Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity
Abstract
1. Introduction
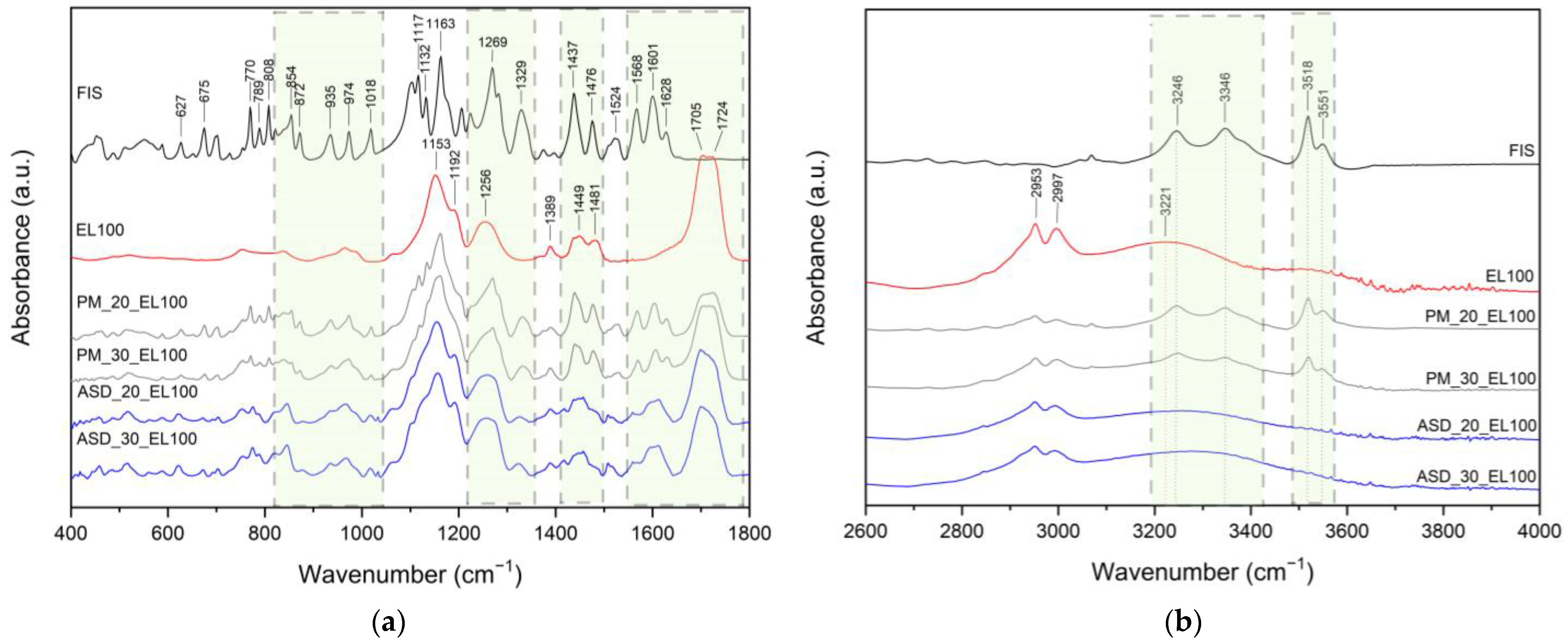
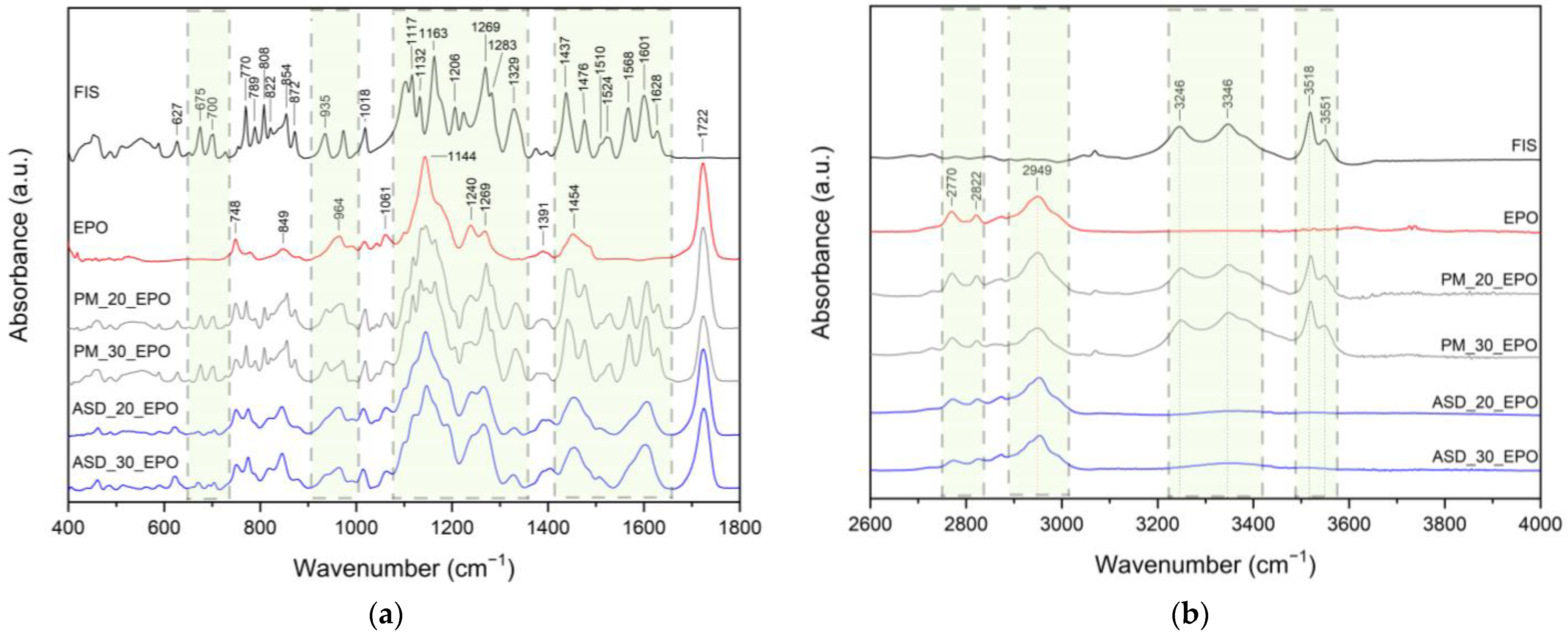
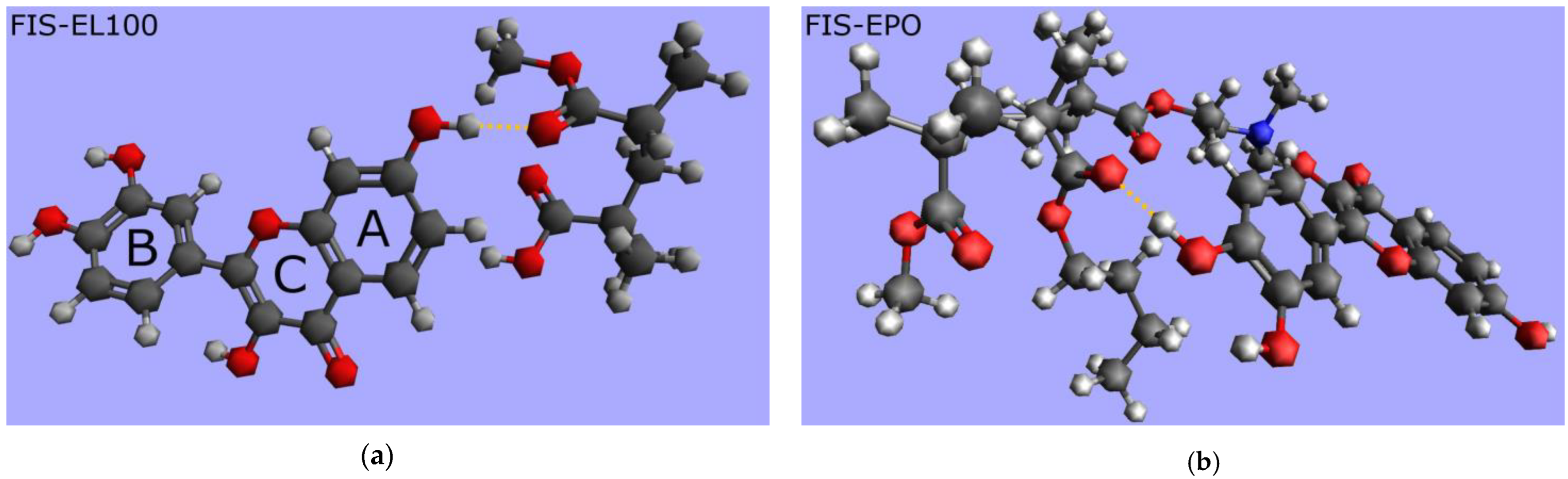
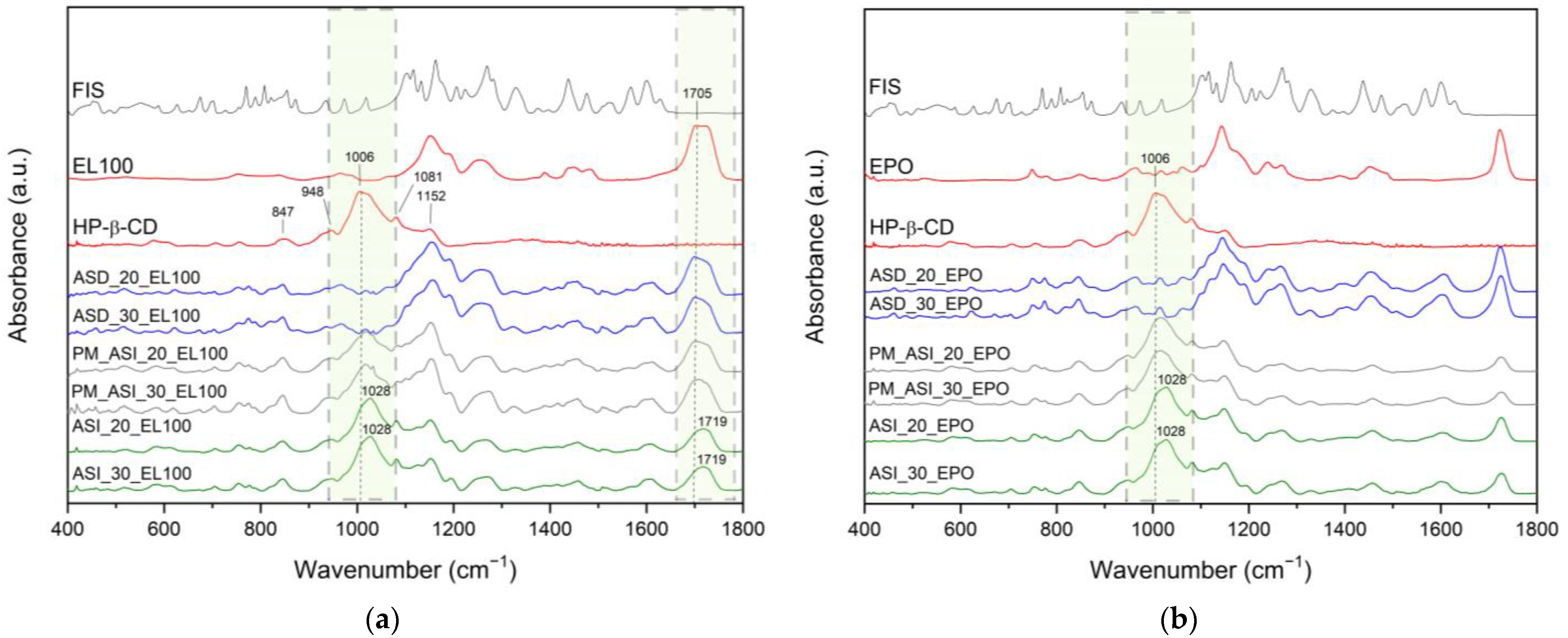
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Amorphous Solid Dispersion (ASD) of FIS
3.3. Preparation of Amorphous Solid Inclusion (ASI) of FIS
3.4. Identification of Neat Compounds, ASDs and ASIs
3.4.1. X-ray Powder Diffraction (XRPD)
3.4.2. Thermogravimetric Analysis (TG)
3.4.3. Differential Scanning Calorimetry (DSC)
- EL100: 30–250 °C; 250 °C; 250–10 °C; 10 °C; 10 °C–200 °C.
- EPO: 30–245 °C; 245 °C; 245–−10 °C; −10 °C; −10–120 °C.
- FIS-EL100 ASD: 30–245 °C; 245 °C; 245–10 °C; 10 °C; 10 °C–200 °C.
- FIS-EPO ASD: 5–120 °C; 120 °C; 120–5 °C; 5 °C; 5 °C–120 °C.
3.4.4. ATR-FTIR Spectroscopy
3.4.5. Molecular Modeling
3.5. Studies of Results Introduction of FIS into ASD/ASI
3.5.1. The Apparent Solubility
3.5.2. Antioxidant Properties
3.5.3. Anticholinesterase Activity
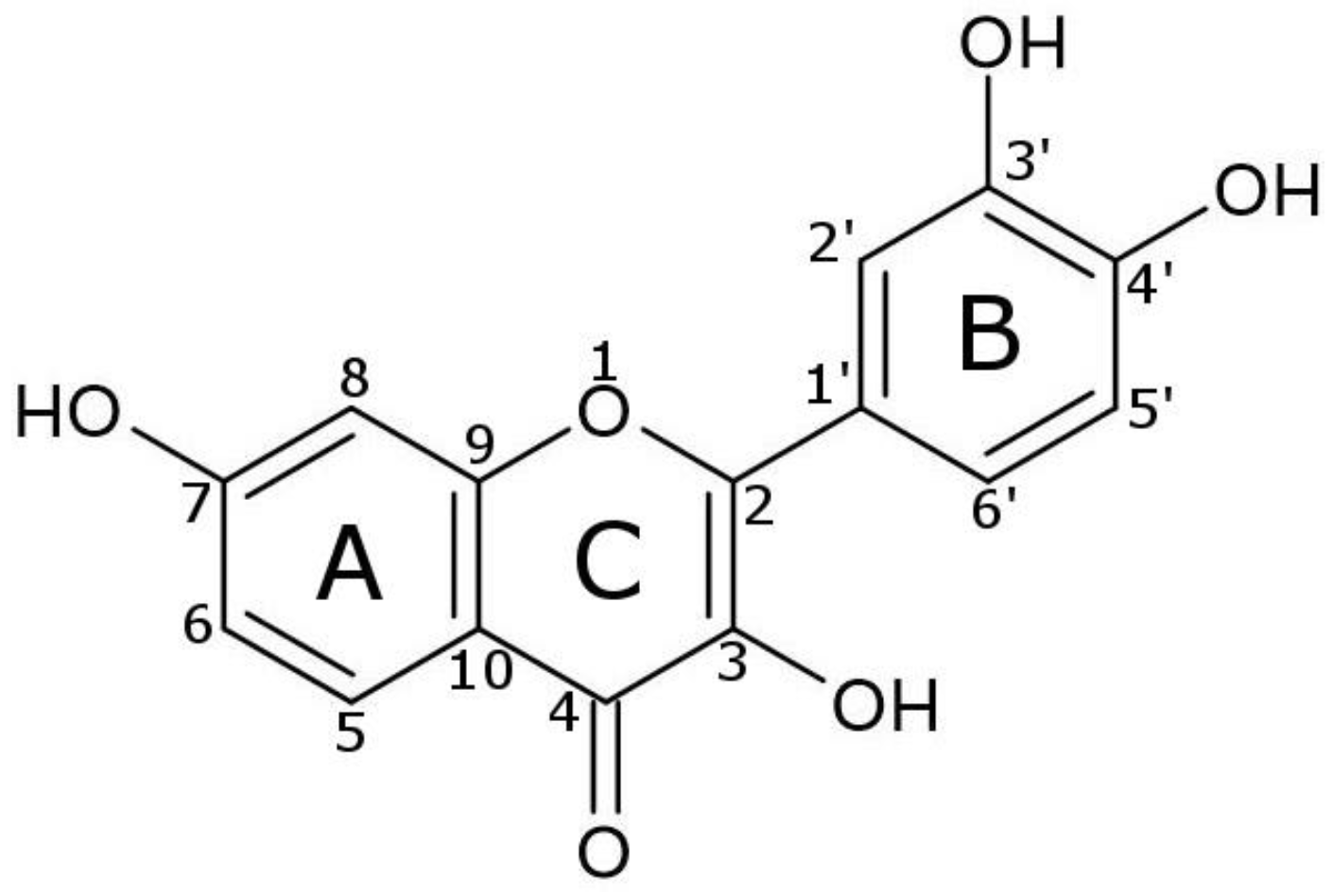
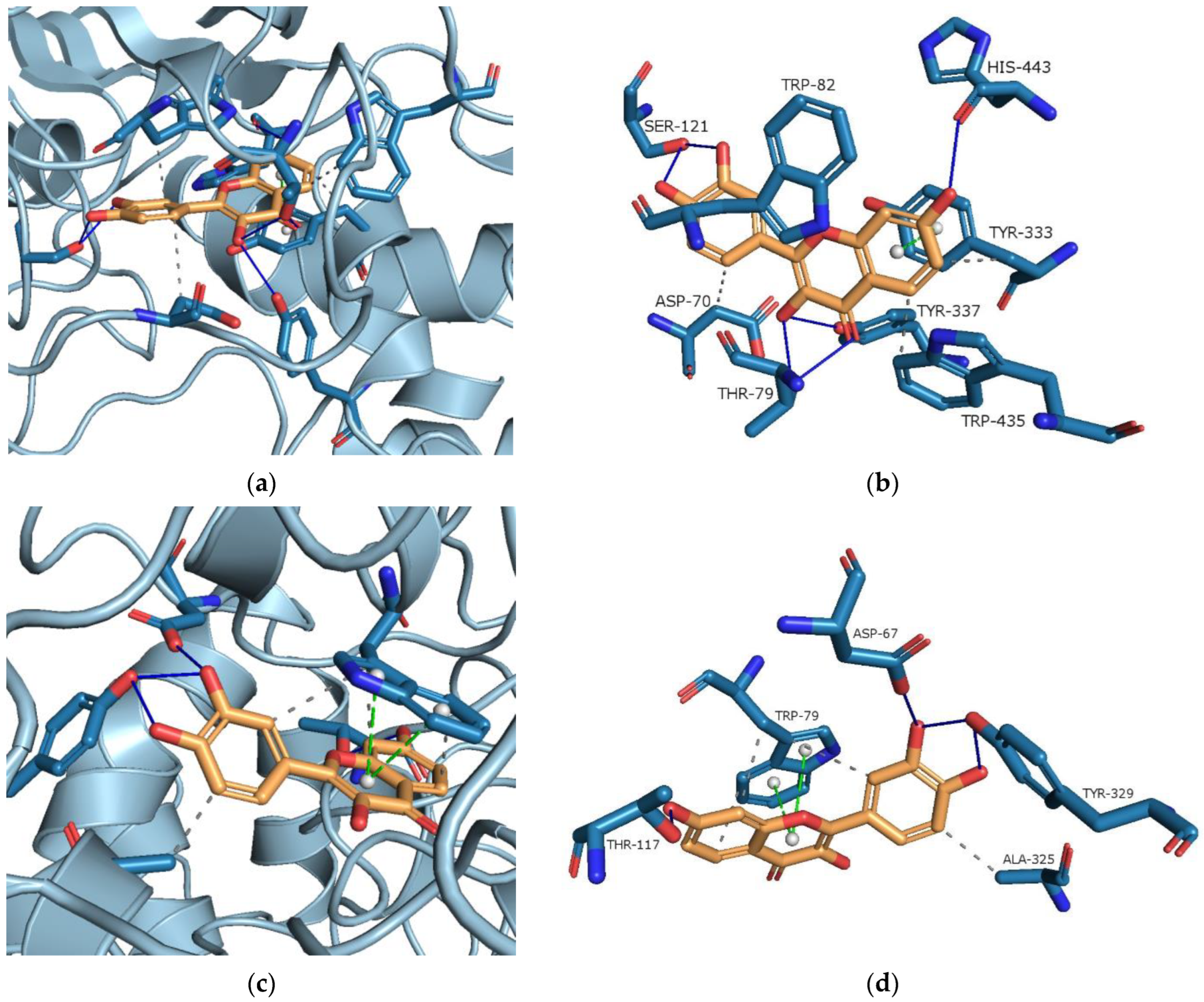
3.5.4. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, R.; Farag, M.A.; Chen, M.; He, C.; Xiao, J. Recent advances in the biosynthesis, structure–activity relationships, formulations, pharmacology, and clinical trials of fisetin. eFood 2022, 3, e3. [Google Scholar] [CrossRef]
- Kumari, S.; Kamboj, A.; Wanjari, M.; Sharma, A.K. Protective Role of Fisetin in STZ Induced Diabetic Nephropathy in Rats. J. Pharm. Res. Int. 2021, 33, 97–111. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Kapoor, B.; Vyas, M.; Khursheed, R. Enhanced oral bioavailability and neuroprotective effect of fisetin through its SNEDDS against rotenone-induced Parkinson’s disease rat model. Food Chem. Toxicol. 2020, 144, 111590. [Google Scholar] [CrossRef]
- Chenxu, G.; Xianling, D.; Qin, K.; Linfeng, H.; Yan, S.; Mingxin, X.; Jun, T.; Minxuan, X. Fisetin protects against high fat diet-induced nephropathy by inhibiting inflammation and oxidative stress via the blockage of iRhom2/NF-κB signaling. Int. Immunopharmacol. 2021, 92, 107353. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, X.; Deng, P.; Jiang, C.; He, Y.; Hao, D.; Yang, H. The Neuroprotective Role of Fisetin in Different Neurological Diseases: A Systematic Review. Mol. Neurobiol. 2023, 60, 6383–6394. [Google Scholar] [CrossRef]
- Maher, P. Preventing and Treating Neurological Disorders with the Flavonol Fisetin. Brain Plast. 2021, 6, 155–166. [Google Scholar] [CrossRef]
- Leclerc, J.L.; Garcia, J.M.; Diller, M.A.; Carpenter, A.-M.; Kamat, P.K.; Hoh, B.L.; Doré, S. A Comparison of Pathophysiology in Humans and Rodent Models of Subarachnoid Hemorrhage. Front. Mol. Neurosci. 2018, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, D.; Wu, H.; Jia, H.; Yang, C.; Zhang, L. Fisetin Prolongs Therapy Window of Brain Ischemic Stroke Using Tissue Plasminogen Activator: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Clin. Appl. Thromb. 2019, 25, 107602961987135. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, H.; Ahn, J.; Jeon, T.-I.; Lee, D.-H.; Ha, T.-Y. Fisetin regulates obesity by targeting mTORC1 signaling. J. Nutr. Biochem. 2013, 24, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Prasath, G.S.; Subramanian, S.P. Modulatory effects of fisetin, a bioflavonoid, on hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in hepatic and renal tissues in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2011, 668, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J. Gerontol. Ser. A 2018, 73, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhou, Y.; Zhu, Y.; Fei, M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem. Int. 2018, 118, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Diamond, A.C.; Strickland, L.R.; Kappes, J.C.; Katiyar, S.K.; Elmets, C.A.; Athar, M.; Afaq, F. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget 2016, 7, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front. Pharmacol. 2021, 12, 612078. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Chen, S.; Wen, Y.; Zhang, S.; Luo, E.; Li, X.; Zhong, W.; Zeng, H. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. CNS Neurosci. Ther. 2022, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Teegala, S.B.; Kalakotla, S.; Pasangulapati, J.P.; Perumal, V.; Boyina, H.K. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. Eur. J. Pharmacol. 2021, 910, 174492. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.-K.; Park, S.-K. Effects of Fisetin, a Plant-Derived Flavonoid, on Response to Oxidative Stress, Aging, and Age-Related Diseases in Caenorhabditis elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin—In Search of Better Bioavailability—From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. [Google Scholar] [CrossRef]
- Vishwas, S.; Singh, S.K.; Gulati, M.; Awasthi, A.; Khursheed, R.; Corrie, L.; Kumar, R.; Collet, T.; Loebenberg, R.; Porwal, O.; et al. Harnessing the therapeutic potential of fisetin and its nanoparticles: Journey so far and road ahead. Chem. Biol. Interact. 2022, 356, 109869. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xu, P.-Y.; Fu, C.-P.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. Fabrication of Supercritical Antisolvent (SAS) Process-Assisted Fisetin-Encapsulated Poly (Vinyl Pyrrolidone) (PVP) Nanocomposites for Improved Anticancer Therapy. Nanomaterials 2020, 10, 322. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, X.; Chu, K.; Zhang, H.; Ji, W.; Rui, M. Preparation and optimization of poly (lactic acid) nanoparticles loaded with fisetin to improve anti-cancer therapy. Int. J. Biol. Macromol. 2019, 125, 700–710. [Google Scholar] [CrossRef]
- Sip, S.; Rosiak, N.; Sip, A.; Żarowski, M.; Hojan, K.; Cielecka-Piontek, J. A Fisetin Delivery System for Neuroprotection: A Co-Amorphous Dispersion Prepared in Supercritical Carbon Dioxide. Antioxidants 2023, 13, 24. [Google Scholar] [CrossRef]
- Kumar, R.; Khursheed, R.; Kumar, R.; Awasthi, A.; Sharma, N.; Khurana, S.; Kapoor, B.; Khurana, N.; Singh, S.K.; Gowthamarajan, K.; et al. Self-nanoemulsifying drug delivery system of fisetin: Formulation, optimization, characterization and cytotoxicity assessment. J. Drug Deliv. Sci. Technol. 2019, 54, 101252. [Google Scholar] [CrossRef]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef]
- Fang, J.; Xu, B.; Jin, X.; Chen, F.; Liu, S.; Liu, S.; Wang, S.; Zhang, F.; Song, K.; Wang, J.; et al. Nerve Guide Conduits Integrated with Fisetin-Loaded Chitosan Hydrogels for Reducing Oxidative Stress, Inflammation, and Nerve Regeneration. Macromol. Biosci. 2024, 2300476. [Google Scholar] [CrossRef] [PubMed]
- Athira, K.; Syam Das, S.; Swick, A.; Krishnakumar, I.M.; Abdul Vahab, A. Oral bioavailability and neuroprotective effect of a novel food-grade formulation of fisetin using fenugreek-galactomannan hydrogel scaffolds. PharmaNutrition 2023, 23, 100329. [Google Scholar] [CrossRef]
- Krishnakumar, I.M.; Jaja-Chimedza, A.; Joseph, A.; Balakrishnan, A.; Maliakel, B.; Swick, A. Enhanced bioavailability and pharmacokinetics of a novel hybrid-hydrogel formulation of fisetin orally administered in healthy individuals: A randomised double-blinded comparative crossover study. J. Nutr. Sci. 2022, 11, e74. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.-K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M. Preparation and optimization of fisetin loaded glycerol based soft nanovesicles by Box-Behnken design. Int. J. Pharm. 2020, 578, 119125. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Iqbal, B.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Development of transethosomes formulation for dermal fisetin delivery: Box–Behnken design, optimization, in vitro skin penetration, vesicles–skin interaction and dermatokinetic studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 755–765. [Google Scholar] [CrossRef]
- Dzakwan, M. Kinetic solubility of fisetin nanocrystal. J. Farm. 2018, 1, 7–10. [Google Scholar] [CrossRef]
- Nutho, B.; Khuntawee, W.; Rungnim, C.; Pongsawasdi, P.; Wolschann, P.; Karpfen, A.; Kungwan, N.; Rungrotmongkol, T. Binding mode and free energy prediction of fisetin/β-cyclodextrin inclusion complexes. Beilstein J. Org. Chem. 2014, 10, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, K.; An, K.; Ren, S.-H.; Xie, X.; Jin, Y.; Lin, J. Novel water-soluble fisetin/cyclodextrins inclusion complexes: Preparation, characterization, molecular docking and bioavailability. Carbohydr. Res. 2015, 418, 20–28. [Google Scholar] [CrossRef]
- Preuksarattanawut, C.; Mangmee, W.; Prousoontorn, M.; Nisarattanaporn, E.; Siraleartmukul, K. Enhancing stability and antioxidant efficacy of fisetin by encapsulating as β-cyclodextrin inclusion complex with porous polylactic acid film from breath figure. J. Met. Mater. Miner. 2021, 31. [Google Scholar] [CrossRef]
- Corina, D.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-chemical and Biological Evaluation of Flavonols: Fisetin, Quercetin and Kaempferol Alone and Incorporated in beta Cyclodextrins. Anticancer. Agents Med. Chem. 2017, 17, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Kadari, A.; Gudem, S.; Kulhari, H.; Bhandi, M.M.; Borkar, R.M.; Kolapalli, V.R.M.; Sistla, R. Enhanced oral bioavailability and anticancer efficacy of fisetin by encapsulating as inclusion complex with HPβCD in polymeric nanoparticles. Drug Deliv. 2017, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ho, C. Amorphous Solid Dispersions: Utilization and Challenges in Drug Discovery and Development. J. Pharm. Sci. 2015, 104, 3237–3258. [Google Scholar] [CrossRef]
- Gilley, A.D.; Arca, H.C.; Nichols, B.L.B.; Mosquera-Giraldo, L.I.; Taylor, L.S.; Edgar, K.J.; Neilson, A.P. Novel cellulose-based amorphous solid dispersions enhance quercetin solution concentrations in vitro. Carbohydr. Polym. 2017, 157, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, N.; Wdowiak, K.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Solid Dispersion of Hesperidin with Polymer Excipients for Enhanced Apparent Solubility as a More Effective Approach to the Treatment of Civilization Diseases. Int. J. Mol. Sci. 2022, 23, 15198. [Google Scholar] [CrossRef] [PubMed]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Amorphous Pterostilbene Delivery Systems Preparation—Innovative Approach to Preparation Optimization. Pharmaceutics 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-J.; Ma, S.-X.; Zhou, S.-Y.; Chen, W.; Yuan, M.-W.; Yin, Y.-Q.; Yang, X.-D.; Guan, M.; Shi, R.; Zheng, Y.; et al. Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-β-Cyclodextrin Inclusion for Pulmonary Delivery. Carbohydr. Polym. 2020, 98, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Teng, J.; Selbo, J. Amorphous solid dispersion of epigallocatechin gallate for enhanced physical stability and controlled release. Pharmaceuticals 2017, 10, 88. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Elzayat, E.M.; Alshehri, S.M.; Mohsin, K.; Ibrahim, M.A.; Al Meanazel, O.T.; Shakeel, F.; Alanazi, F.K.; Alsarra, I.A. Utilizing spray drying technique to improve oral bioavailability of apigenin. Adv. Powder Technol. 2018, 29, 1676–1684. [Google Scholar] [CrossRef]
- Jangid, A.K.; Jain, P.; Medicherla, K.; Pooja, D.; Kulhari, H. Solid-state properties, solubility, stability and dissolution behaviour of co-amorphous solid dispersions of baicalin. CrystEngComm 2020, 22, 6128–6136. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.; Song, J.G.; Han, H.-K. Improved in vivo Effect of Chrysin as an Absorption Enhancer via the Preparation of Ternary Solid Dispersion with Brij®L4 and Aminoclay. Curr. Drug Deliv. 2018, 16, 86–92. [Google Scholar] [CrossRef]
- Panizzon, G.P.; Giacomini Bueno, F.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Manufacturing Different Types of Solid Dispersions of BCS Class IV Polyphenol (Daidzein) by Spray Drying: Formulation and Bioavailability. Pharmaceutics 2019, 11, 492. [Google Scholar] [CrossRef]
- Zaini, E.; Putri, V.Z.; Octavia, M.D.; Ismed, F. Peningkatan Laju Disolusi Dispersi Padat Amorf Genistein dengan PVP K-30. J. Sains Farm. Klin. 2017, 4, 67. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. The Study of Amorphous Kaempferol Dispersions Involving FT-IR Spectroscopy. Int. J. Mol. Sci. 2023, 24, 17155. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, K.; Shimada, Y.; Ohno, A.; Otani, S.; Ago, Y.; Maeda, S.; Lin, B.; Nunomura, K.; Hino, N.; Suzuki, M.; et al. Physicochemical and Biochemical Evaluation of Amorphous Solid Dispersion of Naringenin Prepared Using Hot-Melt Extrusion. Front. Nutr. 2022, 9, 850103. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fan, N.; Zhang, G.; Sun, J.; He, Z.; Li, J. Quercetin amorphous solid dispersions prepared by hot melt extrusion with enhanced solubility and intestinal absorption. Pharm. Dev. Technol. 2020, 25, 472–481. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Zhou, F.; Liang, Q.; Deng, Y. The amorphous quercetin/hydroxypropylmethylcellulose acetate succinate solid dispersions prepared by co-precipitation method to enhance quercetin dissolution. J. Pharm. Sci. 2021, 110, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Hatwar, P.; Pathan, I.B.; Chishti, N.A.H.; Ambekar, W. Pellets containing quercetin amino acid co-amorphous mixture for the treatment of pain: Formulation, optimization, in-vitro and in-vivo study. J. Drug Deliv. Sci. Technol. 2021, 62, 102350. [Google Scholar] [CrossRef]
- Ha, E.-S.; Choi, D.H.; Baek, I.; Park, H.; Kim, M.-S. Enhanced Oral Bioavailability of Resveratrol by Using Neutralized Eudragit E Solid Dispersion Prepared via Spray Drying. Antioxidants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Li, J.; Li, J. Advantages of introducing an effective crystalline inhibitor in curcumin amorphous solid dispersions formulated by Eudragit E100. J. Pharm. Pharmacol. 2021, 73, 185–192. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Zhao, X.; Zhang, S.; Cao, M.; Wang, X.; Li, W. Development and characterization of an amorphous curcumin-Eudragit ® E100 solid dispersions with improved solubility, stability, and pharmacokinetic properties. Pharm. Dev. Technol. 2022, 27, 965–974. [Google Scholar] [CrossRef]
- Budiman, A.; Lailasari, E.; Nurani, N.V.; Yunita, E.N.; Anastasya, G.; Aulia, R.N.; Lestari, I.N.; Subra, L.; Aulifa, D.L. Ternary Solid Dispersions: A Review of the Preparation, Characterization, Mechanism of Drug Release, and Physical Stability. Pharmaceutics 2023, 15, 2116. [Google Scholar] [CrossRef]
- Davis, M.T.; Potter, C.B.; Mohammadpour, M.; Albadarin, A.B.; Walker, G.M. Design of spray dried ternary solid dispersions comprising itraconazole, soluplus and HPMCP: Effect of constituent compositions. Int. J. Pharm. 2017, 519, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wan, W.; Zhu, S.; Liu, Q. Preparation of crystalline nanocellulose/hydroxypropyl β cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Im, D.; ud Din, F.; Kim, J.O.; Youn, Y.S.; Oh, K.T.; et al. New potential application of hydroxypropyl-β-cyclodextrin in solid self-nanoemulsifying drug delivery system and solid dispersion. Carbohydr. Polym. 2021, 271, 118433. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Liang, N.; Li, Q.; Yan, P.; Sun, S. Biotin and arginine modified hydroxypropyl-β-cyclodextrin nanoparticles as novel drug delivery systems for paclitaxel. Carbohydr. Polym. 2019, 216, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.R.; Kwon, J.; Vo, A.Q.; Bhagurkar, A.M.; Bandari, S.; Kim, D.W. Hot-Melt Extruded Amorphous Solid Dispersion for Solubility, Stability, and Bioavailability Enhancement of Telmisartan. Pharmaceuticals 2021, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Alsayad, R. Preparation and in vitro evaluation for amorphous solid dispersion of azithromycin. Azithromycin 2023. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, C.; Guan, X.; Yuan, D. The solid dispersion of resveratrol with enhanced dissolution and good system physical stability. J. Drug Deliv. Sci. Technol. 2023, 84, 104507. [Google Scholar] [CrossRef]
- Zong, S.; Liu, Y.; Park, H.J.; Ye, M.; Li, J. Curcumin solid dispersion based on three model acrylic polymers: Formulation and release properties. Braz. J. Pharm. Sci. 2022, 58, e18946. [Google Scholar] [CrossRef]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Zalewski, P.; Tajber, L.; Cielecka-Piontek, J. Genistein Co-Amorphous Systems with Amino Acids: An Investigation into Enhanced Solubility and Biological Activity. Pharmaceutics 2023, 15, 2653. [Google Scholar] [CrossRef]
- Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Enhanced Antioxidant and Neuroprotective Properties of Pterostilbene (Resveratrol Derivative) in Amorphous Solid Dispersions. Int. J. Mol. Sci. 2024, 25, 2774. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Konecke, S.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr. Polym. 2013, 92, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, L.A.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Crystallization of Amorphous Solid Dispersions of Resveratrol during Preparation and Storage—Impact of Different Polymers. J. Pharm. Sci. 2013, 102, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fatmi, S.; Bournine, L.; Iguer-Ouada, M.; Lahiani-Skiba, M.; Bouchal, F.; Skiba, M. Amorphous solid dispersion studies of camptothecin-cyclodextrin inclusion complexes in PEG 6000. Acta Pol. Pharm 2015, 72, 179–192. [Google Scholar] [PubMed]
- Mane, P.T.; Wakure, B.S.; Wakte, P.S. Ternary inclusion complex of docetaxel using ß-cyclodextrin and hydrophilic polymer: Physicochemical characterization and in-vitro anticancer activity. J. Appl. Pharm. Sci. 2022, 12, 150–161. [Google Scholar] [CrossRef]
- Thiry, J.; Krier, F.; Ratwatte, S.; Thomassin, J.-M.; Jerome, C.; Evrard, B. Hot-melt extrusion as a continuous manufacturing process to form ternary cyclodextrin inclusion complexes. Eur. J. Pharm. Sci. 2017, 96, 590–597. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, E.W.V.; Dupont, S.; Beney, L.; Hoskin, R.T.; da Silva Pedrini, M.R. Osmoporation is a versatile technique to encapsulate fisetin using the probiotic bacteria Lactobacillus acidophilus. Appl. Microbiol. Biotechnol. 2022, 106, 1031–1044. [Google Scholar] [CrossRef]
- Skiba, M.; Gasmi, H.; Milon, N.; Bounoure, F.; Malika, L.S. Water Solubility and Dissolution Enhancement of Fisetin by Spherical Amorphous Solid Dispersion in Polymer of Cyclodextrin. Austin J. Biotechnol. Bioeng. 2021, 8, 1106. [Google Scholar] [CrossRef]
- Sathigari, S.K.; Radhakrishnan, V.K.; Davis, V.A.; Parsons, D.L.; Babu, R.J. Amorphous-State Characterization of Efavirenz—Polymer Hot-Melt Extrusion Systems for Dissolution Enhancement. J. Pharm. Sci. 2012, 101, 3456–3464. [Google Scholar] [CrossRef]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Suwardie, H.; Wang, P.; Gogos, C.G. Miscibility Studies of Indomethacin and Eudragit® E PO by Thermal, Rheological, and Spectroscopic Analysis. J. Pharm. Sci. 2012, 101, 2204–2212. [Google Scholar] [CrossRef]
- Garbiec, E.; Rosiak, N.; Tykarska, E.; Zalewski, P.; Cielecka-Piontek, J. Sinapic Acid Co-Amorphous Systems with Amino Acids for Improved Solubility and Antioxidant Activity. Int. J. Mol. Sci. 2023, 24, 5533. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermal analysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Eudragit: A Novel Carrier for Controlled Drug Delivery in Supercritical Antisolvent Coprecipitation. Polymers 2020, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Dimitrić Marković, J.M.; Marković, Z.S.; Milenković, D.; Jeremić, S. Application of comparative vibrational spectroscopic and mechanistic studies in analysis of fisetin structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Awadeen, R.H.; Boughdady, M.F.; Zaghloul, R.A.; Elsaed, W.M.; Abu Hashim, I.I.; Meshali, M.M. Formulation of lipid polymer hybrid nanoparticles of the phytochemical Fisetin and its in vivo assessment against severe acute pancreatitis. Sci. Rep. 2023, 13, 19110. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Burapapadh, K.; Praphanwittaya, P.; Loftsson, T. Characterization and Evaluation of Ternary Complexes of Ascorbic Acid with γ-Cyclodextrin and Poly(vinyl Alcohol). Int. J. Mol. Sci. 2020, 21, 4399. [Google Scholar] [CrossRef] [PubMed]
- Al-Burtomani, S.K.S.; Suliman, F.O. Inclusion complexes of norepinephrine with β-cyclodextrin, 18-crown-6 and cucurbit [7]uril: Experimental and molecular dynamics study. RSC Adv. 2017, 7, 9888–9901. [Google Scholar] [CrossRef]
- El-Maradny, H.; Mortada, S.; Kamel, O.; Hikal, A. Characterization of ternary complexes of meloxicam-HPβCD and PVP or L-arginine prepared by the spray-drying technique. Acta Pharm. 2008, 58, 455–466. [Google Scholar] [CrossRef]
- Gupta, P.; Bansal, A.K. Molecular interactions in celecoxib-PVP-meglumine amorphous system. J. Pharm. Pharmacol. 2010, 57, 303–310. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta-Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, S.-E.; Pyo, Y.-C.; Tran, P.; Park, J.-S. Solubility enhancement and application of cyclodextrins in local drug delivery. J. Pharm. Investig. 2020, 50, 17–27. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Drug Solubilization and Stabilization by Cyclodextrin Drug Carriers. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Wiley: Hoboken, NJ, USA, 2013; pp. 67–101. [Google Scholar]
- Taupitz, T.; Dressman, J.B.; Buchanan, C.M.; Klein, S. Cyclodextrin-water soluble polymer ternary complexes enhance the solubility and dissolution behaviour of poorly soluble drugs. Case example: Itraconazole. Eur. J. Pharm. Biopharm. 2013, 83, 378–387. [Google Scholar] [CrossRef]
- Ahad, A.; Jardan, Y.A.B.; Raish, M.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Ternary Inclusion Complex of Sinapic Acid with Hydroxypropyl-β-cyclodextrin and Hydrophilic Polymer Prepared by Microwave Technology. Processes 2022, 10, 2637. [Google Scholar] [CrossRef]
- Agrawal, R.; Patel, N.; Raval, M. Novel Amorphous Solid Dispersions of Canagliflozin Hemihydrate In Eudragit® E Po. Int. J. Pharm. Sci. Res. 2019, 10, 2923. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, R.; Chen, Y.; Ke, X.; Hu, D.; Han, M. Application of carrier and plasticizer to improve the dissolution and bioavailability of poorly water-soluble baicalein by hot melt extrusion. AAPS PharmSciTech 2014, 15, 560–568. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Kacprzak, K.; Markowska, J.; Huczyński, A. Role of Fisetin in Selected Malignant Neoplasms in Women. Nutrients 2023, 15, 4686. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef]
- Chiruta, C.; Schubert, D.; Dargusch, R.; Maher, P. Chemical Modification of the Multitarget Neuroprotective Compound Fisetin. J. Med. Chem. 2012, 55, 378–389. [Google Scholar] [CrossRef]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-Induced Morphological Modifications of Endothelial Cells Through Microtubule Stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef]
- Huang, M.-C.; Hsueh, T.Y.; Cheng, Y.-Y.; Lin, L.-C.; Tsai, T.-H. Pharmacokinetics and biliary excretion of fisetin in rats. J. Agric. Food Chem. 2018, 66, 6300–6307. [Google Scholar] [CrossRef]
- Muhammad, A.; Tel-Cayan, G.; Öztürk, M.; Nadeem, S.; Duru, M.E.; Anis, I.; Ng, S.W.; Shah, M.R. Biologically active flavonoids from Dodonaea viscosa and their structure–activity relationships. Ind. Crops Prod. 2015, 78, 66–72. [Google Scholar] [CrossRef]
- Wang, T.; Lin, H.; Tu, Q.; Liu, J.; Li, X. Fisetin Protects DNA Against Oxidative Damage and Its Possible Mechanism. Adv. Pharm. Bull. 2016, 6, 267–270. [Google Scholar] [CrossRef]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Rivera, F.; Urbanavicius, J.; Gervaz, E.; Morquio, A.; Dajas, F. Some aspects of the in vivo neuroprotective capacity of flavonoids: Bioavailability and structure-activity relationship. Neurotox. Res. 2004, 6, 543–553. [Google Scholar] [CrossRef]
- Sokal, A.; Stocerz, K.; Olczyk, P.; Kadela-Tomanek, M. Therapeutic potential of flavonoids used in traditional Chinese medicine—A comparative study of galangin, kaempferol, chrysin and fisetin. In Annales Academiae Medicae Silesiensis; Śląski Uniwersytet Medyczny w Katowicach: Katowice, Poland, 2024; pp. 49–60. [Google Scholar]
- Katalinić, M.; Rusak, G.; Domaćinović Barović, J.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Kumar, V.; Haldar, S.; Ghosh, S.; Chauhan, S.; Sharma, A.; Dhankhar, P.; Kumar, A.; Jaiswal, S.; Saini, S.; Gupta, S.; et al. Pterostilbene-isothiocyanate impedes RANK/TRAF6 interaction to inhibit osteoclastogenesis, promoting osteogenesis in vitro and alleviating glucocorticoid induced osteoporosis in rats. Biochem. Pharmacol. 2022, 206, 115284. [Google Scholar] [CrossRef]
- Lv, C.; Ma, X.; Liang, C.; Chen, Y.; Qin, F.; Zhou, C.; Huang, W.; Liu, Q.; Wang, Y.; Liu, Z.; et al. The interaction of pterostilbene with Kelch-like ECH-associated protein 1 and its regulation on the nuclear factor erythroid 2-related factor 2/antioxidant response element pathway. Process Biochem. 2023, 132, 228–235. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Ban, W.; Liu, H.; Lv, L.; Zhang, B.; Liu, A.; Hou, Z.; Lu, J.; Chen, X.; et al. Pterostilbene alleviated cerebral ischemia/reperfusion-induced blood–brain barrier dysfunction via inhibiting early endothelial cytoskeleton reorganization and late basement membrane degradation. Food Funct. 2023, 14, 8291–8308. [Google Scholar] [CrossRef]
- Tippani, R.; Prakhya, L.J.S.; Porika, M.; Sirisha, K.; Abbagani, S.; Thammidala, C. Pterostilbene as a potential novel telomerase inhibitor: Molecular docking studies and its in vitro evaluation. Curr. Pharm. Biotechnol. 2014, 14, 1027–1035. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; You, S.; Zhang, J.; Zhang, Y.; Akram, Z.; Sun, S. Pterostilbene upregulates MICA/B via the PI3K/AKT signaling pathway to enhance the capability of natural killer cells to kill cervical cancer cells. Exp. Cell Res. 2024, 435, 113933. [Google Scholar] [CrossRef]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef]
- Shi, W.; Han, W.; Liao, Y.; Wen, J.; Zhang, G. Inhibition mechanism of fisetin on acetylcholinesterase and its synergistic effect with galantamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 305, 123452. [Google Scholar] [CrossRef]
- Scotti, L.; Scotti, M.T. In Silico Studies Applied to Natural Products with Potential Activity Against Alzheimer’s Disease. In Computational Modeling of Drugs Against Alzheimer’s Disease; Springer: Berlin/Heidelberg, Germany, 2018; pp. 513–531. [Google Scholar]
- Farihi, A.; Bouhrim, M.; Chigr, F.; Elbouzidi, A.; Bencheikh, N.; Zrouri, H.; Nasr, F.A.; Parvez, M.K.; Alahdab, A.; Ahami, A.O.T. Exploring Medicinal Herbs’ Therapeutic Potential and Molecular Docking Analysis for Compounds as Potential Inhibitors of Human Acetylcholinesterase in Alzheimer’s Disease Treatment. Medicina 2023, 59, 1812. [Google Scholar] [CrossRef]
- Inam, S.; Irfan, M.; Lali, N.U.A.; Khalid Syed, H.; Asghar, S.; Khan, I.U.; Khan, S.-U.-D.; Iqbal, M.S.; Zaheer, I.; Khames, A.; et al. Development and Characterization of Eudragit® EPO-Based Solid Dispersion of Rosuvastatin Calcium to Foresee the Impact on Solubility, Dissolution and Antihyperlipidemic Activity. Pharmaceuticals 2022, 15, 492. [Google Scholar] [CrossRef]
- Linares, V.; Yarce, C.J.; Echeverri, J.D.; Galeano, E.; Salamanca, C.H. Relationship between degree of polymeric ionisation and hydrolytic degradation of Eudragit® E polymers under extreme acid conditions. Polymers 2019, 11, 1010. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Cheng, W.-T.; Wei, Y.-S.; Lin, H.-L. DSC-FTIR microspectroscopy used to investigate the heat-induced intramolecular cyclic anhydride formation between Eudragit E and PVA copolymer. Polym. J. 2011, 43, 577–580. [Google Scholar] [CrossRef]
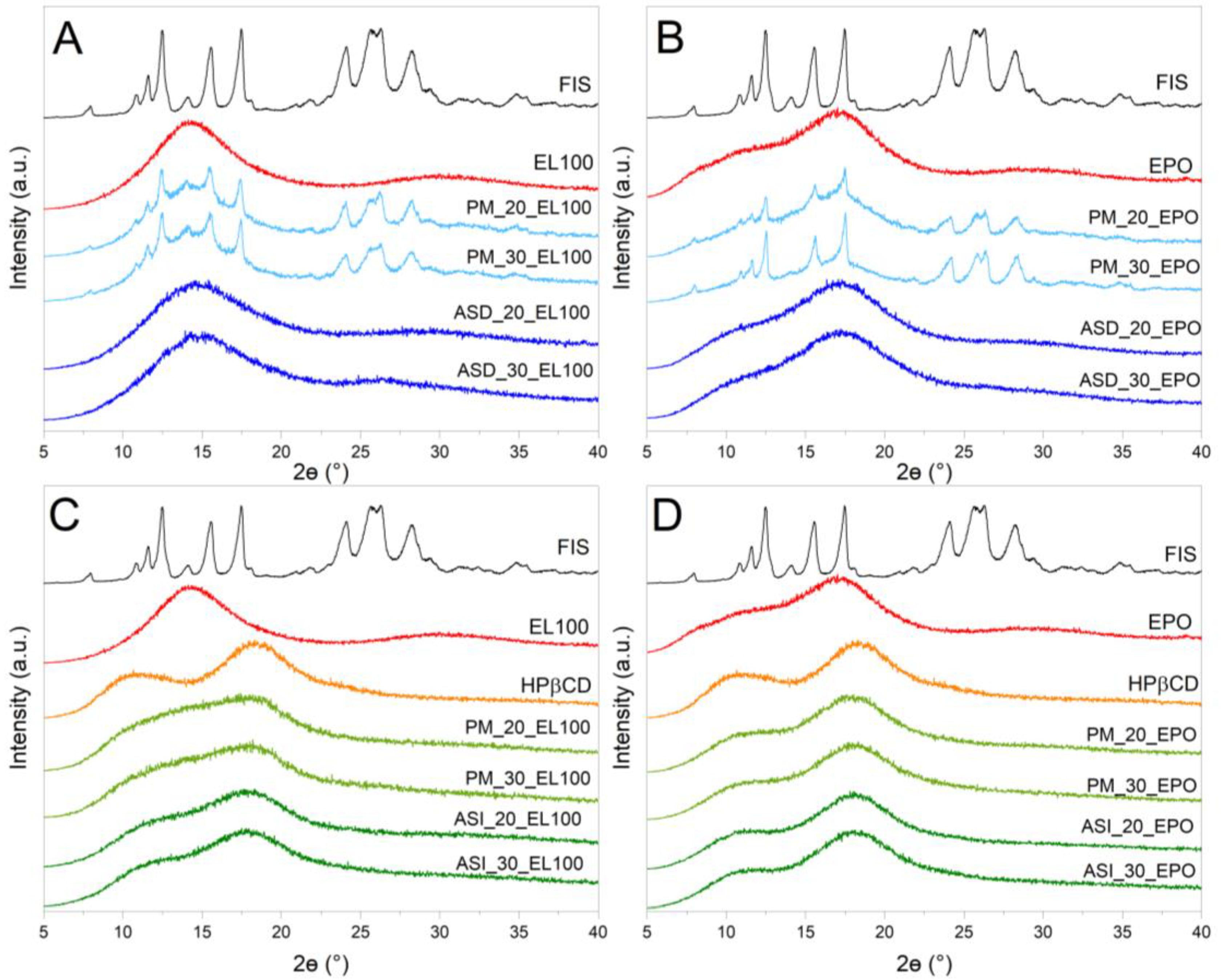
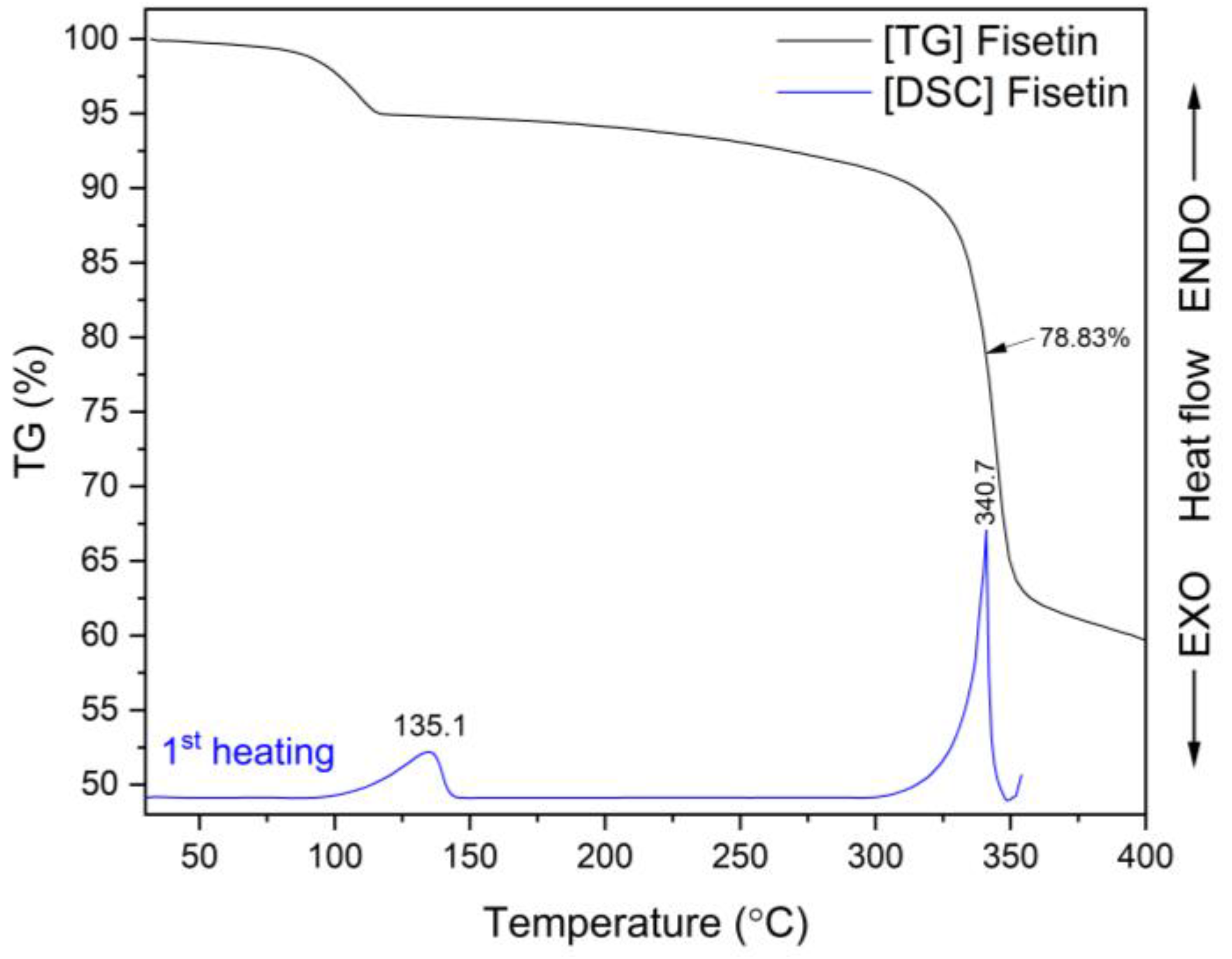
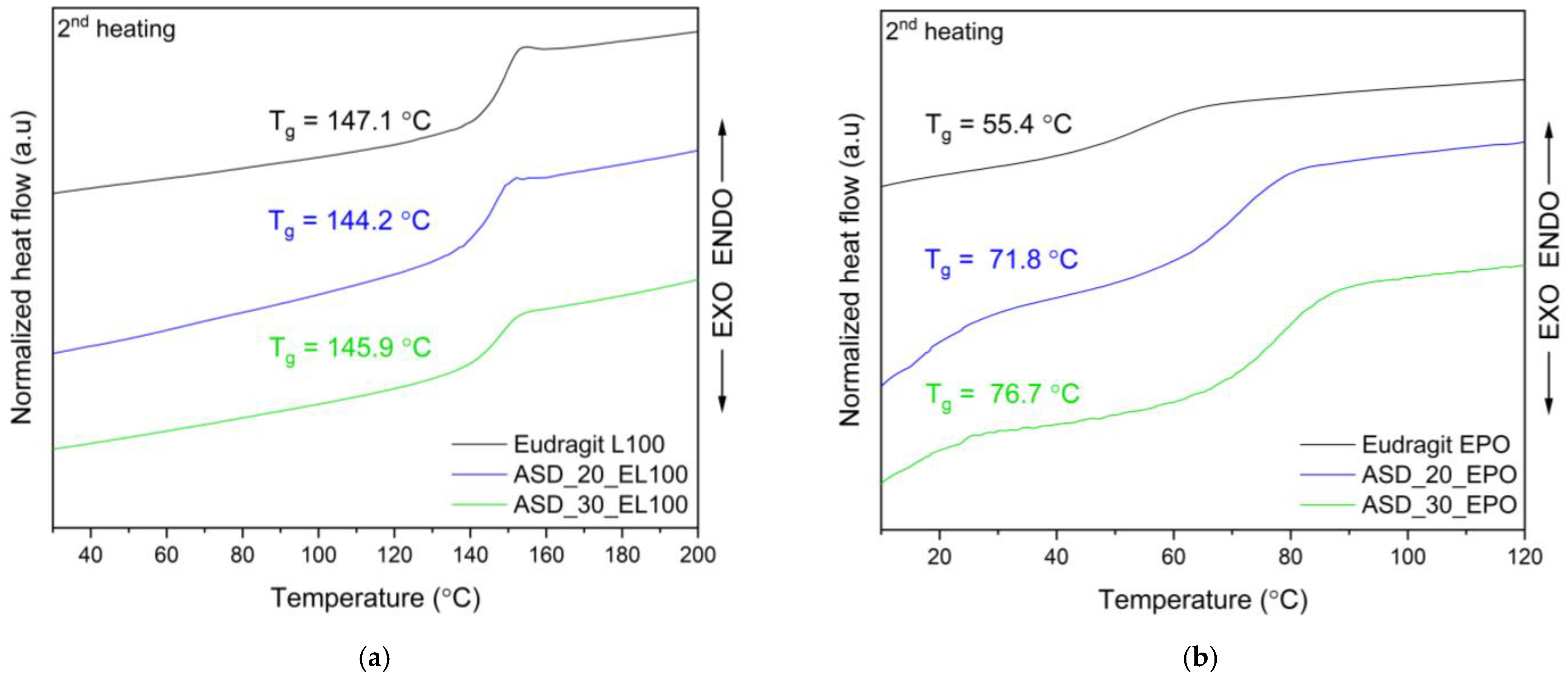
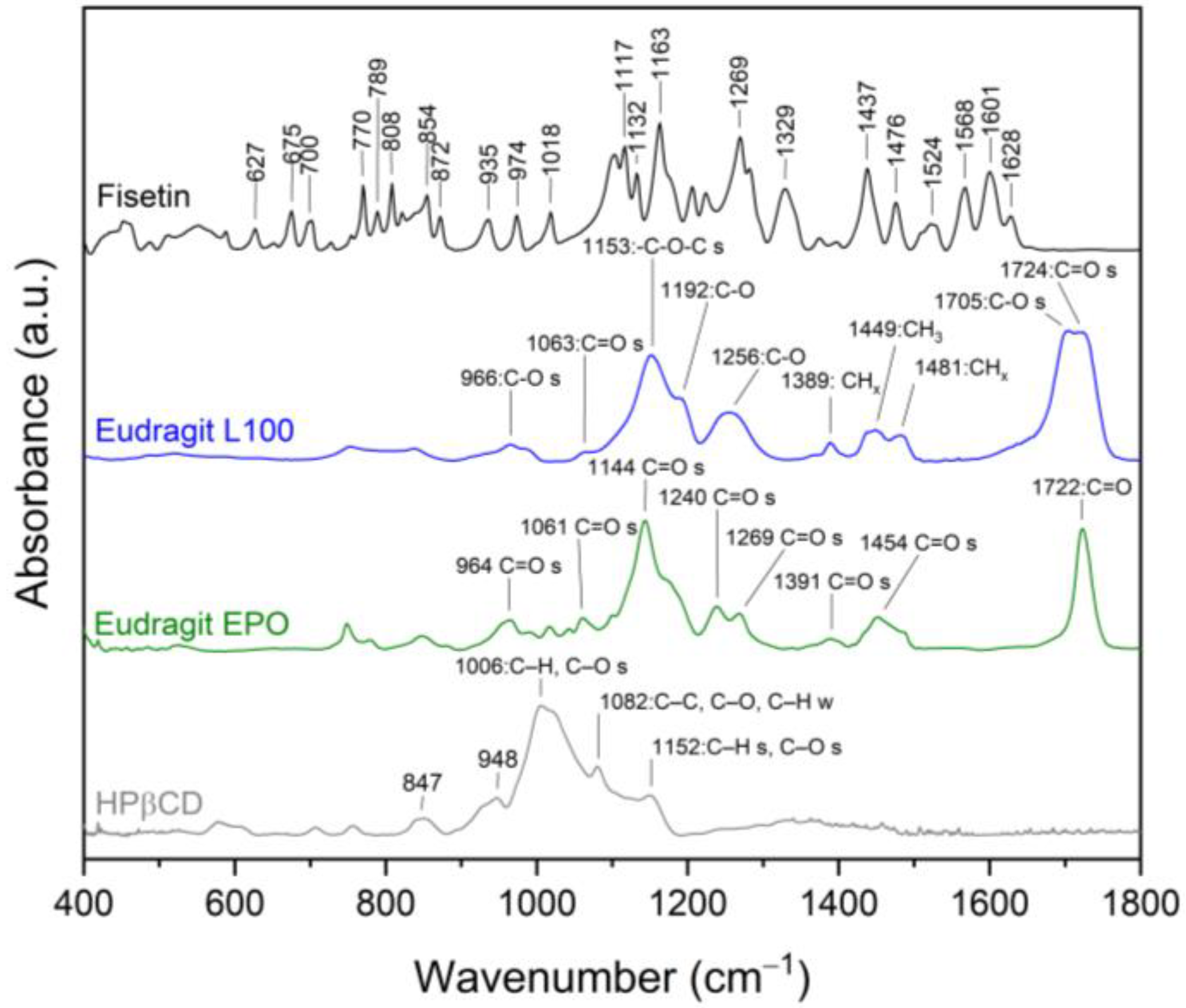
| Content of FIS [%] | System | Concentration [µg mL−1] | Improved Solubility [–Fold] |
|---|---|---|---|
| 20 | ASD_20_EL100 | - | none |
| ASD_20_EPO | - | none | |
| ASI_20_EL100 | 221.7 ± 0.1 | 221.7 | |
| ASI_20_EPO | 77.5 ± 1.3 | 77.5 | |
| 30 | ASD_30_EL100 | - | none |
| ASD_30_EPO | - | none | |
| ASI_30_EL100 | 318.3 ± 17.3 | 318.3 | |
| ASI_30_EPO | 126.5 ± 0.1 | 126.5 |
| Assay | Value | ASI_20_EPO | ASI_30_EPO | ASI_20_EL100 | ASI_30_EL100 | |
|---|---|---|---|---|---|---|
| ABTS | IC50 | [µg∙mL−1] | 10.49 ± 0.70 | 10.25 ± 0.24 | 13.61 ± 0.51 | 15.23 ± 0.44 |
| DPPH | IC50 | 27.52 ± 1.15 | 27.69 ± 1.96 | 33.20 ± 0.64 | 37.90 ± 0.73 | |
| CUPRAC | IC0.5 | 13.97 ± 0.67 | 9.52 ± 0.03 | 24.53 ± 0.30 | 28.56 ± 0.52 | |
| FRAP | IC0.5 | 13.05 ± 0.14 | 8.56 ± 0.07 | 22.37 ± 0.34 | 25.87 ± 0.26 | |
| AChE | AChE inhibition | % | 22.74 ± 3.82 | 39.91 ± 3.47 | 19.43 ± 3.79 | 15.38 ± 3.43 |
| BChE | BChE inhibition | % | 41.37 ± 0.72 | 42.62 ± 1.01 | 27.93 ± 1.26 | 32.71 ± 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiak, N.; Tykarska, E.; Cielecka-Piontek, J. Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity. Int. J. Mol. Sci. 2024, 25, 3648. https://doi.org/10.3390/ijms25073648
Rosiak N, Tykarska E, Cielecka-Piontek J. Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity. International Journal of Molecular Sciences. 2024; 25(7):3648. https://doi.org/10.3390/ijms25073648
Chicago/Turabian StyleRosiak, Natalia, Ewa Tykarska, and Judyta Cielecka-Piontek. 2024. "Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity" International Journal of Molecular Sciences 25, no. 7: 3648. https://doi.org/10.3390/ijms25073648
APA StyleRosiak, N., Tykarska, E., & Cielecka-Piontek, J. (2024). Mechanochemical Approach to Obtaining a Multicomponent Fisetin Delivery System Improving Its Solubility and Biological Activity. International Journal of Molecular Sciences, 25(7), 3648. https://doi.org/10.3390/ijms25073648







