Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid
Abstract
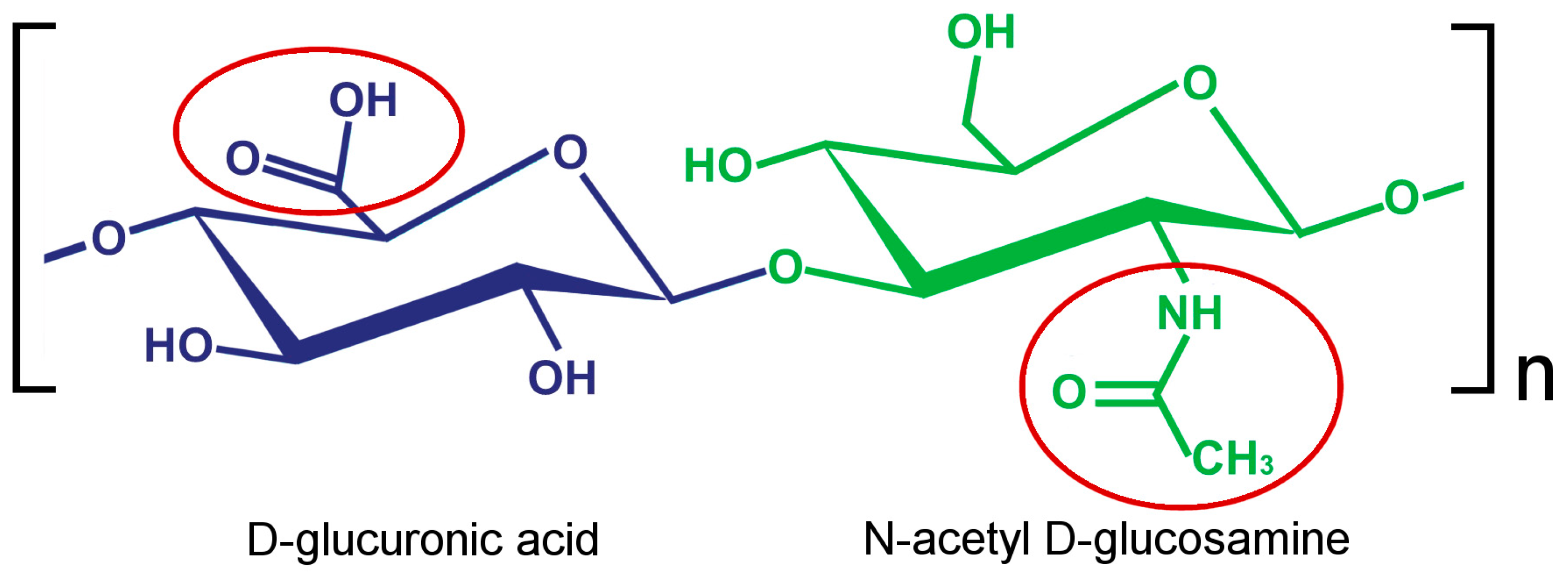
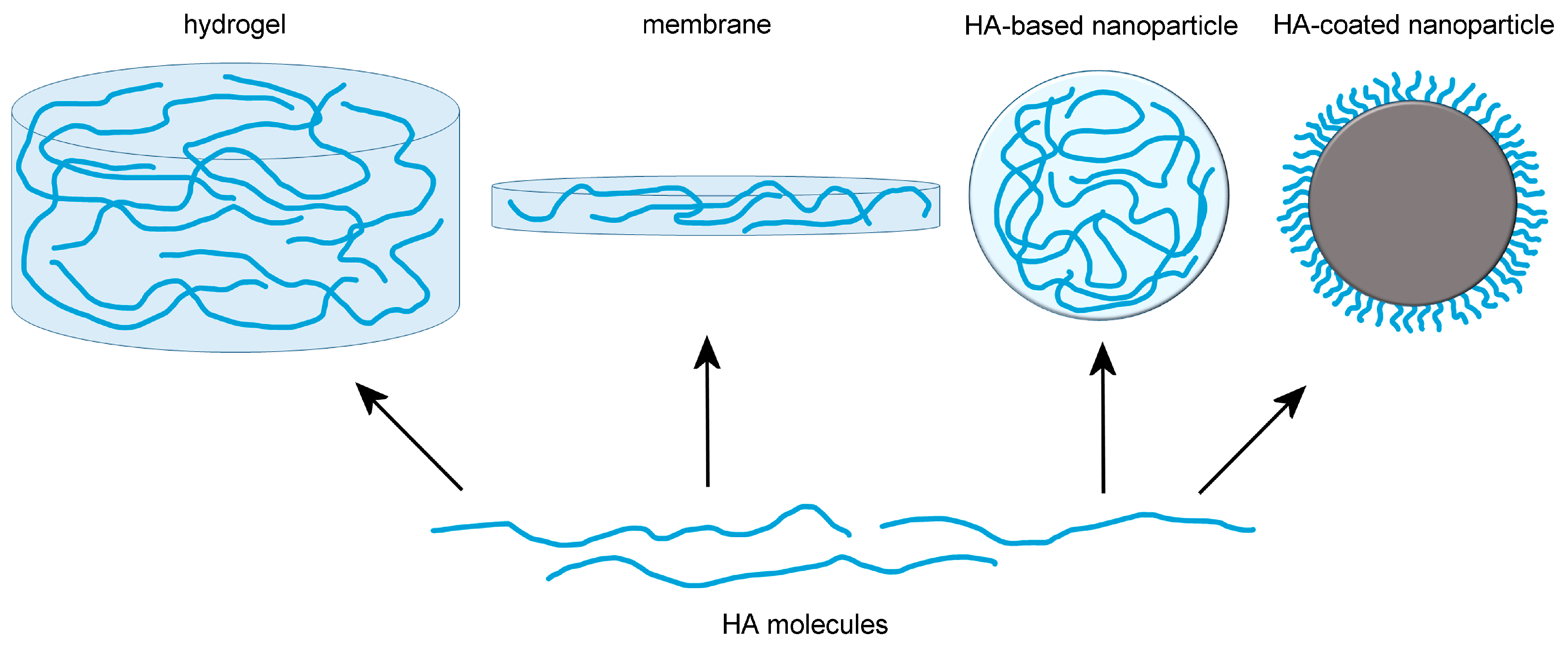
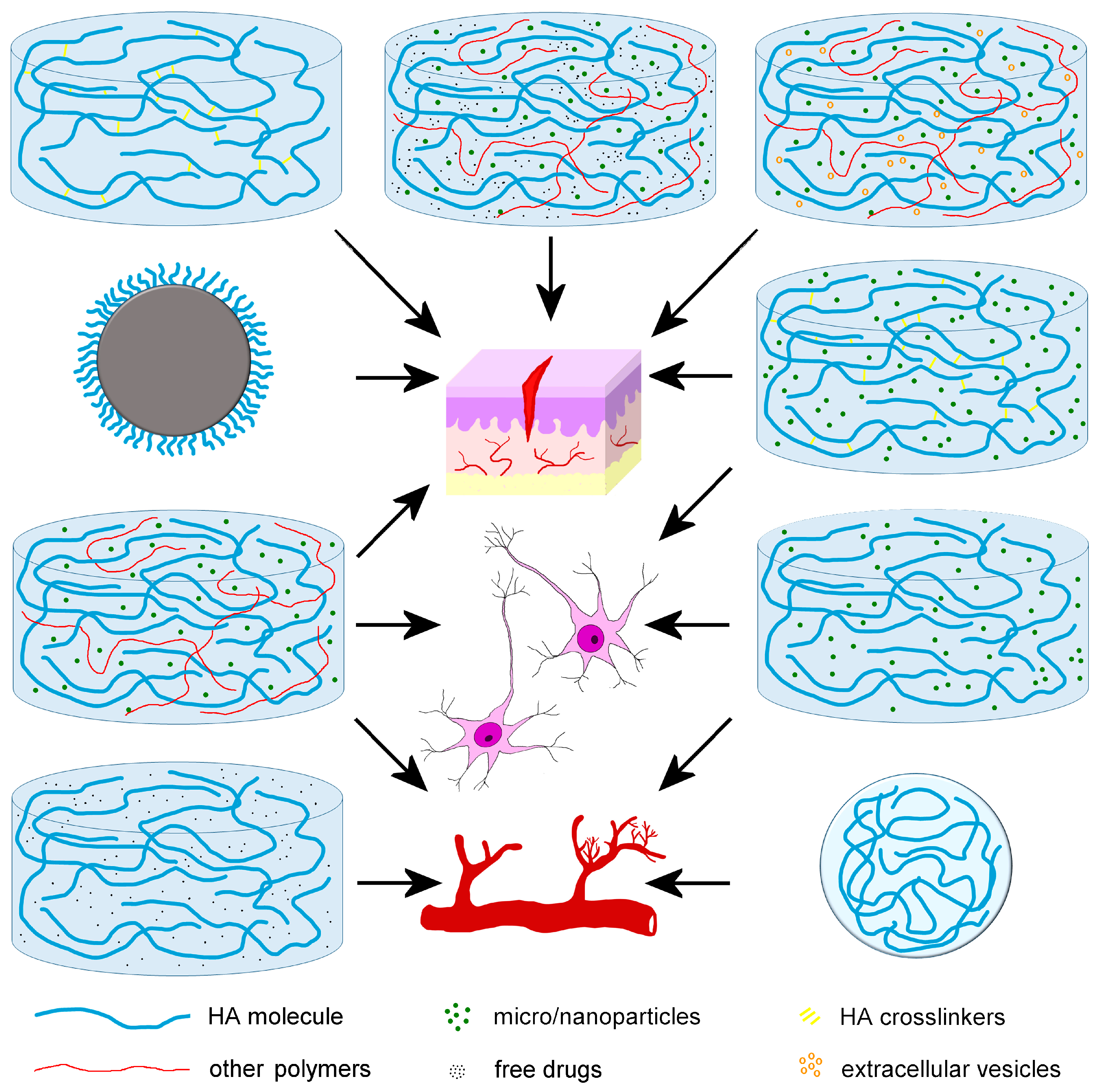
1. Introduction
2. Nanomedical Applications of HA for Cartilage Regeneration
3. Nanomedical Applications of HA for Bone Regeneration
4. Nanomedical Applications of HA for Wound Healing
5. Nanomedical Applications of HA for Angiogenesis
6. Nanomedical Applications of HA for Nervous Tissue Regeneration
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its Nature, Distribution, Functions and Turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Käsdorf, B.T.; Arends, F.; Lieleg, O. Diffusion Regulation in the Vitreous Humor. Biophys. J. 2015, 109, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M. Hyaluronan and Synovial Joint: Function, Distribution and Healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Brecht, M.; Mayer, U.; Schlosser, E.; Prehm, P. Increased Hyaluronate Synthesis Is Required for Fibroblast Detachment and Mitosis. Biochem. J. 1986, 239, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M.J.; Caplan, A.I. Hyaluronic Acid Bonded to Cell-Culture Surfaces Stimulates Chondrogenesis in Stage 24 Limb Mesenchyme Cell Cultures. Dev. Biol. 1986, 114, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M.J.; Pechak, D.G.; Fiszman, M.Y.; Caplan, A.I. Hyaluronic Acid Bonded to Cell Culture Surfaces Inhibits the Program of Myogenesis. Dev. Biol. 1986, 113, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Yamagata, M.; Suzuki, S.; Kimata, K. Hyaluronic Acid Modulates Proliferation of Mouse Dermal Fibroblasts in Culture. J. Cell Sci. 1988, 90 Pt 2, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Atsumi, F.; Sawai, T.; Yamada, Y.; Miyaishi, O.; Senga, T.; Hamaguchi, M.; Kimata, K. Abnormal Accumulation of Hyaluronan Matrix Diminishes Contact Inhibition of Cell Growth and Promotes Cell Migration. Proc. Natl. Acad. Sci. USA 2002, 99, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Cui, Z.; Foley, J.P.; Zhao, H.; Grimm, P.C.; Delisser, H.M.; Savani, R.C. Expression and Role of the Hyaluronan Receptor RHAMM in Inflammation after Bleomycin Injury. Am. J. Respir. Cell Mol. Biol. 2005, 33, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Goomer, R.S.; Harwood, F.; Kubo, T.; Hirasawa, Y.; Amiel, D. The Effects of Hyaluronan on Matrix Metalloproteinase-3 (MMP-3), Interleukin-1beta(IL-1beta), and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Gene Expression during the Development of Osteoarthritis. Osteoarthr. Cartil. 1999, 7, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Prevo, R.; Banerji, S.; Ferguson, D.J.; Clasper, S.; Jackson, D.G. Mouse LYVE-1 Is an Endocytic Receptor for Hyaluronan in Lymphatic Endothelium. J. Biol. Chem. 2001, 276, 19420–19430. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Lin, Y.-T.; Chiang, B.-L.; Lin, Y.-H.; Hou, S.-M. High Molecular Weight Hyaluronic Acid Down-Regulates the Gene Expression of Osteoarthritis-Associated Cytokines and Enzymes in Fibroblast-like Synoviocytes from Patients with Early Osteoarthritis. Osteoarthr. Cartil. 2006, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Pauloin, T.; Dutot, M.; Warnet, J.-M.; Rat, P. In Vitro Modulation of Preservative Toxicity: High Molecular Weight Hyaluronan Decreases Apoptosis and Oxidative Stress Induced by Benzalkonium Chloride. Eur. J. Pharm. Sci. 2008, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Halicka, H.D.; Mitlitski, V.; Heeter, J.; Balazs, E.A.; Darzynkiewicz, Z. Attenuation of the Oxidative Burst-Induced DNA Damage in Human Leukocytes by Hyaluronan. Int. J. Mol. Med. 2009, 23, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant Acitivity of Low Molecular Weight Hyaluronic Acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Hamilton, S.R.; Nakrieko, K.-A.; Kooshesh, F.; Walton, P.; McCarthy, J.B.; Bissell, M.J.; Turley, E.A. Rhamm-/- Fibroblasts Are Defective in CD44-Mediated ERK1,2 Motogenic Signaling, Leading to Defective Skin Wound Repair. J. Cell Biol. 2006, 175, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Hamilton, S.R.; Zalinska, E.; McCulloch, L.; Amin, R.; Akentieva, N.; Winnik, F.; Savani, R.; Bagli, D.J.; Luyt, L.G.; et al. A RHAMM Mimetic Peptide Blocks Hyaluronan Signaling and Reduces Inflammation and Fibrogenesis in Excisional Skin Wounds. Am. J. Pathol. 2012, 181, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- Tolg, C.; Poon, R.; Fodde, R.; Turley, E.A.; Alman, B.A. Genetic Deletion of Receptor for Hyaluronan-Mediated Motility (Rhamm) Attenuates the Formation of Aggressive Fibromatosis (Desmoid Tumor). Oncogene 2003, 22, 6873–6882. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, R.N.; Beebe, D.C. Hyaluronate in Vasculogenesis. Science 1983, 220, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis Induced by Degradation Products of Hyaluronic Acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Singleton, P.A.; Bourguignon, L.Y.W. CD44 Interaction with Ankyrin and IP3 Receptor in Lipid Rafts Promotes Hyaluronan-Mediated Ca2+ Signaling Leading to Nitric Oxide Production and Endothelial Cell Adhesion and Proliferation. Exp. Cell Res. 2004, 295, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Firsanov, D.; Tombline, G.; Ning, H.; Ablaeva, J.; Seluanov, A.; Gorbunova, V. Naked Mole-Rat Very-High-Molecular-Mass Hyaluronan Exhibits Superior Cytoprotective Properties. Nat. Commun. 2020, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Volpi, N.; Stern, R.; Soltes, L. Hyaluronan in Medical Practice. Curr. Med. Chem. 2016, 23, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, D.; Xu, Y.; Zhu, Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef] [PubMed]
- Yasin, A.; Ren, Y.; Li, J.; Sheng, Y.; Cao, C.; Zhang, K. Advances in Hyaluronic Acid for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 910290. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Dunnill, P. A Brief Definition of Regenerative Medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Hachet, E.; Van Den Berghe, H.; Bayma, E.; Block, M.R.; Auzély-Velty, R. Design of Biomimetic Cell-Interactive Substrates Using Hyaluronic Acid Hydrogels with Tunable Mechanical Properties. Biomacromolecules 2012, 13, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Costa Marques, M.R.; Costa, V.C.; Santos, G.S.; Martins, R.A.; Santos, M.D.S.; Santana, M.H.A.; Nallakumarasamy, A.; Jeyaraman, M.; Lana, J.V.B.; et al. Intra-Articular Hyaluronic Acid in Osteoarthritis and Tendinopathies: Molecular and Clinical Approaches. Biomedicines 2023, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; Reis, R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- D’Albis, G.; D’Albis, V.; Palma, M.; Plantamura, M.; Nizar, A.K. Use of Hyaluronic Acid for Regeneration of Maxillofacial Bones. Genesis 2022, 60, e23497. [Google Scholar] [CrossRef]
- Miglani, A.; Vishnani, R.; Reche, A.; Buldeo, J.; Wadher, B. Hyaluronic Acid: Exploring Its Versatile Applications in Dentistry. Cureus 2023, 15, e46349. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Short, W.D.; Keswani, S.G.; Wang, X. Immunologic Roles of Hyaluronan in Dermal Wound Healing. Biomolecules 2021, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The Development of Hyaluronic Acids Used for Skin Tissue Regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, L.; Zou, Y.; Sun, D.; Wang, L.; Yu, Z.; Guo, J. Role of Hyaluronic Acids and Potential as Regenerative Biomaterials in Wound Healing. ACS Appl. Bio Mater. 2021, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.; Mohammadnejad, D.; Karimipour, M.; Rasta, S.H.; Rahbarghazi, R.; Abedelahi, A. Hyaluronic Acid and Regenerative Medicine: New Insights into the Stroke Therapy. Curr. Mol. Med. 2020, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Gilbert, R.J. Extracellular Matrix-Mimetic Hydrogels for Treating Neural Tissue Injury: A Focus on Fibrin, Hyaluronic Acid, and Elastin-Like Polypeptide Hydrogels. Adv. Healthc. Mater. 2021, 10, e2101329. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.G.R.; Pantoja, B.T.D.S.; Almeida, G.H.D.R.; Carreira, A.C.O.; Miglino, M.A. Bacterial Cellulose and ECM Hydrogels: An Innovative Approach for Cardiovascular Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 3955. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-Rich Exosomes Functionalized DEGMA-Modified Hyaluronic Acid Hydrogels for Corneal Epithelial Healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing Functional Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, A.; Amorim, S.; Solanki, A.; Costa, D.S.; Pires, R.A.; Quintero, F.; Del Val, J.; Comesaña, R.; Badaoui, A.; Lusquiños, F.; et al. Hyaluronic Acid Hydrogels Reinforced with Laser Spun Bioactive Glass Micro- and Nanofibres Doped with Lithium. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112124. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Locke, R.C.; Witherel, C.E.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, J.A. Fabrication of MSC-Laden Composites of Hyaluronic Acid Hydrogels Reinforced with MEW Scaffolds for Cartilage Repair. Biofabrication 2021, 14, 014106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, S.; Chen, Z.; Liu, Q.; Cai, Y.; Mei, Y.; Xu, Y.; Guo, R.; Yan, C. Kartogenin (KGN)/Synthetic Melanin Nanoparticles (SMNP) Loaded Theranostic Hydrogel Scaffold System for Multiparametric Magnetic Resonance Imaging Guided Cartilage Regeneration. Bioeng. Transl. Med. 2023, 8, e10364. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, N.; Zhou, R.; Zhao, X.; Han, Y.; Xia, F.; Cheng, J.; Duan, M.; Qian, Q.; Wang, X.; et al. Combination Therapy Using Kartogenin-Based Chondrogenesis and Complex Polymer Scaffold for Cartilage Defect Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 6276–6284. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Lv, Z.; Wu, R.; Yan, W.; Jiang, Q.; Shi, D. An Injectable Hydrogel Scaffold With Kartogenin-Encapsulated Nanoparticles for Porcine Cartilage Regeneration: A 12-Month Follow-up Study. Am. J. Sports Med. 2020, 48, 3233–3244. [Google Scholar] [CrossRef]
- Kim, B.J.; Arai, Y.; Choi, B.; Park, S.; Ahn, J.; Han, I.-B.; Lee, S.-H. Restoration of Articular Osteochondral Defects in Rat by a Bi-Layered Hyaluronic Acid Hydrogel Plug with TUDCA-PLGA Microsphere. J. Ind. Eng. Chem. 2018, 61, 295–303. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-Rich Plasma Preparation for Regenerative Medicine: Optimization and Quantification of Cytokines and Growth Factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.A.; Burnouf, T.; Stakiw, J.; Seghatchian, J. Platelet Microparticle: A Sensitive Physiological “Fine Tuning” Balancing Factor in Health and Disease. Transfus. Apher. Sci. 2015, 52, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Espinosa, N.; Mayoral, L.P.-C.; Perez-Campos, E.; Cabrera Fuentes, H.A.; Mayoral, E.P.-C.; Martínez-Cruz, R.; Canseco, S.P.; Andrade, G.M.; Cruz, M.M.; Velasco, I.G.; et al. Platelets and Platelet-Derived Microvesicles as Immune Effectors in Type 2 Diabetes. Curr. Vasc. Pharmacol. 2017, 15, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Jiang, Q.; Shi, D. Platelet-Rich Plasma Combined with Injectable Hyaluronic Acid Hydrogel for Porcine Cartilage Regeneration: A 6-Month Follow-Up. Regen. Biomater. 2020, 7, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Saeedi Garakani, S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Hashemi Motlagh, G. Injectable PNIPAM/Hyaluronic Acid Hydrogels Containing Multipurpose Modified Particles for Cartilage Tissue Engineering: Synthesis, Characterization, Drug Release and Cell Culture Study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Dennis, S.C.; Whitlow, J.; Detamore, M.S.; Kieweg, S.L.; Berkland, C.J. Hyaluronic-Acid-Hydroxyapatite Colloidal Gels Combined with Micronized Native ECM as Potential Bone Defect Fillers. Langmuir 2017, 33, 206–218. [Google Scholar] [CrossRef]
- Han, F.; Tu, Z.; Zhu, Z.; Liu, D.; Meng, Q.; Yu, Q.; Wang, Y.; Chen, J.; Liu, T.; Han, F.; et al. Targeting Endogenous Reactive Oxygen Species Removal and Regulating Regenerative Microenvironment at Annulus Fibrosus Defects Promote Tissue Repair. ACS Nano 2023, 17, 7645–7661. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Watanabe, C. Stimulation of Osteoinduction in Bone Wound Healing by High-Molecular Hyaluronic Acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Injectable Cuttlefish HAP and Macromolecular Fibroin Protein Hydrogel for Natural Bone Mimicking Matrix for Enhancement of Osteoinduction Progression. React. Funct. Polym. 2021, 160, 104841. [Google Scholar] [CrossRef]
- Noh, I.; Kim, N.; Tran, H.N.; Lee, J.; Lee, C. 3D Printable Hyaluronic Acid-Based Hydrogel for Its Potential Application as a Bioink in Tissue Engineering. Biomater. Res. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Rajabnejadkeleshteri, A.; Basiri, H.; Mohseni, S.S.; Farokhi, M.; Mehrizi, A.A.; Moztarzadeh, F. Preparation of Microfluidic-Based Pectin Microparticles Loaded Carbon Dots Conjugated with BMP-2 Embedded in Gelatin-Elastin-Hyaluronic Acid Hydrogel Scaffold for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 184, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Flegeau, K.; Gauthier, O.; Rethore, G.; Autrusseau, F.; Schaefer, A.; Lesoeur, J.; Veziers, J.; Brésin, A.; Gautier, H.; Weiss, P. Injectable Silanized Hyaluronic Acid Hydrogel/Biphasic Calcium Phosphate Granule Composites with Improved Handling and Biodegradability Promote Bone Regeneration in Rabbits. Biomater. Sci. 2021, 9, 5640–5651. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.; Martino, M.M.; Ventura, M.; Hubbell, J.A.; Hilborn, J.; Ossipov, D.A. Improving the Osteogenic Potential of BMP-2 with Hyaluronic Acid Hydrogel Modified with Integrin-Specific Fibronectin Fragment. Biomaterials 2013, 34, 704–712. [Google Scholar] [CrossRef]
- Zhang, Q.; Pei, Q.; Yang, J.; Guo, S.; Yang, A.; Qian, Y.; Li, C.; Feng, Q.; Lv, H.; Zhou, X.; et al. Vascularized Nanocomposite Hydrogel Mechanically Reinforced by Polyelectrolyte-Modified Nanoparticles. J. Mater. Chem. B 2022, 10, 5439–5453. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.-Z.A.; Eltaher, H.M. 3D Printed Bioinspired Scaffolds Integrating Doxycycline Nanoparticles: Customizable Implants for in Vivo Osteoregeneration. Int. J. Pharm. 2021, 607, 121002. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Tran, T.V.T.; Kang, H.J.; Tripathi, G.; Lee, B.T.; Bae, S.H. Synthesis and Characterization of Biphasic Calcium Phosphate Laden Thiolated Hyaluronic Acid Hydrogel Based Scaffold: Physical and in-Vitro Biocompatibility Evaluations. J. Biomater. Sci. Polym. Ed. 2021, 32, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Jamnezhad, S.; Asefnejad, A.; Motififard, M.; Yazdekhasti, H.; Kolooshani, A.; Saber-Samandari, S.; Khandan, A. Development and Investigation of Novel Alginate-Hyaluronic Acid Bone Fillers Using Freeze Drying Technique for Orthopedic Field. Nanomed. Res. J. 2020, 5, 306–315. [Google Scholar] [CrossRef]
- Zhou, P.; Yan, B.; Wei, B.; Fu, L.; Wang, Y.; Wang, W.; Zhang, L.; Mao, Y. Quercetin-Solid Lipid Nanoparticle-Embedded Hyaluronic Acid Functionalized Hydrogel for Immunomodulation to Promote Bone Reconstruction. Regen. Biomater. 2023, 10, rbad025. [Google Scholar] [CrossRef]
- Amorim, S.; Martins, A.; Neves, N.M.; Reis, R.L.; Pires, R.A. Hyaluronic Acid/Poly-l-Lysine Bilayered Silica Nanoparticles Enhance the Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Mater. Chem. B 2014, 2, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.C.; Vale, A.C.; Costa, R.R.; Reis, R.L.; Alves, N.M. Exploiting Polyelectrolyte Complexation for the Development of Adhesive and Bioactive Membranes Envisaging Guided Tissue Regeneration. J. Funct. Biomater. 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and Adhesive Properties of Multilayered Coatings Based on Catechol-Functionalized Chitosan/Hyaluronic Acid and Bioactive Glass Nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Ji, S.M.; Kummara, M.R.; Han, S.S. Recent Progress on Hyaluronan-Based Products for Wound Healing Applications. Pharmaceutics 2022, 14, 2235. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Long, T.; Wan, Y.; Li, B.; Xu, Z.; Zhao, L.; Mu, C.; Ge, L.; Li, D. Dual-Drug Loaded Polysaccharide-Based Self-Healing Hydrogels with Multifunctionality for Promoting Diabetic Wound Healing. Carbohydr. Polym. 2023, 312, 120824. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolomé, M.; Włodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasa, B.; San Román, J. Development of Bioactive Catechol Functionalized Nanoparticles Applicable for 3D Bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112515. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Nejad, Z.; Torabinejad, B.; Davachi, S.M.; Zamanian, A.; Saeedi Garakani, S.; Najafi, F.; Nezafati, N. Synthesis, Physicochemical, Rheological and in-Vitro Characterization of Double-Crosslinked Hyaluronic Acid Hydrogels Containing Dexamethasone and PLGA/Dexamethasone Nanoparticles as Hybrid Systems for Specific Medical Applications. Int. J. Biol. Macromol. 2019, 126, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Lv, Y.; Zhang, H.; He, Y.; Gao, H.; Chen, C.; Wang, D.; Chen, P.; Tang, S.; Li, J.; et al. A Multifunctional Hydrogel Loaded with Two Nanoagents Improves the Pathological Microenvironment Associated with Radiation Combined with Skin Wounds. Acta Biomater. 2023, 159, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shi, T.; Wang, Y.; He, Z.; Mu, Z.; Cai, X.; Deng, H.; Shen, J.; Liu, F. Hybrid Hydrogel Loaded with Chlorhexidine⊂β-CD-MSN Composites as Wound Dressing. Int. J. Nanomed. 2023, 18, 1725–1740. [Google Scholar] [CrossRef] [PubMed]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of Hyaluronan in Angiogenesis and Its Utility to Angiogenic Tissue Engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, J.; Cullen, M.; Fattah, S.; Murphy, R.; Stefanovic, S.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; et al. Development of a Sustained Release Nano-In-Gel Delivery System for the Chemotactic and Angiogenic Growth Factor Stromal-Derived Factor 1α. Pharmaceutics 2020, 12, 513. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; Cryan, S.A. Translational Studies on the Potential of a VEGF Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779. [Google Scholar] [CrossRef]
- Jian, W.-H.; Wang, H.-C.; Kuan, C.-H.; Chen, M.-H.; Wu, H.-C.; Sun, J.-S.; Wang, T.-W. Glycosaminoglycan-Based Hybrid Hydrogel Encapsulated with Polyelectrolyte Complex Nanoparticles for Endogenous Stem Cell Regulation in Central Nervous System Regeneration. Biomaterials 2018, 174, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, S.; Sivashanmugam, A.; Annapoorna, M.; Janarthanan, R.; Subramania, I.; Shantikumar, V.; Jayakumar, R. Injectable Deferoxamine Nanoparticles Loaded Chitosan-Hyaluronic Acid Coacervate Hydrogel for Therapeutic Angiogenesis. Colloids Surf. B Biointerfaces 2018, 161, 129–138. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and Tunable Hyaluronic Acid Hydrogels Releasing Chemotactic and Angiogenic Growth Factors for Endodontic Regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Mondalek, F.G.; Ashley, R.A.; Roth, C.C.; Kibar, Y.; Shakir, N.; Ihnat, M.A.; Fung, K.-M.; Grady, B.P.; Kropp, B.P.; Lin, H.-K. Enhanced Angiogenesis of Modified Porcine Small Intestinal Submucosa with Hyaluronic Acid-Poly(Lactide-Co-Glycolide) Nanoparticles: From Fabrication to Preclinical Validation. J. Biomed. Mater. Res. A 2010, 94, 712–719. [Google Scholar] [CrossRef]
- Serafin, A.; Rubio, M.C.; Carsi, M.; Ortiz-Serna, P.; Sanchis, M.J.; Garg, A.K.; Oliveira, J.M.; Koffler, J.; Collins, M.N. Electroconductive PEDOT Nanoparticle Integrated Scaffolds for Spinal Cord Tissue Repair. Biomater. Res. 2022, 26, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO2 Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, S.; Xu, J.; Li, W.; Ge, D.; Sun, C.; Liu, T.; Ma, X. Neural Stem Cell Proliferation and Differentiation in the Conductive PEDOT-HA/Cs/Gel Scaffold for Neural Tissue Engineering. Biomater. Sci. 2017, 5, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Führmann, T.; Obermeyer, J.; Tator, C.H.; Shoichet, M.S. Click-Crosslinked Injectable Hyaluronic Acid Hydrogel Is Safe and Biocompatible in the Intrathecal Space for Ultimate Use in Regenerative Strategies of the Injured Spinal Cord. Methods 2015, 84, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.; Jerome, W.; Zaslav, K.; Grande, D. Exosome-Laden Scaffolds for Treatment of Post-Traumatic Cartilage Injury and Osteoarthritis of the Knee: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15178. [Google Scholar] [CrossRef]
- Hutomo, D.I.; Amir, L.; Suniarti, D.F.; Bachtiar, E.W.; Soeroso, Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers 2023, 15, 2591. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Learmonth, D.A.; Gomes, D.B.; Cautela, M.P.; Oliveira, A.C.N.; Andrade, R.; Espregueira-Mendes, J.; Veloso, T.R.; Cunha, C.B.; Sousa, R.A. Mussel-Inspired Catechol Functionalisation as a Strategy to Enhance Biomaterial Adhesion: A Systematic Review. Polymers 2021, 13, 3317. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carton, F.; Malatesta, M. Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3975. https://doi.org/10.3390/ijms25073975
Carton F, Malatesta M. Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid. International Journal of Molecular Sciences. 2024; 25(7):3975. https://doi.org/10.3390/ijms25073975
Chicago/Turabian StyleCarton, Flavia, and Manuela Malatesta. 2024. "Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid" International Journal of Molecular Sciences 25, no. 7: 3975. https://doi.org/10.3390/ijms25073975
APA StyleCarton, F., & Malatesta, M. (2024). Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid. International Journal of Molecular Sciences, 25(7), 3975. https://doi.org/10.3390/ijms25073975







