipid nanoparticle-based AMAD3 antisense oligonucleotides for the inhibition of collagen production in keloid fibroblasts. Eur. J. Pharm. Biopharm. 2012, 82, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, L.X.; Bi, Q.X.; Wang, P.; Wang, X.M.; Liu, J.; Wang, Y.T. Effects of hTERT oligodeoxynucleotide on cell apoptosis and expression of hTERT and bcl-2 mRNA in keloid fibroblasts. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1944–1951. [Google Scholar] [PubMed]
- Edwards, L. The interferons. Dermatol. Clin. 2001, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.R.; Berman, B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J. Exp. Med. 1985, 162, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Duncan, M.R. Short-term keloid treatment in vivo with human interferon alfa-2b results in a selective and persistent normalization of keloidal fibroblast collagen, glycosaminoglycan, and collagenase production in vitro. J. Am. Acad. Dermatol. 1989, 21 Pt 1, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Granstein, R.D.; Rook, A.; Flotte, T.J.; Haas, A.; Gallo, R.L.; Jaffe, H.S.; Amento, E.P. A controlled trial of intralesional recombinant interferon-gamma in the treatment of keloidal scarring. Clinical and histologic findings. Arch. Dermatol. 1990, 126, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Shen, Y.J.; Liu, G.; Forsyth, N.; Smith, C.; Robertson Harrop, A.; Scott, P.G.; Ghahary, A. Regulation of collagen synthesis and messenger RNA levels in normal and hypertrophic scar fibroblasts in vitro by interferon alfa-2b. Wound Repair Regen. 1993, 1, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ghahary, A.; Shen, Y.J.; Nedelec, B.; Scott, P.G.; Ghahary, A. Interferons gamma and alpha-2b differentially regulate the expression of collagenase and tissue inhibitor of metalloproteinase-1 messenger RNA in human hypertrophic and normal dermal fibroblasts. Wound Repair Regen. 1995, 3, 176–184. [Google Scholar] [CrossRef]
- Ghahary, A.; Shen, Q.; Rogers, J.A.; Wang, R.; Fathi-Afshar, A.; Scott, P.G.; Tredget, E.E. Liposome-associated interferon-alpha-2b functions as an anti-fibrogenic factor for human dermal fibroblasts. J. Investig. Dermatol. 1997, 109, 55–60. [Google Scholar] [CrossRef]
- Larrabee, W.F., Jr.; East, C.A.; Jaffe, H.S.; Stephenson, C.; Peterson, K.E. Intralesional interferon gamma treatment for keloids and hypertrophic scars. Arch. Otolaryngol.-Head Neck Surg. 1990, 116, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Low, S.Q.; Moy, R.L. Scar wars strategies. Target collagen. J. Dermatol. Surg. Oncol. 1992, 18, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Broker, B.J.; Rosen, D.; Amsberry, J.; Schmidt, R.; Sailor, L.; Pribitkin, E.A.; Keane, W.M. Keloid excision and recurrence prophylaxis via intradermal interferon-gamma injections: A pilot study. Laryngoscope 1996, 106 Pt 1, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, B.; Shankowsky, H.; Scott, P.G.; Ghahary, A.; Tredget, E.E. Myofibroblasts and apoptosis in human hypertrophic scars: The effect of interferon-alpha2b. Surgery 2001, 130, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Ping, J.A. Calcium antagonists retard extracellular matrix production in connective tissue equivalent. J. Surg. Res. 1990, 49, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Copcu, E.; Sivrioglu, N.; Oztan, Y. Combination of surgery and intralesional verapamil injection in the treatment of the keloid. J. Burn. Care Rehabil. 2004, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palamaras, I.; Kyriakis, K. Calcium antagonists in dermatology: A review of the evidence and research-based studies. Dermatol. Online J. 2005, 11, 8. [Google Scholar] [CrossRef]
- Boggio, R.F.; Freitas, V.M.; Cassiola, F.M.; Urabayashi, M.; Machado-Santelli, G.M. Effect of a calcium-channel blocker (verapamil) on the morphology, cytoskeleton and collagenase activity of human skin fibroblasts. Burns 2011, 37, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.N.; Lee, Y.J.; Kim, K.J.; Lee, S.J.; Choi, J.Y.; Moon, S.H.; Rhie, J.W. Nitric oxide produced by the antioxidant activity of verapamil improves the acute wound healing process. Tissue Eng. Regen. Med. 2021, 18, 179–186. [Google Scholar] [CrossRef]
- Lo, Y.; Lin, L.Y.; Tsai, T.F. Use of calcium channel blockers in dermatology: A narrative review. Expert. Rev. Clin. Pharmacol. 2021, 14, 481–489. [Google Scholar] [CrossRef]
- Doong, H.; Dissanayake, S.; Gowrishankar, T.R.; LaBarbera, M.C.; Lee, R.C. The 1996 Lindberg Award. Calcium antagonists alter cell shape and induce procollagenase synthesis in keloid and normal human dermal fibroblasts. J. Burn. Care Rehabil. 1996, 17 Pt 1, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Verhiel, S.; Piatkowski de Grzymala, A.; van der Hulst, R. Mechanism of action, efficacy, and adverse events of calcium antagonists in hypertrophic scars and keloids: A systematic review. Dermatol. Surg. 2015, 41, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Boggio, R.F.; Boggio, L.F.; Galvão, B.L.; Machado-Santelli, G.M. Topical verapamil as a scar modulator. Aesthetic Plast. Surg. 2014, 38, 968–975. [Google Scholar] [CrossRef]
- Ramos-Gallardo, G.; Miranda-Altamirano, A.; Valdes-López, R.; Figueroa-Jiménez, S.; García-Benavides, L. Verapamil in conjunction with pressure therapy in the treatment of pathologic scar due burn injury. Rev. Med. Inst. Mex Seguro Soc. 2016, 54, 454–457. [Google Scholar] [PubMed]
- Abou-Taleb, D.A.E.; Badary, D.M. Intralesional verapamil in the treatment of keloids: A clinical, histopathological, and immunohistochemical study. J. Cosmet. Dermatol. 2021, 20, 267–273. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, M.F.; Selim, M.K.; Alghobary, M.F. Keloidectomy with core fillet flap and intralesional verapamil injection for recurrent earlobe keloids. Indian. J. Dermatol. Venereol. Leprol. 2016, 82, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Belie, O.; Ugburo, A.O.; Mofikoya, B.O.; Omidiji, O.A.T.; Belie, M.F. A comparison of intralesional verapamil and triamcinolone monotherapy in the treatment of keloids in an African population. Niger. J. Clin. Pract. 2021, 24, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.B.; Chatterjee, P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns 2014, 40, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Liao, X.C.; Liu, M.Z.; Fu, Z.H.; Min, D.H.; Guo, G.H. The safety and efficacy of intralesional verapamil versus intralesional triamcinolone acetonide for keloids and hypertrophic scars: A systematic review and meta-analysis. Adv. Skin Wound Care 2020, 33, 1–7. [Google Scholar] [CrossRef]
- Liu, R.; Yang, B.; Deng, Z.; Liu, L.; Zhao, X. Efficacy and safety of verapamil vs triamcinolone acetonide for keloids and hypertrophic scars: A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13564. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Li, X. Efficacy and safety of verapamil versus triamcinolone acetonide in treating keloids and hypertrophic scars: A systematic review and meta-analysis. Aesthetic Plast. Surg. 2023, 47, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Margaret Shanthi, F.X.; Ernest, K.; Dhanraj, P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian. J. Dermatol. Venereol. Leprol. 2008, 74, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Abedini, R.; Sasani, P.; Mahmoudi, H.R.; Nasimi, M.; Teymourpour, A.; Shadlou, Z. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: A randomized controlled trial. Burns 2018, 44, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Kamesaki, H. Mechanisms involved in chemotherapy-induced apoptosis and their implications in cancer chemotherapy. Int. J. Hematol. 1998, 68, 29–43. [Google Scholar] [CrossRef]
- Wahba, J.; Natoli, M.; Whilding, L.M.; Parente-Pereira, A.C.; Jung, Y.; Zona, S.; Lam, E.W.; Smith, J.R.; Maher, J.; Ghaem-Maghami, S. Chemotherapy-induced apoptosis, autophagy and cell cycle arrest are key drivers of synergy in chemo-immunotherapy of epithelial ovarian cancer. Cancer Immunol. Immunother. 2018, 67, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Mesner, P.W., Jr.; Budihardjo, I.I.; Kaufmann, S.H. Chemotherapy-induced apoptosis. Adv. Pharmacol. 1997, 41, 461–499. [Google Scholar] [PubMed]
- Wang, X.Q.; Liu, Y.K.; Qing, C.; Lu, S.L. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann. Plast. Surg. 2009, 63, 688–692. [Google Scholar] [PubMed]
- Jones, C.D.; Guiot, L.; Samy, M.; Gorman, M.; Tehrani, H. The use of chemotherapeutics for the treatment of keloid scars. Dermatol. Rep. 2015, 7, 5880. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.; Saxena, A.; Lubana, P.S.; Mathur, R.K.; Jain, D.K. Treatment of keloids and hypertrophic scars using bleom. J. Cosmet. Dermatol. 2008, 7, 43–49. [Google Scholar] [CrossRef]
- España, A.; Solano, T.; Quintanilla, E. Bleomycin in the treatment of keloids and hypertrophic scars by multiple needle punctures. Dermatol. Surg. 2001, 27, 23–27. [Google Scholar]
- Manca, G.; Pandolfi, P.; Gregorelli, C.; Cadossi, M.; de Terlizzi, F. Treatment of keloids and hypertrophic scars with bleomycin and electroporation. Plast. Reconstr. Surg. 2013, 132, 621e–630e. [Google Scholar] [CrossRef] [PubMed]
- Bik, L.; Wolkerstorfer, A.; Bekkers, V.; Prens, E.P.; Haedersdal, M.; Bonn, D.; van Doorn, M.B.A. Needle-free jet injection-induced small-droplet aerosol formation during intralesional bleomycin therapy. Lasers Surg. Med. 2022, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Huu, N.D.; Huu, S.N.; Thi, X.L.; Van, T.N.; Minh, P.P.T.; Minh, T.T.; Van, T.H.; Cam, V.T.; Huyen, M.L.; Hau, K.T.; et al. Successful treatment of intralesional bleomycin in keloids of Vietnamese population. Open Access Maced. J. Med. Sci. 2019, 7, 298–299. [Google Scholar] [PubMed]
- Vanhooteghem, O. Remarkable efficiency of surgical shave excision of keloids followed by intralesional injection of Bleomycin. A retrospective study of 314 cases. Dermatol. Ther. 2022, 35, e15425. [Google Scholar] [CrossRef] [PubMed]
- Moravej, H.; Forghanian, A.; Dadkhahfar, S.; Mozafari, N. Intralesional bleomycin versus intralesional triamcinolone in the treatment of keloids and hypertrophic scars. Dermatol. Ther. 2022, 35, e15730. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Sun, H.; Yu, D.; Liang, Y.; Yuan, Z.; Ge, Y. Hydroxycamptothecin induces apoptosis of human tenon’s capsule fibroblasts by activating the PERK signaling pathway. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4749–4758. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, L.; Ni, B.; Liu, J.; Yang, J.; Guo, Q.; Zhou, W. Hydroxycamptothecin liposomes inhibit collagen secretion and induce fibroblast apoptosis in a postlaminectomy rabbit model. Eur. J. Orthop. Surg. Traumatol. 2013, 23 (Suppl. S1), S85–S91. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Chen, H.; Zhu, G.; Liang, Y.; Wang, Q.; Wang, J.; Yan, L. Hydroxycamptothecin induces apoptosis of fibroblasts and prevents intraarticular scar adhesion in rabbits by activating the IRE-1 signal pathway. Eur. J. Pharmacol. 2016, 781, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Sun, Y.; Li, X.; Wang, J.; Yan, L. 10-Hydroxycamptothecin induces apoptosis in human fibroblasts by regulating miRNA-23b-3p expression. Mol. Med. Rep. 2019, 19, 2680–2686. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, X.; Wang, Z.; Wang, J.; Gao, T.; Li, P.; Kong, M.; Chen, X. Transdermal delivery of 10,11-methylenedioxycamptothecin by hyaluronic acid based nanoemulsion for inhibition of keloid fibroblast. Carbohydr. Polym. 2014, 112, 376–386. [Google Scholar] [CrossRef]
- Ezzat Mohamad, N.; Abd El Raheem, T.A.; Mahmoud, R.H.; Osama Hamed, N. Evaluating serum level of thymidylate synthase in post burn keloid patients before and after intralesional injection of 5-fluorouracil. Scars Burns Heal. 2022, 8, 20595131211049043. [Google Scholar] [CrossRef] [PubMed]
- Wendling, J.; Marchand, A.; Mauviel, A.; Verrecchia, F. 5-fluorouracil blocks transforming growth factor-beta-induced alpha 2 type I collagen gene (COL1A2) expression in human fibroblasts via c-Jun NH2-terminal kinase/activator protein-1 activation. Mol. Pharmacol. 2003, 64, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wong, Y.P.; Cai, Y.J.; Lung, I.; Leung, C.S.; Burd, A. Low-dose 5-fluorouracil induces cell cycle G2 arrest and apoptosis in keloid fibroblasts. Br. J. Dermatol. 2010, 163, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Bulstrode, N.W.; Mudera, V.; McGrouther, D.A.; Grobbelaar, A.O.; Cambrey, A.D. 5-Fluorouracil selectively inhibits collagen synthesis. Plast. Reconstr. Surg. 2005, 116, 209–221; discussion 222–223. [Google Scholar] [CrossRef]
- Gupta, S.; Kalra, A. Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology 2002, 204, 130–132. [Google Scholar] [CrossRef]
- Shah, V.V.; Aldahan, A.S.; Mlacker, S.; Alsaidan, M.; Samarkandy, S.; Nouri, K. 5-Fluorouracil in the treatment of keloids and hypertrophic scars: A comprehensive review of the literature. Dermatol. Ther. 2016, 6, 169–183. [Google Scholar] [CrossRef]
- Kontochristopoulos, G.; Stefanaki, C.; Panagiotopoulos, A.; Stefanaki, K.; Argyrakos, T.; Petridis, A.; Katsambas, A. Intralesional 5-fluorouracil in the treatment of keloids: An open clinical and histopathologic study. J. Am. Acad. Dermatol. 2005, 52 Pt 1, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, Y.C. Topical delivery of 5-fluorouracil-loaded carboxymethyl chitosan nanoparticles using microneedles for keloid treatment. Drug Deliv. Transl. Res. 2021, 11, 205–213. [Google Scholar] [CrossRef]
- Erlendsson, A.M.; Rosenberg, L.K.; Lerche, C.M.; Togsverd-Bo, K.; Wiegell, S.R.; Karmisholt, K.; Philipsen, P.A.; Hansen, A.C.N.; Janfelt, C.; Holmes, J.; et al. A one-time pneumatic jet-injection of 5-fluorouracil and triamcinolone acetonide for treatment of hypertrophic scars-A blinded randomized controlled trial. Lasers Surg. Med. 2022, 54, 663–671. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Shen, Y.; Hou, A.; Quan, G.; Pan, X.; Wu, C. Bilayer dissolving microneedle array containing 5-fluorouracil and triamcinolone with biphasic release profile for hypertrophic scar therapy. Bioact. Mater. 2021, 6, 2400–2411. [Google Scholar] [CrossRef]
- Scheithauer, M.O.; Riechelmann, H. Mitomycin C in head and neck surgical procedures. Laryngorhinootologie 2007, 86, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Kunnavatana, S.S.; Koch, R.J. Effects of mitomycin-C on normal dermal fibroblasts. Laryngoscope 2006, 116, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Fde, A.; Guaraldo, L.; Borges Jde, P.; Zacchi, F.F.; Eckley, C.A. Clinical and histological healing of surgical wounds treated with mitomycin C. Laryngoscope 2004, 114, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Talmi, Y.P.; Orenstein, A.; Wolf, M.; Kronenberg, J. Use of mitomycin C for treatment of keloid: A preliminary report. Otolaryngol.-Head Neck Surg. 2005, 132, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.W.; Gage-White, L.; Stucker, F.J. Topical mitomycin C in the prevention of keloid scar recurrence. Arch. Facial Plast. Surg. 2005, 7, 172–175. [Google Scholar] [CrossRef][Green Version]
- Bailey, J.N.; Waite, A.E.; Clayton, W.J.; Rustin, M.H. Application of topical mitomycin C to the base of shave-removed keloid scars to prevent their recurrence. Br. J. Dermatol. 2007, 156, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Sung, H.W. Treatment of keloids and hypertrophic scars using topical and intralesional mitomycin C. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.G.; Kim, J.Y.; Lee, W.J.; Lee, S.J.; Kim, D.W.; Sohn, M.Y.; Kim, G.W.; Kim, M.B.; Kim, B.S. Ear keloids as a primary candidate for the application of mitomycin C after shave excision: In vivo and in vitro study. Dermatol. Surg. 2011, 37, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Narang, T. Role of mitomycin C in reducing keloid recurrence: Patient series and literature review. J. Laryngol. Otol. 2011, 125, 297–300. [Google Scholar] [CrossRef]
- Mandour, Y.; Bake, H.; Mofty, E.; Ramadan, E.; Gomaa, M.; Akl, E.; Elrefae, A. Topical versus interlesional mitomycin C in auricular keloids. Acta Otorrinolaringol. Esp. (Engl. Ed.) 2021, 72, 280–287. [Google Scholar] [CrossRef]
- Wang, T.H.; Wang, H.S.; Soong, Y.K. Paclitaxel-induced cell death: Where the cell cycle and apoptosis come together. Cancer 2000, 88, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, L.; Huang, W.; Jin, M.; Wang, Q.; Gao, Z.; Jin, Z. Improving the anti-keloid outcomes through liposomes loading paclitaxel-cholesterol complexes. Int. J. Nanomed. 2019, 14, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.P.; Wang, G.Q.; Jia, Z.S.; Chen, J.W.; Wang, G.; Wang, X.L. Paclitaxel reduces formation of hypertrophic scars in the rabbit ear model. Ther. Clin. Risk Manag. 2015, 11, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Gragnani, A.; Warde, M.; Furtado, F.; Ferreira, L.M. Topical tamoxifen therapy in hypertrophic scars or keloids in burns. Arch. Dermatol. Res. 2010, 302, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.; Mancoll, J.S.; Lee, S.; Zhao, J.; Phillips, L.G.; Gittes, G.K.; Longaker, M.T. Tamoxifen downregulates TGF-beta production in keloid fibroblasts. Ann. Plast. Surg. 1998, 40, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mikulec, A.A.; Hanasono, M.M.; Lum, J.; Kadleck, J.M.; Kita, M.; Koch, R.J. Effect of tamoxifen on transforming growth factor beta1 production by keloid and fetal fibroblasts. Arch. Facial Plast. Surg. 2001, 3, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Hughes, M.A.; Cherry, G.W. Topical tamoxifen—A potential therapeutic regime in treating excessive dermal scarring? Br. J. Plast. Surg. 1998, 51, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ruffy, M.B.; Kunnavatana, S.S.; Koch, R.J. Effects of tamoxifen on normal human dermal fibroblasts. Arch. Facial Plast. Surg. 2006, 8, 329–332. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Raaiszadeh, M.; Aminseresht, M.; Behjoo, S. Evaluating tamoxifen effect in the prevention of hypertrophic scars following surgical incisions. Dermatol. Surg. 2010, 36, 665–669. [Google Scholar] [CrossRef]
- Soares-Lopes, L.R.; Soares-Lopes, I.M.; Filho, L.L.; Alencar, A.P.; da Silva, B.B. Morphological and morphometric analysis of the effects of intralesional tamoxifen on keloids. Exp. Biol. Med. 2017, 242, 926–929. [Google Scholar] [CrossRef]
- Mehrvarz, S.; Ebrahimi, A.; Sahraei, H.; Bagheri, M.H.; Fazili, S.; Manoochehry, S.; Rasouli, H.R. Effects of topical tamoxifen on wound healing of burned skin in rats. Arch. Plast. Surg. 2017, 44, 378–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, L.J.; Russell, S.B.; Russell, J.D.; Trupin, J.S.; Egbert, B.M.; Shuster, S.; Stern, R. Reduced hyaluronan in keloid tissue and cultured keloid fibroblasts. J. Investig. Dermatol. 2000, 114, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Goldman, A. Improvement of hypertrophic scars with intralesion injections of hyaluronidase. Georgian Med. News. 2020, 301, 41–43. [Google Scholar]
- Kang, N.; Sivakumar, B.; Sanders, R.; Nduka, C.; Gault, D. Intra-lesional injections of collagenase are ineffective in the treatment of keloid and hypertrophic scars. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 693–699. [Google Scholar] [CrossRef]
- Bae-Harboe, Y.S.; Harboe-Schmidt, J.E.; Graber, E.; Gilchrest, B.A. Collagenase followed by compression for the treatment of earlobe keloids. Dermatol. Surg. 2014, 40, 519–524. [Google Scholar] [CrossRef]
- Olaiya, O.R.; Forbes, D.; Humphrey, S.; Beleznay, K.; Mosher, M.; Carruthers, J. Hyaluronidase for treating complications related to HA fillers: A National Plastic Surgeon Survey. Plast. Surg. 2022, 30, 233–237. [Google Scholar] [CrossRef]
- Bertheim, U.; Hellström, S. The distribution of hyaluronan in human skin and mature, hypertrophic and keloid scars. Br. J. Plast. Surg. 1994, 47, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. Hyaluronidase in dermatology: Uses beyond hyaluronic acid fillers. J. Drugs Dermatol. 2020, 19, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.; Levy, P.M. Hyaluronidase offers an efficacious treatment for inaesthetic hyaluronic acid overcorrection. J. Cosmet. Dermatol. 2007, 6, 159–162. [Google Scholar] [CrossRef]
- Alibegashvili, M.; Loladze, M.; Gabisonia, T.; Gabisonia, G.; Tsitsishvili, D. Hyaluronidase ointment in treatment of hypertrophic scars. Georgian Med. News 2020, 308, 140–143. [Google Scholar]
- Aggarwal, A.; Ravikumar, B.C.; Vinay, K.N.; Raghukumar, S.; Yashovardhana, D.P. A comparative study of various modalities in the treatment of keloids. Int. J. Dermatol. 2018, 57, 1192–1200. [Google Scholar] [CrossRef]
- Burgess, C. Topical vitamins. J. Drugs Dermatol. 2008, 7 (Suppl. S7), s2–s6. [Google Scholar]
- Hunt, T.K. Vitamin A and wound healing. J. Am. Acad. Dermatol. 1986, 15 Pt 2, 817–821. [Google Scholar] [CrossRef]
- Demetriou, A.A.; Levenson, S.M.; Rettura, G.; Seifter, E. Vitamin A and retinoic acid: Induced fibroblast differentiation in vitro. Surgery 1985, 98, 931–934. [Google Scholar]
- Polcz, M.E.; Barbul, A. The role of vitamin A in wound healing. Nutr. Clin. Pract. 2019, 34, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Janssen de Limpens, A.M. The local treatment of hypertrophic scars and keloids with topical retinoic acid. Br. J. Dermatol. 1980, 103, 319–323. [Google Scholar] [CrossRef]
- Daly, T.J.; Weston, W.L. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J. Am. Acad. Dermatol. 1986, 15 Pt 2, 900–902. [Google Scholar] [CrossRef]
- Dematte, M.F.; Gemperli, R.; Salles, A.G.; Dolhnikoff, M.; Lanças, T.; Saldiva, P.H.; Ferreira, M.C. Mechanical evaluation of the resistance and elastance of post-burn scars after topical treatment with tretinoin. Clinics 2011, 66, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zitelli, J. Wound healing for the clinician. Adv. Dermatol. 1987, 2, 243–267. [Google Scholar]
- Phillips, J.D.; Kim, C.S.; Fonkalsrud, E.W.; Zeng, H.; Dindar, H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am. J. Surg. 1992, 163, 71–77. [Google Scholar] [CrossRef]
- Haws, M.; Brown, R.E.; Suchy, H.; Roth, A. Vitam A-Soaked Gelfoam Sponges Wound Heal. Steroid-Treat. Anim. Ann. Plast. Surg. 1994, 32, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Anstead, G.M. Steroids, retinoids, and wound healing. Adv. Wound Care 1998, 11, 277–285. [Google Scholar]
- Wicke, C.; Halliday, B.; Allen, D.; Roche, N.S.; Scheuenstuhl, H.; Spencer, M.M.; Roberts, A.B.; Hunt, T.K. Effects of steroids and retinoids on wound healing. Arch. Surg. 2000, 135, 1265–1270. [Google Scholar] [CrossRef]
- Zineb, R.; Zhor, B.; Odile, W.; Marthe, R.R. Distinct, tissue-specific regulation of vitamin D receptor in the intestine, kidney, and skin by dietary calcium and vitamin D. Endocrinology 1998, 139, 1844–1852. [Google Scholar] [CrossRef][Green Version]
- Cooke, G.L.; Chien, A.; Brodsky, A.; Lee, R.C. Incidence of hypertrophic scars among African Americans linked to vitamin D-3 metabolism? J. Natl. Med. Assoc. 2005, 97, 1004–1009. [Google Scholar]
- Ramakrishnan, K.M.; Babu, M.; Madhavi, M.L. Response of keloid fibroblasts to vitamin D3 and quercetin treatment-in vitro study. Ann. Burns Fire Disasters 2015, 28, 187–191. [Google Scholar]
- Hahn, J.M.; Supp, D.M. Abnormal expression of the vitamin D receptor in keloid scars. Burns 2017, 43, 1506–1515. [Google Scholar] [CrossRef]
- Kilmister, E.J.; Paterson, C.; Brasch, H.D.; Davis, P.F.; Tan, S.T. The role of the renin-angiotensin system and vitamin D in keloid disorder—A review. Front. Surg. 2019, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, M.; Omar, G.A.; Hafiz, H.S.A.; Ali, S.M. Role of vitamin D in treatment of keloid. J. Cosmet. Dermatol. 2022, 21, 331–336. [Google Scholar] [CrossRef]
- Hahn, J.M.; Combs, K.A.; Powell, H.M.; Supp, D.M. A role for vitamin D and the vitamin D receptor in keloid disorder. Wound Repair Regen. 2023, 31, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Cheng, T.; Luan, Q.; Liao, T.; Nie, C.L.; Zheng, X.; Xie, X.G.; Gao, W.Y. Vitamin D: A novel therapeutic approach for keloid, an in vitro analysis. Br. J. Dermatol. 2011, 164, 729–737. [Google Scholar] [CrossRef]
- Lee, D.E.; Trowbridge, R.M.; Ayoub, N.T.; Agrawal, D.K. High-mobility group box protein-1, matrix metalloproteinases, and vitamin D in keloids and hypertrophic scars. Plast. Reconstr. Surg. Glob. Open. 2015, 3, e425. [Google Scholar] [CrossRef]
- Mehta, H.; Goyal, A.; Narang, T. Intralesional vitamin D injection for management of keloids. Clin. Exp. Dermatol. 2022, 47, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Gozzi, G.; Palmieri, G. Vitamin E added silicone gel sheets for treatment of hypertrophic scars and keloids. Int. J. Dermatol. 1995, 34, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Skaehill, P.A. Tacrolimus in dermatologic disorders. Ann. Pharmacother. 2001, 35, 582–588. [Google Scholar] [CrossRef]
- Garland, S.M. Imiquimod. Curr. Opin. Infect. Dis. 2003, 16, 85–89. [Google Scholar] [CrossRef]
- Wu, C.S.; Wu, P.H.; Fang, A.H.; Lan, C.C. FK506 inhibits the enhancing effects of transforming growth factor (TGF)-β1 on collagen expression and TGF-β/Smad signalling in keloid fibroblasts: Implication for new therapeutic approach. Br. J. Dermatol. 2012, 167, 532–541. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, R.; Liang, X.; Deng, Z.; He, J.; Ding, Y.; Ding, F.; Lu, L.; Liu, F.; Yang, J. Angiogenesis modulation-mediated inhibitory effects of tacrolimus on hypertrophic scar formation. Microvasc. Res. 2023, 145, 104446. [Google Scholar] [CrossRef]
- Gisquet, H.; Liu, H.; Blondel, W.C.; Leroux, A.; Latarche, C.; Merlin, J.L.; Chassagne, J.F.; Peiffert, D.; Guillemin, F. Intradermal tacrolimus prevent scar hypertrophy in a rabbit ear model: A clinical, histological and spectroscopical analysis. Skin Res. Technol. 2011, 17, 160–166. [Google Scholar] [CrossRef]
- Menezes, M.C.S.; Vasconcellos, L.S.; Nunes, C.B.; Alberti, L.R. Evaluation of the use of tacrolimus ointment for the prevention of hypertrophic scars in experimental model. Bras. Dermatol. 2019, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.C.S.; Buzelin, M.; Nunes, C.B.; Alberti, L.R. Tacrolimus action pathways in an ointment base for hypertrophic scar prevention in a rabbit ear model. Bras. Dermatol. 2021, 96, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, H.; Yao, L.; Zhang, X.; Yu, Q.; Gu, J.; Shi, Y. Efficient local delivery of FK506 using blocking patches in psoriasis. J. Colloid. Interface Sci. 2023, 630 Pt A, 676–687. [Google Scholar] [CrossRef]
- Weeks, C.E.; Gibson, S.J. Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J. Interferon Res. 1994, 14, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, K.; Au, W.C.; Rosztoczy, I.; Raj, N.B.; Miller, R.L.; Tomai, M.A.; Pitha, P.M. Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by Sendai virus utilize similar signal transduction pathways. Mol. Cell Biol. 1995, 15, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.L.; Gerster, J.F.; Imbertson, L.M.; Reiter, M.J.; Miller, R.L.; Gibson, S.J.; Wagner, T.L.; Tomai, M.A. Cytokine induction by the immunomodulators imiquimod and S-27609. J. Leukoc. Biol. 1995, 58, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Reiter, M.J.; Testerman, T.L.; Miller, R.L.; Weeks, C.E.; Tomai, M.A. Cytokine induction in mice by the immunomodulator imiquimod. J. Leukoc. Biol. 1994, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Bottrel, R.L.; Yang, Y.L.; Levy, D.E.; Tomai, M.; Reis, L.F. The immune response modifier imiquimod requires STAT-1 for induction of interferon, interferon-stimulated genes, and interleukin-6. Antimicrob. Agents Chemother. 1999, 43, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.K.; Gupta, S.; Khaitan, B.K.; Sharma, V.K. Imiquimod 5% cream for the prevention of recurrence after excision of presternal keloids. Dermatology 2007, 215, 63–65. [Google Scholar] [CrossRef]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the design of antibody-based therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef]
- Kuwahara, H.; Tosa, M.; Egawa, S.; Murakami, M.; Mohammad, G.; Ogawa, R. Examination of epithelial mesenchymal transition in keloid tissues and possibility of keloid therapy target. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e1138. [Google Scholar] [CrossRef]
- Kurokawa, I.; Layton, A.M.; Ogawa, R. Updated treatment for acne: Targeted therapy based on pathogenesis. Dermatol. Ther. 2021, 11, 1129–1139. [Google Scholar] [CrossRef]
- Matsunaga, K.; Katoh, N.; Fujieda, S.; Izuhara, K.; Oishi, K. Dupilumab: Basic aspects and applications to allergic diseases. Allergol. Int. 2020, 69, 187–196. [Google Scholar] [CrossRef]
- Jia, F.; Zhao, Q.; Shi, P.; Liu, H.; Zhang, F. Dupilumab: Advances in the off-label usage of IL4/IL13 antagonist in dermatoses. Dermatol. Ther. 2022, 35, e15924. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.; Fakhoury, J.; Ozog, D. Nonresponse and progression of diffuse keloids to dupilumab therapy. J. Drugs Dermatol. 2022, 21, 197–199. [Google Scholar] [CrossRef]
- Min, M.S.; Mazori, D.R.; Lee, M.S.; Merola, J.F.; Vleugels, R.A.; Cobos, G.; LaChance, A.H. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J. Drugs Dermatol. 2023, 22, 1220–1222. [Google Scholar] [CrossRef]
- Wittmer, A.; Finklea, L.; Joseph, J. Effects of dupilumab on keloid stabilization and prevention. JAAD Case Rep. 2023, 37, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Muragaki, Y.; Ooshima, A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br. J. Dermatol. 2005, 153, 295–300. [Google Scholar] [CrossRef]
- Lu, L.; Saulis, A.S.; Liu, W.R.; Roy, N.K.; Chao, J.D.; Ledbetter, S.; Mustoe, T.A. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J. Am. Coll. Surg. 2005, 201, 391–397. [Google Scholar] [CrossRef]
- Chodon, T.; Sugihara, T.; Igawa, H.H.; Funayama, E.; Furukawa, H. Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance. Am. J. Pathol. 2000, 157, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Viera, M.H.; Vivas, A.C.; Berman, B. Update on keloid management: Clinical and basic science advances. Adv. Wound Care 2012, 1, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Vascular endothelial growth factor and cutaneous scarring. Adv. Wound Care 2019, 8, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Song, F.; Liu, Y.K. Hypertrophic scar regression is linked to the occurrence of endothelial dysfunction. PLoS ONE 2017, 12, e0176681. [Google Scholar]
- Shi, J.; Wu, Y.; Guo, S.; Zhang, H.; Chen, G.; Xu, X. The efficacy of anti-VEGF antibody-modified liposomes loaded with paeonol in the prevention and treatment of hypertrophic scars. Drug Dev. Ind. Pharm. 2019, 45, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.; Lowman, H.B. Therapeutic anti-VEGF antibodies. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 181, pp. 131–150. [Google Scholar]
- Yeung, Y.A.; Wu, X.; Reyes, A.E., 2nd; Vernes, J.M.; Lien, S.; Lowe, J.; Maia, M.; Forrest, W.F.; Meng, Y.G.; Damico, L.A.; et al. A therapeutic anti-VEGF antibody with increased potency independent of pharmacokinetic half-life. Cancer Res. 2010, 70, 3269–3277. [Google Scholar] [CrossRef] [PubMed]
- Small, R. Botulinum toxin injection for facial wrinkles. Am. Fam. Physician 2014, 90, 168–175. [Google Scholar] [PubMed]
- Kasyanju Carrero, L.M.; Ma, W.W.; Liu, H.F.; Yin, X.F.; Zhou, B.R. Botulinum toxin type A for the treatment and prevention of hypertrophic scars and keloids: Updated review. J. Cosmet. Dermatol. 2019, 18, 10–15. [Google Scholar] [CrossRef]
- Xiaoxue, W.; Xi, C.; Zhibo, X. Effects of botulinum toxin type A on expression of genes in keloid fibroblasts. Aesthet. Surg. J. 2014, 34, 154–159. [Google Scholar] [CrossRef]
- Hao, R.; Li, Z.; Chen, X.; Ye, W. Efficacy and possible mechanisms of botulinum toxin type A on hypertrophic scarring. J. Cosmet. Dermatol. 2018, 17, 340–346. [Google Scholar] [CrossRef]
- Austin, E.; Koo, E.; Jagdeo, J. The cellular response of keloids and hypertrophic scars to botulinum toxin A: A comprehensive literature review. Dermatol. Surg. 2018, 44, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Lei, T.C. Botulinum toxin A promotes the transdifferentiation of primary keloid myofibroblasts into adipocyte-like cells. Basic Clin. Pharmacol. Toxicol. 2021, 129, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Shaarawy, E.; Hegazy, R.A.; Abdel Hay, R.M. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: A randomized controlled trial. J. Cosmet. Dermatol. 2015, 14, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Z.; Liu, X.Y.; Xiao, W.L.; Xu, Y.X. Botulinum toxin type A and the prevention of hypertrophic scars on the maxillofacial area and neck: A meta-analysis of randomized controlled trials. PLoS ONE 2016, 11, e0151627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Li, X. Efficacy and safety of botulinum toxin type A in preventing postoperative scars and improving the cosmetic appearance of scars: A systematic review and meta-analysis. J. Cutan. Med. Surg. 2020, 24, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Gamil, H.D.; Khattab, F.M.; El Fawal, M.M.; Eldeeb, S.E. Comparison of intralesional triamcinolone acetonide, botulinum toxin type A, and their combination for the treatment of keloid lesions. J. Dermatol. Treat. 2020, 31, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mohammed, N.H.K.; Sotohy, M.; Abou-Taleb, D.A.E. Botulinum toxin type A versus 5-fluorouracil in treatment of keloid. Arch. Dermatol. Res. 2021, 313, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.G.; Zhang, D. Evaluation of efficacy of corticosteroid and corticosteroid combined with botulinum toxin type A in the treatment of keloid and hypertrophic scars: A meta-analysis. Aesthetic Plast. Surg. 2021, 45, 3037–3044. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Ali, R.A. Evaluation of botulinum toxin type A for treating post burn hypertrophic scars and keloid in children: An intra-patient randomized controlled study. J. Cosmet. Dermatol. 2023, 22, 1256–1260. [Google Scholar] [CrossRef]
- Ziade, M.; Domergue, S.; Batifol, D.; Jreige, R.; Sebbane, M.; Goudot, P.; Yachouh, J. Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 209–214. [Google Scholar] [CrossRef]
- Khatery, B.H.M.; Hussein, H.A.; Abd-El-Raheem, T.A.; El Hanbuli, H.M.; Yassen, N.N. Assessment of intralesional injection of botulinum toxin type A in hypertrophic scars and keloids: Clinical and pathological study. Dermatol. Ther. 2022, 35, e15748. [Google Scholar] [CrossRef] [PubMed]
- Aviado, D.M.; Porter, J.M. Pentoxifylline: A new drug for the treatment of intermittent claudication. Mechanism of action, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy 1984, 4, 297–307. [Google Scholar] [CrossRef]
- Salhiyyah, K.; Forster, R.; Senanayake, E.; Abdel-Hadi, M.; Booth, A.; Michaels, J.A. Pentoxifylline for intermittent claudication. Cochrane Database Syst. Rev. 2015, 9, CD005262. [Google Scholar] [CrossRef] [PubMed]
- Balazic, E.; Axler, E.; Konisky, H.; Khanna, U.; Kobets, K. Pentoxifylline in dermatology. J. Cosmet. Dermatol. 2023, 22, 410–417. [Google Scholar] [CrossRef]
- Berman, B.; Duncan, M.R. Pentoxifylline inhibits the proliferation of human fibroblasts derived from keloid, scleroderma and morphoea skin and their production of collagen, glycosaminoglycans and fibronectin. Br. J. Dermatol. 1990, 123, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Meng, X.; Zhang, Y.; Zhang, Z. Improvement of surgical scars by early intervention with 5-aminolevulinic acid-mediated photodynamic therapy: A case report. Photodiagn. Photodyn. Ther. 2023, 44, 103811. [Google Scholar] [CrossRef]
- Rawlins, J.M.; Lam, W.L.; Karoo, R.O.; Naylor, I.L.; Sharpe, D.T. Pentoxifylline inhibits mature burn scar fibroblasts in culture. Burns 2006, 32, 42–45. [Google Scholar] [CrossRef]
- Isaac, C.; Mathor, M.B.; Bariani, G.; Paggiaro, A.O.; Herson, M.R.; Goldenstein-Schainberg, C.; Carrasco, S.; Teodoro, W.R.; Yoshinari, N.H.; Ferreira, M.C. Pentoxifylline modifies three-dimensional collagen lattice model contraction and expression of collagen types I and III by human fibroblasts derived from post-burn hypertrophic scars and from normal skin. Burns 2009, 35, 701–706. [Google Scholar] [CrossRef]
- Tan, A.; Martinez Luna, O.; Glass, D.A., 2nd. Pentoxifylline for the prevention of postsurgical keloid recurrence. Dermatol. Surg. 2020, 46, 1353–1356. [Google Scholar] [CrossRef]
- Tsioutsiou, E.E.; Amountzias, V.; Vontzalidou, A.; Dina, E.; Stevanović, Z.D.; Cheilari, A.; Aligiannis, N. Medicinal plants used traditionally for skin related problems in the South Balkan and East Mediterranean Region-A review. Front. Pharmacol. 2022, 13, 936047. [Google Scholar] [CrossRef]
- Isaac, C.; Carvalho, V.F.; Paggiaro, A.O.; de Maio, M.; Ferreira, M.C. Intralesional pentoxifylline as an adjuvant treatment for perioral post-burn hypertrophic scars. Burns 2010, 36, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Serag-Eldin, Y.M.A.; Mahmoud, W.H.; Gamea, M.M.; Hegab, D.S. Intralesional pentoxifylline, triamcinolone acetonide, and their combination for treatment of keloid scars. J. Cosmet. Dermatol. 2021, 20, 3330–3340. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Yang, Y. Research progress of photodynamic therapy in wound healing: A literature review. J. Burn. Care Res. 2023, 44, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, H. 5-Aminolevulinic acid-mediated photodynamic therapy on wound healing: A systemic review of human evidences. J. Am. Podiatr. Med. Assoc. 2023, 1, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ormrod, D.; Jarvis, B. Topical aminolevulinic acid HCl photodynamic therapy. Am. J. Clin. Dermatol. 2000, 1, 133–139; discussion 140–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hu, X.; Zhou, L.; He, Y.; Zhang, X.; Yang, J.; Ju, Z.; Liou, Y.C.; Shen, H.M.; Luo, G.; et al. Photodynamic therapy accelerates skin wound healing through promoting re-epithelialization. Burn. Trauma. 2021, 9, tkab008. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Allan, E.; Allan, D.; Colthurst, J.; Bayat, A. Addition of novel degenerate electrical waveform stimulation with photodynamic therapy significantly enhances its cytotoxic effect in keloid fibroblasts: First report of a potential combination therapy. J. Dermatol. Sci. 2011, 64, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Sebastian, A.; Allan, E.; Allan, D.; Mandal, P.; Alonso-Rasgado, T.; Bayat, A. Differential cytotoxic response in keloid fibroblasts exposed to photodynamic therapy is dependent on photosensitiser precursor, fluence and location of fibroblasts within the lesion. Arch. Dermatol. Res. 2012, 304, 549–562. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Chen, Y.; Li, L.; Lan, P.; He, D.; Song, J.; Zhang, Y. Transdermal delivery of 5-aminolevulinic acid by nanoethosome gels for photodynamic therapy of hypertrophic scars. ACS Appl. Mater. Interfaces 2019, 11, 3704–3714. [Google Scholar] [CrossRef]
- Qu, Z.; Chen, Y.; Du, K.; Qiao, J.; Chen, L.; Chen, J.; Wei, L. ALA-PDT promotes the death and contractile capacity of hypertrophic scar fibroblasts through inhibiting the TGF-β1/Smad2/3/4 signaling pathway. Photodiagn. Photodyn. Ther. 2023, 45, 103915. [Google Scholar] [CrossRef]
- Nie, Z.; Bayat, A.; Behzad, F.; Rhodes, L.E. Positive response of a recurrent keloid scar to topical methyl aminolevulinate-photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2010, 26, 330–332. [Google Scholar] [CrossRef]
- Ud-Din, S.; Thomas, G.; Morris, J.; Bayat, A. Photodynamic therapy: An innovative approach to the treatment of keloid disease evaluated using subjective and objective non-invasive tools. Arch. Dermatol. Res. 2013, 305, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Chen, X.D.; Wu, X.Y. 5-ALA PDT successfully treats facial hidradenitis suppurativa-induced severe hypertrophic scar. Photodiagn. Photodyn. Ther. 2019, 28, 343–345. [Google Scholar] [CrossRef]
- Yang, L.; Deng, H.; Chen, Y.; Chen, Y.; Guo, L.; Feng, M. 5-Aminolevulinic acid-hyaluronic acid complexes enhance skin retention of 5-aminolevulinic acid and therapeutic efficacy in the treatment of hypertrophic scar. AAPS PharmSciTech 2022, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhao, H.; Li, C.; Xia, A.; Zhang, J.; Zhang, S.; Yun, Q.; Li, X.; Huang, F.; Tian, Y. A clinical study of carbon dioxide lattice laser-assisted or microneedle-assisted 5-aminolevulinic acid-based photodynamic therapy for the treatment of hypertrophic acne scars. Photodermatol. Photoimmunol. Photomed. 2022, 38, 53–59. [Google Scholar] [CrossRef]
- Wei, J.; Du, L.; Cao, Z.; Li, M.; Zhang, C.; Zhang, C.; Meng, L. 5-Aminolevulinic acid photodynamic therapy combined with intralesional triamcinolone and 5-fluorouracil to treat acne hypertrophic scar. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3057–3064. [Google Scholar] [CrossRef]
- Agyare, C.; Boakye, Y.D.; Bekoe, E.O.; Hensel, A.; Dapaah, S.O.; Appiah, T. Review: African medicinal plants with wound healing properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Sun, X.Y.; Zhang, J.J.; Giampieri, F.; Jiang, C.J.; Feng, T.T.; Wang, Z.W.; Chen, R.Y.; Battino, M.; Zhou, Y. A six-herb Chinese medicine composition ointment as a promising candidate for treatment of hypertrophic scars. Chin. Herb. Med. 2020, 13, 210–220. [Google Scholar] [CrossRef]
- Chen, D.; Li, Q.; Zhang, H.; Kou, F.; Li, Q.; Lyu, C.; Wei, H. Traditional Chinese medicine for hypertrophic scars-A review of the therapeutic methods and potential effects. Front. Pharmacol. 2022, 13, 1025602. [Google Scholar] [CrossRef]
- Kazancı, C.; Oruç, S.; Mosulishvili, M. Medicinal ethnobotany of wild plants: A cross-cultural comparison around Georgia-Turkey border, the Western Lesser Caucasus. J. Ethnobiol. Ethnomed. 2020, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Treatment of surgical site infection and hypertrophic scars. Kyobu Geka 2012, 65, 409–417. [Google Scholar] [PubMed]
- Suárez, M.E. Medicines in the forest: Ethnobotany of wild medicinal plants in the pharmacopeia of the Wichí people of Salta province (Argentina). J. Ethnopharmacol. 2019, 231, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Solati, K.; Karimi, M.; Rafieian-Kopaei, M.; Abbasi, N.; Abbaszadeh, S.; Bahmani, M. Phytotherapy for wound healing: The most important herbal plants in wound healing based on Iranian ethnobotanical documents. Mini Rev. Med. Chem. 2021, 21, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Razia, S.; Park, H.; Shin, E.; Shim, K.S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of aloe vera flower and aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from Aloe vera: A review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-based scaffolds loaded with Aloe vera extract for the treatment of wounds. Pharmaceutics 2021, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.E., Jr. The stimulation of postdermabrasion wound healing with stabilized aloe vera gel-polyethylene oxide dressing. J. Dermatol. Surg. Oncol. 1990, 16, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Surakunprapha, P.; Winaikosol, K.; Chowchuen, B.; Punyavong, P.; Jenwitheesuk, K.; Jenwitheesuk, K. A prospective randomized double-blind study of silicone gel plus herbal extracts versus placebo in pre-sternal hypertrophic scar prevention and amelioration. Heliyon 2020, 6, e03883. [Google Scholar] [CrossRef]
- Pangkanon, W.; Yenbutra, P.; Kamanamool, N.; Tannirandorn, A.; Udompataikul, M. A comparison of the efficacy of silicone gel containing onion extract and aloe vera to silicone gel sheets to prevent postoperative hypertrophic scars and keloids. J. Cosmet. Dermatol. 2021, 20, 1146–1153. [Google Scholar] [CrossRef]
- Zago, L.R.; Prado, K.; Benedito, V.L.; Pereira, M.M. The use of babosa (Aloe vera) in treating burns: A literature review. Braz. J. Biol. 2021, 83, e249209. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Mandal, S.; Ghorai, M.; Jha, N.K.; Kumar, M.; Radha; Ghosh, A.; Proćków, J.; Pérez de la Lastra, J.M.; Dey, A. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: A review. J. Cell Mol. Med. 2023, 27, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zhu, B.; Liang, Y.; Bi, L.; Hu, Z.; Chen, B.; Zhang, K.; Zhu, J. Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch. Dermatol. Res. 2011, 303, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Zhang, J.; Wu, X.; Dou, Y.; Yang, Y.; Tan, Q.; Xia, Y.; Gong, Z.; Dai, Y. Asiatic acid isolated from Centella asiatica inhibits TGF-β1-induced collagen expression in human keloid fibroblasts via PPAR-γ activation. Int. J. Biol. Sci. 2013, 9, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, H.; Lu, Q.; Xu, Z.; Bian, D.; Xia, Y.; Wei, Z.; Gong, Z.; Dai, Y. Madecassoside suppresses migration of fibroblasts from keloids: Involvement of p38 kinase and PI3K signaling pathways. Burns 2012, 38, 677–2684. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S. Pharmacological effects of Centella asiatica on skin diseases: Evidence and possible mechanisms. Evid. Based Complement. Altern. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef] [PubMed]
- Jenwitheesuk, K.; Rojsanga, P.; Chowchuen, B.; Surakunprapha, P. A prospective randomized, controlled, double-blind trial of the efficacy using Centella cream for scar improvement. Evid. Based Complement. Altern. Med. 2018, 2018, 9525624. [Google Scholar] [CrossRef]
- Cotellese, R.; Hu, S.; Belcaro, G.; Ledda, A.; Feragalli, B.; Dugall, M.; Hosoi, M.; Ippolito, E. Centella asiatica (Centellicum®) facilitates the regular healing of surgical scars in subjects at high risk of keloids. Minerva Chir. 2018, 73, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Opatha, S.A.T.; Chutoprapat, R.; Khankaew, P.; Titapiwatanakun, V.; Ruksiriwanich, W.; Boonpisuttinant, K. Asiatic acid-entrapped transfersomes for the treatment of hypertrophic scars: In vitro appraisal, bioactivity evaluation, and clinical study. Int. J. Pharm. 2023, 651, 123738. [Google Scholar] [CrossRef]
- Kang, J.Y.; Huang, H.; Zhu, F.Q. Effect of curcumin on growth and function of fibroblast in human hyperplastic scar. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009, 29, 1100–1103. [Google Scholar]
- Hsu, Y.C.; Chen, M.J.; Yu, Y.M.; Ko, S.Y.; Chang, C.C. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: Its potential therapeutic use in the chemoprevention of keloid. Arch. Dermatol. Res. 2010, 302, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Xie, P.; Hong, S.J.; Galiano, R.; Singer, A.; Clark, R.A.; Mustoe, T.A. Intravenous curcumin efficacy on healing and scar formation in rabbit ear wounds under nonischemic, ischemic, and ischemia-reperfusion conditions. Wound Repair Regen. 2014, 22, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green tea catechins: Their use in treating and preventing infectious diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kelly, A.P.; Wang, L.; French, S.W.; Tang, X.; Duong, H.S.; Messadi, D.V.; Le, A.D. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J. Investig. Dermatol. 2006, 126, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Yoon, B.S.; Moon, J.H.; Kim, B.; Jun, E.K.; Oh, S.; Kim, H.; Song, H.J.; Noh, J.Y.; Oh, C.; et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J. Investig. Dermatol. 2008, 128, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, T.; Yang, L.; Wu, J.; Chen, L.; Fan, X.; Zhang, Z.; Yang, Q.; Yu, Z.; Song, B. EGCG inhibits hypertrophic scar formation in a rabbit ear model. J. Cosmet. Dermatol. 2023, 22, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Bagabir, R.A.; Paus, R.; Bayat, A. Ex vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab. Investig. 2013, 93, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Tafazoli Moghadam, A.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran J. Basic Med. Sci. 2022, 25, 1045–1058. [Google Scholar]
- Zanoli, P. Role of hyperforin in the pharmacological activities of St. John’s Wort. CNS Drug Rev. 2004, 10, 203–218. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical application of St. John’s wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar] [PubMed]
- Gaid, M.; Füller, J.; Müller-Goymann, C. The petroleum ether extract from Hypericum perforatum root cultures exhibits potent antiproliferative activity in human keratinocytes and fibroblasts. Planta Med. 2019, 85, 591–598. [Google Scholar] [CrossRef]
- Füller, J.; Müller-Goymann, C.C. Anti-proliferative and anti-migratory effects of hyperforin in 2D and 3D artificial constructs of human dermal fibroblasts—A new option for hypertrophic scar treatment? Eur. J. Pharm. Biopharm. 2018, 126, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Khadivzadeh, T.; Emami, A.; Moosavi, N.S.; Tafaghodi, M.; Behnam, H.R. The effect of hypericum perforatum on the wound healing and scar of cesarean. J. Altern. Complement. Med. 2010, 16, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; He, T.; Liu, J.; Wang, Y.; Fan, L.; Tao, K.; Shi, J.; Tang, C.; Su, L.; Hu, D. Loureirin B inhibits fibroblast proliferation and extracellular matrix deposition in hypertrophic scar via TGF-β/Smad pathway. Exp. Dermatol. 2015, 24, 355–360. [Google Scholar]
- He, T.; Bai, X.; Yang, L.; Fan, L.; Li, Y.; Su, L.; Gao, J.; Han, S.; Hu, D. Loureirin B Inhibits Hypertrophic Scar Formation via Inhibition of the TGF-β1-ERK/JNK Pathway. Cell Physiol. Biochem. 2015, 37, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Duan, X.; Zhang, R.; Li, H.; Guo, Y.; Tian, Y.; Huang, M.; Chen, G.; Wang, Z.; Li, L. Loureirin A exerts antikeloid activity by antagonizing the TGF-β1/Smad signalling pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 8661288. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, S.; Xiao, D.; Zheng, X.; Gu, Y.; Guo, S. Evaluation of the wound healing potential of resina draconis (Dracaena cochinchinensis) in animal models. Evid. Based Complement. Altern. Med. 2013, 2013, 709865. [Google Scholar]
- Phan, T.T.; Lim, I.J.; Chan, S.Y.; Tan, E.K.; Lee, S.T.; Longaker, M.T. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J. Trauma 2004, 57, 1032–1037. [Google Scholar] [CrossRef]
- Willital, G.H.; Heine, H. Efficacy of Contractubex gel in the treatment of fresh scars after thoracic surgery in children and adolescents. Int. J. Clin. Pharmacol. Res. 1994, 14, 193–202. [Google Scholar]
- Hosnuter, M.; Payasli, C.; Isikdemir, A.; Tekerekoglu, B. The effects of onion extract on hypertrophic and keloid scars. J. Wound Care 2007, 16, 251–254. [Google Scholar] [CrossRef]
- Cosio, T.; Costanza, G.; Coniglione, F.; Romeo, A.; Iacovelli, F.; Diluvio, L.; Dika, E.; Shumak, R.G.; Rossi, P.; Bianchi, L.; et al. From in silico simulation between TGF-β receptors and quercetin to clinical insight of a medical device containing Allium cepa: Its efficacy and tolerability on post-surgical scars. Life 2023, 13, 1781. [Google Scholar] [CrossRef]
- Sahin, M.T.; Inan, S.; Ozturkcan, S.; Guzel, E.; Bilac, C.; Giray, G.; Muftuoglu, S. Comparison of the effects of Contractubex® gel in an experimental model of scar formation in rats: An immunohistochemical and ultrastructural study. J. Drugs Dermatol. 2012, 11, 74–81. [Google Scholar] [PubMed]
- Ho, W.S.; Ying, S.Y.; Chan, P.C.; Chan, H.H. Use of onion extract, heparin, allantoin gel in prevention of scarring in Chinese patients having laser removal of tattoos: A prospective randomized controlled trial. Dermatol. Surg. 2006, 32, 891–896. [Google Scholar] [CrossRef]
- Beuth, J.; Hunzelmann, N.; Van Leendert, R.; Basten, R.; Noehle, M.; Schneider, B. Safety and efficacy of local administration of contractubex to hypertrophic scars in comparison to corticosteroid treatment. Results of a multicenter, comparative epidemiological cohort study in Germany. In Vivo 2006, 20, 277–283. [Google Scholar] [PubMed]
- Güngör, E.S.; Güzel, D.; Zebitay, A.G.; İlhan, G.; Verit, F.F. The efficacy of onion extract in the management of subsequent abdominal hypertrophic scar formation. J. Wound Care 2020, 29, 612–616. [Google Scholar] [CrossRef]
- Karagoz, H.; Yuksel, F.; Ulkur, E.; Evinc, R. Comparison of efficacy of silicone gel, silicone gel sheeting, and topical onion extract including heparin and allantoin for the treatment of postburn hypertrophic scars. Burns 2009, 35, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Li, X.; Wu, X.; Hui, B.; Han, S.; Gao, J.; Li, Y.; Shi, J.; Zhu, H.; Zhao, B.; et al. Simultaneous deactivation of FAK and Src improves the pathology of hypertrophic scar. Sci. Rep. 2016, 6, 26023. [Google Scholar] [CrossRef]
- Hassanpour, S.E.; Farnoush, N.; Karami, M.Y.; Makarem, A. The effect of silicone gel versus contractubex gel on the upper-extremity postsurgical scars: A randomized, double-blinded, controlled trial. Med. J. Islam. Repub. Iran 2020, 34, 146. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of resveratrol on the immune response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Zeng, G.; Zhong, F.; Li, J.; Luo, S.; Zhang, P. Resveratrol-mediated reduction of collagen by inhibiting proliferation and producing apoptosis in human hypertrophic scar fibroblasts. Biosci. Biotechnol. Biochem. 2013, 77, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Torigoe, T.; Matsumoto, Y.; Fujita, T.; Sato, N.; Yotsuyanagi, T. Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen. 2013, 21, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Zhang, M.; Guan, E.; Han, Q.; Liu, Y.; Long, X.; Long, F.; Zhao, R.C.; Huang, J.; Liu, Z.; et al. Resveratrol inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting HIF-1α. J. Plast. Surg. Hand Surg. 2020, 54, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hayashi, S.; Kitahara, Y.; Nagasawa, K.; Aono, H.; Shibata, J.; Utsumi, D.; Amagase, K.; Kadowaki, M. Saireito (TJ-114), a Japanese traditional herbal medicine, reduces 5-fluorouracil-induced intestinal mucositis in mice by inhibiting cytokine-mediated apoptosis in intestinal crypt cells. PLoS ONE 2015, 10, e0116213. [Google Scholar] [CrossRef] [PubMed]
- Soen, H.; Tanabe, A.; Fujita, D.; Yamashita, Y.; Terai, Y.; Kamegai, H.; Ohmichi, M. The Inhibitory Effect of Sairei-to on Hypertrophic Scar Formation through TGF-β Signaling. Scar Manag. 2015, No. 9, 1–7. (J-GLOBAL ID: 201502219777621523). Available online: http://www.scar-keloid.com/en/9thmeeting/abs014.html (accessed on 22 April 2024).
- Hiramatsu, Y.; Asai, S.; Kato, Y.; Kato, T.; Hide, A. The effect of saireito for the treatment of keloids and hypertrophic scars. Nikkei Kaishi 2008, 28, 549–553. [Google Scholar]
- Mu, Z.; Guo, J.; Zhang, D.; Xu, Y.; Zhou, M.; Guo, Y.; Hou, Y.; Gao, X.; Han, X.; Geng, L. Therapeutic effects of shikonin on skin diseases: A review. Am. J. Chin. Med. 2021, 49, 1871–1895. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, Q.; Qiang, L.; Chi, M.; Xie, N.; Wu, Y.; Yao, M.; Zhao, D.; Ma, J.; Zhang, N.; et al. Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of shikonin on hypertrophic scar remediation. Front. Pharmacol. 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Deng, X.; Zhao, D.; Zhao, F.; Xiao, J.; Ma, J.; Pan, X. Shinkonin promotes hypertrophic scar repair by autophagy of hypertrophic scar-derived fibroblasts. Acta Cir. Bras. 2023, 38, e384623. [Google Scholar] [CrossRef]
- Fan, C.; Dong, Y.; Xie, Y.; Su, Y.; Zhang, X.; Leavesley, D.; Upton, Z. Shikonin reduces TGF-β1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int. J. Mol. Med. 2015, 36, 985–991. [Google Scholar] [CrossRef]
- Vinaik, R.; Barayan, D.; Auger, C.; Abdullahi, A.; Jeschke, M.G. Regulation of glycolysis and the Warburg effect in wound healing. JCI Insight 2020, 5, e138949. [Google Scholar] [CrossRef]
- Ning, X.; Wiraja, C.; Chew, W.T.S.; Fan, C.; Xu, C. Transdermal delivery of Chinese herbal medicine extract using dissolvable microneedles for hypertrophic scar treatment. Acta Pharm. Sin. B 2021, 11, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, N.N.; Li, H.Y.; Jiang, M.; Gao, J.; Bai, G. Active ingredients in rhubarb with anti-roliferative effects on scar fibroblasts. Yao Xue Xue Bao 2012, 47, 1618–1622. [Google Scholar]
- Liu, C. Inhibition of mechanical stress-induced hypertrophic scar inflammation by emodin. Mol. Med. Rep. 2015, 11, 4087–4092. [Google Scholar] [CrossRef]
- Tao, Y.; Qu, Y. Influence of Emodin Gel on the Fibroblasts of Hypertrophic Scars in Rabbit Ear Model. J. Biomed. Eng. 2015, 32, 862–866. [Google Scholar]
- Guan, R.; Zhao, X.; Wang, X.; Song, N.; Guo, Y.; Yan, X.; Jiang, L.; Cheng, W.; Shen, L. Emodin alleviates bleomycin-induced pulmonary fibrosis in rats. Toxicol. Lett. 2016, 262, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, J.; Yang, S.; Liu, C.; Qin, S.; Li, W.; Cheng, Y.; Hu, H.; Qian, J.; Liu, Y.; et al. Emodine alleviates hypertrophic scar formation by suppressing macrophage polarization and inhibiting the Notch and TGF-β pathways in macrophages. Braz. J. Med. Biol. Res. 2021, 54, e11184. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, D.; Tang, D.D.; Liu, J.; Wang, L.Y.; Chen, W.; Wu, C.J.; Peng, W. Glabridin from glycyrrhiza glabra possesses a therapeutic role against keloid via attenuating PI3K/Akt and transforming growth factor-β1/SMAD signaling pathways. J. Agric. Food Chem. 2022, 70, 10782–10793. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, L.; Zhang, Y.; Gao, Z. Kaempferol inhibits fibroblast collagen synthesis, proliferation and activation in hypertrophic scar via targeting TGF-β receptor type I. Biomed. Pharmacother. 2016, 83, 967–974. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, E.; Lei, Y.; Yan, Q.; Xue, C. Tripterine inhibits proliferation and promotes apoptosis of keloid fibroblasts by targeting ROS/JNK signaling. J. Burn. Care Res. 2024, 45, 104–111. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, J.; Wang, P. Tripterine emerges as a potential anti-scarring agent in NIH/3T3 cells by repressing ANRIL. Gen. Physiol. Biophys. 2020, 39, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.X.; Ding, J.-C.; Tang, Z.-M.; Li, J.-G.; Chen, X.-H.; Zhang, C.-X. Effect of Wubeizi ointment aqueous solution on the expression of type I and III procollagen genes in keloid fibroblasts. Exp. Ther. Med. 2017, 13, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Cao, Y.; Ding, J.; Zhai, X.; Jing, M.; Wang, M.; Lu, L. Wubeizi ointment suppresses keloid formation through modulation of the mTOR pathway. BioMed Res. Int. 2020, 2020, 3608372. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.C.; Tang, Z.M.; Zhai, X.X.; Chen, X.H.; Li, J.G.; Zhang, C.X. The effects of Wubeizi ointment on the proliferation of keloid-derived fibroblasts. Cell Biochem. Biophys. 2015, 71, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Haj Hussein, B.; Kasabri, V.; Al-Hiari, Y.; Arabiyat, S.; Ikhmais, B.; Alalawi, S.; Al-Qirim, T. Selected statins as dual antiproliferative-antiinflammatory compounds. Asian Pac. J. Cancer Prev. 2022, 23, 4047–4062. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.H.; Kim, Y.M.; Kim, B.S.; Kim, J.H.; Kim, M.B.; Ko, H.C. Simvastatin inhibits transforming growth factor-β1-induced expression of type I collagen, CTGF, and α-SMA in keloid fibroblasts. Wound Repair Regen. 2014, 22, 125–133. [Google Scholar] [CrossRef]
- Chen, B.; Kang, C.; Yu, D.; Zhao, X.; An, Y.; Qin, Z. Different effects of simvastatin on keloid fibroblasts under hypoxia and TGF-β1 treatment. Zhonghua Zheng Xing Wai Ke Za Zhi 2016, 32, 130–135. [Google Scholar] [PubMed]
- Dolivo, D.; Rodrigues, A.; Sun, L.; Hou, C.; Li, Y.; Chung, E.; Leung, K.; Galiano, R.; Mustoe, T.; Hong, S.J. Simvastatin cream alleviates dermal fibrosis in a rabbit ear hypertrophic scar model. J. Cosmet. Dermatol. 2023, 22, 534–541. [Google Scholar] [CrossRef]
- van den Broek, L.J.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Development, validation and testing of a human tissue engineered hypertrophic scar model. ALTEX 2012, 29, 389–402. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Wang, R.; Wang, Y.; Wang, X.; Feng, Z. Effects of atorvastatin on the function of Tenon’s capsule fibroblasts in human eyes. Int. Ophthalmol. 2023, 43, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Kim, P.S.; Zhao, Y.; Hong, S.J.; Mustoe, T.A. HMG-CoA reductase inhibitors (statins) reduce hypertrophic scar formation in a rabbit ear wounding model. Plast. Reconstr. Surg. 2012, 129, 252e–261e. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Dolivo, D.M.; Jia, S.; Cheng, X.; Salcido, J.; Galiano, R.D.; Hong, S.J.; Mustoe, T.A. Liposome-encapsulated statins reduce hypertrophic scarring through topical application. Wound Repair Regen. 2020, 28, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Mumby, S. Glucocorticoids. Handb. Exp. Pharmacol. 2017, 237, 171–196. [Google Scholar] [PubMed]
- Timmermans, S.; Souffriau, J.; Libert, C. A general introduction to glucocorticoid biology. Front. Immunol. 2019, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Bayat, A. Current use of steroids in management of abnormal raised skin scars. Surgeon 2007, 5, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.A.; Hanasono, M.M.; Mikulec, A.A.; Kita, M.; Koch, R.J. Triamcinolone stimulates bFGF production and inhibits TGF-beta1 production by human dermal fibroblasts. Dermatol. Surg. 2002, 28, 704–709. [Google Scholar] [PubMed]
- Yang, T.H.; Gingery, A.; Thoreson, A.R.; Larson, D.R.; Zhao, C.; Amadio, P.C. Triamcinolone acetonide affects TGF-β signaling regulation of fibrosis in idiopathic carpal tunnel syndrome. BMC Musculoskelet. Disord. 2018, 19, 342. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, D.; Xie, J.; Li, M.; Chen, P.; Yu, Z. Triamcinolone acetonide suppressed scar formation in mice and human hypertrophic scar fibroblasts in a dose-dependent manner. Cell Mol. Biol. 2023, 69, 226–231. [Google Scholar]
- Chen, A.D.; Chen, R.F.; Li, Y.T.; Huang, Y.T.; Lin, S.D.; Lai, C.S.; Kuo, Y.R. Triamcinolone acetonide suppresses keloid formation through enhancing apoptosis in a nude mouse model. Ann. Plast. Surg. 2019, 83 (Suppl. S1), S50–S54. [Google Scholar] [CrossRef]
- Kauh, Y.C.; Rouda, S.; Mondragon, G.; Tokarek, R.; di Leonardo, M.; Tuan, R.S.; Tan, E.M. Major suppression of pro-alpha1(I) type I collagen gene expression in the dermis after keloid excision and immediate intrawound injection of triamcinolone acetonide. J. Am. Acad. Dermatol. 1997, 37, 586–589. [Google Scholar] [CrossRef]
- Cai, L.; Hu, M.; Lin, L.; Zheng, T.; Liu, J.; Li, Z. Evaluation of the efficacy of triamcinolone acetonide in the treatment of keloids by high-frequency ultrasound. Skin Res. Technol. 2020, 26, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, Y.H.; Lee, H.J.; Lee, G.Y.; Kim, W.S. Auricular keloid management in Asian skin: Clinical outcome of intralesional excision and postoperative triamcinolone acetonide intralesional injection. J. Cosmet. Dermatol. 2020, 19, 3041–3047. [Google Scholar] [CrossRef]
- Lembo, F.; Cecchino, L.R.; Parisi, D.; Portincasa, A. The objective evaluation of triamcinolone acetonide efficacy in keloids management using Antera3D® imaging system. Scars Burn. Heal. 2022, 8, 20595131221137768. [Google Scholar] [CrossRef]
- Chua, S.C.; Gidaszewski, B.; Khajehei, M. Efficacy of surgical excision and sub-dermal injection of triamcinolone acetonide for treatment of keloid scars after caesarean section: A single blind randomised controlled trial protocol. Trials 2019, 20, 363. [Google Scholar] [CrossRef]
- Wu, W.S.; Wang, F.S.; Yang, K.D.; Huang, C.C.; Kuo, Y.R. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J. Investig. Dermatol. 2006, 126, 1264–1271. [Google Scholar] [CrossRef]
- Bagabir, R.; Syed, F.; Paus, R.; Bayat, A. Long-term organ culture of keloid disease tissue. Exp. Dermatol. 2012, 21, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, R.; Li, L.; Yang, G.; Ding, S.; Zhong, Z.; Zhou, S. Co-delivery of dexamethasone and green tea polyphenols using electrospun ultrafine fibers for effective treatment of keloid. Pharm. Res. 2014, 31, 1632–1643. [Google Scholar] [CrossRef]
- Joglar, A.; Song, J.; Golovko, G.; Jay, J.; Wolf, S.; El Ayadi, A. Comparing the effectiveness of glucocorticoids in preventing hypertrophic scar diagnosis in burn patients. Medicina 2023, 59, 1970. [Google Scholar] [CrossRef] [PubMed]
- Çalıskan, E.; Gamsızkan, M.; Açıkgöz, G.; Durmuş, M.; Toklu, S.; Doğrul, A.; Kurt, A.; Tunca, M. Intralesional treatments for hypertrophic scars: Comparison among corticosteroid, 5-fluorouracil and botulinum toxin in rabbit ear hypertrophic scar model. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1603–1608. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.M.; Kim, Y.B. The effects of platelet-rich plasma on hypertrophic scars fibroblasts. Int. Wound J. 2018, 15, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Alser, O.H.; Goutos, I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: A literature review. Scars Burn. Heal. 2018, 4, 2059513118808773. [Google Scholar] [CrossRef]
- Jin, P.; Pan, Q.; Lin, Y.; Dong, Y.; Zhu, J.; Liu, T.; Zhu, W.; Cheng, B. Platelets facilitate wound healing by mitochondrial transfer and reducing oxidative stress in endothelial cells. Oxid. Med. Cell. Longev. 2023, 2023, 2345279. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; PourMohammad, A.; Goodarzi, A. A systematic review of the efficacy, safety and satisfaction of regenerative medicine treatments, including platelet-rich plasma, stromal vascular fraction and stem cell-conditioned medium for hypertrophic scars and keloids. Int. Wound J. 2024, 21, e14557. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, A.; Martins, P.N.; Solez, K.; Uygun, B.E.; Gorantla, V.S.; Orlando, G. Regenerative medicine applications: An overview of clinical trials. Front. Bioeng. Biotechnol. 2022, 10, 942750. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Carias, R.B.; Teixeira, M.V.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M.; Caviggioli, F.; Klinger, F.M.; Giannasi, S.; Bandi, V.; Banzatti, B.; Forcellini, D.; Maione, L.; Catania, B.; Vinci, V. Autologous fat graft in scar treatment. J. Craniofac. Surg. 2013, 24, 1610–1615. [Google Scholar] [CrossRef]
- Gentile, P.; De Angelis, B.; Pasin, M.; Cervelli, G.; Curcio, C.B.; Floris, M.; Di Pasquali, C.; Bocchini, I.; Balzani, A.; Nicoli, F.; et al. Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical evaluation for cell-based therapies in patients with scars on the face. J. Craniofac. Surg. 2014, 25, 267–272. [Google Scholar] [CrossRef]
- Sultan, S.M.; Barr, J.S.; Butala, P.; Davidson, E.H.; Weinstein, A.L.; Knobel, D.; Saadeh, P.B.; Warren, S.M.; Coleman, S.R.; Hazen, A. Fat grafting accelerates revascularisation and decreases fibrosis following thermal injury. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 219–227. [Google Scholar] [CrossRef]
- Huang, S.H.; Wu, S.H.; Chang, K.P.; Lin, C.H.; Chang, C.H.; Wu, Y.C.; Lee, S.S.; Lin, S.D.; Lai, C.S. Alleviation of neuropathic scar pain using autologous fat grafting. Ann. Plast. Surg. 2015, 74 (Suppl. S2), S99–S104. [Google Scholar] [CrossRef]
- Byrne, M.; O’Donnell, M.; Fitzgerald, L.; Shelley, O.P. Early experience with fat grafting as an adjunct for secondary burn reconstruction in the hand: Technique, hand function assessment and aesthetic outcomes. Burns 2016, 42, 356–365. [Google Scholar] [CrossRef]
- Fredman, R.; Edkins, R.E.; Hultman, C.S. Fat grafting for neuropathic pain after severe burns. Ann. Plast. Surg. 2016, 76 (Suppl. S4), S298–S303. [Google Scholar] [CrossRef]
- De Jongh, F.; Pouwels, S.; Tan, L.T. Autologous fat grafting for the treatment of a painful neuroma of the hand: A case report and review of literature. Cureus 2020, 12, e10381. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, N.S.; Piccolo, M.S.; Piccolo, M.T. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin. Plast. Surg. 2015, 42, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, N.S.; Piccolo, M.S.; de Paula Piccolo, N.; de Paula Piccolo, P.; de Paula Piccolo, N.; Daher, R.P.; Lobo, R.P.; Daher, S.P.; Sarto Piccolo, M.T. Fat grafting for treatment of facial burns and burn scars. Clin. Plast. Surg. 2020, 47, 119–130. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahmad, I.; Khurram, M.F.; Chaudhury, G.; Karad, S.; Tripathi, S.; Sharma, A. The role of platelet-rich plasma in reducing pain, pruritis, and improving wound healing of skin graft donor site. Indian. J. Plast. Surg. 2022, 55, 376–382. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Picard, F.; Hermeziu, O.; Rodriguez, A.M.; Ezzedine, K.; Meningaud, J.P. Efficacy of autologous platelet concentrates as adjuvant therapy to surgical excision in the treatment of keloid scars refractory to conventional treatments: A pilot prospective study. Ann. Plast. Surg. 2018, 81, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Neinaa, Y.M.E.; Elsayed, T.A.; Mohamed, D.A.; Elfar, N.N. Botulinum toxin and platelet rich plasma as innovative therapeutic modalities for keloids. Dermatol. Ther. 2021, 34, e14900. [Google Scholar] [CrossRef]
- Willemsen, J.C.N.; Van Dongen, J.; Spiekman, M.; Vermeulen, K.M.; Harmsen, M.C.; van der Lei, B.; Stevens, H.P.J. The addition of platelet-rich plasma to facial lipofilling: A double-blind, placebo-controlled, randomized trial. Plast. Reconstr. Surg. 2018, 141, 331–343. [Google Scholar] [CrossRef]
- Albalat, W.; Nabil, S.; Khattab, F. Assessment of various intralesional injections in keloid: Comparative analysis. J. Dermatol. Treat. 2022, 33, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Domergue, S.; Bony, C.; Maumus, M.; Toupet, K.; Frouin, E.; Rigau, V.; Vozenin, M.C.; Magalon, G.; Jorgensen, C.; Noël, D. Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars. PLoS ONE 2016, 11, e0156161. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wei, W.; Pan, T.; Lu, J.; Wei, Y. Comparison research on the therapeutic effects of botulinum toxin type A and stromal vascular fraction gel on hypertrophic scars in the rabbit ear model. Burns 2024, 50, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Giudice, G.; Filoni, A.; Maggio, G.; Bonamonte, D.; Maruccia, M.; Nacchiero, E.; Ribatti, D.; Annese, T.; Vestita, M. Use of the stromal vascular fraction in intermediate-deep acute burns: A case with its own control. J. Burn. Care Res. 2018, 39, 846–849. [Google Scholar] [CrossRef]
- Mbiine, R.; Kayiira, A.; Wayengera, M.; Guyton, M.I.; Kiwanuka, N.; Alenyo, R.; Kalanzi, E.W.; Muwonge, H.; Nakanwagi, C.; Joloba, M.; et al. Safety and feasibility of autologous adipose-derived stromal vascular fraction in the treatment of keloids: A phase one randomized controlled pilot trial. Am. J. Stem Cells. 2023, 12, 23–36. [Google Scholar]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, Y.; Zou, J.; Zhang, E.; Li, Q.; Chen, L.; Li, J. The adipose-derived stem cell peptide ADSCP2 alleviates hypertrophic scar fibrosis via binding with pyruvate carboxylase and remodeling the metabolic landscape. Acta Physiol. 2023, 238, e14010. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; He, L.; Chen, M. Adipose-derived stem cells inhibit dermal fibroblast growth and induce apoptosis in keloids through the arachidonic acid-derived cyclooxygenase-2/prostaglandin E2 cascade by paracrine. Burn. Trauma 2021, 9, tkab020. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, H.J.; Zhou, Z.H.; Li, Z.P.; Liao, S.M.; Wu, Z.Y.; Huang, H.H.; Shi, Y.C. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland. Surg. 2021, 10, 1046–1056. [Google Scholar] [CrossRef]
- Xie, F.; Teng, L.; Xu, J.; Lu, J.; Zhang, C.; Yang, L.; Ma, X.; Zhao, M. Adipose-derived mesenchymal stem cells inhibit cell proliferation and migration and suppress extracellular matrix synthesis in hypertrophic-scar and keloid fibroblasts. Exp. Ther. Med. 2021, 21, 139. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Hu, X.; Zhang, J.; Wang, Z.H.; Wu, S.; Yi, Y.Y. Extracellular vesicles derived from human adipose-derived stem cell prevent the formation of hypertrophic scar in a rabbit model. Ann. Plast. Surg. 2020, 84, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Putri, K.T.; Prasetyono, T.O.H. A critical review on the potential role of adipose-derived stem cells for future treatment of hypertrophic scars. J. Cosmet. Dermatol. 2022, 21, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K.; Kerpedjieva, S.S. Acceleration of wound healing by multiple growth factors and cytokines secreted from multipotential stromal cells/mesenchymal stem cells. Adv. Wound Care 2012, 1, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Huang, R.-L.; Zheng, Y.; Liu, M.; Huo, R. Bone marrow derived mesenchymal stem cells inhibit the proliferative and profibrotic phenotype of hypertrophic scar fibroblasts and keloid fibroblasts through paracrine signaling. J. Dermatol. Sci. 2016, 83, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Sánchez, Á.; Montero-Vilchez, T.; Quiñones-Vico, M.I.; Sanchez-Diaz, M.; Arias-Santiago, S. Current advanced therapies based on human mesenchymal stem cells for skin diseases. Front. Cell Dev. Biol. 2021, 9, 643125. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Yamamoto, Y.; Funayama, E.; Furukawa, H.; Oyama, A.; Murao, N.; Hosono, H.; Kawakubo, K.; Sakamoto, N.; Ohnishi, S. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plast. Reconstr. Surg. 2018, 141, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, J.; Su, L.; Cheng, S.; Zhou, J.; Ye, X.; Dong, Y.; Sun, S.; Qi, F.; Liu, Z.; et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2018, 44, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Harsono, A.D.; Dilogo, I.H.; Prasetyono, T.O.H.; Prasetyo, M.; Werdhani, R.A.; Jusman, S.W.A.; Siregar, N.C.; Soedjana, H. Clinical evaluation of intralesional umbilical cord-derived mesenchymal stem cells, conditioned medium and triamcinolone acetonide injection for keloid treatment: A pilot study. Int. Wound J. 2023, 21, e14460. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192–5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar]
- Bojanic, C.; To, K.; Hatoum, A.; Shea, J.; Seah, K.T.M.; Khan, W.; Malata, C.M. Mesenchymal stem cell therapy in hypertrophic and keloid scars. Cell Tissue Res. 2021, 383, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, P.; Sarkozyova, N.; Ferancikova, N.; Bukovcan, P.; Danisovic, L.; Bohac, M.; Tomas, M.; Koller, J. Autologous mesenchymal stem cells application in post-burn scars treatment: A preliminary study. Cell Tissue Bank. 2021, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Sierra-Sánchez, Á.; Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria-de-la-Torre, R.; Martinez-Lopez, A.; Arias-Santiago, S. Mesenchymal stromal cell-conditioned medium for skin diseases: A systematic review. Front. Cell Dev. Biol. 2021, 9, 654210. [Google Scholar] [CrossRef]
- Galus, R.; Antiszko, M.; Włodarski, P. Clinical applications of hyaluronic acid. Pol. Merkur. Lekarski 2006, 20, 606–608. [Google Scholar] [PubMed]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120 Pt B, 1682–1695. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2016, 28, 78–88. [Google Scholar] [PubMed]
- Vinshtok, Y.; Cassuto, D. Biochemical and physical actions of hyaluronic acid delivered by intradermal jet injection route. J. Cosmet. Dermatol. 2020, 19, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Hoing, J.L.; Newman, M.; Simman, R. Role of hyaluronic acid treatment in the prevention of keloid scarring. J. Am. Coll. Clin. Wound Spec. 2013, 4, 23–31. [Google Scholar] [CrossRef] [PubMed]
- DI Stadio, A. Ear keloid treated with infiltrated non-cross-linked hyaluronic acid and cortisone therapy. In Vivo 2016, 30, 695–699. [Google Scholar]
- Xie, Y.; Wang, H.; Mao, J.; Li, Y.; Hussain, M.; Zhu, J.; Li, Y.; Zhang, L.; Tao, J.; Zhu, J. Enhanced in vitro efficacy for inhibiting hypertrophic scar by bleomycin-loaded dissolving hyaluronic acid microneedles. J. Mater. Chem. B 2019, 7, 6604–6611. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Y.; Fan, H.; Zhang, Y.; Min, P. Microneedle delivery of botulinum toxin type A combined with hyaluronic acid for the synergetic management of multiple sternal keloids with oily skin: A retrospective clinical investigation. J. Cosmet. Dermatol. 2022, 21, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—A review. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef] [PubMed]
- Chello, C.; Nenna, A.; Chello, M.; Satriano, U.M.; Cardetta, F.; Lusini, M.; Nappi, F.; Dianzani, C. Statin treatment and hypertrophic scarring after cardiac surgery. Wound Repair Regen. 2021, 29, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Mukhopadhyay, M. A comparative clinical study on role of 5-flurouracil versus triamcinolone in the treatment of keloids. Indian J. Surg. 2012, 74, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Hietanen, K.E.; Järvinen, T.A.; Huhtala, H.; Tolonen, T.T.; Kuokkanen, H.O.; Kaartinen, I.S. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections—A randomized controlled trial. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.L.; Rea, S.M.; Wood, F.M.; Fear, M.W.; Viola, H.M.; Hool, L.C.; Gankande, T.; Alghamdi, M.; Stevenson, A.W.; Manzur, M.; et al. Verapamil is less effective than triamcinolone for prevention of keloid scar recurrence after excision in a randomized controlled trial. Acta Derm. Venereol. 2016, 96, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Saki, N.; Mokhtari, R.; Nozari, F. Comparing the efficacy of intralesional triamcinolone acetonide with verapamil in treatment of keloids: A randomized controlled trial. Dermatol. Pract. Concept. 2019, 9, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Flores, F. Recurrence rates of excised keloids treated with postoperative triamcinolone acetonide injections or interferon alfa-2b injections. J. Am. Acad. Dermatol. 1997, 37 Pt 1, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Sahibzada, M.N.; Paracha, M.M. Comparison of the efficacy of intralesional bleomycin versus intralesional triamcinolone acetonide in the treatment of keloids. Dermatol. Ther. 2019, 32, e13036. [Google Scholar] [CrossRef]
- Bi, M.; Sun, P.; Li, D.; Dong, Z.; Chen, Z. Intralesional injection of botulinum toxin type A compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: A systematic review and meta-analysis. Med. Sci. Monit. 2019, 25, 2950–2958. [Google Scholar] [CrossRef]
- Hamada Mohamed, B.; Eltahlawy, S.; Marzouk, W.A.; Mohamad, N.E. Safety and efficacy of intralesional injection of enalapril versus triamcinolone acetonide in the treatment of keloids. Acta Dermatovenerol. Alp. Pannonica Adriat. 2023, 32, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, Y.J.; Lung, I.; Leung, B.C.; Burd, A. A study of the combination of triamcinolone and 5-fluorouracil in modulating keloid fibroblasts in vitro. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, e251–e259. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, T.C.; Kao, S.C.; Kuo, Y.F.; Chien, L.F. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol. 1993, 129, 322–327. [Google Scholar] [CrossRef]
- Moreira, V.M.; Leite, J.M.D.S.; Medeiros, K.A.; Assis, K.M.A.; Borges, J.C.; Santana, L.M.B.; Moreira, L.M.C.C.; Alves, L.P.; Oliveira, T.K.B.; Silveira, J.W.S.D.; et al. Pentoxifylline/chitosan films on wound healing: In vitro/in vivo evaluation. Pharmaceutics 2023, 15, 1122. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.E. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol. Surg. 1999, 25, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Asilian, A.; Darougheh, A.; Shariati, F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol. Surg. 2006, 32, 907–915. [Google Scholar]
- Darougheh, A.; Asilian, A.; Shariati, F. Intralesional triamcinolone alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. Clin. Exp. Dermatol. 2009, 34, 219–223. [Google Scholar] [CrossRef]
- Davison, S.P.; Dayan, J.H.; Clemens, M.W.; Sonni, S.; Wang, A.; Crane, A. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthet. Surg. J. 2009, 29, 40–46. [Google Scholar] [CrossRef]
- Alexandrescu, D.; Fabi, S.; Yeh, L.C.; Fitzpatrick, R.E.; Goldman, M.P. Comparative results in treatment of keloids with intralesional 5-FU/Kenalog, 5-FU/verapamil, enalapril alone, verapamil alone, and laser: A case report and review of the literature. J. Drugs Dermatol. 2016, 15, 1442–1447. [Google Scholar]
- Sharma, S.; Vinay, K.; Bassi, R. Treatment of small keloids using intralesional 5-fluorouracil and triamcinolone acetonide versus intralesional bleomycin and triamcinolone acetonide. J. Clin. Aesthet. Dermatol. 2021, 14, 17–21. [Google Scholar]
- Monteiro, R.C.; Bhat, M.R.; Martis, J.; Kamath, H.G. A Comparative Study of the Efficacy of Intralesional 5 Fluorouracil vs Combination of 5 Fluorouracil with Triamcinolone Acetonide in Keloids. Indian J. Dermatol. 2022, 67, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Mavilakandy, A.K.; Vayalapra, S.; Minty, I.; Parekh, J.N.; Charles, W.N.; Khajuria, A. Comparing the efficacy and safety of combination triamcinolone acetonide and 5-fluorouracil versus monotherapy triamcinolone acetonide or 5-fluorouracil in the treatment of hypertrophic scars and keloids: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2023, 10, A1–A11. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Wei, Z.; Lin, W.; Fan, B.; Feng, S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: A systematic review and meta-analysis. Int. Wound J. 2017, 14, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, M.; Guertler, A.; Schwaiger, H.; Poetschke, J.; Gauglitz, G.G. Treatment of keloids using 5-fluorouracil in combination with crystalline triamcinolone acetonide suspension: Evaluating therapeutic effects by using non-invasive objective measures. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, R.M.; Won, P.; Lin, J.; Pham, C.; Madrigal, P.; Yenikomshian, H.; Gillenwater, T.J. Combining scar-modulating agents for the treatment of hypertrophic scars and keloids: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2024, 88, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Stromps, J.P.; Dunda, S.; Eppstein, R.J.; Babic, D.; Har-Shai, Y.; Pallua, N. Intralesional cryosurgery combined with topical silicone gel sheeting for the treatment of refractory keloids. Dermatol. Surg. 2014, 40, 996–1003. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yang, J.Y.; Hsiao, Y.C. Comparison of combination therapy (steroid, calcium channel blocker, and interferon) with steroid monotherapy for treating human hypertrophic scars in an animal model. Ann. Plast. Surg. 2015, 74 (Suppl. S2), S162–S167. [Google Scholar] [CrossRef]
- Klomparens, K.; Simman, R. Treatment of keloids: A meta-analysis of intralesional triamcinolone, verapamil, and their combination. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4075. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, L.; Younas, A.; Liu, F.; Sun, J.; Dong, Z.; Zhao, Y. Co-delivery of triamcinolone acetonide and verapamil for synergistic treatment of hypertrophic scars via carboxymethyl chitosan and Bletilla striata polysaccharide-based microneedles. Carbohydr. Polym. 2022, 284, 119219. [Google Scholar] [CrossRef]
- Haghani-Dogahe, Z.; Hadadi, R.; Esmailzadeh, M.; Mobayen, M. Comparing intralesional triamcinolone and verapamil-triamcinolone injections in keloids: A single-blinded randomised clinical trial. Int. Wound J. 2023, 20, 4166–4174. [Google Scholar] [CrossRef]
- Camacho-Martínez, F.M.; Rey, E.R.; Serrano, F.C.; Wagner, A. Results of a combination of bleomycin and triamcinolone acetonide in the treatment of keloids and hypertrophic scars. Bras. Dermatol. 2013, 88, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ledon, J.A.; Savas, J.; Franca, K.; Chacon, A.; Nouri, K. Intralesional treatment for keloids and hypertrophic scars: A review. Dermatol. Surg. 2013, 39, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.F. The combined application of bleomycin and triamcinolone for the treatment of keloids and hypertrophic scars: An effective therapy for treating refractory keloids and hypertrophic scars. Skin Res. Technol. 2023, 29, e13389. [Google Scholar] [CrossRef]
- Mozafari, N.; Mollaabasi, F.; Mansouri, P.; Robati, R.M. The combined application of bleomycin and triamcinolone for treating refractory keloids. Dermatol. Surg. 2023, 50, 271. [Google Scholar] [CrossRef] [PubMed]
- Tran, J.V.; Lultschik, S.D.; Ho, J.S.; Sapra, S.; Dong, K.; Gusic, K. Concomitant therapy of surgical shave excision and intralesional injections for ear keloids: Early results from a retrospective cohort study. Scars Burn. Heal. 2022, 8, 20595131221098531. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Valesky, E.M.; Meissner, M.; Hrgovic, I.; Kaufmann, R.; Kippenberger, S.; Zöller, N.N. Normal and keloid fibroblasts are differentially influenced by IFN-γ and triamcinolone as well as by their combination. Wound Repair Regen. 2019, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, E.S.; Sabaa, B.E.I.; Mohamed, W.S.; Hegab, D.S. Combined intralesional triamcinolone acetonide and platelet rich plasma versus intralesional triamcinolone acetonide alone in treatment of keloids. J. Dermatol. Treat. 2022, 33, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, Z.; Alimohamadi, Y.; Janani, M.; Hejazi, P.; Kamali, M.; Goodarzi, A. Platelet-rich plasma in the treatment of scars, to suggest or not to suggest? A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2022, 16, 875–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Luo, Y.J.; Luo, C. Network meta-analysis of different clinical commonly used drugs for the treatment of hypertrophic scar and keloid. Front. Med. 2021, 8, 691628. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Y.; Chen, Y.; Zhong, A. Comparing the efficacy of multiple drugs injection for the treatment of hypertrophic scars and keloid: A network meta-analysis. Aesthetic Plast. Surg. 2023, 47, 465–472. [Google Scholar] [CrossRef]
- Stewart, C.E., 4th; Kim, J.Y. Application of mitomycin-C for head and neck keloids. Otolaryngol.-Head Neck Surg. 2006, 135, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Bijlard, E.; Timman, R.; Verduijn, G.M.; Niessen, F.B.; Hovius, S.E.R.; Mureau, M.A.M. Intralesional cryotherapy versus excision with corticosteroid injections or brachytherapy for keloid treatment: Randomised controlled trials. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 847–856. [Google Scholar] [CrossRef]
- Stern, R.S.; Dover, J.S.; Levin, J.A.; Arndt, K.A. Laser therapy versus cryotherapy of lentigines: A comparative trial. J. Am. Acad. Dermatol. 1994, 30, 985–987. [Google Scholar]
- van Leeuwen, M.C.; Bulstra, A.E.; van Leeuwen, P.A.; Niessen, F.B. A new argon gas-based device for the treatment of keloid scars with the use of intralesional cryotherapy. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, M.C.E.; van der Wal, M.B.A.; Bulstra, A.J.; Galindo-Garre, F.; Molier, J.; van Zuijlen, P.P.M.; van Leeuwen, P.A.M.; Niessen, F.B. Intralesional cryotherapy for treatment of keloid scars: A prospective study. Plast. Reconstr. Surg. 2015, 135, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Tawaranurak, N.; Pliensiri, P.; Tawaranurak, K. Combination of fractional carbon dioxide laser and topical triamcinolone vs intralesional triamcinolone for keloid treatment: A randomised clinical trial. Int. Wound J. 2022, 19, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Reissis, D.; Tickunas, T.; Agha, R.A.; Greig, A. Intralesional excision with topical intralesional cryotherapy improves the treatment of keloid scarring in a paediatric patient. Ann. R. Coll. Surg. Engl. 2017, 99, e233–e335. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Kim, J.; Yang, C.E.; Hong, J.W.; Lee, W.J.; Lee, J.H. Combined therapeutic strategies for keloid treatment. Dermatol. Surg. 2019, 45, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Muthanna, A.M.; Al-Qubati, Y.A. Cryotherapy: A successful monotherapy for earlobe keloids. Malays. Fam. Physician. 2020, 15, 83–85. [Google Scholar]
- Thornton, N.J.; Garcia, B.A.; Hoyer, P.; Wilkerson, M.G. Keloid scars: An updated review of combination therapies. Cureus 2021, 13, e12999. [Google Scholar] [CrossRef]
- Park, T.H.; Cho, H.J.; Lee, J.W.; Kim, C.W.; Chong, Y.; Chang, C.H.; Park, K.S. Could −79 °C spray-type cryotherapy be an effective monotherapy for the treatment of keloid? Int. J. Mol. Sci. 2017, 18, 2536. [Google Scholar] [CrossRef] [PubMed]
- Manuskiatti, W.; Fitzpatrick, R.E. Treatment response of keloidal and hypertrophic sternotomy scars: Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch. Dermatol. 2002, 138, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.S.; Wulkan, A.J.; Shumaker, P.R. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg. Med. 2013, 45, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Cavalié, M.; Sillard, L.; Montaudié, H.; Bahadoran, P.; Lacour, J.P.; Passeron, T. Treatment of keloids with laser-assisted topical steroid delivery: A retrospective study of 23 cases. Dermatol. Ther. 2015, 28, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kraeva, E.; Ho, D.; Jagdeo, J. Successful treatment of keloid with fractionated carbon dioxide (CO2) laser and laser-assisted drug delivery of triamcinolone acetonide ointment in an African-American man. J. Drugs Dermatol. 2017, 16, 925–927. [Google Scholar] [PubMed]
- Alegre-Sánchez, A.; Jiménez-Gómez, N.; Boixeda, P. Laser-assisted drug delivery. Actas Dermosifiliogr. (Engl. Ed.) 2018, 109, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Khetarpal, S.; Kaw, U.; Dover, J.S.; Arndt, K.A. Laser advances in the treatment of burn and traumatic scars. Semin. Cutan. Med. Surg. 2017, 36, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumari, H.; Singh, A. Comparison of fractional CO2 laser, verapamil, and triamcinolone for the treatment of keloid. Adv. Wound Care 2019, 8, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sabry, H.H.; Abdel Rahman, S.H.; Hussein, M.S.; Sanad, R.R.; Abd El Azez, T.A. The efficacy of combining fractional carbon dioxide laser with verapamil hydrochloride or 5-fluorouracil in the treatment of hypertrophic scars and keloids: A clinical and immunohistochemical study. Dermatol. Surg. 2019, 45, 536–546. [Google Scholar] [CrossRef]
- Tawfic, S.O.; El-Tawdy, A.; Shalaby, S.; Foad, A.; Shaker, O.; Sayed, S.S.; Metwally, D. Evaluation of fractional CO2 versus long pulsed Nd:YAG lasers in treatment of hypertrophic scars and keloids: A randomized clinical trial. Lasers Surg. Med. 2020, 52, 959–965. [Google Scholar] [CrossRef]
- Sabry, H.H.; Ibrahim, E.A.; Hamed, A.M. Assessment of laser-assisted delivery vs intralesional injection of botulinum toxin A in treatment of hypertrophic scars and keloids. Dermatol. Ther. 2020, 33, e13980. [Google Scholar] [CrossRef] [PubMed]
- Oosterhoff, T.C.H.; Beekman, V.K.; van der List, J.P.; Niessen, F.B. Laser treatment of specific scar characteristics in hypertrophic scars and keloid: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Etman, Y.; AbdElhameed, A.; Elsharaby, R.; Tawfik, A. Comparative study between Nd-YAG laser, fractional CO2 laser, and combined Nd-YAG with fractional CO2 laser in the management of keloid: Clinical and molecular study. J. Cosmet. Dermatol. 2021, 20, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lou, X.; Shen, T.; Sun, Y.; Xiao, Y.; Zheng, X.; Wang, X.; Peng, Y.; Guo, Y.; Guo, Y.; et al. Combination of ablative fractional carbon dioxide laser and platelet-rich plasma treatment to improve hypertrophic scars: A retrospective clinical observational study. Burn. Trauma 2021, 9, tkab016. [Google Scholar] [CrossRef] [PubMed]
- El-Hamid El-Azhary, E.A.; Abd Al-Salam, F.M.; El-Hafiz, H.S.A.; Maghraby, H.M. Fractional carbon dioxide (CO2) laser alone versus fractional CO2 laser combined with triamcinolone acetonide or trichloroacetic acid in keloid treatment: A comparative clinical and radiological study. Dermatol. Pract. Concept. 2022, 12, e2022072. [Google Scholar] [PubMed]
- Nishi, N.; Rajashekar, T.S. A comparative study of effectiveness of cryotherapy with intralesional triamcinolone vs fractional CO2 laser with topical betamethasone for the treatment of keloids. J. Cutan. Aesthet. Surg. 2022, 15, 254–259. [Google Scholar] [PubMed]
- Liu, Z.; Zhang, J.; Guo, X. Clinical effects of pulsed dye laser dynamically combined with triamcinolone acetonide in the treatment of postoperative recurrence keloids. Indian J. Dermatol. 2023, 68, 486. [Google Scholar] [PubMed]
- Wang, W.; Zhao, J.; Zhang, C.; Zhang, W.; Jin, M.; Shao, Y. Current advances in the selection of adjuvant radiotherapy regimens for keloid. Front. Med. 2022, 9, 1043840. [Google Scholar] [CrossRef]
- Dong, W.; Qiu, B.; Fan, F. Adjuvant Radiotherapy for Keloids. Aesthetic Plast. Surg. 2022, 46, 489–499. [Google Scholar] [CrossRef]
- Berman, B.; Bieley, H.C. Adjunct therapies to surgical management of keloids. Dermatol. Surg. 1996, 22, 126–130. [Google Scholar] [CrossRef]
- Kal, H.B.; Veen, R.E. Biologically effective doses of postoperative radiotherapy in the prevention of keloids. Dose-effect relationship. Strahlenther. Onkol. 2005, 181, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.; Reznik, R.; Orgel, M.; Li, Q.; Mirhadi, A.; Kulber, D.A. Surgical excision and adjuvant brachytherapy vs external beam radiation for the effective treatment of keloids: 10-Year institutional retrospective analysis. Aesthet. Surg. J. 2017, 37, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, K.N.; Venkatesh, M.S.; Alva, R.; Koushik, K.; Waiker, V.; Mohan, K.; Shivalingappa, S. Efficacy of surgical excision and adjuvant high-dose rate brachytherapy in treatment of keloid: Our experience. J. Cutan. Aesthet. Surg. 2021, 14, 337–343. [Google Scholar] [CrossRef]
- Masoodi, Z.; Ahmad, I.; Khurram, M.F.; Haq, A. Excision, skin grafting, corticosteroids, adjuvant radiotherapy, pressure therapy, and emancipation: The ESCAPE model for successful taming of giant auricular keloids. Adv. Skin Wound Care 2014, 27, 404–412. [Google Scholar] [CrossRef]
- Deng, K.; Xiao, H.; Liu, X.; Ogawa, R.; Xu, X.; Liu, Y. Strontium-90 brachytherapy following intralesional triamcinolone and 5-fluorouracil injections for keloid treatment: A randomized controlled trial. PLoS ONE 2021, 16, e0248799. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, E.T.; Zhou, Y.; Tong, S.; Zhou, K.J.; Guo, S. Individualized surgery combined with radiotherapy and triamcinolone acetonide injection for the treatment of auricular keloids. BMC Surg. 2021, 21, 256. [Google Scholar] [CrossRef]
- Berman, B.; Flores, F. Comparison of a silicone gel-filled cushion and silicon gel sheeting for the treatment of hypertrophic or keloid scars. Dermatol. Surg. 1999, 25, 484–486. [Google Scholar] [CrossRef]
- Meaume, S.; Le Pillouer-Prost, A.; Richert, B.; Roseeuw, D.; Vadoud, J. Management of scars: Updated practical guidelines and use of silicones. Eur. J. Dermatol. 2014, 24, 435–443. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.; Jones, D.J. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2013, 2013, CD003826. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Nunes, T.A.; Magna, L.A.; Cintra, M.L.; Kitten, G.T.; Zarpellon, S.; Raposo Do Amaral, C.M. Silicone versus nonsilicone gel dressings: A controlled trial. Dermatol. Surg. 2001, 27, 721–726. [Google Scholar] [CrossRef]
- Musgrave, M.A.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. J. Burn. Care Rehabil. 2002, 23, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, T.A. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast. Surg. 2008, 32, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Bleasdale, B.; Finnegan, S.; Murray, K.; Kelly, S.; Percival, S.L. The use of silicone adhesives for scar reduction. Adv. Wound Care 2015, 4, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hong, J.P.; Choi, J.W.; Seo, D.K.; Lee, E.S.; Lee, H.S. The efficacy of a silicone sheet in postoperative scar management. Adv. Skin Wound Care 2016, 29, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Meseci, E.; Kayatas, S.; Api, M.; Boza, A.; Cikman, M.S. Comparison of the effectiveness of topical silicone gel and corticosteroid cream on the Pfannenstiel scar prevention—A randomized controlled trial. Ginekol. Pol. 2017, 88, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.P.; Mess, S.; Kauffman, L.C.; Al-Attar, A. Ineffective treatment of keloids with interferon alpha-2b. Plast. Reconstr. Surg. 2006, 117, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.; Harrison-Balestra, C.; Perez, O.A.; Viera, M.; Villa, A.; Zell, D.; Ramirez, C. Treatment of keloid scars post-shave excision with imiquimod 5% cream: A prospective, double-blind, placebo-controlled pilot study. J. Drugs Dermatol. 2009, 8, 455–458. [Google Scholar]
- Shin, J.Y.; Yun, S.K.; Roh, S.G.; Lee, N.H.; Yang, K.M. Efficacy of 2 representative topical agents to prevent keloid recurrence after surgical excision. J. Oral. Maxillofac. Surg. 2017, 75, 401. [Google Scholar] [CrossRef]
- Aljodah, M.A.; Alfeehan, M.J.; Al-Zajrawee, M.Z. Outcome of recurrent auricular keloid treatment with a combination of surgical excision and perioperative corticosteroid injection. J. Cutan. Aesthet. Surg. 2021, 14, 392–396. [Google Scholar] [CrossRef]
- Yıldız, E. Triple treatment in ear keloids: Comparison of post-excisional intralesional steroid and platelet-rich plasma treatment. Am. J. Otolaryngol. 2021, 42, 102935. [Google Scholar] [CrossRef]
- Rimmer, S.N.; Chandy, R.J.; Khan, D.; Feldman, S.R. Recurrence rates in the treatment of keloids and hypertrophic scars with intralesional triamcinolone combined with other intralesional agents. Arch. Dermatol. Res. 2023, 315, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Park, S.D.; Park, K. Comparative effect of topical silicone gel and topical tretinoin cream for the prevention of hypertrophic scar and keloid formation and the improvement of scars. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Hardy, C.; Ridgway, J. Keloid management: A retrospective case review on a new approach using surgical excision, platelet-rich plasma, and in-office superficial photon X-ray radiation therapy. Adv. Skin Wound Care 2016, 29, 303–307. [Google Scholar] [CrossRef]
- Ogawa, R.; Miyashita, T.; Hyakusoku, H.; Akaishi, S.; Kuribayashi, S.; Tateno, A. Postoperative radiation protocol for keloids and hypertrophic scars: Statistical analysis of 370 sites followed for over 18 months. Ann. Plast. Surg. 2007, 59, 688–691. [Google Scholar] [CrossRef]
- Sakuraba, M.; Takahashi, N.; Akahoshi, T.; Miyasaka, Y.; Suzuki, K. Use of silicone gel sheets for prevention of keloid scars after median sternotomy. Surg. Today 2011, 41, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.Z.; Omar, S.S. Treatment of auricular keloids by triple combination therapy: Surgical excision, platelet-rich plasma, and cryosurgery. J. Cosmet. Dermatol. 2018, 17, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Tan, J.; Fu, Q.; Huang, L.; Ao, M. Comparative efficacy of intralesional triamcinolone acetonide injection during early and static stage of pathological scarring. J. Cosmet. Dermatol. 2019, 18, 874–878. [Google Scholar]
- Zhuang, Z.; Li, Y.; Wei, X. The safety and efficacy of intralesional triamcinolone acetonide for keloids and hypertrophic scars: A systematic review and meta-analysis. Burns 2021, 47, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Maeda, R.; Furuya, K.; Isamu, M.; Masayuki, K.; Kanamoto, I. Relationship between the usability and physicochemical properties of triamcinolone acetonide ointments. Results Pharma Sci. 2013, 3, 15–19. [Google Scholar] [CrossRef][Green Version]
- Qin, Z.; Chen, F.; Chen, D.; Wang, Y.; Tan, Y.; Ban, J. Transdermal permeability of triamcinolone acetonide lipid nanoparticles. Int. J. Nanomed. 2019, 14, 2485–2495. [Google Scholar] [CrossRef]
- Kaur, A.; Garg, R.; Mittal, R.K.; Shah, S.; Patial, T.; Addiwal, R. Comparative efficacy of intralesional triamcinolone acetonide and 5-fluorouracil for keloid scars. Plast. Aesthet. Nurs. 2022, 42, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Quan, G.; Hou, A.; Yang, P.; Peng, T.; Gu, Y.; Qin, W.; Liu, R.; Ma, X.; Pan, X.; et al. Strategy for hypertrophic scar therapy: Improved delivery of triamcinolone acetonide using mechanically robust tip-concentrated dissolving microneedle array. J. Control Release 2019, 306, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ono, N. Pain-free intralesional injection of triamcinolone for the treatment of keloid. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1999, 33, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Tosa, M.; Murakami, M.; Hyakusoku, H. Effect of lidocaine tape on pain during intralesional injection of triamcinolone acetonide for the treatment of keloid. J. Nippon. Med. Sch. 2009, 76, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Usanakornkul, A.; Burusapat, C. A topical anesthetic and lidocaine mixture for pain relief during keloid treatment: A double-blind, randomized controlled trial. Dermatol. Surg. 2017, 43, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Shin, S.H.; Koh, Y.G.; Kim, G.H.; Rho, N.K.; Park, K.Y. Cold anesthesia for pain reduction during intralesional steroid injection for nodulocystic acne. J. Cosmet. Dermatol. 2023, 22, 3375–3378. [Google Scholar] [CrossRef] [PubMed]
- Disphanurat, W.; Sivapornpan, N.; Srisantithum, B.; Leelawattanachai, J. Efficacy of a triamcinolone acetonide-loaded dissolving microneedle patch for the treatment of hypertrophic scars and keloids: A randomized, double-blinded, placebo-controlled split-scar study. Arch. Dermatol. Res. 2023, 315, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Shigeki, S.; Murakami, T.; Kiyonaka, G.; Yata, N.; Ikuta, Y. Transdermal iontophoretic delivery of triamcinolone acetonide: A preliminary study in hairless rats. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1996, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, X.; Zhu, Y.; Chen, H.; Xu, H. Nanostructured lipid carriers as vehicles for transdermal iontophoretic drug delivery. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 1236–1239. [Google Scholar]
- Liu, W.; Hu, M.; Liu, W.; Xue, C.; Xu, H.; Yang, X. Investigation of the carbopol gel of solid lipid nanoparticles for the transdermal iontophoretic delivery of triamcinolone acetonide acetate. Int. J. Pharm. 2008, 364, 135–141. [Google Scholar] [CrossRef]
- Yang, J.H.; Kim, D.K.; Kim, T.Y.; Kim, G.Y.; Shin, S.C. Anti-inflammatory effects by transdermal application of triamcinolone acetonide gel using phonophoresis in rats. Int. J. Pharm. 2005, 302, 39–46. [Google Scholar] [CrossRef]
- Shin, J.U.; Park, J.; Lee, J.H.; Lee, K.H.; Kim, Y.O.; Yun, C.O.; Lee, W.J. Extramarginal excision is preferable for hypertrophic scars. Int. J. Dermatol. 2014, 53, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
| Keloids (KDs) | Hypertrophic Scars (HTSs) | Refs. | |
|---|---|---|---|
| Common future | During the healing process of the cutaneous injury, growth factors such as TGF-β are released and they activate fibroblasts. The overexpression of TGF-β1/β2 and ECM such as collagen can cause raised and firm scars with increased vascularization and cellularity. | [3,12] | |
| Onset | KD typically arise from 3 to 12 months, or from 3 months to several years. | HTSs appear within 1 month, or 4–8 weeks, of injury and grow over 6–8 months. | [13] |
| Scar formation | The raised scar of the KD extends beyond the boundaries of the original wounds. | The raised scar of the HTS is contained within the boundaries of the original injury. | [13,14] |
| Scar sites | Predominant anatomical sites of KDs are chest, shoulder, upper back, posterior neck, cheeks, knees, and earlobes. | No predominant anatomical sites of HTSs. | [16,17] |
| Incidence | Less common. Association with age ≥ 22 years, family history and genetic factors. | More common in males than females. | [15] |
| Genetic factor | A genetic predisposition is associated with skin color and familial disposition: African > Asian and Hispanic >> Caucasian populations. | No evidence of genetic factors’ influence. Less association between skin color and familial disposition. | [17,18,19] |
| Collagen | Approximately 20-fold higher collagen production. Increase in type I/III collagen ratio in parallel with the increase in α1(I) procollagen mRNA compared to normal skin tissue. | Approximately 3-fold higher collagen production than normal scars. A higher collagen III scar/normal ratio, with no difference in collagen I scar/normal ratio. | [11,12,22,23] |
| Proinflammatory factors | Upregulation of interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor (TNF)-α expression levels. | Increase in IL-31, IL-31 receptor α, and oncostatin M receptor expression levels. | [3,24] |
| Regression | KD growth continues with time and there is no spontaneous regression. | HTS growth is limited for months, and regression (contraction) occurs spontaneously. | [25] |
| Recurrence after surgical excision | The recurrence rate of KDs is high, 50–100%, after surgical excision without preventive treatments. If excised, preventive treatment is necessary. | The recurrence rate of HTSs is none or low after surgical excision. | [26,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Shigeki, S. Pharmacotherapy for Keloids and Hypertrophic Scars. Int. J. Mol. Sci. 2024, 25, 4674. https://doi.org/10.3390/ijms25094674
Murakami T, Shigeki S. Pharmacotherapy for Keloids and Hypertrophic Scars. International Journal of Molecular Sciences. 2024; 25(9):4674. https://doi.org/10.3390/ijms25094674
Chicago/Turabian StyleMurakami, Teruo, and Sadayuki Shigeki. 2024. "Pharmacotherapy for Keloids and Hypertrophic Scars" International Journal of Molecular Sciences 25, no. 9: 4674. https://doi.org/10.3390/ijms25094674
APA StyleMurakami, T., & Shigeki, S. (2024). Pharmacotherapy for Keloids and Hypertrophic Scars. International Journal of Molecular Sciences, 25(9), 4674. https://doi.org/10.3390/ijms25094674