Functionalization of Ceramic Scaffolds with Exosomes from Bone Marrow Mesenchymal Stromal Cells for Bone Tissue Engineering
Abstract
1. Introduction
2. Results
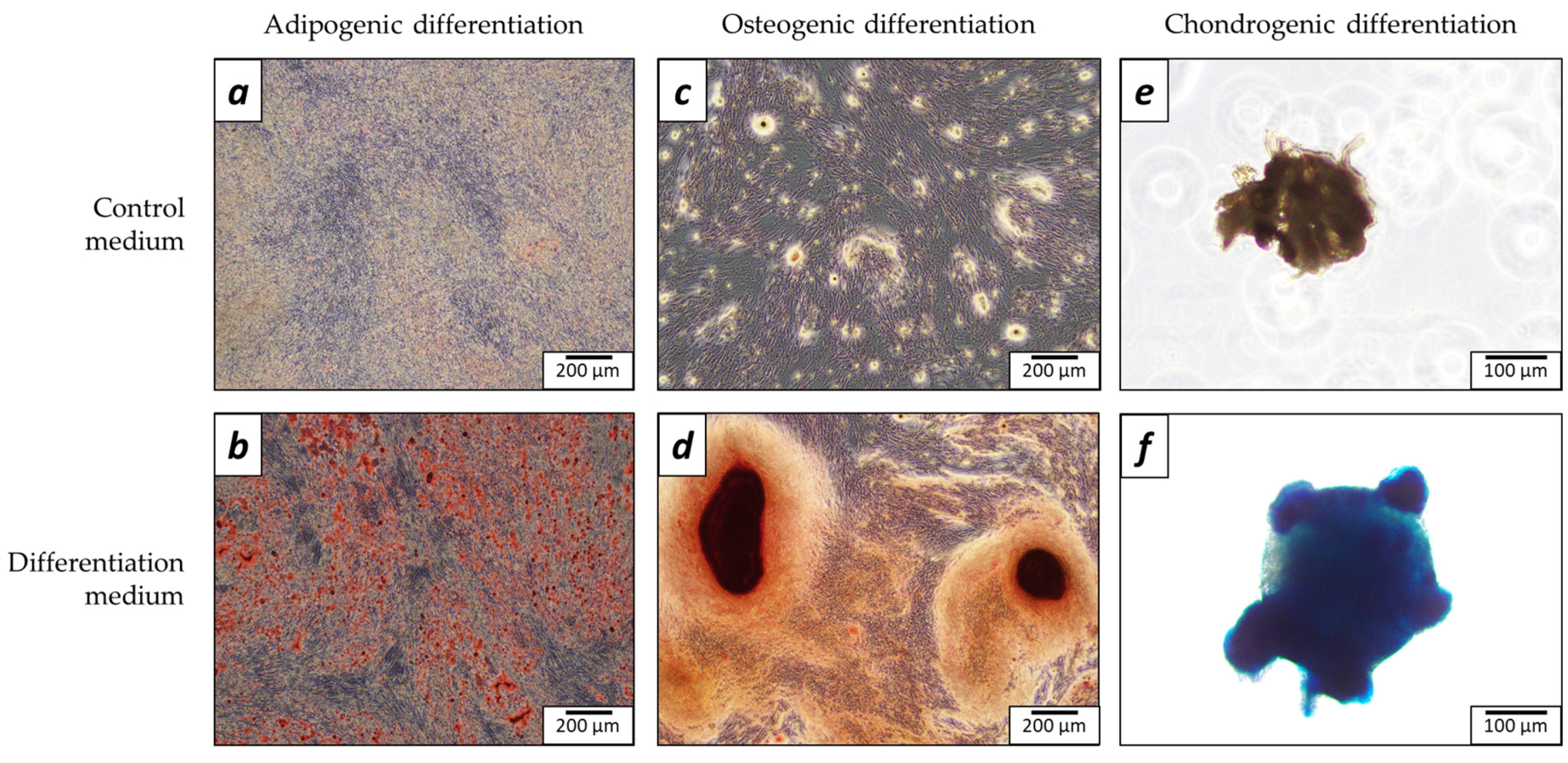
2.1. Characterization of Cells
2.2. Nanoparticle Tracking Analysis (NTA)
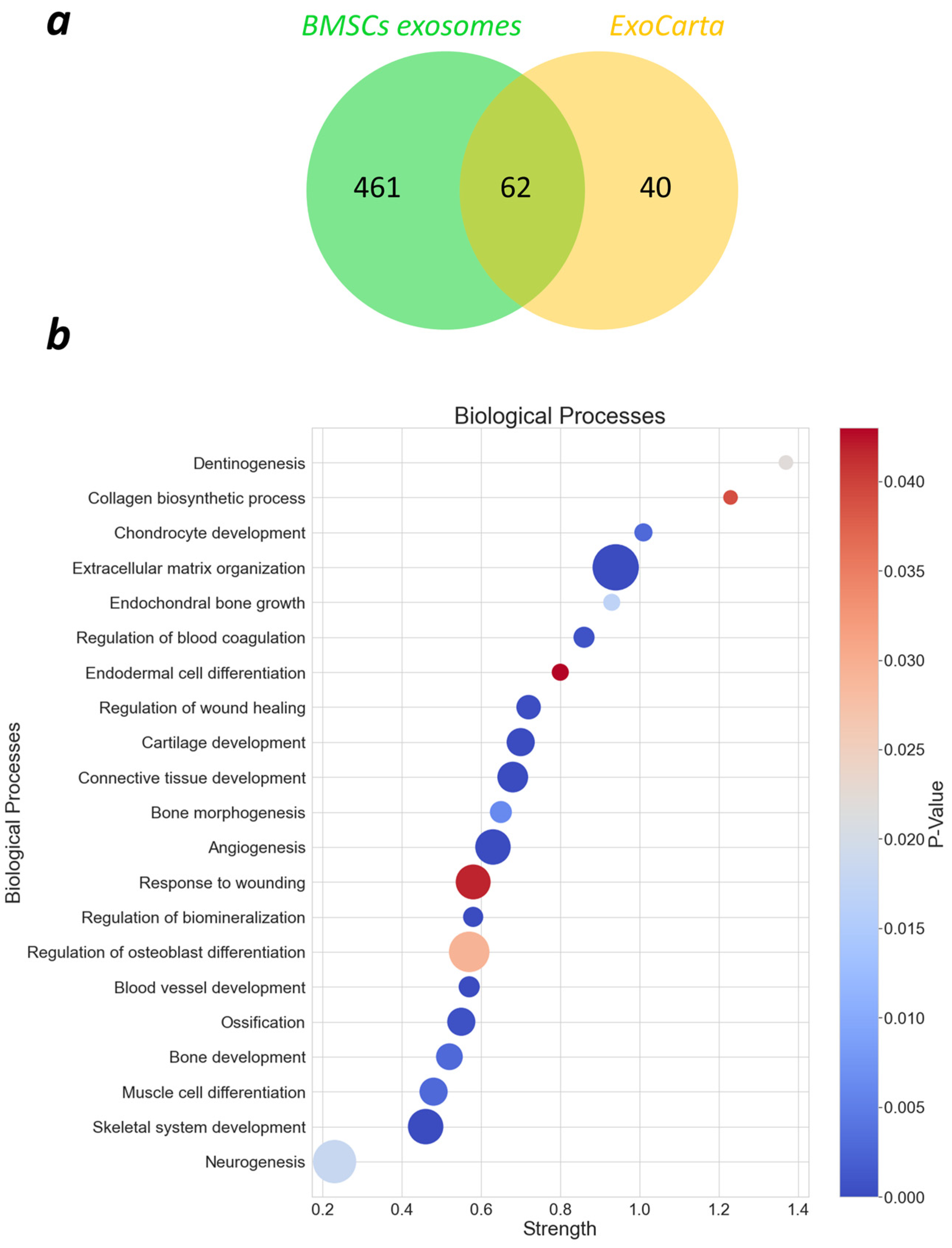
2.3. Proteomics
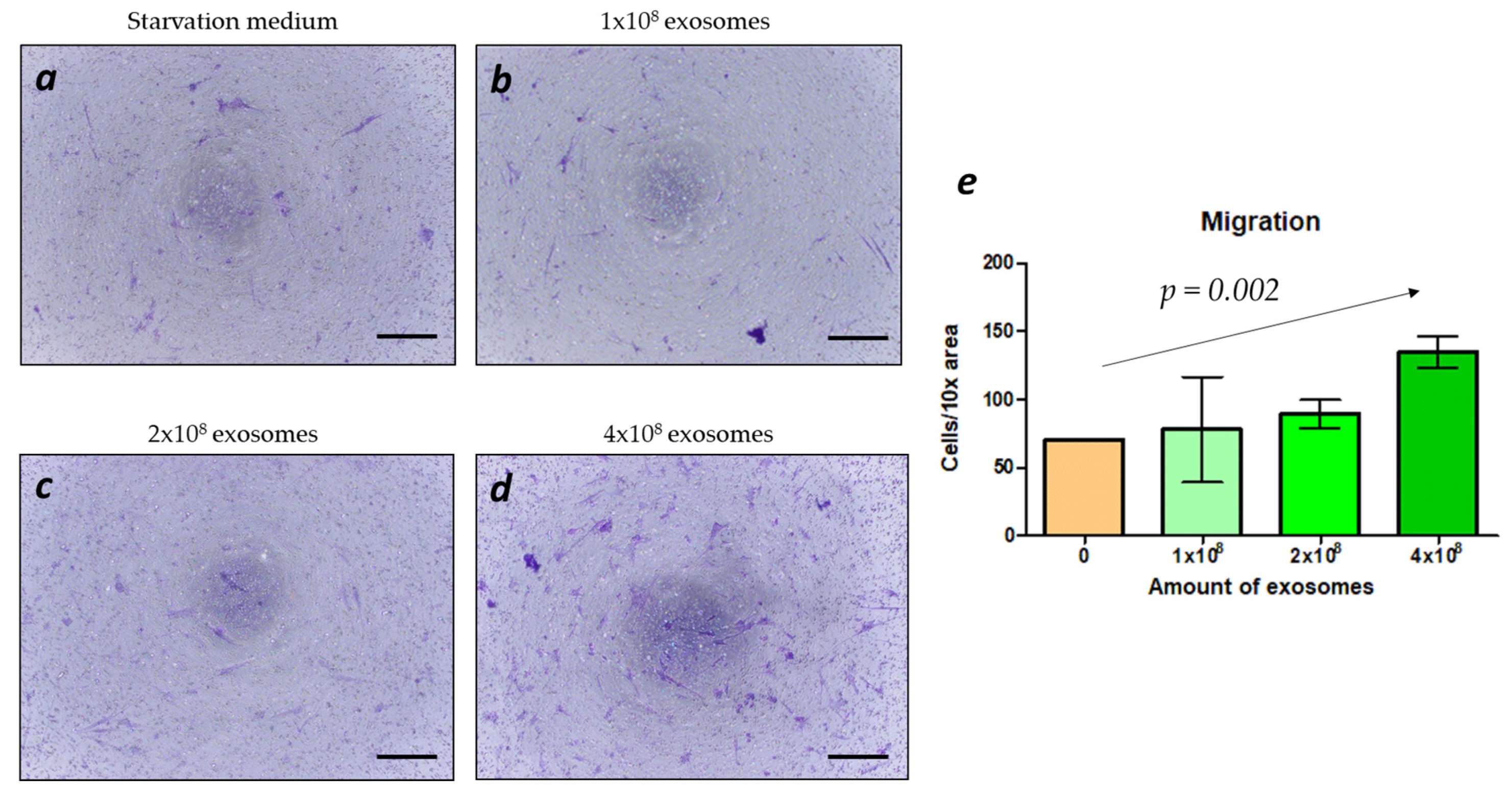
2.4. Migration
2.5. Release of Exosomes from the Scaffolds
2.6. BMSC Metabolism and Differentiation
2.7. Implantation of Scaffolds with Exosomes
3. Discussion
4. Materials and Methods
4.1. Extraction of BMSCs
4.2. Characterization of BMSCs
4.2.1. Adipogenic Differentiation
4.2.2. Osteogenic Differentiation
4.2.3. Chondrogenic Differentiation
4.3. Extraction of Exosomes
4.4. Characterization of Exosomes
4.4.1. Nanoparticle Tracking Analysis (NTA)
4.4.2. Proteomics
4.5. Migration
4.6. Scaffold Production
4.7. Culture of BMSCs with Exosomes
4.8. Release of Exosomes from the Scaffolds
4.9. Implantation of Scaffolds
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahdavi, S.; Amirsadeghi, A.; Jafari, A.; Niknezhad, S.V.; Bencherif, S.A. Avian Egg: A Multifaceted Biomaterial for Tissue Engineering. Ind. Eng. Chem. Res. 2021, 60, 17348–17364. [Google Scholar] [CrossRef]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Jafari, A.; Farahani, M.; Sedighi, M.; Rabiee, N.; Savoji, H. Carrageenans for tissue engineering and regenerative medicine applications: A review. Carbohydr. Polym. 2021, 281, 119045. [Google Scholar] [CrossRef]
- Chogan, F.; Chen, Y.; Wood, F.; Jeschke, M.G. Skin Tissue Engineering Advances in Burns: A Brief Introduction to the Past, the Present, and the Future Potential. J. Burn. Care Res. 2022, 44, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Galaxy, C. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res. 2022, 50, W345–W351. [Google Scholar]
- Ranganath, S.K.; Schlund, M.; Delattre, J.; Ferri, J.; Chai, F. Bilateral double site (calvarial and mandibular) critical-size bone defect model in rabbits for evaluation of a craniofacial tissue engineering constructs. Mater. Today Bio 2022, 14, 100267. [Google Scholar] [CrossRef]
- Trueman, R.P.; Ahlawat, A.S.; Phillips, J.B. A Shock to the (Nervous) System: Bioelectricity Within Peripheral Nerve Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 1137–1150. [Google Scholar] [CrossRef]
- Chimerad, M.; Barazesh, A.; Zandi, M.; Zarkesh, I.; Moghaddam, A.; Borjian, P.; Chimehrad, R.; Asghari, A.; Akbarnejad, Z.; Khonakdar, H.A.; et al. Tissue engineered scaffold fabrication methods for medical applications. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1455–1479. [Google Scholar] [CrossRef]
- Samat, A.A.; Hamid, Z.A.A.; Mariatti Jaafar, M.; Yahaya, B.H. Tissue Engineering for Tracheal Replacement: Strategies and Challenges. Adv. Exp. Med. Biol. 2022. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Zamboulis, A.; Koumentakou, I.; Michailidou, G.; Noordam, M.J.; Bikiaris, D.N. Biocompatible Synthetic Polymers for Tissue Engineering Purposes. Biomacromolecules 2022, 23, 1841–1863. [Google Scholar] [CrossRef]
- Alonzo, M.; Primo, F.A.; Kumar, S.A.; Mudloff, J.A.; Dominguez, E.; Fregoso, G.; Ortiz, N.; Weiss, W.M.; Joddar, B. Bone tissue engineering techniques, advances and scaffolds for treatment of bone defects. Curr. Opin. Biomed. Eng. 2021, 17, 100248. [Google Scholar] [CrossRef] [PubMed]
- Arifin, N.; Sudin, I.; Ngadiman, N.H.A.; Ishak, M.S.A. A Comprehensive Review of Biopolymer Fabrication in Additive Manufacturing Processing for 3D-Tissue-Engineering Scaffolds. Polymers 2022, 14, 2119. [Google Scholar] [CrossRef] [PubMed]
- Yeong, W.-Y.; Chua, C.-K.; Leong, K.-F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Maevskaia, E.; Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Triply Periodic Minimal Surface-Based Scaffolds for Bone Tissue Engineering: A Mechanical, In Vitro and In Vivo Study. Tissue Eng. Part A 2023, 29, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Wang, C.; Liu, J.; Chen, S.; Liu, C.; Huang, X.; Wu, J.; Pan, Y.; Xie, Y.; Wang, M. Low temperature hybrid 3D printing of hierarchically porous bone tissue engineering scaffolds within situdelivery of osteogenic peptide and mesenchymal stem cells. Biofabrication 2022, 14, 045006. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Hang, R.; Sun, R.; Ding, Y.; Yao, X.; Hang, R.; Sun, H.; Bai, L. Multifunctional exosomes derived from bone marrow stem cells for fulfilled osseointegration. Front. Chem. 2022, 10, 984131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Hu, Y.; Chen, R.; Hao, Z.; Wang, Y.; Li, J. Exosome-Hydrogel System in Bone Tissue Engineering: A Promising Therapeutic Strategy. Macromol. Biosci. 2023, 23, e2200496. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, X.; Zheng, K.; Ge, G.; Chen, X.; Xu, Y.; Bai, J.; Pan, G.; Geng, D. Horizon of exosome-mediated bone tissue regeneration: The all-rounder role in biomaterial engineering. Mater. Today Bio 2022, 16, 100355. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Hua, Y.; Mei, P.; Zeng, D.; Jiang, S.; Liao, C. Black phosphorus thermosensitive hydrogels loaded with bone marrow mesenchymal stem cell-derived exosomes synergistically promote bone tissue defect repair. J. Mater. Chem. B 2023, 11, 4396–4407. [Google Scholar] [CrossRef]
- Huber, J.L.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 101–113. [Google Scholar] [CrossRef]
- Hazrati, A.; Mirsanei, Z.; Heidari, N.; Malekpour, K.; Rahmani-Kukia, N.; Abbasi, A.; Soudi, S. The potential application of encapsulated exosomes: A new approach to increase exosomes therapeutic efficacy. Biomed. Pharmacother. 2023, 162, 114615. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wu, Y.; Huang, M.; Zhou, X.; Liu, J.; Yi, Y.; Wang, J.; Liu, J. A new frontier in temporomandibular joint osteoarthritis treatment: Exosome-based therapeutic strategy. Front. Bioeng. Biotechnol. 2022, 10, 1074536. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.B.; Zhang, Y.; Lan, B.B.; Wang, J.J.; Zhang, Z.W.; Zhang, L.L.; Xiao, P.L.; Meng, Q.Y.; Geng, Y.J.; Yu, X.Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed. Res. Int. 2017, 2017, 4150705. [Google Scholar] [CrossRef]
- Yao, Y.; Jiang, Y.; Song, J.; Wang, R.; Li, Z.; Yang, L.; Wu, W.; Zhang, L.; Peng, Q. Exosomes as Potential Functional Nanomaterials for Tissue Engineering. Adv. Healthc. Mater. 2022, 12, e2201989. [Google Scholar] [CrossRef]
- Zhai, M.; Zhu, Y.; Yang, M.; Mao, C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv. Sci. 2020, 7, 2001334. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, P.; Su, Z.; Zhang, X.; Nie, L.; Chang, Y. Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem. Biophys. Res. Commun. 2020, 531, 559–565. [Google Scholar] [CrossRef]
- Liu, H.; Gu, R.; Li, W.; Zeng, L.; Zhu, Y.; Heng, B.C.; Liu, Y.; Zhou, Y. Engineering 3D-Printed Strontium-Titanium Scaffold-Integrated Highly Bioactive Serum Exosomes for Critical Bone Defects by Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2023, 15, 27486–27501. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lin, D.; Zhao, H.; Chen, L.; Cai, B.; Lin, K.; Shen, S.G. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials 2021, 272, 120718. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, S.; Kim, M.; Jeong, Y.; Lee, S. Exosome-coated silk fibroin 3D-scaffold for inducing osteogenic differentiation of bone marrow derived mesenchymal stem cells. Chem. Eng. J. 2020, 406, 127080. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic effects of bone marrow mesenchymal stem cells-derived exosomes on osteoarthritis. J. Cell Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, A.; Wang, M.; Arshaghi, T.E.; Shaw, G.; Alves, J.; Byrne, A.; Butterworth, S.; Chandler, R.; Cuddy, L.; Dunne, J.; et al. Manufacturing Mesenchymal Stromal Cells for the Treatment of Osteoarthritis in Canine Patients: Challenges and Recommendations. Front. Veter-Sci. 2022, 9, 897150. [Google Scholar] [CrossRef]
- Wu, L.; Tian, X.; Zuo, H.; Zheng, W.; Li, X.; Yuan, M.; Tian, X.; Song, H. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia–reperfusion injury in steatotic grafts. J. Nanobiotechnol. 2022, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Chen, Y.-C.; Yu, S.-N.; Lai, W.-L.; Shen, Y.-S.; Shen, P.-C.; Lin, S.-H.; Chang, C.-H.; Lee, S.-M. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: A first-in-human study with evaluation of the potency marker. Cytotherapy 2021, 24, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Orfei, C.P.; Kouroupis, D.; Ragni, E.; De Luca, P.; Viganò, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Li, G. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef]
- Heyman, E.; Meeremans, M.; Devriendt, B.; Olenic, M.; Chiers, K.; De Schauwer, C. Validation of a color deconvolution method to quantify MSC tri-lineage differentiation across species. Front. Veter-Sci. 2022, 9, 987045. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.C.; Phinney, D.G.; Lacey, M.R.; Barrilleaux, B.L.; Meyertholen, K.E.; O’Connor, K.C. In Vitro High-Capacity Assay to Quantify the Clonal Heterogeneity in Trilineage Potential of Mesenchymal Stem Cells Reveals a Complex Hierarchy of Lineage Commitment. Stem Cells 2010, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2022, 119, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Kastelowitz, N.; Yin, H. Exosomes and Microvesicles: Identification and Targeting by Particle Size and Lipid Chemical Probes. ChemBioChem 2014, 15, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Cox, A.; Rodriguez-Menocal, L.; Salgado, M.; Van Badiavas, E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015, 24, 1635–1647. [Google Scholar] [CrossRef]
- Gu, H.; Ji, R.; Zhang, X.; Wang, M.; Zhu, W.; Qian, H.; Chen, Y.; Jiang, P.; Xu, W. Exosomes derived from human mesenchymal stem cells promote gastric cancer cell growth and migration via the activation of the Akt pathway. Mol. Med. Rep. 2016, 14, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part A 2021, 27, 962–976. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Ghayor, C.; Siegenthaler, B.; Schuler, F.; Rüegg, J.; De Wild, M.; Weber, F.E. Lattice Microarchitecture for Bone Tissue Engineering from Calcium Phosphate Compared to Titanium. Tissue Eng. Part A 2018, 24, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Mohamed-Ahmed, S.; Shanbhag, S.; Kampleitner, C.; Elnour, R.; Yamada, S.; Rana, N.; Birkeland, E.; Tangl, S.; Gruber, R.; et al. Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model. Stem Cell Res. Ther. 2024, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Zhao, F.; Cao, C.; Wu, T.; Fan, Y.; Ao, Y.; Hu, X. 3D Printing of Microenvironment-Specific Bioinspired and Exosome-Reinforced Hydrogel Scaffolds for Efficient Cartilage and Subchondral Bone Regeneration. Adv. Sci. 2023, 10, e2303650. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.S.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal Stem Cell Exosomes for Cartilage Regeneration: A Systematic Review of Preclinical In Vivo Studies. Tissue Eng. Part B Rev. 2021, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stokovic, N.; Ivanjko, N.; Maticic, D.; Luyten, F.P.; Vukicevic, S. Bone Morphogenetic Proteins, Carriers, and Animal Models in the Development of Novel Bone Regenerative Therapies. Materials 2021, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Ivica, A.; Zehnder, M.; E Weber, F. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in regenerative endodontics. Eur. Cells Mater. 2021, 41, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Pishavar, E.; Luo, H.; Naserifar, M.; Hashemi, M.; Toosi, S.; Atala, A.; Ramakrishna, S.; Behravan, J. Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 6203. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, M.N.-K.; Sadroddiny, E.; Ai, J.; Balalaie, S.; Asgari, Y. Targeted expression of a designed fusion protein containing BMP2 into the lumen of exosomes. Biochim. Biophys. Acta (BBA) Gen. Subj. 2024, 1868, 130505. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Zhang, J.; Chiang, C.; Pan, J.; Wang, X.; Kwak, K.J.; Li, H.; Zhao, R.; Rima, X.Y.; et al. Exosomal mRNAs for Angiogenic–Osteogenic Coupled Bone Repair. Adv. Sci. 2023, 10, e2302622. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, H.; Han, H.; Song, S.; Zhang, X.; Ouyang, L.; Qian, C.; Hong, Y.; Qiu, Y.; Zhou, W.; et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene 2018, 37, 5508–5519. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, F.; Hu, Y.; Luo, Y.; Wei, Y.; Xu, K.; Zhang, H.; Liu, H.; Bo, L.; Lv, S.; et al. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep. Med. 2023, 4, 100881. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Li, F.-X.; Liu, Y.-W.; Rao, S.-S.; Yin, H.; Huang, J.; Chen, C.-Y.; Hu, Y.; Zhang, Y.; Tan, Y.-J.; et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale 2019, 11, 20884–20892. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Bhattacharya, I.; Guerrero, J.; Özcan, M.; Weber, F.E. 3D-Printed HA-Based Scaffolds for Bone Regeneration: Microporosity, Osteoconduction and Osteoclastic Resorption. Materials 2022, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Chen, T.-H.; Bhattacharya, I.; Özcan, M.; Weber, F.E. Microporosities in 3D-Printed Tricalcium-Phosphate-Based Bone Substitutes Enhance Osteoconduction and Affect Osteoclastic Resorption. Int. J. Mol. Sci. 2020, 21, 9270. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, M.C.; Malpasso, G.; Musarò, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol. Biol. 2016, 1416, 149–158. [Google Scholar] [CrossRef]
- Croft, A.S.; Guerrero, J.; Oswald, K.A.C.; Häckel, S.; Albers, C.E.; Gantenbein, B. Effect of different cryopreservation media on human nucleus pulposus cells’ viability and trilineage potential. JOR Spine 2021, 4, e1140. [Google Scholar] [CrossRef]
- Guerrero, J.; Häckel, S.; Croft, A.S.; Albers, C.E.; Gantenbein, B. The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency. JOR Spine 2020, 4, e1131. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.E.; Ku, H.-C.; Dluzen, D.F.; Xing, C.; Zhou, Z. A nonparametric alternative to the Cochran-Armitage trend test in genetic case-control association studies: The Jonckheere-Terpstra trend test. PLoS ONE 2023, 18, e0280809. [Google Scholar] [CrossRef] [PubMed]

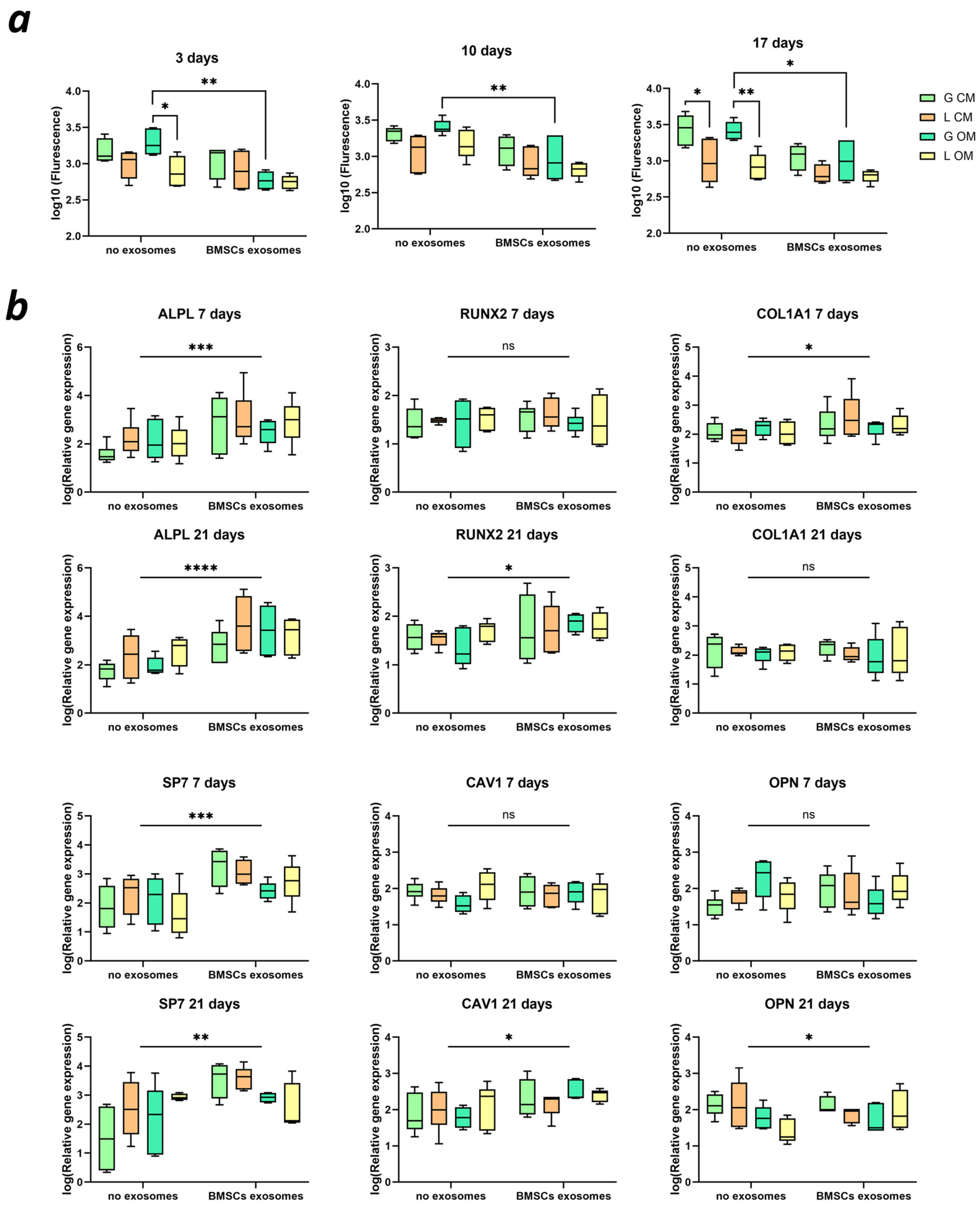

| Source of Variation | 3 Days | 10 Days | 17 Days |
|---|---|---|---|
| Exosomes | *** | **** | *** |
| Scaffold | ** | ** | **** |
| Medium | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maevskaia, E.; Guerrero, J.; Ghayor, C.; Bhattacharya, I.; Weber, F.E. Functionalization of Ceramic Scaffolds with Exosomes from Bone Marrow Mesenchymal Stromal Cells for Bone Tissue Engineering. Int. J. Mol. Sci. 2024, 25, 3826. https://doi.org/10.3390/ijms25073826
Maevskaia E, Guerrero J, Ghayor C, Bhattacharya I, Weber FE. Functionalization of Ceramic Scaffolds with Exosomes from Bone Marrow Mesenchymal Stromal Cells for Bone Tissue Engineering. International Journal of Molecular Sciences. 2024; 25(7):3826. https://doi.org/10.3390/ijms25073826
Chicago/Turabian StyleMaevskaia, Ekaterina, Julien Guerrero, Chafik Ghayor, Indranil Bhattacharya, and Franz E. Weber. 2024. "Functionalization of Ceramic Scaffolds with Exosomes from Bone Marrow Mesenchymal Stromal Cells for Bone Tissue Engineering" International Journal of Molecular Sciences 25, no. 7: 3826. https://doi.org/10.3390/ijms25073826
APA StyleMaevskaia, E., Guerrero, J., Ghayor, C., Bhattacharya, I., & Weber, F. E. (2024). Functionalization of Ceramic Scaffolds with Exosomes from Bone Marrow Mesenchymal Stromal Cells for Bone Tissue Engineering. International Journal of Molecular Sciences, 25(7), 3826. https://doi.org/10.3390/ijms25073826







