Stem Cell Therapy against Ischemic Heart Disease
Abstract
1. Introduction
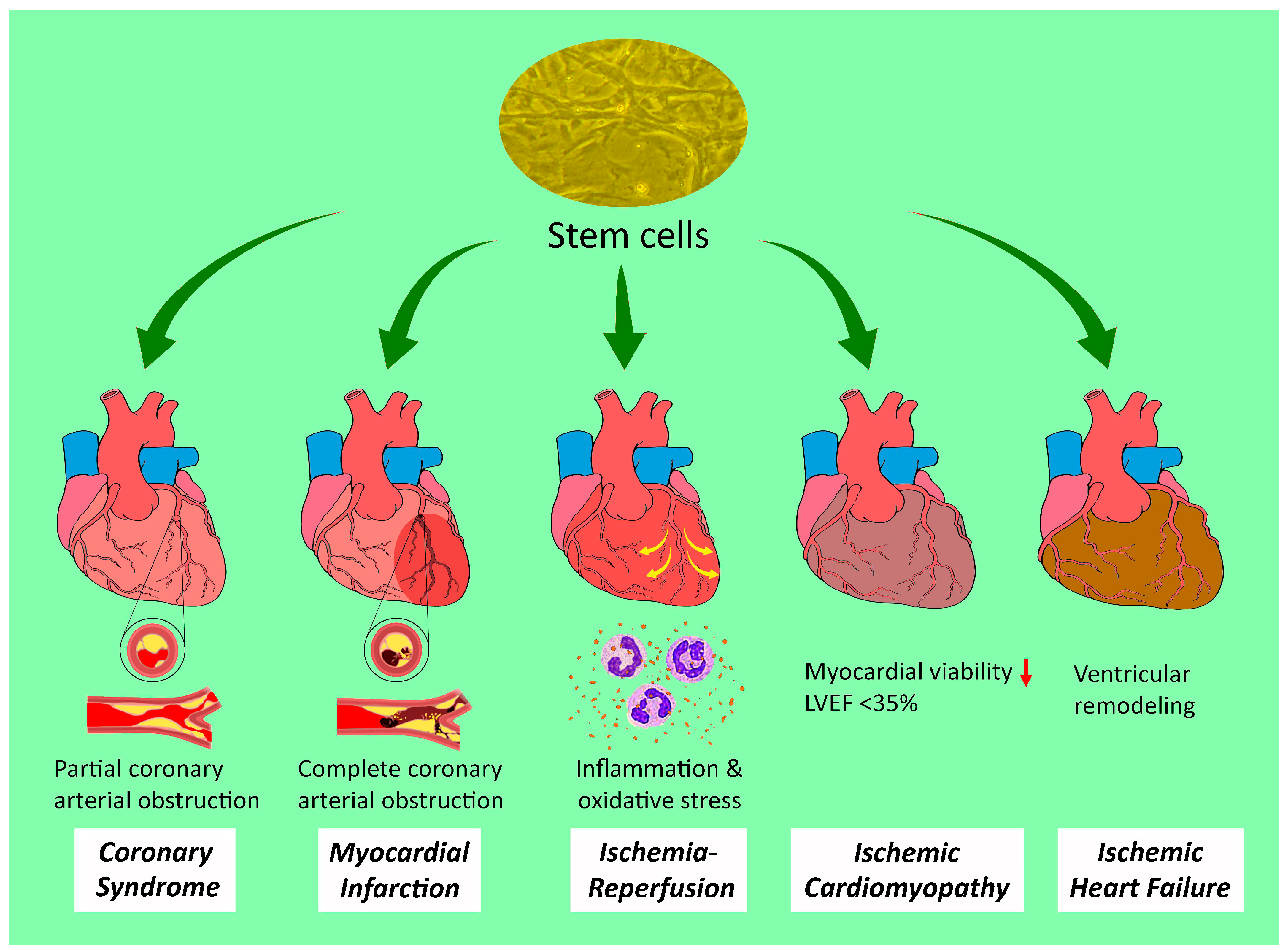
2. Stem Cell Therapies against Different Entities of Ischemic Heart Disease
2.1. Acute and Chronic Coronary Syndromes
2.2. Myocardial Infarction
2.3. Ischemia-Reperfusion (IR) Injury
2.4. Ischemic Cardiomyopathy
2.5. Ischemic Heart Failure
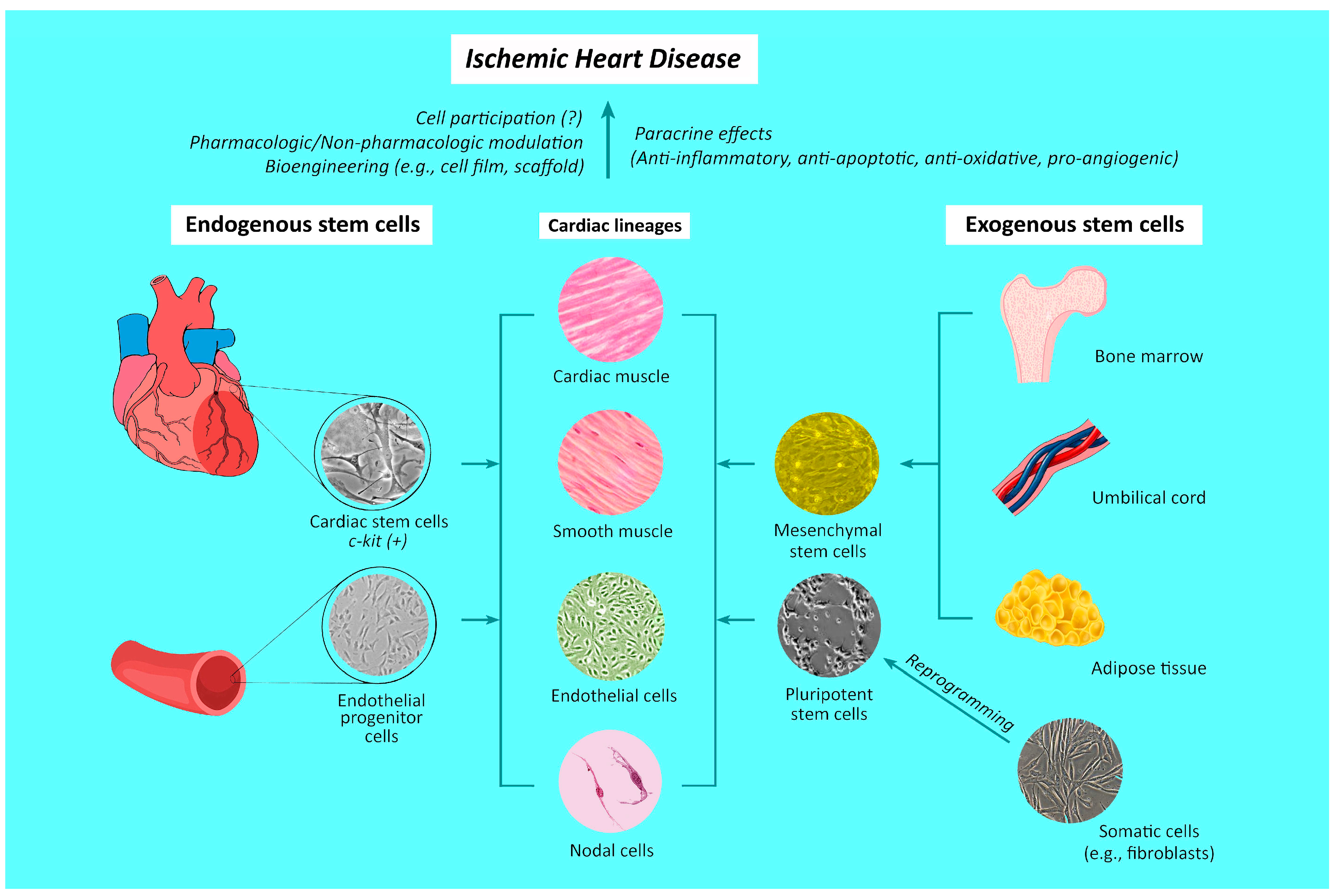
3. Origins of Stem Cells and Mechanisms Underlying Their Therapeutic Effects against Ischemic Heart Disease
3.1. Endogenous Stem Cell Differentiation
3.2. Differentiation and Incorporation of Exogenous Stem Cells
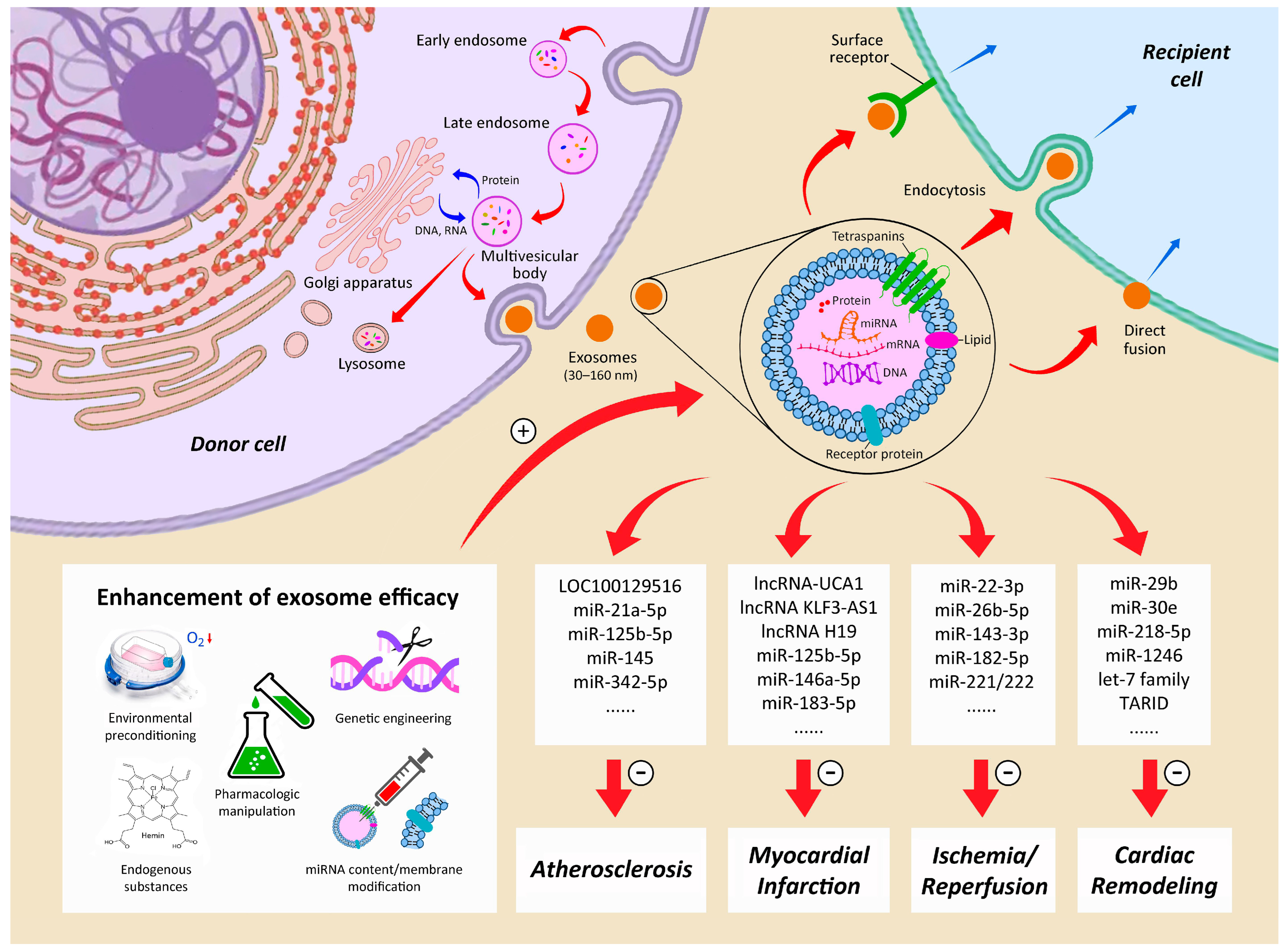
3.3. Paracrine Effects and Exosomes
4. Directions of Future Research and Speculations
4.1. Pluripotent Stem Cells
4.2. Scaffold and Cell Sheet Approaches to Stem Cell Application
4.3. Combination of Stem Cell with Other Strategies
4.4. Refinements of Paracrine and Exosome Treatments
4.5. Allogeneic and Xenogeneic Stem Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Winchester, D.E.; Maron, D.J.; Blankstein, R.; Chang, I.C.; Kirtane, A.J.; Kwong, R.Y.; Pellikka, P.A.; Prutkin, J.M.; Russell, R.; Sandhu, A.T. ACC/AHA/ASE/ASNC/ASPC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2023 Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Chronic Coronary Disease. J. Cardiovasc. Magn. Reson. 2023, 25, 58. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Ye, F.; Hou, C.C.; Pi, L.; Chen, F. Mesenchymal Stem Cell Therapy for Patients with Ischemic Heart Failure- Past, Present, and Future. Curr. Stem Cell Res. Ther. 2021, 16, 608–621. [Google Scholar]
- Manfroi, W.C.; Peukert, C.; Berti, C.B.; Noer, C.; Gutierres Dde, A.; Silva, F.T. Acute myocardial infarction: The first manifestation of ischemic heart disease and relation to risk factors. Arq. Bras. Cardiol. 2002, 78, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Makikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; Mogensen, L.J.H.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, M.; Winslow, B.T.; Mills, K.; Dauber, I.M. Medical management of stable coronary artery disease. Am. Fam. Physician 2011, 83, 819–826. [Google Scholar] [PubMed]
- Safiri, S.; Karamzad, N.; Singh, K.; Carson-Chahhoud, K.; Adams, C.; Nejadghaderi, S.A.; Almasi-Hashiani, A.; Sullman, M.J.M.; Mansournia, M.A.; Bragazzi, N.L.; et al. Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Itescu, S.; Schuster, M.D.; Kocher, A.A. New directions in strategies using cell therapy for heart disease. J. Mol. Med. 2003, 81, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Anversa, P.; Kajstura, J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ. Res. 1998, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, X.; Wang, K.; Ju, J.; Yu, X.; Yu, W.; Liu, C.; Wang, Y. Cardiac regeneration: Pre-existing cardiomyocyte as the hub of novel signaling pathway. Genes. Dis. 2024, 11, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A brief review: The therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Tura-Ceide, O.; Hunter, A.; Mitchell, A.; Vesey, A.; Medine, C.; Gallogly, S.; Hadoke, P.W.F.; Keith, C.; Sproul, A.; et al. Endothelial Progenitor Cells Do Not Originate From the Bone Marrow. Circulation 2019, 140, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, D.; Lei, I.; Li, W.; Liu, L.; Li, L.; Ni, J.; Liu, Z.; Fan, G. The origin, progress, and application of cell-based cardiac regeneration therapy. J. Cell Physiol. 2023, 238, 1732–1755. [Google Scholar] [CrossRef] [PubMed]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef]
- Orlic, D.; Hill, J.M.; Arai, A.E. Stem cells for myocardial regeneration. Circ. Res. 2002, 91, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.D.; Giordano, F.J. Stem cells and cardiovascular disease. J. Nucl. Cardiol. 2003, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hassink, R.J.; Dowell, J.D.; Brutel de la Riviere, A.; Doevendans, P.A.; Field, L.J. Stem cell therapy for ischemic heart disease. Trends Mol. Med. 2003, 9, 436–441. [Google Scholar] [CrossRef]
- Muller-Ehmsen, J.; Kedes, L.H.; Schwinger, R.H.; Kloner, R.A. Cellular cardiomyoplasty—A novel approach to treat heart disease. Congest. Heart Fail. 2002, 8, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S. Cardiac stem cells. J. Pathol. 2002, 197, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Dimmeler, S. Endothelial progenitor cells: Regulation and contribution to adult neovascularization. Herz 2002, 27, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Leu, S.; Sun, C.K.; Yen, C.H.; Kao, Y.H.; Chang, L.T.; Tsai, T.H.; Chua, S.; Fu, M.; Ko, S.F.; et al. Early combined treatment with sildenafil and adipose-derived mesenchymal stem cells preserves heart function in rat dilated cardiomyopathy. J. Transl. Med. 2010, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Kao, Y.H.; Sun, C.K.; Lin, Y.C.; Tsai, T.H.; Chang, L.T.; Chua, S.; Yeh, K.H.; Wu, C.J.; Fu, M.; et al. Myocardium-derived conditioned medium improves left ventricular function in rodent acute myocardial infarction. J. Transl. Med. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Sun, C.K.; Sheu, J.J.; Chang, L.T.; Yuen, C.M.; Yen, C.H.; Chiang, C.H.; Ko, S.F.; Pei, S.N.; Chua, S.; et al. Autologous bone marrow cell implantation attenuates left ventricular remodeling and improves heart function in porcine myocardial infarction: An echocardiographic, six-month angiographic, and molecular-cellular study. Int. J. Cardiol. 2011, 150, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Q.; Leu, S.; Sheu, J.J.; Zhen, Y.Y.; Tsai, T.H.; Chen, Y.L.; Chung, S.Y.; Chai, H.T.; Sun, C.K.; Yang, J.L.; et al. Prompt bone marrow-derived mesenchymal stem cell therapy enables early porcine heart function recovery from acute myocardial infarction. Int. Heart J. 2014, 55, 362–371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheu, J.J.; Lee, F.Y.; Yuen, C.M.; Chen, Y.L.; Huang, T.H.; Chua, S.; Chen, C.H.; Chai, H.T.; Sung, P.H.; Chang, H.W.; et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int. J. Cardiol. 2015, 193, 69–83. [Google Scholar]
- Cambria, E.; Pasqualini, F.S.; Wolint, P.; Gunter, J.; Steiger, J.; Bopp, A.; Hoerstrup, S.P.; Emmert, M.Y. Translational cardiac stem cell therapy: Advancing from first-generation to next-generation cell types. NPJ Regen. Med. 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Sheng, M.; Ye, J.H.; Tang, Z.X.; Hu, S.; Wang, B.; Yuan, J.L.; Yang, Y.H.; Zhong, Y.M.; Liao, Y.L. Research trends in cardiovascular tissue engineering from 1992 to 2022: A bibliometric analysis. Front Cardiovasc. Med. 2023, 10, 1208227. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ (accessed on 20 November 2023).
- Baaten, C.; Nagy, M.; Bergmeier, W.; Spronk, H.M.H.; van der Meijden, P.E.J. Platelet biology and function: Plaque erosion vs. rupture. Eur. Heart J. 2023, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.; Serino, F.; Esposito, G. Cardiovascular mortality in patients with acute and chronic coronary syndrome: Insights from the clinical evidence on ticagrelor. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2524–2542. [Google Scholar] [PubMed]
- Braun, M.M.; Stevens, W.A.; Barstow, C.H. Stable Coronary Artery Disease: Treatment. Am. Fam. Physician 2018, 97, 376–384. [Google Scholar] [PubMed]
- Cartlidge, T.; Kovacevic, M.; Navarese, E.P.; Werner, G.; Kunadian, V. Role of percutaneous coronary intervention in the modern-day management of chronic coronary syndrome. Heart 2023, 109, 1429–1435. [Google Scholar] [CrossRef]
- Kohsaka, S.; Ejiri, K.; Takagi, H.; Watanabe, I.; Gatate, Y.; Fukushima, K.; Nakano, S.; Hirai, T. Diagnostic and Therapeutic Strategies for Stable Coronary Artery Disease following the ISCHEMIA Trial. JACC Asia 2023, 3, 15–30. [Google Scholar] [CrossRef]
- Perera, D.; Morgan, H.P.; Ryan, M.; Dodd, M.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Walsh, S.J.; Weerackody, R.; McDiarmid, A.; et al. Arrhythmia and Death following Percutaneous Revascularization in Ischemic Left Ventricular Dysfunction: Prespecified Analyses from the REVIVED-BCIS2 Trial. Circulation 2023, 148, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Drexler, H. The clinical significance of endothelial dysfunction. Curr. Opin. Cardiol. 2005, 20, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Wojakowski, W.; Tendera, M. Mobilization of bone marrow-derived progenitor cells in acute coronary syndromes. Folia Histochem. Cytobiol. 2005, 43, 229–232. [Google Scholar] [PubMed]
- Wojakowski, W.; Kucia, M.; Kazmierski, M.; Ratajczak, M.Z.; Tendera, M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: Relevant reparatory mechanism? Heart 2008, 94, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Pompili, V.J.; Das, H. Hematopoietic stem cells: Ex-vivo expansion and therapeutic potential for myocardial ischemia. Stem Cells Cloning 2010, 3, 57–68. [Google Scholar] [PubMed]
- Erbs, S.; Linke, A.; Adams, V.; Lenk, K.; Thiele, H.; Diederich, K.W.; Emmrich, F.; Kluge, R.; Kendziorra, K.; Sabri, O.; et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: First randomized and placebo-controlled study. Circ. Res. 2005, 97, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, P.; Turagam, M.; Kolte, D.; Khera, S.; Hyder, O.; Gordon, P.; Aronow, H.D.; Leopold, J.; Abbott, J.D. Intramyocardial autologous CD34+ cell therapy for refractory angina: A meta-analysis of randomized controlled trials. Cardiovasc. Revascularization Med. 2019, 20, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.H.; Chiang, H.J.; Li, Y.C.; Chiang, J.Y.; Chu, C.H.; Shao, P.L.; Lee, F.Y.; Lee, M.S.; Yip, H.K. Baseline factors identified for the prediction of good responders in patients with end-stage diffuse coronary artery disease undergoing intracoronary CD34+ cell therapy. Stem Cell Res. Ther. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.F.; Lau, C.P. Therapeutic angiogenesis with bone marrow—Derived stem cells. J. Cardiovasc. Pharmacol. Ther. 2007, 12, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, N.; Hirose, M.; Takakura, Y.; Ohgushi, H. Cultured autologous human cells for hard tissue regeneration: Preparation and characterization of mesenchymal stem cells from bone marrow. Artif. Organs 2004, 28, 33–39. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, J.; Bax, J.J.; Beeres, S.L.; Dibbets-Schneider, P.; Roes, S.D.; Stokkel, M.P.; de Roos, A.; Fibbe, W.E.; Zwaginga, J.J.; Boersma, E.; et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: A randomized controlled trial. JAMA 2009, 301, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Chen, Y.L.; Sung, P.H.; Ma, M.C.; Pei, S.N.; Wu, C.J.; Yang, C.H.; Fu, M.; Ko, S.F.; Leu, S.; et al. Intracoronary Transfusion of Circulation-Derived CD34+ Cells Improves Left Ventricular Function in Patients with End-Stage Diffuse Coronary Artery Disease Unsuitable for Coronary Intervention. Crit. Care Med. 2015, 43, 2117–2132. [Google Scholar] [CrossRef]
- Sung, P.H.; Li, Y.C.; Lee, M.S.; Hsiao, H.Y.; Ma, M.C.; Pei, S.N.; Chiang, H.J.; Lee, F.Y.; Yip, H.K. Intracoronary Injection of Autologous CD34+ Cells Improves One-Year Left Ventricular Systolic Function in Patients with Diffuse Coronary Artery Disease and Preserved Cardiac Performance-A Randomized, Open-Label, Controlled Phase II Clinical Trial. J. Clin. Med. 2020, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Samanta, A.; Shah, Z.I.; Jeevanantham, V.; Abdel-Latif, A.; Zuba-Surma, E.K.; Dawn, B. Adult Bone Marrow Cell Therapy for Ischemic Heart Disease: Evidence and Insights from Randomized Controlled Trials. Circ. Res. 2015, 117, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Soetisna, T.W.; Thamrin, A.M.H.; Permadijana, D.; Ramadhani, A.N.E.; Santoso, A.; Mansyur, M. Intramyocardial Stem Cell Transplantation during Coronary Artery Bypass Surgery Safely Improves Cardiac Function: Meta-Analysis of 20 Randomized Clinical Trials. J. Clin. Med. 2023, 12, 4430. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Vasquez, A.; Kereiakes, D.J.; Klapholz, M.; Schaer, G.L.; Abdel-Latif, A.; Frohwein, S.; Henry, T.D.; Schatz, R.A.; Dib, N.; et al. PreSERVE-AMI: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Intracoronary Administration of Autologous CD34+ Cells in Patients with Left Ventricular Dysfunction Post STEMI. Circ. Res. 2017, 120, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Apple, F.S.; Lindahl, B.; Morrow, D.A.; et al. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar] [CrossRef]
- Yusuf, S.; Rangarajan, S.; Teo, K.; Islam, S.; Li, W.; Liu, L.; Bo, J.; Lou, Q.; Lu, F.; Liu, T.; et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014, 371, 818–827. [Google Scholar] [CrossRef]
- Murray, C.J.; Barber, R.M.; Foreman, K.J.; Abbasoglu Ozgoren, A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; Abu-Raddad, L.J.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Rev. Esp. Cardiol. 2021, 74, 544. [Google Scholar]
- Omer, M.A.; Tyler, J.M.; Henry, T.D.; Garberich, R.; Sharkey, S.W.; Schmidt, C.W.; Henry, J.T.; Eckman, P.; Megaly, M.; Brilakis, E.S.; et al. Clinical Characteristics and Outcomes of STEMI Patients with Cardiogenic Shock and Cardiac Arrest. JACC Cardiovasc. Interv. 2020, 13, 1211–1219. [Google Scholar] [CrossRef]
- Yamada, Y.; Minatoguchi, S.; Kanamori, H.; Mikami, A.; Okura, H.; Dezawa, M. Stem cell therapy for acute myocardial infarction—Focusing on the comparison between Muse cells and mesenchymal stem cells. J. Cardiol. 2022, 80, 80–87. [Google Scholar] [CrossRef]
- Yip, H.K.; Chang, L.T.; Wu, C.J.; Sheu, J.J.; Youssef, A.A.; Pei, S.N.; Lee, F.Y.; Sun, C.K. Autologous bone marrow-derived mononuclear cell therapy prevents the damage of viable myocardium and improves rat heart function following acute anterior myocardial infarction. Circ. J. 2008, 72, 1336–1345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Onate, B.; Vilahur, G. Adipose-derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease. Rev. Esp. Cardiol. 2015, 68, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Naaijkens, B.A.; van Dijk, A.; Kamp, O.; Krijnen, P.A.; Niessen, H.W.; Juffermans, L.J. Therapeutic application of adipose derived stem cells in acute myocardial infarction: Lessons from animal models. Stem Cell Rev. Rep. 2014, 10, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qin, F.; Ge, M.; Shu, Q.; Xu, J. Application of adipose-derived stem cells in heart disease. J. Cardiovasc. Transl. Res. 2014, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.F.; Yip, H.K.; Lee, C.C.; Sheu, J.J.; Sun, C.K.; Ng, S.H.; Huang, C.C.; Lin, Y.C.; Chang, L.T.; Chen, M.C. Immediate intramyocardial bone marrow-derived mononuclear cells implantation in minipig myocardium after permanent coronary artery ligation: Magnetic resonance imaging with histopathologic and immunochemical correlation. Investig. Radiol. 2011, 46, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Haenel, A.; Ghosn, M.; Karimi, T.; Vykoukal, J.; Shah, D.; Valderrabano, M.; Schulz, D.G.; Raizner, A.; Schmitz, C.; Alt, E.U. Unmodified autologous stem cells at point of care for chronic myocardial infarction. World J. Stem Cells 2019, 11, 831–858. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.K. Editorial: [Application of stem cells in the treatment of myocardial infarction]. Front. Cardiovasc. Med. 2023, 10, 1333732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, H.; Zhai, C.; Jing, L.; Tian, H. Cardioprotective Strategies After Ischemia-Reperfusion Injury. Am. J. Cardiovasc. Drugs 2023, 24, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Barrere-Lemaire, S.; Vincent, A.; Jorgensen, C.; Piot, C.; Nargeot, J.; Djouad, F. Mesenchymal stromal cells for improvement of cardiac function following acute myocardial infarction: A matter of timing. Physiol. Rev. 2024, 104, 659–725. [Google Scholar] [CrossRef] [PubMed]
- Syriga, M.; Mavroidis, M. Complement system activation in cardiac and skeletal muscle pathology: Friend or foe? Adv. Exp. Med. Biol. 2013, 735, 207–218. [Google Scholar] [PubMed]
- Yang, F.; Smith, M.J. Metal profiling in coronary ischemia-reperfusion injury: Implications for KEAP1/NRF2 regulated redox signaling. Free Radic. Biol. Med. 2024, 210, 158–171. [Google Scholar] [CrossRef]
- Kaminski, K.A.; Bonda, T.A.; Korecki, J.; Musial, W.J. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002, 86, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Cai, R.P.; Su, Y.M.; Wu, Q.; Su, Q. Mesenchymal Stem Cell-Derived Exosomal Noncoding RNAs as Alternative Treatments for Myocardial Ischemia-Reperfusion Injury: Current Status and Future Perspectives. J. Cardiovasc. Transl. Res. 2023, 16, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Liang, Y.; Wang, C.; Naruse, K.; Takahashi, K. Treatment of Oxidative Stress with Exosomes in Myocardial Ischemia. Int. J. Mol. Sci. 2021, 22, 1729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Fang, T.; Cheng, Z. Mechanism of heart failure after myocardial infarction. J. Int. Med. Res. 2023, 51, 3000605231202573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, E.; Zhang, J.; Zhang, Y. CaMKII, ‘jack of all trades’ in inflammation during cardiac ischemia/reperfusion injury. J. Mol. Cell Cardiol. 2023, 184, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, Y.; Peng, L.; Rong, X.; Liu, Y.; Li, J.; Luo, J. Ferroptosis in cardiovascular diseases: Role and mechanism. Cell Biosci. 2023, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, W.; Liu, J.; Wan, J.; Wang, M. Scavenger Receptors in Myocardial Infarction and Ischemia/Reperfusion Injury: The Potential for Disease Evaluation and Therapy. J. Am. Heart Assoc. 2023, 12, e027862. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, D. Research progress on the effects of novel hypoglycemic drugs in diabetes combined with myocardial ischemia/reperfusion injury. Ageing Res. Rev. 2023, 86, 101884. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, F.; Wang, Y.; Li, W.; Wu, J.; Hu, X.; Tang, T.; Liu, X. Multiple delivery strategies of nanocarriers for myocardial ischemia-reperfusion injury: Current strategies and future prospective. Drug Deliv. 2024, 31, 2298514. [Google Scholar] [CrossRef]
- Ko, S.F.; Sung, P.H.; Yang, C.C.; Chiang, J.Y.; Yip, H.K. Combined therapy with dapagliflozin and entresto offers an additional benefit on improving the heart function in rat after ischemia-reperfusion injury. Biomed. J. 2023, 46, 100546. [Google Scholar] [CrossRef]
- Souidi, N.; Stolk, M.; Seifert, M. Ischemia-reperfusion injury: Beneficial effects of mesenchymal stromal cells. Curr. Opin. Organ. Transplant. 2013, 18, 34–43. [Google Scholar] [CrossRef]
- Chai, H.T.; Sheu, J.J.; Chiang, J.Y.; Shao, P.L.; Wu, S.C.; Chen, Y.L.; Li, Y.C.; Sung, P.H.; Lee, F.Y.; Yip, H.K. Early administration of cold water and adipose derived mesenchymal stem cell derived exosome effectively protects the heart from ischemia-reperfusion injury. Am. J. Transl. Res. 2019, 11, 5375–5389. [Google Scholar] [PubMed]
- Mokhtari, B.; Badalzadeh, R.; Aboutaleb, N. Modulation of autophagy as the target of mesenchymal stem cells-derived conditioned medium in rat model of myocardial ischemia/reperfusion injury. Mol. Biol. Rep. 2021, 48, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, D.; Bernardini, C.; Zannoni, A.; Salaroli, R.; Wang, C.; Bencivenni, S.; Forni, M. Efficacy of Stem Cell Therapy in Large Animal Models of Ischemic Cardiomyopathies: A Systematic Review and Meta-Analysis. Animals 2022, 12, 749. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, Y.; Ito, E.; Takeda, M.; Mikami, T.; Taguchi, T.; Mochizuki-Oda, N.; Sasai, M.; Shimamoto, T.; Nitta, Y.; et al. Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: First three case reports. Front. Cardiovasc. Med. 2023, 10, 1182209. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef]
- Stewart, S.; MacIntyre, K.; Hole, D.J.; Capewell, S.; McMurray, J.J. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur. J. Heart Fail. 2001, 3, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in Heart Failure Management: A Comprehensive Narrative Review of Emerging Therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef] [PubMed]
- Rosenstrauch, D.; Poglajen, G.; Zidar, N.; Gregoric, I.D. Stem celltherapy for ischemic heart failure. Tex. Heart Inst. J. 2005, 32, 339–347. [Google Scholar] [PubMed]
- Perin, E.C.; Dohmann, H.F.; Borojevic, R.; Silva, S.A.; Sousa, A.L.; Mesquita, C.T.; Rossi, M.I.; Carvalho, A.C.; Dutra, H.S.; Dohmann, H.J.; et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003, 107, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Mann, I.; Tseng, C.C.S.; Rodrigo, S.F.; Koudstaal, S.; van Ramshorst, J.; Beeres, S.L.; Dibbets-Schneider, P.; de Geus-Oei, L.F.; Lamb, H.J.; Wolterbeek, R.; et al. Intramyocardial bone marrow cell injection does not lead to functional improvement in patients with chronic ischaemic heart failure without considerable ischaemia. Neth. Heart J. 2019, 27, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Marvasti, T.B.; Alibhai, F.J.; Yang, G.J.; Li, S.H.; Wu, J.; Yau, T.; Li, R.K. Heart Failure Impairs Bone Marrow Hematopoietic Stem Cell Function and Responses to Injury. J. Am. Heart Assoc. 2023, 12, e027727. [Google Scholar] [CrossRef]
- Mauretti, A.; Spaans, S.; Bax, N.A.M.; Sahlgren, C.; Bouten, C.V.C. Cardiac Progenitor Cells and the Interplay with Their Microenvironment. Stem Cells Int. 2017, 2017, 7471582. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.F.; Almeida, T.R.; Lopes, C.S.; Dias da Silva, V.J. Multipotent stem cells of the heart-do they have therapeutic promise? Front. Physiol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, V.; De Luca, A.; Colorito, M.L.; Montalbano, A.; Ardizzone, N.M.; Macaluso, F.; Gammazza, A.M.; Cappello, F.; Zummo, G. Cardiac stem cell research: An elephant in the room? Anat. Rec. 2009, 292, 449–454. [Google Scholar] [CrossRef]
- Vagnozzi, R.J.; Molkentin, J.D.; Houser, S.R. New Myocyte Formation in the Adult Heart: Endogenous Sources and Therapeutic Implications. Circ. Res. 2018, 123, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.L.; Molkentin, J.D. The Elusive Progenitor Cell in Cardiac Regeneration: Slip Slidin’ Away. Circ. Res. 2017, 120, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Marban, E. Cardiac cell therapy: Where we’ve been, where we are, and where we should be headed. Br. Med. Bull. 2011, 98, 161–185. [Google Scholar] [CrossRef]
- Lee, S.H.; Hong, J.H.; Cho, K.H.; Noh, J.W.; Cho, H.J. Discrepancy between short-term and long-term effects of bone marrow-derived cell therapy in acute myocardial infarction: A systematic review and meta-analysis. Stem Cell Res. Ther. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hou, C.H.; Chen, Y.L.; Shen, H.H.; Lin, C.H.; Wu, C.Y.; Lin, M.H.; Liao, C.C.; Huang, J.J.; Yang, C.Y.; et al. Safety and efficacy of intracoronary artery administration of human bone marrow-derived mesenchymal stem cells in STEMI of Lee-Sung pigs-A preclinical study for supporting the feasibility of the OmniMSC-AMI phase I clinical trial. Front. Cardiovasc. Med. 2023, 10, 1153428. [Google Scholar] [CrossRef] [PubMed]
- Gorjipour, F.; Hosseini Gohari, L.; Hajimiresmaiel, S.J.; Janani, L.; Moradi, Y.; Pazoki-Toroudi, H. Amniotic Membrane-Derived Mesenchymal Stem Cells for Heart Failure: A Systematic Review and Meta-Analysis of the Published Preclinical Studies. Med. J. Islam. Repub. Iran 2021, 35, 187. [Google Scholar] [CrossRef] [PubMed]
- Jansen Of Lorkeers, S.J.; Eding, J.E.; Vesterinen, H.M.; van der Spoel, T.I.; Sena, E.S.; Duckers, H.J.; Doevendans, P.A.; Macleod, M.R.; Chamuleau, S.A. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ. Res. 2015, 116, 80–86. [Google Scholar] [CrossRef]
- Gai, H.; Leung, E.L.; Costantino, P.D.; Aguila, J.R.; Nguyen, D.M.; Fink, L.M.; Ward, D.C.; Ma, Y. Generation and characterization of functional cardiomyocytes using induced pluripotent stem cells derived from human fibroblasts. Cell Biol. Int. 2009, 33, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuche, K.; Liang, H.; Hannes, T.; Xi, J.; Fatima, A.; Nguemo, F.; Matzkies, M.; Wernig, M.; Jaenisch, R.; Pillekamp, F.; et al. Cardiac myocytes derived from murine reprogrammed fibroblasts: Intact hormonal regulation, cardiac ion channel expression and development of contractility. Cell Physiol. Biochem. 2009, 24, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nose, N.; Iida, T.; Akazawa, K.; Kanno, T.; Fujimoto, Y.; Sasaki, T.; Akehi, M.; Higuchi, T.; Akagi, S.; et al. In vivo tracking transplanted cardiomyocytes derived from human induced pluripotent stem cells using nuclear medicine imaging. Front. Cardiovasc. Med. 2023, 10, 1261330. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Lee, M.S.; Wallace, C.G.; Chen, K.H.; Sung, P.H.; Chua, S.; Lee, F.Y.; Chung, S.Y.; Chen, Y.L.; Li, Y.C.; et al. Therapeutic effects of adipose derived fresh stromal vascular fraction-containing stem cells versus cultured adipose derived mesenchymal stem cells on rescuing heart function in rat after acute myocardial infarction. Am. J. Transl. Res. 2019, 11, 67–86. [Google Scholar] [PubMed]
- Jiang, Q.; Huang, K.; Lu, F.; Deng, S.; Yang, Z.; Hu, S. Modifying strategies for SDF-1/CXCR4 interaction during mesenchymal stem cell transplantation. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; Bouten, C.V.C. A Brief History in Cardiac Regeneration, and How the Extra Cellular Matrix May Turn the Tide. Front. Cardiovasc. Med. 2021, 8, 682342. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Benseler, V.; Kroemer, A.; Popp, F.C.; Geissler, E.K.; Schlitt, H.J.; Baan, C.C.; Dahlke, M.H.; Hoogduijn, M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012, 3, 297. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control Release 2022, 347, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Chen, S.; Wu, Y.; Geng, D.; Sun, G.; Zhang, N.; et al. Advances in the Study of Exosomes in cardiovascular diseases. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 463–487. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol. 2012, 22, R116–R120. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008, 20, 4–11. [Google Scholar] [CrossRef]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; van Meer, G. Cell biology. No ESCRTs for exosomes. Science 2008, 319, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jankovicova, J.; Secova, P.; Michalkova, K.; Antalikova, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, S.; Guo, W.Z.; Li, X.K. The Unique Immunomodulatory Properties of MSC-Derived Exosomes in Organ Transplantation. Front. Immunol. 2021, 12, 659621. [Google Scholar] [CrossRef]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Choo, A.B.H.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-sEV). Pharmaceuticals 2021, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Eguchi, S.; Takefuji, M.; Sakaguchi, T.; Ishihama, S.; Mori, Y.; Tsuda, T.; Takikawa, T.; Yoshida, T.; Ohashi, K.; Shimizu, Y.; et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J. Biol. Chem. 2019, 294, 11665–11674. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339–353. [Google Scholar] [CrossRef]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic Application of Exosomes in Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Zhao, J.; Geng, J.; Xie, J.; Xu, B. Bone marrow mesenchymal stem cell-derived exosomes attenuate cardiac hypertrophy and fibrosis in pressure overload induced remodeling. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1alpha overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.K.; Zhen, Y.Y.; Leu, S.; Tsai, T.H.; Chang, L.T.; Sheu, J.J.; Chen, Y.L.; Chua, S.; Chai, H.T.; Lu, H.I.; et al. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int. J. Cardiol. 2014, 173, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, R.; Huang, P.; Wu, Y.; Fan, W.; He, X.; Dong, Y.; Liu, C. Modified Exosomes: A Good Transporter for miRNAs within Stem Cells to Treat Ischemic Heart Disease. J. Cardiovasc. Transl. Res. 2022, 15, 514–523. [Google Scholar] [CrossRef]
- Green, D.; Dalmay, T.; Chapman, T. Microguards and micromessengers of the genome. Heredity 2016, 116, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Thakur, N.; Monga, I.; Thakur, A.; Kumar, M. VIRmiRNA: A comprehensive resource for experimentally validated viral miRNAs and their targets. Database 2014, 2014, bau103. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, W.; Jiang, X.; Liu, Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: From mechanisms to therapy. Biomed. Pharmacother. 2023, 163, 114817. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, X.; Zhang, T.; Han, Y.; Tao, G. Knockdown of mesenchymal stem cell-derived exosomal LOC100129516 suppresses the symptoms of atherosclerosis via upregulation of the PPARgamma/LXRalpha/ABCA1 signaling pathway. Int. J. Mol. Med. 2021, 48, 208. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, L.; Zhu, X.; Li, Q.; Hu, L.; Li, H. Mesenchymal stem cell-derived exosomal miR-21a-5p promotes M2 macrophage polarization and reduces macrophage infiltration to attenuate atherosclerosis. Acta Biochim. Biophys. Sin. 2021, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, S.; Liu, X.; Wu, M. Mouse bone marrow derived mesenchymal stem cells-secreted exosomal microRNA-125b-5p suppresses atherosclerotic plaque formation via inhibiting Map4k4. Life Sci. 2021, 274, 119249. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Li, Z.; Yang, X.; Li, M.; Liu, C.; Pang, Y.; Zhang, L.; Li, X.; Liu, G.; Xiao, Y. Adipose-derived mesenchymal stem cells-derived exosome-mediated microRNA-342-5p protects endothelial cells against atherosclerosis. Aging 2020, 12, 3880–3898. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yin, R.; Zhu, X.; Yang, S.; Wang, J.; Zhou, Z.; Pan, X.; Ma, A. Mesenchymal stem-cell-derived exosomal miR-145 inhibits atherosclerosis by targeting JAM-A. Mol. Ther. Nucleic Acids 2021, 23, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhu, W.; Zhao, P.; Wang, Q.; Fan, B.; Zhu, Y.; Lu, Y.; Chen, Q.; Zhang, J.; Zhang, F. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: A novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 2020, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tang, R.; Xu, J.; Jiang, W.; Gong, Z.; Zhang, L.; Ning, Y.; Huang, P.; Chen, G.; Li, X.; et al. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-kappaB p65 pathway. Stem Cell Res. Ther. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Liang, X.L.; Zhang, C.L.; Pang, Y.H.; Lu, Y.X. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res. Ther. 2019, 10, 393. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liang, X.; Han, Q.; Shao, Z.; Zhang, Y.; Shi, L.; Hong, Y.; Li, W.; Mai, C.; Mo, Q.; et al. Hemin enhances the cardioprotective effects of mesenchymal stem cell-derived exosomes against infarction via amelioration of cardiomyocyte senescence. J. Nanobiotechnol. 2021, 19, 332. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, L.; Li, Q.; Tian, X.; Xu, J.; Xiong, Y.; Chen, G.; Qian, H.; Jin, C.; Yu, Y.; et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc. Res. 2020, 116, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Su, M.; Wang, X.; Xie, C. Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res. Ther. 2021, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, M.; Ruan, Z.; Zhu, L.; Tang, C. Mesenchymal stem cell-derived exosomal miR-143-3p suppresses myocardial ischemia-reperfusion injury by regulating autophagy. Life Sci. 2021, 280, 119742. [Google Scholar] [CrossRef]
- Lai, T.C.; Lee, T.L.; Chang, Y.C.; Chen, Y.C.; Lin, S.R.; Lin, S.W.; Pu, C.M.; Tsai, J.S.; Chen, Y.L. MicroRNA-221/222 Mediates ADSC-Exosome-Induced Cardioprotection Against Ischemia/Reperfusion by Targeting PUMA and ETS-1. Front. Cell Dev. Biol. 2020, 8, 569150. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Lu, S.; Luo, Y.; Zeng, J.; Liang, H.; Qin, D.; Wang, X.; Wang, T.; Pu, J.; Hu, H. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discov. 2022, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef]
- Pu, L.; Kong, X.; Li, H.; He, X. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am. J. Transl. Res. 2021, 13, 4007–4025. [Google Scholar] [PubMed]
- Wang, Z.; Gao, D.; Wang, S.; Lin, H.; Wang, Y.; Xu, W. Exosomal microRNA-1246 from human umbilical cord mesenchymal stem cells potentiates myocardial angiogenesis in chronic heart failure. Cell Biol. Int. 2021, 45, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.T.; Guo, H.D. Small Extracellular Vesicles Derived from Induced Pluripotent Stem Cells in the Treatment of Myocardial Injury. Int. J. Mol. Sci. 2023, 24, 4577. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.J.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Srivastava, D. Direct cardiac reprogramming: From developmental biology to cardiac regeneration. Circ. Res. 2013, 113, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.J.; Martinez-Fernandez, A.; Yamada, S.; Perez-Terzic, C.; Ikeda, Y.; Terzic, A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2009, 120, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.H.; Chen, I.C.; Tseng, S.Y.; Li, Y.H. Pluripotent Stem Cell Therapy in Ischemic Cardiovascular Disease. Acta Cardiol. Sin. 2014, 30, 365–374. [Google Scholar] [PubMed]
- Rosales, R.M.; Mountris, K.A.; Olivan-Viguera, A.; Perez-Zabalza, M.; Cedillo-Servin, G.; Iglesias-Garcia, O.; Hrynevich, A.; Castilho, M.; Malda, J.; Prosper, F.; et al. Experimentally-guided in silico design of engineered heart tissues to improve cardiac electrical function after myocardial infarction. Comput. Biol. Med. 2024, 171, 108044. [Google Scholar] [CrossRef] [PubMed]
- Park, N.K.; Park, S.J.; Park, Y.G.; Moon, S.H.; Woo, J.; Kim, H.J.; Kim, S.J.; Choi, S.W. Translation reinitiation in c.453delC frameshift mutation of KCNH2 producing functional hERG K+ channels with mild dominant negative effect in the heterozygote patient-derived iPSC cardiomyocytes. Hum. Mol. Genet. 2024, 33, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Maurissen, T.L.; Kawatou, M.; Lopez-Davila, V.; Minatoya, K.; Yamashita, J.K.; Woltjen, K. Modeling mutation-specific arrhythmogenic phenotypes in isogenic human iPSC-derived cardiac tissues. Sci. Rep. 2024, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, F.; Yin, L.; Tang, Y.; Wang, X.; Huang, C. Cadherin-5 facilitated the differentiation of human induced pluripotent stem cells into sinoatrial node-like pacemaker cells by regulating beta-catenin. J. Cell Physiol. 2024, 239, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.D.; Saito, Y.; Nakamura, K.; Iida, T.; Yuasa, S. Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 987. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, N.; Ale-Ebrahim, M.; Asadi, Y.; Barikrow, N.; Salimi, A.; Roholah, F. Combined Application of Human Amniotic Membrane Mesenchymal Stem Cells and a Modified PGS-co-PCL Film in an Experimental Model of Myocardial Ischemia-Reperfusion Injury. Appl. Biochem. Biotechnol. 2023, 195, 7502–7519. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Yang, X.; Fan, S.; Fan, T.; Zhang, M.; Ono, M. Engineering of MSCs sheet for the prevention of myocardial ischemia and for left ventricle remodeling. Stem Cell Res. Ther. 2023, 14, 102. [Google Scholar] [CrossRef]
- Wang, Y.; Tagil, K.; Ripa, R.S.; Nilsson, J.C.; Carstensen, S.; Jorgensen, E.; Sondergaard, L.; Hesse, B.; Johnsen, H.E.; Kastrup, J. Effect of mobilization of bone marrow stem cells by granulocyte colony stimulating factor on clinical symptoms, left ventricular perfusion and function in patients with severe chronic ischemic heart disease. Int. J. Cardiol. 2005, 100, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Moazzami, K.; Roohi, A.; Moazzami, B. Granulocyte colony stimulating factor therapy for acute myocardial infarction. Cochrane Database Syst. Rev. 2013, 2013, CD008844. [Google Scholar] [PubMed]
- Steppich, B.; Hadamitzky, M.; Ibrahim, T.; Groha, P.; Schunkert, H.; Laugwitz, K.L.; Kastrati, A.; Ott, I. Stem cell mobilisation by granulocyte-colony stimulating factor in patients with acute myocardial infarction. Long-term results of the REVIVAL-2 trial. Thromb. Haemost. 2016, 115, 864–868. [Google Scholar] [PubMed]
- Leone, A.M.; D’Amario, D.; Cannata, F.; Graziani, F.; Borovac, J.A.; Leone, G.; De Stefano, V.; Basile, E.; Siracusano, A.; Galiuto, L.; et al. The Effects of Granulocyte Colony-Stimulating Factor in Patients with a Large Anterior Wall Acute Myocardial Infarction to Prevent Left Ventricular Remodeling: A 10-Year Follow-Up of the RIGENERA Study. J. Clin. Med. 2020, 9, 1214. [Google Scholar] [CrossRef]
- Sung, P.H.; Lin, H.S.; Lin, W.C.; Chang, C.C.; Pei, S.N.; Ma, M.C.; Chen, K.H.; Chiang, J.Y.; Chang, H.W.; Lee, F.Y.; et al. Intra-carotid arterial transfusion of autologous circulatory derived CD34+ cells for old ischemic stroke patients—A phase I clinical trial to evaluate safety and tolerability. Am. J. Transl. Res. 2018, 10, 2975–2989. [Google Scholar] [PubMed]
- Herrmann, J.L.; Abarbanell, A.M.; Weil, B.R.; Manukyan, M.C.; Poynter, J.A.; Brewster, B.J.; Wang, Y.; Meldrum, D.R. Optimizing stem cell function for the treatment of ischemic heart disease. J. Surg. Res. 2011, 166, 138–145. [Google Scholar] [CrossRef]
- Huang, P.; Tian, X.; Li, Q.; Yang, Y. New strategies for improving stem cell therapy in ischemic heart disease. Heart Fail. Rev. 2016, 21, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.; Yang, Y.J.; Dong, Q.T.; Wang, T.J.; Qian, H.Y.; Li, N.; Wang, X.M.; Jin, C. Atorvastatin treatment improves the effects of mesenchymal stem cell transplantation on acute myocardial infarction: The role of the RhoA/ROCK/ERK pathway. Int. J. Cardiol. 2014, 176, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, Y.J.; Dong, Q.T.; Qian, H.Y.; Gao, R.L.; Qiao, S.B.; Shen, R.; He, Z.X.; Lu, M.J.; Zhao, S.H.; et al. Atorvastatin enhance efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS ONE 2013, 8, e65702. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, Y.J.; Qian, H.Y.; Li, Q.; Zhang, Q.; Li, X.D.; Dong, Q.T.; Xu, H.; Song, L.; Zhang, H. Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: Role of CXCR4. Am. J. Transl. Res. 2015, 7, 1058–1070. [Google Scholar] [PubMed]
- Sun, C.K.; Shao, P.L.; Wang, C.J.; Yip, H.K. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: A review. Am. J. Transl. Res. 2011, 3, 259–268. [Google Scholar] [PubMed]
- Sheu, J.J.; Sun, C.K.; Chang, L.T.; Fang, H.Y.; Chung, S.Y.; Chua, S.; Fu, M.; Lee, F.Y.; Kao, Y.H.; Ko, S.F.; et al. Shock wave-pretreated bone marrow cells further improve left ventricular function after myocardial infarction in rabbits. Ann. Vasc. Surg. 2010, 24, 809–821. [Google Scholar] [CrossRef]
- Ding, S.; Fan, Z.; Lin, C.; Dai, Q.; Zhou, J.; Huang, H.; Xu, Y.; Zhong, C. Therapeutic Effects of Ischemic-Preconditioned Exosomes in Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 998, 271–281. [Google Scholar] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.I.; Penninger, J.M. Functional Recovery of a Human Neonatal Heart after Severe Myocardial Infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Lv, X.; Liu, W.; Zhu, S.; Wang, Y.; Xu, H. Unraveling the Intricate Roles of Exosomes in Cardiovascular Diseases: A Comprehensive Review of Physiological Significance and Pathological Implications. Int. J. Mol. Sci. 2023, 24, 15677. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Lin, L.; Fang, F.; Zhang, B.; Shen, C. Mechanisms and Optimization Strategies of Paracrine Exosomes from Mesenchymal Stem Cells in Ischemic Heart Disease. Stem Cells Int. 2023, 2023, 6500831. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Gurusamy, N.; Ulaganathan, T.; Paluck, A.J.; Ramalingam, S.; Rajasingh, J. Therapeutic potentials of stem cell-derived exosomes in cardiovascular diseases. Exp. Biol. Med. 2023, 248, 434–444. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, I.-T.; Sun, C.-K. Stem Cell Therapy against Ischemic Heart Disease. Int. J. Mol. Sci. 2024, 25, 3778. https://doi.org/10.3390/ijms25073778
Tsai I-T, Sun C-K. Stem Cell Therapy against Ischemic Heart Disease. International Journal of Molecular Sciences. 2024; 25(7):3778. https://doi.org/10.3390/ijms25073778
Chicago/Turabian StyleTsai, I-Ting, and Cheuk-Kwan Sun. 2024. "Stem Cell Therapy against Ischemic Heart Disease" International Journal of Molecular Sciences 25, no. 7: 3778. https://doi.org/10.3390/ijms25073778
APA StyleTsai, I.-T., & Sun, C.-K. (2024). Stem Cell Therapy against Ischemic Heart Disease. International Journal of Molecular Sciences, 25(7), 3778. https://doi.org/10.3390/ijms25073778







