The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers
Abstract
1. Introduction
2. Anticancer Activities of Natural Terpenoids
2.1. Eucalyptol
2.2. Eugenol
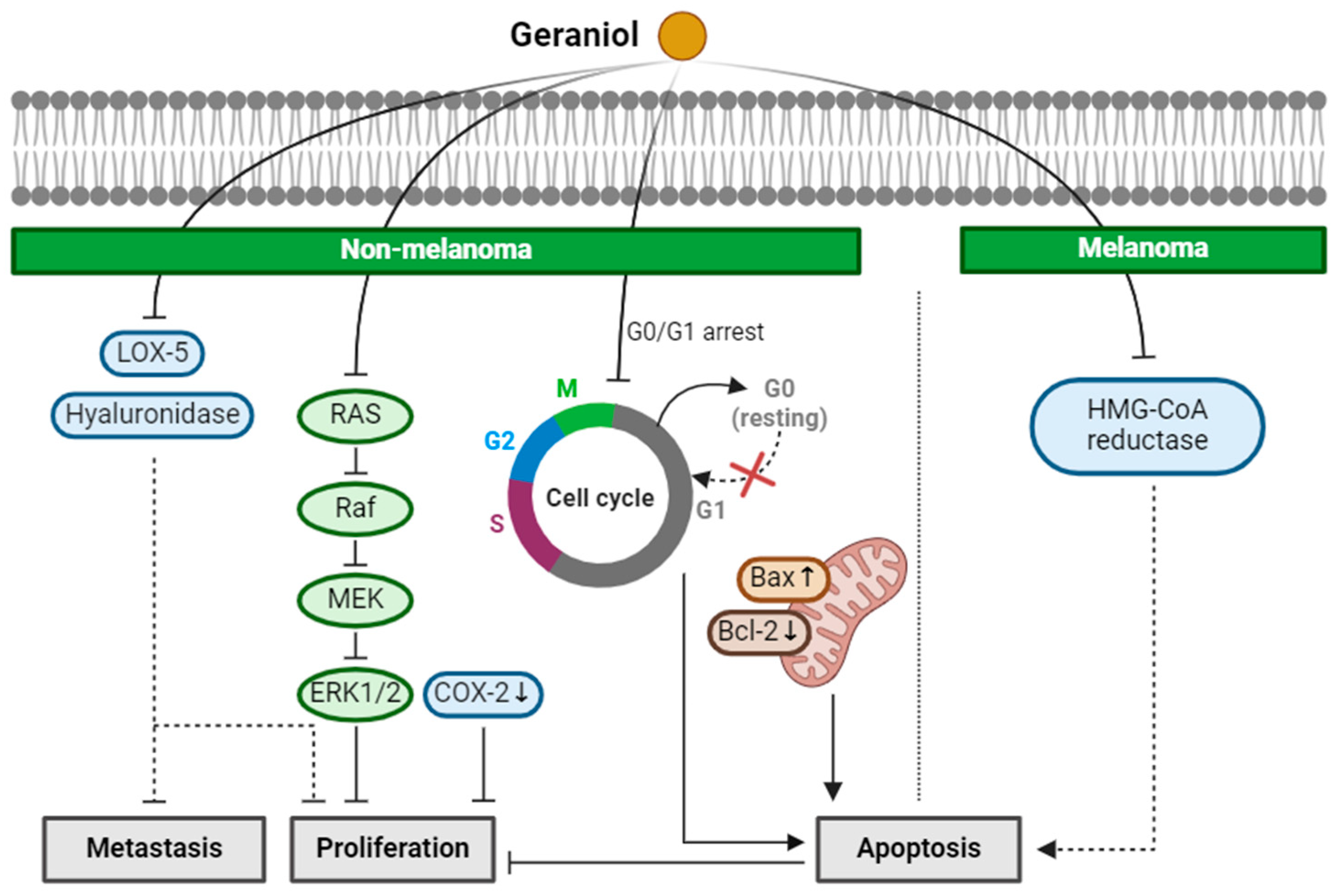
2.3. Geraniol
2.4. Linalool
2.5. Ursolic Acid
3. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Madison, K.C. Barrier function of the skin: “La raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Saladi, R.N.; Persaud, A.N. The causes of skin cancer: A comprehensive review. Drugs Today 2005, 41, 37–53. [Google Scholar] [CrossRef]
- Sandru, A.; Voinea, S.; Panaitescu, E.; Blidaru, A. Survival rates of patients with metastatic malignant melanoma. J. Med. Life 2014, 7, 572–576. [Google Scholar]
- Bradford, P.T.; Goldstein, A.M.; McMaster, M.L.; Tucker, M.A. Acral lentiginous melanoma: Incidence and survival patterns in the United States, 1986–2005. Arch. Dermatol. 2009, 145, 427–434. [Google Scholar] [CrossRef]
- Auluck, A.; Zhang, L.; Desai, R.; Rosin, M.P. Primary malignant melanoma of maxillary gingiva—A case report and review of the literature. J. Can. Dent. Assoc. 2008, 74, 367–371. [Google Scholar]
- Firnhaber, J.M. Diagnosis and treatment of Basal cell and squamous cell carcinoma. Am. Fam. Physician 2012, 86, 161–168. [Google Scholar]
- Suarez, B.; Lopez-Abente, G.; Martinez, C.; Navarro, C.; Tormo, M.J.; Rosso, S.; Schraub, S.; Gafa, L.; Sancho-Garnier, H.; Wechsler, J.; et al. Occupation and skin cancer: The results of the HELIOS-I multicenter case-control study. BMC Public Health 2007, 7, 180. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- O’Driscoll, L.; McMorrow, J.; Doolan, P.; McKiernan, E.; Mehta, J.P.; Ryan, E.; Gammell, P.; Joyce, H.; O’Donovan, N.; Walsh, N.; et al. Investigation of the molecular profile of basal cell carcinoma using whole genome microarrays. Mol. Cancer 2006, 5, 74. [Google Scholar] [CrossRef]
- Katalinic, A.; Kunze, U.; Schafer, T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br. J. Dermatol. 2003, 149, 1200–1206. [Google Scholar] [CrossRef]
- Loh, T.Y.; Ortiz, A.; Goldenberg, A.; Brian Jiang, S.I. Prevalence and Clinical Characteristics of Nonmelanoma Skin Cancers Among Hispanic and Asian Patients Compared With White Patients in the United States: A 5-Year, Single-Institution Retrospective Review. Dermatol. Surg. 2016, 42, 639–645. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer. Adv. Exp. Med. Biol. 2017, 996, 27–40. [Google Scholar] [CrossRef]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef]
- Bandarchi, B.; Jabbari, C.A.; Vedadi, A.; Navab, R. Molecular biology of normal melanocytes and melanoma cells. J. Clin. Pathol. 2013, 66, 644–648. [Google Scholar] [CrossRef]
- Bonerandi, J.J.; Beauvillain, C.; Caquant, L.; Chassagne, J.F.; Chaussade, V.; Clavere, P.; Desouches, C.; Garnier, F.; Grolleau, J.L.; Grossin, M.; et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J. Eur. Acad. Dermatol. Venereol. 2011, 25 (Suppl. S5), 1–51. [Google Scholar] [CrossRef]
- Frank, A.; Groll, M. The Methylerythritol Phosphate Pathway to Isoprenoids. Chem. Rev. 2017, 117, 5675–5703. [Google Scholar] [CrossRef]
- Keshavarz, F.; Soltanshahi, M.; Khosravani, F.; Bakhshiyan, F.; Ghanbari, A.; Hassanzadeh, S.; Amirpour, M.; Ghalamfarsa, G. Thymol-loaded liposomes effectively induced apoptosis and decreased EGFR expression in colorectal cancer cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Boonyanugomol, W.; Rukseree, K.; Prapatpong, P.; Reamtong, O.; Baik, S.C.; Jung, M.; Shin, M.K.; Kang, H.L.; Lee, W.K. Endoplasmic Reticulum Stress and Impairment of Ribosome Biogenesis Mediate the Apoptosis Induced by Ocimum x africanum Essential Oil in a Human Gastric Cancer Cell Line. Medicina 2022, 58, 799. [Google Scholar] [CrossRef]
- Ho, Y.; Suphrom, N.; Daowtak, K.; Potup, P.; Thongsri, Y.; Usuwanthim, K. Anticancer Effect of Citrus hystrix DC. Leaf Extract and Its Bioactive Constituents Citronellol and, Citronellal on the Triple Negative Breast Cancer MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 476. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Yadav, K.; Singh, K.; Rehman, A.; Mazumder, A.; Khan, M.A. Screen natural terpenoids to identify potential Jab1 inhibitors for treating breast cancer. Trends Immunother. 2023, 7, 2055. [Google Scholar] [CrossRef]
- Efferth, T.; Kaina, B. Toxicity of the antimalarial artemisinin and its dervatives. Crit. Rev. Toxicol. 2010, 40, 405–421. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Efferth, T.; Dunstan, H.; Sauerbrey, A.; Miyachi, H.; Chitambar, C.R. The anti-malarial artesunate is also active against cancer. Int. J. Oncol. 2001, 18, 767–773. [Google Scholar] [CrossRef]
- Klos, P.; Chlubek, D. Plant-Derived Terpenoids: A Promising Tool in the Fight against Melanoma. Cancers 2022, 14, 502. [Google Scholar] [CrossRef]
- Wroblewska-Luczka, P.; Cabaj, J.; Bargiel, J.; Luszczki, J.J. Anticancer effect of terpenes: Focus on malignant melanoma. Pharmacol. Rep. 2023, 75, 1115–1125. [Google Scholar] [CrossRef]
- Grudzinska, M.; Stachnik, B.; Galanty, A.; Soltys, A.; Podolak, I. Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules 2023, 28, 7763. [Google Scholar] [CrossRef]
- Kowalski, S.; Karska, J.; Tota, M.; Skinderowicz, K.; Kulbacka, J.; Drag-Zalesinska, M. Natural Compounds in Non-Melanoma Skin Cancer: Prevention and Treatment. Molecules 2024, 29, 728. [Google Scholar] [CrossRef]
- Rosen, L.B.; Williams, W.D.; Benson, J.; Rywlin, A.M. A malignant neoplasm with features of both squamous cell carcinoma and malignant melanoma. Am. J. Dermatopathol. 1984, 6, 213–219. [Google Scholar]
- Satter, E.K.; Metcalf, J.; Lountzis, N.; Elston, D.M. Tumors composed of malignant epithelial and melanocytic populations: A case series and review of the literature. J. Cutan. Pathol. 2009, 36, 211–219. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.J.; Park, J.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Kim, Y.; Song, K.M.; Jung, S.K. 1,8-cineole prevents UVB-induced skin carcinogenesis by targeting the aryl hydrocarbon receptor. Oncotarget 2017, 8, 105995–106008. [Google Scholar] [CrossRef]
- Sampath, S.; Subramani, S.; Janardhanam, S.; Subramani, P.; Yuvaraj, A.; Chellan, R. Bioactive compound 1,8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomedicine 2018, 46, 57–68. [Google Scholar] [CrossRef]
- Rahaman, A.; Chaudhuri, A.; Sarkar, A.; Chakraborty, S.; Bhattacharjee, S.; Mandal, D.P. Eucalyptol targets PI3K/Akt/mTOR pathway to inhibit skin cancer metastasis. Carcinogenesis 2022, 43, 571–583. [Google Scholar] [CrossRef]
- Kaur, G.; Athar, M.; Alam, M.S. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol. Carcinog. 2010, 49, 290–301. [Google Scholar] [CrossRef]
- Pal, D.; Banerjee, S.; Mukherjee, S.; Roy, A.; Panda, C.K.; Das, S. Eugenol restricts DMBA croton oil induced skin carcinogenesis in mice: Downregulation of c-Myc and H-ras, and activation of p53 dependent apoptotic pathway. J. Dermatol. Sci. 2010, 59, 31–39. [Google Scholar] [CrossRef]
- Ghosh, R.; Nadiminty, N.; Fitzpatrick, J.E.; Alworth, W.L.; Slaga, T.J.; Kumar, A.P. Eugenol causes melanoma growth suppression through inhibition of E2F1 transcriptional activity. J. Biol. Chem. 2005, 280, 5812–5819. [Google Scholar] [CrossRef]
- Fatima, K.; Wani, Z.A.; Meena, A.; Luqman, S. Geraniol exerts its antiproliferative action by modulating molecular targets in lung and skin carcinoma cells. Phytother. Res. 2021, 35, 3861–3874. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Siddiqui, M.S.; Athar, M.; Alam, M.S. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J. Appl. Toxicol. 2013, 33, 828–837. [Google Scholar] [CrossRef]
- Yu, S.G.; Hildebrandt, L.A.; Elson, C.E. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J. Nutr. 1995, 125, 2763–2767. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Balupillai, A.; Govindasamy, K.; Muthusamy, G.; Ramasamy, K.; Shanmugam, M.; Prasad, N.R. The preventive effect of linalool on acute and chronic UVB-mediated skin carcinogenesis in Swiss albino mice. Photochem. Photobiol. Sci. 2016, 15, 851–860. [Google Scholar] [CrossRef]
- Cerchiara, T.; Straface, S.V.; Brunelli, E.; Tripepi, S.; Gallucci, M.C.; Chidichimo, G. Antiproliferative effect of linalool on RPMI 7932 human melanoma cell line: Ultrastructural studies. Nat. Prod. Commun. 2015, 10, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Jana, S.; Biswas, I.; Mandal, D.P.; Bhattacharjee, S. Biphasic effect of the dietary phytochemical linalool on angiogenesis and metastasis. Mol. Cell. Biochem. 2022, 477, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.C.; Walaszek, Z.; Kowalczyk, P.; Kinjo, T.; Hanausek, M.; Slaga, T.J. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: Implications for skin cancer prevention. Carcinogenesis 2009, 30, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ramirez, C.N.; Su, Z.Y.; Kong, A.N. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R.; et al. DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef]
- Manu, K.A.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Rabe, S.Z.; Balali-Mood, M.; Karimi, G.; Tabasi, N.; Riahi-Zanjani, B. Ursolic acid induced apoptotic cell death following activation of caspases in isolated human melanoma cells. Cell. Biol. Int. 2015, 39, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells: In vitro and in vivo assays. Int. J. Oncol 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-inflammatory mechanisms of eucalyptol rich Eucalyptus globulus essential oil alone and in combination with flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef]
- Jaradat, N.; Abdallah, S.; Al-Maharik, N.; Altamimi, M.; Hawash, M.; Qneibi, M.; Abu Khair, A.; Zetawi, A.; Jabarin, L. Constituents, Antibacterial Adhesion, Cytotoxic and In Vitro Metastasis Blocking Properties of Salvia fruticosa Essential Oils from Three Palestinian Localities. Chem. Biodivers. 2022, 19, e202100872. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.P.; Gomes, A.S.; Lima, F.J.; Brito, T.S.; Soares, P.M.; Pinho, J.P.; Silva, C.S.; Santos, A.A.; Souza, M.H.; Magalhaes, P.J. Inhaled 1,8-cineole reduces inflammatory parameters in airways of ovalbumin-challenged Guinea pigs. Basic Clin. Pharmacol. Toxicol. 2011, 108, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R.; Stober, M.; Vetter, H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur. J. Med. Res. 1998, 3, 508–510. [Google Scholar] [PubMed]
- Lima, P.R.; de Melo, T.S.; Carvalho, K.M.; de Oliveira, I.B.; Arruda, B.R.; de Castro Brito, G.A.; Rao, V.S.; Santos, F.A. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-kappaB activity in mice. Life Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cao, Z.; Wang, X. Role of aryl hydrocarbon receptor in cancer. Biochim. Biophys. Acta 2013, 1836, 197–210. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Miyamoto, A.; Nguyen, H.T.; Pham, H.T.; Hoang, H.T.; Tong, N.T.M.; Truong, L.T.N.; Nguyen, H.T.T. Short communication: Antibacterial effects of essential oils from Cinnamomum cassia bark and Eucalyptus globulus leaves-The involvements of major constituents. PLoS ONE 2023, 18, e0288787. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Courreges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Azuine, M.A.; Amonkar, A.J.; Bhide, S.V. Chemopreventive efficacy of betel leaf extract and its constituents on 7,12-dimethylbenz(a)anthracene induced carcinogenesis and their effect on drug detoxification system in mouse skin. Indian J. Exp. Biol. 1991, 29, 346–351. [Google Scholar] [PubMed]
- Singh, A.; Singh, S.P.; Bamezai, R. Modulatory potential of clocimum oil on mouse skin papillomagenesis and the xenobiotic detoxication system. Food Chem. Toxicol. 1999, 37, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lockniskar, M.F.; Fischer, S.M. Interleukin-1 alpha mediates phorbol ester-induced inflammation and epidermal hyperplasia. FASEB J. 1994, 8, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Attwooll, C.; Lazzerini Denchi, E.; Helin, K. The E2F family: Specific functions and overlapping interests. EMBO J. 2004, 23, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Halaban, R.; Cheng, E.; Smicun, Y.; Germino, J. Deregulated E2F transcriptional activity in autonomously growing melanoma cells. J. Exp. Med. 2000, 191, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, A.C.; Ruszkowski, A.M.; DeLeo, V.A. Allergic contact dermatitis resulting from sensitivity to citrus peel, geraniol, and citral. J. Am. Acad. Dermatol. 1989, 21, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Miladinovic, D.L.; Ilic, B.S.; Kocic, B.D.; Miladinovic, M.D. An in vitro antibacterial study of savory essential oil and geraniol in combination with standard antimicrobials. Nat. Prod. Commun. 2014, 9, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- de Menezes-Filho, J.E.; Gondim, A.N.; Cruz, J.S.; de Souza, A.A.; Santos, J.N.; Conde-Garcia, E.A.; de Sousa, D.P.; Santos, M.S.; de Oliveira, E.D.; de Vasconcelos, C.M. Geraniol blocks calcium and potassium channels in the mammalian myocardium: Useful effects to treat arrhythmias. Basic Clin. Pharmacol. Toxicol. 2014, 115, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Ringleb, J.; Strack, E.; Angioni, C.; Geisslinger, G.; Steinhilber, D.; Weigert, A.; Brune, B. Apoptotic Cancer Cells Suppress 5-Lipoxygenase in Tumor-Associated Macrophages. J. Immunol. 2018, 200, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.; Yates, T.J.; Lopez, L.E.; Cerwinka, W.H.; Bakkar, A.; Lokeshwar, V.B. Targeting hyaluronidase for cancer therapy: Antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011, 71, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Bouga, H.; Tsouros, I.; Bounias, D.; Kyriakopoulou, D.; Stavropoulos, M.S.; Papageorgakopoulou, N.; Theocharis, D.A.; Vynios, D.H. Involvement of hyaluronidases in colorectal cancer. BMC Cancer 2010, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-kappaB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sala, D.; Mollinedo, F. Inhibition of isoprenoid biosynthesis induces apoptosis in human promyelocytic HL-60 cells. Biochem. Biophys. Res. Commun. 1994, 199, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Vaculova, A.; Zhivotovsky, B. Caspases: Determination of their activities in apoptotic cells. Methods Enzymol. 2008, 442, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Vomund, S.; Schafer, A.; Parnham, M.J.; Brune, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, H.W.; Lee, H.Y.; Son, C.G. Systematic review on herb-induced liver injury in Korea. Food Chem. Toxicol. 2015, 84, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zarybnicky, T.; Bousova, I.; Ambroz, M.; Skalova, L. Hepatotoxicity of monoterpenes and sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.T.; Chen, T. Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450. Int. J. Mol. Sci. 2017, 18, 2353. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Soo, W.N.; Chen, Y.H.; Shyur, L.F. Essential Oil of Mentha aquatica var. Kenting Water Mint Suppresses Two-Stage Skin Carcinogenesis Accelerated by BRAF Inhibitor Vemurafenib. Molecules 2019, 24, 2344. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupre, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The Use of ss-Elemene to Enhance Radio Sensitization of A375 Human Melanoma Cells. Cell J. 2020, 21, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Al Bitar, S.; Ballout, F.; Monzer, A.; Kanso, M.; Saheb, N.; Mukherji, D.; Faraj, W.; Tawil, A.; Doughan, S.; Hussein, M.; et al. Thymoquinone Radiosensitizes Human Colorectal Cancer Cells in 2D and 3D Culture Models. Cancers 2022, 14, 1363. [Google Scholar] [CrossRef] [PubMed]




| Terpenoids | Structure | Source | Effects and Mechanisms of Action | |
|---|---|---|---|---|
| Eucalyptol (Monoterpenoid) | 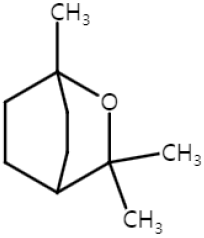 | Salvia fruticosa Eucalyptus globulus Rosmarinus officinalis | Anti-non-melanoma effects | Mechanism of action |
| anti-carcinogenesis pro-apoptotic anti-proliferation anti-metastasis | In vitro COX-2, PGE2 [31] G2/M cell cycle arrest, Bax/Bcl-2, Cyt-c, caspase 3, 9 [32], PI3K/Akt/mTOR , vimentin, snail, slug, twist, MMP2, MMP9 , n-cadherin , E-cadherin [33] | |||
| Anti-melanoma effects | Mechanism of action | |||
| anti-metastasis | In vitro PI3K/Akt/mTOR , vimentin, snail, slug, twist, MMP2, MMP9, n-cadherin , E-cadherin [33] In vivo Vimentin [33] | |||
| Eugenol (Monoterpenoid) | 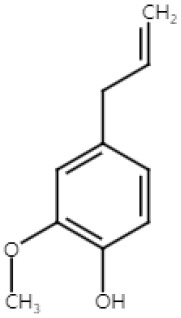 | nutmeg cinnamon clove basil | Anti-non-melanoma effects | Mechanism of action |
| anti-inflammation anti-proliferation pro-apoptotic | In vivo P53, P21WAF1 , NF-κB, iNOS, COX-2, phospho-IkBα, IL-6, TNF-α, PGE2 [34] c-Myc, H-ras, Bcl-2 , P53, Bax, caspase-3 [35] | |||
| Anti-melanoma effects | Mechanism of action | |||
| pro-apoptotic anti-proliferation anti-metastasis | In vitro E2F1 , S-phase cell cycle arrest [36] In vivo Tumor growth delay, tumor size [36] | |||
| Geraniol (Monoterpenoid) | 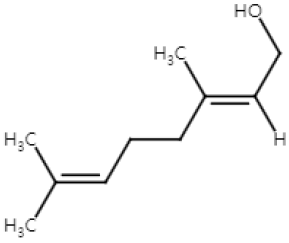 | Cinnamomum tenuipilum Phyla scaberrima lemon grapefruit | Anti-non-melanoma effects | Mechanism of action |
| pro-apoptotic anti-proliferation anti-metastasis | In vitro LOX-5, hyaluronidase , G0/G1 cell cycle arrest [37] In vivo Edema, hyperplasia, COX-2, oxida-tive stress [38] Tumor incidence, number [38] RAS/Raf/ERK1/2 , Bcl-2/Bax [38] | |||
| Anti-melanoma effects | Mechanism of action | |||
| pro-apoptotic | In vivo HMG CoA [39] | |||
| Linalool (Monoterpenoid) | 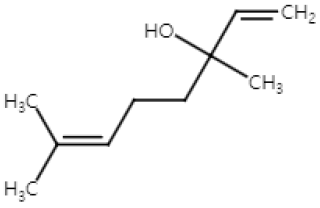 | Cinnamomum tenuipilum Coriandrum sativum Lavandula angustifolia | Anti-non-melanoma effects | Mechanism of action |
| anti-proliferation | In vivo NF-κB, TNF-, IL-6 [40] COX-2, VEGF, TGF-1, Bcl-2 [40] | |||
| Anti-melanoma effects | Mechanism of action | |||
| pro-apoptotic anti-proliferation anti-angiogenesis anti-metastasis | In vitro Caspase-3 [41] HIF-1, VEGF , vimentin, MMP2, MMP9 [42] E-cadherin [42] | |||
| Ursolic acid (Triterpenoid) | 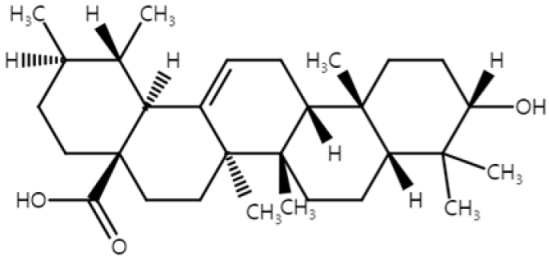 | blueberry cranberry apple Salvia rosmarinus | Anti-non-melanoma effects | Mechanism of action |
| pro-apoptotic anti-proliferation anti-neoplastic transformation | In vitro ROS , caspase-3, -7 [43] Nrf2 , HO-1, NQO1, UGT, GST [44] In vivo Nrf2, Nqo1 [45] | |||
| Anti-melanoma effects | Mechanism of action | |||
| pro-apoptotic anti-inflammation | In vitro P53, caspase-3 , Bcl-2 [46] NF-κB, c-FOS, ATF-2, CREB-1 [46] TNF-α, IL-1β, IL-6, GM-CSF [46] Caspase-3, -8 , Bax , Bcl [47,48] S-phase arrest [49] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.E.; Jung, Y.J.; Lee, S.-J. The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2024, 25, 4423. https://doi.org/10.3390/ijms25084423
Yoon YE, Jung YJ, Lee S-J. The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers. International Journal of Molecular Sciences. 2024; 25(8):4423. https://doi.org/10.3390/ijms25084423
Chicago/Turabian StyleYoon, Ye Eun, Young Jae Jung, and Sung-Joon Lee. 2024. "The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers" International Journal of Molecular Sciences 25, no. 8: 4423. https://doi.org/10.3390/ijms25084423
APA StyleYoon, Y. E., Jung, Y. J., & Lee, S.-J. (2024). The Anticancer Activities of Natural Terpenoids That Inhibit Both Melanoma and Non-Melanoma Skin Cancers. International Journal of Molecular Sciences, 25(8), 4423. https://doi.org/10.3390/ijms25084423







