The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets
Abstract
1. Introduction
2. Results
2.1. Effect of a Rotating Magnetic Field on the Concentration of Platelet-Derived Growth Factor BB
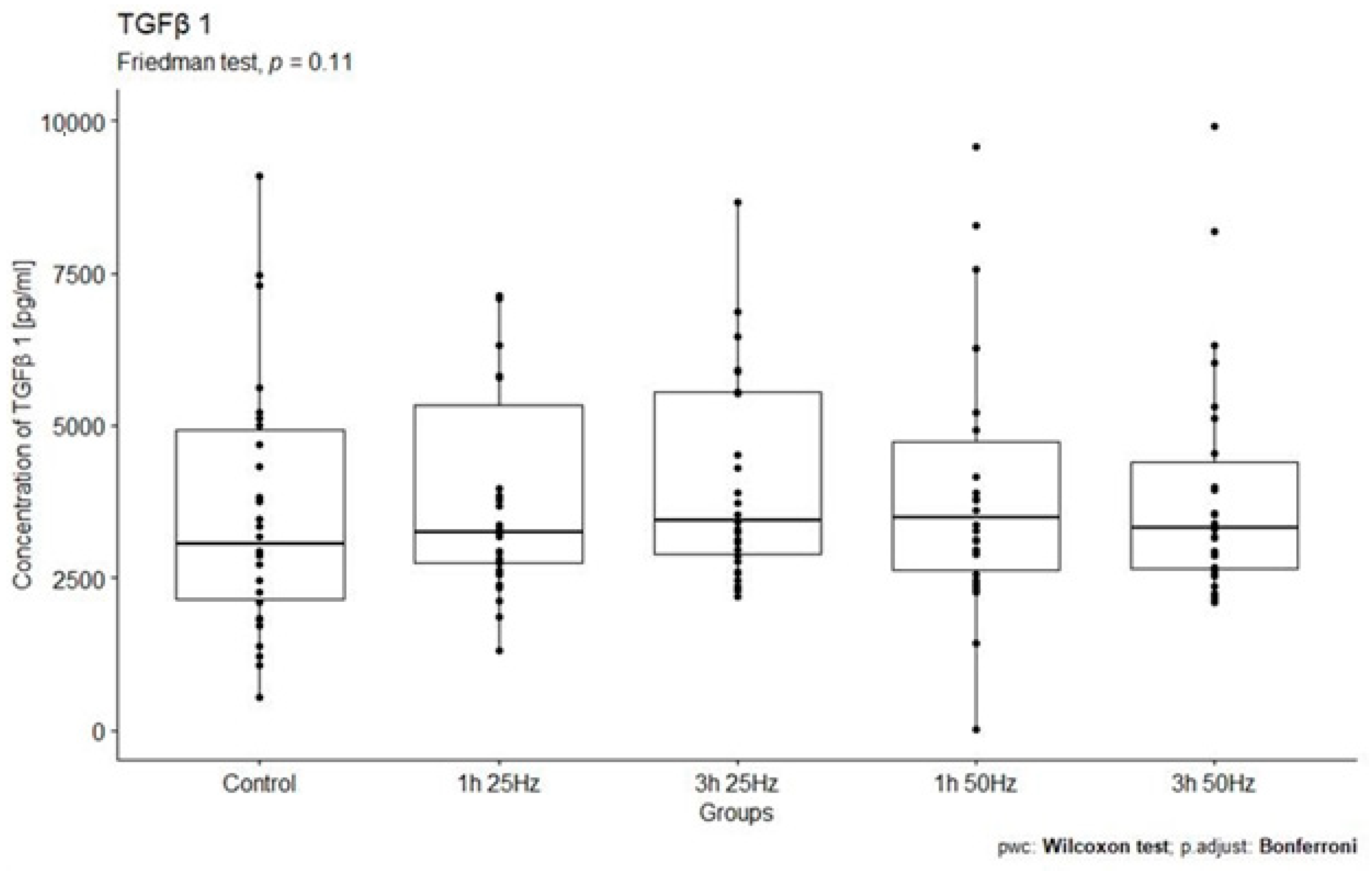
2.2. Effect of a Rotating Magnetic Field on the Concentration of Transforming Growth Factor Beta 1
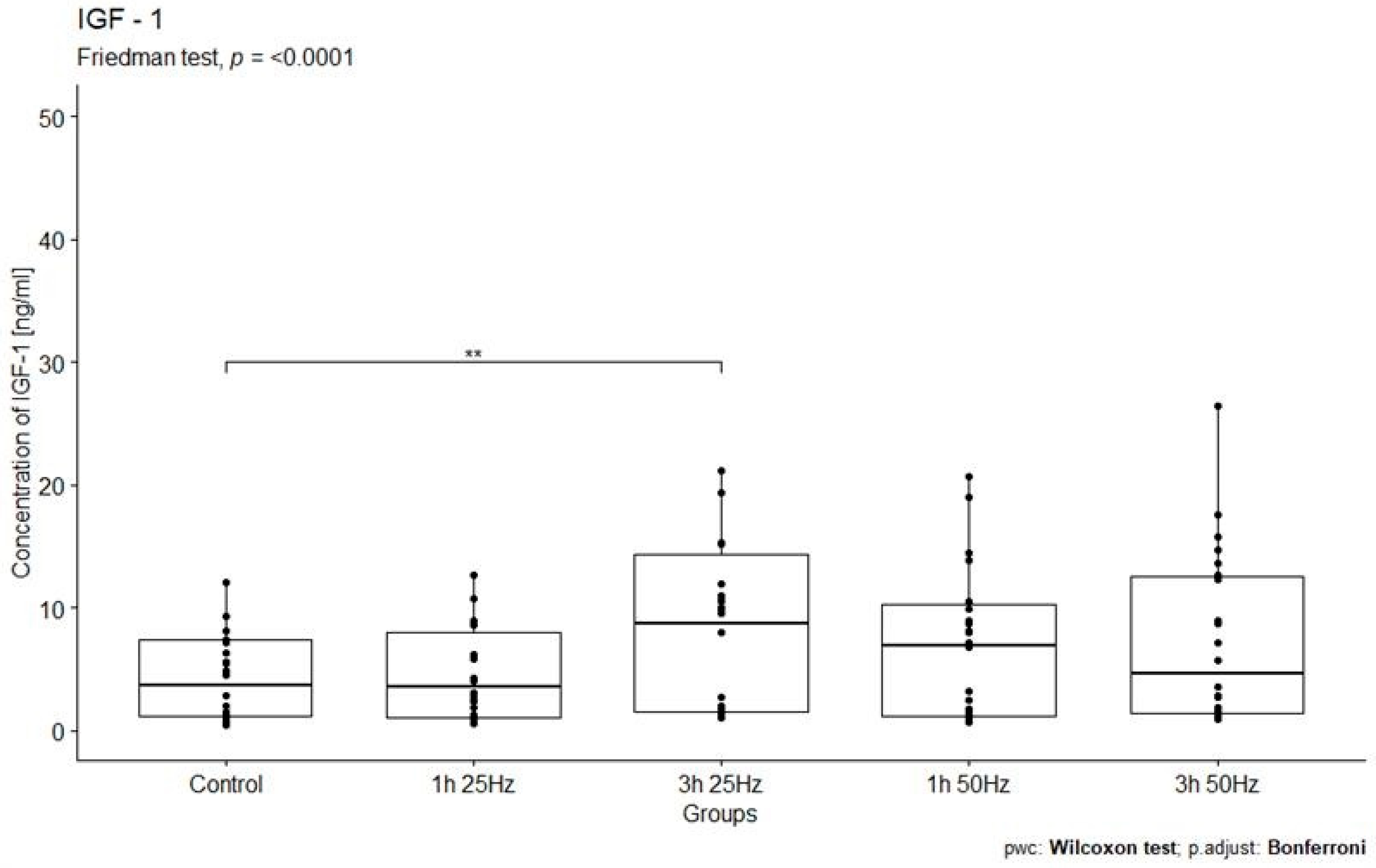
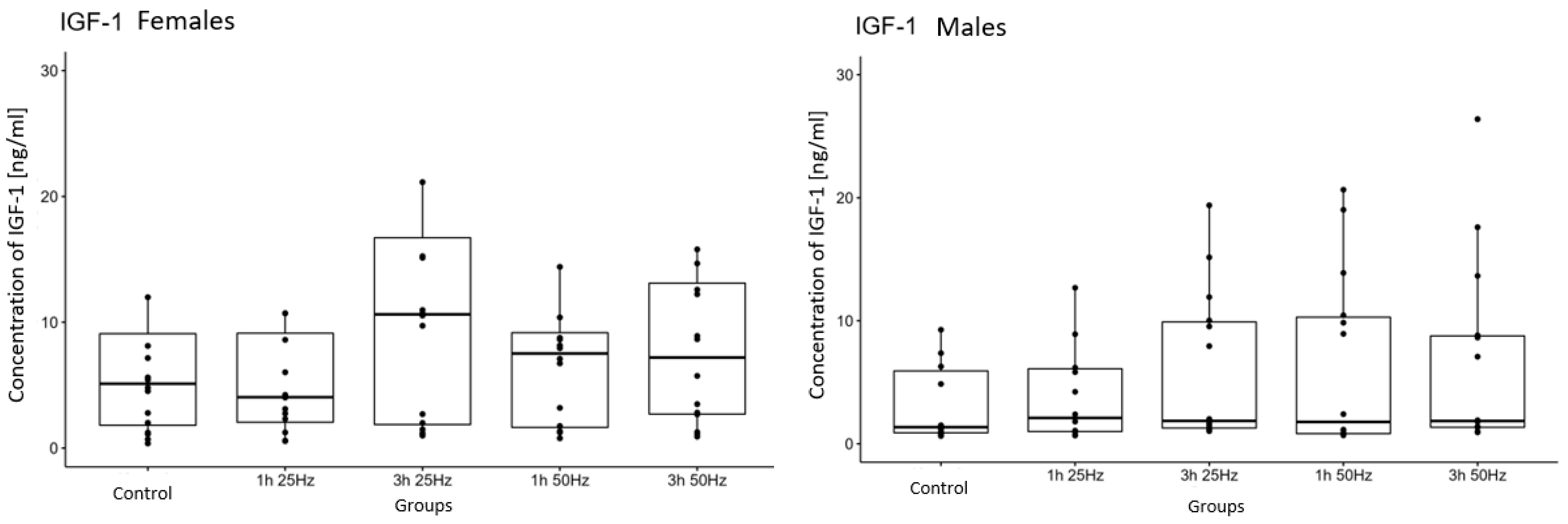
2.3. The Influence of a Rotating Magnetic Field on the Concentration of Insulin-Derived Growth Factor 1
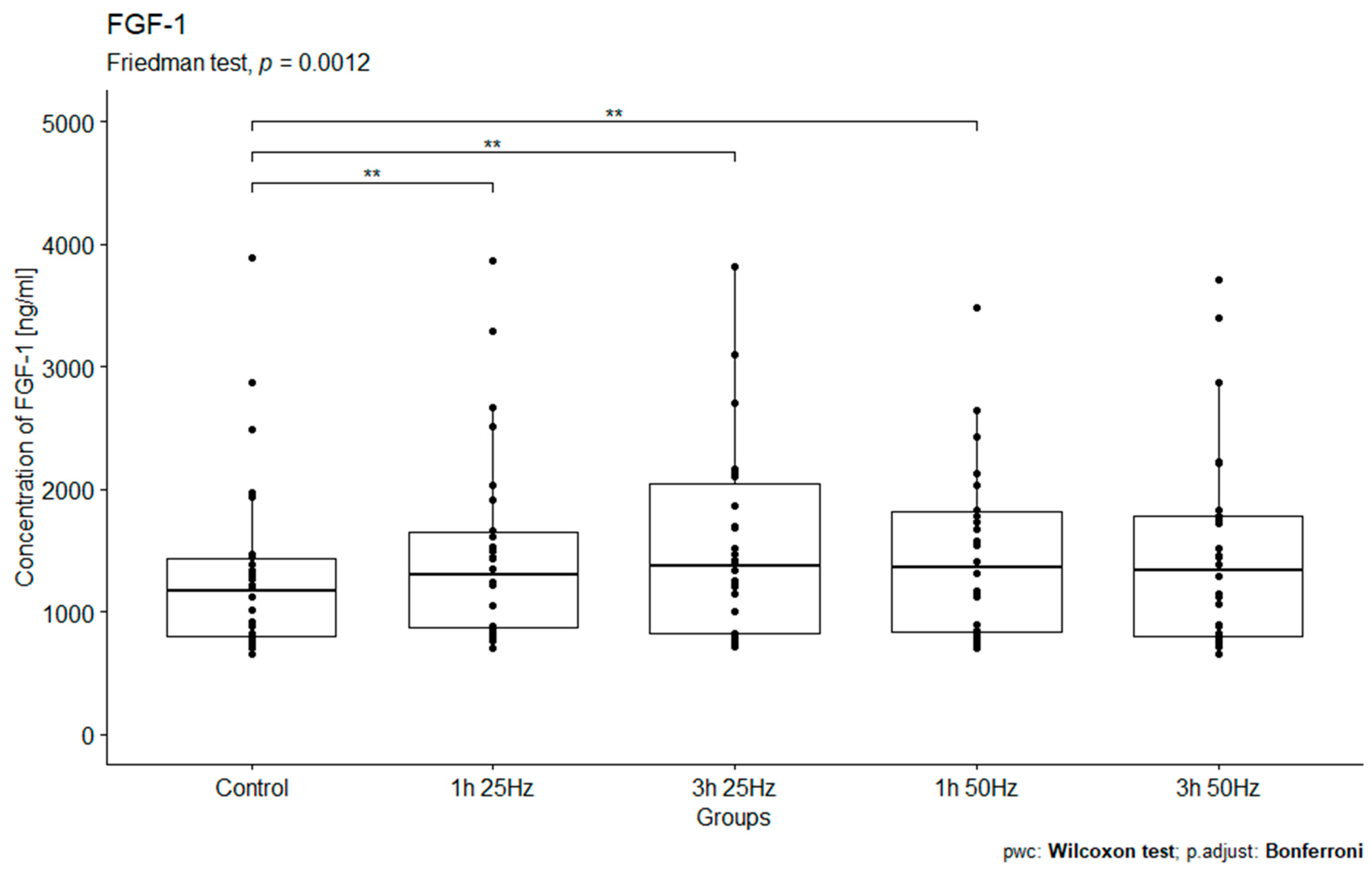
2.4. The Influence of a Rotating Magnetic Field on the Concentration of Fibroblast Growth Factor 1
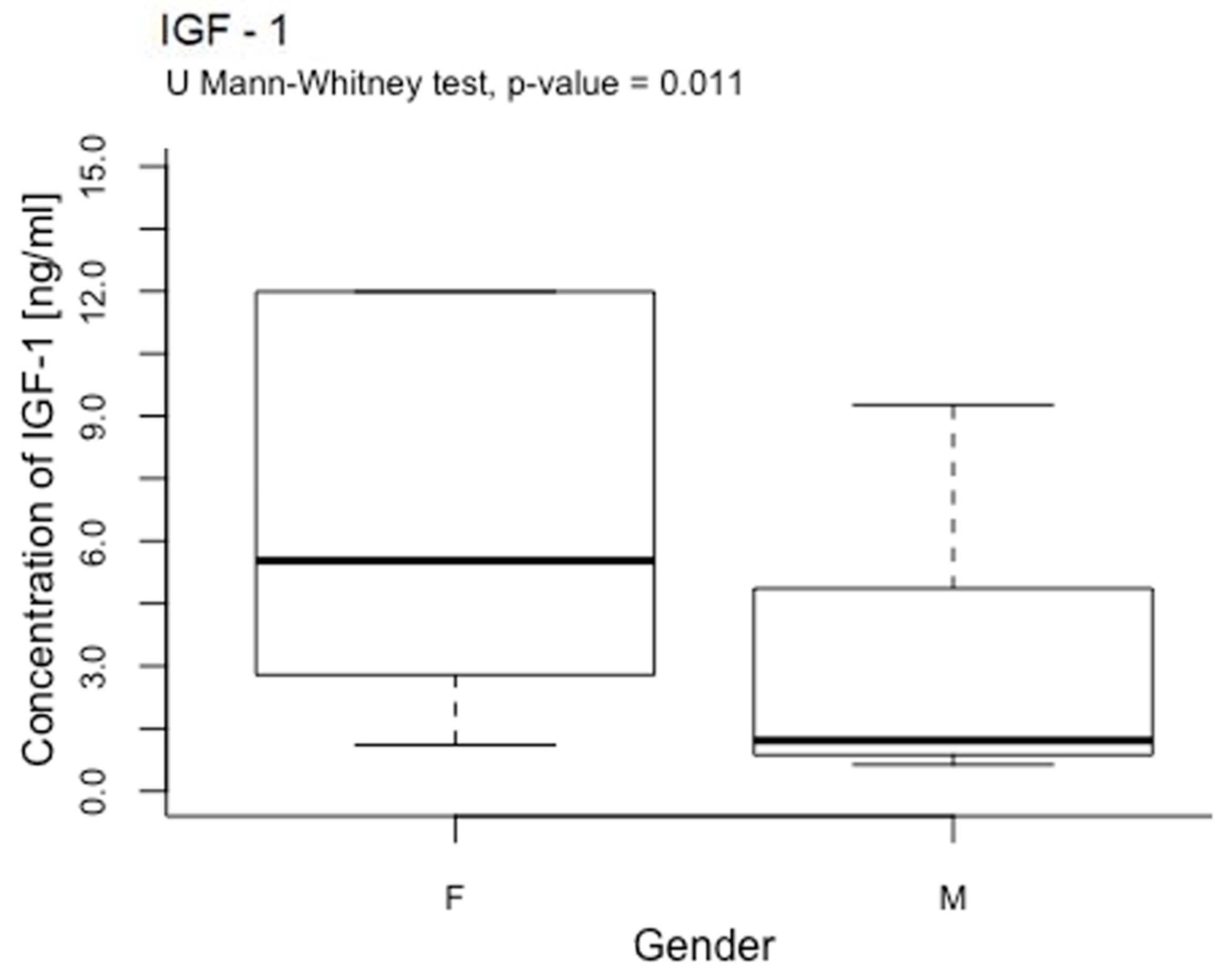
2.5. The Influence of a Rotating Magnetic Field on the Concentration of Platelet Growth Factors Depending on Sex
2.6. Platelet Concentrations in the Samples
2.7. The Strength of the Correlation between the Parameters
2.8. Multi-Factor Evaluation of Relationships between the Tested Parameters
3. Discussion
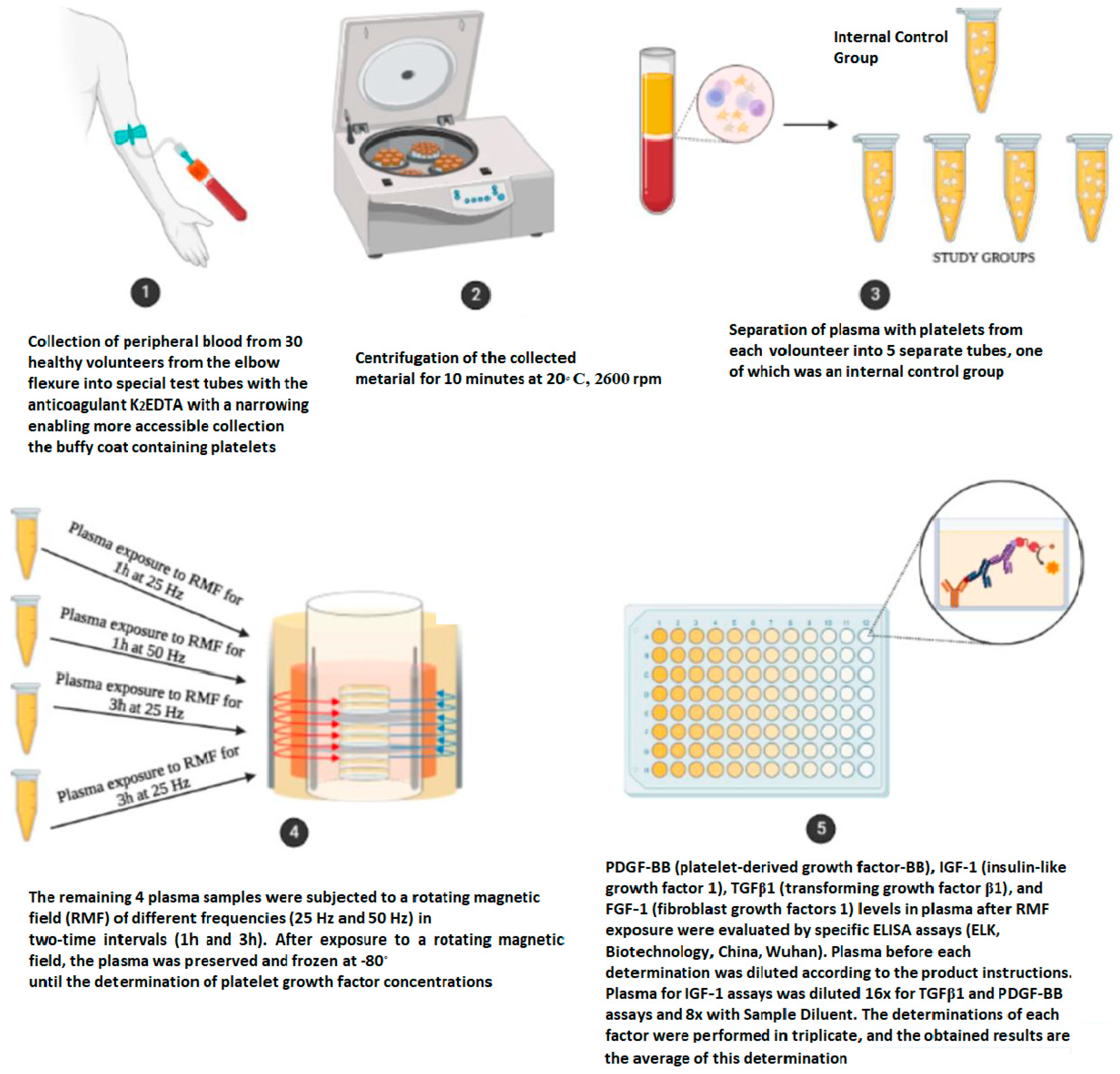
4. Materials and Methods
4.1. Characteristics of the Study Group
4.2. Materials
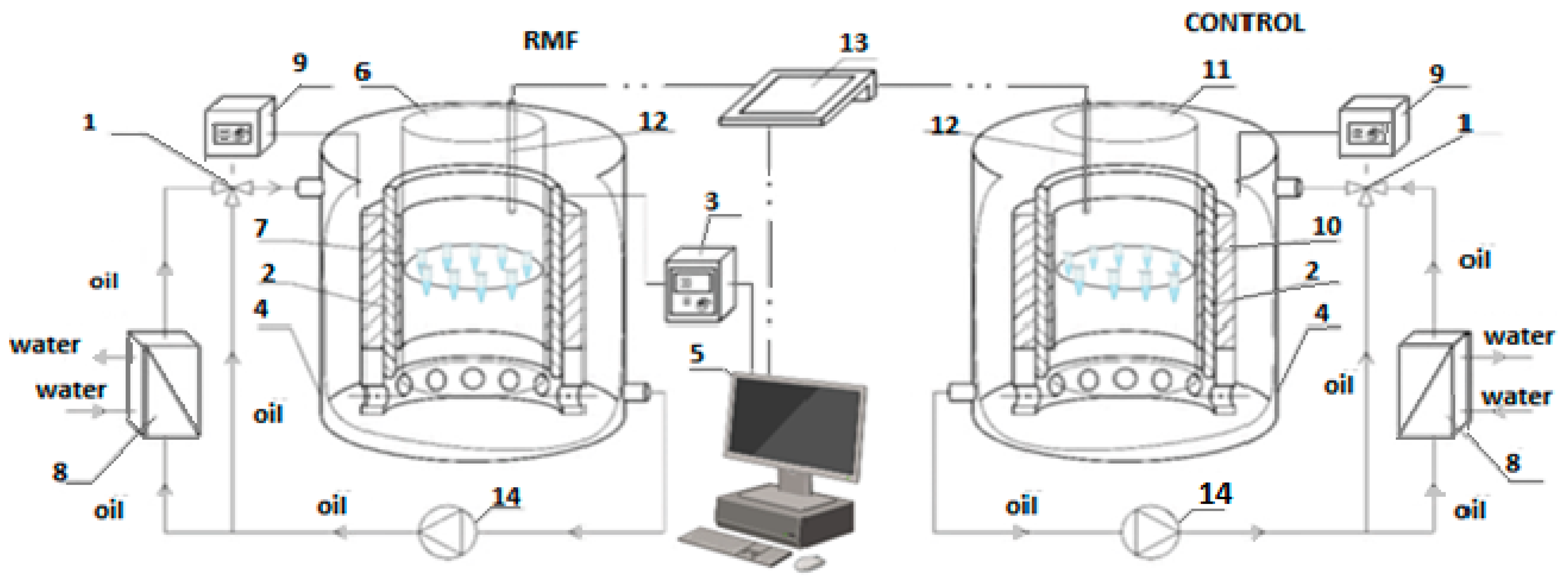
4.3. Experimental Setup
4.4. Methods of Determination of Platelet Growth Factors
4.5. Statistical Analysis
5. Conclusions
- The rotating magnetic field can cause increased production of platelet growth factors such as IGF-1, PDGF-BB, and FGF-1.
- Women’s platelets may react more intensely to rotating magnetic fields than men’s due to the higher concentrations of IGF-1 obtained in the material collected from women. However, considering that the concentration of IGF-1 depends on various factors, such as diet, hormones, BMI, total cholesterol, aerobic capacity, body fat, and muscular endurance, increased IGF-1 concentration after RMF exposure might be more personalized and indicative of overall health.
- Elevated levels of growth factors after exposure to RMF indicate that the rotating magnetic field may activate blood platelets, enhancing their regenerative potential. Achieving higher concentrations of growth factors may regulate the complex wound-healing process, reducing its duration, accelerating tissue reconstruction and regeneration, improving angiogenesis and vascular integrity, and reducing inflammatory states.
- The results of this study may be used as a new alternative method of platelet activation in the future. However, further research is needed, which will involve using RFM against PRP to check whether platelet-rich plasma can be used to obtain platelet growth factors even more effectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, W. Histologia. Wyd. 4 Popr. i Uzup; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2003. [Google Scholar]
- Szczeklik, A. Choroby Nerek Dróg Moczowych. Ostre Uszkodzenie Nerek i Przewlekła Choroba Nerek; Interna Szczeklika, Medycyna Praktyczna: Kraków, Poland, 2017; pp. 1443–1461. [Google Scholar]
- Cecerska, E. Aktywność Enzymów Antyoksydacyjnych w Płytkach Krwi u Pacjentów z Przewlekłą Chorobą nerek. Master’s Thesis, Pomorski Uniwersytet Medyczny, Szczecin, Poland, 2013. [Google Scholar]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.S.; de Sousa Faria, A.V.; de Paulo Queluz, D.; Ferreira-Halder, C.V. Platelets as a ‘natural factory’ for growth factor production that sustains normal (and pathological) cell biology. Biol. Chem. 2020, 401, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Ahola-Olli, A.V.; Würtz, P.; Havulinna, A.S.; Aalto, K.; Pitkänen, N.; Lehtimäki, T.; Kähönen, M.; Lyytikäinen, L.-P.; Raitoharju, E.; Seppälä, I.; et al. Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am. J. Hum. Genet. 2017, 100, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak-Silicka, M.; Kordas, M.; Konopacki, M.; Rakoczy, R. Modulation of Cellular Response to Different Parameters of the Rotating Magnetic Field (RMF)—An In Vitro Wound Healing Study. Int. J. Mol. Sci. 2021, 22, 5785. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, K.; Chen, T.; Zhan, T.; Ouyang, Z.; Wang, Y.; Liu, W.; Zhang, X.; Sun, Y.; Xu, G.; et al. Rotating magnetic field delays human umbilical vein endothelial cell aging and prolongs the lifespan of Caenorhabditis elegans. Aging 2019, 11, 10385–10408. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Nawrotek, P.; Struk, M.; Kordas, M.; Rakoczy, R. Effects of rotating magnetic field exposure on the functional parameters of different species of bacteria. Electromagn. Biol. Med. 2015, 34, 48–55. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. Rotating magnetic field as tool for enhancing enzymes properties—Laccase case study. Sci. Rep. 2019, 9, 3707. [Google Scholar] [CrossRef]
- Pasek, J.; Pasek, T.; Sieroń-Stołtny, K.; Cieślar, G.; Sieroń, A. Electromagnetic fields in medicine—The state of art. Electromagn. Biol. Med. 2016, 35, 170–175. [Google Scholar] [CrossRef]
- Tofani, S. Magnetic fields and apoptosis: A possible mechanism. Electromagn. Biol. Med. 2022, 41, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.Z.; Feng, S.W.; Lee, S.Y.; Huang, H.M. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells 2021, 10, 2662. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Röcken, M. Static magnetic field (SMF) as a regulator of stem cell fate—New perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev. Rep. 2018, 14, 785–792. [Google Scholar] [CrossRef]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagnetics 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Kim, E.-C.; Leesungbok, R.; Lee, S.-W.; Lee, H.-W.; Park, S.H.; Mah, S.-J.; Ahn, S.-J. Effects of moderate intensity static magnetic fields on human bone marrow-derived mesenchymal stem cells. Bioelectromagnetics 2015, 36, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, X.; Yang, X.; Zhang, X. Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 2017, 8, 13126–13141. [Google Scholar] [CrossRef]
- Marędziak, M.; Smieszek, A.; Tomaszewski, K.A.; Lewandowski, D.; Marycz, K. The effect of low static magnetic field on osteogenic and adipogenic differentiation potential of human adipose stromal/stem cells. J. Magn. Magn. Mater. 2016, 398, 235–245. [Google Scholar] [CrossRef]
- Vadalà, M.; Morales-Medina, J.C.; Vallelunga, A.; Palmieri, B.; Laurino, C.; Iannitti, T. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 2016, 5, 3128–3139. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Fano, G.; Mariggio, M.A. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell. Physiol. Biochem. 2010, 26, 947–958. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, C.C.; Tu, W.; Cheng, Y.T.; Tseng, F.G. Design and fabrication of a microplatform for the proximity effect study of localized ELF-EMF on the growth of in vitro HeLa and PC-12 cells. J. Micromech. Microeng. 2010, 20, 125023. [Google Scholar] [CrossRef]
- Sadeghipour, R.; Ahmadian, S.; Bolouri, B.; Pazhang, Y.; Shafiezadeh, M. Effects of extremely low-frequency pulsed electromagnetic fields on morphological and biochemical properties of human breast carcinoma cells (T47D). Electromagn. Biol. Med. 2012, 31, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Loja, T.; Stehlikova, O.; Palko, L.; Vrba, K.; Rampl, I.; Klabusay, M. Influence of pulsed electromagnetic and pulsed vector magnetic potential field on the growth of tumor cells. Electromagn. Biol. Med. 2014, 33, 190–197. [Google Scholar] [CrossRef]
- Filipovic, N.D.; Peulic, A.S.; Zdravkovic, N.D.; Grbovic-Markovic, V.M.; Jurisic-Skevin, A.J. Transient finite element modeling of functional electrical stimulation. Gen. Physiol. Biophys. 2011, 30, 59–65. [Google Scholar] [CrossRef]
- Peng, L.; Fu, C.; Wang, L.; Zhang, Q.; Liang, Z.; He, C.; Wei, Q. The Effect of Pulsed Electromagnetic Fields on Angiogenesis. Bioelectromagnetics. 2021, 42, 250–258. [Google Scholar] [CrossRef]
- Berg, H.; Gunther, B.; Hilger, I.; Radeva, M.; Traitcheva, N.; Wollweber, L. Bioelectromagnetic field effects on cancer cells and mice tumors. Electromagn. Biol. Med. 2010, 29, 132–143. [Google Scholar] [CrossRef]
- Zimmerman, J.W.; Pennison, M.J.; Brezovich, I.; Yi, N.; Yang, C.T.; Ramaker, R.; Absher, D.; Myers, R.M.; Kuster, N.; Costa, F.P.; et al. Cancer cell proliferation is inhibited by specific modulation frequencies. Br. J. Cancer 2012, 106, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, S.; Piantelli, F.; Leonzio, C. Selective destabilization of tumor cells with pulsed electric and magnetic sequences: A preliminary report. Electromagn. Biol. Med. 2011, 30, 128–135. [Google Scholar] [CrossRef]
- Costa, F.P.; de Oliveira, A.C.; Meirelles, R.; Machado, M.C.; Zanesco, T.; Surjan, R.; Chammas, M.C.; Rocha, M.d.S.; Morgan, D.; Cantor, A.; et al. Treatment of advanced hepatocellular carcinoma with very low levels of amplitude-modulated electromagnetic fields. Br. J. Cancer 2011, 105, 640–648. [Google Scholar] [CrossRef]
- Junka, A.F.; Rakoczy, R.; Szymczyk, P.; Bartoszewicz, M.; Sedghizadeh, P.P.; Fijałkowski, K. Application of Rotating Magnetic Fields Increase the Activity of Antimicrobials against Wound Biofilm Pathogens. Sci. Rep. 2018, 8, 167. [Google Scholar] [CrossRef]
- Sağdilek, E.; Sebik, O.; Celebi, G. Investigation of the effects of 50 Hz magnetic fields on platelet aggregation using a modified aggregometer. Electromagn. Biol. Med. 2012, 31, 382–393. [Google Scholar] [CrossRef]
- Tschon, M.; Veronesi, F.; Contartese, D.; Sartori, M.; Martini, L.; Vincenzi, F.; Ravani, A.; Varani, K.; Fini, M. Effects of pulsed electromagnetic fields and platelet rich plasma in preventing osteoclastogenesis in an in vitro model of osteolysis. J. Cell Physiol. 2018, 233, 2645–2656. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Fini, M.; Sartori, M.; Parrilli, A.; Martini, L.; Tschon, M. Pulsed electromagnetic fields and platelet rich plasma alone and combined for the treatment of wear-mediated periprosthetic osteolysis: An in vivo study. Acta Biomater. 2018, 77, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kapi, E.; Bozkurt, M.; Selcuk, C.T.; Celik, M.S.; Akpolat, V.; Isik, F.B.; Bozarslan, B.H.; Celik, Y. Comparison of Effects of Pulsed Electromagnetic Field Stimulation on Platelet-Rich Plasma and Bone Marrow Stromal Stem Cell Using Rat Zygomatic Bone Defect Model. Ann. Plast. Surg. 2015, 75, 565–571. [Google Scholar] [CrossRef]
- Arslan, U.; Özmert, E. Management of Retinitis Pigmentosa via Platelet-Rich Plasma or Combination with Electromagnetic Stimulation: Retrospective Analysis of 1-Year Results. Adv. Ther. 2020, 37, 2390–2412. [Google Scholar] [CrossRef] [PubMed]
- Özmert, E.; Arslan, U. Management of Deep Retinal Capillary Ischemia by Electromagnetic Stimulation and Platelet-Rich Plasma: Preliminary Clinical Results. Adv. Ther. 2019, 36, 2273–2286. [Google Scholar] [CrossRef] [PubMed]
- Anna, D.; Tadeusz, R. Basics of Hematology; Wydawnictwo Czelej: Lublin, Poland, 2003; ISBN 83-88063-94-4. [Google Scholar]
- Alvarez, R.H.; Kantarjian, H.M.; Cortes, J.E. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 2006, 81, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Platelet-derived growth factor. Annu. Rev. Med. 1987, 38, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Joint Surg. Am. 2008, 90 (Suppl. S1), 48–54. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.M.; Park, H.J.; Nam, M.H.; Seo, Y.K. Extremely Low-Frequency Electromagnetic Fields Increase Cytokines in Human Hair Follicles through Wnt/β-Catenin Signaling. Biomedicines 2022, 10, 924. [Google Scholar] [CrossRef]
- Obayashi-Ishii, M.; Saito, S.; Omagari, D.; Asano, M.; Ishigami, T. A static magnetic field inhibits the expression of platelet-derived growth factor-AA in human oral squamous cell carcinoma. J. Oral Sci. 2018, 60, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Rolfe, K.J.; Richardson, J.; Vigor, C.; Irvine, L.M.; Grobbelaar, A.O.; Linge, C. A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J. Investig. Dermatol. 2007, 127, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, M.K.; Otero-Vinas, M.; Falanga, V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair. Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-b family in wound healing, burns and scarring: A review. Int. J. Burns Trauma. 2012, 2, 18–28. [Google Scholar]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Shen, W.W.; Zhao, J.H. Pulsed electromagnetic fields stimulation affects BMD and local factor production of rats with disuse osteoporosis. Bioelectromagnetics 2010, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Wang, W.; Seldes, R.M.; Tahernia, A.D.; Fan, H.; Brighton, C.T. Electrical stimulation induces the level of TGF-β1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem. Biophys. Res. Commun. 1997, 237, 225–229. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Lee, J.W.; Lee, K.Y.; Li, Z.; Simon, B.J.; Sylvia, V.L.; Dean, D.D.; Bonewald, L.F.; Donahue, H.J.; et al. Magnetic field-responsive release of transforming growth factor beta 1 from heparin-modified alginate ferrogels. Carbohydr. Polym. 2016, 151, 467–473. [Google Scholar] [CrossRef]
- Streit, A.; Watson, B.C.; Granata, J.D.; Philbin, T.M.; Lin, H.N.; O’Connor, J.P.; Lin, S. Effect on Clinical Outcome and Growth Factor Synthesis with Adjunctive Use of Pulsed Electromagnetic Fields for Fifth Metatarsal Nonunion Fracture: A Double-Blind Randomized Study. Foot Ankle Int. 2016, 37, 919–923. [Google Scholar] [CrossRef]
- Lohmann, C.H.; Schwartz, Z.; Liu, Y.; Li, Z.; Simon, B.J.; Sylvia, V.L.; Dean, D.D.; Bonewald, L.F.; Donahue, H.J.; Boyan, B.D. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J. Orthop. Res. 2003, 21, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kimsa-Dudek, M.; Synowiec-Wojtarowicz, A.; Krawczyk, A. Phenolic acids and a static magnetic field change the expression of transforming growth factor β isoforms in amelanotic melanoma cells. Mol. Biol. Rep. 2023, 50, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Halmos, T.; Suba, I. A növekedési hormon és az inzulinszerű növekedési faktorok élettani szerepe. [The physiological role of growth hormone and insulin-like growth factors]. Orvosi Hetil. 2019, 160, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Miescher, I.; Rieber, J.; Calcagni, M.; Buschmann, J. In Vitro and In Vivo Effects of IGF-1 Delivery Strategies on Tendon Healing: A Review. Int. J. Mol. Sci. 2023, 24, 2370. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef]
- Fitzsimmons, R.J.; Strong, D.D.; Mohan, S.; Baylink, D.J. Low-amplitude, lowfrequency electric field-stimulated bone cell proliferation may in part be mediated by increased IGF-II release. J. Cell Physiol. 1992, 150, 84–89. [Google Scholar] [CrossRef]
- Fitzsimmons, R.J.; Ryaby, J.T.; Magee, F.P.; Baylink, D.J. IGF-II receptor number is increased in TE-85 osteosarcoma cells by combined magnetic fields. J. Bone Miner. Res. 1995, 10, 812–819. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.N.; Gao, Y.H.; Yan, J.L.; Shi, W.G.; Xian, C.J.; Chen, K.M. Sinusoidal electromagnetic fields promote bone formation and inhibit bone resorption in rat femoral tissues in vitro. Electromagn. Biol. Med. 2016, 35, 75–83. [Google Scholar] [CrossRef]
- Song, C.X.; Fan, J.Z.; Wu, H.Y.; Wei, Y.; Zhen, J.R. Effects of pulsed magnetic field on insulin-like growth factor-1 (IGF-1) in cerebrospinal fluid and effects of IGF-1 on functional recovery. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 2288–2290+2294. (In Chinese) [Google Scholar]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 744868. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004, 18, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, C.; Liang, Z.; Zhang, Q.; Xiong, F.; Chen, L.; He, C.; Wei, Q. Pulsed Electromagnetic Fields Increase Angiogenesis and Improve Cardiac Function after Myocardial Ischemia in Mice. Circ. J. 2020, 84, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Budkowska, M.; Lebiecka, A.; Marcinowska, Z.; Woźniak, J.; Jastrzębska, M.; Dołęgowska, B. The circadian rhythm of selected parameters of the hemostasis system in healthy people. Thromb. Res. 2019, 182, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.; Segev, Y.; Landau, D. Growth Hormone and IGF1 Actions in Kidney Development and Function. Cells 2021, 10, 3371. [Google Scholar] [CrossRef]
- Nindl, B.C.; Santtila, M.; Vaara, J.; Hakkinen, K.; Kyrolainen, H. Circulating IGF-I is associated with fitness and health outcomes in a population of 846 young healthy men. Growth Horm. IGF Res. 2011, 21, 124–128. [Google Scholar]
- Maggio, M.; De Vita, F.; Lauretani, F.; Butto, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G.P. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef]
- Puche, J.E.; Castilla-Cortazar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.M.; Gronowicz, G.; Zhu, L.; Kuhn, L.T.; Rodner, C.; Xiao, L. Age-Related Changes in FGF-2, Fibroblast Growth Factor Receptors and β-Catenin Expression in Human Mesenchyme-Derived Progenitor Cells. J. Cell Biochem. 2016, 117, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Dang, E.; Chen, Y.; Wang, W.; Zhang, L.; An, N.; Yin, W.; Yi, J.; Chen, Y. A comparative study of platelet storage lesion in platelet-rich plasma under cryopreservation. Ann. Hematol. 2023, 103, 631–643. [Google Scholar] [CrossRef]
- Kim, J.I.; Bae, H.C.; Park, H.J.; Lee, M.C.; Han, H.S. Effect of Storage Conditions and Activation on Growth Factor Concentration in Platelet-Rich Plasma. J. Orthop. Res. 2020, 38, 777–784. [Google Scholar] [CrossRef] [PubMed]
- McClain, A.K.; McCarrel, T.M. The effect of four different freezing conditions and time in frozen storage on the concentration of commonly measured growth factors and enzymes in equine platelet-rich plasma over six months. BMC Vet. Res. 2019, 15, 292. [Google Scholar] [CrossRef] [PubMed]
- Grygorcewicz, B.; Rakoczy, R.; Roszak, M.; Konopacki, M.; Kordas, M.; Piegat, A.; Serwin, N.; Cecerska-Heryć, E.; El Fray, M.; Dołęgowska, B. Rotating Magnetic Field-Assisted Reactor Enhances Mechanisms of Phage Adsorption on Bacterial Cell Surface. Curr. Issues Mol. Biol. 2022, 44, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Augustyniak, A.; Kordas, M.; Dubrowska, K.; Sołoducha, D.; Borowski, T.; Konopacki, M.; Grygorcewicz, B.; Roszak, M.; Dołęgowska, B.; et al. Evaluation of ferrofluid-coated rotating magnetic field-assisted bioreactor for biomass production. Chem. Eng. J. 2021, 431, 133913. [Google Scholar] [CrossRef]

| Parameter [pg/mL] | n | Min | Max | Median | IQR | Mean | SD |
|---|---|---|---|---|---|---|---|
| PDGF-BB control | 30 | 905.68 | 376,680.00 | 2242.84 | 2767.37 | 28,018.03 | 92,363.65 |
| PDGF-BB 1 h 25 Hz | 30 | 813.32 | 352,680.00 | 2895.97 | 2986.43 | 15,668.34 | 63,870.67 |
| PDGF-BB 1 h 50 Hz | 30 | 1013.44 | 32,000.00 | 2149.49 | 2397.97 | 4744.49 | 7595.15 |
| PDGF-BB 3 h 25 Hz | 30 | 759.44 | 324,280.00 | 2402.75 | 2395.30 | 15,088.64 | 58,776.32 |
| PDGF-BB 3 h 50 Hz | 30 | 1054.49 | 12,060.80 | 2524.85 | 2425.92 | 3522.31 | 2880.52 |
| Parameter [pg/mL] | n | Min | Max | Median | IQR | Mean | SD |
|---|---|---|---|---|---|---|---|
| TGF-β1 control | 30 | 541.61 | 11,319.84 | 3058.98 | 2790.61 | 3780.59 | 2454.98 |
| TGF-β1 1 h 25 Hz | 30 | 1299.46 | 13,062.08 | 3248.65 | 2572.69 | 4392.48 | 2914.41 |
| TGF-β1 1 h 50 Hz | 30 | 9.98 | 2,227,244.10 | 3486.75 | 2107.59 | 78,269.29 | 405,886.01 |
| TGF-β1 3 h 25 Hz | 30 | 2203.43 | 17,337.60 | 3441.18 | 2649.07 | 4646.24 | 3142.56 |
| TGF-β1 3 h 50 Hz | 30 | 2102.54 | 11,973.61 | 3331.38 | 1771.63 | 4114.96 | 2330.23 |
| Parameter [ng/mL] | n | Min | Max | Median | IQR | Mean | SD |
|---|---|---|---|---|---|---|---|
| IGF-1 control | 30 | 0.37 | 1063.52 | 3.66 | 6.18 | 77.34 | 247.61 |
| IGF-1 1 h 25 Hz | 30 | 0.55 | 261.64 | 3.56 | 6.94 | 19.59 | 54.82 |
| IGF-1 1 h 50 Hz | 30 | 0.69 | 180.98 | 6.91 | 9.08 | 17.19 | 42.34 |
| IGF-1 3 h 25 Hz | 30 | 0.99 | 462.99 | 8.74 | 12.87 | 34.40 | 94.10 |
| IGF-1 3 h 50 Hz | 30 | 0.92 | 15,752.00 | 4.62 | 11.11 | 1055.52 | 3991.58 |
| Parameter [ng/mL] | n | Min | Max | Median | IQR | Mean | SD |
|---|---|---|---|---|---|---|---|
| FGF-1 control | 30 | 654.30 | 5494.30 | 1165.65 | 655.09 | 1431.96 | 1052.94 |
| FGF-1 1 h 25 Hz | 30 | 704.07 | 5503.60 | 1295.40 | 794.07 | 1596.83 | 1062.08 |
| FGF-1 1 h 50 Hz | 30 | 703.07 | 10,037.00 | 1357.35 | 1000.09 | 1831.36 | 1835.66 |
| FGF-1 3 h 25 Hz | 30 | 718.03 | 8895.70 | 1378.20 | 1285.93 | 1902.93 | 1805.91 |
| FGF-1 3 h 50 Hz | 30 | 653.30 | 14,897.00 | 1336.75 | 996.89 | 2579.48 | 1900.86 |
| Group | n | Mean | Median | SD | Min | Max |
|---|---|---|---|---|---|---|
| For all cases | 30 | 210,533.3 | 196,500 | 75,773.13 | 132,000 | 441,000 |
| Women | 15 | 187,500 | 179,000 | 52,033.32 | 132,000 | 343,000 |
| Men | 15 | 236,857.1 | 208,000 | 91,021.43 | 137,000 | 441,000 |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| Sex (Rs = −0.232; p = 0.004) | Age (Rs = 0.213; p = 0.009) | Age (Rs = 0.212; p = 0.009) | Age (Rs = 0.515; p < 0.001) |
| Height (Rs = −0.188; p = 0.021) | Weigt (Rs = −0.270; p = 0.001) | Height (Rs = −0.186; p = 0.023) | Height (Rs = −0.219; p = 0.007) |
| Weight (Rs = −0.257; p = 0.001) | PDGF-BB (Rs = 0.648; p < 0.001) | Weight (Rs = −0.180; p = 0.027) | IGF-1 (Rs = 0.464; p = 0.000) |
| PDGF-BB (Rs = 0.234; p = 0.004) | FGF-1 (Rs = 0.317; p = 0.000) | IGF-1 (Rs = 0.234; p = 0.004) | TGF-β1 (Rs = 0.317; p = 0.000) |
| FGF-1 (Rs = 0.464; p = 0.000) | - | TGF-β1 (Rs = 0.648; p = 0.000) | PDGF-BB (Rs = 0.564; p = 0.000) |
| - | - | FGF-1 (Rs = 0.564; p = 0.000) | - |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| Weight (Rs = −0.430; p = 0.018) | PDGF-BB (Rs = 0.696; p = 0.000) | TGF-β1 (Rs = 0.696; p = 0.000) | Age (Rs = 0.498; p = 0.005) |
| - | FGF-1 (Rs = 0.415; p = 0.022) | FGF-1 (Rs = 0.600; p = 0.001) | Total protein (Rs = 0.445; p = 0.014) |
| - | - | - | TGF-β1 (Rs = 0.415; p = 0.022) |
| - | - | - | PDGF-BB (Rs = 0.600; p = 0.001) |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| FGF-1 (Rs = 0.426; p = 0.019) | PDGF-BB (Rs = 0.450; p = 0.013) | TGF-β1 (Rs = 0.450; p = 0.013) | Age (Rs = 0.475; p = 0.008) |
| - | - | FGF-1 (Rs = 0.657; p = 0.000) | IGF-1 (Rs = 0.426; p = 0.019) |
| - | - | - | PDGF-BB (Rs = 0.657; p = 0.000) |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| FGF-1 (Rs = 0.600; p = 0.001) | PDGF-BB (Rs = 0.714; p = 0.000) | TGF-β1 (Rs = 0.714; p = 0.000) | Age (Rs = 0.497; p = 0.005) |
| - | - | FGF-1 (Rs = 0.600; p = 0.001) | IGF-1 (Rs = 0.600; p = 0.001) |
| - | - | - | PDGF-BB (Rs = 0.600; p = 0.001) |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| FGF-1 (Rs = 0.592; p = 0.001) | PDGF-BB (Rs = 0.519; p = 0.003) | TGF-β1 (Rs = 0.519; p = 0.003) | Age (Rs = 0545; p = 0.002) |
| - | - | FGF-1 (Rs = 0.526; p = 0.003) | IGF-1 (Rs = 0.592; p = 0.001) |
| - | - | - | PDGF-BB (Rs = 0.526; p = 0.003) |
| IGF-1 [ng/mL] | TGF-β1 [pg/mL] | PDGF-BB [pg/mL] | FGF-1 [ng/mL] |
|---|---|---|---|
| FGF-1 (Rs = 0.564; p = 0.001) | PDGF-BB (Rs = 0.818; p = 0.000) | TGF-β1 (Rs = 0.818; p = 0.000) | Age (Rs = 0.518; p = 0.003) |
| - | FGF-1 (Rs = 0.387; p = 0.035) | FGF-1 (Rs = 0.494; p = 0.006) | IGF-1 (Rs = 0.564; p = 0.001) |
| - | - | - | TGF-β1 (Rs = 0.387; p = 0.035) |
| - | - | - | PDGF-BB (Rs = 0.494; p = 0.006) |
| Dependent Variable | Independent Variable | β | R2 | p | p for Model | F |
|---|---|---|---|---|---|---|
| PDGF-BB [pg/mL] | Group | −0.15 | 0.12 | NS | 0.040 | 2.55 |
| Age | 0.19 | 0.03 | ||||
| High | −0.07 | 0.56 | ||||
| Weight | 0.06 | 0.62 | ||||
| TGF-β1 [pg/mL] | Group | 0.06 | 0.004 | NS | NS | 0.74 |
| Age | 0.06 | NS | ||||
| High | 0.19 | NS | ||||
| Weight | −0.09 | NS | ||||
| IGF-1 [ng/mL] | Group | 0.15 | 0.02 | NS | 0.049 | 2.45 |
| Age | −0.08 | NS | ||||
| High | 0.14 | NS | ||||
| Weight | −0.26 | 0.03 | ||||
| FGF-1 [ng/mL] | Group | 0.095 | 0.18 | NS | <0.001 | 12.04 |
| Age | 0.443 | <0.001 | ||||
| High | −0.159 | NS | ||||
| Weight | 0.316 | 0.004 |
| Parameter | Median | Mean | Lower Quartile | Upper Quartile | SD |
|---|---|---|---|---|---|
| Cholesterol [mg/dL] | 154.42 | 158.75 | 136.28 | 184.51 | 28.90 |
| HDL [mg/dL] | 40.23 | 42.47 | 33.86 | 50.18 | 14.17 |
| LDL [mg/dL] | 94.06 | 97.42 | 76.49 | 119.90 | 28.60 |
| Glucose [mg/dL] | 97.33 | 97.94 | 92.00 | 101.78 | 7.63 |
| Triglycerides [mg/dL] | 132.84 | 135.92 | 114.43 | 153.23 | 25.96 |
| Albumin [g/dL] | 3.88 | 3.93 | 3.74 | 4.18 | 0.36 |
| Iron [μg/dL] | 70.09 | 68.16 | 50.47 | 82.24 | 19.49 |
| Total protein [g/dL] | 6.30 | 6.35 | 6.00 | 6.63 | 0.46 |
| Uric acid [mg/dL] | 4.47 | 4.51 | 3.68 | 5.26 | 1.10 |
| Creatinine [mg/dL] | 0.83 | 0.86 | 0.76 | 1.02 | 0.19 |
| Prevalence of Chronic Diseases | Number | Percent |
|---|---|---|
| No | 23 | 76.67 |
| Epilepsy | 1 | 3.33 |
| Asthma | 2 | 6.67 |
| Hashimoto’s | 3 | 10.00 |
| JIA | 1 | 3.33 |
| Medications Taken | Number | Percent |
|---|---|---|
| No | 23 | 76.67 |
| Levetivacetam, Lamotigrinum | 1 | 3.33 |
| Budesonide, formoterol fumarate dihydrate | 1 | 3.33 |
| levothyroxine | 1 | 3.33 |
| levothyroxine, desogestrel | 1 | 3.33 |
| Bisoprololi fumarans, levothyroxine | 1 | 3.33 |
| Dienogest | 1 | 3.33 |
| Methotrexate | 1 | 3.33 |
| Factor | Class | Number | Percent |
|---|---|---|---|
| Smoking | Yes | 24 | 80.00 |
| No | 6 | 20.00 | |
| Unanswered | 0 | 0.00 | |
| Taking hormonal contraception | Yes | 14 | 46.67 |
| No | 3 | 10.00 | |
| Unanswered | 13 | 43.33 |
| Past Operations within 6 Months | Number | Percent |
|---|---|---|
| No | 27 | 90.00 |
| Acute pancreatitis | 1 | 3.33 |
| Jaw surgery | 1 | 3.33 |
| Bone marrow donor | 1 | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecerska-Heryć, E.; Goszka, M.; Gliźniewicz, M.; Grygorcewicz, B.; Serwin, N.; Stodolak, P.; Słodzińska, W.; Birger, R.; Polikowska, A.; Budkowska, M.; et al. The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets. Int. J. Mol. Sci. 2024, 25, 3644. https://doi.org/10.3390/ijms25073644
Cecerska-Heryć E, Goszka M, Gliźniewicz M, Grygorcewicz B, Serwin N, Stodolak P, Słodzińska W, Birger R, Polikowska A, Budkowska M, et al. The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets. International Journal of Molecular Sciences. 2024; 25(7):3644. https://doi.org/10.3390/ijms25073644
Chicago/Turabian StyleCecerska-Heryć, Elżbieta, Małgorzata Goszka, Marta Gliźniewicz, Bartłomiej Grygorcewicz, Natalia Serwin, Patrycja Stodolak, Weronika Słodzińska, Radosław Birger, Aleksandra Polikowska, Marta Budkowska, and et al. 2024. "The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets" International Journal of Molecular Sciences 25, no. 7: 3644. https://doi.org/10.3390/ijms25073644
APA StyleCecerska-Heryć, E., Goszka, M., Gliźniewicz, M., Grygorcewicz, B., Serwin, N., Stodolak, P., Słodzińska, W., Birger, R., Polikowska, A., Budkowska, M., Rakoczy, R., & Dołęgowska, B. (2024). The Effect of a Rotating Magnetic Field on the Regenerative Potential of Platelets. International Journal of Molecular Sciences, 25(7), 3644. https://doi.org/10.3390/ijms25073644







