Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist’s Key Role
Abstract
1. Introduction
2. Diagnostic Approach to Idiopathic Interstitial Pneumonias: A Challenge in Pathology Practice
- Acute lung injury;
- Fibrosis;
- Cellular infiltrates;
- Alveolar filling;
- Nodules—small or large, single or multiple;
- Minimal changes.
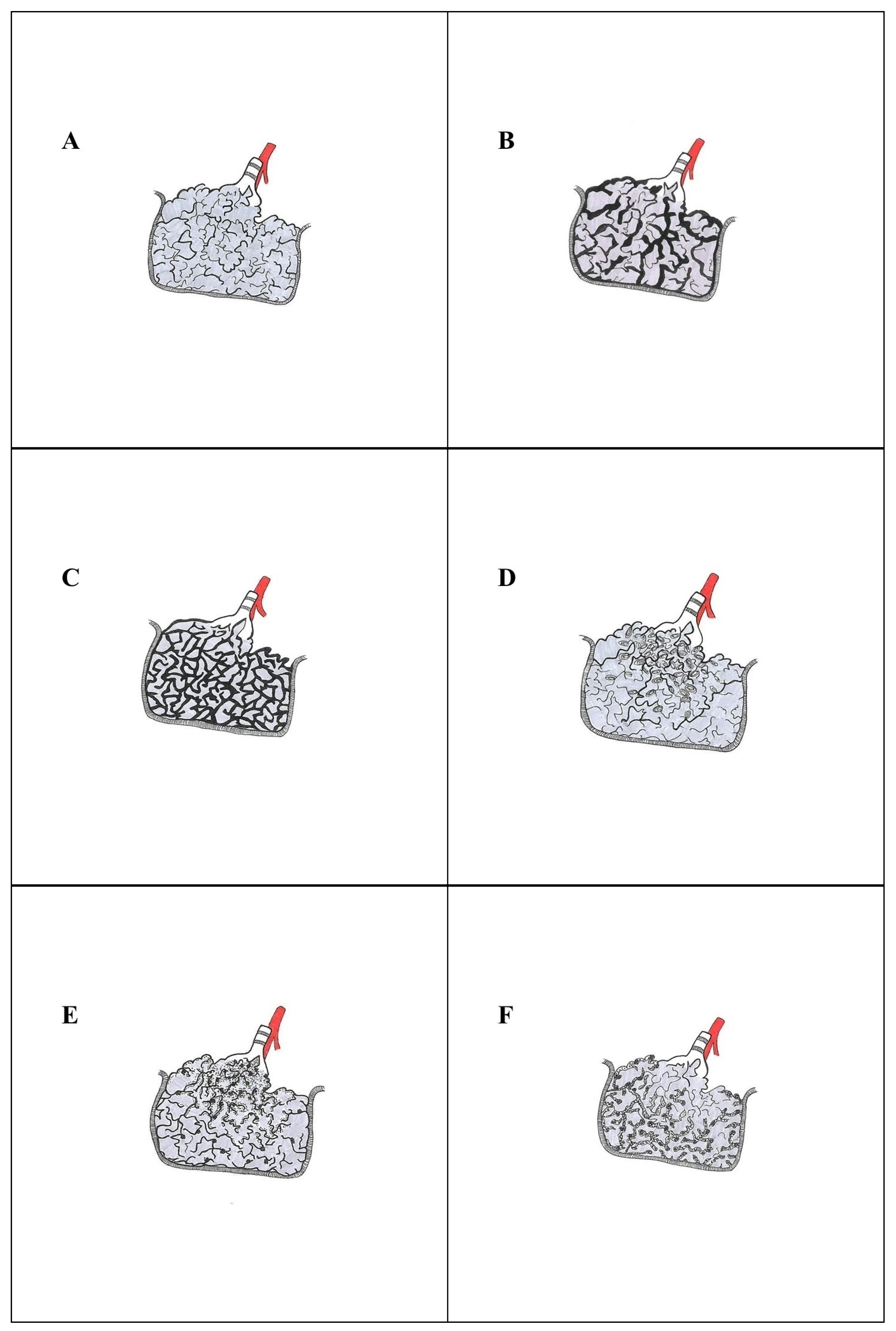
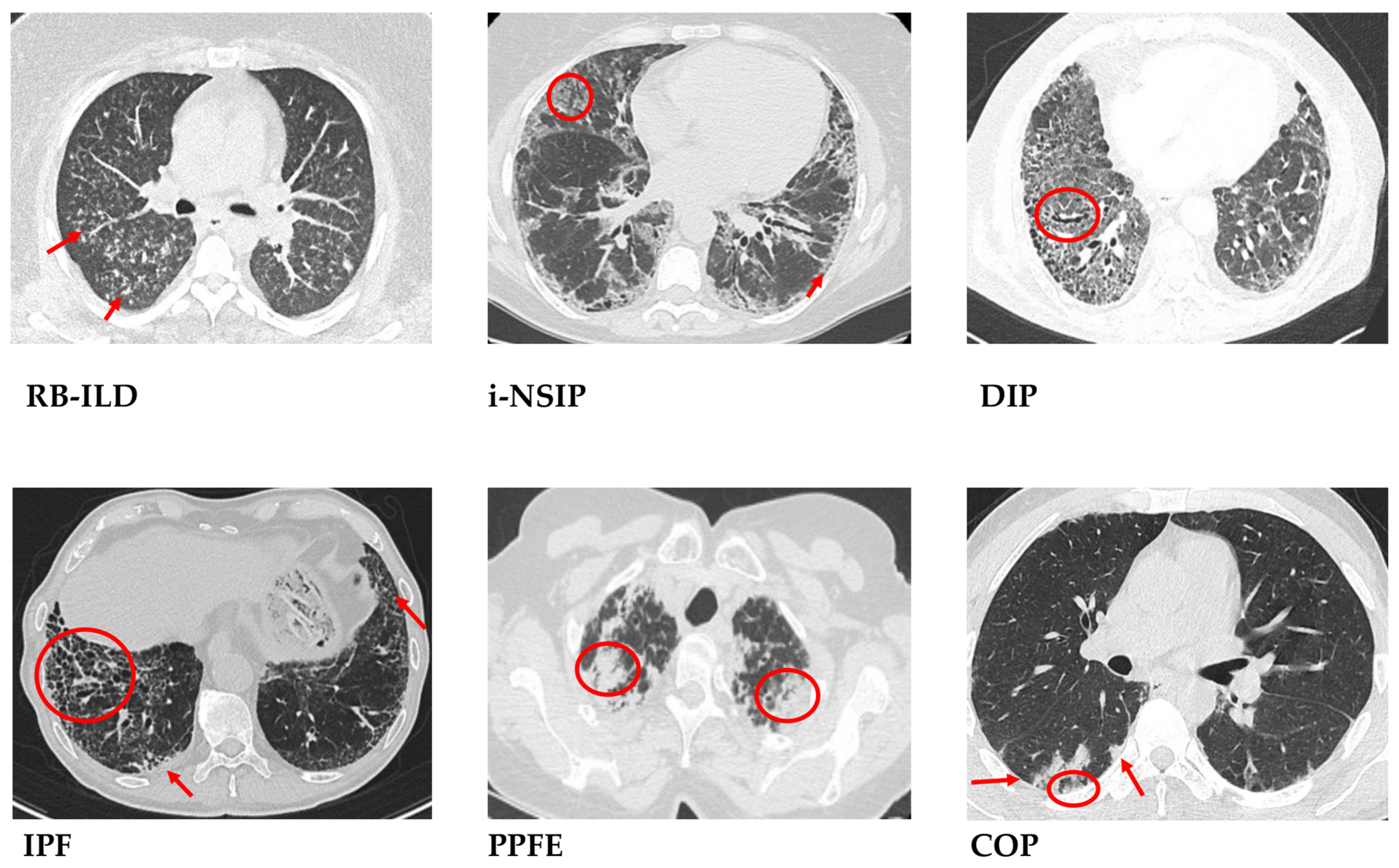
3. Histological Diagnosis of Idiopathic Interstitial Pneumonias
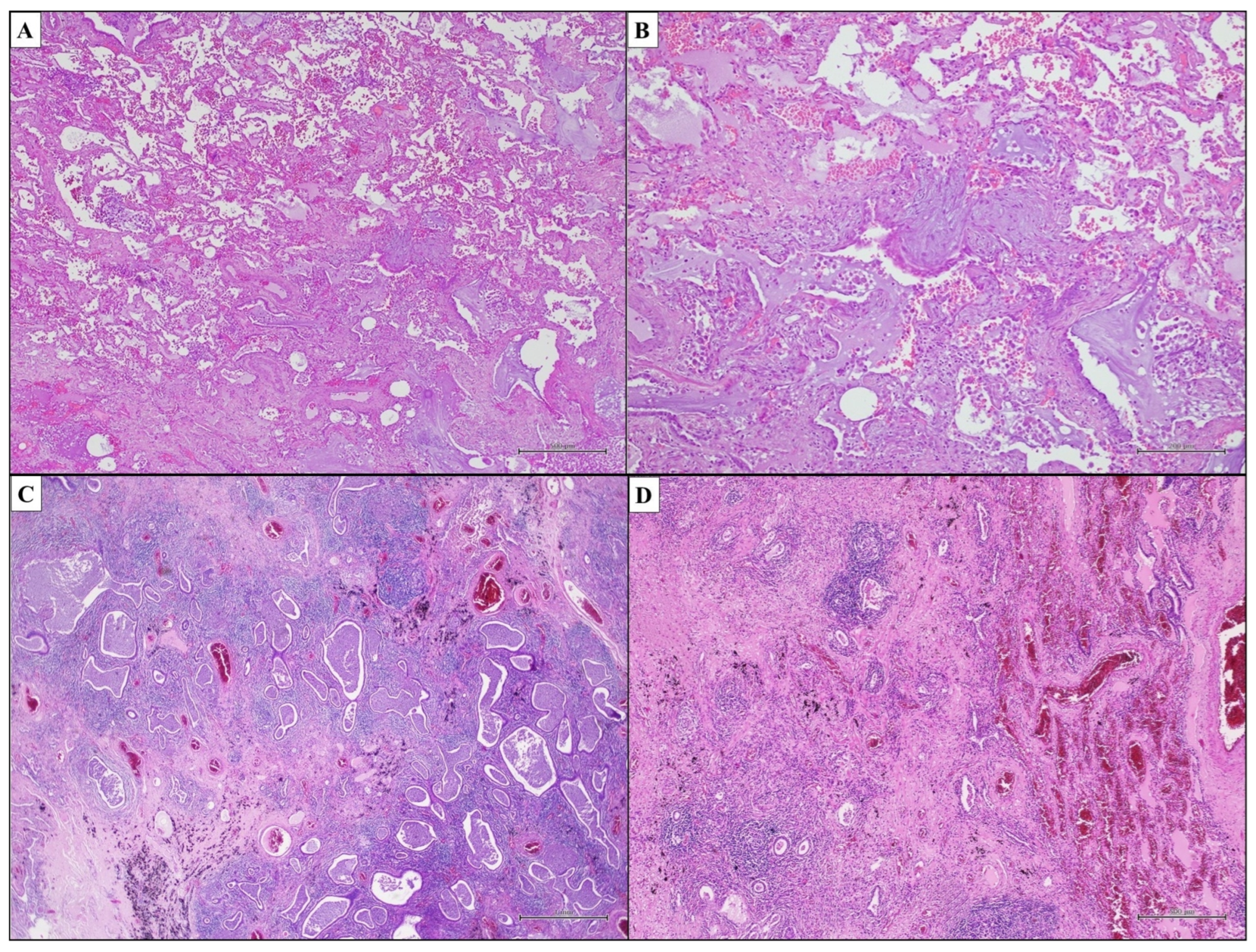
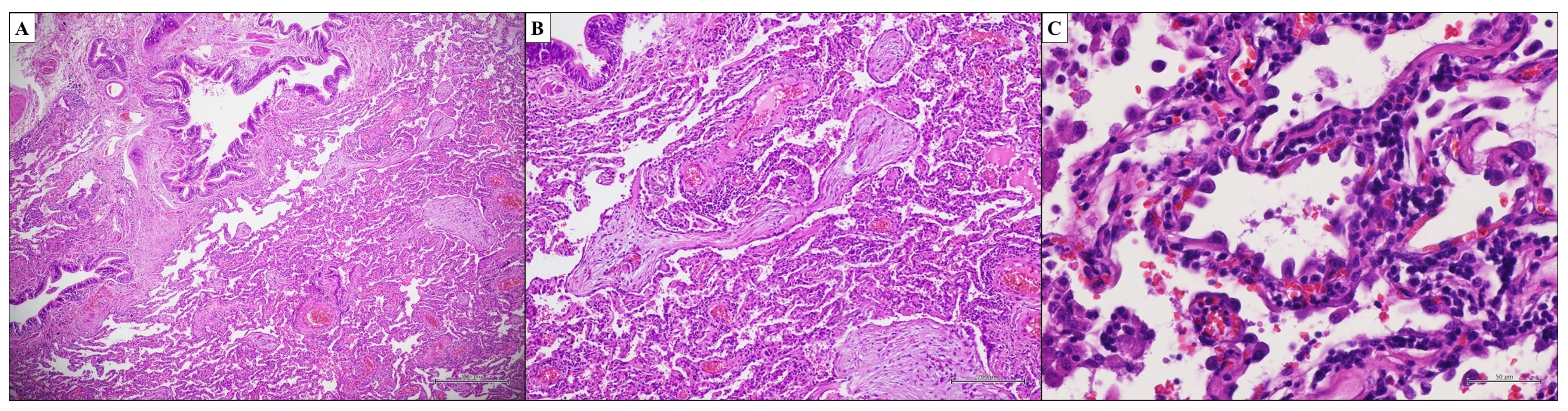
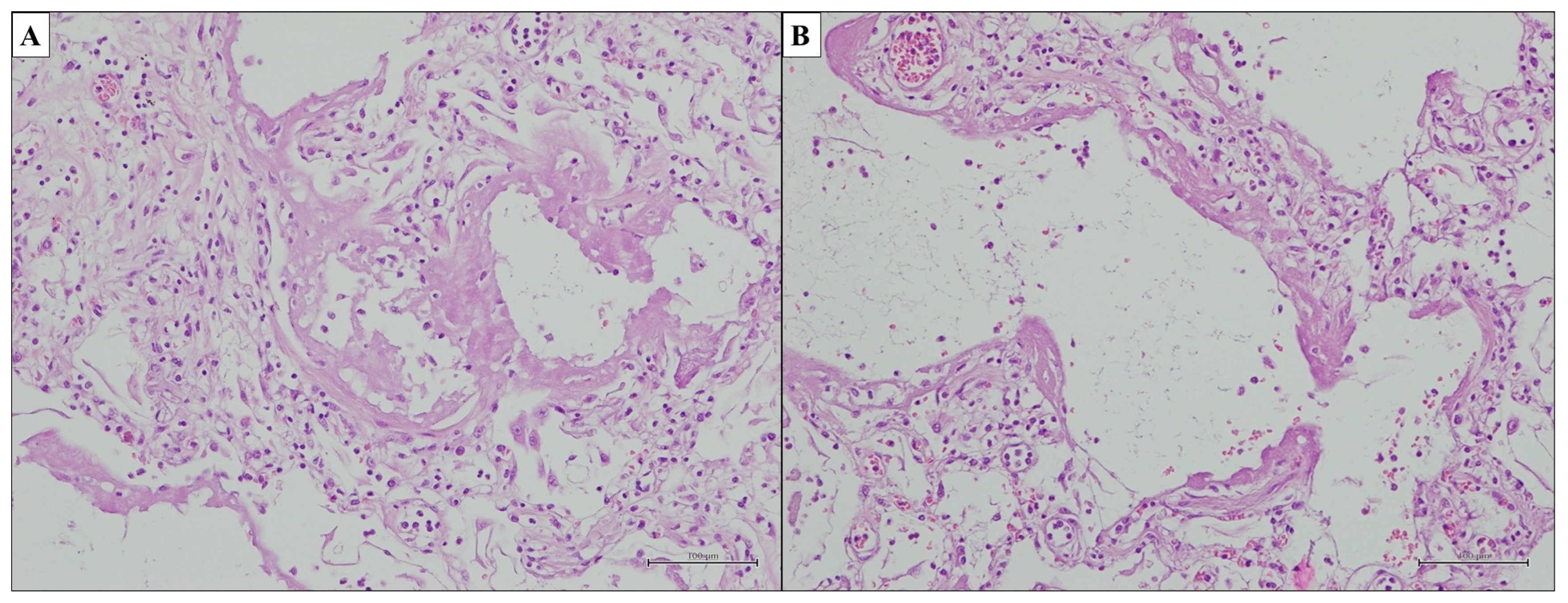
3.1. Idiopathic Pulmonary Fibrosis—Usual Interstitial Pneumonia
- Dense fibrosis with architectural distortion;
- Predominant peripheral involvement of the secondary pulmonary lobule by fibrosis;
- Patchy involvement of lung parenchyma by fibrosis;
- The presence of fibroblast foci at the edges of the dense scars;
- The absence of features to suggest an alternative diagnosis.
| UIP Pattern |
|---|
| Dense interstitial fibrosis with lung architectural distortion (scar-like fibrosis and/or honeycomb change) |
| Patchy lung involvement (spatial heterogeneity) |
| Mainly subpleural and paraseptal involvement |
| Temporal heterogeneity of fibrosis (fibroblast foci and scar-like fibrosis) |
| Minimal interstitial inflammation |
| UIP | Probable UIP | Indeterminate for UIP | Alternative Diagnosis |
|---|---|---|---|
|
|
|
|
|
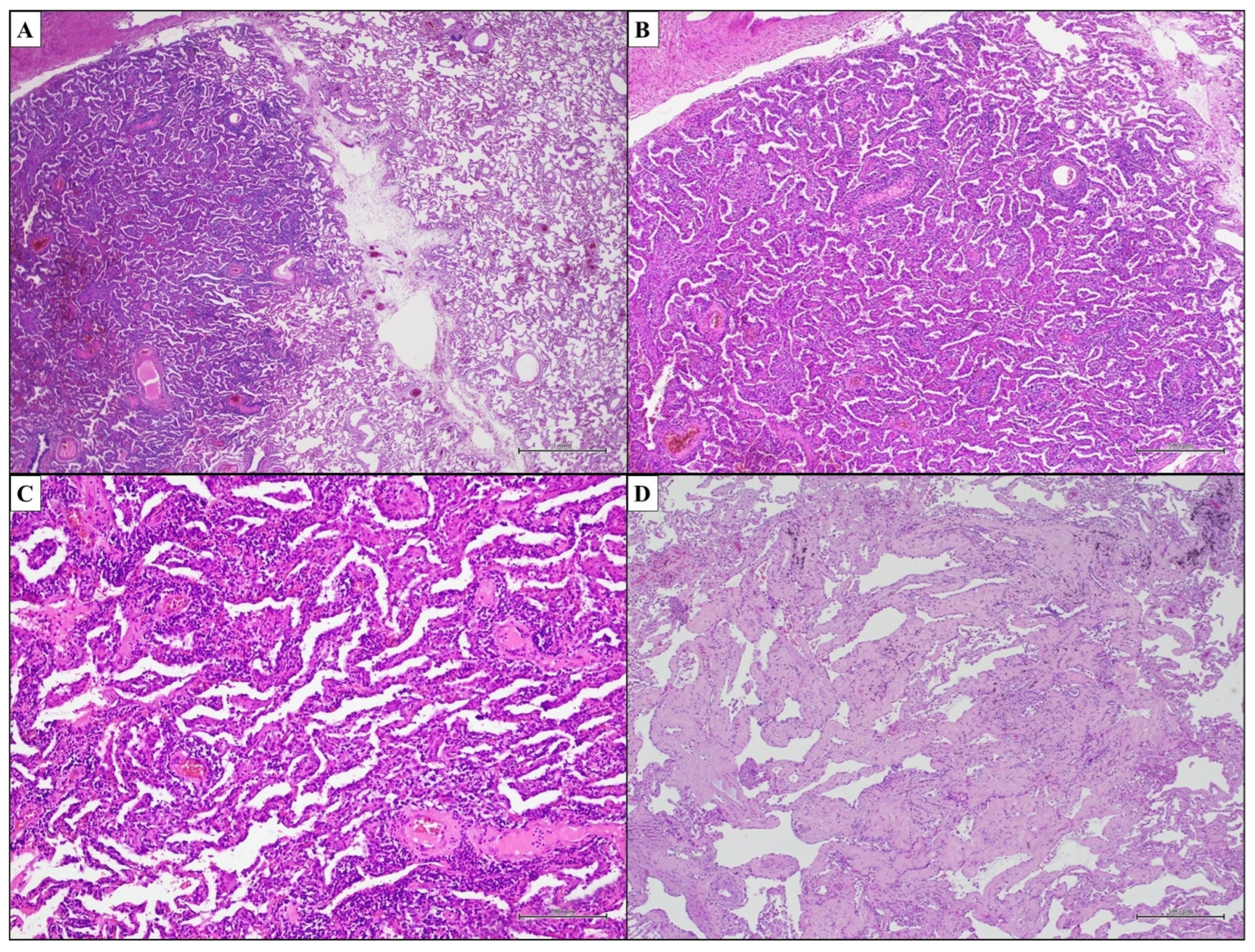
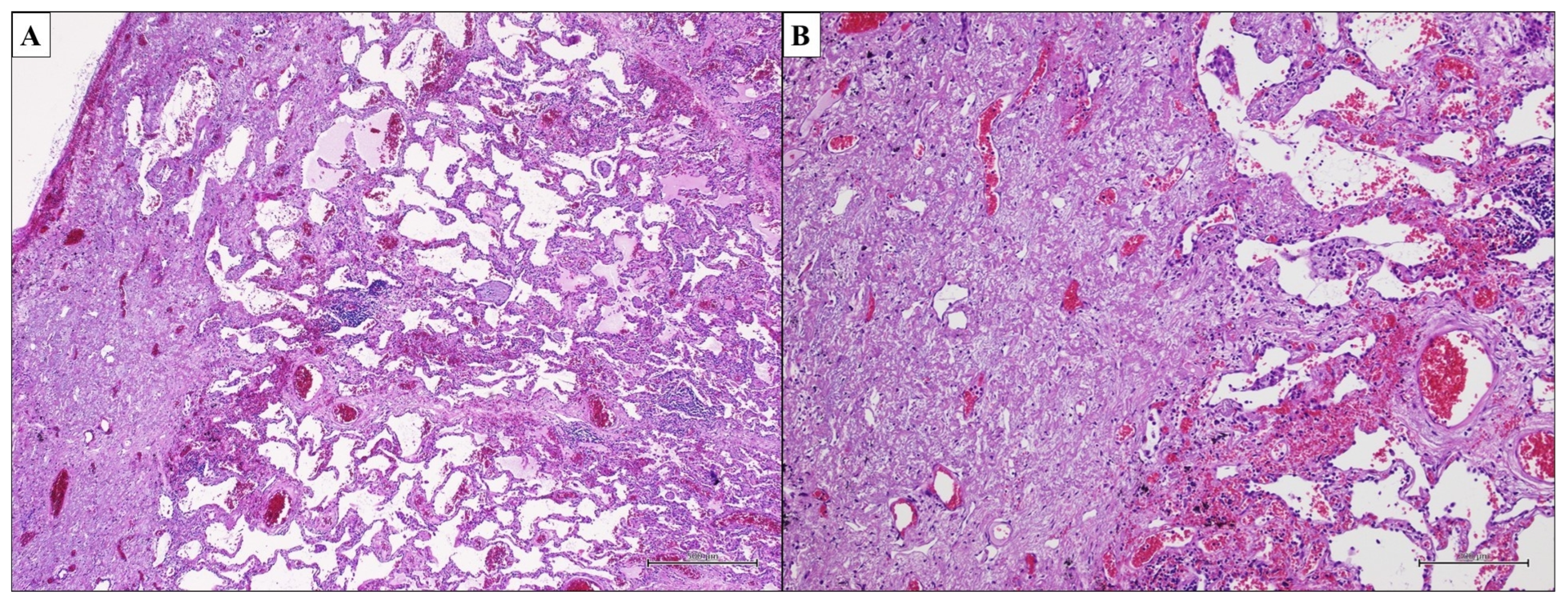
3.2. Idiopathic Nonspecific Interstitial Pneumonia—Nonspecific Interstitial Pneumonia
3.3. Cryptogenic Organizing Pneumonia—Organizing Pneumonia
3.4. Acute Interstitial Pneumonia—Diffuse Alveolar Damage
3.5. Desquamative Interstitial Pneumonia—Desquamative Interstitial Pneumonia
3.6. Respiratory Bronchiolitis-Interstitial Lung Disease—Respiratory Bronchiolitis
3.7. Idiopathic Lymphoid Interstitial Pneumonia—Lymphoid Interstitial Pneumonia
3.8. Idiopathic Pleuroparenchymal Fibroelastosis—Pleuroparenchymal Fibroelastosis
3.9. Unclassifiable Idiopathic Interstitial Pneumonia
3.10. Rare Histological Patterns of Idiopathic Interstitial Pneumonia
4. Combined Pulmonary Fibrosis and Emphysema: A Newly and Still Poorly Understood Clinico-Pathological Entity
5. Clinico-Radiological and Prognostic Features
6. Lung Biopsy in Interstitial Lung Disease: A Multidisciplinary Choice
7. Molecular Testing as a Useful Partner of Morphology: The Beginning of a New Era
8. Multidisciplinary Team: Sharing and Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guler, S.A.; Corte, T.J. Interstitial Lung Disease in 2020: A History of Progress. Clin. Chest Med. 2021, 42, 229–239. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Margaritopoulos, G.A.; Tomassetti, S.; Bonella, F.; Costabel, U.; Poletti, V. Interstitial lung disease. Eur. Respir. Rev. 2014, 23, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Carvajalino, S.; Reigada, C.; Johnson, M.J.; Dzingina, M.; Bajwah, S. Symptom prevalence of patients with fibrotic interstitial lung disease: A systematic literature review. BMC Pulm. Med. 2018, 18, 78. [Google Scholar] [CrossRef]
- Lucà, S.; Zannini, G.; Morgillo, F.; Della Corte, C.M.; Fiorelli, A.; Zito Marino, F.; Campione, S.; Vicidomini, G.; Guggino, G.; Ronchi, A.; et al. The prognostic value of histopathology in invasive lung adenocarcinoma: A comparative review of the main proposed grading systems. Expert. Rev. Anticancer Ther. 2023, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Lucà, S.; Franco, R.; Napolitano, A.; Soria, V.; Ronchi, A.; Zito Marino, F.; Della Corte, C.M.; Morgillo, F.; Fiorelli, A.; Luciano, A.; et al. PATZ1 in Non-Small Cell Lung Cancer: A New Biomarker That Negatively Correlates with PD-L1 Expression and Suppresses the Malignant Phenotype. Cancers 2023, 15, 2190. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Suzuki, A.; Maher, T.M. Interstitial lung diseases. Lancet 2022, 400, 769–786. [Google Scholar] [CrossRef]
- Hilberg, O.; Hoffmann-Vold, A.M.; Smith, V.; Bouros, D.; Kilpeläinen, M.; Guiot, J.; Morais, A.; Clemente, S.; Daniil, Z.; Papakosta, D.; et al. Epidemiology of interstitial lung diseases and their progressive-fibrosing behaviour in six European countries. ERJ Open Res. 2022, 8, 00597–02021. [Google Scholar] [CrossRef]
- George, P.M.; Spagnolo, P.; Kreuter, M.; Altinisik, G.; Bonifazi, M.; Martinez, F.J.; Molyneaux, P.L.; Renzoni, E.A.; Richeldi, L.; Tomassetti, S.; et al. Progressive fibrosing interstitial lung disease: Clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir. Med. 2020, 8, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Ryerson, C.J.; Collard, H.R. Update on the diagnosis and classification of ILD. Curr. Opin. Pulm. Med. 2013, 19, 453–459. [Google Scholar] [CrossRef]
- Neurohr, C.; Behr, J. Changes in the current classification of IIP: A critical review. Respirology 2015, 20, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A. Definition and classification of interstitial pneumonias in human pathology. In Alveolar Interstitium of the Lung; Karger Publishers: Basel, Switzerland, 1975; Volume 8, pp. 1–33. [Google Scholar]
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2002, 165, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A., Jr.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Bergin, C.; Roggli, V.; Coblentz, C.; Chiles, C. The secondary pulmonary lobule: Normal and abnormal CT appearances. AJR Am. J. Roentgenol. 1988, 151, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S. The Lung, 2nd ed.; Charles C. Thomas: Springfield, IL, USA, 1947. [Google Scholar]
- Webb, W.R. Thin-section CT of the secondary pulmonary lobule: Anatomy and the image—The 2004 Fleischner lecture. Radiology 2006, 239, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Zaizen, Y.; Fukuoka, J. Pathology of Idiopathic Interstitial Pneumonias. Surg. Pathol. Clin. 2020, 13, 91–118. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, A.; Sato, S.; Tanaka, T.; Hashisako, M.; Kashima, Y.; Tsuchiya, T.; Yamasaki, N.; Nagayasu, T.; Yamamoto, H.; Fukuoka, J. Breakdown of lung framework and an increase in pores of Kohn as initial events of emphysema and a cause of reduction in diffusing capacity. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Canzian, M.; de Matos Soeiro, A.; de Lima Taga, M.F.; Farhat, C.; Barbas, C.S.; Capelozzi, V.L. Semiquantitative assessment of surgical lung biopsy: Predictive value and impact on survival of patients with diffuse pulmonary infiltrate. Clinics 2007, 62, 23–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchiori, D.M. Introduction to chest radiography. In Clinical Imaging, 3rd ed.; Marchiori, D.M., Ed.; Mosby: Maryland Heights, MO, USA, 2013; pp. 1153–1165. [Google Scholar]
- Leslie, K.O. My approach to interstitial lung disease using clinical, radiological and histopathological patterns. J. Clin. Pathol. 2009, 62, 387–401. [Google Scholar] [CrossRef]
- Leslie, K.; Wick, M. Practical Pulmonary Pathology. A Diagnostic Approach, 1st ed.; Churchill-Livingstone: Philadelphia, PA, USA, 2005. [Google Scholar]
- Leslie, K.O. Pulmonary pathology for the clinician. Clin. Chest Med. 2006, 27 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Jawad, H.; Chung, J.H.; Lynch, D.A.; Newell, J.D., Jr. Radiological approach to interstitial lung disease: A guide for the nonradiologist. Clin. Chest Med. 2012, 33, 11–26. [Google Scholar] [CrossRef]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Ebner, L.; Christodoulidis, S.; Stathopoulou, T.; Geiser, T.; Stalder, O.; Limacher, A.; Heverhagen, J.T.; Mougiakakou, S.G.; Christe, A. Meta-analysis of the radiological and clinical features of Usual Interstitial Pneumonia (UIP) and Nonspecific Interstitial Pneumonia (NSIP). PLoS ONE 2020, 15, e0226084. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Usual interstitial pneumonia (UIP): A clinically significant pathologic diagnosis. Mod. Pathol. 2022, 35, 580–588. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Mukhopadhyay, S.; Myers, J.L. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum. Pathol. 2008, 39, 1275–1294. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Zisman, D.A.; Litzky, L.A.; Nguyen, B.T.; Kotloff, R.M. Usual interstitial pneumonia: Histologic study of biopsy and explant specimens. Am. J. Surg. Pathol. 2002, 26, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Myers, J.L. Idiopathic pulmonary fibrosis: Clinical relevance of pathologic classification. Am. J. Respir. Crit. Care Med. 1998, 157, 1301–1315. [Google Scholar] [CrossRef]
- Hashisako, M.; Fukuoka, J. Pathology of Idiopathic Interstitial Pneumonias. Clin. Med. Insights Circ. Respir. Pulm. Med. 2016, 9 (Suppl. S1), 123–133. [Google Scholar] [CrossRef]
- Smith, M.; Dalurzo, M.; Panse, P.; Parish, J.; Leslie, K. Usual interstitial pneumonia-pattern fibrosis in surgical lung biopsies. Clinical, radiological and histopathological clues to aetiology. J. Clin. Pathol. 2013, 66, 896–903. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Larsen, B.T. Usual interstitial pneumonia: A clinically significant pattern, but not the final word. Mod. Pathol. 2022, 35, 589–593. [Google Scholar] [CrossRef]
- Katzenstein, A.L. Smoking-related interstitial fibrosis (SRIF), pathogenesis and treatment of usual interstitial pneumonia (UIP), and transbronchial biopsy in UIP. Mod. Pathol. 2012, 25 (Suppl. S1), S68–S78. [Google Scholar] [CrossRef]
- Coward, W.R.; Saini, G.; Jenkins, G. The pathogenesis of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 2010, 4, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Mehrad, B. New mechanisms of pulmonary fibrosis. Chest 2009, 136, 1364–1370. [Google Scholar] [CrossRef]
- Blumhagen, R.Z.; Kurche, J.S.; Cool, C.D.; Walts, A.D.; Heinz, D.; Fingerlin, T.E.; Yang, I.V.; Schwartz, D.A. Spatially distinct molecular patterns of gene expression in idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 287. [Google Scholar] [CrossRef]
- Tabaj, G.C.; Fernandez, C.F.; Sabbagh, E.; Leslie, K.O. Histopathology of the idiopathic interstitial pneumonias (IIP): A review. Respirology 2015, 20, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Genereux, G.P. The end-stage lung: Pathogenesis, pathology, and radiology. Radiology 1975, 116, 279–289. [Google Scholar] [CrossRef]
- Xu, L.; Kligerman, S.; Burke, A. End-stage sarcoid lung disease is distinct from usual interstitial pneumonia. Am. J. Surg. Pathol. 2013, 37, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.L.; Katzenstein, A.L. Beyond a consensus classification for idiopathic interstitial pneumonias: Progress and controversies. Histopathology 2009, 54, 90–103. [Google Scholar] [CrossRef]
- Tanabe, N.; McDonough, J.E.; Vasilescu, D.M.; Ikezoe, K.; Verleden, S.E.; Xu, F.; Wuyts, W.A.; Vanaudenaerde, B.M.; Colby, T.V.; Hogg, J.C. Pathology of Idiopathic Pulmonary Fibrosis Assessed by a Combination of Microcomputed Tomography, Histology, and Immunohistochemistry. Am. J. Pathol. 2020, 190, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Staats, P.; Kligerman, S.; Todd, N.; Tavora, F.; Xu, L.; Burke, A. A comparative study of honeycombing on high resolution computed tomography with histologic lung remodeling in explants with usual interstitial pneumonia. Pathol. Res. Pract. 2015, 211, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rabeyrin, M.; Thivolet, F.; Ferretti, G.R.; Chalabreysse, L.; Jankowski, A.; Cottin, V.; Pison, C.; Cordier, J.F.; Lantuejoul, S. Usual interstitial pneumonia end-stage features from explants with radiologic and pathological correlations. Ann. Diagn. Pathol. 2015, 19, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L. Katzenstein and Askin’s Surgical Pathology of Non-Neoplastic Lung Disease, 4th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2006. [Google Scholar]
- Katzenstein, A.L.A. Diagnostic Atlas of Non-Neoplastic Lung Disease: A Practical Guide for Surgical Pathologists; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E.; Lasky, J.A., Jr.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643. [Google Scholar] [CrossRef]
- Collard, H.R.; Ryerson, C.J.; Corte, T.J.; Jenkins, G.; Kondoh, Y.; Lederer, D.J.; Lee, J.S.; Maher, T.M.; Wells, A.U.; Antoniou, K.M.; et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am. J. Respir. Crit. Care Med. 2016, 194, 265–275. [Google Scholar] [CrossRef]
- Kondoh, Y.; Taniguchi, H.; Kawabata, Y.; Yokoi, T.; Suzuki, K.; Takagi, K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993, 103, 1808–1812. [Google Scholar] [CrossRef]
- Parambil, J.G.; Myers, J.L.; Ryu, J.H. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005, 128, 3310–3315. [Google Scholar] [CrossRef]
- Ambrosini, V.; Cancellieri, A.; Chilosi, M.; Zompatori, M.; Trisolini, R.; Saragoni, L.; Poletti, V. Acute exacerbation of idiopathic pulmonary fibrosis: Report of a series. Eur. Respir. J. 2003, 22, 821–826. [Google Scholar] [CrossRef]
- Silva, C.I.; Müller, N.L.; Fujimoto, K.; Kato, S.; Ichikado, K.; Taniguchi, H.; Kondoh, Y.; Johkoh, T.; Churg, A. Acute exacerbation of chronic interstitial pneumonia: High-resolution computed tomography and pathologic findings. J. Thorac. Imaging 2007, 22, 221–229. [Google Scholar] [CrossRef]
- Sakamoto, K.; Taniguchi, H.; Kondoh, Y.; Ono, K.; Hasegawa, Y.; Kitaichi, M. Acute exacerbation of idiopathic pulmonary fibrosis as the initial presentation of the disease. Eur. Respir. Rev. 2009, 18, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Taniguchi, H.; Kataoka, K.; Kato, K.; Suzuki, R.; Ogura, T.; Johkoh, T.; Yokoi, T.; Wells, A.U.; Kitaichi, M.; et al. Prognostic factors in rapidly progressive interstitial pneumonia. Respirology 2010, 15, 257–264. [Google Scholar] [CrossRef]
- Travis, W.D.; Hunninghake, G.; King, T.E.; Lynch, D.A., Jr.; Colby, T.V.; Galvin, J.R.; Brown, K.K.; Chung, M.P.; Cordier, J.F.; du Bois, R.M.; et al. Idiopathic nonspecific interstitial pneumonia: Report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Perelas, A.; Silver, R.M.; Arrossi, A.V.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef]
- Konopka, K.E.; Myers, J.L. Interstitial lung disease pathology in systemic sclerosis. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211032437. [Google Scholar] [CrossRef] [PubMed]
- Theegarten, D.; Müller, H.M.; Bonella, F.; Wohlschlaeger, J.; Costabel, U. Diagnostic approach to interstitial pneumonias in a single centre: Report on 88 cases. Diagn. Pathol. 2012, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.W.; Marciniak, E.T.; Sachdeva, A.; Kligerman, S.J.; Galvin, J.R.; Luzina, I.G.; Atamas, S.P.; Burke, A.P. Organizing pneumonia/non-specific interstitial pneumonia overlap is associated with unfavorable lung disease progression. Respir. Med. 2015, 109, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.Y. Pathological interpretation of connective tissue disease-associated lung diseases. Yeungnam Univ. J. Med. 2019, 36, 8–15. [Google Scholar] [CrossRef]
- Mehrian, P.; Cheraghvandi, A.; Droudnia, A.; Talischi, F.; Fallah Tafti, S.; Kahkouee, S.; Jamaati, H. Nonspecific Interstitial pneumonia (NSIP)/Overlap or Distinct Entity: A case report from the National Research Institute of Tuberculosis and Lung Disease (NRITLD). Casp. J. Intern. Med. 2014, 5, 118–122. [Google Scholar]
- Enomoto, N.; Sumikawa, H.; Sugiura, H.; Kitani, M.; Tanaka, T.; Hozumi, H.; Fujisawa, T.; Suda, T. Clinical, radiological, and pathological evaluation of “NSIP with OP overlap” pattern compared with NSIP in patients with idiopathic interstitial pneumonias. Respir. Med. 2020, 174, 106201. [Google Scholar] [CrossRef]
- Tafti, S.F.; Cheraghvandi, A.; Mokri, B.; Talischi, F. Cases non-specific interstitial pneumonia and hypersensitivity pneumonia: A new pathologic diagnosis or overlap syndrome. Respir. Med. Case Rep. 2012, 5, 45–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kondoh, Y.; Taniguchi, H.; Tabata, K.; Kimura, T.; Kataoka, K.; Ono, K.; Hashisako, M.; Fukuoka, J. Lung histopathological pattern in a survivor with rapidly progressive interstitial lung disease and anti-melanoma differentiation-associated gene 5 antibody-positive clinically amyopathic dermatomyositis. Respir. Med. Case Rep. 2016, 19, 5–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katzenstein, A.L.; Fiorelli, R.F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994, 18, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.L. Nonspecific interstitial pneumonia: Pathologic features and clinical implications. Semin. Diagn. Pathol. 2007, 24, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Colby, T.V.; Wells, A.U. Histopathological approach to patterns of interstitial pneumonia in patient with connective tissue disorders. Sarcoidosis Vasc. Diffuse Lung Dis. 2002, 19, 10–17. [Google Scholar] [PubMed]
- Belloli, E.A.; Beckford, R.; Hadley, R.; Flaherty, K.R. Idiopathic non-specific interstitial pneumonia. Respirology 2016, 21, 259–268. [Google Scholar] [CrossRef]
- Kligerman, S.J.; Groshong, S.; Brown, K.K.; Lynch, D.A. Nonspecific interstitial pneumonia: Radiologic, clinical, and pathologic considerations. Radiographics 2009, 29, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Kitaichi, M.; Itoh, H.; Nishimura, K.; Izumi, T.; Colby, T.V. Idiopathic nonspecific interstitial pneumonia/fibrosis: Comparison with idiopathic pulmonary fibrosis and BOOP. Eur. Respir. J. 1998, 12, 1010–1019. [Google Scholar] [CrossRef]
- Huo, Z.; Li, J.; Li, S.; Zhang, H.; Jin, Z.; Pang, J.; Liu, H.; Shi, J.; Feng, R. Organizing pneumonia components in non-specific interstitial pneumonia (NSIP): A clinicopathological study of 33 NSIP cases. Histopathology 2016, 68, 347–355. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Travis, W.D.; Colby, T.V.; Toews, G.B.; Kazerooni, E.A.; Gross, B.H.; Jain, A.; Strawderman, R.L.; Flint, A.; Lynch, J.P.; et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2001, 164, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M.; Wells, A.U. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 2000, 162, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Matsui, K.; Moss, J.; Ferrans, V.J. Idiopathic nonspecific interstitial pneumonia: Prognostic significance of cellular and fibrosing patterns: Survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am. J. Surg. Pathol. 2000, 24, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Amenomori, M.; Mukae, H.; Sakamoto, N.; Kakugawa, T.; Hayashi, T.; Hara, A.; Hara, S.; Fujita, H.; Ishimoto, H.; Ishimatsu, Y.; et al. HSP47 in lung fibroblasts is a predictor of survival in fibrotic nonspecific interstitial pneumonia. Respir. Med. 2010, 104, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P. Some moot points in the pathology and clinical history of pneumonia. Lancet 1912, 1, 1665–1670. [Google Scholar]
- Sulavik, S.B. The concept of “organizing pneumonia”. Chest 1989, 96, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Milne, L.S. Chronic pneumonia (including a discussion of two cases of syphilis of the lung). Am. J. Med. Sci. 1911, 142, 408–438. [Google Scholar] [CrossRef]
- Floyd, R. Organization of pneumonic exudates. Am. J. Med. Sci. 1922, 163, 527–548. [Google Scholar] [CrossRef]
- Miskoff, J.A.; Ali, R.; Chaudhri, M.A. Case of Dermatomyositis Causing Cryptogenic Organizing Pneumonia. Cureus 2019, 11, e6296. [Google Scholar] [CrossRef]
- Henriet, A.C.; Diot, E.; Marchand-Adam, S.; de Muret, A.; Favelle, O.; Crestani, B.; Diot, P. Organising pneumonia can be the inaugural manifestation in connective tissue diseases, including Sjogren’s syndrome. Eur. Respir. Rev. 2010, 19, 161–163. [Google Scholar] [CrossRef]
- Radzikowska, E.; Fijolek, J. Update on cryptogenic organizing pneumonia. Front. Med. 2023, 10, 1146782. [Google Scholar] [CrossRef]
- Zizzo, G.; Caruso, S.; Ricchiuti, E.; Turato, R.; Stefani, I.; Mazzone, A. Amiodarone-induced organizing pneumonia mimicking COVID-19: A case report. Eur. J. Med. Res. 2021, 26, 62. [Google Scholar] [CrossRef]
- Golbets, E.; Kaplan, A.; Shafat, T.; Yagel, Y.; Jotkowitz, A.; Awesat, J.; Barski, L. Secondary organizing pneumonia after recovery of mild COVID-19 infection. J. Med. Virol. 2022, 94, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Yokoi, T.; Nishiyama, N.; Koizumi, Y.; Sakanashi, D.; Kato, H.; Hagihara, M.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. Secondary organizing pneumonia following viral pneumonia caused by severe influenza B: A case report and literature reviews. BMC Infect. Dis. 2017, 17, 572. [Google Scholar] [CrossRef]
- Bai, J.S.; Zhang, Q.; Liu, J.X.; Wang, J.M.; Chen, Q.C.; Zhou, X.Y.; Liu, R.X.; Gao, S.; Fu, A.S.; Ge, Y.L. Organizing Pneumonia Secondary to Infection with Coxiella Burnetii: A Case Report. Clin. Lab. 2023, 69, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.M.; Narayana Gowda, S.; Sharma, R.; Ellithi, M.; Oberoi, M. Temozolomide-Associated Organizing Pneumonia. Am. J. Ther. 2022, 29, e781–e783. [Google Scholar] [CrossRef]
- Choi, K.J.; Yoo, E.H.; Kim, K.C.; Kim, E.J. Comparison of clinical features and prognosis in patients with cryptogenic and secondary organizing pneumonia. BMC Pulm. Med. 2021, 21, 336. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.L.; Limper, A.H.; Swensen, S.J. Drug-induced lung disease: A pragmatic classification incorporating HRCT appearances. Semin. Respir. Crit. Care Med. 2003, 24, 445–454. [Google Scholar] [CrossRef]
- Nambu, A.; Araki, T.; Ozawa, K.; Kanazawa, M.; Ohki, Z.; Miyata, K. Bronchiolitis obliterans organizing pneumonia after tangential beam irradiation to the breast: Discrimination from radiation pneumonitis. Radiat. Med. 2002, 20, 151–154. [Google Scholar]
- Miwa, S.; Morita, S.; Suda, T.; Suzuki, K.; Hayakawa, H.; Chida, K.; Nakamura, H. The incidence and clinical characteristics of bronchiolitis obliterans organizing pneumonia syndrome after radiation therapy for breast cancer. Sarcoidosis Vasc. Diffus. Lung Dis. 2004, 21, 212–218. [Google Scholar]
- Falk, L.; Broman, L.M. Extracorporeal membrane oxygenation rescue in adolescent with bronchiolitis obliterans-organizing pneumonia like Wegener’s granulomatosis. Clin. Case Rep. 2016, 5, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, D.J.; Theodorou, S.J.; Mpougias, K.; Mastora, M.; Stefanaki, S.; Akritidis, N.C. Wegener granulomatosis masquerading as pneumonia. J. Belg. Soc. Radiol. 2010, 93, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Jang, Y.R.; Yoon, H.H.; Kim, S.; Kim, E.Y.; Ha, S.Y.; Park, J.W. A Case of Balsalazide-Induced Limited Form of Granulomatosis with Polyangiitis with Bronchiolitis Obliterans Organizing Pneumonia-like Variant in Ulcerative Colitis. Tuberc. Respir. Dis. 2012, 72, 323–327. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Lee, J.S. Cryptogenic Organizing Pneumonia. N. Engl. J. Med. 2022, 386, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Cordier, J.F. Cryptogenic organising pneumonia. Eur. Respir. J. 2006, 28, 422–446. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.V.; Patel, D.; Machnicki, S.; Naidich, D.; Stover, D.; Travis, W.D.; Brown, K.K.; Naidich, J.J.; Mahajan, A.; Esposito, M.; et al. Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features. Chest 2022, 162, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Colby, T.V.; Koss, M.N.; Rosado-de-Christenson, M.L.; Müller, N.L.; King, T.E., Jr. Cryptogenic organizing pneumonia (bronchiolitis obliterans organizing pneumonia). In Non-Neoplastic Disorders of the Lower Respiratory Tract; American Registry of Pathology: Arlington, VA, USA, 2002; pp. 82–88. [Google Scholar]
- Raghu, G.; Meyer, K.C. Cryptogenic organising pneumonia: Current understanding of an enigmatic lung disease. Eur. Respir. Rev. 2021, 30, 210094. [Google Scholar] [CrossRef]
- Cottin, V.; Cordier, J.F. Cryptogenic organizing pneumonia. Semin. Respir. Crit. Care Med. 2012, 33, 462–475. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Myers, J.L.; Mazur, M.T. Acute interstitial pneumonia. A clinicopathologic, ultrastructural, and cell kinetic study. Am. J. Surg. Pathol. 1986, 10, 256–267. [Google Scholar] [CrossRef]
- Schwarz, M.A. Acute lung injury: Cellular mechanisms and derangements. Paediatr. Respir. Rev. 2001, 2, 3–9. [Google Scholar] [CrossRef]
- Hamman, L.; Rich, A.R. Fulminating Diffuse Interstitial Fibrosis of the Lungs. Trans. Am. Clin. Climatol. Assoc. 1935, 51, 154–163. [Google Scholar]
- Hamman, L.; Rich, A.R. Acute diffuse interstitial fibrosis of the lungs. Bull. Johns Hopkins Hosp. 1944, 74, 177–212. [Google Scholar]
- Beasley, M.B. The pathologist’s approach to acute lung injury. Arch. Pathol. Lab. Med. 2010, 134, 719–727. [Google Scholar] [CrossRef]
- Tomashefski, J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000, 21, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Kondoh, Y. Acute and subacute idiopathic interstitial pneumonias. Respirology 2016, 21, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin. Respir. Crit. Care Med. 2006, 27, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Bouros, D.; Nicholson, A.C.; Polychronopoulos, V.; du Bois, R.M. Acute interstitial pneumonia. Eur. Respir. J. 2000, 15, 412–418. [Google Scholar] [CrossRef]
- Dawod, Y.T.; Cook, N.E.; Graham, W.B.; Madhani-Lovely, F.; Thao, C. Smoking-associated interstitial lung disease: Update and review. Expert Rev. Respir. Med. 2020, 14, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.A.; Steer, A.; Billingsley, J.G. Desquamative interstitial pneumonia. Am. J. Med. 1965, 39, 369–404. [Google Scholar] [CrossRef]
- Carrington, C.B.; Gaensler, E.A.; Coutu, R.E.; Fitzgerald, M.X.; Gupta, R.G. Usual and desquamative interstitial pneumonia. Chest 1976, 69 (Suppl. S2), 261–263. [Google Scholar] [CrossRef]
- Bak, S.H.; Lee, H.Y. Overlaps and uncertainties of smoking-related idiopathic interstitial pneumonias. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Medenica, M.; Medenica, M. Desquamative interstitial pneumonia with clinical, radiological and histologic correlation. Radiol. Case Rep. 2019, 14, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Takemura, T.; Hebisawa, A.; Ogura, T.; Yamaguchi, T.; Kuriyama, T.; Nagai, S.; Sakatani, M.; Chida, K.; Sakai, F.; et al. Eosinophilia in bronchoalveolar lavage fluid and architectural destruction are features of desquamative interstitial pneumonia. Histopathology 2008, 52, 194–202. [Google Scholar] [CrossRef]
- Portnoy, J.; Veraldi, K.L.; Schwarz, M.I.; Cool, C.D.; Curran-Everett, D.; Cherniack, R.M.; King, T.E., Jr.; Brown, K.K. Respiratory bronchiolitis-interstitial lung disease: Long-term outcome. Chest 2007, 131, 664–671. [Google Scholar] [CrossRef]
- Ryu, J.H.; Myers, J.L.; Capizzi, S.A.; Douglas, W.W.; Vassallo, R.; Decker, P.A. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest 2005, 127, 178–184. [Google Scholar] [CrossRef]
- Elkin, S.L.; Nicholson, A.G.; du Bois, R.M. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Semin. Respir. Crit. Care Med. 2001, 22, 387–398. [Google Scholar] [CrossRef]
- Chakraborty, R.K.; Basit, H.; Sharma, S. Desquamative Interstitial Pneumonia; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tazelaar, H.D.; Wright, J.L.; Churg, A. Desquamative interstitial pneumonia. Histopathology 2011, 58, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.J.; Wells, A.U.; Doffman, S.; Rassl, D.; Colby, T.V.; Hansell, D.M.; Du Bois, R.M.; Nicholson, A.G. Desquamative interstitial pneumonia, respiratory bronchiolitis and their relationship to smoking. Histopathology 2004, 45, 275–282. [Google Scholar] [CrossRef]
- Diken, Ö.E.; Şengül, A.; Beyan, A.C.; Ayten, Ö.; Mutlu, L.C.; Okutan, O. Desquamative interstitial pneumonia: Risk factors, laboratory and bronchoalveolar lavage findings, radiological and histopathological examination, clinical features, treatment and prognosis. Exp. Ther. Med. 2019, 17, 587–595. [Google Scholar] [CrossRef]
- Godbert, B.; Wissler, M.P.; Vignaud, J.M. Desquamative interstitial pneumonia: An analytic review with an emphasis on aetiology. Eur. Respir. Rev. 2013, 22, 117–123. [Google Scholar] [CrossRef]
- Wells, A.U.; Nicholson, A.G.; Hansell, D.M. Challenges in pulmonary fibrosis· 4: Smoking-induced diffuse interstitial lung diseases. Thorax 2007, 62, 904–910. [Google Scholar] [CrossRef]
- Sieminska, A.; Kuziemski, K. Respiratory bronchiolitis-interstitial lung disease. Orphanet J. Rare Dis. 2014, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, D.E.; Kleinerman, J.; Rice, D.B. Pathologic changes in the peripheral airways of young cigarette smokers. N. Engl. J. Med. 1974, 291, 755–758. [Google Scholar] [CrossRef]
- Myers, J.L.; Veal, C.F., Jr.; Shin, M.S.; Katzenstein, A.L. Respiratory bronchiolitis causing interstitial lung disease. A clinicopathologic study of six cases. Am. Rev. Respir. Dis. 1987, 135, 880–884. [Google Scholar] [CrossRef]
- Woo, O.H.; Yong, H.S.; Oh, Y.W.; Lee, S.Y.; Kim, H.K.; Kang, E.Y. Respiratory bronchiolitis-associated interstitial lung disease in a nonsmoker: Radiologic and pathologic findings. AJR Am. J. Roentgenol. 2007, 188, W412–W414. [Google Scholar] [CrossRef] [PubMed]
- Strimlan, C.V.; Rosenow, E.C., 3rd; Weiland, L.H.; Brown, L.R. Lymphocytic interstitial pneumonitis. Review of 13 cases. Ann. Intern. Med. 1978, 88, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.A.; Carrington, C.B. The interstitial pneumonias. In Frontiers of Pulmonary Radiology, 1st ed.; Simon, M., Potchen, E.J., LeMay, M., Eds.; Grune & Stratton: New York, NY, USA, 1969; pp. 102–141. [Google Scholar]
- Sirajuddin, A.; Raparia, K.; Lewis, V.A.; Franks, T.J.; Dhand, S.; Galvin, J.R.; White, C.S. Primary Pulmonary Lymphoid Lesions: Radiologic and Pathologic Findings. Radiographics 2016, 36, 53–70. [Google Scholar] [CrossRef]
- Chung, A.; Wilgus, M.L.; Fishbein, G.; Lynch, J.P., 3rd. Pulmonary and Bronchiolar Involvement in Sjogren’s Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 235–254. [Google Scholar] [CrossRef]
- Garcia, D.; Young, L. Lymphocytic interstitial pneumonia as a manifestation of SLE and secondary Sjogren’s syndrome. BMJ Case Rep. 2013, 2013, bcr2013009598. [Google Scholar] [CrossRef]
- Yoo, H.; Hino, T.; Hwang, J.; Franks, T.J.; Han, J.; Im, Y.; Lee, H.Y.; Chung, M.P.; Hatabu, H.; Lee, K.S. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): Evolving concept of CT findings, pathology and management. Eur. J. Radiol. Open. 2022, 9, 100419. [Google Scholar] [CrossRef]
- Maeda, A.; Kinjo, M.; Kinjo, K.; Kishaba, T. Systemic lupus erythematosus presenting with lymphoid interstitial pneumonia as an initial manifestation. BMJ Case Rep. 2021, 14, e243539. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A. Lung disease related to collagen vascular disease. J. Thorac. Imaging 2009, 24, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Carmier, D.; Marchand-Adam, S.; Diot, P.; Diot, E. Respiratory involvement in systemic lupus erythematosus. Rev. Mal. Respir. 2010, 27, e66–e78. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, A.; Chidiac, C.; Ferry, T. HIV-associated lymphoid interstitial pneumonia mimicking miliary tuberculosis. Acta Clin. Belg. 2011, 66, 397–398. [Google Scholar] [PubMed]
- Kurosu, K.; Weiden, M.D.; Takiguchi, Y.; Rom, W.N.; Yumoto, N.; Jaishree, J.; Nakata, K.; Kasahara, Y.; Tanabe, N.; Tatsumi, K.; et al. BCL-6 mutations in pulmonary lymphoproliferative disorders: Demonstration of an aberrant immunological reaction in HIV-related lymphoid interstitial pneumonia. J. Immunol. 2004, 172, 7116–7122. [Google Scholar] [CrossRef] [PubMed]
- van Zyl-Smit, R.N.; Naidoo, J.; Wainwright, H.; Said-Hartley, Q.; Davids, M.; Goodman, H.; Rogers, S.; Dheda, K. HIV associated Lymphocytic Interstitial Pneumonia: A clinical, histological and radiographic study from an HIV endemic resource-poor setting. BMC Pulm. Med. 2015, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, M.N.; Aslan, S. Lymphocytic Interstitial Pneumonia in a Man with Human Immunodeficiency Virus Infection. Rev. Soc. Bras. Med. Trop. 2023, 56, e0055. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.T. Pneumonia in the immunocompromised child. Semin. Respir. Infect. 1987, 2, 177–183. [Google Scholar]
- Kokosi, M.A.; Nicholson, A.G.; Hansell, D.M.; Wells, A.U. Rare idiopathic interstitial pneumonias: LIP and PPFE and rare histologic patterns of interstitial pneumonias: AFOP and BPIP. Respirology 2016, 21, 600–614. [Google Scholar] [CrossRef]
- Tian, X.; Yi, E.S.; Ryu, J.H. Lymphocytic interstitial pneumonia and other benign lymphoid disorders. Semin. Respir. Crit. Care Med. 2012, 33, 450–461. [Google Scholar] [CrossRef]
- Arcadu, A.; Moua, T.; Yi, E.S.; Ryu, J.H. Lymphoid Interstitial Pneumonia and Other Benign Lymphoid Disorders. Semin. Respir. Crit. Care Med. 2016, 37, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Ravaglia, C.; Tomassetti, S.; Gurioli, C.; Casoni, G.; Asioli, S.; Dubini, A.; Piciucchi, S.; Chilosi, M. Lymphoproliferative lung disorders: Clinicopathological aspects. Eur. Respir. Rev. 2013, 22, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Galvin, J.R. Non-neoplastic pulmonary lymphoid lesions. Thorax 2001, 56, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Berry, G.J.; Raffin, T.A.; Kuschner, W.G. Lymphoid interstitial pneumonia: A narrative review. Chest 2002, 122, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Panchabhai, T.S.; Farver, C.; Highland, K.B. Lymphocytic Interstitial Pneumonia. Clin. Chest Med. 2016, 37, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K. Pleuroparenchymal Fibroelastosis: Its Clinical Characteristics. Curr. Respir. Med. Rev. 2013, 9, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Amitani, R.; Niimi, A.; Kuze, F. Idiopathic pulmonary upper lobe fibrosis (IPUF). Kokyu 1992, 11, 693–699. [Google Scholar]
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004, 126, 2007–2013. [Google Scholar] [CrossRef]
- Becker, C.D.; Gil, J.; Padilla, M.L. Idiopathic pleuroparenchymal fibroelastosis: An unrecognized or misdiagnosed entity? Mod. Pathol. 2008, 21, 784–787. [Google Scholar] [CrossRef]
- Tanizawa, K.; Handa, T.; Kubo, T.; Chen-Yoshikawa, T.F.; Aoyama, A.; Motoyama, H.; Hijiya, K.; Yoshizawa, A.; Oshima, Y.; Ikezoe, K.; et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: A retrospective cohort study. Respir. Res. 2018, 19, 162. [Google Scholar] [CrossRef]
- Ishii, H.; Watanabe, K.; Kushima, H.; Baba, T.; Watanabe, S.; Yamada, Y.; Arai, T.; Tsushima, K.; Kondoh, Y.; Nakamura, Y.; et al. Pleuroparenchymal fibroelastosis diagnosed by multidisciplinary discussions in Japan. Respir. Med. 2018, 141, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Shioya, M.; Otsuka, M.; Yamada, G.; Umeda, Y.; Ikeda, K.; Nishikiori, H.; Kuronuma, K.; Chiba, H.; Takahashi, H. Poorer Prognosis of Idiopathic Pleuroparenchymal Fibroelastosis Compared with Idiopathic Pulmonary Fibrosis in Advanced Stage. Can. Respir. J. 2018, 2018, 6043053. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Sasaki, S.; Kurokawa, K.; Nakamura, T.; Yamada, T.; Sasano, H.; Arano, N.; Komura, M.; Ihara, H.; Nagashima, O.; et al. Usual Interstitial Pneumonia Pattern in the Lower Lung Lobes as a Prognostic Factor in Idiopathic Pleuroparenchymal Fibroelastosis. Respiration 2019, 97, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Thangakunam, B.; Isaac, B.T.; Christopher, D.J.; Burad, D. Idiopathic pleuroparenchymal fibroelastosis—A rare idiopathic interstitial pneumonia. Respir. Med. Case Rep. 2015, 17, 8–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavakolian, K.; Udongwo, N.; Douedi, S.; Odak, M.; Ilagan, J.; Khan, T.; Salam, N.; Chaughtai, S.; Asif, A. Idiopathic Pleuroparenchymal Fibroelastosis. J. Med. Cases 2022, 13, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Li, H.; Cai, H.R. Idiopathic pleuroparenchymal fibroelastosis confirmed by pathology: A case report. J. Int. Med. Res. 2021, 49, 300060521992217. [Google Scholar] [CrossRef] [PubMed]
- Dbouk, S.; Bazzi, N.; Mansour, S.; Masry, A.; Mansour, B. Corrigendum to: Idiopathic pleuroparenchymal fibroelastosis: First case report in Lebanon. J. Surg. Case Rep. 2022, 2022, rjac027. [Google Scholar] [CrossRef]
- von der Thüsen, J.H.; Hansell, D.M.; Tominaga, M.; Veys, P.A.; Ashworth, M.T.; Owens, C.M.; Nicholson, A.G. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod. Pathol. 2011, 24, 1633–1639. [Google Scholar] [CrossRef]
- Parish, J.M.; Muhm, J.R.; Leslie, K.O. Upper lobe pulmonary fibrosis associated with high-dose chemotherapy containing BCNU for bone marrow transplantation. Mayo Clin. Proc. 2003, 78, 630–634. [Google Scholar] [CrossRef]
- Mariani, F.; Gatti, B.; Rocca, A.; Bonifazi, F.; Cavazza, A.; Fanti, S.; Tomassetti, S.; Piciucchi, S.; Poletti, V.; Zompatori, M. Pleuroparenchymal fibroelastosis: The prevalence of secondary forms in hematopoietic stem cell and lung transplantation recipients. Diagn. Interv. Radiol. 2016, 22, 400–406. [Google Scholar] [CrossRef]
- Pakhale, S.S.; Hadjiliadis, D.; Howell, D.N.; Palmer, S.M.; Gutierrez, C.; Waddell, T.K.; Chaparro, C.; Davis, R.D.; Keshavjee, S.; Hutcheon, M.A.; et al. Upper lobe fibrosis: A novel manifestation of chronic allograft dysfunction in lung transplantation. J. Heart Lung Transplant. 2005, 24, 1260–1268. [Google Scholar] [CrossRef]
- Konen, E.; Weisbrod, G.L.; Pakhale, S.; Chung, T.; Paul, N.S.; Hutcheon, M.A. Fibrosis of the upper lobes: A newly identified late-onset complication after lung transplantation? AJR Am. J. Roentgenol. 2003, 181, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Beynat-Mouterde, C.; Beltramo, G.; Lezmi, G.; Pernet, D.; Camus, C.; Fanton, A.; Foucher, P.; Cottin, V.; Bonniaud, P. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur. Respir. J. 2014, 44, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, Y.; Nakamura, Y.; Colby, T.V.; Johkoh, T.; Sumikawa, H.; Nishimoto, K.; Yoshimura, K.; Matsushima, S.; Oyama, Y.; Hozumi, H.; et al. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PLoS ONE 2017, 12, e0180283. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Odink, A.; Brun, A.L.; Macaluso, C.; de Lauretis, A.; Kokosi, M.; Devaraj, A.; Desai, S.; Renzoni, E.; Wells, A.U. Functional associations of pleuroparenchymal fibroelastosis and emphysema with hypersensitivity pneumonitis. Respir. Med. 2018, 138, 95–101. [Google Scholar] [CrossRef]
- Xu, L.; Rassaei, N.; Caruso, C. Pleuroparenchymal fibroelastosis with long history of asbestos and silicon exposure. Int. J. Surg. Pathol. 2018, 26, 190–193. [Google Scholar] [CrossRef]
- Chua, F.; Desai, S.R.; Nicholson, A.G.; Devaraj, A.; Renzoni, E.; Rice, A.; Wells, A.U. Pleuroparenchymal Fibroelastosis. A Review of Clinical, Radiological, and Pathological Characteristics. Ann. Am. Thorac. Soc. 2019, 16, 1351–1359. [Google Scholar] [CrossRef]
- Lagstein, A. Pulmonary Apical Cap-What’s Old Is New Again. Arch. Pathol. Lab. Med. 2015, 139, 1258–1262. [Google Scholar] [CrossRef]
- Hirota, T.; Yoshida, Y.; Kitasato, Y.; Yoshimi, M.; Koga, T.; Tsuruta, N.; Minami, M.; Harada, T.; Ishii, H.; Fujita, M.; et al. Histological evolution of pleuroparenchymal fibroelastosis. Histopathology 2015, 66, 545–554. [Google Scholar] [CrossRef]
- Ali, M.S.; Ramalingam, V.S.; Haasler, G.; Presberg, K. Pleuroparenchymal fibroelastosis (PPFE) treated with lung transplantation and review of the literature. BMJ Case Rep. 2019, 12, e229402. [Google Scholar] [CrossRef]
- Rosenbaum, J.N.; Butt, Y.M.; Johnson, K.A.; Meyer, K.; Batra, K.; Kanne, J.P.; Torrealba, J.R. Pleuroparenchymal fibroelastosis: A pattern of chronic lung injury. Hum. Pathol. 2015, 46, 137–146. [Google Scholar] [CrossRef] [PubMed]
- von der Thüsen, J.H. Pleuroparenchymal Fibroelastosis: Its Pathological Characteristics. Curr. Respir. Med. Rev. 2013, 9, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.K.; Chuah, K.L. Pleuroparenchymal Fibroelastosis of the Lung: A Review. Arch. Pathol. Lab. Med. 2016, 140, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Ishii, H.; Nabeshima, K.; Watanabe, K. The pathogenesis and pathology of idiopathic pleuroparenchymal fibroelastosis. Histol. Histopathol. 2021, 36, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.T.; Colby, T.V. Update for pathologists on idiopathic interstitial pneumonias. Arch. Pathol. Lab. Med. 2012, 136, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ishii, H.; Kiyomi, F.; Terasaki, Y.; Hebisawa, A.; Kawabata, Y.; Johkoh, T.; Sakai, F.; Kondoh, Y.; Inoue, Y.; et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: A proposal. Respir. Investig. 2019, 57, 312–320. [Google Scholar] [CrossRef]
- Enomoto, N.; Kusagaya, H.; Oyama, Y.; Kono, M.; Kaida, Y.; Kuroishi, S.; Hashimoto, D.; Fujisawa, T.; Yokomura, K.; Inui, N.; et al. Quantitative analysis of lung elastic fibers in idiopathic pleuroparenchymal fibroelastosis (IPPFE): Comparison of clinical, radiological, and pathological findings with those of idiopathic pulmonary fibrosis (IPF). BMC Pulm. Med. 2014, 14, 91. [Google Scholar] [CrossRef]
- Beasley, M.B.; Franks, T.J.; Galvin, J.R.; Gochuico, B.; Travis, W.D. Acute fibrinous and organizing pneumonia: A histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch. Pathol. Lab. Med. 2002, 126, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Yousem, S.A.; Dacic, S. Idiopathic bronchiolocentric interstitial pneumonia. Mod. Pathol. 2002, 15, 1148–1153. [Google Scholar] [CrossRef]
- Churg, A.; Myers, J.; Suarez, T.; Gaxiola, M.; Estrada, A.; Mejia, M.; Selman, M. Airway-centered interstitial fibrosis: A distinct form of aggressive diffuse lung disease. Am. J. Surg. Pathol. 2004, 28, 62–68. [Google Scholar] [CrossRef]
- Fukuoka, J.; Franks, T.J.; Colby, T.V.; Flaherty, K.R.; Galvin, J.R.; Hayden, D.; Gochuico, B.R.; Kazerooni, E.A.; Martinez, F.; Travis, W.D. Peribronchiolar metaplasia: A common histologic lesion in diffuse lung disease and a rare cause of interstitial lung disease: Clinicopathologic features of 15 cases. Am. J. Surg. Pathol. 2005, 29, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Kuranishi, L.T.; Leslie, K.O.; Ferreira, R.G.; Coletta, E.A.; Storrer, K.M.; Soares, M.R.; de Castro Pereira, C.A. Airway-centered interstitial fibrosis: Etiology, clinical findings and prognosis. Respir. Res. 2015, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Nunes, H.; Brillet, P.Y.; Delaval, P.; Devouassoux, G.; Tillie-Leblond, I.; Israel-Biet, D.; Court-Fortune, I.; Valeyre, D.; Cordier, J.F.; et al. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. Eur. Respir. J. 2005, 26, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Cordier, J.F. The syndrome of combined pulmonary fibrosis and emphysema. Chest 2009, 136, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, C.J.; Hartman, T.; Elicker, B.M.; Ley, B.; Lee, J.S.; Abbritti, M.; Jones, K.D.; King, T.E.; Ryu, J., Jr.; Collard, H.R. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest 2013, 144, 234–240. [Google Scholar] [CrossRef]
- Sangani, R.; Ghio, A.; Culp, S.; Patel, Z.; Sharma, S. Combined Pulmonary Fibrosis Emphysema: Role of Cigarette Smoking and Pulmonary Hypertension in a Rural Cohort. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Ikushima, S.; Awano, N.; Kondoh, K.; Satake, K.; Masuo, M.; Kusunoki, Y.; Moriya, A.; Kamiya, H.; Ando, T. An autopsy study of combined pulmonary fibrosis and emphysema: Correlations among clinical, radiological, and pathological features. BMC Pulm. Med. 2014, 14, 104. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Watanabe, K.; Ishii, H.; Kushima, H.; Fujita, M.; Nabeshima, K. Distribution of emphysema and fibrosis in idiopathic pulmonary fibrosis with coexisting emphysema. Histopathology 2019, 74, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Snider, G.L. Chronic obstructive pulmonary disease: A definition and implications of structural determinants of airflow obstruction for epidemiology. Am. Rev. Respir. Dis. 1989, 140 Pt 2, S3–S8. [Google Scholar] [CrossRef]
- Takahashi, M.; Fukuoka, J.; Nitta, N.; Takazakura, R.; Nagatani, Y.; Murakami, Y.; Otani, H.; Murata, K. Imaging of pulmonary emphysema: A pictorial review. Int. J. Chronic Obstr. Pulm. Dis. 2008, 3, 193–204. [Google Scholar] [CrossRef]
- Katzenstein, A.L. Smoking-related interstitial fibrosis (SRIF): Pathologic findings and distinction from other chronic fibrosing lung diseases. J. Clin. Pathol. 2013, 66, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.J.; Jin, G.Y.; Jung, H.N.; Kwon, K.S.; Choi, H.; Lee, Y.C.; Chung, M.J.; Park, H.S. Differentiating Smoking-Related Interstitial Fibrosis (SRIF) from Usual Interstitial Pneumonia (UIP) with Emphysema Using CT Features Based on Pathologically Proven Cases. PLoS ONE 2016, 11, e0162231. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Selman, M.; Inoue, Y.; Wong, A.W.; Corte, T.J.; Flaherty, K.R.; Han, M.K.; Jacob, J.; Johannson, K.A.; Kitaichi, M.; et al. Syndrome of Combined Pulmonary Fibrosis and Emphysema: An Official ATS/ERS/JRS/ALAT Research Statement. Am. J. Respir. Crit. Care Med. 2022, 206, e7–e41. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Nakamura, Y.; Ito, T.; Isshiki, T.; Sakamoto, S.; Homma, S. Comparison of clinical characteristics and outcomes between combined pulmonary fibrosis and emphysema associated with usual interstitial pneumonia pattern and non-usual interstitial pneumonia. Sarcoidosis Vasc. Diffus. Lung Dis. 2015, 32, 129–137. [Google Scholar]
- Ryerson, C.J.; Collard, H.R.; Pantilat, S.Z. Management of Dyspnea in Interstitial Lung Disease. Curr. Opin. Support. Palliat. Care 2010, 4, 69–75. [Google Scholar] [CrossRef] [PubMed]
- van Manen, M.J.G.; Birring, S.S.; Vancheri, C.; Cottin, V.; Renzoni, E.A.; Russell, A.M.; Wijsenbeek, M.S. Cough in Idiopathic Pulmonary Fibrosis. Eur. Respir. Rev. 2016, 25, 278–286. [Google Scholar] [CrossRef] [PubMed]
- van Manen, M.J.G.; Wijsenbeek, M.S. Cough, an Unresolved Problem in Interstitial Lung Diseases. Curr. Opin. Support. Palliat. Care 2019, 13, 143–151. [Google Scholar] [CrossRef]
- Watanabe, T.; Tanahashi, M.; Suzuki, E.; Yoshii, N.; Tsuchida, H.; Yobita, S.; Iguchi, K.; Uchiyama, S.; Nakamura, M. Treatment of Secondary Pneumothorax with Interstitial Lung Disease: The Surgical Indications at the Start of Treatment Is Important. J. Thorac. Dis. 2022, 14, 1393–1400. [Google Scholar] [CrossRef]
- Martinez, F.J.; Flaherty, K. Pulmonary Function Testing in Idiopathic Interstitial Pneumonias. Proc. Am. Thorac. Soc. 2006, 3, 315–321. [Google Scholar] [CrossRef]
- Maher, T.M.; Stowasser, S.; Voss, F.; Bendstrup, E.; Kreuter, M.; Martinez, F.J.; Sime, P.J.; Stock, C. Decline in Forced Vital Capacity as a Surrogate for Mortality in Patients with Pulmonary Fibrosis. Respirology 2023, 28, 1147–1153. [Google Scholar] [CrossRef]
- Salvatore, M.; Singh, A.; Yip, R.; Fevrier, E.; Henschke, C.I.; Yankelevitz, D.; Padilla, M. Progression of probable UIP and UIP on HRCT. Clin. Imaging 2019, 58, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hino, T.; Lee, K.S.; Yoo, H.; Han, J.; Franks, T.J.; Hatabu, H. Interstitial lung abnormality (ILA) and nonspecific interstitial pneumonia (NSIP). Eur. J. Radiol. Open 2021, 8, 100336. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lynch, D.A.; Sharma, S.; Brown, K.K.; Müller, N.L. Organizing Pneumonia: Prognostic Implication of High-Resolution Computed Tomography Features. J. Comput. Assist. Tomogr. 2003, 27, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Margallo Iribarnegaray, J.; Churruca Arróspide, M.; Matesanz López, C.; Pérez Rojo, R. Interstitial Lung Disease. Open Respir. Arch. 2023, 5, 277–289. [Google Scholar] [CrossRef]
- Morshid, A.; Moshksar, A.; Das, A.; Duarte, A.G.; Palacio, D.; Villanueva-Meyer, J. HRCT Diagnosis of Pleuroparenchymal fibroelastosis: Report of two cases. Radiol. Case Rep. 2021, 16, 1564–1569. [Google Scholar] [CrossRef]
- Esteves, C.; Costa, F.R.; Redondo, M.T.; Moura, C.S.; Guimarães, S.; Morais, A.; Pereira, J.M. Pleuroparenchymal fibroelastosis: Role of high-resolution computed tomography (HRCT) and CT-guided transthoracic core lung biopsy. Insights Imaging 2016, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Ryerson, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, C.J.; Vittinghoff, E.; Ley, B.; Lee, J.S.; Mooney, J.J.; Jones, K.D.; Elicker, B.M.; Wolters, P.J.; Koth, L.L.; King, T.E.J.; et al. Predicting Survival across Chronic Interstitial Lung Disease: The ILD-GAP Model. Chest 2014, 145, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; King, C.S.; Ignacio, R.V.; Pastre, J.; Shlobin, O.A.; Khangoora, V.; Aryal, S.; Nyquist, A.; Singhal, A.; Flaherty, K.R.; et al. External Validation and Longitudinal Application of the DO-GAP Index to Individualise Survival Prediction in Idiopathic Pulmonary Fibrosis. ERJ Open Res. 2023, 9, 00124-2023. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chen, C.-Y.; Kuo, Y.-T.; Ko, C.-C.; Wu, W.-J.; Liang, C.-H.; Yun, C.-H.; Huang, W.-M. Radiomics for the Prediction of Response to Antifibrotic Treatment in Patients with Idiopathic Pulmonary Fibrosis: A Pilot Study. Diagnostics 2022, 12, 1002. [Google Scholar] [CrossRef]
- Budzikowski, J.D.; Foy, J.J.; Rashid, A.A.; Chung, J.H.; Noth, I.; Armato, S.G., 3rd. Radiomics-Based Assessment of Idiopathic Pulmonary Fibrosis Is Associated with Genetic Mutations and Patient Survival. J. Med. Imaging 2021, 8, 31903. [Google Scholar] [CrossRef]
- Hunninghake, G.W.; Zimmerman, M.B.; Schwartz, D.A.; King, T.E.; Lynch, J., Jr.; Hegele, R.; Waldron, J.; Colby, T.; Müller, N.; Lynch, D.; et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001, 164, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Diette, G.B.; Scatarige, J.C.; Haponik, E.F.; Merriman, B.; Fishman, E.K. Do high-resolution CT findings of usual interstitial pneumonitis obviate lung biopsy? Views of pulmonologists. Respiration 2005, 72, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sanduzzi Zamparelli, S.; Sanduzzi Zamparelli, A.; Bocchino, M. The Evolving Concept of the Multidisciplinary Approach in the Diagnosis and Management of Interstitial Lung Diseases. Diagnostics 2023, 13, 2437. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, B.; Verschakelen, J.; Coolen, J.; Verbeken, E.; Verleden, G.; Wuyts, W. Diagnostic workup for diffuse parenchymal lung disease: Schematic flowchart, literature review, and pitfalls. Lung 2013, 191, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ergun, E.; Ates, U.; Bahadir, K.; Gollu, G.; Bingol-Kologlu, M.; Cakmak, M.; Dindar, H.; Yagmurlu, A. A Safe and Minimally Invasive Method for Thoracoscopic Lung Biopsy in Interstitial Lung Disease. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.C.; Duggal, A.; Bice, T.; Lederer, D.J.; Wilson, K.C.; Raghu, G. 2018 Clinical practice guideline summary for clinicians: Diagnosis of idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2019, 16, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, W.; Meyer, K.C. Surgical lung biopsy for the diagnosis of interstitial lung disease: A review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc. Diffus. Lung Dis. 2013, 30, 3–16. [Google Scholar] [PubMed]
- Carnochan, F.M.; Walker, W.S.; Cameron, E.W. Efficacy of video assisted thoracoscopic lung biopsy: An historical comparison with open lung biopsy. Thorax 1994, 49, 361–363. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; Fogarty, A.W.; McKeever, T.M.; Hubbard, R.B. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am. J. Respir. Crit. Care Med. 2016, 193, 1161–1167. [Google Scholar] [CrossRef]
- Utz, J.P.; Ryu, J.H.; Douglas, W.W.; Hartman, T.E.; Tazelaar, H.D.; Myers, J.L.; Allen, M.S.; Schroeder, D.R. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur. Respir. J. 2001, 17, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, Y.; Taniguchi, H.; Kitaichi, M.; Yokoi, T.; Johkoh, T.; Oishi, T.; Kimura, T.; Nishiyama, O.; Kato, K.; du Bois, R.M. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir. Med. 2006, 100, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.A.; Mahar, A.; Myers, J.L.; Grainge, C.; Corte, T.J.; Williamson, J.P.; Vallely, M.P.; Lai, S.; Mulyadi, E.; Torzillo, P.J.; et al. Cryobiopsy for Identification of Usual Interstitial Pneumonia and Other Interstitial Lung Disease Features. Further Lessons from COLDICE, a Prospective Multicenter Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, F.; Lucena, C.M.; Ramírez, J.; Sánchez, M.; Jimenez, M.J.; Xaubet, A.; Sellares, J.; Agustí, C. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: Yield and cost-effectiveness analysis. Arch. Bronconeumol. 2015, 51, 261–267. [Google Scholar] [CrossRef] [PubMed]
- DeMaio, A.; Thiboutot, J.; Yarmus, L. Applications of cryobiopsy in airway, pleural, and parenchymal disease. Expert. Rev. Respir. Med. 2022, 16, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Colby, T.V.; Dubini, A.; Tomassetti, S.; Ravaglia, C.; Poletti, V.; Mengoli, M.C.; Tagliavini, E.; Rossi, G. Transbronchial Cryobiopsy in the Diagnosis of Diffuse Lung Disease. Surg. Pathol. Clin. 2020, 13, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Ronaghi, R.; Oh, S. Transbronchial Lung Cryobiopsy for Diffuse Parenchymal Lung Disease. Semin. Respir. Crit. Care Med. 2022, 43, 536–540. [Google Scholar] [CrossRef]
- Rodrigues, I.; Estêvão Gomes, R.; Coutinho, L.M.; Rego, M.T.; Machado, F.; Morais, A.; Novais Bastos, H. Diagnostic yield and safety of transbronchial lung cryobiopsy and surgical lung biopsy in interstitial lung diseases: A systematic review and meta-analysis. Eur. Respir. Rev. 2022, 31, 210280. [Google Scholar] [CrossRef] [PubMed]
- Kronborg-White, S.; Ravaglia, C.; Dubini, A.; Piciucchi, S.; Tomassetti, S.; Bendstrup, E.; Poletti, V. Cryobiopsies are diagnostic in Pleuroparenchymal and Airway-centered Fibroelastosis. Respir. Res. 2018, 19, 135. [Google Scholar] [CrossRef]
- Poletti, V.; Casoni, G.L.; Gurioli, C.; Ryu, J.H.; Tomassetti, S. Lung cryobiopsies: A paradigm shift in diagnostic bronchoscopy? Respirology 2014, 19, 645–654. [Google Scholar] [CrossRef]
- Colella, S.; Haentschel, M.; Shah, P.; Poletti, V.; Hetzel, J. Transbronchial Lung Cryobiopsy in Interstitial Lung Diseases: Best Practice. Respiration 2018, 95, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Casoni, G.L.; Tomassetti, S.; Cavazza, A.; Colby, T.V.; Dubini, A.; Ryu, J.H.; Carretta, E.; Tantalocco, P.; Piciucchi, S.; Ravaglia, C.; et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS ONE 2014, 9, e86716. [Google Scholar] [CrossRef]
- Hagmeyer, L.; Theegarten, D.; Wohlschläger, J.; Treml, M.; Matthes, S.; Priegnitz, C.; Randerath, W.J. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin. Respir. J. 2016, 10, 589–595. [Google Scholar] [CrossRef]
- Fortin, M.; Liberman, M.; Delage, A.; Dion, G.; Martel, S.; Rolland, F.; Soumagne, T.; Trahan, S.; Assayag, D.; Albert, E.; et al. Transbronchial Lung Cryobiopsy and Surgical Lung Biopsy: A Prospective Multi-Centre Agreement Clinical Trial (CAN-ICE). Am. J. Respir. Crit. Care Med. 2023, 207, 1612–1619. [Google Scholar] [CrossRef]
- Ravaglia, C.; Nicholson, A.G. Biopsy in interstitial lung disease: Specific diagnosis and the identification of the progressive fibrotic phenotype. Curr. Opin. Pulm. Med. 2021, 27, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kashkash, F.; Khorri, A. Observational findings of transbronchial lung biopsy in patients with interstitial lung disease: A retrospective study in Aleppo University Hospital. Ann. Med. Surg. 2023, 85, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.N.; Batra, K.; Silhan, L.; Anand, V.; Joerns, E.K.; Moore, S.; Butt, Y.M.; Torrealba, J.; Newton, C.A.; Glazer, C.S. Utility of Bronchoalveolar Lavage and Transbronchial Biopsy in Patients with Interstitial Lung Disease. Lung 2020, 198, 803–810. [Google Scholar] [CrossRef]
- Lee, W.; Chung, W.S.; Hong, K.S.; Huh, J. Clinical usefulness of bronchoalveolar lavage cellular analysis and lymphocyte subsets in diffuse interstitial lung diseases. Ann. Lab. Med. 2015, 35, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schildge, J.; Frank, J.; Klar, B. The role of bronchoalveolar lavage in the diagnosis of idiopathic pulmonary fibrosis: An investigation of the relevance of the protein content. Pneumologie 2016, 70, 435–441. (In German) [Google Scholar]
- Welker, L.; Jörres, R.A.; Costabel, U.; Magnussen, H. Predictive value of BAL cell differentials in the diagnosis of interstitial lung diseases. Eur. Respir. J. 2004, 24, 1000–1006. [Google Scholar] [CrossRef]
- Efared, B.; Ebang-Atsame, G.; Rabiou, S.; Diarra, A.S.; Tahiri, L.; Hammas, N.; Smahi, M.; Amara, B.; Benjelloun, M.C.; Serraj, M.; et al. The diagnostic value of the bronchoalveolar lavage in interstitial lung diseases. J. Negat. Results Biomed. 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Hatabu, H.; Hunninghake, G.M.; Lynch, D.A. Interstitial Lung Abnormality: Recognition and Perspectives. Radiology 2019, 291, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, G.P.; Bianchi, P.; Danese, S.; Lederer, D.J. Barriers to timely diagnosis of interstitial lung disease in the real world: The INTENSITY survey. BMC Pulm. Med. 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.D.; Mosher, C.; Holtze, C.H.; Lancaster, L.H.; Flaherty, K.R.; Noth, I.; Neely, M.L.; Hellkamp, A.S.; Bender, S.; Conoscenti, C.S.; et al. Time to diagnosis of idiopathic pulmonary fibrosis in the IPF-PRO Registry. BMJ Open Respir. Res. 2020, 7, e000567. [Google Scholar] [CrossRef] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Raghu, G.; Anstrom, K.J.; King, T.E.; Lasky, J.A., Jr.; Martinez, F.J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Richeldi, L.; Behr, J.; Maher, T.M.; Tang, W.; Stowasser, S.; Hallmann, C.; du Bois, R.M. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax 2017, 72, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Albera, C.; Costabel, U.; Fagan, E.A.; Glassberg, M.K.; Gorina, E.; Lancaster, L.; Lederer, D.J.; Nathan, S.D.; Spirig, D.; Swigris, J.J. Efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis with more preserved lung function. Eur. Respir. J. 2016, 48, 843–851. [Google Scholar] [CrossRef]
- Nathan, S.D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Glaspole, I.; Glassberg, M.K.; Kardatzke, D.R.; Daigl, M.; Kirchgaessler, K.U.; Lancaster, L.H.; et al. Effect of pirfenidone on mortality: Pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis. Lancet Respir. Med. 2017, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Wirtz, H.; Koschel, D.; Pittrow, D.; Held, M.; Klotsche, J.; Andreas, S.; Claussen, M.; Grohé, C.; et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: Long-term results of the INSIGHTS-IPF registry. Eur. Respir. J. 2020, 56, 1902279. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997–2008. Eur. Respir. J. 2016, 48, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.H.; Shapera, S.; To, T.; Marras, T.K.; Gershon, A.; Dell, S. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur. Respir. J. 2019, 53, 1801164. [Google Scholar] [CrossRef]
- Kim, S.Y.; Diggans, J.; Pankratz, D.; Huang, J.; Pagan, M.; Sindy, N.; Tom, E.; Anderson, J.; Choi, Y.; Lynch, D.A.; et al. Classification of usual interstitial pneumonia in patients with interstitial lung disease: Assessment of a machine learning approach using high-dimensional transcriptional data. Lancet Respir. Med. 2015, 3, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Pankratz, D.G.; Choi, Y.; Imtiaz, U.; Fedorowicz, G.M.; Anderson, J.D.; Colby, T.V.; Myers, J.L.; Lynch, D.A.; Brown, K.K.; Flaherty, K.R.; et al. Usual Interstitial Pneumonia Can Be Detected in Transbronchial Biopsies Using Machine Learning. Ann. Am. Thorac. Soc. 2017, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Liu, T.T.; Pankratz, D.G.; Colby, T.V.; Barth, N.M.; Lynch, D.A.; Walsh, P.S.; Raghu, G.; Kennedy, G.C.; Huang, J. Identification of usual interstitial pneumonia pattern using RNA-Seq and machine learning: Challenges and solutions. BMC Genom. 2018, 19 (Suppl. S2), 101. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.K.; Choi, Y.; Colby, T.V.; Flaherty, K.R.; Groshong, S.; Imtiaz, U.; Lynch, D.A.; Myers, J.L.; Steele, M.P.; Martinez, F.J.; et al. Prospective validation of a genomic classifier for usual interstitial pneumonia in transbronchial biopsies. Am. J. Respir. Crit. Care Med. 2017, 195, A6792. [Google Scholar]
- Raghu, G.; Flaherty, K.R.; Lederer, D.J.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Larsen, B.T.; Chung, J.H.; Steele, M.P.; et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: A prospective validation study. Lancet Respir. Med. 2019, 7, 487–496. [Google Scholar] [CrossRef]
- Richeldi, L.; Scholand, M.B.; Lynch, D.A.; Colby, T.V.; Myers, J.L.; Groshong, S.D.; Chung, J.H.; Benzaquen, S.; Nathan, S.D.; Davis, J.R.; et al. Utility of a Molecular Classifier as a Complement to High-Resolution Computed Tomography to Identify Usual Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 203, 211–220. [Google Scholar] [CrossRef]
- Kheir, F.; Alkhatib, A.; Berry, G.J.; Daroca, P.; Diethelm, L.; Rampolla, R.; Saito, S.; Smith, D.L.; Weill, D.; Bateman, M.; et al. Using Bronchoscopic Lung Cryobiopsy and a Genomic Classifier in the Multidisciplinary Diagnosis of Diffuse Interstitial Lung Diseases. Chest 2020, 158, 2015–2025. [Google Scholar] [CrossRef]
- Choi, Y.; Lu, J.; Hu, Z.; Pankratz, D.G.; Jiang, H.; Cao, M.; Marchisano, C.; Huiras, J.; Fedorowicz, G.; Wong, M.G.; et al. Analytical performance of Envisia: A genomic classifier for usual interstitial pneumonia. BMC Pulm. Med. 2017, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Kheir, F.; Uribe Becerra, J.P.; Bissell, B.; Ghazipura, M.; Herman, D.; Hon, S.M.; Hossain, T.; Khor, Y.H.; Knight, S.L.; Kreuter, M.; et al. Use of a Genomic Classifier in Patients with Interstitial Lung Disease: A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2022, 19, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.A.; Case, A.; Unterman, A.; Kreuter, M.; Scholand, M.B.; Chaudhary, S.; Lofaro, L.R.; Johnson, M.; Huang, J.; Bhorade, S.M.; et al. The Impact of the Envisia Genomic Classifier in the Diagnosis and Management of Patients with Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2022, 19, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Glenn, L.M.; Troy, L.K.; Corte, T.J. Novel diagnostic techniques in interstitial lung disease. Front. Med. 2023, 10, 1174443. [Google Scholar] [CrossRef] [PubMed]
- Testa, L.C.; Jule, Y.; Lundh, L.; Bertotti, K.; Merideth, M.A.; O’Brien, K.J.; Nathan, S.D.; Venuto, D.C.; El-Chemaly, S.; Malicdan, M.C.V.; et al. Automated Digital Quantification of Pulmonary Fibrosis in Human Histopathology Specimens. Front. Med. 2021, 8, 607720. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, K.; Mäyränpää, M.I.; Sihvo, H.K.; Bergman, P.; Sutinen, E.; Ollila, H.; Kaarteenaho, R.; Myllärniemi, M. Artificial intelligence identifies inflammation and confirms fibroblast foci as prognostic tissue biomarkers in idiopathic pulmonary fibrosis. Hum. Pathol. 2021, 107, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.J.; Tarmey, T.; Ferreira, A.; Mangaonkar, A.A.; Ferrer, A.; Patnaik, M.M.; Wylam, M.E.; Jenkins, S.M.; Spears, G.M.; Yi, E.S.; et al. Pathology, Radiology, and Genetics of Interstitial Lung Disease in Patients With Shortened Telomeres. Am. J. Surg. Pathol. 2021, 45, 871–884. [Google Scholar] [CrossRef]
- Stainer, A.; Faverio, P.; Busnelli, S.; Catalano, M.; Della Zoppa, M.; Marruchella, A.; Pesci, A.; Luppi, F. Molecular Biomarkers in Idiopathic Pulmonary Fibrosis: State of the Art and Future Directions. Int. J. Mol. Sci. 2021, 22, 6255. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.F.; Wells, A.U.; Desai, S.R.; Poletti, V.; Piciucchi, S.; Dubini, A.; Nunes, H.; Valeyre, D.; Brillet, P.Y.; Kambouchner, M.; et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: A case-cohort study. Lancet Respir. Med. 2016, 4, 557–565. [Google Scholar] [CrossRef]
- Ryerson, C.J.; Corte, T.J.; Lee, J.S.; Richeldi, L.; Walsh, S.L.F.; Myers, J.L.; Behr, J.; Cottin, V.; Danoff, S.K.; Flaherty, K.R.; et al. A Standardized Diagnostic Ontology for Fibrotic Interstitial Lung Disease. An International Working Group Perspective. Am. J. Respir. Crit. Care Med. 2017, 196, 1249–1254. [Google Scholar] [CrossRef]
- Prasad, J.D.; Mahar, A.; Bleasel, J.; Ellis, S.J.; Chambers, D.C.; Lake, F.; Hopkins, P.M.A.; Corte, T.J.; Allan, H.; Glaspole, I.N. The interstitial lung disease multidisciplinary meeting: A position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology 2017, 22, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Martinez, F.J.; Smith, V.; Walsh, S.L.F. Multidisciplinary teams in the clinical care of fibrotic interstitial lung disease: Current perspectives. Eur. Respir. Rev. 2022, 31, 220003. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Thwaite, E.L.; Kazerooni, E.A.; Gross, B.H.; Toews, G.B.; Colby, T.V.; Travis, W.D.; Mumford, J.A.; Murray, S.; Flint, A.; et al. Radiological versus histological diagnosis in UIP and NSIP: Survival implications. Thorax 2003, 58, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Mageto, Y.N.; Lockhart, D.; Schmidt, R.A.; Wood, D.E.; Godwin, J.D. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: A prospective study. Chest 1999, 116, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.L.; Rubens, M.B.; Hansell, D.M.; Copley, S.J.; Desai, S.R.; du Bois, R.M.; Nicholson, A.G.; Colby, T.V.; Wells, A.U. Nonspecific interstitial pneumonia and usual interstitial pneumonia: Comparative appearances at and diagnostic accuracy of thin-section CT. Radiology 2001, 221, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Wells, A. Clinical usefulness of high resolution computed tomography in cryptogenic fibrosing alveolitis. Thorax 1998, 53, 1080–1087. [Google Scholar] [CrossRef]
- Fraune, C.; Churg, A.; Yi, E.S.; Khoor, A.; Kelemen, K.; Larsen, B.T.; Butt, Y.M.; Smith, M.L.; Gotway, M.B.; Ryu, J.H.; et al. Lymphoid Interstitial Pneumonia (LIP) Revisited: A Critical Reappraisal of the Histologic Spectrum of “Radiologic” and “Pathologic” LIP in the Context of Diffuse Benign Lymphoid Proliferations of the Lung. Am. J. Surg. Pathol. 2023, 47, 281–295. [Google Scholar] [CrossRef]
- Lyberis, P.; Verri, G.; Solidoro, P.; Femia, F.; Perotti, C.; Limerutti, G.; Delsedime, L.; Della Beffa, E.; Papotti, M.G.; Ruffini, E.; et al. Correlation between high-resolution computed tomography appearance and histopathological features in the diagnosis of interstitial lung diseases. A real-life study. Minerva Surg. 2023. [Google Scholar] [CrossRef]
| Category | Clinical–Radiological–Pathologic Diagnosis | Pathological Patterns |
|---|---|---|
| Major IIPs | ||
| Chronic Fibrosing Interstitial Pneumonia | Idiopathic Pulmonary Fibrosis | Usual interstitial pneumonia |
| Idiopathic Nonspecific Interstitial Pneumonia | Nonspecific interstitial pneumonia | |
| Acute/Subacute Interstitial Pneumonia | Cryptogenic Organizing Pneumonia | Organizing Pneumonia |
| Acute Interstitial Pneumonia | Diffuse Alveolar Damage | |
| Smoking-Related Interstitial Pneumonia | Respiratory Bronchiolitis–Interstitial Lung Disease | Respiratory Bronchiolitis |
| Desquamative Interstitial Pneumonia | Desquamative Interstitial Pneumonia | |
| Rare IIPs | ||
| Idiopathic Lymphoid Interstitial Pneumonia | Lymphocytic Interstitial Pneumonia | |
| Idiopathic Pleuroparenchymal Fibroelastosis | Pleuroparenchymal Fibroelastosis | |
| Unclassifiable Idiopathic Interstitial Pneumonia | Variable | |
| Rare Histologic Patterns | ||
| Acute Fibrinous and Organizing Pneumonia | ||
| Bronchiolocentric Patterns of Interstitial Pneuomnia | ||
| cNSIP Pattern | fNSIP Pattern |
|---|---|
| Uniform mild-to-moderate interstitial lymphoplasmacellular inflammation | Dense or loose interstitial fibrosis with uniform appearance (temporal homogeneity) |
| Reactive Type 2 pneumocyte hyperplasia in areas of inflammation | Basic architecture of the lung preserved (also demonstrated by elastic fibers staining) |
| Basic architecture of the lung preserved (also demonstrated by elastic fibers staining) | Variable degree of interstitial chronic inflammation |
| OP pattern not the prominent, if present (<20% of biopsy specimen) | Microscopic honeycombing often inconspicuous, if present |
| Lack of temporal heterogeneity (scar-like fibrosis—fibroblastic foci—normal lung) | |
For both types
| |
| OP Pattern |
|---|
| Airspace filled by Masson bodies |
| Basic architecture of the lung preserved (also demonstrated by elastic fibers staining) |
| Patchy distribution |
| Temporal uniformity of fibrosis (young fibroplasia) |
| Mild to moderate interstitial chronic inflammation |
| DAD Pattern |
|---|
| Focal or diffuse hyaline membranes |
| Diffuse involvement of lung parenchyma |
| Temporal uniformity |
| Alveolar septal thickening due to organisation |
| Diffuse type II pneumocytes hyperplasia also with reactive atypia |
| DIP Pattern |
|---|
| Prominent and diffuse airspace filed by smokers’ macrophages |
| Uniform involvement of lung parenchyma |
| Basic architecture of the lung preserved (also demonstrated by elastic fibers staining) |
| Evidence of multiple lymphoid follicles, also with germinal centers |
| Lack of prominent interstitial fibrosis and inflammatory infiltrate |
| Diffuse type II pneumocytes hyperplasia |
| LIP Pattern |
|---|
| Diffuse interstitial lymphoplasmacellular infiltrate |
| Predominantly alveolar septal distribution |
| Marked widening of alveolar septa with compression and distortion of alveolar spaces |
| Evidence of lymphoid follicles, also with germinal centers, close to the pulmonary lymphatics |
| Definite iPPFE | ||
|---|---|---|
| Multiple subpleural foci of airspace consolidation with traction bronchiectasis located predominantly in the bilateral upper lobes on HRCT scans | Subpleural zonal or wedge-shaped dense fibrosis consisting of collapsed alveoli and collagen-filled alveoli with septal elastosis, with or without collagenous thickening of visceral pleura in surgical lung biopsy specimens | Exclusion of other diseases with known causes or conditions showing radiological and/or histological PPFE patterns such as chronic hypersensitivity pneumonia, connective tissue diseases, occupational diseases, hematopoietic stem cell or lung transplantation-related lung diseases |
| Definite iPPFE is diagnosed when all three criteria are met. If the lower lobes are affected by fibrosis, multidisciplinary discussion is necessary for the final diagnosis. | ||
| PPFE Pattern |
|---|
| Prominent and homogenous (lack of temporal heterogeneity) subpleural elastofibrosis of the upper lobe/s |
| Hyalinized fibrosis of overlying pleura |
| Traction bronchiolectasis and interstitial emphysema |
| At most, small numbers of fibroblastic foci at the interface |
| At most, mild lymphoplasmocytic infiltrates |
| Reference | No of Patients | Population | Reference Standard | Sensitivity [95% Cl] | Specificity [95% Cl] | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Pankratz et al. [264] | 31 | Suspected ILD | Histopathology | 0.59 [0.33, 0.82] | 1.00 [0.66, 1.00] | 100% | 56.2% |
| Kheir et al. [269] | 24 | Suspected ILD | MD | 0.80 [0.52, 0.96] | 0.78 [0.40, 0.97] | 85% | 70% |
| Raghu et al. [267] | 49 | Suspected ILD | MD | 0.70 [0.47, 0.87] | 0.88 [0.70, 0.98] | 84.2% | 76.6% |
| Richeldi et al. [268] | 96 | Suspected ILD | MD | 0.60 [0.47, 0.73] | 0.92 [0.79, 0.98] | 92% | 60.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà, S.; Pagliuca, F.; Perrotta, F.; Ronchi, A.; Mariniello, D.F.; Natale, G.; Bianco, A.; Fiorelli, A.; Accardo, M.; Franco, R. Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist’s Key Role. Int. J. Mol. Sci. 2024, 25, 3618. https://doi.org/10.3390/ijms25073618
Lucà S, Pagliuca F, Perrotta F, Ronchi A, Mariniello DF, Natale G, Bianco A, Fiorelli A, Accardo M, Franco R. Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist’s Key Role. International Journal of Molecular Sciences. 2024; 25(7):3618. https://doi.org/10.3390/ijms25073618
Chicago/Turabian StyleLucà, Stefano, Francesca Pagliuca, Fabio Perrotta, Andrea Ronchi, Domenica Francesca Mariniello, Giovanni Natale, Andrea Bianco, Alfonso Fiorelli, Marina Accardo, and Renato Franco. 2024. "Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist’s Key Role" International Journal of Molecular Sciences 25, no. 7: 3618. https://doi.org/10.3390/ijms25073618
APA StyleLucà, S., Pagliuca, F., Perrotta, F., Ronchi, A., Mariniello, D. F., Natale, G., Bianco, A., Fiorelli, A., Accardo, M., & Franco, R. (2024). Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist’s Key Role. International Journal of Molecular Sciences, 25(7), 3618. https://doi.org/10.3390/ijms25073618








