1. Introduction
Breast cancer represents a worldwide health problem, being the most common form of malignancy in women, ranking second in terms of mortality. In the period 2021–2022, 281,550 new cases of breast cancer were reported, which represents approximately 30% of newly detected cancer cases among women worldwide [
1,
2,
3]. Numerous potentially predictive factors for breast cancer are described in the literature, such as the histological type, histological grade, lymphovascular invasion, tumor size, axillary lymph node status, and hormone receptors. All these factors are currently classified as the gold standard in breast cancer staging [
4]. There are biomarkers with prognostic and predictive potential, such as the lipid profile (HDL, LDL, VLDL, Triglycerides, Total Cholesterol, Apolipoprotein A1, Apolipoprotein B), p53, and ki-67, which should be included in routine investigations and which, not infrequently, can influence therapeutic conduct [
5,
6,
7,
8]. Dyslipidemia is a component of metabolic syndrome, having an important role in the carcinogenesis of different tumor types, such as prostate, ovarian, or renal cancer [
9].
The number of studies on the predictive potential of the different components of the lipid profile with a predictive potential in breast cancer is quite low [
10,
11]. The studies identified in the literature provide heterogeneous data regarding the predictive impact of the lipid profile in breast cancer in relation to molecular subtypes and neoadjuvant therapy [
12,
13,
14,
15]. Relatively sparse studies have evaluated the relationship between novel lipid biomarkers, such as apolipoprotein A-I (Apo A-I) and apolipoprotein B-100 (Apo B-100), and cancer risk Apo A-I, the major protein components of HDL cholesterol, and Apo B-100, the major protein components of low-density lipoprotein (LDL) cholesterol, and antiatherogenic and proatherogenic effect manifestation of the HDL and LDL cholesterol, respectively. The analysis of potential biomarkers in breast cancer has been a highly debated topic in recent years, however, with heterogeneous results in the literature [
16]. The relation of these potential biomarkers with the molecular subtypes of breast cancer represents an important objective, and once some predictive directives are certified, we could develop new therapeutic guidelines. Another important biomarker in carcinogenesis is represented by P53 or TP53. The alteration of p53 has been investigated with particular interest in recent years and studies have shown that the p53 gene represents the most frequent mutation in carcinogenesis processes [
17]. The frequency of these mutations in the case of breast carcinoma is quite heterogeneous in relation to molecular subtypes. The mutation of the
P53 gene may represent an early event in tumor progress because it is obvious in the in situ phase of cancer growth. Additionally, the
P53 mutation probably stimulates cell proliferation and renders the phenotype aggressive. The availability of detecting mutant p53 protein on formalin-fixed paraffin-embedded (FFPE) tissue has allowed retrospective studies of patients with a long follow-up. Ki-67 represents another important parameter in tumor carcinogenesis, being a non-histone nuclear protein that correlates with cell growth. The Ki-67 expression varies through cell cycle, with different expression levels in the G1, G2/M, and S phases, but undetectable in the G0 phase. Ki-67 associates with cell cycle progress and the short half-life confers it as an effective biomarker for assessing the growth fraction of tumor cells [
18,
19,
20,
21].
2. Results
We have evaluated 313 cases of BC followed by surgical resection between 2020 and 2022, in the Ist Clinic of Oncological Surgery, Oncological Institute “Prof Dr I Chiricuta” Cluj Napoca. We applied the inclusion criteria for our study and defined a study batch of 233 cases. Of the 233 cases, 142 benefited from neoadjuvant therapy (NAC), and the remaining 91 cases benefited from primary surgical therapy. The stratification of the study group was made according to the St. Gallen classification; therefore, 40.77% (n = 95 cases) were included in the luminal subtype A, 24.89% (n = 58 cases) in the luminal type B, 6% (n = 14 cases) in the HER2+ subtype, and 28.32% (n = 66 cases) in the TNBC subtype. A total of 21% of the patients enrolled in the study had a family history of malignancy and approximately 70% of them had a BMI over 25 kg/m2. From a histological point of view, ductal carcinoma had an incidence of approximately 88.02%, followed by lobular carcinoma with a percentage of 11.15%.
Among the patients who benefited from neoadjuvant therapy (n = 142 cases), 88.02% (n = 125 cases) had between 4 and 8 cycles of neoadjuvant therapy, while 11.97% (n = 17 cases) had less than 4 cycles of therapy. Regarding the response rate to NAC according to the Response Evaluation Criteria in Solid Tumors (RECIST-Miller-Payne Classification) guidelines, version 1.1, 64.78% presented a partial response (PR) or complete response (pCR) to NAC therapy (
Table 1).
If we refer to the tumor size, the test carried out shows that 36.48% (n = 85) of the patients were staged as cT2, and 33.9% (n = 79) were staged as cT3. Regarding tumor grading, 41.63% (n = 97) were moderately differentiated G2 tumors, followed by poorly differentiated G3 tumors in approximately 29.61% (n = 69) of the cases, while 28.75% (n = 67) were classified as well-differentiated G1 tumors.
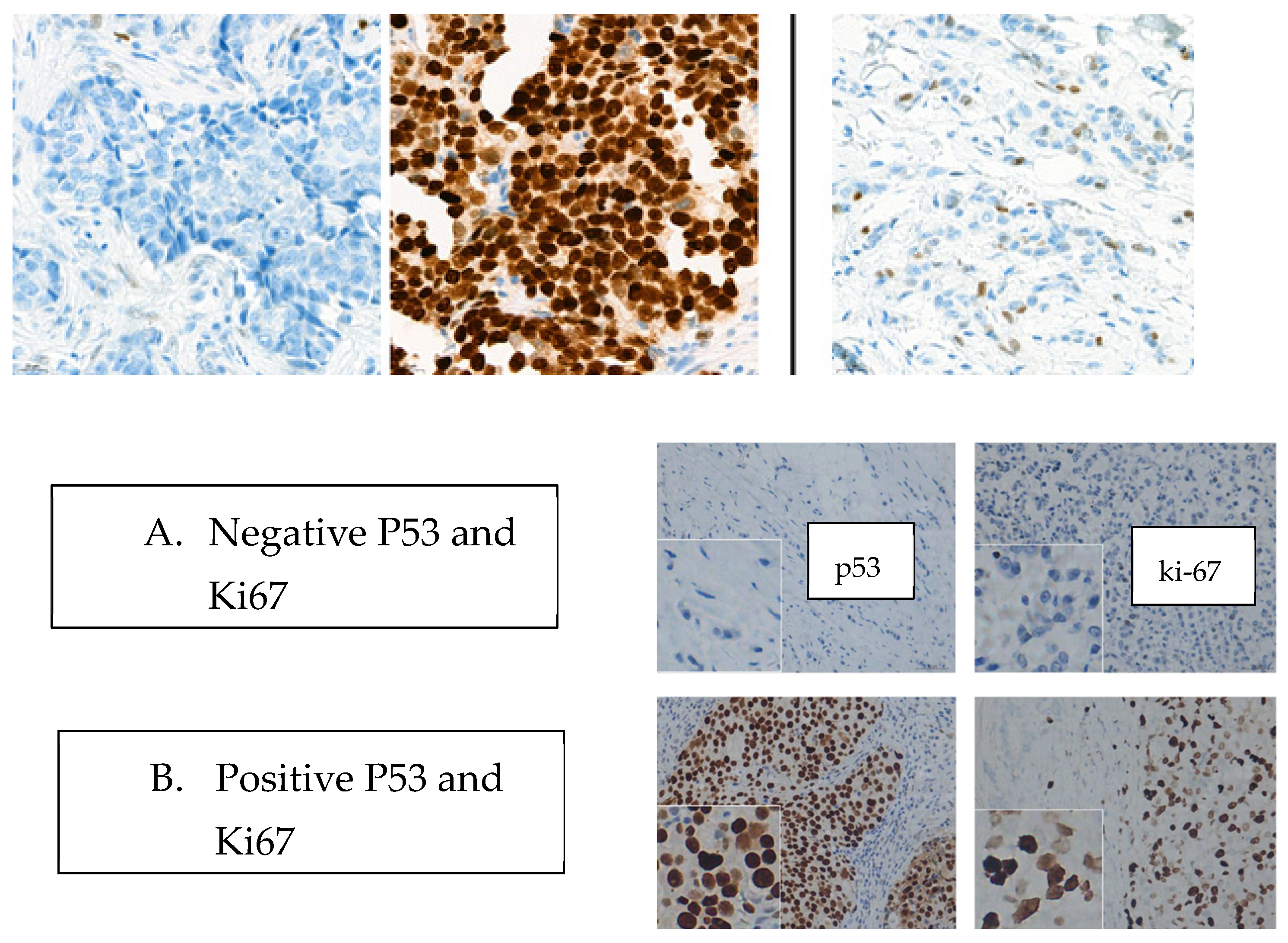
The analysis of the genetic and molecular profile of the study group reveals that 71.67% (n = 167 cases) have a Ki-67 above 14%, while 39% (n = 91 cases) of the patients present a positive P53 mutation. Among the patients with positive P53 mutation (n = 91), 15 (16.48%) were included in the luminal A subtype, 9 (9.89%) in the luminal B subtype, 10 (10.98%) in the HER2+ subtype, and 57 patients (62.63%) in the TNBC subtype. Another eloquent aspect of our evaluation is related to the presence of the BRCA1/2 mutation, which was reported as positive in 18.8% (n = 44 cases) of the cases, thus confirming the hereditability of the tumor. Genomic DNA was purified from EDTA-anticoagulated blood using the QiaSymphony instrument (Qiagen, Hilden, Germany). Genotyping of a panel of 20 pathogenic BRCA1 and 10 pathogenic BRCA2 variants was carried out using TaqMan Low-Density Arrays on the ABI 9700 instrument (Applied Biosystems, Foster City, CA, USA) as recommended by the manufacturer.
There are five simple cancer-based criteria for BRCA: (1) ovarian cancer; (2) breast cancer diagnosed when patients are 45 years or younger; (3) two primary breast cancers, both diagnosed when patients are 60 years or younger; (4) triple-negative breast cancer; and (5) male breast cancer. A sixth criterion—breast cancer plus a parent, sibling, or child with any of the other criteria—can be added to address family history. Criteria 1 through 5 are considered the MCG criteria, and criteria 1 through 6 are considered the MCGplus criteria. In the literature, approximately 20% of breast neoplasias have a hereditary nature, the BRCA1/2 genes having a cardinal role in their transmission.
Regarding neoadjuvant therapy regimens, 40.14% of the cases benefited from Carboplatin therapy, while 59.85% benefited from Anthracycline/Taxane therapy (
Table 2). Analyzing the mutant status of p53 at the level of the general group (n = 233) it was found that 91 patients (39.05%) presented a positive mutation. Referring to the molecular subtype according to the St Gallen classification, 61 cases (67.03%) with a p53 mutation were included in the TNBC subtype (See
Table 3), 11 cases (12.08%) were classified as HER2+, 10 cases (10.98%) as Luminal B, and 9 cases as Luminal A (9.89%).
We analyzed the p53 status at the level of the study group and the possibility of its association with the tumor response rate to the NAC therapy (See
Figure 1 and
Table 4). In the case of patients included in the TNBC subtype (n = 61), the mutant status of p53 was identified in 49 cases, while the wildtype was identified in 12 cases. In the case of TNBC patients, no impact of the p53 status on the complete tumor response rate was identified (
p = 0.797). A statistically significant correlation was found in the case of TNBC patients, classified as cT1-2, having neoadjuvant therapy with Carboplatin; they presented pCR in 57.3% of cases (
p < 0.00001). In the case of patients belonging to the Luminal A (n = 9), Luminal B (n = 10), and HER2+ (n = 11) subtypes, we recorded a p53 mutant status in 13 cases, and a p53 wildtype status in 17 cases, without a statistically significant impact of the p53 status on the tumor response rate to NAC (
p = 0.325). The hormonal status is an important aspect in terms of tumor response rate, therefore in the case of patients with negative hormonal status, significant therapeutic responses were recorded (
p = 0.0001).
We compared the average values of the lipid parameters in the group that benefited from neoadjuvant therapy (n = 142) and we obtained statistically significant values regarding the TG and LDL-C values, which presented a significant increase post-NAC compared to the time of diagnosis (before -NAC) and which maintained their increased values in the dynamics 6 months post-NAC (p < 0.05).
According to a multivariate analysis (see
Table 5), the larger tumor size (
p = 0.011), high HDL-C level (
p = 0.005), and LDL-C (
p = 0.003) were independent predictive factors of the efficacy of NAC. A high HDL-C level before NAC and increased LDL-C level after NAC were associated with better treatment response in ER-positive and HER2+ breast cancer patients.
A study published by Wei Tian et al. on the analysis of the lipid profile in the case of patients who benefit from neoadjuvant therapy concludes increased values of TC, TG, and LDL-C, but without analyzing them based on the molecular subtype of the tumor. In 2022, Xinru Wang et al. published an analysis including 220 patients regarding the association between the histological type of the tumor and lipid profile, concluding the association between the HDL-C and LDL-C values and the absence of positive hormonal status (PR, ER), thus the low values of HDL-C and LD-C being associated with the ER- and PR- subtypes [
22,
23].
The multivariate analysis in the case of patients included in the TNBC subtype showed that the increased tumor volume (
p = 0.002) and increased level of HDL (
p = 0.004) represent predictive factors for the tumor response rate to NAC. In the case of LDL-C values, we did not obtain statistically significant data regarding tumor response to therapy (See
Table 6 and
Table 7).
The TC levels were slightly increased before the last cycles of chemotherapy compared to the prechemotherapy levels in all three regimen subgroups. 6 months after chemotherapy, TC levels returned to baseline levels (0.145 [0.084, 0.234]; z = 5.72;
p < 0.05). The TG levels increased significantly during chemotherapy in all chemotherapy regimen groups. 6 months after chemotherapy completion, TG levels were slightly decreased but were still higher than the baseline levels (prechemotherapy level) in all groups. No significant difference in changes in the TG levels was noticed between the groups. The LDL-C levels were significantly increased during chemotherapy (0.137 [0.071, 0.198]; z = 5.71;
p < 0.05), but were restored to baseline levels 6 months after chemotherapy completion. Significantly lower LDL-C levels were noticed 6 months after chemotherapy completion compared to the baseline values in the Carboplatin group (0.127 [0.088, 0.218]; z = 5.82;
p < 0.05). The HDL-C levels decreased significantly during chemotherapy in all regimen groups, and 6 months after chemotherapy completion the levels were nearly restored to baseline levels. The levels of APO-A and APO-B showed minimal fluctuations post-NAC and 6 months after the completion of NAC (
p > 0.05) regardless of the type of chemotherapy undertaken. A study carried out by Youzhao Ma et al. reveals increased values of triglycerides (TG), total cholesterol (TC), and LDL-C, and low values of HDL-C after NAC (
p < 0.001) [
24].
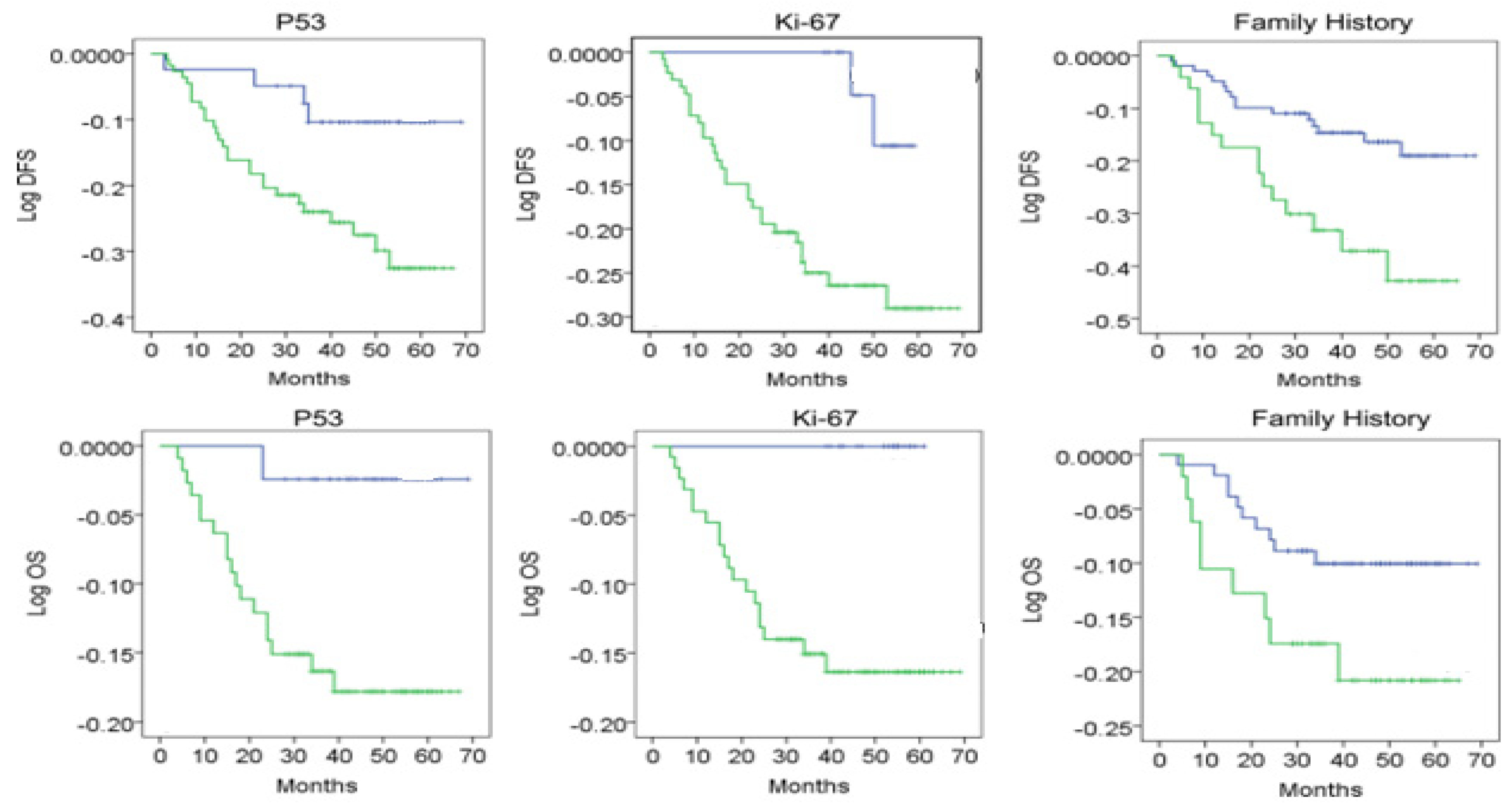
In order to evaluate the predictive nature of p53 (positive) and Ki-67 (increased values > 14) of the disease-free interval (DFS) and long-term survival (OS-overall survival) we applied a univariate and multivariate analysis in the general group; the analysis included the following variables: age, presence or absence of menopause, family history of the patients, tumor size, tumor grading, Ki-67, lymphatic metastases (LN metastases), and p53.
The univariate and multivariate analysis reveals that age has a predictive nature regarding OS (
p = 0.001, 0.117 [0.071, 0.212]; z = 5.71;) but not for DFS (0.127 [0.061, 0.162]; z = 5.58;
p < 0.05). The menopausal status, tumor size (0.145 [0.081, 0.182]; z = 5.30;
p < 0.05), family history, grading, Ki-67, p53, and LN metastasis have a predictive nature regarding OS (0.177 [0.081, 0.212]; z = 5.11;
p < 0.05), while for DFS, only tumor size, tumor grading, Ki-67>14, and p53+ are of predictive nature (0.187 [0.094, 0.262]; z = 5.92;
p < 0.05) (
Table 8/
Figure 2).
In the logistic regression, tumor size, lymph node metastases, and p53 positivity were associated with recurrence with a more prominent predictive effect (
p < 0.05). P53/Ki-67 levels must be divided into categorical variables (positive vs. negative, high index vs. low index, and negative) in our logistic regression and Cox regression models (
Table 9).
3. Discussions
Our study performs an exhaustive analysis of genetic and molecular parameters of a predictive nature regarding the response rate to the NAC therapy. The literature presents absolute heterogeneity regarding the existence of biomarkers with predictive capacity in the case of breast cancer patients who benefit from neoadjuvant therapy. The genetic and molecular analysis carried out in our group indicates that 71.67% have a Ki-67 score higher than 14%, and 39% of the patients have the positive P53 mutation. Among the patients with positive P53 mutation, 16.48% were included in the luminal A subtype, 9.89% in the luminal B subtype, 10.98% in the HER2+ subtype, and 57 patients, i.e., 62.63%, in the TNBC subtype.
A special category is represented by the patients included in the TNBC subtype; the mutant status of p53 was recorded in 49 cases, and the wildtype in 12 cases. In the case of TNBC patients, no impact of the p53 status on the complete tumor response rate (p = 0.797) was identified. In the case of patients belonging to the Luminal A, Luminal B, and HER2+ subtypes, we recorded a p53 mutant status in 13 cases, and a p53 wildtype status in 17 cases without a statistically significant impact on the tumor response rate to NAC (p = 0.325).
Bertheau reported that TP53 might be predictive for chemotherapy response in a particular setting: In a pooled analysis of 144 breast cancers from three series, this group found that pCR rates in TP53 mutant tumors strongly depended on the type of chemotherapy, and were significantly higher after high-dose cyclophosphamide (36%) as opposed to standard-dose (4%) or no cyclophosphamide (12%). The effect was most pronounced in ER-negative tumors, where the pCR rate after high-dose (not lower dose) cyclophosphamide among TP53 mutant cases reached 71% [
25].
The menopausal status, tumor size, family history, grading, Ki-67, p53, and LN metastases have a predictive nature regarding OS (p < 0.05), while for DFS, only tumor size, tumor grading, Ki-67 > 14, and p53+ are of predictive nature. Pan Y, Yuan Y, Liu G, Wei Y (2017) conducted a study on the immunohistochemical analysis of p53 and Ki-67 in the case of patients with TNBC, which revealed their possible stratification according to the level of tumor aggressiveness and integrated Ki-67 and p53 as potential prognostic biomarkers.
In a recent report, Carey et al. described that in the CALGB 40601 trial, the p53 signature was independently associated with high pCR rates in 305 patients with HER2-positive breast cancer. There are further previous studies on the predictive effect of TP53 status in breast cancer that were summarized in a meta-analysis by Chen in 2012 [
26]. Investigating 26 studies comprising 3.476 cases, the authors concluded that TP53 aberrations were associated with a higher response to neoadjuvant chemotherapy, particularly for anthracycline-based regimes [
27].
The multivariate analysis in the case of patients included in the TNBC subtype showed that the increased tumor volume (
p = 0.002) and increased level of HDL (
p = 0.004) represent predictive factors for the tumor response rate to NAC. High HDL-C levels before NAC and increased LDL-C levels after NAC were associated with a better treatment response in ER-positive and HER2+ breast cancer patients. In the literature, there are heterogeneous results regarding the impact of the lipid profile on the tumor response rate to NAC according to the tumor subtype. Xinru Wang et al. published a study comparing the lipid profile between 2 study subgroups that include 70 ER (estrogen receptor) negative patients and 73 PR (progesterone receptor) negative patients, respectively [
28,
29]. In the case of negative PR patients, increased values of LDL-C and lipoprotein (LP) levels were revealed compared to the positive PR patients. In the case of negative PR patients, increased values of LDL-C and LP were recorded compared to positive PR patients (
p < 0.05). Another study published in 2020 by Sung Mi Jung et al. underlines the impact of lipid profile values in the breast tumor carcinogenesis process [
30]. The TC and TG levels are generally increased in breast cancer patients, and increased levels of LDL-C and VLDL-C and low levels of HDL-C are associated with the tumor development process. Fanli Qu et al. published a retrospective study enrolling 533 patients with breast tumors who benefited from NAC therapy [
31]. During the study, the analysis of the lipid profile (TC, TG, HDL-C, LDL-C) was performed before and after the NAC therapy. High HDL-C levels before NAC and increased LDL-C levels after NAC were associated with a better treatment response in ER-positive breast cancer patients.