Abstract
PD-L1 is one of the two programmed cell death 1 (PD-1) ligands and a part of an immune checkpoint system (PD-1/PD-L1) with widespread clinical application. The aim of this study was to investigate PD-L1 expression and its association with clinicopathological and prognostic significance in non-clear cell renal cell carcinoma (non-ccRCC) patients. A total of 41 papillary (pRCC) and 20 chromophobe (chRCC) RCC tumors were examined for PD-L1 expression by immunohistochemistry in the cancer cells and tumor-infiltrating mononuclear cells (TIMCs). PD-L1 positivity was detected in 36.6% pRCC and 85.0% chRCC cancer cells, while PD-L1 positivity was observed in 73.2% pRCC and 50.0% chRCC TIMCs. PD-L1 positivity in both pRCC and chRCC tumor cells was not correlated with any of the examined clinicopathological features, while PD-L1 positivity in TIMCs was associated with the age of patients with pRCC. During follow-up, the death was documented among 6 patients with pRCC. Papillary RCC patients with PD-L1-positive tumor cells were significantly associated with an increased risk of death compared with patients with PD-L1-negative cancer cells. A similar trend was observed when comparing PD-L1 expression in TIMCs. However, no differences in overall survival for PD-L1-positive pRCC patients with compared to PD-L1-negative patients were observed in tumor cells or TIMCs.
1. Introduction
Kidney cancer is the third most common genitourinary malignancy among both sexes [1]. Renal cell carcinoma (RCC) is the most common solid lesion within the kidney, accounting for nearly 90% of all kidney malignancies. It is a heterogenous disease encompassing different histological subtypes. Clear-cell RCC (ccRCC) is the most common subtype, accounting for more than 80% of all RCCs. The remaining renal epithelial malignancies, collectively named non-clear cell RCCs (non-ccRCCs), include several subtypes such as papillary RCC (10–15%), chromophobe RCC (5%) and the more rare forms [2]. Localized tumors can be generally treated by total or partial nephrectomy. However, about 30% of patients treated with nephrectomy will still develop systemic metastases [3,4]. Some studies suggested that localized non-ccRCC cases are more likely to have a favorable prognosis than ccRCC [4,5]. In contrast, several reports claimed that some types of metastatic non-ccRCC such as papillary RCC, may have an aggressive clinical course and a shorter overall survival [6,7].
Treatment of patients with advanced RCC has progressed through the development of systemic therapy, such as immunotherapy and targeted therapy. As in other cancer types, immune checkpoint inhibitors are the focus of current research, because the immune checkpoint pathway is an important immune evasion mechanism utilized by cancer cells. PD-L1 is one of the two programmed cell death 1 (PD-1) ligands and, thus, a part of an immune checkpoint system (PD-1/PD-L1) with widespread clinical application [1,3,8].
PD-1 is a T cell co-inhibitory receptor with two ligands: PD-L1 (B7-H1) and PD-L2 (B7-DC). PD-1 is an inhibitor of both adaptive and innate immune responses, and, under normal conditions, is expressed on activated T cells, natural killer (NK) T cells, B cells, macrophages, dendritic cells and monocytes [9,10]. Of note, PD-1 is highly expressed on tumor-specific T cells. PD-1 plays two opposite roles, because it can be both beneficial and harmful. As for its beneficial effects, it reduces the regulation of ineffective or harmful immune responses and maintains immune tolerance. On the other hand, PD-1 causes the dilation of malignant cells by interfering with the protective immune response [10,11]. PD-L1 is usually expressed by macrophages, some activated T cells and B cells, dendritic cells and some epithelial cells, particularly under inflammatory conditions [12]. In addition, PD-L1 is expressed by tumor cells as an “adaptive immune mechanism” to escape anti-tumor responses [13]. Interferon gamma (IFN-γ) induces protein kinase D isoform 2 (PKD2), which is important for the regulation of PD-L1. The inhibition of PKD2 activity inhibits the expression of PD-L1 and promotes a strong antitumor immune response. T and NK cells secrete IFN-γ, increasing the expression of PD-L1 on the surface of the target cells, including tumor cells [14,15].
The PD-1/PD-L1 pathway controls the induction and maintenance of immune tolerance within the tumor microenvironment. The activity of PD-1 and its ligands PD-L1 or PD-L2 are responsible for T cell activation, proliferation and cytotoxic secretion in cancer to degenerating anti-tumor immune responses [10]. The PD-1/PD-L1 signaling axis is considered an important immunological escape mechanism exploited by neoplasms [16,17,18]. Thus, the inhibition of this pathway can enhance antitumor immune responses, leading to enhanced effector T cell activity in tissues and tumor microenvironments [8,9,17]. Based on these findings, PD-1 and its ligand PD-L1 have been investigated as novel targets in oncology [19]. Although the predictive markers for the PD-1/PD-L1 blockade remain vague, some researchers demonstrated that responsiveness and clinical outcome is better in patients with PD-L1-positive tumors detected by immunohistochemistry (IHC) [8,19,20]. Several studies reported that PD-L1 expression was associated with poor prognosis of ccRCC [8]. However, because of a relatively low occurrence and distinct biology, a significant proportion of patients with non-ccRCC have generally been excluded from the clinical trials of tumor-targeted agents. To date, only a few studies have investigated the utility of PD-L1 as a prognostic marker in non-ccRCC [3,4,8,9,21,22,23]. Thus, the prognostic value and clinical significance of PD-L1 expression in non-ccRCC subtypes still remains unclear.
Therefore, the aim of this study was to investigate PD-L1 expression and its association with clinicopathologic features and prognostic significance in non-ccRCC patients with a long-term follow-up.
2. Results
2.1. Patient Characteristics
Between 2015 and 2020, we identified 61 non-ccRCC patients with available tissue. The histological subtypes included pRCC (n = 41) and chRCC (n = 20). The clinicopathological characteristics of the patients were summarized in Table 1.

Table 1.
Non-clear cell renal cell carcinoma (RCC) patient characteristics.
2.2. PD-L1 Expression in Tumor Cells and Clinicopathological Features
PD-L1 staining in tumor cells was either diffuse or focal within the tumor and showed a mosaic pattern with a random-appearing mixture of positive and negative cells. Representative images of PD-L1 expression in tumor cells were given in Figure 1 and Figure 2.
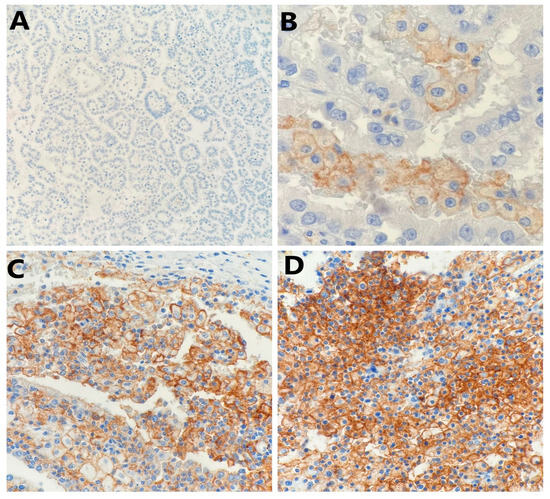
Figure 1.
Representative photomicrography showing immunohistochemical staining of PD-L1 in papillary renal cell carcinoma tumor cells (membranous brown color): (A) no expression (×100), (B) weak expression (×400), (C) moderate expression (×200), and (D) strong expression (×200).
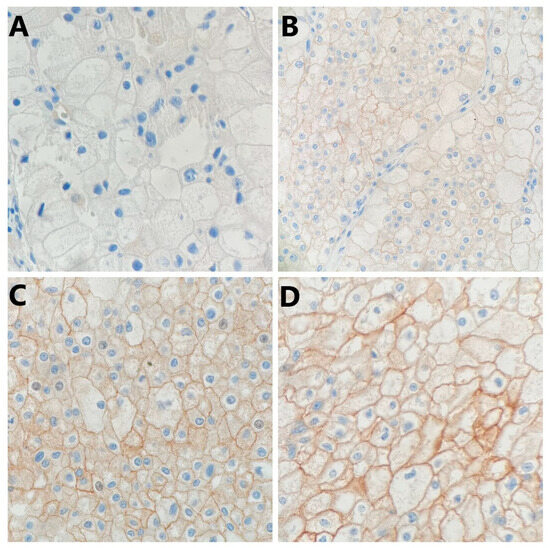
Figure 2.
Representative photomicrography showing immunohistochemical staining of PD-L1 in chromophobe renal cell carcinoma tumor cells (membranous brown color): (A) no expression (×400), (B) weak expression (×200), (C) moderate expression (×400), and (D) strong expression (×400).
Among 61 patients with non-ccRCC, the PD-L1 expression in cancer cell membranes was negative in 29 tumors (47.5%) and positive in 32 tumors (52.5%). Specifically, PD-L1 positivity in cancer cell membranes was detected in 15 of 41 (36.6%) pRCC and 17 of 20 (85.0%) chRCC. The mean positivity of PD-L1 in non-ccRCC cells was 10.9%: 3.4% in pRCC and 27.5% in chRCC.
PD-L1 positivity in both pRCC and chRCC tumor cells was not correlated with any of the examined clinicopathological features such as age at surgery, sex, pathological tumor stage, WHO/ISUP grade, presence of tumor necrosis, angioinvasion, neuroinvasion, renal fibrous capsule invasion, perinephric fat invasion and risk of death (Table 2 and Table 3).

Table 2.
Association of PD-L1 expression in cancer cells and immune cells (TIMCs) with the clinicopathological features in papillary renal cell carcinoma.

Table 3.
Association of PD-L1 expression in cancer cells and immune cells (TIMCs) with the clinicopathological features in chromophobe renal cell carcinoma.
2.3. PD-L1 Expression in Tumor-Infiltrating Mononuclear Cells (TIMCs) and Clinicopathological Features
Among 61 patients with non-ccRCC, PD-L1 expression in TIMCs was negative in 21 tumors (34.4%) and positive in 40 tumors (65.6%). Specifically, PD-L1 positivity in TIMCs was detected in 30 of 41 (73.2%) pRCC and 10 of 20 (50.0%) chRCC. The mean positivity of PD-L1 in TIMCs was 7.4%: 9.6% in pRCC and 2.3% in chRCC. Representative images of PD-L1 expression in TIMCs are given in Figure 3.
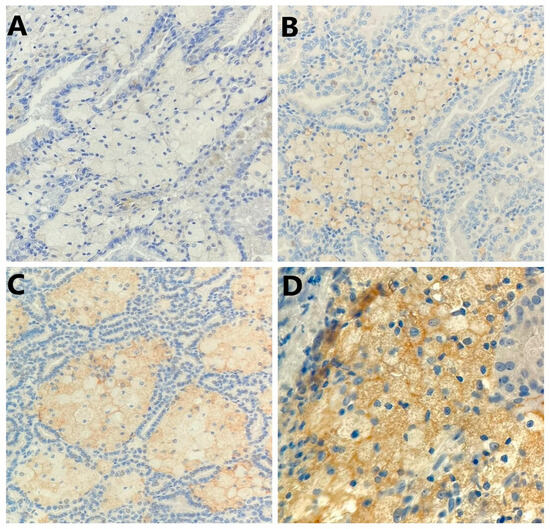
Figure 3.
Representative photomicrography showing immunohistochemical staining of PD-L1 in the tumor-infiltrating mononuclear cells (membranous brown color): (A) no expression (×200), (B) weak expression (×200), (C) moderate expression (×200), and (D) strong expression (×400).
Among all examined clinicopathological features, there was only a significant association of age at surgery and PD-L1 expression levels in TIMCs in pRCC (Table 2).
In the case of chRCC, PD-L1 positivity in TIMCs did not correlate with any of the examined clinicopathological features such as age during surgery, sex, pathological tumor stage, WHO/ISUP grade, presence of tumor necrosis, angioinvasion, neuroinvasion, renal fibrous capsule invasion, perinephric fat invasion and risk of death (Table 3).
2.4. PD-L1 Expression and Clinical Outcome in Non-ccRCC Patients
The mean duration of follow-up was 47.88 months (SD = 27.81), with a median of 43.80 months (interquartile range = 25.25 to 66.22 months).
At last follow-up, 55 (90.2%) patients were alive, while 6 (9.8%) patients died—all these cases concerned the patients with pRCC (14.6%).
Papillary RCC patients with PD-L1-positive tumor cells were significantly associated with an increased risk of death (HR = 8.20, 95% CI 1.22–6.27; p = 0.0012) compared with patients with PD-L1-negative tumor cells. A similar trend was observed when comparing the PD-L1 expression in TIMCs (HR = 1.33, 95% CI 1.53–12.94; p = 0.00032).
Kaplan–Meier analysis demonstrated no differences in overall survival (OS) for PD-L1 positive pRCC patients compared to those with PD-L1-negative tumor cells (Figure 4) and TIMCs (Figure 5).
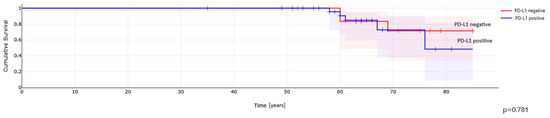
Figure 4.
PD-L1 expression in cancer cells and overall survival in patients with papillary renal cell carcinoma.

Figure 5.
PD-L1 expression in immune cells (TIMCs) and overall survival in patients with papillary renal cell carcinoma.
3. Discussion
The main aim of cancer immunotherapies targeting the PD-1/PD-L1 pathway is to normalize the immune system instead of simply enhancing the function of immune cells in tumors. Based on the molecular mechanisms of PD-1/PD-L1 signaling, many researchers investigated various types of anti-PD-1 and anti-PD-L1 antibodies due to its clinical successful efficacy, long-lasting response and low toxicity [24,25]. Many anti-PD-1 antibodies such as nivolumab, pembrolizumab and cemiplimab have been produced to date and already approved by the FDA, while other antibodies are still in the experimental phase of development. Also several anti-PD-L1 monoclonal antibodies are commercially available. Atezolizumab, avelumab and duravulumab were approved by the FDA, but a few others are still in the experimental phase of development [24]. Since the usefulness of such immune checkpoint therapy appears limited to a specific subset of patients, there is a need to develop predictive markers to facilitate patient selection. When using anti-PD-1 or PD-L1 antibodies to treat neoplasms, some patients with low PD-L1 expression might be poor responders. Therefore, to personalize treatments and obtain an optimal treatment effect, biomarkers need to be identified. Although the FDA has approved a PD-L1 IHC test as a companion diagnostic for the immunotherapeutic targeting of the PD-1/PD-L1 pathway in some neoplasms, the expression pattern of PD-1 and PD-L1 is not a good predictive biomarker for all types of cancer [24].
Currently, there is an urgent need in particular to develop non-ccRCC-specific therapeutic approaches [26,27]. PD-L1 expression is relatively well documented in ccRCC and is reported to be associated with aggressive features such as high TNM stage, tumor size and tumor grade, as well as an increased risk of cancer-specific mortality [4,28,29,30]. The correlation between PD-L1 positivity and unfavorable prognostic factors was identified with PD-L1 expression in both tumor cells and TIMCs. Based on these studies, PD-L1 positivity may be considered an independent predictor of poor prognosis in ccRCC. However, in non-ccRCC the role of PD-L1 remains controversial.
Choueiri et al. [4] were the first to describe the PD-L1 expression in 101 non-ccRCC, including 50 pRCC and 36 chRCC, and its correlation with clinical outcome. Among these patients, PD-L1 expression in tumor cells was detected in 10.9% of cases, specifically in 10% of pRCC and 5% of chRCC. Consistent with previously published studies regarding ccRCC, they found that PD-L1 expression in non-ccRCC cells was correlated with higher tumor grade and TNM stage and shorter OS. In addition, they reported a trend of shorter OS in patients with PD-L1 positivity in TIMCs. Moreover, they observed that PD-L1 positivity in both tumor cell membranes and TIMCs was associated with a shorter time to recurrence. These observations were not confirmed in our research. We observed a much higher percentage of PD-L1 positivity in both cancer cells and TIMCs. We also did not find any associations described by Choueiri et al. [4].
Since the first research regarding non-ccRCC in 2014, only a few subsequent studies have examined PD-L1 expression in these histologic subtypes of RCC [3,8,9,21,22,23]. These studies included from 14 to 165 pRCC, from 18 to 64 chRCC and single cases of other non-ccRCC subtypes.
Chandrasekaran et al. [22] observed PD-L1 positivity in 28.6% of pRCC and 31.8% of chRCC. In their study, PD-L1 expression was significantly associated with the WHO/ISUP grading; however, it was not associated with age, gender, stage and tumor histology.
Similarly, Walter et al. [23] reported PD-L1 positivity in 32.2% of pRCC and 35.0% of chRCC. They also investigated the number of positive intratumoral cells for PD-L1 in each subtype of non-ccRCC and found that in pRCC the mean was 41.1% and in chRCC was 25.7%. In our study the mean positivity of PD-L1 was 3.4% in pRCC and 27.5% in chRCC.
Another study by Abbas et al. [9] found that the intratumoral expression of PD-L1 was observed in 46.4% non-ccRCC cases. There was no association between an intratumoral expression of PD-L1 and either patients or tumor characteristics, such as age, sex, stage, grade and lymph node or distant metastasis. Moreover, PD-L1 positivity in cancer cells was not significantly associated with cancer-specific survival (CSS) and OS in non-ccRCC; however, a trend for improved survival was seen for PD-L1-positive tumors. These observations are consistent with ours.
In the research conducted by Chipollini et al. [21], PD-L1 positivity in non-ccRCC cells was noted generally in 20% cases and in 18% of pRCC. PD-L1 positivity was significantly associated with higher nuclear grade and perineural invasion. Although not statistically significant, patients with PD-L1-positive tumors had higher AJCC pathologic tumor stages, a higher AJCC clinical M1 stage of disease, larger tumor size, more lymphovascular invasion and more tumor necrosis. Moreover, they found that PD-L1 expression was not significantly associated with 5-year CSS and 5-year relapse-free survival (RFS); however, there was a trend toward worse oncological outcomes in patients with PD-L1-positive tumors.
Shin et al. [8] investigated PD-L1 expression in a group of 201 pRCC and 10 chRCC patients and found it to be expressed in tumor cells in 6% and 10% of cases, respectively. In pRCC, PD-L1 expression did not show any significant relationship with clinicopathological variables such as age, sex, tumor size, nuclear grade, vascular invasion, necrosis, sarcomatoid transformation, lymph node metastasis and pathologic tumor stage, which corresponds with our observations.
In the latest research regarding PD-L1 expression, Möller et al. [3] investigated a large cohort of patients with RCC. In their study, PD-L1 immunostaining varied significantly between kidney cancer subtypes. This proves the well-known biological differences between different RCC subtypes. PD-L1 expression in tumor cells was 18.8% in 64 chRCC and 18.2% in 165 pRCC, which is definitely lower than in our study. In immune cells, PD-L1 expression was seen in 13.9% of pRCC and in no cases of chRCC. These results also differ significantly from ours. In pRCC, no association was found between PD-L1 expression and this cancer subtype or patient prognosis. Additionally, no association was found between PD-L1 expression in pRCC tumor cells and CD8+ density. However, there was a link between PD-L1 expression in immune cells and a high CD8+ density in pRCC.
As the above observations show, some studies concerning PD-L1 expression in cancer cells and TIMCs in non-ccRCC presented different conclusions. These partially discrepant study results are likely to have been caused by a lack of standardized procedures for PD-L1 measurement. There is no definitive cut-off value for PD-L1 positivity, with more than one threshold being reported in the literature. The variability in test cut-offs and standards for PD-L1 testing suggests that there is presently no standardized approach. According to Festino et al. [31], there is no definitive threshold result that can be universally applied to predict clinical response to PD-L1-targeted precision treatments. Published data for PD-L1 tests are mainly focused on IHC for lung cancer, while data on RCC are limited. Thereby, in our study we followed the approach verified by many researchers and based on guidelines for lung cancer where PD-L1 was considered positive when membranous tumor cell staining was observed in at least 1% of the tumor cells at any intensity [32].
Our study has several limitations. First, the non-ccRCC is a very heterogeneous disease in terms of origin and prognosis. In addition, a low incidence of the neoplasms and relatively small number of patients with these histologic subtypes have been represented, limiting our conclusions. Given the small group size for patients with number of events (deaths), a statistical analysis may not properly adjust the association of PD-L1 expression and clinical outcome for potential adverse factors. Moreover, intratumoral diversity has been described in RCC. Although we have evaluated the most representative whole tissue sections, our results may not represent the PD-L1 expression in the entire tumor. Finally, comparisons with other studies should be performed carefully, because many different methodologies and antibodies have been applied to assess PD-L1 expression.
4. Materials and Methods
4.1. Patients and Tumor Characteristics
Sixty-one patients with non-ccRCC (41 pRCC and 20 chRCC) who underwent radical or partial nephrectomy between 2015 and 2020 were included in this study. Formalin-fixed paraffin-embedded blocks were retrieved and corresponding slides from all cases were re-reviewed by two pathologists who assigned both a WHO/ISUP grade and eighth edition of the American Joint Committee on Cancer (AJCC) TNM pathological staging category. Baseline clinicopathological characteristics such as age, gender, tumor size, WHO/ISUP grade (rated only for pRCC), the presence of necrosis, sarcomatoid and rhabdoid differentiation, small vessel lymphovascular invasion, neuroinvasion, fibrous renal capsule invasion, perinephric fat invasion, renal sinus fat and vascular invasion of renal sinus vessels, macroscopic main-renal-vein invasion and AJCC TNM pathologic stage of the primary tumor (pT) were retrospectively collected for patients. The follow-up data included: date of nephrectomy, survival status, date of death and/or date of last follow-up. Anonymized and deidentified information was used for the analyses.
4.2. Immunohistochemical Staining
Four-micron sections were cut on glass slides and air-dried during the night. Following deparaffinization in xylene and rehydration in alcohol, heat-induced epitope retrieval was achieved by immersing the slides in buffer EnVision FLEX Target Retrieval Solution (Perlan, Beaverton, OR, USA) (pH 6.0 or 9.0) and boiling at 95 °C for 20 min. Then, slides were pre-incubated with blocking solution EnVision FLEX Peroxidase-blocking Reagent (Perlan, Beaverton, OR, USA) for 5 min. The staining was performed in an automated immunostainer according to the manufacturer’s instructions using anti-PD-L1 rabbit monoclonal antibody clone ZR3 (Zeta Corporation, Arcadia, CA, USA). The optimal dilution for antibodies was 1:100. Incubation lasted 30 min at room temperature. After this step, the sections were rinsed in buffer EnVision FLEX Wash Buffer (Perlan, Beaverton, OR, USA) and incubated with visualization system EnVision FLEX/HRP (Perlan, Beaverton, OR, USA) for 20 min. Staining patterns were visualized by exposure to 3′3-diaminobenzidine DAB (Perlan, Beaverton, OR, USA) to achieve visualization of the antigens and counterstaining with hematoxylin. Finally, the slides were dehydrated in alcohol, cleared in xylenes and mounted for examination. In each run of the experiment, replacement of the primary antibody with Tris-buffered saline was used as a negative control. The human tonsillar tissue was used as a positive control.
4.3. Interpretation of IHC Staining
The membranous PD-L1 staining of the cancer cells and membranous or cytoplasmic PD-L1 staining of the tumor-infiltrating mononuclear cells (TIMCs) (i.e., lymphocytes and macrophages) were separately interpreted. In normal kidney tissue, PD-L1 expression was not observed. The immunohistochemical results were independently evaluated by two pathologists blinded to the clinical outcome. PD-L1 was considered positive when IHC staining was observed in at least 1% of the cells at any intensity. Moreover, we recorded the proportion of cells stained positive in all tumor areas.
4.4. Statistical Analysis
The quantitative data included numbers and case percentages (%), the mean and the standard deviation (SD). The association between PD-L1 expression status and histopathological characteristics was assessed using the chi-square test and Fisher’s exact test for numerical and categorical variables, respectively. The follow-up duration was calculated from the date of surgery to the date of death or last date of follow-up. An estimator predicting the survival function was used to illustrate the Kaplan–Meier statistical analysis. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) and Statistica 13.1 (StatSoft Inc., Tulsa, OK, USA).
The standard methodology was reported in this study according to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines [33].
5. Conclusions
In conclusion, in the era of the evolving targeted therapies and immunotargeted therapies for RCC, we have analyzed in this study PD-L1 as a novel biomarker. Our study demonstrated that in non-ccRCC, neither PD-L1-positive TIMCs nor intratumoral PD-L1 expression were associated with aggressive or advanced disease. However, the association of PD-L1 expression and risk of death was statistically significant for papillary RCC patients. This suggest that blocking the PD-1/PD-L1 pathway may be an effective method in cancer immunotherapy, and targeted therapies for PD-L1 can be of potential therapeutic benefit in the future. Therefore, further assessment within larger non-ccRCC cohorts is warranted to ascertain the relevance of PD-1/PD-L1 and prove that PD-L1 can become an independent marker for the prognosis of non-ccRCC.
Author Contributions
Conceptualization, M.C.; methodology, M.C., N.S.-G. and M.R.; validation, M.C., D.H. and N.S.-G.; formal analysis, M.C. and N.S.-G.; investigation, M.C., D.H., M.R. and N.S.-G.; resources, M.C., N.S.-G. and M.R.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, D.H., N.S.-G. and B.D.; visualization, M.C. and N.S.-G.; supervision, B.D.; project administration, M.C.; funding acquisition, M.C. and B.D. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Medical University of Silesia.
Institutional Review Board Statement
The Institutional Review Board of Medical University of Silesia opined that this study does not require the consent of the bioethics committee (BNW/NWN/0052/KB/257/23).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shen, M.; Chen, G.; Xie, Q.; Li, X.; Xu, H.; Wang, H.; Zhao, S. Association between PD-L1 Expression and the Prognosis and Clinicopathologic Features of Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Urol. Int. 2020, 104, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016.
- Möller, K.; Fraune, C.; Blessin, N.C.; Lennartz, M.; Kluth, M.; Hube-Magg, C.; Lindhorst, L.; Dahlem, R.; Fisch, M.; Eichenauer, T.; et al. Tumor cell PD-L1 expression is a strong predictor of unfavorable prognosis in immune checkpoint therapy-naive clear cell renal cell cancer. Int. Urol. Nephrol. 2021, 53, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Fay, A.P.; Gray, K.P.; Callea, M.; Ho, T.H.; Albiges, L.; Bellmunt, J.; Song, J.; Carvo, I.; Lampron, M.; et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann. Oncol. 2014, 25, 2178–2184. [Google Scholar] [CrossRef]
- Heng, D.Y.; Choueiri, T.K. Non-clear cell renal cancer: Features and medical management. J. Natl. Compr. Cancer Netw. 2009, 7, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Bacik, J.; Mariani, T.; Russo, P.; Mazumdar, M.; Reuter, V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J. Clin. Oncol. 2002, 20, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Choueiri, T.K.; Fougeray, R.; Schutz, F.A.; Salhi, Y.; Winquist, E.; Culine, S.; von der Maase, H.; Vaughn, D.J.; Rosenberg, J.E. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J. Clin. Oncol. 2010, 28, 1850–1855. [Google Scholar] [CrossRef]
- Shin, S.J.; Jeon, Y.K.; Kim, P.J.; Cho, Y.M.; Koh, J.; Chung, D.H.; Go, H. Clinicopathologic Analysis of PD-L1 and PD-L2 Expression in Renal Cell Carcinoma: Association with Oncogenic Proteins Status. Ann. Surg. Oncol. 2016, 23, 694–702. [Google Scholar] [CrossRef]
- Abbas, M.; Steffens, S.; Bellut, M.; Becker, J.U.; Großhennig, A.; Eggers, H.; Wegener, G.; Kuczyk, M.A.; Kreipe, H.H.; Grünwald, V.; et al. Do programmed death 1 (PD-1) and its ligand (PD-L1) play a role in patients with non-clear cell renal cell carcinoma? Med. Oncol. 2016, 33, 59. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Watari, H. Tumor-intrinsic PD-L1 signaling in cancer initiation, development and treatment: Beyond immune evasion. Front. Oncol. 2018, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Chipollini, J.; Azizi, M.; Peyton, C.C.; Tang, D.H.; Dhillon, J.; Spiess, P.E. Implications of Programmed Death Ligand-1 Positivity in Non-Clear Cell Renal Cell Carcinoma. J. Kidney Cancer VHL 2018, 5, 6–13. [Google Scholar] [CrossRef]
- Chandrasekaran, D.; Sundaram, S.; Kadhiresan, N.; Padmavathi, R. Programmed Death Ligand 1; An Immunotarget for Renal Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.; Gil, S.; Naizhen, X.; Kruhlak, M.J.; Linehan, W.M.; Srinivasan, R.; Merino, M.J. Determination of the Expression of PD-L1 in the Morphologic Spectrum of Renal Cell Carcinoma. J. Cancer 2020, 11, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccin. Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Chen, L. A paradigm shift in cancer immunotherapy: From enhancement to normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Schrader, A.J.; Varga, Z.; Hegele, A.; Pfoertner, S.; Olbert, P.; Hofmann, R. Second-line strategies for metastatic renal cell carcinoma: Classics and novel approaches. J. Cancer Res. Clin. Oncol. 2006, 132, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Vera-Badillo, F.E.; Templeton, A.J.; Duran, I.; Ocana, A.; de Gouveia, P.; Aneja, P.; Knox, J.J.; Tannock, I.F.; Escudier, B.; Amir, E. Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. Eur. Urol. 2015, 67, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Sullivan, D.C.; Brookland, R.K. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Krejci, K.G.; Lobo, J.R.; Sengupta, S.; Chen, L.; et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA 2004, 101, 17174–17179. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Kuntz, S.M.; Leibovich, B.C.; Dong, H.; Lohse, C.M.; Webster, W.S.; Sengupta, S.; Frank, I.; Parker, A.S.; Zincke, H.; et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006, 66, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Festino, L.; Botti, G.; Lorigan, P.; Masucci, G.V.; Hipp, J.D.; Horak, C.E.; Melero, I.; Ascierto, P.A. Cancer Treatment with Anti-PD-1/PD-L1 Agents: Is PD-L1 Expression a Biomarker for Patient Selection? Drugs 2016, 76, 925–945. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).