Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells
Abstract
1. Introduction
2. Results
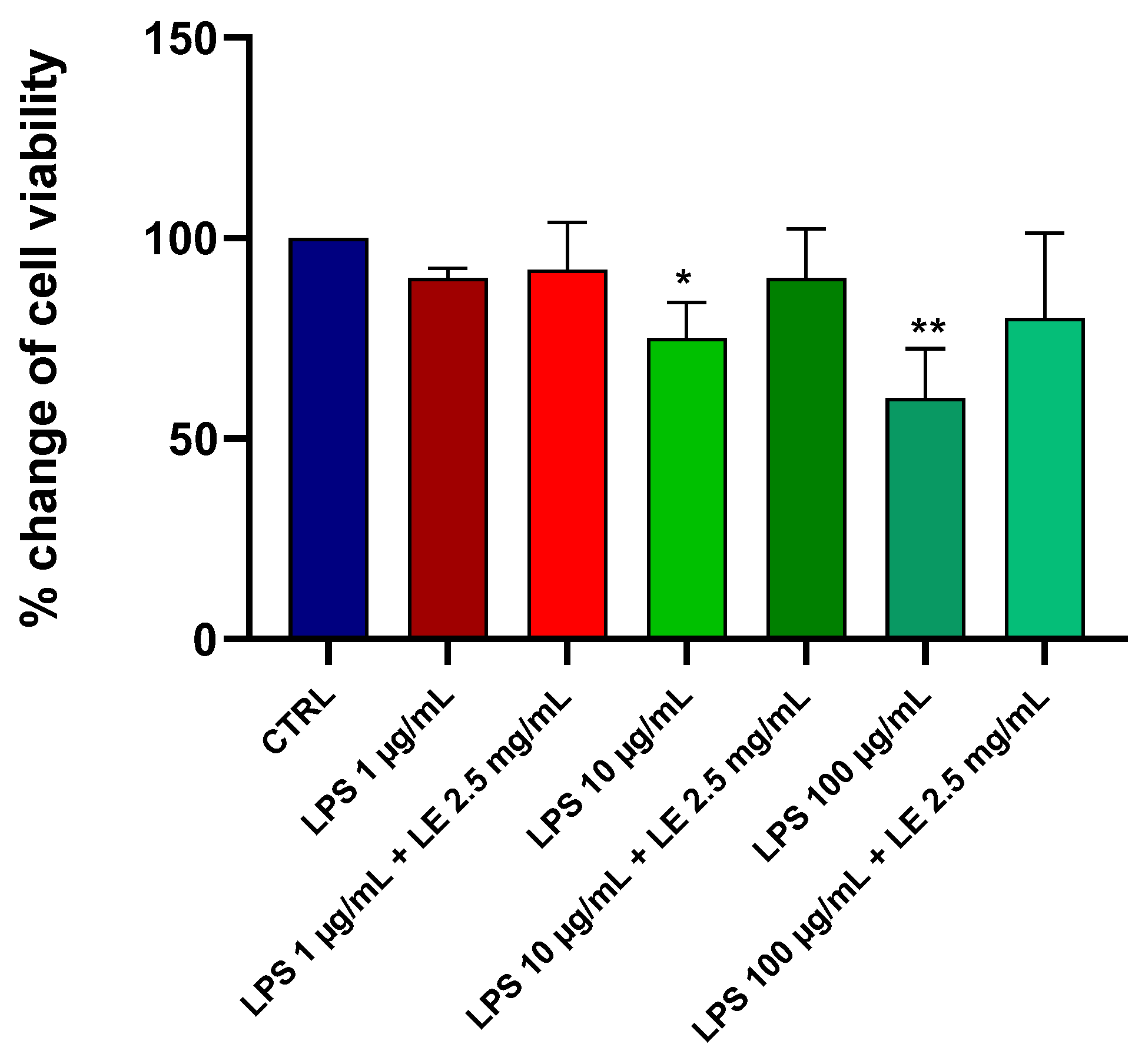
2.1. Effects of LE on the Viability of Caco-2 Cells under Different LPS Treatments
2.2. Preliminary Data and Choice of Concentration of LE and LPS
2.3. Effects of LE on TEER in LPS-Induced Intestinal Epithelial Barrier Dysfunction
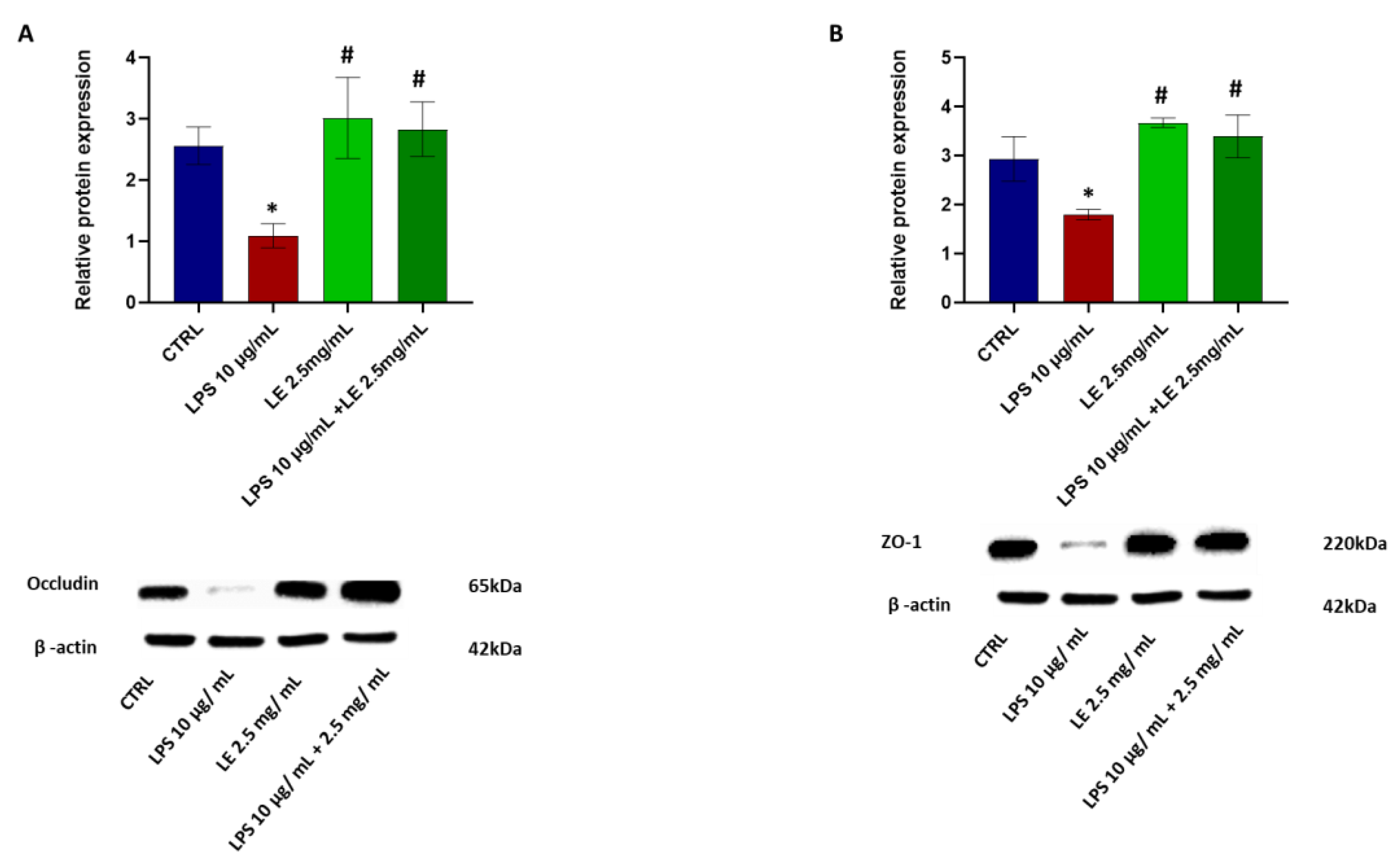
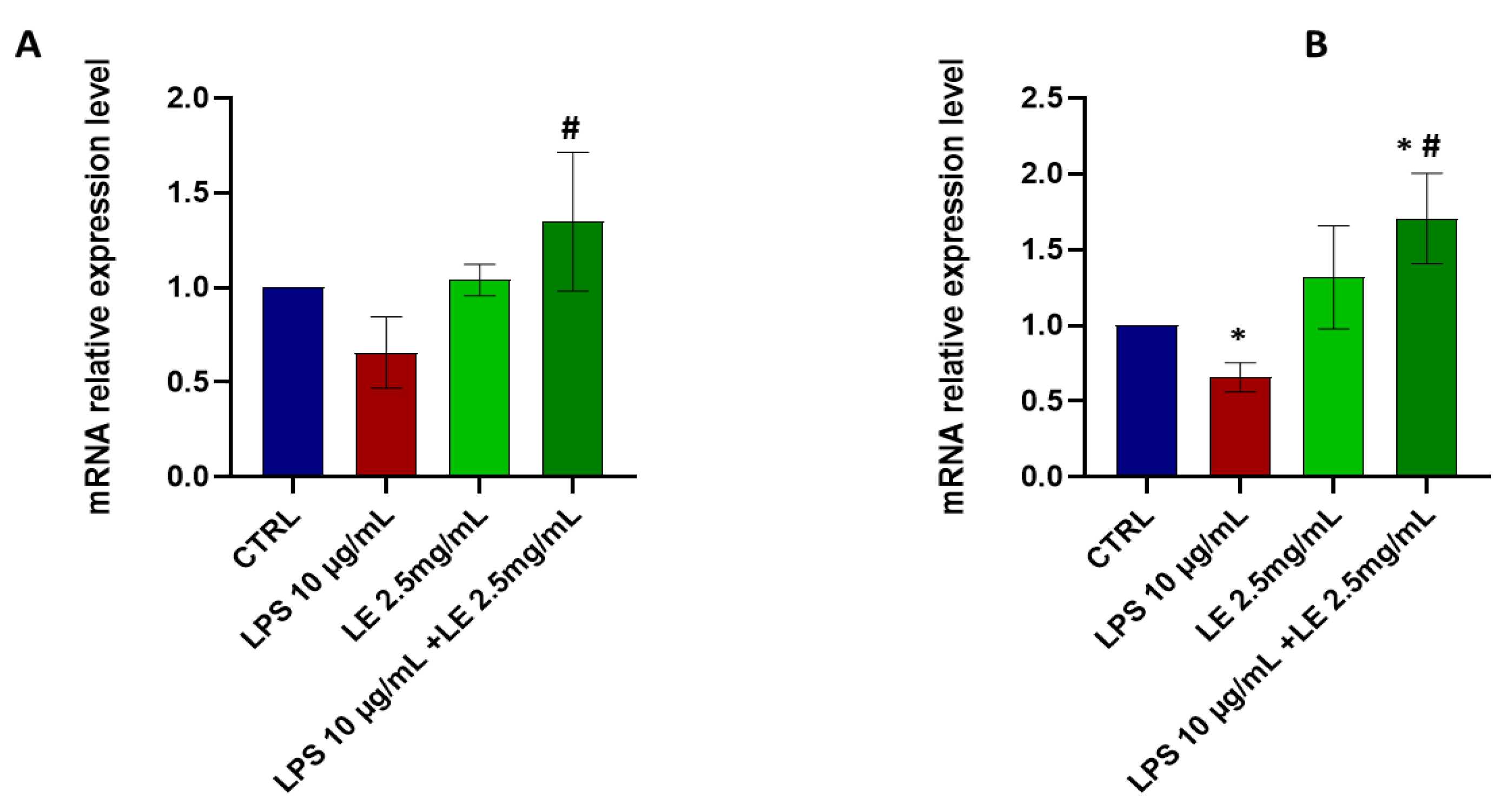
2.4. The Impact of LEon the Expression of TJ Proteins during LPS-Induced Intestinal Epithelial Barrier Dysfunction
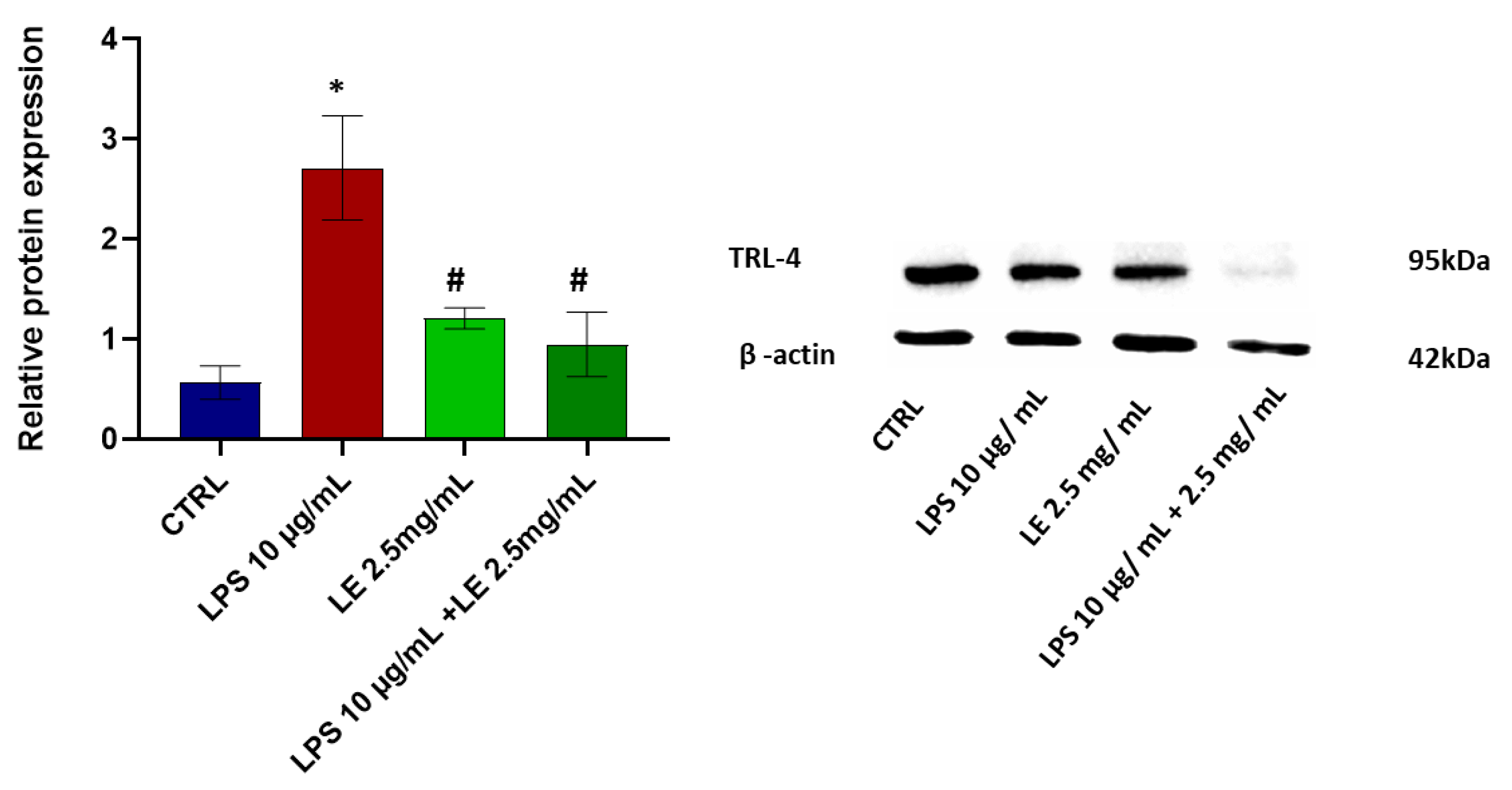
2.5. LE Treatment Effect on TLR-4 Modulation
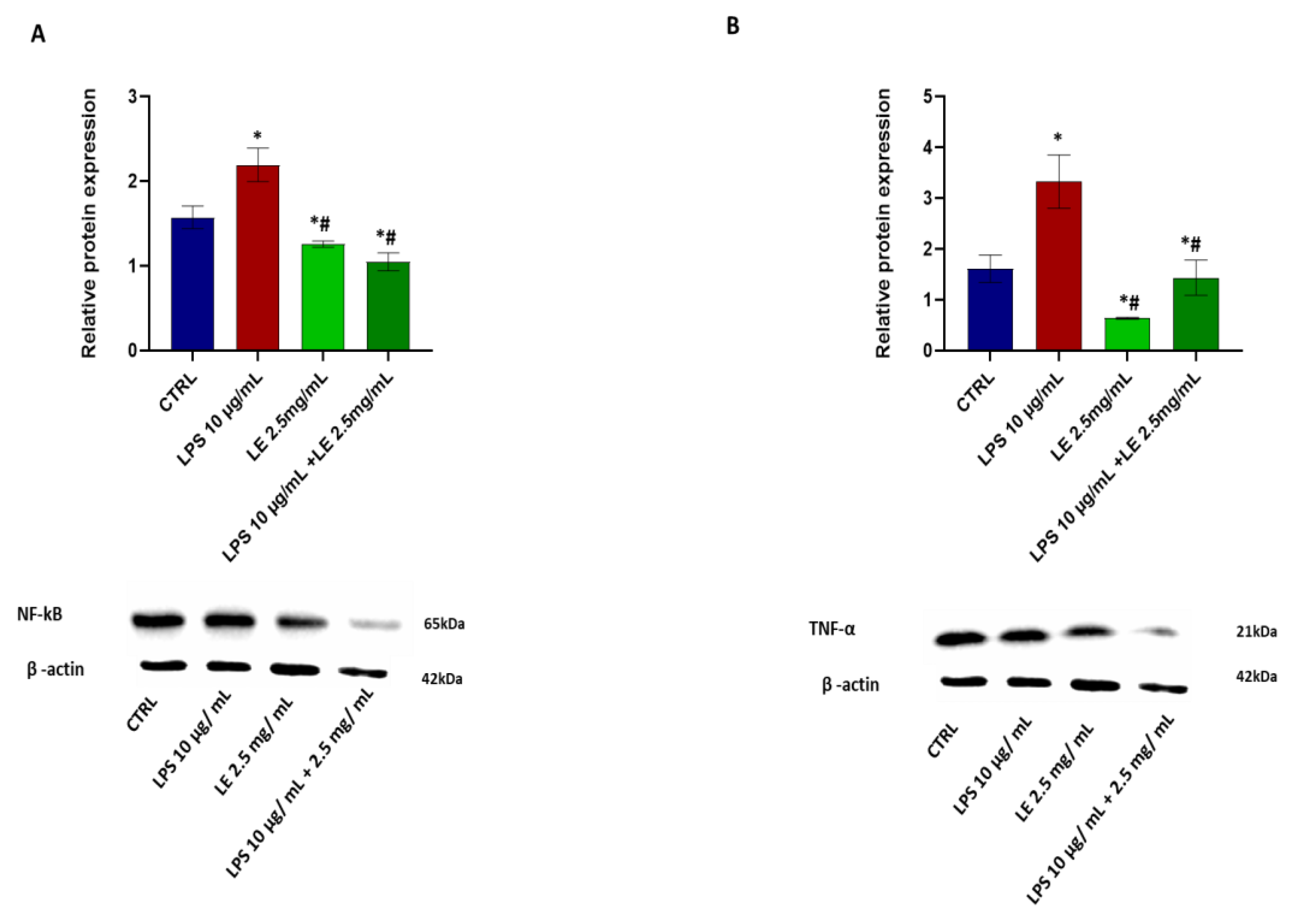
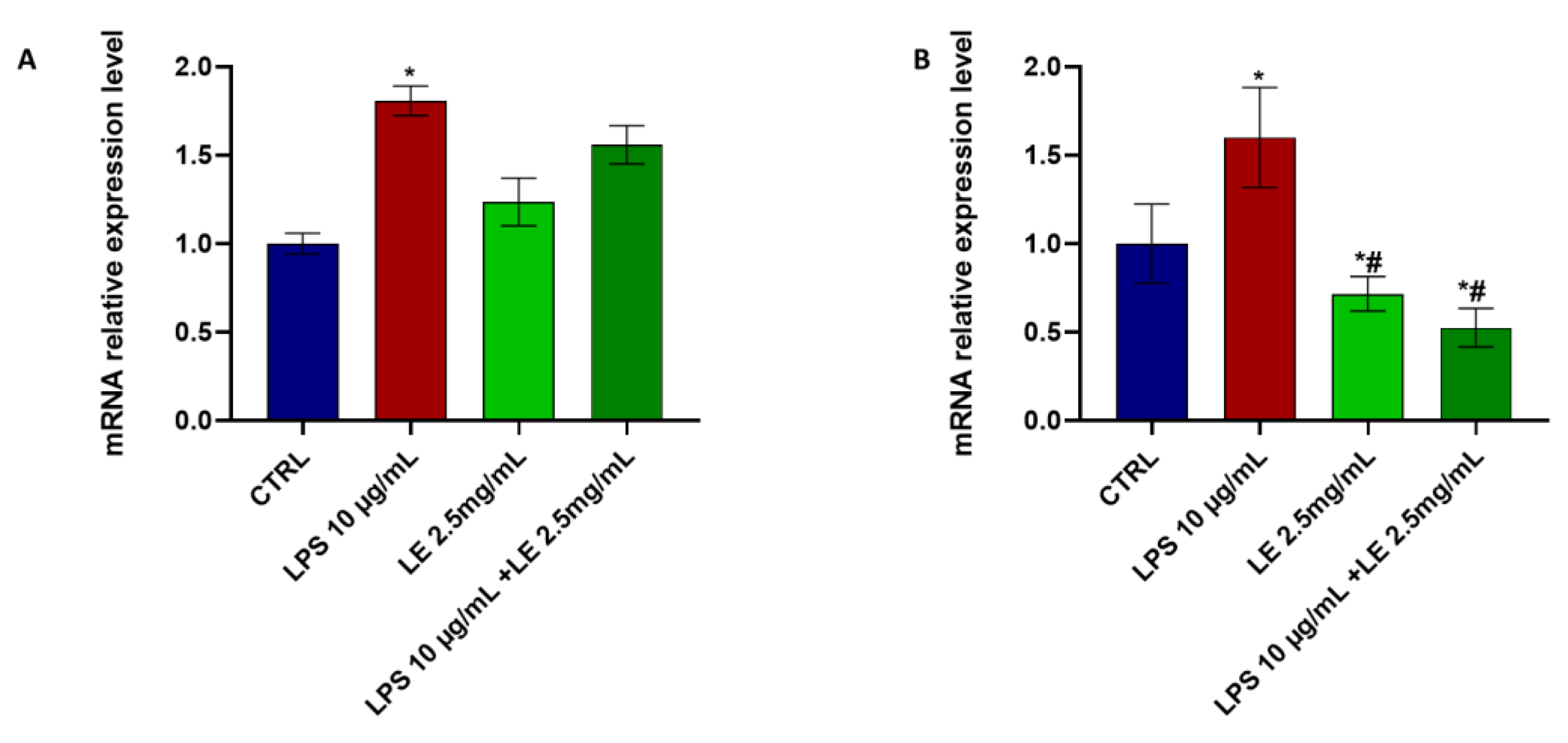
2.6. Effects of LE Treatment on LPS-Induced Inflammation
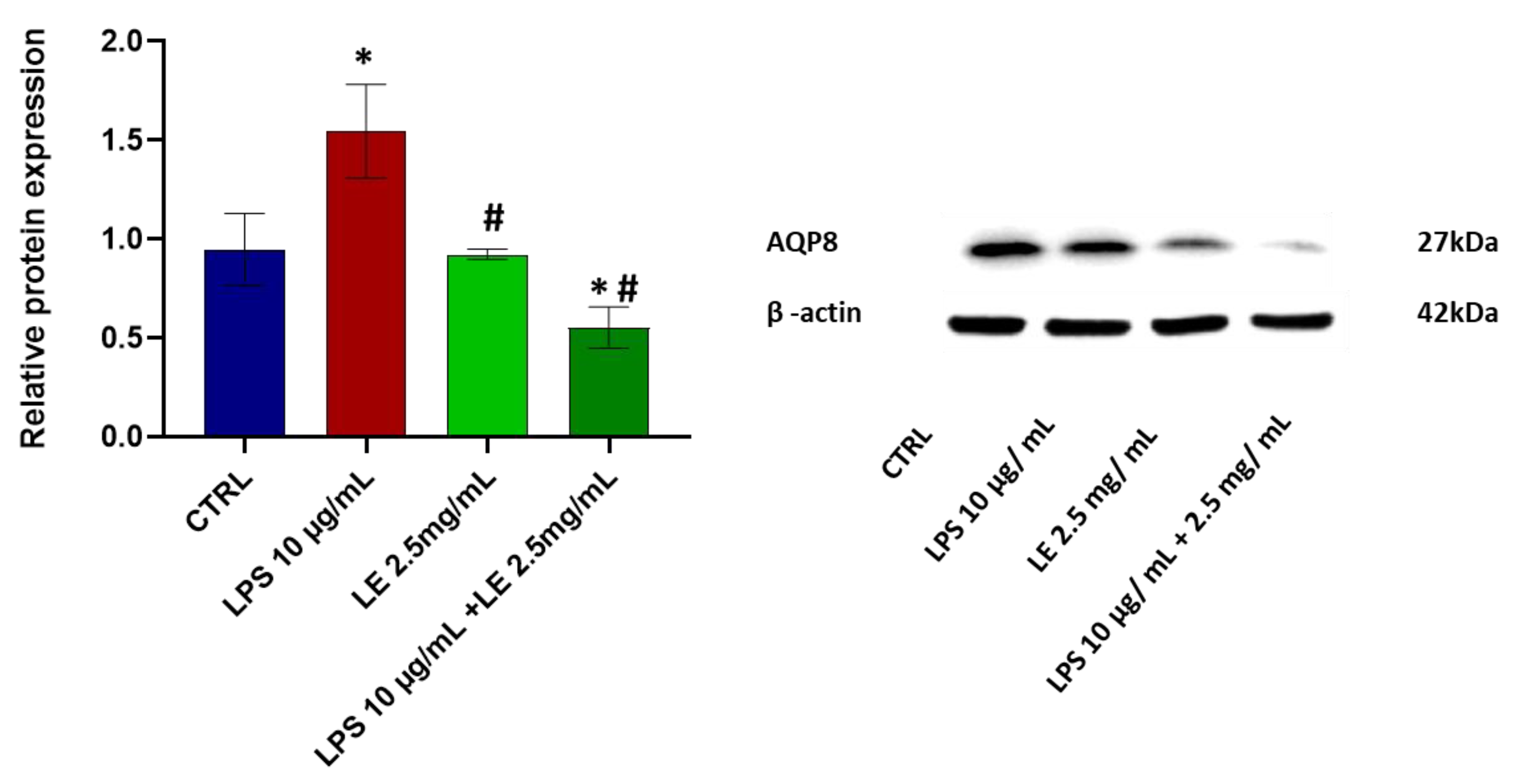
2.7. Effects of LE Treatment on the Protein Expression of AQP8
2.8. LE Treatment Modulates TRPV1 and TRPA1 mRNA Expression in Caco-2 Cells
2.9. Ion Channel Currents in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Line and Culture Conditions
4.3. Viability Assay on Caco-2
4.4. Measurement of Trans-Epithelial Electrical Resistance (TEER)
4.5. Western Blotting Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Patch-Clamp Experiments
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, K.E. New ways of thinking about (and teaching about) intestinal epithelial function. Adv. Physiol. Educ. 2008, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Runkle, E.A.; Mu, D. Tight junction proteins: From barrier to tumorigenesis. Cancer Lett. 2013, 337, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hodges, K.; Gill, R. Infectious diarrhea: Cellular and molecular mechanisms. Gut Microbes 2010, 1, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Maqoud, F.; Tricarico, D.; Mallamaci, R.; Orlando, A.; Russo, F. The Role of Ion Channels in Functional Gastrointestinal Disorders (FGID): Evidence of Channelopathies and Potential Avenues for Future Research and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 11074. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Kon, R.; Sugiyama, K. Aquaporins in the Colon as a New Therapeutic Target in Diarrhea and Constipation. Int. J. Mol. Sci. 2016, 17, 1172. [Google Scholar] [CrossRef] [PubMed]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt inhibits intestinal barrier dysfunction in Caco-2 cells by increasing tight junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Holst, O.; Ulmer, A.J.; Brade, H.; Flad, H.-D.; Rietschel, E.T. Biochemistry and cell biology of bacterial endotoxins. FEMS Immunol. Med. Microbiol. 1996, 16, 83–104. [Google Scholar] [CrossRef]
- Straub, R.H. TRPV1, TRPA1, and TRPM8 channels in inflammation, energy redirection, and water retention: Role in chronic inflammatory diseases with an evolutionary perspective. J. Mol. Med. 2014, 92, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Kanda, Y.; Yamasaki, Y.; Sasaki-Yamaguchi, Y.; Ida-Koga, N.; Kamisuki, S.; Sugawara, F.; Nagumo, Y.; Usui, T. TRPA1-dependent reversible opening of tight junction by natural compounds with an α,β-unsaturated moiety and capsaicin. Sci. Rep. 2018, 8, 2251. [Google Scholar] [CrossRef]
- Noda, S.; Tanabe, S.; Suzuki, T. Naringenin enhances intestinal barrier function through the expression and cytoskeletal association of tight junction proteins in Caco-2 cells. Mol. Nutr. Food Res. 2013, 57, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef]
- Toschi, A.; Rossi, B.; Tugnoli, B.; Piva, A.; Grilli, E. Nature-Identical Compounds and Organic Acids Ameliorate and Prevent the Damages Induced by an Inflammatory Challenge in Caco-2 Cell Culture. Molecules 2020, 25, 4296. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, S.; Toschi, A.; Yu, L.-e.; Schlitzkus, L.; Mann, P.; Grilli, E.; Li, Y. Effects of microencapsulated blend of organic acids and botanicals on growth performance, intestinal barrier function, inflammatory cytokines, and endocannabinoid system gene expression in broiler chickens. Poult. Sci. 2023, 102, 102460. [Google Scholar] [CrossRef] [PubMed]
- Di Turi, A.; Antonacci, M.; Dibenedetto, J.R.; Maqoud, F.; Leonetti, F.; Centoducati, G.; Colonna, N.; Tricarico, D. Molecular Composition and Biological Activity of a Novel Acetonitrile–Water Extract of Lens Culinaris Medik in Murine Native Cells and Cell Lines Exposed to Different Chemotherapeutics Using Mass Spectrometry. Cells 2023, 12, 575. [Google Scholar] [CrossRef]
- Antonacci, M.; Dibenedetto, J.R.; Maqoud, F.; Centoducati, G.; Colonna, N.; Leonetti, F.; Tricarico, D. Counteractions of a Novel Hydroalcoholic Extract from Lens Culinaria against the Dexamethasone-Induced Osteoblast Loss of Native Murine Cells. Cells 2022, 11, 2936. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.; Fioramonti, J. Protease-activated receptor 2 and gut permeability: A review. Neurogastroenterol. Motil. 2008, 20, 580–587. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.-R.; Zhang, Q.; Zhao, X.-H.; Wang, L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem. Toxicol. 2021, 147, 111896. [Google Scholar] [CrossRef] [PubMed]
- Felix, K.; Tobias, S.; Jan, H.; Nicolas, S.; Michael, M. Measurements of transepithelial electrical resistance (TEER) are affected by junctional length in immature epithelial monolayers. Histochem. Cell Biol. 2021, 156, 609–616. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Kim, H.S.; Kwon, T.H.; Palikhe, A.; Zaw, T.S.; Jeong, J.H.; Sohn, U.D. Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 43–50. [Google Scholar] [CrossRef]
- Amasheh, M.; Schlichter, S.; Amasheh, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Quercetin Enhances Epithelial Barrier Function and Increases Claudin-4 Expression in Caco-2 Cells. J. Nutr. 2008, 138, 1067–1073. [Google Scholar] [CrossRef]
- DiGuilio, K.M.; Rybakovsky, E.; Valenzano, M.C.; Nguyen, H.H.; Del Rio, E.A.; Newberry, E.; Spadea, R.; Mullin, J.M. Quercetin improves and protects Calu-3 airway epithelial barrier function. Front. Cell Dev. Biol. 2023, 11, 1271201. [Google Scholar] [CrossRef]
- Junyuan, Z.; Hui, X.; Chunlan, H.; Junjie, F.; Qixiang, M.; Yingying, L.; Lihong, L.; Xingpeng, W.; Yue, Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef]
- Li, J.; Tian, R.; Liang, G.; Shi, R.; Hu, J.; Jiang, Z. Interaction mechanism of flavonoids with whey protein isolate: A spectrofluorometric and theoretical investigation. Food Chem. 2021, 355, 129617. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tu, L.; Pan, D.; Gao, X.; Du, L.; Cai, Z.; Wu, J.; Dang, Y. A Comparative Study of Binding Interactions between Proteins and Flavonoids in Angelica Keiskei: Stability, α-Glucosidase Inhibition and Interaction Mechanisms. Int. J. Mol. Sci. 2023, 24, 6582. [Google Scholar] [CrossRef]
- Tunisi, L.; Forte, N.; Fernández-Rilo, A.C.; Mavaro, I.; Capasso, R.; D’Angelo, L.; Milić, N.; Cristino, L.; Di Marzo, V.; Palomba, L. Orexin-A Prevents Lipopolysaccharide-Induced Neuroinflammation at the Level of the Intestinal Barrier. Front. Endocrinol. 2019, 10, 219. [Google Scholar] [CrossRef]
- He, C.; Deng, J.; Hu, X.; Zhou, S.; Wu, J.; Xiao, D.; Darko, K.O.; Huang, Y.; Tao, T.; Peng, M.; et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019, 10, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. (Landmark Ed.) 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Jiang, Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int. J. Mol. Sci. 2016, 17, 1399. [Google Scholar] [CrossRef] [PubMed]
- Krüger, C.; Jörns, A.; Kaynert, J.; Waldeck-Weiermair, M.; Michel, T.; Elsner, M.; Lenzen, S. The importance of aquaporin-8 for cytokine-mediated toxicity in rat insulin-producing cells. Free Radic. Biol. Med. 2021, 174, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure–function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; Zizzo, N.; Passantino, G.; Mele, A.; Camerino, G.M.; McClenaghan, C.; Harter, T.M.; Nichols, C.G.; Tricarico, D. Consequences of SUR2[A478V] Mutation in Skeletal Muscle of Murine Model of Cantu Syndrome. Cells 2021, 10, 1791. [Google Scholar] [CrossRef]
- Scala, R.; Maqoud, F.; Antonacci, M.; Dibenedetto, J.R.; Perrone, M.G.; Scilimati, A.; Castillo, K.; Latorre, R.; Conte, D.; Bendahhou, S.; et al. Bisphosphonates Targeting Ion Channels and Musculoskeletal Effects. Front. Pharmacol. 2022, 13, 837534. [Google Scholar] [CrossRef]
- Conte, E.; Romano, A.; De Bellis, M.; de Ceglia, M.; Carratù, M.R.; Gaetani, S.; Maqoud, F.; Tricarico, D.; Camerino, C. Oxtr/TRPV1 expression and acclimation of skeletal muscle to cold-stress in male mice. J. Endocrinol. 2021, 249, 135–148. [Google Scholar] [CrossRef]
- Maqoud, F.; Scala, R.; Tragni, V.; Pierri, C.L.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K+ Channels: Role in the Adverse Drug Reactions. Pharmaceutics 2021, 13, 1350. [Google Scholar] [CrossRef]
- Maqoud, F.; Cetrone, M.; Mele, A.; Tricarico, D. Molecular structure and function of big calcium-activated potassium channels in skeletal muscle: Pharmacological perspectives. Physiol. Genom. 2017, 49, 306–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maqoud, F.; Vacca, E.; Tommaseo-Ponzetta, M. From Morocco to Italy: How Women’s Bodies Reflect their Change of Residence. Coll. Antropol. 2016, 40, 9–15. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqoud, F.; Orlando, A.; Tricarico, D.; Antonacci, M.; Di Turi, A.; Giannelli, G.; Russo, F. Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells. Int. J. Mol. Sci. 2024, 25, 3802. https://doi.org/10.3390/ijms25073802
Maqoud F, Orlando A, Tricarico D, Antonacci M, Di Turi A, Giannelli G, Russo F. Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells. International Journal of Molecular Sciences. 2024; 25(7):3802. https://doi.org/10.3390/ijms25073802
Chicago/Turabian StyleMaqoud, Fatima, Antonella Orlando, Domenico Tricarico, Marina Antonacci, Annamaria Di Turi, Gianluigi Giannelli, and Francesco Russo. 2024. "Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells" International Journal of Molecular Sciences 25, no. 7: 3802. https://doi.org/10.3390/ijms25073802
APA StyleMaqoud, F., Orlando, A., Tricarico, D., Antonacci, M., Di Turi, A., Giannelli, G., & Russo, F. (2024). Anti-Inflammatory Effects of a Novel Acetonitrile–Water Extract of Lens Culinaris against LPS-Induced Damage in Caco-2 Cells. International Journal of Molecular Sciences, 25(7), 3802. https://doi.org/10.3390/ijms25073802








