Pelvic Organ Prolapse Reconstruction with the Chitosan-Based Novel Haemostatic Agent in Ovine Model—Preliminary Report
Abstract
1. Introduction
2. Results
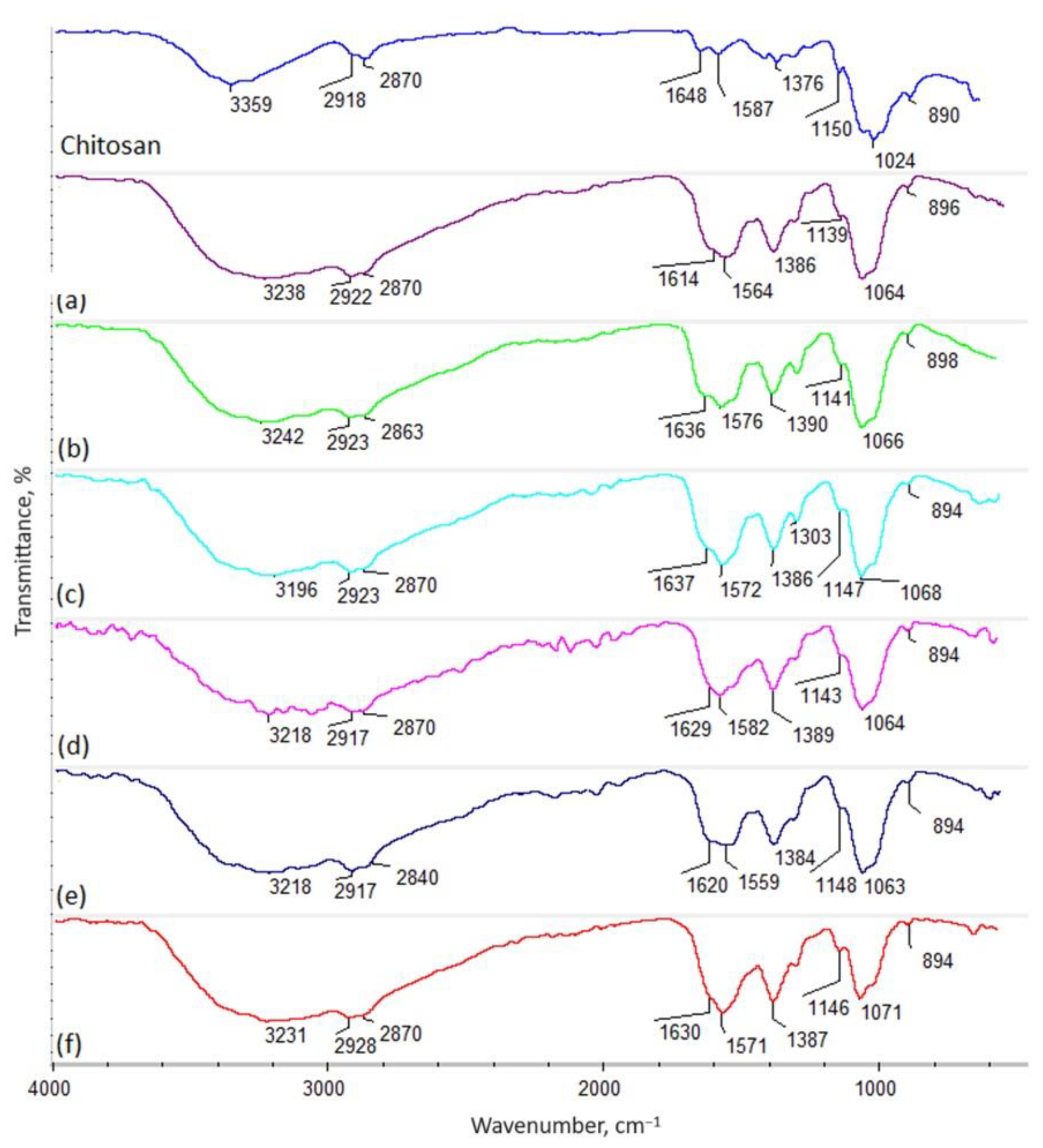
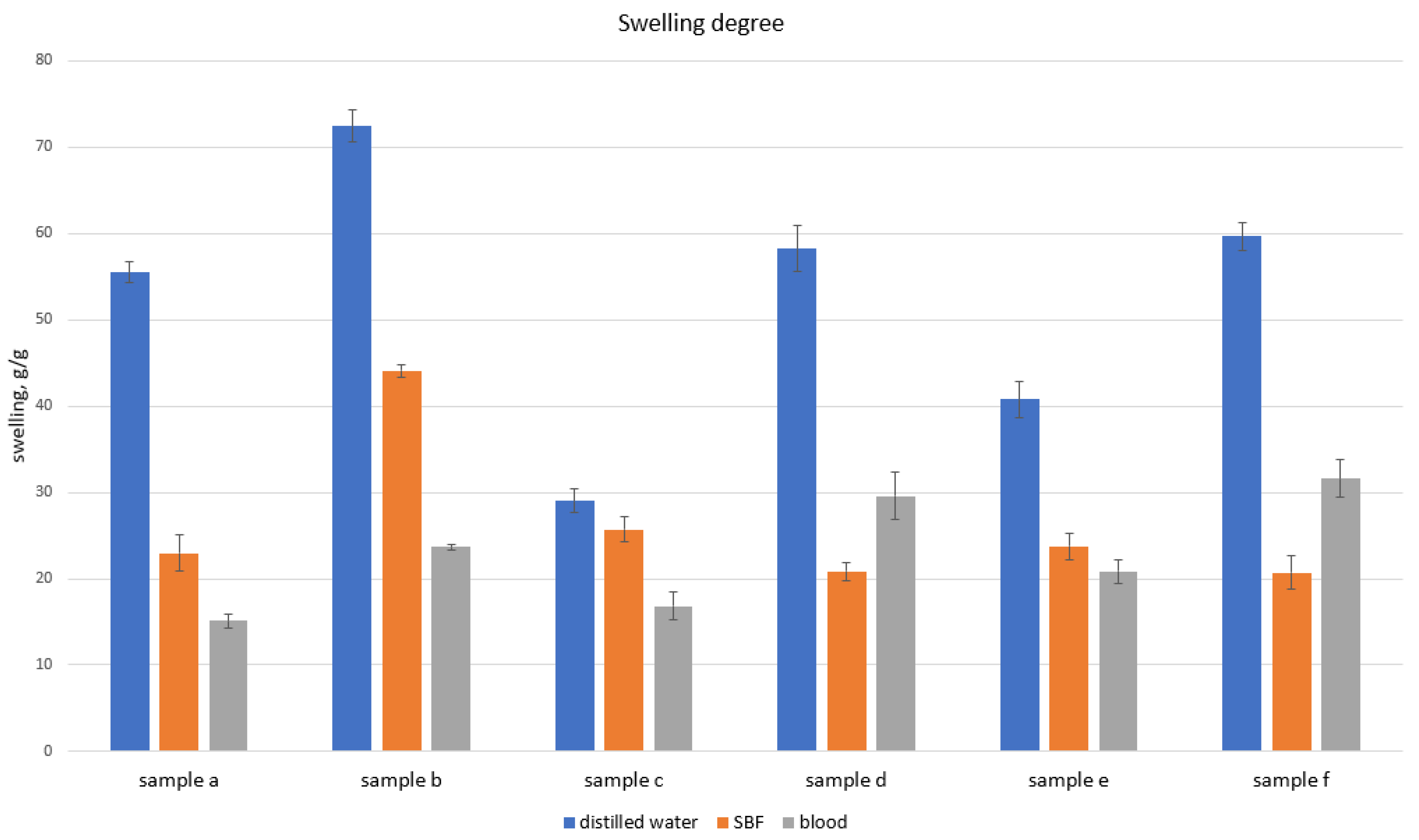
2.1. Chitosan Biomaterials: Characterization
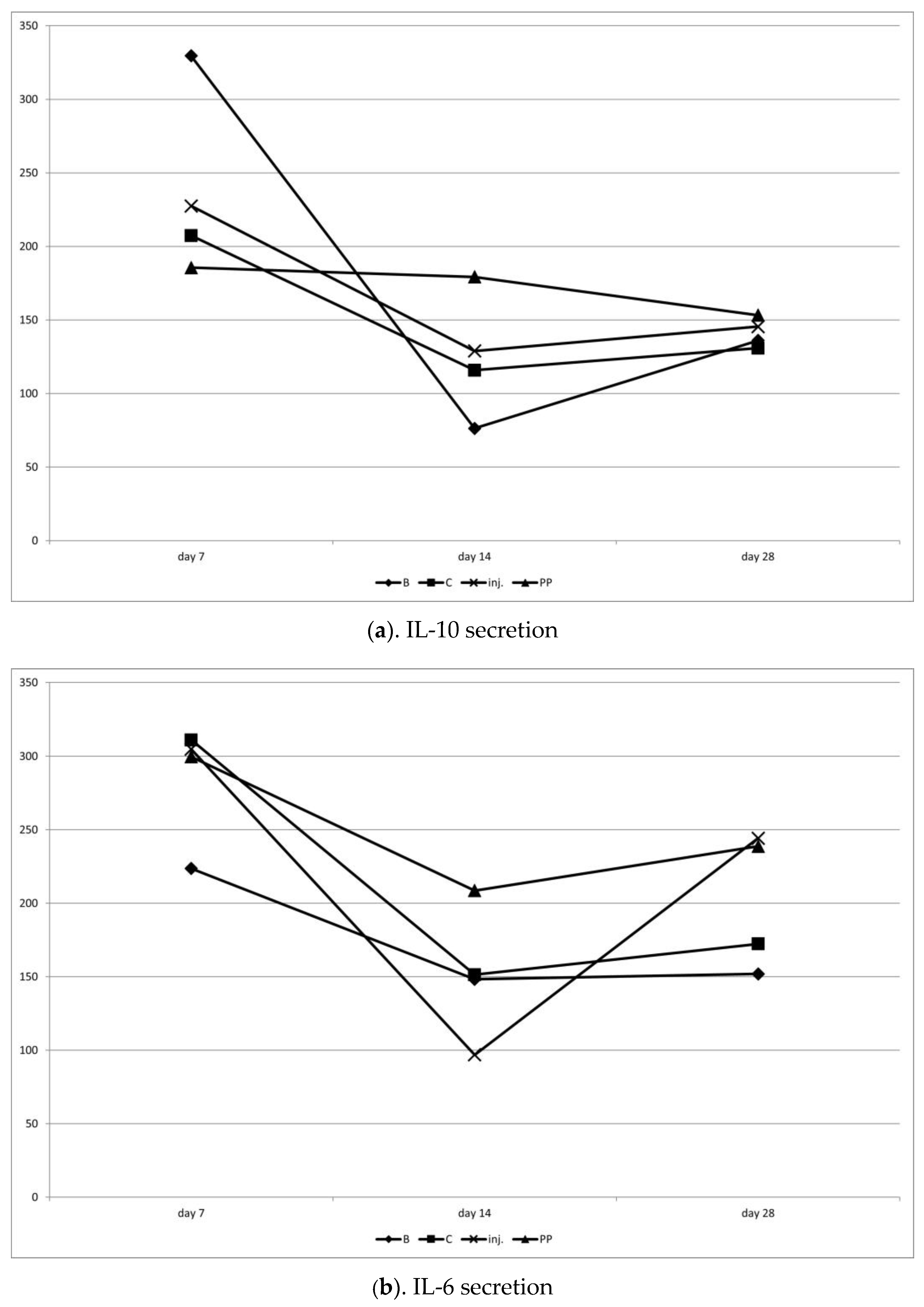
2.2. Evaluation of Serum Levels of IL-6 and IL-10
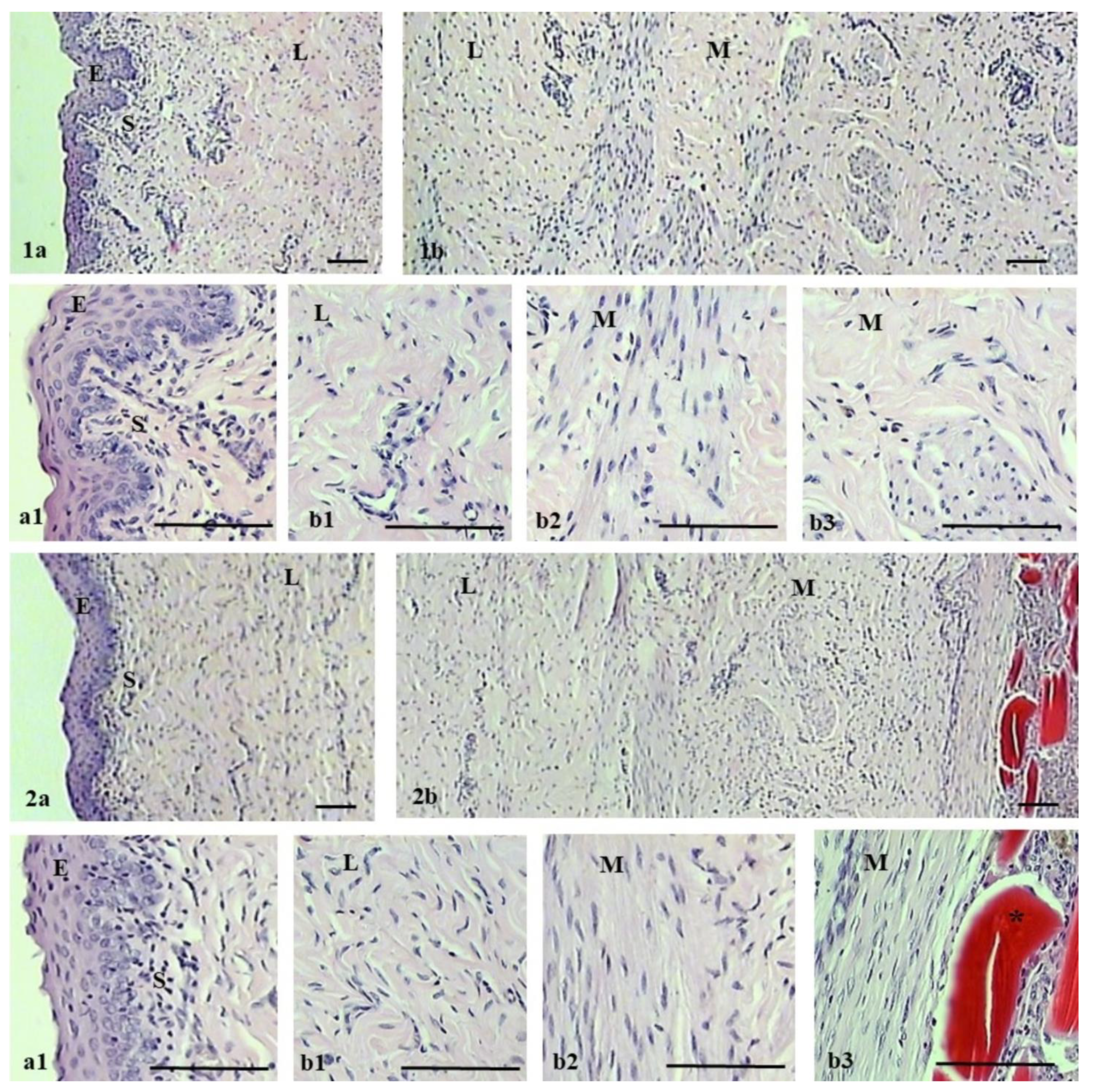
2.3. Pathomorphological Outcome
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Searching for a New Materials in Urogynaecology
4.3. Preparation for the Surgery, Proper Lining and Anaesthesia
4.4. Surgery
4.5. Chitosan Biomaterials: Characterization
4.6. ELISA Assay: IL-6, IL-10
4.7. Pathomorphology
4.8. Statistical Analyses of Data
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aboseif, C.; Liu, P. Pelvic Organ Prolapse. Lancet 2023, 369, 1027–1038. [Google Scholar]
- Bozkurt, M.; Yumru, A.E.; Şahin, L. Pelvic floor dysfunction, and effects of pregnancy and mode of delivery on pelvic floor. Taiwan J. Obstet. Gynecol. 2014, 53, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef]
- Reid, F.M.; Elders, A.; Breeman, S.; Freeman, R.M.; PROSPECT Study Group. How common are complications following polypropylene mesh, biological xenograft and native tissue surgery for pelvic organ prolapse? A secondary analysis from the PROSPECT trial. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Udpa, N.; Iyer, S.R.; Rajoria, R.; Breyer, K.E.; Valentine, H.; Singh, B.; McDonough, S.P.; Brown, B.N.; Bonassar, L.J.; Gao, Y. Effects of chitosan coatings on polypropylene mesh for implantation in a rat abdominal wall model. Tissue Eng. Part A 2013, 19, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Deineka, V.; Sulaieva, O.; Pernakov, M.; Radwan-Pragłowska, J.; Janus, Ł.; Kornienko, V.; Husak, E.; Yanovska, A.; Liubchak, I.; Yusupova, A.; et al. Hemostatic performance and biocompatibility of chitosan-based agents in experimental parenchymal bleeding. Mater. Sci. Eng. C 2021, 120, 111740. [Google Scholar] [CrossRef]
- Margarit, S.C.; Vasian, H.N.; Balla, E.; Vesa, S.; Ionescu, D.C. The influence of total intravenous anaesthesia and isoflurane anaesthesia on plasma interleukin-6 and interleukin-10 concentrations after colorectal surgery for cancer: A randomised controlled trial. Eur. J. Anaesthesiol. 2014, 31, 678–684. [Google Scholar] [CrossRef]
- Fernández, A.; Marteles, D.; de Arcaute Rivero, M.; Lacasta, D.; Conde, T.; Loste, A. Relationship between Pro-Inflammatory Cytokines, IL-10 Anti-Inflammatory Cytokine and Serum Proteins in Healthy Lambs and with Diarrhea. Pak. Vet. J. 2016, 36, 63–67. [Google Scholar]
- Balaram, P.; Kien, P.K.; Ismail, A. Toll-like receptors and cytokines in immune responses to persistent mycobacterial and Salmonella infections. Int. J. Med. Microbiol. 2009, 299, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-G.; Kawazoe, N.; Tateishi, T.; Chen, G. Preparation of chitosan scaffolds with a hierarchical porous structure. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Alshiek, J.; Bar-El, L.; Shobeiri, S.A. Vaginal Robotic Supracervical Hysterectomy in an Ovine Animal Model: The Proof of Concept. Open J. Obstet. Gynecol. 2019, 9, 1114–1129. [Google Scholar] [CrossRef]
- Mansoor, A.; Curinier, S.; Campagne-Loiseau, S.; Platteeuw, L.; Jacquetin, B.; Rabischong, B. Development of an ovine model for training in vaginal surgery for pelvic organ prolapse. Int. Urogynecol. J. 2017, 28, 1595–1597. [Google Scholar] [CrossRef] [PubMed]
- Couri, B.M.; Lenis, A.T.; Borazjani, A.; Paraiso, M.F.R.; Damaser, M.S. Animal models of female pelvic organ prolapse: Lessons learned. Expert Rev. Obstet. Gynecol. 2012, 7, 249–260. [Google Scholar] [CrossRef] [PubMed]
- McCraken, T.O.; Kainer, R.A. Spurgeon’s Color Atlas of Large Animal Anatomy: The Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999. [Google Scholar]
- Supakorn, C.; Stock, J.D.; Hostetler, C.; Stalder, K.J. Prolapse Incidence in Swine Breeding Herds Is a Cause for Concern. Open J. Vet. Med. 2014, 7, 85–97. [Google Scholar]
- Middelkoop, E.; van den Bogaerdt, A.J.; Lamme, E.N.; Hoekstra, M.J.; Brandsma, K.; Ulrich, M.M.W. Porcine wound models for skin substitution and burn treatment. Biomaterials 2004, 25, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matýsek, D. Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering. Polymers 2020, 12, 159. [Google Scholar] [CrossRef] [PubMed]


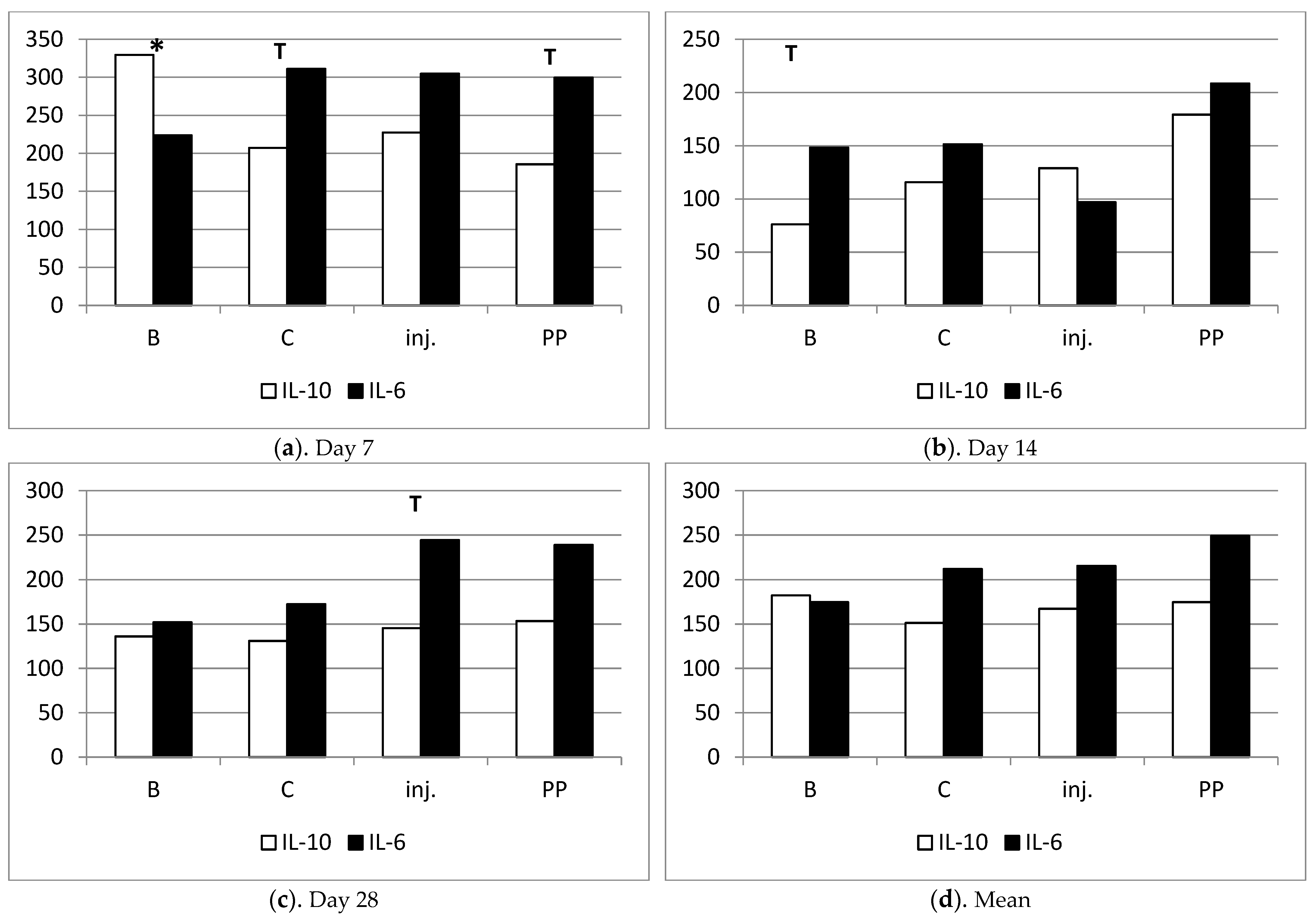


| Item | IL-10 | IL-6 | IL-10/IL6 | IL-6/IL-10 |
|---|---|---|---|---|
| Chitosan B | 182.28 ± 32.36 | 174.65 ± 13.53 | 1.23 ± 0.20 | 0.88 ± 0.14 |
| Chitosan C | 151.35 ±16.19 | 211.64 ± 34.02 | 0.99 ± 0.40 | 1.53 ± 0.29 |
| Chitosan injection | 167.29 ± 32.69 | 215.64 ± 28.48 | 0.86 ± 0.23 | 1.60 ± 0.34 |
| Polypropylene | 174.80 ± 36.40 | 248.99 ± 49.72 | 0.93 ± 0.36 | 1.98 ± 0.69 |
| Type/Group | Chitosan Type, g | Crosslinking Agent, g | High Boiling Solvent, mL | Nanoadditive, mg | Power, W | Time, min |
|---|---|---|---|---|---|---|
| A | Shrimp, 85% DD, −80 °C freeze | Levulinic acid, aspartic acid; 0.5:0.5 | Propylene glycol, 10 | --- | 800 | 3 |
| B * | Shrimp, 95% DD | Aspartic acid, glutamic acid; 0.5:0.5 | --- | |||
| C * | Shrimp, 90% DD | Glutamic acid; 0.84 | ZnO NPs, 8 | |||
| D | Shrimp, 85% DD | Aspartic acid, 0.84 | Fe3O4 NPs, 8 | |||
| E | Shrimp, 85% DD | Levulinic acid, aspartic acid; 0.5:0.5 | --- | |||
| F | Squid, 85% DD | Aspartic acid, 0.84 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stangel-Wójcikiewicz, K.; Murawski, M.; Schwarz, T.; Skotniczny, K.; Fuchs, A.; Wolski, J.; Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Kot, M.; et al. Pelvic Organ Prolapse Reconstruction with the Chitosan-Based Novel Haemostatic Agent in Ovine Model—Preliminary Report. Int. J. Mol. Sci. 2024, 25, 3801. https://doi.org/10.3390/ijms25073801
Stangel-Wójcikiewicz K, Murawski M, Schwarz T, Skotniczny K, Fuchs A, Wolski J, Radwan-Pragłowska J, Janus Ł, Piątkowski M, Kot M, et al. Pelvic Organ Prolapse Reconstruction with the Chitosan-Based Novel Haemostatic Agent in Ovine Model—Preliminary Report. International Journal of Molecular Sciences. 2024; 25(7):3801. https://doi.org/10.3390/ijms25073801
Chicago/Turabian StyleStangel-Wójcikiewicz, Klaudia, Maciej Murawski, Tomasz Schwarz, Krzysztof Skotniczny, Agnieszka Fuchs, Jan Wolski, Julia Radwan-Pragłowska, Łukasz Janus, Marek Piątkowski, Marta Kot, and et al. 2024. "Pelvic Organ Prolapse Reconstruction with the Chitosan-Based Novel Haemostatic Agent in Ovine Model—Preliminary Report" International Journal of Molecular Sciences 25, no. 7: 3801. https://doi.org/10.3390/ijms25073801
APA StyleStangel-Wójcikiewicz, K., Murawski, M., Schwarz, T., Skotniczny, K., Fuchs, A., Wolski, J., Radwan-Pragłowska, J., Janus, Ł., Piątkowski, M., Kot, M., Wróbel, A., Wojtysiak, D., & Urbaniec, P. (2024). Pelvic Organ Prolapse Reconstruction with the Chitosan-Based Novel Haemostatic Agent in Ovine Model—Preliminary Report. International Journal of Molecular Sciences, 25(7), 3801. https://doi.org/10.3390/ijms25073801





