The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases
Abstract
1. Introduction and Objectives of This Review
2. Alzheimer’s Disease
2.1. Iron Dysregulation in AD
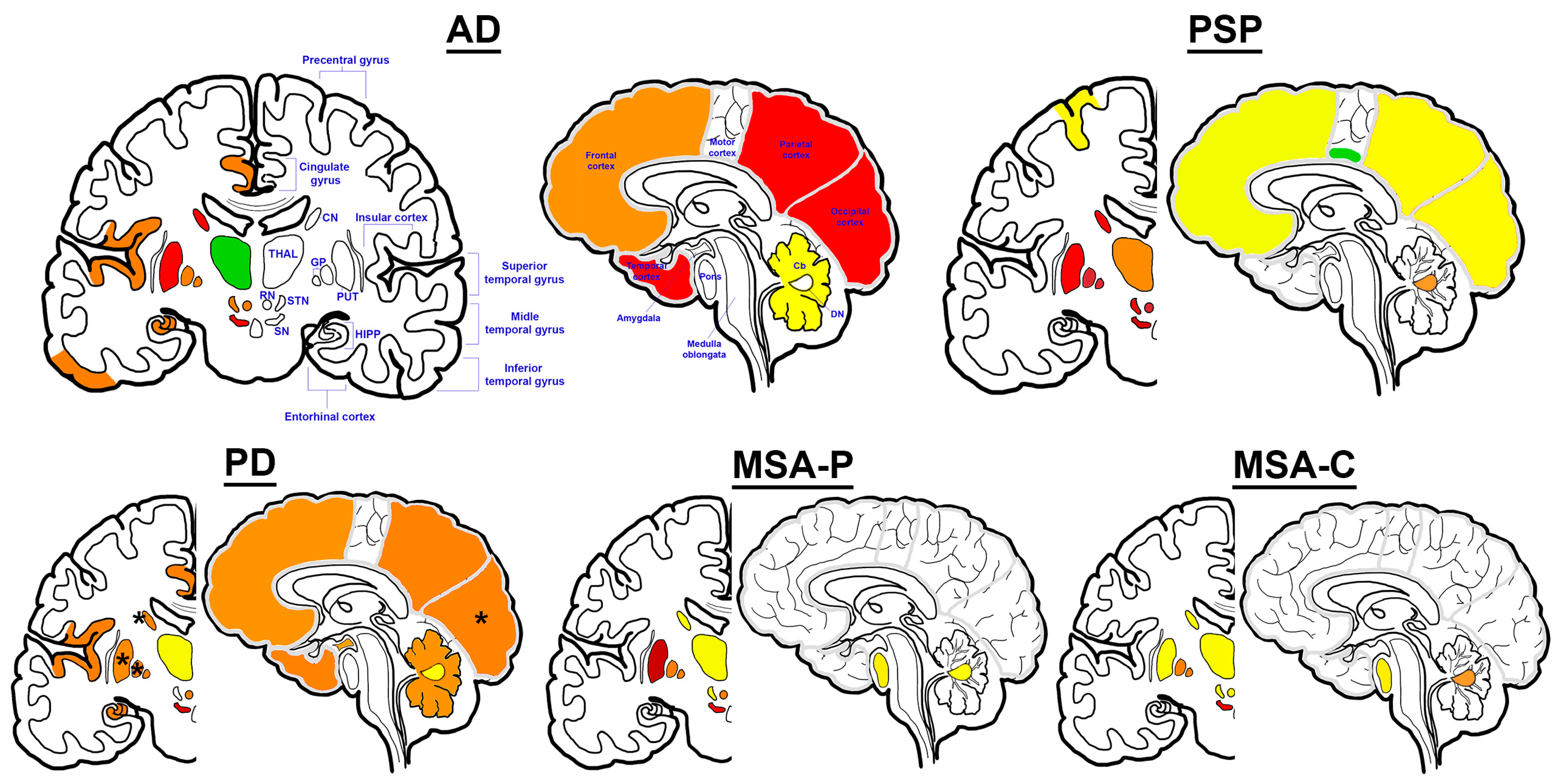
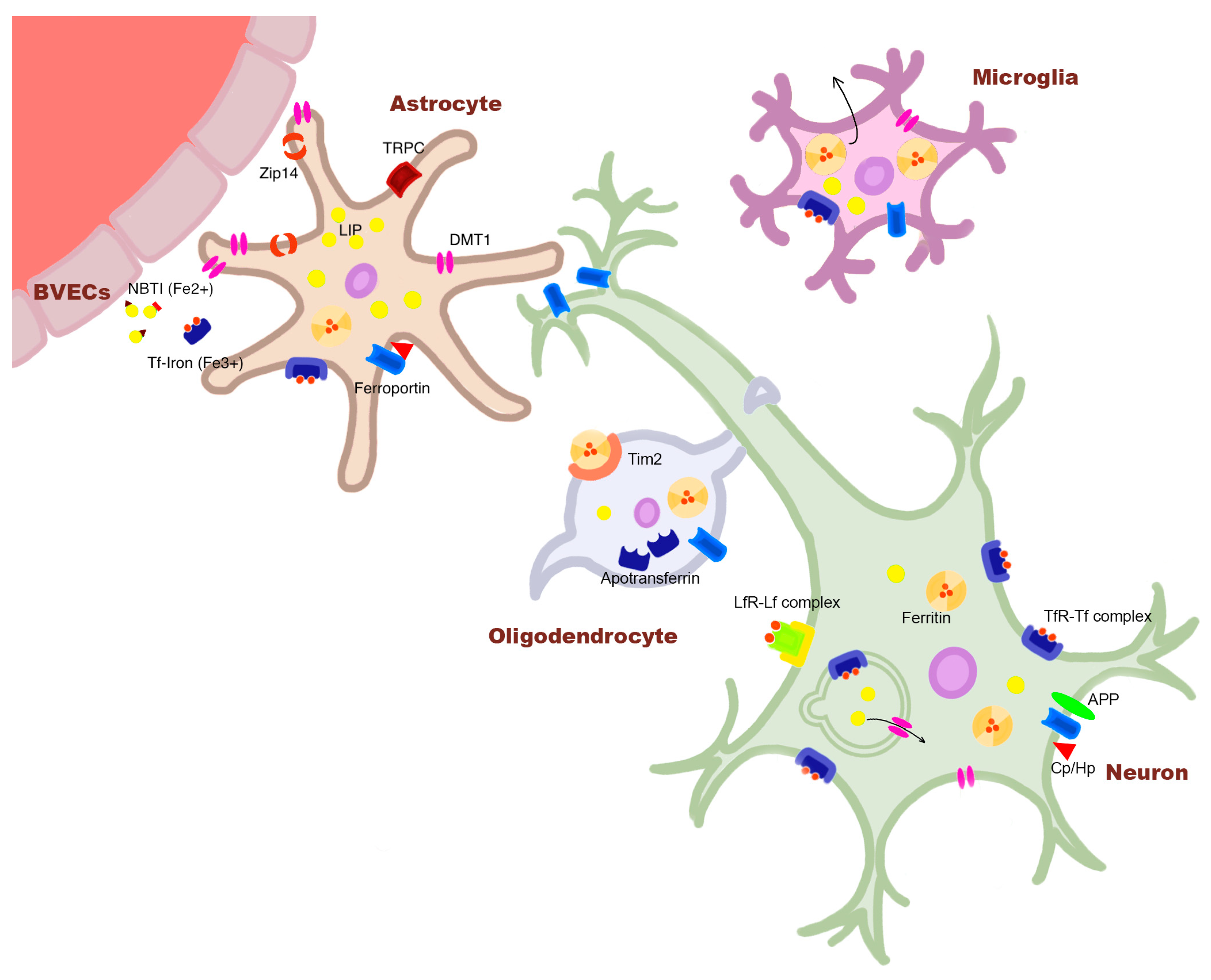
2.2. Cellular Iron Dysregulation in AD
2.3. Hypothesis 1
2.3.1. Iron Accumulation Is a Consequence of Pathological Alterations Related to Aβ
2.3.2. Iron Accumulation Is a Consequence of Pathological Alterations in Tau
2.4. Hypothesis 2
2.4.1. Iron Accumulation Promotes Aβ Pathology
2.4.2. Iron Accumulation Promotes Tau Pathology
2.5. Hypothesis 3: Iron Accumulation Protects from or Hinders the Accumulation of Misfolded Protein Pathology
2.6. Hypothesis 4: Iron Dyshomeostasis and Protein Accumulation Are Parallel and Converging Pathways
2.7. Conclusion: Iron Dysregulation in AD Pathogenesis
3. Progressive Supranuclear Palsy
3.1. Iron Dysregulation in PSP
3.2. Cellular Iron Dysregulation in PSP
3.3. Hypothesis 2: Iron Accumulation Promotes PSP-Related Tau Pathology
3.4. Conclusion: Iron Dysregulation in PSP Pathogenesis
4. Parkinson’s Disease
4.1. Iron Dysregulation in PD
4.2. Cellular Iron Dysregulation in PD
4.3. Hypothesis 1: Iron Accumulation Is a Consequence of Pathological Alterations in α-Synuclein
4.4. Hypothesis 2: Iron Accumulation Promotes α-Synuclein Pathology
4.5. Hypothesis 3: Iron Accumulation Protects from or Hinders the Accumulation of Misfolded Protein Pathology
4.6. Hypothesis 4: Iron Dyshomeostasis and Protein Accumulation Are Parallel and Converging Pathways
4.7. Conclusion: Iron Dysregulation in PD Pathogenesis
5. Multiple System Atrophy
5.1. Iron Dysregulation in MSA
5.2. Cellular Iron Dysregulation in MSA
5.3. MSA-Specific Evidence of Iron Involvement in Disease Pathogenesis
5.4. Conclusion: Iron Dysregulation in MSA Pathogenesis
6. Synthesis and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Heo, J.; Youk, T.-M.; Seo, K.-D. Anemia Is a Risk Factor for the Development of Ischemic Stroke and Post-Stroke Mortality. J. Clin. Med. 2021, 10, 2556. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Świtońska, M.; Żekanowska, E. Hepcidin Levels Are Increased in Patients with Acute Ischemic Stroke: Preliminary Report. J. Stroke Cerebrovasc. Dis. 2015, 24, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ren, Q.; Meng, J.; Gao, W.-J.; Chang, Y.-Z. Brain Iron Homeostasis and Mental Disorders. Antioxidants 2023, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, G.; Fang, X.; Bai, S.; Yuan, G.; Pan, Y. Ferroptosis and Its Potential Role in Glioma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants 2022, 11, 2123. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Loi, S.M.; Syeda, W.T.; Van Rheenen, T.E.; Bush, A.I.; Desmond, P.; Cropley, V.L.; Lane, D.J.R.; Opazo, C.M.; Moffat, B.A.; et al. Systematic Review: Quantitative Susceptibility Mapping (QSM) of Brain Iron Profile in Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 618435. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Song, N.; Wang, J.; Jiang, H.; Xie, J. New Progress on the Role of Glia in Iron Metabolism and Iron-Induced Degeneration of Dopamine Neurons in Parkinson’s Disease. Front. Mol. Neurosci. 2017, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Prousek, J. Fenton Chemistry in Biology and Medicine. Pure Appl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.F.; Maes, M.; Walker, A.J.; Puri, B.K. Why Should Neuroscientists Worry about Iron? The Emerging Role of Ferroptosis in the Pathophysiology of Neuroprogressive Diseases. Behav. Brain Res. 2018, 341, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kovacs, G.G. Astrocytic-Neuronal Teamwork Against External Iron Attacks: Does It Always Work? Function 2021, 2, zqab009. [Google Scholar] [CrossRef]
- Loy, C.T.; Schofield, P.R.; Turner, A.M.; Kwok, J.B.J. Genetics of Dementia. Lancet 2014, 383, 828–840. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular Pathological Classification of Neurodegenerative Diseases: Turning towards Precision Medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The Preclinical Phase of the Pathological Process Underlying Sporadic Alzheimer’s Disease. Brain 2015, 138, 2814–2833. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rüb, U.; Ferretti, R.E.L.; Nitrini, R.; Farfel, J.M.; Polichiso, L.; Gierga, K.; Jacob-Filho, W.; Heinsen, H.; Brazilian Brain Bank Study Group. The Dorsal Raphe Nucleus Shows Phospho-Tau Neurofibrillary Changes before the Transentorhinal Region in Alzheimer’s Disease. A Precocious Onset? Neuropathol. Appl. Neurobiol. 2009, 35, 406–416. [Google Scholar] [CrossRef]
- Attems, J.; Thomas, A.; Jellinger, K. Correlations between Cortical and Subcortical Tau Pathology. Neuropathol. Appl. Neurobiol. 2012, 38, 582–590. [Google Scholar] [CrossRef]
- Yang, A.; Du, L.; Gao, W.; Liu, B.; Chen, Y.; Wang, Y.; Liu, X.; Lv, K.; Zhang, W.; Xia, H.; et al. Associations of Cortical Iron Accumulation with Cognition and Cerebral Atrophy in Alzheimer’s Disease. Quant. Imaging Med. Surg. 2022, 12, 4570–4586. [Google Scholar] [CrossRef]
- Spotorno, N.; Acosta-Cabronero, J.; Stomrud, E.; Lampinen, B.; Strandberg, O.T.; van Westen, D.; Hansson, O. Relationship between Cortical Iron and Tau Aggregation in Alzheimer’s Disease. Brain 2020, 143, 1341–1349. [Google Scholar] [CrossRef]
- Damulina, A.; Pirpamer, L.; Soellradl, M.; Sackl, M.; Tinauer, C.; Hofer, E.; Enzinger, C.; Gesierich, B.; Duering, M.; Ropele, S.; et al. Cross-Sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease Using 3-T MRI. Radiology 2020, 296, 619–626. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.; Yang, J.H.; Moon, Y.; Lee, J.; Moon, W.-J. Cortical Iron Accumulation as an Imaging Marker for Neurodegeneration in Clinical Cognitive Impairment Spectrum: A Quantitative Susceptibility Mapping Study. Korean J. Radiol. 2023, 24, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Portbury, S.; Kalinowski, P.; Agarwal, P.; Diouf, I.; Schneider, J.A.; Morris, M.C.; Bush, A.I. Regional Brain Iron Associated with Deterioration in Alzheimer’s Disease: A Large Cohort Study and Theoretical Significance. Alzheimer’s Dement. 2021, 17, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.M.; Wiste, H.J.; Senjem, M.L.; Gunter, J.L.; Weigand, S.D.; Schwarz, C.G.; Arani, A.; Therneau, T.M.; Lowe, V.J.; Knopman, D.S.; et al. Associations of Quantitative Susceptibility Mapping with Alzheimer’s Disease Clinical and Imaging Markers. Neuroimage 2021, 224, 117433. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Guo, T.; Zhou, C.; Wu, J.; Zeng, Q.; Li, K.; Luo, X.; Bai, X.; Wu, H.; Gao, T.; et al. Altered Brain Iron Depositions from Aging to Parkinson’s Disease and Alzheimer’s Disease: A Quantitative Susceptibility Mapping Study. Neuroimage 2022, 264, 119683. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, P.M.; Fan, A.P. Multimodal Comparisons of QSM and PET in Neurodegeneration and Aging. Neuroimage 2023, 273, 120068. [Google Scholar] [CrossRef] [PubMed]
- Ayton, S.; Faux, N.G.; Bush, A.I. Alzheimer’s Disease Neuroimaging Initiative Ferritin Levels in the Cerebrospinal Fluid Predict Alzheimer’s Disease Outcomes and Are Regulated by APOE. Nat. Commun. 2015, 6, 6760. [Google Scholar] [CrossRef] [PubMed]
- Boelmans, K.; Holst, B.; Hackius, M.; Finsterbusch, J.; Gerloff, C.; Fiehler, J.; Münchau, A. Brain Iron Deposition Fingerprints in Parkinson’s Disease and Progressive Supranuclear Palsy. Mov. Disord. 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain Iron Is Associated with Accelerated Cognitive Decline in People with Alzheimer Pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef]
- Ahmed, M.; Chen, J.; Arani, A.; Senjem, M.L.; Cogswell, P.M.; Jack, C.R.; Liu, C. The Diamagnetic Component Map from Quantitative Susceptibility Mapping (QSM) Source Separation Reveals Pathological Alteration in Alzheimer’s Disease-Driven Neurodegeneration. Neuroimage 2023, 280, 120357. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Ayton, S.; Fazlollahi, A.; Bourgeat, P.; Raniga, P.; Ng, A.; Lim, Y.Y.; Diouf, I.; Farquharson, S.; Fripp, J.; Ames, D.; et al. Cerebral Quantitative Susceptibility Mapping Predicts Amyloid-β-Related Cognitive Decline. Brain 2017, 140, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- van Bergen, J.M.G.; Li, X.; Quevenco, F.C.; Gietl, A.F.; Treyer, V.; Meyer, R.; Buck, A.; Kaufmann, P.A.; Nitsch, R.M.; van Zijl, P.C.M.; et al. Simultaneous Quantitative Susceptibility Mapping and Flutemetamol-PET Suggests Local Correlation of Iron and β-Amyloid as an Indicator of Cognitive Performance at High Age. Neuroimage 2018, 174, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Bulk, M.; Abdelmoula, W.M.; Nabuurs, R.J.A.; van der Graaf, L.M.; Mulders, C.W.H.; Mulder, A.A.; Jost, C.R.; Koster, A.J.; van Buchem, M.A.; Natté, R.; et al. Postmortem MRI and Histology Demonstrate Differential Iron Accumulation and Cortical Myelin Organization in Early- and Late-Onset Alzheimer’s Disease. Neurobiol. Aging 2018, 62, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Sromek, S.M.; Grahovac, I.; Harik, S.I. Transferrin Receptors of Rat and Human Brain and Cerebral Microvessels and Their Status in Alzheimer’s Disease. Brain Res. 1992, 585, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Meadowcroft, M.D.; Connor, J.R.; Yang, Q.X. Cortical Iron Regulation and Inflammatory Response in Alzheimer’s Disease and APPSWE/PS1ΔE9 Mice: A Histological Perspective. Front. Neurosci. 2015, 9, 255. [Google Scholar] [CrossRef]
- Kenkhuis, B.; Somarakis, A.; de Haan, L.; Dzyubachyk, O.; IJsselsteijn, M.E.; de Miranda, N.F.C.C.; Lelieveldt, B.P.F.; Dijkstra, J.; van Roon-Mom, W.M.C.; Höllt, T.; et al. Iron Loading Is a Prominent Feature of Activated Microglia in Alzheimer’s Disease Patients. Acta Neuropathol. Commun. 2021, 9, 27. [Google Scholar] [CrossRef]
- Quintana, C.; Bellefqih, S.; Laval, J.Y.; Guerquin-Kern, J.L.; Wu, T.D.; Avila, J.; Ferrer, I.; Arranz, R.; Patiño, C. Study of the Localization of Iron, Ferritin, and Hemosiderin in Alzheimer’s Disease Hippocampus by Analytical Microscopy at the Subcellular Level. J. Struct. Biol. 2006, 153, 42–54. [Google Scholar] [CrossRef]
- Baringer, S.L.; Lukacher, A.S.; Palsa, K.; Kim, H.; Lippmann, E.S.; Spiegelman, V.S.; Simpson, I.A.; Connor, J.R. Amyloid-β Exposed Astrocytes Induce Iron Transport from Endothelial Cells at the Blood-Brain Barrier by Altering the Ratio of Apo- and Holo-Transferrin. J. Neurochem. 2023, 167, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; He, Q.; Yang, S.; Sun, H.; Liu, Y.; Li, W.; Tang, Y.; Zheng, Y.; Wu, T. FTH1- and SAT1-Induced Astrocytic Ferroptosis Is Involved in Alzheimer’s Disease: Evidence from Single-Cell Transcriptomic Analysis. Pharmaceuticals 2022, 15, 1177. [Google Scholar] [CrossRef]
- Duce, J.A.; Tsatsanis, A.; Cater, M.A.; James, S.A.; Robb, E.; Wikhe, K.; Leong, S.L.; Perez, K.; Johanssen, T.; Greenough, M.A.; et al. Iron-Export Ferroxidase Activity of β-Amyloid Precursor Protein Is Inhibited by Zinc in Alzheimer’s Disease. Cell 2010, 142, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Ayton, S.; Finkelstein, D.I.; Spoerri, L.; Ciccotosto, G.D.; Wright, D.K.; Wong, B.X.W.; Adlard, P.A.; Cherny, R.A.; Lam, L.Q.; et al. Tau Deficiency Induces Parkinsonism with Dementia by Impairing APP-Mediated Iron Export. Nat. Med. 2012, 18, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.X.; Tsatsanis, A.; Lim, L.Q.; Adlard, P.A.; Bush, A.I.; Duce, J.A. β-Amyloid Precursor Protein Does Not Possess Ferroxidase Activity but Does Stabilize the Cell Surface Ferrous Iron Exporter Ferroportin. PLoS ONE 2014, 9, e114174. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Park, Y.-H.; Kosman, D.J. SAPP Modulates Iron Efflux from Brain Microvascular Endothelial Cells by Stabilizing the Ferrous Iron Exporter Ferroportin. EMBO Rep. 2014, 15, 809–815. [Google Scholar] [CrossRef]
- Takahashi, M.; Doré, S.; Ferris, C.D.; Tomita, T.; Sawa, A.; Wolosker, H.; Borchelt, D.R.; Iwatsubo, T.; Kim, S.H.; Thinakaran, G.; et al. Amyloid Precursor Proteins Inhibit Heme Oxygenase Activity and Augment Neurotoxicity in Alzheimer’s Disease. Neuron 2000, 28, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Tsatsanis, A.; Wong, B.X.; Gunn, A.P.; Ayton, S.; Bush, A.I.; Devos, D.; Duce, J.A. Amyloidogenic Processing of Alzheimer’s Disease β-Amyloid Precursor Protein Induces Cellular Iron Retention. Mol. Psychiatry 2020, 25, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Lee, S.; Nassir, N.; Martinez-Valbuena, I.; Sackmann, V.; Li, J.; Ahmed, A.; Tartaglia, M.C.; Ittner, L.M.; Lang, A.E.; et al. Cell-Specific MAPT Gene Expression Is Preserved in Neuronal and Glial Tau Cytopathologies in Progressive Supranuclear Palsy. Acta Neuropathol. 2023, 146, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Dekens, D.W.; De Deyn, P.P.; Sap, F.; Eisel, U.L.M.; Naudé, P.J.W. Iron Chelators Inhibit Amyloid-β-Induced Production of Lipocalin 2 in Cultured Astrocytes. Neurochem. Int. 2020, 132, 104607. [Google Scholar] [CrossRef] [PubMed]
- Everett, J.; Céspedes, E.; Shelford, L.R.; Exley, C.; Collingwood, J.F.; Dobson, J.; van der Laan, G.; Jenkins, C.A.; Arenholz, E.; Telling, N.D. Ferrous Iron Formation Following the Co-Aggregation of Ferric Iron and the Alzheimer’s Disease Peptide β-Amyloid (1–42). J. R Soc. Interface 2014, 11, 20140165. [Google Scholar] [CrossRef]
- McIntosh, A.; Mela, V.; Harty, C.; Minogue, A.M.; Costello, D.A.; Kerskens, C.; Lynch, M.A. Iron Accumulation in Microglia Triggers a Cascade of Events That Leads to Altered Metabolism and Compromised Function in APP/PS1 Mice. Brain Pathol. 2019, 29, 606–621. [Google Scholar] [CrossRef]
- Li, X.; Lei, P.; Tuo, Q.; Ayton, S.; Li, Q.-X.; Moon, S.; Volitakis, I.; Liu, R.; Masters, C.L.; Finkelstein, D.I.; et al. Enduring Elevations of Hippocampal Amyloid Precursor Protein and Iron Are Features of β-Amyloid Toxicity and Are Mediated by Tau. Neurotherapeutics 2015, 12, 862–873. [Google Scholar] [CrossRef] [PubMed]
- van Rensburg, S.J.; Carstens, M.E.; Potocnik, F.C.; Aucamp, A.K.; Taljaard, J.J. Increased Frequency of the Transferrin C2 Subtype in Alzheimer’s Disease. Neuroreport 1993, 4, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Ali-Rahmani, F.; Schengrund, C.-L.; Connor, J.R. HFE Gene Variants, Iron, and Lipids: A Novel Connection in Alzheimer’s Disease. Front. Pharmacol. 2014, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W. Dementia with Lewy Bodies: Neuropathology. J. Geriatr. Psychiatry Neurol. 2002, 15, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-H.; Cahill, C.M.; Vanderburg, C.R.; Scherzer, C.R.; Wang, B.; Huang, X.; Rogers, J.T. Selective Translational Control of the Alzheimer Amyloid Precursor Protein Transcript by Iron Regulatory Protein-1. J. Biol. Chem. 2010, 285, 31217–31232. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Randall, J.D.; Cahill, C.M.; Eder, P.S.; Huang, X.; Gunshin, H.; Leiter, L.; McPhee, J.; Sarang, S.S.; Utsuki, T.; et al. An Iron-Responsive Element Type II in the 5′-Untranslated Region of the Alzheimer’s Amyloid Precursor Protein Transcript. J. Biol. Chem. 2002, 277, 45518–45528. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Camaschella, C. A Potential Pathogenetic Role of Iron in Alzheimer’s Disease. J. Cell. Mol. Med. 2008, 12, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Sahoo, A.; Anand, S.; Ganguly, A.; Righi, G.; Bovicelli, P.; Saso, L.; Chakrabarti, S. Multiple Mechanisms of Iron-Induced Amyloid Beta-Peptide Accumulation in SHSY5Y Cells: Protective Action of Negletein. Neuromolecular. Med. 2014, 16, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Chen, W.-Y.; Huang, X.-T.; Xu, Y.-C.; Zhang, H.-Y. Iron Dysregulates APP Processing Accompanying with SAPPα Cellular Retention and β-Secretase Inhibition in Rat Cortical Neurons. Acta Pharmacol. Sin. 2018, 39, 177–183. [Google Scholar] [CrossRef]
- Becerril-Ortega, J.; Bordji, K.; Fréret, T.; Rush, T.; Buisson, A. Iron Overload Accelerates Neuronal Amyloid-β Production and Cognitive Impairment in Transgenic Mice Model of Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 2288–2301. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Liu, G.; Zeng, C.; Xu, E.; Zhu, W.; Anderson, G.J.; Chen, H. High Dietary Iron Disrupts Iron Homeostasis and Induces Amyloid-β and Phospho-τ Expression in the Hippocampus of Adult Wild-Type and APP/PS1 Transgenic Mice. J. Nutr. 2019, 149, 2247–2254. [Google Scholar] [CrossRef]
- Tahmasebinia, F.; Emadi, S. Effect of Metal Chelators on the Aggregation of Beta-Amyloid Peptides in the Presence of Copper and Iron. Biometals 2017, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Moloney, A.; Meehan, S.; Morris, K.; Thomas, S.E.; Serpell, L.C.; Hider, R.; Marciniak, S.J.; Lomas, D.A.; Crowther, D.C. Iron Promotes the Toxicity of Amyloid Beta Peptide by Impeding Its Ordered Aggregation. J. Biol. Chem. 2011, 286, 4248–4256. [Google Scholar] [CrossRef]
- Galante, D.; Cavallo, E.; Perico, A.; D’Arrigo, C. Effect of Ferric Citrate on Amyloid-Beta Peptides Behavior. Biopolymers 2018, 109, e23224. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Martinez-Valbuena, I.; de Andrea, C.E.; Villalba-Esparza, M.; Ilaalagan, S.; Couto, B.; Visanji, N.P.; Lang, A.E.; Kovacs, G.G. Cell-Specific Dysregulation of Iron and Oxygen Homeostasis as a Novel Pathophysiology in PSP. Ann. Neurol. 2023, 93, 431–445. [Google Scholar] [CrossRef]
- Wan, W.; Cao, L.; Kalionis, B.; Murthi, P.; Xia, S.; Guan, Y. Iron Deposition Leads to Hyperphosphorylation of Tau and Disruption of Insulin Signaling. Front. Neurol. 2019, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, P.; Zhong, M.-L.; Wang, T.; Huang, X.-S.; Li, J.-Y.; Wang, Z.-Y. Deferoxamine Inhibits Iron Induced Hippocampal Tau Phosphorylation in the Alzheimer Transgenic Mouse Brain. Neurochem. Int. 2013, 62, 165–172. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shin, R.-W.; Hasegawa, K.; Naiki, H.; Sato, H.; Yoshimasu, F.; Kitamoto, T. Iron (III) Induces Aggregation of Hyperphosphorylated Tau and Its Reduction to Iron (II) Reverses the Aggregation: Implications in the Formation of Neurofibrillary Tangles of Alzheimer’s Disease. J. Neurochem. 2002, 82, 1137–1147. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ebralidze, I.I.; She, Z.; Kraatz, H.-B. Electrochemical Studies of Tau Protein-Iron Interactions—Potential Implications for Alzheimer’s Disease. Electrochim. Acta 2017, 236, 384–393. [Google Scholar] [CrossRef]
- Chen, L.; Soldan, A.; Oishi, K.; Faria, A.; Zhu, Y.; Albert, M.; van Zijl, P.C.M.; Li, X. Quantitative Susceptibility Mapping of Brain Iron and β-Amyloid in MRI and PET Relating to Cognitive Performance in Cognitively Normal Older Adults. Radiology 2021, 298, 353–362. [Google Scholar] [CrossRef]
- Rottkamp, C.A.; Raina, A.K.; Zhu, X.; Gaier, E.; Bush, A.I.; Atwood, C.S.; Chevion, M.; Perry, G.; Smith, M.A. Redox-Active Iron Mediates Amyloid-Beta Toxicity. Free Radic Biol. Med. 2001, 30, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Collingwood, J.F.; Chong, R.K.K.; Kasama, T.; Cervera-Gontard, L.; Dunin-Borkowski, R.E.; Perry, G.; Pósfai, M.; Siedlak, S.L.; Simpson, E.T.; Smith, M.A.; et al. Three-Dimensional Tomographic Imaging and Characterization of Iron Compounds within Alzheimer’s Plaque Core Material. J. Alzheimer’s Dis. 2008, 14, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, L.; Contarino, V.E.; Siggillino, S.; Carandini, T.; Fumagalli, G.G.; Pietroboni, A.M.; Arcaro, M.; Fenoglio, C.; Orunesu, E.; Castellani, M.; et al. Banks of the Superior Temporal Sulcus in Alzheimer’s Disease: A Pilot Quantitative Susceptibility Mapping Study. J. Alzheimer’s Dis. 2023, 93, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Invited Review: Neuropathology of Tauopathies: Principles and Practice. Neuropathol. Appl. Neurobiol. 2015, 41, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution Patterns of Tau Pathology in Progressive Supranuclear Palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamada, M. Early-Stage Progressive Supranuclear Palsy with Degenerative Lesions Confined to the Subthalamic Nucleus and Substantia Nigra. Neuropathology 2011, 31, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Yamazaki, M.; Saito, Y.; Hatsuta, H.; Sakiyama, Y.; Takao, M.; Kimura, K.; Murayama, S. Early Stage of Progressive Supranuclear Palsy: A Neuropathological Study of 324 Consecutive Autopsy Cases. J. Nippon Med. Sch. 2015, 82, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, P.; Murray, M.E.; Fujioka, S.; Schweitzer, K.J.; Dickson, D.W.; Wszolek, Z.K.; Grant, S.C. Progressive Supranuclear Palsy: High-Field-Strength MR Microscopy in the Human Substantia Nigra and Globus Pallidus. Radiology 2013, 266, 280–288. [Google Scholar] [CrossRef]
- Ferrer, I.; Vidal, N. Neuropathology of Cerebrovascular Diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 145, pp. 79–114. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Iron Levels in the Human Brain: A Post-Mortem Study of Anatomical Region Differences and Age-Related Changes. J. Trace Elem. Med. Biol. 2014, 28, 13–17. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, M.-S. Brain Iron Accumulation in Atypical Parkinsonian Syndromes: In Vivo MRI Evidences for Distinctive Patterns. Front. Neurol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Krishnan, S.; George, S.S.; Radhakrishnan, V.; Raghavan, S.; Thomas, B.; Thulaseedharan, J.V.; Puthenveedu, D.K. Quantitative Susceptibility Mapping from Basal Ganglia and Related Structures: Correlation with Disease Severity in Progressive Supranuclear Palsy. Acta Neurol. Belg. 2024, 124, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, H.; Surova, Y.; Nilsson, M.; Granberg, T.; Westman, E.; van Westen, D.; Svenningsson, P.; Hansson, O. Mapping of Apparent Susceptibility Yields Promising Diagnostic Separation of Progressive Supranuclear Palsy from Other Causes of Parkinsonism. Sci. Rep. 2019, 9, 6079. [Google Scholar] [CrossRef]
- Mazzucchi, S.; Frosini, D.; Costagli, M.; Del Prete, E.; Donatelli, G.; Cecchi, P.; Migaleddu, G.; Bonuccelli, U.; Ceravolo, R.; Cosottini, M. Quantitative Susceptibility Mapping in Atypical Parkinsonisms. Neuroimage Clin. 2019, 24, 101999. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Weigand, S.D.; Pham, N.T.T.; Ali, F.; Arani, A.; Senjem, M.L.; Jack, C.R.; Whitwell, J.L.; Josephs, K.A. Magnetic Susceptibility in Progressive Supranuclear Palsy Variants, Parkinson’s Disease, and Corticobasal Syndrome. Mov. Disord. 2023, 38, 2282–2290. [Google Scholar] [CrossRef]
- Satoh, R.; Ali, F.; Botha, H.; Lowe, V.J.; Josephs, K.A.; Whitwell, J.L. Direct Comparison between 18F-Flortaucipir Tau PET and Quantitative Susceptibility Mapping in Progressive Supranuclear Palsy. Neuroimage 2024, 286, 120509. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, M.J.; Kim, E.-J.; Huh, G.Y.; Lee, J.-H.; Cho, H. Iron Accumulation in the Oculomotor Nerve of the Progressive Supranuclear Palsy Brain. Sci. Rep. 2021, 11, 2950. [Google Scholar] [CrossRef]
- Tanaka, H.; Martinez-Valbuena, I.; Forrest, S.L.; Couto, B.; Reyes, N.G.; Morales-Rivero, A.; Lee, S.; Li, J.; Karakani, A.M.; Tang-Wai, D.F.; et al. Distinct Involvement of the Cranial and Spinal Nerves in Progressive Supranuclear Palsy. Brain 2023, 147, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lyoo, C.H.; Ahn, S.J.; Rinne, J.O.; Lee, M.S. Brain Regional Iron Contents in Progressive Supranuclear Palsy. Parkinsonism Relat. Disord. 2017, 45, 28–32. [Google Scholar] [CrossRef]
- Lee, J.-H.; Han, Y.-H.; Kang, B.-M.; Mun, C.-W.; Lee, S.-J.; Baik, S.-K. Quantitative Assessment of Subcortical Atrophy and Iron Content in Progressive Supranuclear Palsy and Parkinsonian Variant of Multiple System Atrophy. J. Neurol. 2013, 260, 2094–2101. [Google Scholar] [CrossRef]
- Beliveau, V.; Müller, C.; Steiger, R.; Gizewski, E.R.; Poewe, W.; Seppi, K.; Scherfler, C. Characterization and Diagnostic Potential of R2* in Early-Stage Progressive Supranuclear Palsy Variants. Parkinsonism Relat. Disord. 2022, 101, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Valpuesta, J.M.; de Garcini, E.M.; Quintana, C.; Arrasate, M.; López Carrascosa, J.L.; Rábano, A.; García de Yébenes, J.; Avila, J. Ferritin Is Associated with the Aberrant Tau Filaments Present in Progressive Supranuclear Palsy. Am. J. Pathol. 1998, 152, 1531–1539. [Google Scholar] [PubMed]
- Shibuya, K.; Yagishita, S.; Nakamura, A.; Uchihara, T. Perivascular Orientation of Astrocytic Plaques and Tuft-Shaped Astrocytes. Brain Res. 2011, 1404, 50–54. [Google Scholar] [CrossRef]
- Whitney, K.; Song, W.-M.; Sharma, A.; Dangoor, D.K.; Farrell, K.; Krassner, M.M.; Ressler, H.W.; Christie, T.D.; Walker, R.H.; Nirenberg, M.J.; et al. Single-Cell Transcriptomic and Neuropathologic Analysis Reveals Dysregulation of the Integrated Stress Response in Progressive Supranuclear Palsy. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mukherjee, S.; Panda, D. Contrasting Effects of Ferric and Ferrous Ions on Oligomerization and Droplet Formation of Tau: Implications in Tauopathies and Neurodegeneration. ACS Chem. Neurosci. 2021, 12, 4393–4405. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.W.; Brown, G.L.; Lewis, M.M.; Jellen, L.C.; Pu, C.; Johnson, M.L.; Chen, H.; Kong, L.; Du, G.; Huang, X. Susceptibility Magnetic Resonance Imaging Correlates with Glial Density and Tau in the Substantia Nigra Pars Compacta. Mov. Disord. 2023, 38, 464–473. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s Disease: Substantia Nigra Regional Selectivity. Brain 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef]
- Váradi, C. Clinical Features of Parkinson’s Disease: The Evolution of Critical Symptoms. Biology 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s Disease: Separating the Wheat from the Chaff. J. Parkinson’s Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef]
- Dexter, D.T.; Wells, F.R.; Agid, F.; Agid, Y.; Lees, A.J.; Jenner, P.; Marsden, C.D. Increased Nigral Iron Content in Postmortem Parkinsonian Brain. Lancet 1987, 2, 1219–1220. [Google Scholar] [CrossRef] [PubMed]
- Péran, P.; Barbagallo, G.; Nemmi, F.; Sierra, M.; Galitzky, M.; Traon, A.P.-L.; Payoux, P.; Meissner, W.G.; Rascol, O. MRI Supervised and Unsupervised Classification of Parkinson’s Disease and Multiple System Atrophy. Mov. Disord. 2018, 33, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the Levels of Iron, Ferritin and Other Trace Metals in Parkinson’s Disease and Other Neurodegenerative Diseases Affecting the Basal Ganglia. Brain 1991, 114 Pt 4, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Marxreiter, F.; Lambrecht, V.; Mennecke, A.; Hanspach, J.; Jukic, J.; Regensburger, M.; Herrler, J.; German, A.; Kassubek, J.; Grön, G.; et al. Parkinson’s Disease or Multiple System Atrophy: Potential Separation by Quantitative Susceptibility Mapping. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221143834. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.C.; Leyland, L.A.; Schrag, A.-E.; Lees, A.J.; Acosta-Cabronero, J.; Weil, R.S. Brain Iron Deposition Is Linked with Cognitive Severity in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kan, H.; Sakurai, K.; Arai, N.; Kato, D.; Kawashima, S.; Ueki, Y.; Matsukawa, N. Voxel-Based Quantitative Susceptibility Mapping in Parkinson’s Disease with Mild Cognitive Impairment. Mov. Disord. 2019, 34, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, S.; Kim, D.; Cho, J.W.; Kim, J.S.; Ahn, J.H.; Mun, J.K.; Choi, I.; Lee, S.-K.; Youn, J. Extra-Basal Ganglia Iron Content and Non-Motor Symptoms in Drug-Naïve, Early Parkinson’s Disease. Neurol. Sci. 2021, 42, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Kan, H.; Sakurai, K.; Inui, S.; Kobayashi, S.; Akagawa, Y.; Shibuya, K.; Ueki, Y.; Matsukawa, N. Magnetic Susceptibility Associates with Dopaminergic Deficits and Cognition in Parkinson’s Disease. Mov. Disord. 2020, 35, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Alushaj, E.; Hemachandra, D.; Kuurstra, A.; Menon, R.S.; Ganjavi, H.; Sharma, M.; Kashgari, A.; Barr, J.; Reisman, W.; Khan, A.R.; et al. Subregional Analysis of Striatum Iron in Parkinson’s Disease and Rapid Eye Movement Sleep Behaviour Disorder. Neuroimage Clin. 2023, 40, 103519. [Google Scholar] [CrossRef]
- Li, K.R.; Avecillas-Chasin, J.; Nguyen, T.D.; Gillen, K.M.; Dimov, A.; Chang, E.; Skudin, C.; Kopell, B.H.; Wang, Y.; Shtilbans, A. Quantitative Evaluation of Brain Iron Accumulation in Different Stages of Parkinson’s Disease. J. Neuroimaging 2022, 32, 363–371. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Wei, W.; Yang, Z.; Guo, L.; Wang, Z.; Wei, X. Correlation of Brain Iron Deposition and Freezing of Gait in Parkinson’s Disease: A Cross-Sectional Study. Quant. Imaging Med. Surg. 2023, 13, 7961–7972. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, L.-F.; Huang, L.; Liu, Y.; Xia, Z.-C.; Zhou, X.-X.; Tang, B.-S.; Guo, J.-F.; Lei, L.-F. Different Iron Deposition Patterns in Akinetic/Rigid-Dominant and Tremor-Dominant Parkinson’s Disease. Clin. Neurol. Neurosurg. 2020, 198, 106181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Huang, J.; Liang, J.; Ma, M.; Zhao, Q.; Lei, X.; Shi, C.; Luo, L. Evaluation of Abnormal Iron Distribution in Specific Regions in the Brains of Patients with Parkinson’s Disease Using Quantitative Susceptibility Mapping and R2* Mapping. Exp. Ther. Med. 2020, 19, 3778–3786. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Biological Fluid Levels of Iron and Iron-Related Proteins in Parkinson’s Disease: Review and Meta-Analysis. Eur. J. Neurol. 2021, 28, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, X.; Geng, X.; Wang, F. Meta-Analysis of Iron Metabolism Markers Levels of Parkinson’s Disease Patients Determined by Fluid and MRI Measurements. J. Trace Elem. Med. Biol. 2023, 78, 127190. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Y.; Zhang, C.; Li, J.; Li, Z.; Guan, X.; Bao, J.; Zhang, Y.; Cheng, J.; Wei, H. Free Water and Iron Content in the Substantia Nigra at Different Stages of Parkinson’s Disease. Eur. J. Radiol. 2023, 167, 111030. [Google Scholar] [CrossRef]
- He, N.; Langley, J.; Huddleston, D.E.; Chen, S.; Huang, P.; Ling, H.; Yan, F.; Hu, X. Increased Iron-Deposition in Lateral-Ventral Substantia Nigra Pars Compacta: A Promising Neuroimaging Marker for Parkinson’s Disease. Neuroimage Clin. 2020, 28, 102391. [Google Scholar] [CrossRef]
- Schwarz, S.T.; Mougin, O.; Xing, Y.; Blazejewska, A.; Bajaj, N.; Auer, D.P.; Gowland, P. Parkinson’s Disease Related Signal Change in the Nigrosomes 1-5 and the Substantia Nigra Using T2* Weighted 7T MRI. Neuroimage Clin. 2018, 19, 683–689. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Ghassaban, K.; Huang, P.; Jokar, M.; Wang, Y.; Cheng, Z.; Jin, Z.; Li, Y.; Sethi, S.K.; He, Y.; et al. Imaging Iron and Neuromelanin Simultaneously Using a Single 3D Gradient Echo Magnetization Transfer Sequence: Combining Neuromelanin, Iron and the Nigrosome-1 Sign as Complementary Imaging Biomarkers in Early Stage Parkinson’s Disease. Neuroimage 2021, 230, 117810. [Google Scholar] [CrossRef]
- Ariz, M.; Martínez, M.; Alvarez, I.; Fernández-Seara, M.A.; Castellanos, G.; Catalonian Neuroimaging Parkinson’s Disease Consortium; Pastor, P.; Pastor, M.A.; Ortiz de Solórzano, C. Automatic Segmentation and Quantification of Nigrosome-1 Neuromelanin and Iron in MRI: A Candidate Biomarker for Parkinson’s Disease. J. Magn. Reason. Imaging, 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Fu, X.; Deng, W.; Cui, X.; Zhou, X.; Song, W.; Pan, M.; Chi, X.; Xu, J.; Jiang, Y.; Wang, Q.; et al. Time-Specific Pattern of Iron Deposition in Different Regions in Parkinson’s Disease Measured by Quantitative Susceptibility Mapping. Front. Neurol. 2021, 12, 631210. [Google Scholar] [CrossRef]
- Du, G.; Lewis, M.M.; Sica, C.; He, L.; Connor, J.R.; Kong, L.; Mailman, R.B.; Huang, X. Distinct Progression Pattern of Susceptibility MRI in the Substantia Nigra of Parkinson’s Patients. Mov. Disord. 2018, 33, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.C.; Zarkali, A.; Ryten, M.; Shmueli, K.; Gil-Martinez, A.L.; Leyland, L.-A.; McColgan, P.; Acosta-Cabronero, J.; Lees, A.J.; Weil, R.S. Regional Brain Iron and Gene Expression Provide Insights into Neurodegeneration in Parkinson’s Disease. Brain 2021, 144, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, L.; Mao, H.; Chen, K.; Shi, Y.; Meng, X.; Wang, F.; Hu, X.; Fang, X. The Impact of Iron Deposition on the Fear Circuit of the Brain in Patients with Parkinson’s Disease and Anxiety. Front. Aging Neurosci. 2023, 15, 1116516. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Wang, E.; Sica, C.; Chen, H.; De Jesus, S.; Lewis, M.M.; Kong, L.; Connor, J.; Mailman, R.B.; Huang, X. Dynamics of Nigral Iron Accumulation in Parkinson’s Disease: From Diagnosis to Late Stage. Mov. Disord. 2022, 37, 1654–1662. [Google Scholar] [CrossRef]
- Guo, J.-J.; Yue, F.; Song, D.-Y.; Bousset, L.; Liang, X.; Tang, J.; Yuan, L.; Li, W.; Melki, R.; Tang, Y.; et al. Intranasal Administration of α-Synuclein Preformed Fibrils Triggers Microglial Iron Deposition in the Substantia Nigra of Macaca Fascicularis. Cell Death Dis. 2021, 12, 81. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Connor, J.R.; Juneau, P.L.; Snyder, B.S.; Kanaley, L.; DeMaggio, A.J.; Nguyen, H.; Brickman, C.M.; LeWitt, P.A. Transferrin and Iron in Normal, Alzheimer’s Disease, and Parkinson’s Disease Brain Regions. J. Neurochem. 1995, 65, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.E.; Collingwood, J.F.; Dobson, J.; Love, G.; Perrott, H.R.; Edwardson, J.A.; Elstner, M.; Morris, C.M. Individual Dopaminergic Neurons Show Raised Iron Levels in Parkinson Disease. Neurology 2007, 68, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Brammerloh, M.; Morawski, M.; Friedrich, I.; Reinert, T.; Lange, C.; Pelicon, P.; Vavpetič, P.; Jankuhn, S.; Jäger, C.; Alkemade, A.; et al. Measuring the Iron Content of Dopaminergic Neurons in Substantia Nigra with MRI Relaxometry. Neuroimage 2021, 239, 118255. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Huang, J.; Tang, D.; Qian, H.; Yan, K.; Shi, X.; Li, Y.; Zhang, J. Application Value of Multiparametric MRI for Evaluating Iron Deposition in the Substantia Nigra in Parkinson’s Disease. Front. Neurol. 2022, 13, 1096966. [Google Scholar] [CrossRef]
- Visanji, N.P.; Collingwood, J.F.; Finnegan, M.E.; Tandon, A.; House, E.; Hazrati, L.-N. Iron Deficiency in Parkinsonism: Region-Specific Iron Dysregulation in Parkinson’s Disease and Multiple System Atrophy. J. Parkinsons. Dis. 2013, 3, 523–537. [Google Scholar] [CrossRef]
- Howitt, J.; Gysbers, A.M.; Ayton, S.; Carew-Jones, F.; Putz, U.; Finkelstein, D.I.; Halliday, G.M.; Tan, S.-S. Increased Ndfip1 in the Substantia Nigra of Parkinsonian Brains Is Associated with Elevated Iron Levels. PLoS ONE 2014, 9, e87119. [Google Scholar] [CrossRef] [PubMed]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.R.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Prudent, M.; Fauvet, B.; Lashuel, H.A.; Girault, H.H. Phosphorylation of α-Synuclein at Y125 and S129 Alters Its Metal Binding Properties: Implications for Understanding the Role of α-Synuclein in the Pathogenesis of Parkinson’s Disease and Related Disorders. ACS Chem. Neurosci. 2011, 2, 667–675. [Google Scholar] [CrossRef]
- Mi, X.; Li, Q.; Wen, X.; Xie, J.; Wang, Y.; Song, N. Extracellular α-Synuclein Modulates Iron Metabolism Related Proteins via Endoplasmic Reticulum Stress in MES23.5 Dopaminergic Cells. Neurochem. Res. 2021, 46, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Carmona, A.; Roudeau, S.; Perrin, L.; Dučić, T.; Carboni, E.; Bohic, S.; Cloetens, P.; Lingor, P. α-Synuclein Over-Expression Induces Increased Iron Accumulation and Redistribution in Iron-Exposed Neurons. Mol. Neurobiol. 2016, 53, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Biondetti, E.; Santin, M.D.; Valabrègue, R.; Mangone, G.; Gaurav, R.; Pyatigorskaya, N.; Hutchison, M.; Yahia-Cherif, L.; Villain, N.; Habert, M.-O.; et al. The Spatiotemporal Changes in Dopamine, Neuromelanin and Iron Characterizing Parkinson’s Disease. Brain 2021, 144, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, X.; Huang, S.; Li, G.; Mo, M.; Zhang, L.; Chen, C.; Guo, W.; Zhou, M.; Wu, Z.; et al. Iron Promotes α-Synuclein Aggregation and Transmission by Inhibiting TFEB-Mediated Autophagosome-Lysosome Fusion. J. Neurochem. 2018, 145, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, H.; Song, N.; Xie, J. Oxidative Stress Partially Contributes to Iron-Induced α-Synuclein Aggregation in SK-N-SH Cells. Neurotox. Res. 2011, 19, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Febbraro, F.; Giorgi, M.; Caldarola, S.; Loreni, F.; Romero-Ramos, M. α-Synuclein Expression Is Modulated at the Translational Level by Iron. Neuroreport 2012, 23, 576–580. [Google Scholar] [CrossRef]
- Rogers, J.T.; Mikkilineni, S.; Cantuti-Castelvetri, I.; Smith, D.H.; Huang, X.; Bandyopadhyay, S.; Cahill, C.M.; Maccecchini, M.L.; Lahiri, D.K.; Greig, N.H. The Alpha-Synuclein 5′untranslated Region Targeted Translation Blockers: Anti-Alpha Synuclein Efficacy of Cardiac Glycosides and Posiphen. J. Neural Transm. 2011, 118, 493–507. [Google Scholar] [CrossRef]
- Friedlich, A.L.; Tanzi, R.E.; Rogers, J.T. The 5′-Untranslated Region of Parkinson’s Disease Alpha-Synuclein MessengerRNA Contains a Predicted Iron Responsive Element. Mol. Psychiatry 2007, 12, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, P.; Bonnet, A.-M.; Débarges, B.; Lohmann, E.; Tison, F.; Pollak, P.; Agid, Y.; Dürr, A.; Brice, A. Causal Relation between Alpha-Synuclein Gene Duplication and Familial Parkinson’s Disease. Lancet 2004, 364, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Uchihara, T.; Giasson, B.I. Propagation of Alpha-Synuclein Pathology: Hypotheses, Discoveries, and yet Unresolved Questions from Experimental and Human Brain Studies. Acta Neuropathol. 2016, 131, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. Alpha-Synuclein Locus Triplication Causes Parkinson’s Disease. Science 2003, 302, 841. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-S.; Reale, M.; Kamal, M.A.; Holloway, H.W.; Luo, W.; Sambamurti, K.; Ray, B.; Lahiri, D.K.; Rogers, J.T.; Greig, N.H. Synthesis of the Alzheimer Drug Posiphen into Its Primary Metabolic Products (+)-N1-NorPosiphen, (+)-N8-NorPosiphen and (+)-N1, N8-BisnorPosiphen, Their Inhibition of Amyloid Precursor Protein, α-Synuclein Synthesis, Interleukin-1β Release, and Cholinergic Action. Antiinflamm Antiallergy Agents Med. Chem. 2013, 12, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tao, Y.; Zhao, K.; Ma, Y.; Xu, Q.; Liu, C.; Zhang, S.; Li, D. Structural Insights of Fe3+ Induced α-Synuclein Fibrillation in Parkinson’s Disease. J. Mol. Biol. 2023, 435, 167680. [Google Scholar] [CrossRef] [PubMed]
- Abeyawardhane, D.L.; Fernández, R.D.; Murgas, C.J.; Heitger, D.R.; Forney, A.K.; Crozier, M.K.; Lucas, H.R. Iron Redox Chemistry Promotes Antiparallel Oligomerization of α-Synuclein. J. Am. Chem. Soc. 2018, 140, 5028–5032. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-Triggered Structural Transformations, Aggregation, and Fibrillation of Human Alpha-Synuclein. A Possible Molecular NK between Parkinson’s Disease and Heavy Metal Exposure. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef] [PubMed]
- Kostka, M.; Högen, T.; Danzer, K.M.; Levin, J.; Habeck, M.; Wirth, A.; Wagner, R.; Glabe, C.G.; Finger, S.; Heinzelmann, U.; et al. Single Particle Characterization of Iron-Induced Pore-Forming Alpha-Synuclein Oligomers. J. Biol. Chem. 2008, 283, 10992–11003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Wang, S.; Yang, D.; Zhang, Y.; Xu, L.; Ma, L.; Zheng, J.; Petersen, R.B.; Zheng, L.; et al. Copper and Iron Ions Accelerate the Prion-like Propagation of α-Synuclein: A Vicious Cycle in Parkinson’s Disease. Int. J. Biol. Macromol. 2020, 163, 562–573. [Google Scholar] [CrossRef]
- Ostrerova-Golts, N.; Petrucelli, L.; Hardy, J.; Lee, J.M.; Farer, M.; Wolozin, B. The A53T Alpha-Synuclein Mutation Increases Iron-Dependent Aggregation and Toxicity. J. Neurosci. 2000, 20, 6048–6054. [Google Scholar] [CrossRef]
- Agostini, F.; Bubacco, L.; Chakrabarti, S.; Bisaglia, M. α-Synuclein Toxicity in Drosophila Melanogaster Is Enhanced by the Presence of Iron: Implications for Parkinson’s Disease. Antioxidants 2023, 12, 261. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Qu, L.; Chen, B.; Jiang, H.; Song, N.; Xie, J. Iron-Induced Oxidative Stress Contributes to α-Synuclein Phosphorylation and up-Regulation via Polo-like Kinase 2 and Casein Kinase 2. Neurochem. Int. 2019, 125, 127–135. [Google Scholar] [CrossRef]
- Smith, W.W.; Margolis, R.L.; Li, X.; Troncoso, J.C.; Lee, M.K.; Dawson, V.L.; Dawson, T.M.; Iwatsubo, T.; Ross, C.A. Alpha-Synuclein Phosphorylation Enhances Eosinophilic Cytoplasmic Inclusion Formation in SH-SY5Y Cells. J. Neurosci. 2005, 25, 5544–5552. [Google Scholar] [CrossRef]
- Guan, H.; Yang, H.; Yang, M.; Yanagisawa, D.; Bellier, J.-P.; Mori, M.; Takahata, S.; Nonaka, T.; Zhao, S.; Tooyama, I. Mitochondrial Ferritin Protects SH-SY5Y Cells against H2O2-Induced Oxidative Stress and Modulates α-Synuclein Expression. Exp. Neurol. 2017, 291, 51–61. [Google Scholar] [CrossRef]
- Dauer Née Joppe, K.; Tatenhorst, L.; Caldi Gomes, L.; Zhang, S.; Parvaz, M.; Carboni, E.; Roser, A.-E.; El DeBakey, H.; Bähr, M.; Vogel-Mikuš, K.; et al. Brain Iron Enrichment Attenuates α-Synuclein Spreading after Injection of Preformed Fibrils. J. Neurochem. 2021, 159, 554–573. [Google Scholar] [CrossRef]
- Mahoney-Sanchez, L.; Bouchaoui, H.; Boussaad, I.; Jonneaux, A.; Timmerman, K.; Berdeaux, O.; Ayton, S.; Krüger, R.; Duce, J.A.; Devos, D.; et al. Alpha Synuclein Determines Ferroptosis Sensitivity in Dopaminergic Neurons via Modulation of Ether-Phospholipid Membrane Composition. Cell Rep. 2022, 40, 111231. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular Pathology of Neurodegenerative Diseases: Principles and Practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef]
- Gilman, S.; Low, P.A.; Quinn, N.; Albanese, A.; Ben-Shlomo, Y.; Fowler, C.J.; Kaufmann, H.; Klockgether, T.; Lang, A.E.; Lantos, P.L.; et al. Consensus Statement on the Diagnosis of Multiple System Atrophy. J. Neurol. Sci. 1999, 163, 94–98. [Google Scholar] [CrossRef]
- Lantos, P.L. The Definition of Multiple System Atrophy: A Review of Recent Developments. J. Neuropathol. Exp. Neurol. 1998, 57, 1099–1111. [Google Scholar] [CrossRef]
- Graham, J.G.; Oppenheimer, D.R. Orthostatic Hypotension and Nicotine Sensitivity in a Case of Multiple System Atrophy. J. Neurol. Neurosurg. Psychiatry 1969, 32, 28–34. [Google Scholar] [CrossRef]
- Rohan, Z.; Rahimi, J.; Weis, S.; Kapas, I.; Auff, E.; Mitrovic, N.; Liberski, P.P.; Sikorska, B.; Matej, R.; Kovacs, G.G. Screening for α-Synuclein Immunoreactive Neuronal Inclusions in the Hippocampus Allows Identification of Atypical MSA (FTLD-Synuclein). Acta Neuropathol. 2015, 130, 299–301. [Google Scholar] [CrossRef]
- Aoki, N.; Boyer, P.J.; Lund, C.; Lin, W.-L.; Koga, S.; Ross, O.A.; Weiner, M.; Lipton, A.; Powers, J.M.; White, C.L.; et al. Atypical Multiple System Atrophy Is a New Subtype of Frontotemporal Lobar Degeneration: Frontotemporal Lobar Degeneration Associated with α-Synuclein. Acta Neuropathol. 2015, 130, 93–105. [Google Scholar] [CrossRef]
- Brettschneider, J.; Suh, E.; Robinson, J.L.; Fang, L.; Lee, E.B.; Irwin, D.J.; Grossman, M.; Van Deerlin, V.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Converging Patterns of α-Synuclein Pathology in Multiple System Atrophy. J. Neuropathol. Exp. Neurol. 2018, 77, 1005–1016. [Google Scholar] [CrossRef]
- Brettschneider, J.; Irwin, D.J.; Boluda, S.; Byrne, M.D.; Fang, L.; Lee, E.B.; Robinson, J.L.; Suh, E.; Van Deerlin, V.M.; Toledo, J.B.; et al. Progression of Alpha-Synuclein Pathology in Multiple System Atrophy of the Cerebellar Type. Neuropathol. Appl. Neurobiol. 2017, 43, 315–329. [Google Scholar] [CrossRef]
- Lancione, M.; Cencini, M.; Costagli, M.; Donatelli, G.; Tosetti, M.; Giannini, G.; Zangaglia, R.; Calandra-Buonaura, G.; Pacchetti, C.; Cortelli, P.; et al. Diagnostic Accuracy of Quantitative Susceptibility Mapping in Multiple System Atrophy: The Impact of Echo Time and the Potential of Histogram Analysis. Neuroimage Clin. 2022, 34, 102989. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, T.-H.; Mun, C.-W.; Kim, T.-H.; Han, Y.-H. Progression of Subcortical Atrophy and Iron Deposition in Multiple System Atrophy: A Comparison between Clinical Subtypes. J. Neurol. 2015, 262, 1876–1882. [Google Scholar] [CrossRef]
- Lee, S.; Martinez-Valbuena, I.; Lang, E.A.; Kovacs, G.G. Regional and Cellular Iron Deposition Patterns Predict Clinical Subtypes of Multiple System Atrophy. PREPRINT (Version 1) Available at Research Square. 2023. Available online: https://www.researchsquare.com/article/rs-3296997/v1 (accessed on 11 April 2024).
- Sugiyama, A.; Sato, N.; Kimura, Y.; Fujii, H.; Maikusa, N.; Shigemoto, Y.; Suzuki, F.; Morimoto, E.; Koide, K.; Takahashi, Y.; et al. Quantifying Iron Deposition in the Cerebellar Subtype of Multiple System Atrophy and Spinocerebellar Ataxia Type 6 by Quantitative Susceptibility Mapping. J. Neurol. Sci. 2019, 407, 116525. [Google Scholar] [CrossRef]
- Chen, L.; Mao, L.; Lu, H.; Liu, P. Detecting Ferroptosis and Immune Infiltration Profiles in Multiple System Atrophy Using Postmortem Brain Tissue. Front. Neurosci. 2023, 17, 1269996. [Google Scholar] [CrossRef]
- Barbagallo, G.; Sierra-Peña, M.; Nemmi, F.; Traon, A.P.-L.; Meissner, W.G.; Rascol, O.; Péran, P. Multimodal MRI Assessment of Nigro-Striatal Pathway in Multiple System Atrophy and Parkinson Disease. Mov. Disord. 2016, 31, 325–334. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, T.-H.; Mun, C.-W.; Shin, H.K.; Son, J.; Lee, J.-H. Spatial Correlation and Segregation of Multimodal MRI Abnormalities in Multiple System Atrophy. J. Neurol. 2018, 265, 1540–1547. [Google Scholar] [CrossRef]
- Matsusue, E.; Fujii, S.; Kanasaki, Y.; Sugihara, S.; Miyata, H.; Ohama, E.; Ogawa, T. Putaminal Lesion in Multiple System Atrophy: Postmortem MR-Pathological Correlations. Neuroradiology 2008, 50, 559–567. [Google Scholar] [CrossRef]
- Shukla, J.J.; Stefanova, N.; Bush, A.I.; McColl, G.; Finkelstein, D.I.; McAllum, E.J. Therapeutic Potential of Iron Modulating Drugs in a Mouse Model of Multiple System Atrophy. Neurobiol. Dis. 2021, 159, 105509. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, T.-H.; Kim, S.J.; Mun, C.-W.; Shin, J.-H.; Lee, G.-H.; Lee, J.-H. Speculating the Timing of Iron Deposition in the Putamen in Multiple System Atrophy. Parkinsonism Relat. Disord. 2019, 63, 106–110. [Google Scholar] [CrossRef]
- Han, Y.-H.; Lee, J.-H.; Kang, B.-M.; Mun, C.-W.; Baik, S.-K.; Shin, Y.-I.; Park, K.-H. Topographical Differences of Brain Iron Deposition between Progressive Supranuclear Palsy and Parkinsonian Variant Multiple System Atrophy. J. Neurol. Sci. 2013, 325, 29–35. [Google Scholar] [CrossRef]
- Lee, J.-H.; Baik, S.-K. Putaminal Hypointensity in the Parkinsonian Variant of Multiple System Atrophy: Simple Visual Assessment Using Susceptibility-Weighted Imaging. J. Mov. Disord. 2011, 4, 60–63. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Shibata, M.; Hirayanagi, K.; Nagashima, K.; Mihara, B.; Ikeda, Y. Putaminal Iron Deposition Precedes MSA-P Onset by 2 Years. Neurology 2018, 90, 1071–1072. [Google Scholar] [CrossRef]
- Kaindlstorfer, C.; Jellinger, K.A.; Eschlböck, S.; Stefanova, N.; Weiss, G.; Wenning, G.K. The Relevance of Iron in the Pathogenesis of Multiple System Atrophy: A Viewpoint. J. Alzheimer’s Dis. 2018, 61, 1253–1273. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Martin-Bastida, A.; Crichton, R.R. Is Chelation Therapy a Potential Treatment for Parkinson’s Disease? Int. J. Mol. Sci. 2021, 22, 3338. [Google Scholar] [CrossRef]
- Devos, D.; Labreuche, J.; Rascol, O.; Corvol, J.-C.; Duhamel, A.; Guyon Delannoy, P.; Poewe, W.; Compta, Y.; Pavese, N.; Růžička, E.; et al. Trial of Deferiprone in Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 2045–2055. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Lee, S.; Santamaria, E.; Irigoyen, J.F.; Forrest, S.; Li, J.; Tanaka, H.; Couto, B.; Reyes, N.G.; Qamar, H.; et al. 4R-Tau Seeding Activity Unravels Molecular Subtypes in Patients with Progressive Supranuclear Palsy. bioRxiv 2023. [Google Scholar] [CrossRef]
| Hypothesis | Study | Sample Model | Relevant Findings |
|---|---|---|---|
| Hypothesis 1 (Aβ) | Dekens et al. [48] | Primary astrocytic culture | Addition of human recombinant Aβ in culture medium resulted in increased ferritin expression. |
| Baringer et al. [39] | iPSC-derived endothelial cells and astrocytes | Aβ exposure led to increased iron uptake by astrocytes and iron transport across the blood–brain barrier model of endothelial cells. | |
| McIntosh et al. [50] | Primary microglial culture and APPswe/PS1dE9 mice | IFNγ and Aβ exposure led to increased glial iron retention. | |
| Li et al. [51] | WT C57/B16 mice | Intrahippocampal injection of Aβ oligomers induced increased APP expression and iron accumulation. | |
| Tsatsanis et al. [46] | Primary neuronal culture | Inhibition of α-secretase activity led to increased cellular iron retention. | |
| Hypothesis 1 (tau) | Lei et al. [42] | Primary neuronal culture and Tau KO C57BL/6/SV129 mice | Tau KO led to cellular iron retention and iron accumulation in the cortex, hippocampus, and the substantia nigra. |
| Hypothesis 2 (Aβ) | Banerjee et al. [58] | SHSY5Y cells and monomeric Aβ42 | Ferric ammonium citrate (FAC) treatment induced increased β-secretase activity and Aβ42 secretion, and oligomerization of monomeric Aβ42. |
| Chen et al. [59] | Primary neuronal culture | FAC treatment induced abnormal cellular localization of soluble APP α. | |
| Becerill-Ortega et al. [60] | Primary neuronal and astrocytic culture and APP/PS1 mice | Iron exposure led to neuronal-specific increase in KPI-APP (pro-amyloidogenic form) expression and secretion of Aβ42. | |
| Chen et al. [61] | APP/PS1 mice | Dietary iron treatment led to increased Aβ in the hippocampus. | |
| Tahmasebinia and Emadi [62] | Aβ40 and Aβ42 peptides | Ferric chloride treatment promoted the aggregation of Aβ40 and Aβ42 peptides. | |
| Hypothesis 2 (tau) | Wan et al. [66] | Primary neuronal culture and C57BL/6 mice | Iron treatment led to increased phosphorylation at Ser202/Thr205, Thr181, and Ser396 sites of tau. |
| Yamamoto et al. [68] | PHF-tau from post-mortem AD tissue | Ferric iron bound to hyperphosphorylated tau and induced aggregation in a dose-dependent manner. | |
| Ahmadi et al. [69] | Tau-410 | Ferrous and ferric iron bound to tau and induced structural changes. | |
| Guo et al. [67] | APP/PS1 mice | Iron-rich diet resulted in increased phosphorylation of tau at Thr205, Thr231, and Ser396 sites. | |
| Hypothesis 3 | - | - | - |
| Hypothesis 4 | - | - | - |
| Hypothesis | Study | Sample Model | Relevant Findings |
|---|---|---|---|
| Hypothesis 1 | Please refer to Table 1, rows under “Hypothesis 1 (tau)”. | ||
| Hypothesis 2 | Mukherjee and Panda [96] | 4R2N recombinant tau | Ferric iron induced tau oligomerization and fibrillization in a dose-dependent manner. |
| For more information, please refer to Table 1, rows under “Hypothesis 2 (tau)”. | |||
| Hypothesis 3 | - | - | - |
| Hypothesis 4 | - | - | - |
| Hypothesis | Study | Sample Model | Relevant Findings |
|---|---|---|---|
| Hypothesis 1 | Guo et al. [127] | Macaca fascicularis | Administration of pre-formed α-syn fibrils resulted in robust iron deposition in the SN and GP, localized to microglia, and altered iron homeostatic protein expression in dopaminergic neurons. |
| Deas et al. [134] | iPSC-derived neuron with SNCA triplication mutation | Exposure to exogenous α-syn oligomeric species resulted in iron-induced oxidative stress. | |
| Mi et al. [136] | MES23.5 dopaminergic cells | Addition of recombinant α-syn to culture media induced dysregulation of iron homeostatic genes. | |
| Ortega et al. [137] | Primary neuronal culture | Overexpression of human α-syn in an iron-rich environment resulted in increased intracellular iron retention. | |
| Hypothesis 2 | Zhao et al. [148] | Human recombinant α-syn | Incubation with ferric iron promoted α-syn fibrillization at low concentrations. |
| Abeyawardhane et al. [149] | Human recombinant α-syn | Under aerobic conditions, ferrous iron incubation induced a soluble α-syn oligomer structure, and ferric iron induced a fibril structure with β-sheets, both of elevated toxic species. | |
| Uversky et al. [150] | Human recombinant α-syn | Incubation with ferric iron induced accelerated and increased aggregation of α-syn. | |
| Kostka et al. [151] | Human recombinant α-syn | Incubation with ferric iron enhanced α-syn aggregation, producing larger and toxic oligomers. | |
| Li et al. [152] | Human recombinant α-syn and HEK293 cells | Incubation with ferric iron enhanced the seeded aggregation of α-syn in a dose-dependent manner, demonstrating enhanced intracellular aggregation and transcellular propagation of α-syn; iron-seeded fibrils were more cytotoxic. | |
| Ostrerova-Golts et al. [153] | Human BE-M17 neuroblastoma cells transfected with WT, A53T, A30P α-syn | Treatment with ferrous iron induced a dose-dependent formation of high-molecular-weight α-syn aggregates in A53T α-syn-expressing cells. | |
| Agostini et al. [154] | α-syn-overexpressing dopaminergic neuron Drosophila model | Administration of ferric ammonium citrate accelerated α-syn pathology formation in dopaminergic neurons and reduced fly life span. | |
| Xiao et al. [139] | Dopaminergic cell line SN4741 | Ferrous iron promoted α-syn aggregation and secretion via inhibition of autophagosome–lysosome fusion. | |
| Wang et al. [155] | Sprague Dawley rats and SH-SY5Y cells | Iron upregulated α-syn phosphorylation at Ser129 and high-molecular-weight-α-syn levels in the SN and dopaminergic cells. | |
| Li et al. [140] | SK-N-SH cells | Ferrous and ferric iron induced cell loss and α-syn aggregation, both directly and via oxidative stress. | |
| Hypothesis 3 | Dauer Née Joppe et al. [156] | C57BI/6J mice and primary neuronal culture | Neonatal iron-enriched mice showed significantly less α-syn pathology in connectome-specific regions. |
| Hypothesis 4 | Ortega et al. [137] | Primary neuronal culture | Overexpression of α-syn in an iron-rich environment resulted in increased intracellular iron retention, which could not be induced via either α-syn overexpression or iron enrichment alone. |
| Osterova-Golts et al. [153] | Human BE-M17 neuroblastoma cells transfected with WT, A53T, A30P α-syn | Cells overexpressing all types of α-syn were more vulnerable to iron-induced toxicity. | |
| Mahoney-Sanchez et al. [159] | LUHMES cells (human dopaminergic neuronal model) | α-syn KO prevented ferroptosis. |
| Hypothesis 1 | Hypothesis 2 | Hypothesis 3 | Hypothesis 4 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI | PM | AM | IV | MRI | PM | AM | IV | MRI | PM | AM | IV | MRI | PM | AM | IV | |
| AD (Aβ) | ||||||||||||||||
| AD (tau) | ||||||||||||||||
| PSP | ||||||||||||||||
| PD | ||||||||||||||||
| MSA | ||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kovacs, G.G. The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 4269. https://doi.org/10.3390/ijms25084269
Lee S, Kovacs GG. The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases. International Journal of Molecular Sciences. 2024; 25(8):4269. https://doi.org/10.3390/ijms25084269
Chicago/Turabian StyleLee, Seojin, and Gabor G. Kovacs. 2024. "The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases" International Journal of Molecular Sciences 25, no. 8: 4269. https://doi.org/10.3390/ijms25084269
APA StyleLee, S., & Kovacs, G. G. (2024). The Irony of Iron: The Element with Diverse Influence on Neurodegenerative Diseases. International Journal of Molecular Sciences, 25(8), 4269. https://doi.org/10.3390/ijms25084269






