Molecular Mechanism of Resveratrol and Its Therapeutic Potential on Female Infertility
Abstract
1. Introduction
2. What Is Resveratrol?
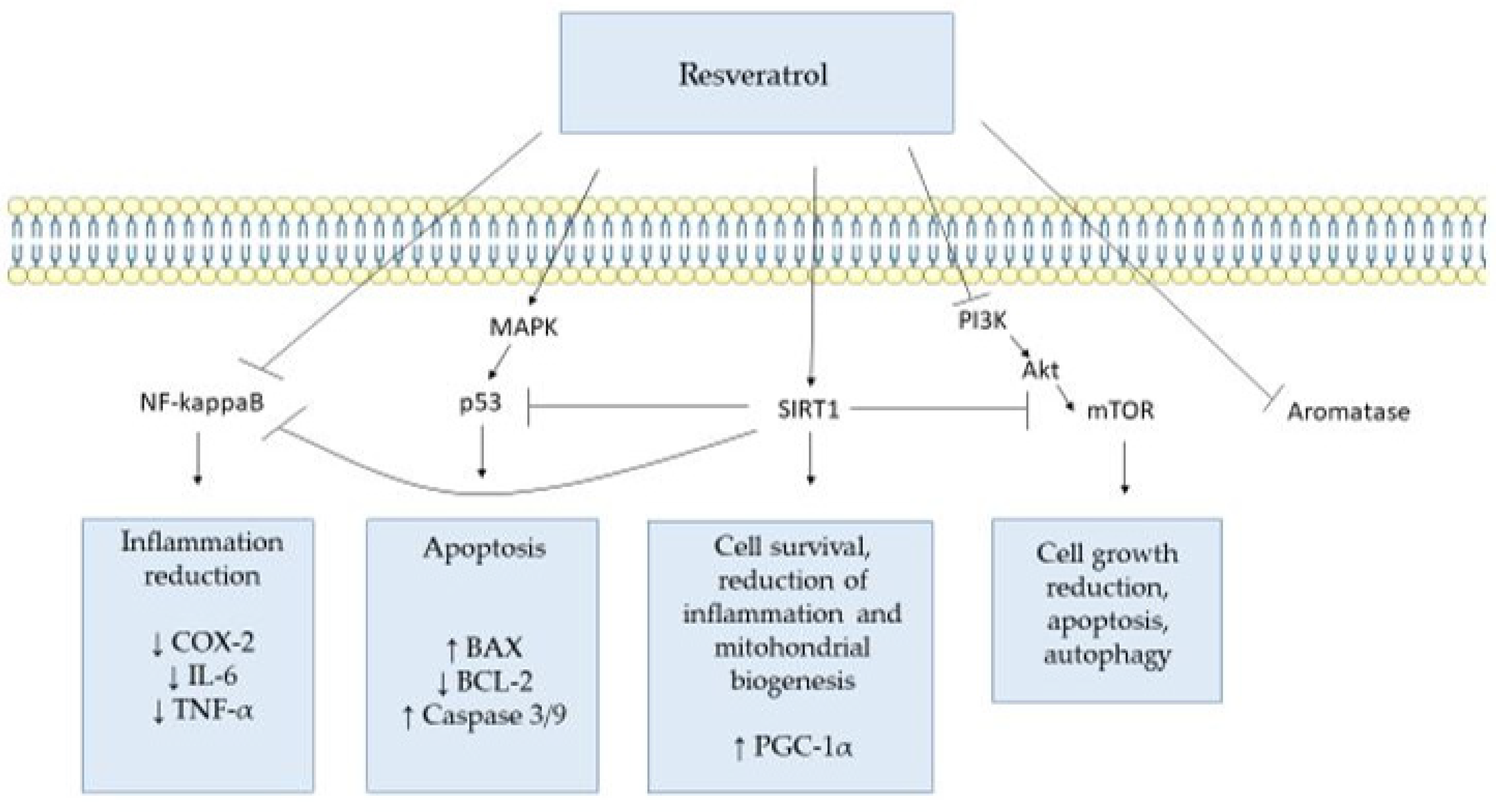
3. Targets of Resveratrol and Its Molecular Mechanisms
3.1. Prostaglandin-Endoperoxide Synthase 1 and 2 (PTGS, PTGS2)
3.2. Tumor Protein p53 (TP53)
3.3. Sirtuin 1 (SIRT1)
3.4. Mammalian Target of Rapamycin (mTOR)
3.5. Tumor Necrosis Factor (TNF)
3.6. Aromatase
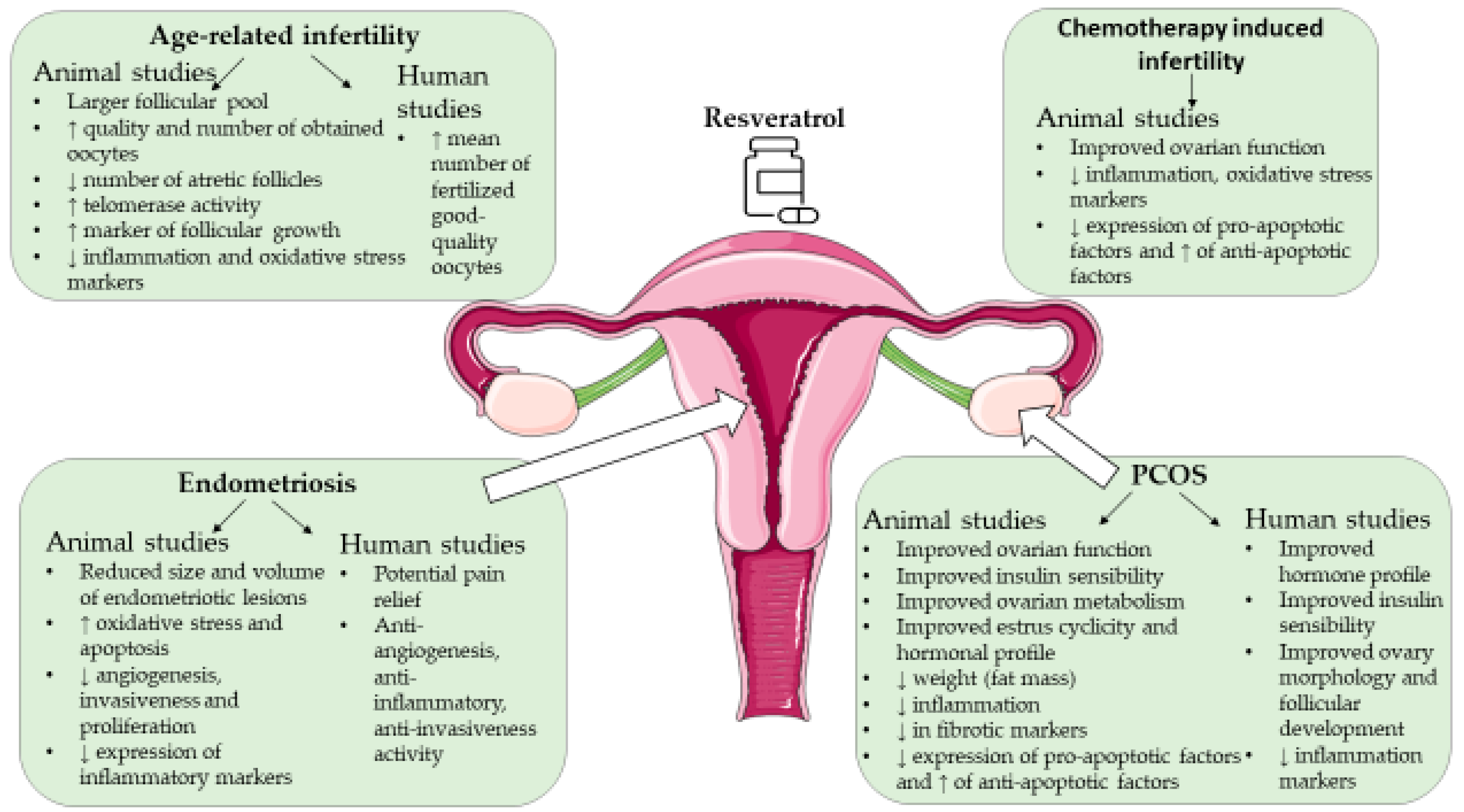
4. Resveratrol and Female Infertility
4.1. Resveratrol and Age-Associated Infertility
4.1.1. Animal Studies
4.1.2. Human Studies
4.2. Resveratrol and Polycystic Ovary Syndrome
4.2.1. Animal Studies
4.2.2. Human Studies
4.3. Resveratrol and Endometriosis
4.3.1. Animal Studies
4.3.2. Cell Models
4.3.3. Human Studies
4.4. Resveratrol and Female Infertility Due to Other Causes
4.4.1. Animal Studies
4.4.2. Human Studies
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vander-Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Deshpande, P.S.; Gupta, A.S. Causes and Prevalence of Factors Causing Infertility in a Public Health Facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar] [CrossRef]
- Farhi, J.; Ben-Haroush, A. Distribution of causes of infertility in patients attending primary fertility clinics in Israel. Isr. Med. Assoc. J. 2011, 13, 51–54. [Google Scholar]
- Dhandapani, K.; Kodavanji, B.; Nithyananda-Madom-Anantharaya, V.; Arun-Kumar, N. Prevalence and distribution of causes of infertility according to women’s age—A cross-sectional study in a tertiary healthcare hospital setup. J. Basic. Clin. Physiol. Pharmacol. 2021, 34, 27–32. [Google Scholar] [CrossRef]
- Ehsani, M.; Mohammadnia-Afrouzi, M.; Mirzakhani, M.; Esmaeilzadeh, S.; Shahbazi, M. Female Unexplained Infertility: A Disease with Imbalanced Adaptive Immunity. J. Hum. Reprod. Sci. 2019, 12, 274–282. [Google Scholar] [CrossRef]
- Koshak, E.; Atwah, A.; Aljedani, R.; Aljaied, Y.; Gaddoury, M.A. Common Autoimmune Antibodies in Unexplained Infertile Female Patients in Saudi Arabia. Cureus 2022, 14, e31724. [Google Scholar] [CrossRef]
- Eskenazi, S.; Bachelot, A.; Hugon-Rodin, J.; Plu-Bureau, G.; Gompel, A.; Catteau-Jonard, S.; Molina-Gomes, D.; Dewailly, D.; Dodé, C.; Christin-Maitre, S.; et al. Next Generation Sequencing Should Be Proposed to Every Woman With “Idiopathic” Primary Ovarian Insufficiency. J. Endocr. Soc. 2021, 5, bvab032. [Google Scholar] [CrossRef]
- Tarín, J.; Ten, J.; Vendrell, F.J.; de Oliveira, M.N.; Cano, A. Effects of maternal ageing and dietary antioxidant supplementation on ovulation, fertilisation and embryo development in vitro in the mouse. Reprod. Nutr. Dev. 1998, 38, 499–508. [Google Scholar] [CrossRef]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 5529741. [Google Scholar] [CrossRef]
- Battaglia, C.; Salvatori, M.; Maxia, N.; Petraglia, F.; Facchinetti, F.; Volpe, A. Adjuvant L-arginine treatment for in-vitro fertilization in poor responder patients. Hum. Reprod. 1999, 14, 1690–1697. [Google Scholar] [CrossRef][Green Version]
- So, S.; Yamaguchi, W.; Murabayashi, N.; Miyano, N.; Tawara, F.; Kanayama, N. Beneficial effect of l-arginine in women using assisted reproductive technologies: A small-scale randomized controlled trial. Nutr. Res. 2020, 82, 67–73. [Google Scholar] [CrossRef]
- Ozkaya, M.O.; Nazıroğlu, M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil. Steril. 2010, 94, 2465–2466. [Google Scholar] [CrossRef]
- Morgante, G.; Orvieto, R.; Di Sabatino, A.; Musacchio, M.C.; De Leo, V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil. Steril. 2011, 95, 2642–2644. [Google Scholar] [CrossRef]
- Firouzabadi, R.d.; Aflatoonian, A.; Modarresi, S.; Sekhavat, L.; Mohammad-Taheri, S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement. Ther. Clin. Pract. 2012, 18, 85–88. [Google Scholar] [CrossRef]
- Nishihara, T.; Hashimoto, S.; Ito, K.; Nakaoka, Y.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol. Endocrinol. 2014, 30, 359–362. [Google Scholar] [CrossRef]
- Özcan, P.; Fıçıcıoğlu, C.; Kizilkale, O.; Yesiladali, M.; Tok, O.E.; Ozkan, F.; Esrefoglu, M. Can Coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J. Assist. Reprod. Genet. 2016, 33, 1223–1230. [Google Scholar] [CrossRef]
- Ma, L.; Cai, L.; Hu, M.; Wang, J.; Xie, J.; Xing, Y.; Shen, J.; Cui, Y.; Liu, X.J.; Liu, J. Coenzyme Q10 supplementation of human oocyte in vitro maturation reduces postmeiotic aneuploidies. Fertil. Steril. 2020, 114, 331–337. [Google Scholar] [CrossRef]
- Kitano, Y.; Hashimoto, S.; Matsumoto, H.; Yamochi, T.; Yamanaka, M.; Nakaoka, Y.; Fukuda, A.; Inoue, M.; Ikeda, T.; Morimoto, Y. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol. Endocrinol. 2018, 34, 684–688. [Google Scholar] [CrossRef]
- Safiyeh, F.D.; Mojgan, M.; Parviz, S.; Sakineh, M.A.; Behnaz, S.O. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: A randomized controlled clinical trial. Complement. Ther. Med. 2021, 56, 102533. [Google Scholar] [CrossRef]
- Novielli, C.; Anelli, G.M.; Lisso, F.; Marzorati, A.; Parrilla, B.; Oneta, M.; Savasi, V.M.; Cetin, I.; Mandò, C. Effects of α-lipoic acid and myo-inositol supplementation on the oocyte environment of infertile obese women: A preliminary study. Reprod. Biol. 2020, 20, 541–546. [Google Scholar] [CrossRef]
- Kadir, M.; Hood, R.B.; Mínguez-Alarcón, L.; Maldonado-Cárceles, A.B.; Ford, J.B.; Souter, I.; Chavarro, J.E.; Gaskins, A.J.; EARTH Study Team. Folate intake and ovarian reserve among women attending a fertility center. Fertil. Steril. 2022, 117, 171–180. [Google Scholar] [CrossRef]
- Cirillo, M.; Fucci, R.; Rubini, S.; Coccia, M.E.; Fatini, C. 5-Methyltetrahydrofolate and Vitamin B12 Supplementation Is Associated with Clinical Pregnancy and Live Birth in Women Undergoing Assisted Reproductive Technology. Int. J. Environ. Res. Public Health 2021, 18, 12280. [Google Scholar] [CrossRef]
- Stanhiser, J.; Jukic, A.M.Z.; Mc Connaughey, D.R.; Steiner, A.Z. Omega-3 fatty acid supplementation and fecundability. Hum. Reprod. 2022, 37, 1037–1046. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, D.; Feng, H.; Wang, X.; Su, J.; Yao, Y.; Ng, E.H.Y.; Yeung, W.S.B.; Li, R.H.W.; et al. Identification of curcumin as a novel potential drug for promoting the development of small ovarian follicles for infertility treatment. PNAS Nexus 2022, 1, pgac108. [Google Scholar] [CrossRef]
- Vaez, S.; Parivr, K.; Amidi, F.; Rudbari, N.H.; Moini, A.; Amini, N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am. J. Reprod. Immunol. 2023, 89, e13644. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, T.; Wang, L.; Cui, M.; Liu, C.; Wang, Z.; Cheng, X. To investigate the role of rutin on bisphenol—A induced female infertility in rats. Comb. Chem. High. Throughput Screen. 2022. [Google Scholar] [CrossRef]
- Dull, A.M.; Moga, M.A.; Dimienescu, O.G.; Sechel, G.; Burtea, V.; Anastasiu, C.V. Therapeutic Approaches of Resveratrol on Endometriosis via Anti-Inflammatory and Anti-Angiogenic Pathways. Molecules 2019, 24, 667. [Google Scholar] [CrossRef]
- Cai, X.; Liu, M.; Zhang, B.; Zhao, S.J.; Jiang, S.W. Phytoestrogens for the Management of Endometriosis: Findings and Issues. Pharmaceuticals 2021, 14, 569. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. The Role of Selected Dietary Factors in the Development and Course of Endometriosis. Nutrients 2023, 15, 2773. [Google Scholar] [CrossRef]
- Banaszewska, B.; Pawelczyk, L.; Spaczynski, R. Current and future aspects of several adjunctive treatment strategies in polycystic ovary syndrome. Reprod. Biol. 2019, 19, 309–315. [Google Scholar] [CrossRef]
- Ali Fadlalmola, H.; Elhusein, A.M.; Al-Sayaghi, K.M.; Albadrani, M.S.; Swamy, D.V.; Mamanao, D.M.; El-Amin, E.I.; Ibrahim, S.E.; Abbas, S.M. Efficacy of resveratrol in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized clinical trials. Pan Afr. Med. J. 2023, 44, 134. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K. Preconception resveratrol intake against infertility: Friend or foe? Reprod. Med. Biol. 2019, 19, 107–113. [Google Scholar] [CrossRef]
- National Library of Medicine. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Resveratrol (accessed on 16 March 2024).
- Neves, A.R.; Lucio, M.; Lima, J.L.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Rizvi, A. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tang, K.; Zhan, J.C.; Yang, H.R.; Huang, W.D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef]
- Kang, J.E.; Yoo, N.; Jeon, B.J.; Kim, B.S.; Chung, E.H. Resveratrol Oligomers, Plant-Produced Natural Products With Anti-virulence and Plant Immune-Priming Roles. Front. Plant Sci. 2022, 13, 885625. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. J. Chem. Soc. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Meiliana, A.; Mustika-Dewi, N.; Wijaya, A. Resveratrol: A Sirtuin Activator and The Fountain of Youth. Indones. Biomed. J. 2015, 7, 1–14. [Google Scholar] [CrossRef][Green Version]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef]
- Szewczuk, L.M.; Forti, L.; Stivala, L.A.; Penning, T.M. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: A mechanistic approach to the design of COX-1 selective agents. J. Biol. Chem. 2004, 279, 22727–22737. [Google Scholar] [CrossRef]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef]
- Adelizzi, R.A. COX-1 and COX-2 in health and disease. J. Am. Osteopath. Assoc. 1999, 99 (Suppl. 11), S7–S12. [Google Scholar] [CrossRef]
- Arias-Negrete, S.; Keller, K.; Chadee, K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem. Biophys. Res. Commun. 1995, 208, 582–589. [Google Scholar] [CrossRef]
- Steinauer, K.K.; Gibbs, I.; Ning, S.; French, J.N.; Armstrong, J.; Knox, S.J. Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 325–328. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, L.Z.; Dixon, D.A.; Zheng, J.Z.; Chandran, B.; Jiang, B.H. Insulin-like growth factor-I induces cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling pathways in human ovarian cancer cells. Cell Signal 2007, 19, 1542–1553. [Google Scholar] [CrossRef]
- Sheng, H.; Williams, C.S.; Shao, J.; Liang, P.; DuBois, R.N.; Beauchamp, R.D. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J. Biol. Chem. 1998, 273, 22120–22127. [Google Scholar] [CrossRef]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y.J. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar] [CrossRef]
- Yi, C.O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol activates AMPK and suppresses LPS-induced NF-κB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat. Cell Biol. 2011, 44, 194–203. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Cavallo, P.; Dragone, T.; Carofiglio, V.; Panaro, M.A. Modulation of NF-κB activation by resveratrol in LPS treated human intestinal cells results in downregulation of PGE2 production and COX-2 expression. Toxicol. Vitr. 2012, 26, 1122–1128. [Google Scholar] [CrossRef]
- Whynott, R.M.; Manahan, P.; Geisler, J.P. Vascular endothelial growth factor (VEGF) and cyclooxygenase 2 (COX 2) immunostaining in ovarian cancer. Eur. J. Gynaecol. Oncol. 2016, 37, 164–166. [Google Scholar]
- Li, X.; Li, F.; Wang, F.; Li, J.; Lin, C.; Du, J. Resveratrol inhibits the proliferation of A549 cells by inhibiting the expression of COX-2. Onco Targets Ther. 2018, 11, 2981–2989. [Google Scholar] [CrossRef]
- Gong, W.H.; Zhao, N.; Zhang, Z.M.; Zhang, Y.X.; Yan, L.; Li, J.B. The inhibitory effect of resveratrol on COX-2 expression in human colorectal cancer: A promising therapeutic strategy. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1136–1143. [Google Scholar]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Simone, T.M.; Wu, Y.H.; Grandis, J.R.; Cao, H.J.; Davis, P.J.; Davis, F.B. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J. Cell. Biochem. 2008, 104, 2131–2142. [Google Scholar] [CrossRef]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef]
- Reese, J.; Zhao, X.; Ma, W.G.; Brown, N.; Maziasz, T.J.; Dey, S.K. Comparative analysis of pharmacologic and/or genetic disruption of cyclooxygenase-1 and cyclooxygenase-2 function in female reproduction in mice. Endocrinology 2001, 142, 3198–3206. [Google Scholar] [CrossRef]
- Takahashi, T.; Morrow, J.D.; Wang, H.; Dey, S.K. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J. Biol. Chem. 2006, 281, 37117–37129. [Google Scholar] [CrossRef]
- Olivares, C.; Bilotas, M.; Buquet, R.; Borghi, M.; Sueldo, C.; Tesone, M.; Meresman, G. Effects of a selective cyclooxygenase-2 inhibitor on endometrial epithelial cells from patients with endometriosis. Hum. Reprod. 2008, 23, 2701–2708. [Google Scholar] [CrossRef]
- Parent, S.; Van Themsche, C.; Leblanc, V.; Asselin, E. In Vivo Molecular Modulation of P-AKT, XIAP, and COX-2 Pathways, and Cellular Proliferation by Resveratrol in Uteri of Immature Rats. Biol. Reprod. 2008, 78, 137. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 mutations in human cancers: Origins, consequences, and clinical use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef]
- Soussi, T. The p53 tumor suppressor gene: From molecular biology to clinical investigation. Ann. N. Y. Acad. Sci. 2000, 910, 121–137; discussion 137–139. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Teresky, A.K.; Levine, A.J. p53 regulates maternal reproduction through LIF. Nature 2007, 450, 721–724. [Google Scholar] [CrossRef]
- Fraga, L.R.; Dutra, C.G.; Boquett, J.A.; Vianna, F.S.; Gonçalves, R.O.; Paskulin, D.D.; Costa, O.L.; Ashton-Prolla, P.; Sanseverino, M.T.; Schuler-Faccini, L. p53 signaling pathway polymorphisms associated to recurrent pregnancy loss. Mol. Biol. Rep. 2014, 41, 1871–1877. [Google Scholar] [CrossRef]
- Palomares, A.R.; Castillo-Domínguez, A.A.; Ruiz-Galdón, M.; Rodriguez-Wallberg, K.A.; Reyes-Engel, A. Genetic variants in the p53 pathway influence implantation and pregnancy maintenance in IVF treatments using donor oocytes. J. Assist. Reprod. Genet. 2021, 38, 3267–3275. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.Y.; Goranson, A.; Dong, Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis 1999, 20, 237–242. [Google Scholar] [CrossRef]
- Lin, H.Y.; Shih, A.; Davis, F.B.; Tang, H.Y.; Martino, L.J.; Bennett, J.A.; Davis, P.J. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J. Urol. 2002, 168, 748–755. [Google Scholar] [CrossRef]
- Kuo, P.L.; Chiang, L.C.; Lin, C.C. Resveratrol- induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Life Sci. 2002, 72, 23–34. [Google Scholar] [CrossRef]
- Shih, A.; Davis, F.B.; Lin, H.Y.; Davis, P.J. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J. Clin. Endocrinol. Metab. 2002, 87, 1223–1232. [Google Scholar] [CrossRef]
- Kim, Y.A.; Choi, B.T.; Lee, Y.T.; Park, D.I.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol. Rep. 2004, 11, 441–446. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng-Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal 2011, 9, 11. [Google Scholar] [CrossRef]
- Duntas, L.H. Resveratrol and its impact on aging and thyroid function. J. Endocrinol. Investig. 2011, 34, 788–792. [Google Scholar] [CrossRef]
- Olmos, Y.; Brosens, J.J.; Lam, E.W. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist. Updat. 2011, 14, 35–44. [Google Scholar] [CrossRef]
- Auley, M.T.; Mooney, K.M.; Angell, P.J.; Wilkinson, S.J. Mathematical modelling of metabolic regulation in aging. Metabolites 2015, 5, 232–251. [Google Scholar] [CrossRef]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef]
- Di Emidio, G.; Falone, S.; Vitti, M.; D’Alessandro, A.M.; Vento, M.; Di Pietro, C.; Amicarelli, F.; Tatone, C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum. Reprod. 2014, 29, 2006–2017. [Google Scholar] [CrossRef]
- Kim, T.H.; Young, S.L.; Sasaki, T.; Deaton, J.L.; Schammel, D.P.; Palomino, W.A.; Jeong, J.W.; Lessey, B.A. Role of SIRT1 and Progesterone Resistance in Normal and Abnormal Endometrium. J. Clin. Endocrinol. Metab. 2022, 107, 788–800. [Google Scholar] [CrossRef]
- Sansone, A.M.; Hisrich, B.V.; Young, R.B.; Abel, W.F.; Bowens, Z.; Blair, B.B.; Funkhouser, A.T.; Schammel, D.P.; Green, L.J.; Lessey, B.A.; et al. Evaluation of BCL6 and SIRT1 as Non-Invasive Diagnostic Markers of Endometriosis. Curr. Issues Mol. Biol. 2021, 43, 1350–1360. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Sung, G.J.; Marquardt, R.; Young, S.L.; Lessey, B.A.; Kim, T.H.; Cheon, Y.P.; Jeong, J.W. SIRT1 plays an important role in implantation and decidualization during mouse early pregnancy. Biol. Reprod. 2022, 106, 1072–1082. [Google Scholar] [CrossRef]
- Sirotkin, A.; Alexa, R.; Kádasi, A.; Adamcová, E.; Alwasel, S.; Harrath, A.H. Resveratrol directly affects ovarian cell sirtuin, proliferation, apoptosis, hormone release and response to follicle-stimulating hormone (FSH) and insulin-like growth factor I (IGF-I). Reprod. Fertil. Dev. 2019, 31, 1378–1385. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Pospelov, V.A. Resveratrol-induced p53 activation is associated with autophagy in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2018, 503, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Buhrmann, C.; Shayan, P.; Shakibaei, M. Resveratrol induces apoptosis by modulating the reciprocal crosstalk between p53 and Sirt-1 in the CRC tumor microenvironment. Front. Immunol. 2023, 14, 1225530. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Fröjdö, S.; Cozzone, D.; Vidal, H.; Pirola, L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem. J. 2007, 406, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Brito, P.M.; Devillard, R.; Nègre-Salvayre, A.; Almeida, L.M.; Dinis, T.C.; Salvayre, R.; Augé, N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis 2009, 205, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J. Exp. Ther. Oncol. 2009, 8, 25–33. [Google Scholar] [PubMed]
- Gong, C.; Xia, H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 19, 1878–1886. [Google Scholar] [CrossRef]
- Kueck, A.; Opipari, A.W., Jr.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Y.; Li, Q.; Guo, X.; Gu, L.; Ma, Z.G.; Zhu, Y.P. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed. Environ. Sci. 2013, 26, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lei, X.; Zhao, G.; Guo, R.; Cui, N. mTOR in programmed cell death and its therapeutic implications. Cytokine Growth Factor. Rev. 2023, 71–72, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Gräb, J.; Rybniker, J. The Expanding Role of p38 Mitogen-Activated Protein Kinase in Programmed Host Cell Death. Microbiol. Insights 2019, 12, 1178636119864594. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, F. Resveratrol inhibits mTOR signaling by targeting DEPTOR. Commun. Integr. Biol. 2011, 4, 382–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leconte, M.; Nicco, C.; Ngô, C.; Chéreau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Su, Y.; He, Y.; Zhang, J.; Liu, W.; Zhang, H.; Hou, Z.; Liu, J.; Li, J. New strategy for in vitro activation of primordial follicles with mTOR and PI3K stimulators. Cell Cycle 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Sun, X.; Kong, F.; Zhu, Y.; Lei, Y.; Su, Y.; Su, Y.; Li, J. Inhibition of mTOR Signaling Pathway Delays Follicle Formation in Mice. J. Cell Physiol. 2017, 232, 585–595. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Guo, Y.; Sun, T.; Li, H.; Zhang, X.; Yin, H.; Cao, G.; Yin, Y.; Wang, H.; et al. Oocyte stage-specific effects of MTOR determine granulosa cell fate and oocyte quality in mice. Proc. Natl. Acad. Sci. USA 2018, 115, E5326–E5333. [Google Scholar] [CrossRef]
- Chu, W.M. Tumor necrosis factor. Cancer Lett. 2013, 328, 222–225. [Google Scholar] [CrossRef]
- Wang, X.M.; Ma, Z.Y.; Song, N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2513–2518. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, S.K. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J. Nutr. 2005, 135, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Q.; Wang, M.; Liang, M.; Yang, X.; Xu, X.; Zou, H.; Qiu, J. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE 2011, 6, e27081. [Google Scholar] [CrossRef] [PubMed]
- Okan, A.; Doğanyiğit, Z.; Yilmaz, S.; Uçar, S.; Arikan-Söylemez, E.S.; Attar, R. Evaluation of the protective role of resveratrol against sepsis caused by LPS via TLR4/NF-κB/TNF-α signaling pathways: Experimental study. Cell Biochem. Funct. 2023, 41, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.L.; Yang, J.Y.; Dong, Y.X.; Wang, J.M.; Cui, Y.H.; Ikeshima, T.; Zhao, Y.Q.; Wu, C.F. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005, 5, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.J.; Petrossian, K.; Chen, S. Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2016, 161, 73–83. [Google Scholar] [CrossRef]
- Mitwally, M.F.; Casper, R.F. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil. Steril. 2001, 75, 305–309. [Google Scholar] [CrossRef]
- Casper, R.F.; Mitwally, M.F. Use of the aromatase inhibitor letrozole for ovulation induction in women with polycystic ovarian syndrome. Clin. Obstet. Gynecol. 2011, 54, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Abushahin, F.; Goldman, K.N.; Barbieri, E.; Milad, M.; Rademaker, A.; Bulun, S.E. Aromatase inhibition for refractory endometriosis-related chronic pelvic pain. Fertil. Steril. 2011, 96, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lee, K.W.; Chan, F.L.; Chen, S.; Leung, L.K. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol. Sci. 2006, 92, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chottanapund, S.; Van Duursen, M.B.; Navasumrit, P.; Hunsonti, P.; Timtavorn, S.; Ruchirawat, M.; Van den Berg, M. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. Vitr. 2014, 28, 1215–1221. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil. Steril. 2014, 101, 633–634. [Google Scholar] [CrossRef]
- Maheshwari, A.; Hamilton, M.; Bhattacharya, S. Effect of female age on the diagnostic categories of infertility. Hum. Reprod. 2008, 23, 538–542. [Google Scholar] [CrossRef]
- Adebayo, F.O.; Ameh, N.; Adesiyun, A.G.; Ekele, B.A.; Wada, I. Correlation of female age with outcome of IVF in a low-resource setting. Int. J. Gynaecol. Obstet. 2023, 161, 283–288. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, M.J.; Wei, C.F.; Yang, J.; Song, J.Y.; Dong, L.; Xiang, S.; Zhang, L.; Qiu, Y.; Lian, F. Age-related differential gene expression in granulosa cells and its effects on fertility using high-throughput transcriptomics. Syst. Biol. Reprod. Med. 2022, 68, 190–202. [Google Scholar] [CrossRef]
- Smits, M.A.J.; Schomakers, B.V.; van Weeghel, M.; Wever, E.J.M.; Wüst, R.C.I.; Dijk, F.; Janssens, G.E.; Goddijn, M.; Mastenbroek, S.; Houtkooper, R.H.; et al. Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction. Hum. Reprod. 2023, 38, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, Y.; Ye, X.; Zeng, M.; Zhao, Q.; Keefe, D.L.; Liu, L. Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 2013, 28, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.A.; Herranz, D.; De Bonis, M.L.; Velasco, S.; Serrano, M.; Blasco, M.A. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J. Cell Biol. 2010, 191, 1299–1313. [Google Scholar] [CrossRef]
- Aguiar, S.S.; Rosa, T.S.; Neves, R.V.P.; Leite, P.L.A.; Maciel, L.A.; Gutierrez, S.D.; Rosa, E.C.; Andrade, R.V.; Degens, H.; Korhonen, M.T.; et al. Telomere Length, SIRT1, and Insulin in Male Master Athletes: The Path to Healthy Longevity? Int. J. Sports Med. 2022, 43, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Sato, Y.; Kawagoe, Y.; Shimizu, T.; Kawamura, K. Short-term resveratrol treatment restored the quality of oocytes in aging mice. Aging 2022, 14, 5628–5640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef]
- Muskhelishvili, L.; Wingard, S.K.; Latendresse, J.R. Proliferating cell nuclear antigen--a marker for ovarian follicle counts. Toxicol. Pathol. 2005, 33, 365–368. [Google Scholar] [CrossRef]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Huang, Y.; Wu, M.; Li, Y.; Li, Y.; Zhu, X.; Wu, M. Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies. Int. J. Mol. Sci. 2023, 24, 13327. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef]
- Nishitoh, H. CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef]
- Battaglia, R.; Caponnetto, A.; Caringella, A.M.; Cortone, A.; Ferrara, C.; Smirni, S.; Iannitti, R.; Purrello, M.; D’Amato, G.; Fioretti, B.; et al. Resveratrol Treatment Induces Mito-miRNome Modification in Follicular Fluid from Aged Women with a Poor Prognosis for In Vitro Fertilization Cycles. Antioxidants 2022, 11, 1019. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Benrick, A.; Maliqueo, M.; Miao, S.; Villanueva, J.A.; Feng, Y.; Ohlsson, C.; Duleba, A.J.; Stener-Victorin, E. Resveratrol is not as effective as physical exercise for improving reproductive and metabolic functions in rats with dihydrotestosterone-induced polycystic ovary syndrome. Evid. Based Complement. Altern. Med. 2013, 2013, 964070. [Google Scholar] [CrossRef]
- Ergenoglu, M.; Yildirim, N.; Yildirim, A.G.; Yeniel, O.; Erbas, O.; Yavasoglu, A.; Taskiran, D.; Karadadas, N. Effects of Resveratrol on Ovarian Morphology, Plasma Anti-Mullerian Hormone, IGF-1 Levels, and Oxidative Stress Parameters in a Rat Model of Polycystic Ovary Syndrome. Reprod. Sci. 2015, 22, 942–947. [Google Scholar] [CrossRef]
- Furat-Rencber, S.; Kurnaz-Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Ghowsi, M.; Khazali, H.; Sisakhtnezhad, S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Ghowsi, M.; Khazali, H.; Sisakhtnezhad, S. Evaluation of TNF-α and IL-6 mRNAs expressions in visceral and subcutaneous adipose tissues of polycystic ovarian rats and effects of resveratrol. Iran. J. Basic Med. Sci. 2018, 21, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Wang, R.; Zhang, X.; Wang, L.; Xiang, Z.; Zhuang, L.; Shen, S.; Wang, H.; Gao, Q.; et al. Suppression of p66Shc prevents hyperandrogenism-induced ovarian oxidative stress and fibrosis. J. Transl. Med. 2020, 18, 84. [Google Scholar] [CrossRef]
- Ashkar, F.; Eftekhari, M.H.; Tanideh, N.; Koohpeyma, F.; Mokhtari, M.; Irajie, C.; Iraji, A. Effect of hydroalcoholic extract of Berberis integerrima and resveratrol on ovarian morphology and biochemical parameters in Letrozole-induced polycystic ovary syndrome rat model: An experimental study. Int. J. Reprod. Biomed. 2020, 18, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Huang, L.; Liu, H.; He, W.; Lei, X.; Li, M.; Li, S.; Liang, H.; Chen, G.; Tang, J.; et al. Resveratrol Improves Follicular Development of PCOS Rats by Regulating the Glycolytic Pathway. Mol. Nutr. Food Res. 2021, 65, e2100457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhuang, L.; Gai, S.; Shan, Y.; Wang, S.; Li, F.; Chen, L.; Zhao, D.; Liu, X. Beneficial phytoestrogenic effects of resveratrol on polycystic ovary syndromein rat model. Gynecol. Endocrinol. 2021, 37, 337–341. [Google Scholar] [CrossRef]
- Yarmolinskaya, M.; Bulgakova, O.; Abashova, E.; Borodina, V.; Tral, T. The effectiveness of resveratrol in treatment of PCOS on the basis of experimental model in rats. Gynecol. Endocrinol. 2021, 37 (Suppl. 1), 54–57. [Google Scholar] [CrossRef]
- Chen, M.; He, C.; Zhu, K.; Chen, Z.; Meng, Z.; Jiang, X.; Cai, J.; Yang, C.; Zuo, Z. Resveratrol ameliorates polycystic ovary syndrome via transzonal projections within oocyte-granulosa cell communication. Theranostics 2022, 12, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Zhang, W.; Wang, Q.; Huang, L.; Zhang, J.; Ma, D.; Liu, K.; Li, S.; Chen, X.; Li, S.; et al. Resveratrol regulates insulin resistance to improve the glycolytic pathway by activating SIRT2 in PCOS granulosa cells. Front. Nutr. 2023, 9, 1019562. [Google Scholar] [CrossRef]
- Huo, P.; Li, M.; Le, J.; Zhu, C.; Yao, J.; Zhang, S. Resveratrol improves follicular development of PCOS rats via regulating glycolysis pathway and targeting SIRT1. Syst. Biol. Reprod. Med. 2023, 69, 153–165. [Google Scholar] [CrossRef]
- Yuan, B.; Luo, S.; Feng, L.; Wang, J.; Mao, J.; Luo, B. Resveratrol regulates the inflammation and oxidative stress of granulosa cells in PCOS via targeting TLR2. J. Bioenerg. Biomembr. 2022, 54, 191–201. [Google Scholar] [CrossRef]
- Marks, K.E.; Cho, K.; Stickling, C.; Reynolds, J.M. Toll-like Receptor 2 in Autoimmune Inflammation. Immune Netw. 2021, 21, e18. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 9282484. [Google Scholar] [CrossRef]
- Raja-Khan, N.; Urbanek, M.; Rodgers, R.J.; Legro, R.S. The role of TGF-β in polycystic ovary syndrome. Reprod. Sci. 2014, 21, 20–31. [Google Scholar] [CrossRef]
- Banaszewska, B.; Wrotyńska-Barczyńska, J.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A.J. Effects of Resveratrol on Polycystic Ovary Syndrome: A Double-blind, Randomized, Placebo-controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 4322–4328. [Google Scholar] [CrossRef]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef]
- Brenjian, S.; Moini, A.; Yamini, N.; Kashani, L.; Faridmojtahedi, M.; Bahramrezaie, M.; Khodarahmian, M.; Amidi, F. Resveratrol treatment in patients with polycystic ovary syndrome decreased pro-inflammatory and endoplasmic reticulum stress markers. Am. J. Reprod. Immunol. 2020, 83, e13186. [Google Scholar] [CrossRef]
- Mansour, A.; Samadi, M.; Sanginabadi, M.; Gerami, H.; Karimi, S.; Hosseini, S.; Shirzad, N.; Hekmatdoost, A.; Mahdavi-Gorabi, A.; Mohajeri-Tehrani, M.R.; et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin. Nutr. 2021, 40, 4106–4112. [Google Scholar] [CrossRef]
- Hashemi-Taheri, A.P.; Moradi, B.; Radmard, A.R.; Sanginabadi, M.; Qorbani, M.; Mohajeri-Tehrani, M.R.; Shirzad, N.; Hosseini, S.; Hekmatdoost, A.; Asadi, S.; et al. Effect of resveratrol administration on ovarian morphology, determined by transvaginal ultrasound for the women with polycystic ovary syndrome (PCOS). Br. J. Nutr. 2021, 128, 211–216. [Google Scholar] [CrossRef]
- Ye, W.; Xie, T.; Song, Y.; Zhou, L. The role of androgen and its related signals in PCOS. J. Cell Mol. Med. 2021, 25, 1825–1837. [Google Scholar] [CrossRef]
- Zhai, Y.; Pang, Y. Systemic and ovarian inflammation in women with polycystic ovary syndrome. J. Reprod. Immunol. 2022, 151, 103628. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Pascuali, N.; Parborell, F.; Abramovich, D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction 2018, 155, R199–R209. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, M.; Woodruff, T.K. Involvement of androgens in ovarian health and disease. Mol. Hum. Reprod. 2013, 19, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Shah, M.; Malik, M.O.; Ehtesham, E.; Habib, S.H.; Rauf, B. Treatment with combined resveratrol and myoinositol ameliorates endocrine, metabolic alterations and perceived stress response in women with PCOS: A double-blind randomized clinical trial. Endocrine 2023, 79, 208–220. [Google Scholar] [CrossRef]
- Malvasi, A.; Tinelli, A.; Lupica, G.; Vimercati, A.; Spyropoulou, K.; Dellino, M.; Mynbaev, O. Effects of a combination of resveratrol and alpha-lipoic acid on body weight and adipose composition in women with PCOS: A preliminary pilot study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6578–6582. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.M.; Jee, B.C.; Kim, Y.B.; Suh, C.S. Various anatomic locations of surgically proven endometriosis: A single-center experience. Obstet. Gynecol. Sci. 2015, 58, 53–58. [Google Scholar] [CrossRef]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: A review with comparison of 8 guidelines. BMC Womens Health 2021, 21, 397. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef]
- Rudzitis-Auth, J.; Menger, M.D.; Laschke, M.W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 2013, 28, 1339–1347. [Google Scholar] [CrossRef]
- Ergenoğlu, A.M.; Yeniel, A.Ö.; Erbaş, O.; Aktuğ, H.; Yildirim, N.; Ulukuş, M.; Taskiran, D. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod. Sci. 2013, 20, 1230–1236. [Google Scholar] [CrossRef]
- Amaya, S.C.; Savaris, R.F.; Filipovic, C.J.; Wise, J.D.; Hestermann, E.; Young, S.L.; Lessey, B.A. Resveratrol and endometrium: A closer look at an active ingredient of red wine using in vivo and in vitro models. Reprod. Sci. 2014, 21, 1362–1369. [Google Scholar] [CrossRef]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef]
- Ozcan-Cenksoy, P.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A potential novel treatment strategy: Inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol. Endocrinol. 2015, 31, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Bayoglu-Tekin, Y.; Guven, S.; Kirbas, A.; Kalkan, Y.; Tumkaya, L.; Guvendag-Guven, E.S. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, X.; Zhou, L.; Zhu, M.; Yao, S.; Ding, Y.; Liu, T.; Wang, Y.; Zhang, Y.; Li, R. MTA1, a Target of Resveratrol, Promotes Epithelial-Mesenchymal Transition of Endometriosis via ZEB2. Mol. Ther. Methods Clin. Dev. 2020, 19, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Ayen, E.; Razi, M.; Behfar, M. Effects of atorvastatin and resveratrol against the experimental endometriosis; evidence for glucose and monocarboxylate transporters, neoangiogenesis. Life Sci. 2021, 272, 119230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Lin, C.; Zhang, L.; Zheng, H.; Zhou, Y.; Li, X.; Li, C.; Zhang, X.; Yang, X.; et al. Lipidomic Alterations and PPARα Activation Induced by Resveratrol Lead to Reduction in Lesion Size in Endometriosis Models. Oxid. Med. Cell Longev. 2021, 2021, 9979953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Z.; Zhao, X.; Lin, C.; Hong, S.; Lou, Y.; Shi, X.; Zhao, M.; Yang, X.; Guan, M.X.; et al. Transcriptome-Based Analysis Reveals Therapeutic Effects of Resveratrol on Endometriosis in a Rat Model. Drug Des. Devel. Ther. 2021, 15, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wang, X.; Xia, X.; Zhang, T.; Nie, M.; Xiong, J.; Fang, X. Resveratrol protected against the development of endometriosis by promoting ferroptosis through miR-21-3p/p53/SLC7A11 signaling pathway. Biochem. Biophys. Res. Commun. 2024, 692, 149338. [Google Scholar] [CrossRef]
- Béliard, A.; Noël, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Ye, J.; Li, M.; Zhu, Z. The Role of Matrix Metalloproteinases in Endometriosis: A Potential Target. Biomolecules 2021, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.E.; Berardo, P.T.; Palmero, C.Y.; Nasciutti, L.E. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J. Exp. Clin. Cancer Res. 2010, 29, 4. [Google Scholar] [CrossRef]
- Bouet, P.E.; Chao de la Barca, J.M.; Boucret, L.; Descamps, P.; Legendre, G.; Hachem, H.E.; Blanchard, S.; Jeannin, P.; Reynier, P.; May-Panloup, P. Elevated Levels of Monocyte Chemotactic Protein-1 in the Follicular Fluid Reveals Different Populations among Women with Severe Endometriosis. J. Clin. Med. 2020, 9, 1306. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Vallée, J.N.; Le Blanche, A.; Lecarpentier, Y. PPARγ Agonists: Emergent Therapy in Endometriosis. Pharmaceuticals 2021, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.A.; Sokalska, A.; Cress, A.B.; Ortega, I.; Bruner-Tran, K.L.; Osteen, K.G.; Duleba, A.J. Resveratrol potentiates effect of simvastatin on inhibition of mevalonate pathway in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2013, 98, E455–E462. [Google Scholar] [CrossRef]
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol suppresses inflammatory responses in endometrial stromal cells derived from endometriosis: A possible role of the sirtuin 1 pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol Enhances Apoptosis in Endometriotic Stromal Cells. Am. J. Reprod. Immunol. 2016, 75, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Rashidi, Z.; Chobsaz, F.; Niromand, E.; Khazaei, M. Inhibitory effect of resveratrol on the growth and angiogenesis of human endometrial tissue in an In Vitro three-dimensional model of endometriosis. Reprod. Biol. 2020, 20, 484–490. [Google Scholar] [CrossRef]
- Kolahdouz-Mohammadi, R.; Delbandi, A.A.; Khodaverdi, S.; Arefi, S.; Arablou, T.; Shidfar, F. The Effects of Resveratrol Treatment on Bcl-2 and Bax Gene Expression in Endometriotic Compared with Non-Endometriotic Stromal Cells. Iran. J. Public Health 2020, 49, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A.A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.A. The effects of resveratrol on the expression of VEGF, TGF-β, and MMP-9 in endometrial stromal cells of women with endometriosis. Sci. Rep. 2021, 11, 6054. [Google Scholar] [CrossRef] [PubMed]
- Madanes, D.; Meresman, G.; Valla, S.A.; Hassan, N.; Kiesel, L.; Greve, B.; Barañao, R.I.; Götte, M.; Ricci, A.G. Resveratrol impairs cellular mechanisms associated with the pathogenesis of endometriosis. Reprod. Biomed. Online 2022, 44, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek-Grenda, A.; Kaczmarek, M.; Juzwa, W.; Olejnik, A. Natural resveratrol analogs differentially target endometriotic cells into apoptosis pathways. Sci. Rep. 2023, 13, 11468. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int. J. Womens Health 2012, 4, 543–549. [Google Scholar] [CrossRef]
- Mendes da Silva, D.; Gross, L.A.; Neto, E.P.G.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani-Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Gynecol. Endocrinol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-Mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Reprod. Immunol. 2021, 143, 103248. [Google Scholar] [CrossRef]
- Tang, J.; Xu, Y.; Wang, Z.; Ji, X.; Qiu, Q.; Mai, Z.; Huang, J.; Ouyang, N.; Chen, H. Association between metabolic healthy obesity and female infertility: The national health and nutrition examination survey, 2013-2020. BMC Public Health 2023, 23, 1524. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Zolfaghari, Z.; Arabipoor, A. Abdominal obesity can induce both systemic and follicular fluid oxidative stress independent from polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 184, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Schube, U.; Nowicki, M.; Jogschies, P.; Blumenauer, V.; Bechmann, I.; Serke, H. Resveratrol and desferoxamine protect human OxLDL-treated granulosa cell subtypes from degeneration. J. Clin. Endocrinol. Metab. 2014, 99, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Tuck, A.R.; Sjödin, M.O.D.; Lindberg, J.; Sand, A.; Niklasson, B.; Argyraki, M.; Hovatta, O.; Damdimopoulou, P. Resveratrol supports and alpha-naphthoflavone disrupts growth of human ovarian follicles in an in vitro tissue culture model. Toxicol. Appl. Pharmacol. 2018, 338, 73–82. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Kőszegi, T.; Gödöny, K.; Prémusz, V.; Várnagy, Á. Serum and follicular fluid levels of sirtuin 1, sirtuin 6, and resveratrol in women undergoing in vitro fertilization: An observational, clinical study. J. Int. Med. Res. 2019, 47, 772–782. [Google Scholar] [CrossRef]
- Han, J.; Wang, H.; Zhang, T.; Chen, Z.; Zhao, T.; Lin, L.; Xia, G.; Wang, C. Resveratrol attenuates doxorubicin-induced meiotic failure through inhibiting oxidative stress and apoptosis in mouse oocytes. Aging 2020, 12, 7717–7728. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, M.L.; Gao, X.; Wang, Y.; Zhang, L.N.; Li, Y.C.; Guo, Q. Resveratrol improves ovarian state by inhibiting apoptosis of granulosa cells. Gynecol. Endocrinol. 2023, 39, 2181652. [Google Scholar] [CrossRef]
- Cabello, E.; Garrido, P.; Morán, J.; González del Rey, C.; Llaneza, P.; Llaneza-Suárez, D.; Alonso, A.; González, C. Effects of resveratrol on ovarian response to controlled ovarian hyperstimulation in ob/ob mice. Fertil. Steril. 2015, 103, 570–579.e1. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Arosh, J.A.; Burghardt, R.C. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 2016, 303, 65–78. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.L.; He, S.W.; Chen, M.H.; Zhang, Z.; Fu, X.P.; Fu, B.B.; Liao, B.Q.; Lin, Y.H.; Qi, Z.Q.; et al. Protective effects of resveratrol against mancozeb induced apoptosis damage in mouse oocytes. Oncotarget 2017, 8, 6233–6245. [Google Scholar] [CrossRef]
- Atli, M.; Engin-Ustun, Y.; Tokmak, A.; Caydere, M.; Hucumenoglu, S.; Topcuoglu, C. Dose dependent effect of resveratrol in preventing cisplatin-induced ovarian damage in rats: An experimental study. Reprod. Biol. 2017, 17, 274–280. [Google Scholar] [CrossRef]
- Jia, Z.; Feng, Z.; Wang, L.; Li, H.; Wang, H.; Xu, D.; Zhao, X.; Feng, D.; Feng, X. Resveratrol reverses the adverse effects of a diet-induced obese murine model on oocyte quality and zona pellucida softening. Food Funct. 2018, 9, 2623–2633. [Google Scholar] [CrossRef]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging 2019, 11, 1030–1044. [Google Scholar] [CrossRef]
- Said, R.S.; Mantawy, E.M.; El-Demerdash, E. Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: Emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1225–1238. [Google Scholar] [CrossRef]
- Demirel, M.A.; Han, S.; Tokmak, A.; Ercan-Gokay, N.; Uludag, M.O.; Yildirir-Ustun, T.; Cicek, A.F. Therapeutic effects of resveratrol in Escherichia coli-induced rat endometritis model. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Herrero, Y.; Velázquez, C.; Pascuali, N.; May, M.; Abramovich, D.; Scotti, L.; Parborell, F. Resveratrol alleviates doxorubicin-induced damage in mice ovary. Chem. Biol. Interact. 2023, 376, 110431. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplementation on IVF-embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Gerli, S.; Della-Morte, C.; Ceccobelli, M.; Mariani, M.; Favilli, A.; Leonardi, L.; Lanti, A.; Iannitti, R.G.; Fioretti, B. Biological and clinical effects of a resveratrol-based multivitamin supplement on intracytoplasmic sperm injection cycles: A single-center, randomized controlled trial. J. Matern. Fetal Neonatal Med. 2022, 35, 7640–7648. [Google Scholar] [CrossRef]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef]
- Huang, J.; Kuang, Y. Is it too early to deny resveratrol supplementation in embryo transfer cycles? Reprod. Biomed. Online 2019, 39, 177. [Google Scholar] [CrossRef]
| Animal Model | Number of Samples | Dosage and Duration | Results | Study |
|---|---|---|---|---|
| Rat | Control + vehicle n = 10 Control + resveratrol n = 10 PCOS + vehicle n = 10 PCOS- + resveratrol n = 9 PCOS + exercise n = 9–10 | 400 mg/kg 5 days a week for 4 weeks and 7 days of the final treatment week | ↓ smaller adipocytes, ↑ estrogen-related receptor α gene expression in subcutaneous fat, and improved estrus cyclicity after resveratrol and exercise No differences in body weight, Insulin sensitivity, expression of adiponectin, Fndc5, Foxo1, Nrf1, Pparg, Ppargc1a, Sirt1, CYP17a1, and Hsd3b1 | [136] |
| Rat | Control n = 7 PCOS + saline n = 7 PCSO + Resveratrol n = 7 | 10 mg/kg daily for 4 weeks | ↓ number of antral follicles and ↓ concentration of plasma insulin-like growth factor 1 (IGF-1) and anti-Mullerian hormone (AMH) Significantly ↓ superoxide dismutase (SOD) activity and ↑ glutathione peroxidase levels | [137] |
| Rat | Control n = 9 PCOS n = 9 PCOS + resveratrol n = 9 PCOS + resveratrol solvent n = 9 PCOS + metformin n = 9 PCOS + metformin solvent n = 9 PCOS + metformin + resveratrol n = 9 | 20 mg/kg daily for 28 days; 20 mg/kg resveratrol + 300 mg metformin daily for 28 days | ↓ levels of testosterone, TNF-α, LH, LH/FSH, AMH, and malondialdehyde (MDA); a lipid peroxidation marker ↑ SIRT1 immunoreactivity Combined treatment ↓ the body and ovary weight Single or combined treatment ↓ the elevated number of secondary and atretic follicles, ↑ number of primordial and Graafian follicles, and Corpus luteum | [138] |
| Rat | Control n = 5 PCOS + saline n = 5 PCSOS+ resveratrol n = 5 | 10 mg/kg daily for 28 days | ↓ HOMA-IR level and fasting serum glucose ↑ serum total antioxidant capacity ↓ serum MDA concentrations insulin levels were not statistically different | [139] |
| Rat | Control n = 5 PCOS + saline n = 5 PCSOS + resveratrol n = 5 | 10 mg/kg daily for 28 days | ↓ expression of subcutaneous adipose tissue Tnf-α mRNA ↓ expression of visceral adipose tissue Tnf- α and Il-6 mRNAs | [140] |
| Rat | Control n = 7 PCOS n = 7 PCOS + resveratrol n = 7 | 100 mg/kg daily for 35 days | ↓ in body weight ↓ in androgen-induced thick fibrotic capsules, the number of multiple immature follicles, and ovarian interstitial fibrosis ↑ number of luteal cells and antral follicles and serum and ovarian SOD levels ↓ ovarian oxidative stress and serum and ovary MDA levels ↓ expression of P-p66Shc, TGF-β, β-catenin, α-SMA protein, CTGF, collagen IV, and collagen IA1 ↑ expression of SIRT1 protein ↓ phosphorylation of p66Shc | [141] |
| Rat | Control n = 10 Vehicle n = 10 PCOS n = 10 PCOS + metformin n = 10 PCOS + resveratrol n = 10 PCOS + barberry n = 10 PCOS + barberry + resveratrol n = 10 | 20 mg/kg daily for 42 days; 20 mg/kg resveratrol + 3 gr/kg barberry daily for 42 days | ↓ concentration of low-density lipoprotein (LDL), triglycerides, ovarian weight, MDA, TNF-α, insulin resistance, number of atretic and cystic follicles after single or combinatory treatment (alone or in combination with barberry) ↑ total antioxidant activity, levels of high-density lipoprotein (HDL), and superoxide dismutase (groups 4–7) ↓ number of cystic follicles (resveratrol, barberry, and combination group) No significant differences between serum glucose levels | [142] |
| Rat | Control n = 8 PCOS n = 8 PCOS + resveratrol n = 8 | 20 mg/kg daily for 30 days | ↓ weight and concentration of serum FSH, LH, and testosterone Restoration of the estrous cycle and improved follicular development (↑ number of follicles and corpus luteum; ↓ cyst-expanded antral follicles) ↑ number of granular cells and thickness of granular layer ↑ lactate, ATP, dihydroxyacetone, beta-D-fructose 6-phosphate, and ↓ pyruvate. ↑ in glycolysis-involved genes (LDHA, HK2, PKM2, SIRT2) after resveratrol treatment | [143] |
| Rat | Control n = 10 PCOS n = 10 PCOS + resveratrol (40 mg/kg) n = 10 PCOS + resveratrol (80 mg/kg) n = 10 PCOS + resveratrol (160 mg/kg) n = 10 | 40, 80, or 160 mg/kg daily for 30 days | Normalization of plasma adiponectin and estradiol levels (↑ levels) restoration of normal ovarian morphology (↑ numbers of granule cell layers and the presence of oocytes within follicles) restoration of aromatase and nesfatin-1 expression (↑ expression) | [144] |
| Rat | Control n = 4 PCOS + saline n = 4 PCOS + resveratrol (20 mg/kg) n = 6 PCOS + resveratrol (30 mg/kg) n = 8 | 20 mg/kg and 30 mg/kg daily for 30 days | Improved ovarian tissue morphology (↑ number of granulosa cells and oocytes) ↓ weight and positive regulation of the estrous cycle ↓ cystic changes in ovary | [145] |
| Rat | Control n = 13 PCOS n = 18 PCOS + resveratrol n = 14 Resveratrol n = 12 | 20 mg/kg daily for 28 days | Improved estrus cycles and hormonal profile Improved ovarian function, ↓ AMH concentration to a normal level Protective effect on the primordial follicle pool | [146] |
| Rat | Control n = 8 PCOS n = 8 PCOS + resveratrol n = 8 | 20 mg/kg daily for 30 days | ↑ increased ovarian insulin sensibility (↓ blood glucose, serum insulin, and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)) ↑ IGF1R and ↓ IGF1 of mRNA and protein levels ↑ expression of glycolytic genes (HK2, LDHA, PKM2), confirmed on protein level ↑ expression of SIRT2 (mRNA and protein) | [147] |
| Rat | Control n = 6 PCOS n = 6 PCOS + resveratrol n = 6 | 20 mg/kg/ daily for 21 days | Improved estrous cycle and granular cell layer Reversion of decreased proliferation and increased apoptosis of granulosa cells ↑ expression of LDHA, PKM2, and SIRT1 in ovarian tissue ↓ body weight ↓ HOMA-IR level ↓ testosterone Restored PCNA expression ↓ expression of Bax, caspase-3, apaf1, and cytochrome C; ↑ expression of Bcl-2 (↓ apoptosis) Restored AMP/ATP ratio | [148] |
| Study Type | Number of Analyzed Patients | Dosage and Duration | Nationality | Age | Results | Study |
|---|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled trial | PCOS + placebo n = 15 PCOS + resveratrol n = 15 | 1500 mg daily for 3 months | Poland | Placebo = 26.8 ± 1.5 Resveratrol = 26.8 ± 1.1 | Significant ↓ in dehydroepiandrosterone sulfate, total testosterone, fasting insulin, and ↑ Insulin Sensitivity Index. No significant changes in BMI, ovarian volume, gonadotropins, inflammation, or lipid profile | [153] |
| Randomized, triple-blind, placebo-controlled trial | PCOS + placebo n = 31 PCOS + resveratrol n = 30 | 800 mg daily for 40 days | Iran | Placebo = 30.84 ± 3.30 Resveratrol = 29.30 ± 4.44 | Mean difference in LH, TSH, FSH, and testosterone (LH and testosterone ↓, FSH and TSH ↑) ↑ rate of high-quality oocytes and embryos ↓ expression of VEGF and HIF1 (pathologic angiogenesis) No significant differences between AMH levels, fertility, fertilization and cleavage rate, and oocyte maturation between groups | [154] |
| Randomized, double-blind, placebo-controlled trial | PCOS + placebo n = 20 PCOS + resveratrol n = 20 | 800 mg daily for 40 days | Iran | Placebo = 30.35 ± 4.00 Resveratrol = 29.55 ± 3.28 | ↓ in inflammatory markers, like IL-18, CRP, and borderline TNF-α in comparison to the placebo group. IL-6 and IL-1β levels also ↓ after resveratrol treatment, but not statistically significant ↓ Level of NF-κappaB differences in gene expression (↑ expression of ATF4 and ATF6, involved with unfolding protein response due to ER stress, ↓ expression of CHOP, GRP78, and XBP1) | [155] |
| Randomized, double-blind, placebo-controlled trial | PCOS + placebo n = 39 PCOS + resveratrol n = 39 | 1000 mg of resveratrol daily for 3 months | Iran | Placebo = 27.87 ± 6.24 Resveratrol = 26.33 ± 5.62 | ↑ menstruation regularities, ↓ hair loss No significant differences in ovarian and adrenal androgens, sex hormone binding globulin (SHBG) levels, free androgen index (FAI), lipid, and glycoinsulinemic profile | [156] |
| Randomized, double-blind, placebo-controlled trial | PCOS + placebo n = 17 PCOS + resveratrol n = 19 | 1000 mg daily for 3 months | Iran | Placebo = 27.30 ± 5.22 Resveratrol = 29.79 ± 4.61 | Improvement of the polycystic ovarian morphology ↓ ovarian volume No significant differences between the number of follicle count per ovary (FNPO), stromal area (SA), ovarian echogenicity, and distribution of follicles | [157] |
| Animal Model | Number of Samples | Dosage and Duration | Results | Study |
|---|---|---|---|---|
| Mouse | Control n = 16 Resveratrol n= 20 | 6 mg/mouse for 10–12 or 18–20 days | ↓ in the number, size, and total volume of endometriotic implants | [169] |
| Mouse | Control n = 8 Resveratrol (10 mg/kg) n = 10 Resveratrol (25 mg/kg) n = 10 | 10 mg/kg or 25 mg/kg daily for 4 weeks | ↓ in the mean number and volume of endometriotic lesions ↓ vascular density and cell proliferation and ↑ apoptosis in endometriotic lesions | [170] |
| Mouse | Control n = 10 Resveratrol n = 10 | 40 mg/kg daily for 4 weeks | ↓ microvessel density (angiogenesis inhibition in endometriotic lesions; ↓ proliferation of endothelial cells) ↓ growth and size of endometriotic lesions (↓ proliferation of stromal and glandular cells) | [171] |
| Rat | Control n = 6 Resveratrol n = 6 | 10 mg/kg daily for 14 days | ↓ in the endometriotic implant size ↓ levels of vascular endothelial growth factor (VEGF) in the plasma and peritoneal fluid ↓ levels of monocyte chemotactic protein 1 (MCP-1) in the peritoneal fluid ↑ suppression of VEGF expression in the endometriotic tissue Histological changes (↓ vascularization) | [172] |
| Mouse | Estradiol n = 4 Estradiol + progesterone n = 4 Estradiol + resveratrol (6 mg/kg) n = 4 Estradiol + resveratrol (30 mg/kg) n = 4 Estradiol + resveratrol (60 mg/kg) n = 4 | 6, 30, or 60 mg daily for 30 days | ↓ expression of ESR1 and ↓ proliferative activity (60 mg/kg) | [173] |
| Rat | Control n = 8 Resveratrol (1 mg/kg) n = 8 Resveratrol (10 mg/kg) n = 8 | 1 mg/kg or 10 mg/kg daily for 7 days | ↓ endometriotic implant volume ↑ (dose-dependent) activity of superoxide dismutase and glutathione peroxidase in serum and tissue in resveratrol groups ↑ MDA levels and catalase levels in serum and tissue in 10 mg/kg resveratrol group ↓ proliferating cell nuclear antigen expression and histological scores in resveratrol groups | [174] |
| Rat | Control n = 7 Leuprolide acetate n = 8 Resveratrol n =7 | 60 mg/kg daily for 21 days | ↓ mean size of endometriotic implants and histopathological score ↓ VEGF-staining scores and peritoneal fluid levels of VEGF and MCP-1 ↓ serum VEGF and MCP-1 | [175] |
| Rat | Control n = 8 Resveratrol n = 9 Leuprolide acetate n = 8 Resveratrol + leuprolide acetate n = 8 | 30 mg/kg daily for 14 days | ↓ endometriotic implant volume ↓ histopathological score ↓ levels of IL-6, IL-8, and TNF-α in plasma and peritoneal fluid ↓ expression of MMP-2, MMP-9 and VEGF | [176] |
| Mouse | Control (PBS) n = 6 Blank n = 6 Resveratrol n = 6 | 25 mg/kg daily for 4 weeks | ↓ growth of ectopic endometriotic lesions ↓ expression of MTA1 and ZEB2 (involved with epithelial-mesenchymal transition) | [177] |
| Rat | Endometriosis control n = 6 Atorvastatin n = 6 Resveratrol n = 6 Resveratrol + atorvastatin n = 6 | 40 mg kg daily for 28 days | ↓ in ectopic endometrial tissue size and neovasculature ↓ expression of GLUT-1, GLUT-3, MCT-1, and MCT-4 ↓ distribution of GLUT-1+, GLUT-3+, and MCT-4+ cells per mm2 of tissue | [178] |
| Rat | Control n = 10 Endometriosis n = 10 Resveratrol (15 mg/kg) n = 10 Resveratrol (45 mg/kg) n = 10 | 15 mg/kg and 45 mg/kg daily for 28 days | ↓ endometriotic lesion size in both groups Better histology (↓ glandular tubes and endometrial epithelial thickness at histology) in both groups ↓ cholesterol, HDL, and LDL levels in the medium dose group (15 mg/kg) ↓ cholesterol and HDL in high-dose group (45 mg/kg) ↓ expression of MMP-2, VEGF, and BCL-2 Induction of PPARα | [179] |
| Rat | Control n = 10 Endometriosis n = 10 Resveratrol (15 mg/kg) n = 10 Resveratrol (45 mg/kg) n = 10) | 15 mg/kg and 45 mg/kg daily for 28 days | ↓ in the volume of endometriotic lesions and adhesion 2123 differentially expressed genes (↑ expression of genes involved with blood vessel morphogenesis, transmembrane transport, ↓ expression of genes involved with immunity activation and regulation) ↓ in INF-γ, IL-6 TNF-α, and ↑ of IL10 Induction of PPARγ Changes in glucose tolerance, adipocyte size, and macrophage polarization | [180] |
| Mouse | / | 25 mg/kg daily for 14 days | ↓ volume of the endometriotic lesions ↓ cell density of the endometriosis cyst tissue ↓ serum GSH and ↑ MDA levels ↑ ROS ↑ expression of p53 and ↓ expression of miR-21-3p and SLC7A11 ↑ expression of Chac1 and Ptgs2 Promotion of ferroptosis | [181] |
| Study Type | Number of Analyzed Patients | Dosage and Duration | Nationality | Age | Results | Study |
|---|---|---|---|---|---|---|
| Experimental | Endometriosis: Office-based study n = 12 Immunohistochemistry study n = 42 | 30 mg daily for 2 months + oral contraceptives | Brazil | Office-based study = 30 ± 5 Immunohistochemistry study = 31 ± 4 | ↓ endometriosis-associated dysmenorrhea, potentiation of the effect of oral contraceptives ↓ aromatase and cyclooxygenase-2 expression in the endometrium | [198] |
| Randomized double-blind, placebo-controlled trial | Resveratrol n = 22 Placebo n = 22 | 40 mg daily for 42 days + monophasic oral contraceptives | Brazil | Placebo = 32.4 ± 7 Resveratrol 35.4 ± 7.1 | No significant differences between pain relief in comparison to placebo | [199] |
| Randomized double-blind, placebo-controlled trial | Resveratrol n = 17 Control n = 17 | 400 mg twice daily for 12–14 weeks | Iran | Control = 31.32 ± 1.71 Resveratrol 30.19 ± 2.40 | ↓ in mRNA and protein levels of MMP-2 and MMP-9 in the endometrium, serum, and endometrial fluid | [200] |
| Randomized double-blind, placebo-controlled trial | Resveratrol n = 17 Control n = 17 | 400 mg daily for 12–14 weeks | Iran | Control = 31.32 ± 1.71 Resveratrol 30.19 ± 2.40 | ↓ gene and protein level of VEGF and TNF-α in the eutopic endometrium | [201] |
| Animal Model | Number of Samples | Dosage and Duration | Results | Study |
|---|---|---|---|---|
| Mouse | Placebo n = 8 Resveratrol n = 8 Obesity placebo n = 8 Obesity resveratrol n = 8 | 3.75 mg/kg daily for 20 days | Ovarian hyperstimulation in obesity-related infertility: ↓ levels of plasma insulin and testosterone in obese mice Improvement of Homeostatic Index of Insulin Resistance in obese mice Normalization of IL-6 and TNF-α levels in obese mice ↓ number of primary, growing, preovulatory, and atretic follicles in obese mice ↑ number of retrieved oocytes in non-obese mice | [209] |
| Rat | Control n = 10 Hexavalent chromium n = 10 Hexavalent chromium + Resveratrol n = 10 | 10 mg/kg daily for 21 days | Chromium-toxicity: Protective effect against chromium toxicity in the ovarium ↑ expression of cell survival proteins: Bcl-2, HIF1α (↓ apoptosis) ↓ expression of cytochrome C and cleaved caspase-3 ↓ in oocyte and granulosa cell apoptosis ↓ oxidative stress (↑ antioxidants) Restoration of estradiol levels (↑) | [210] |
| Mouse | Control n = 60 Mancozed n = 60 Mancozed + resveratrol (100 mg/L) n = 60 Mancozed + resveratrol (200 mg/L) n = 60 | 100 mg/L or 200 mg/L daily for 4 weeks | Mancozeb (fungicide) toxicity: improvement of mancozeb-induced decrease in fertility, ovary weight, and primary follicles ↑ litter size and weight ↓ ROS, apoptosis Improved oocyte quality and development potential Improvement of abnormal epigenetic modification | [211] |
| Rat | Control n = 7 Cisplatin + resveratrol (5 mg/kg) n = 7 Cisplatin + resveratrol (25 mg/kg) n =7 Cisplatin + saline n = 7 | 5 mg/kg + cisplatin or 25 mg/kg + cisplatin daily for 21 days | Cisplatin (chemotherapeutic) toxicity: prevention of cisplatin-induced ovarian damage ↑ numbers of primary and primordial follicles (5 mg/kg) no significant differences in AMH levels | [212] |
| Mouse | Control n = 15 High fat diet + resveratrol = 15 High-fat diet + saline = 15 | 10 mg/kg/daily for 3 weeks | Diet-induced obesity: Protective effect on ovary ↓ in the negative effect of diet-associated obesity on oocyte quality ↓ number of destroyed follicles Restoration of oocyte zona pellucida (proper hardness) ↓ in the abnormal lipid distribution ↓ oocyte ROS levels Protective effect on mitochondrial damage ↓ in obesity-related abnormal spindle morphology and chromosomal abnormalities | [213] |
| Mouse | Conntrol n = 15 Cyclophosphamide + busulfan n =15 Cyclophosphamid + busulfan + resveratrol (30 mg/kg) n = 15 Cyclophosphamid + busulfan + resveratrol (100 mg/kg) n = 15 | 30 or 100 mg/kg/daily for 2 weeks. | Chemotherapy toxicity: Improvement of chemotherapy-induced ovarian aging (30 mg dose) ↓ oogonial stem cell loss (30 mg dose) ↑ expression of c-KIT, Oct4, Sox2, Nanog, Gdf9 and Ddx4 (30 mg dose) ↑ SOD2 and ↓ oxidative damage markers (NTY and 4-HNE) (30 mg dose) ↑ expression of SIRT1, FOXO1, and ↓ expression of NF-κappaB (30 and 100 mg dose) Improvement of oogonial stem cell viability at low doses (2 and 5 μM) at decreased at low doses (50, 100, and 200 μM) ↑ expression of SOD2 and Nrf2 and ↓ expression of caspase-3 and bax ↓ oogonial stem cell apoptosis | [214] |
| Rat | Control n = 15 Cisplatin n = 15 Resveratrol n = 15 Resveratrol + cisplatin n = 15 | 10 mg/kg daily for 17 days | Cisplatin (chemotherapeutic) toxicity: Improvement of follicle morphology ↑ levels AMH (decreased due to cisplatin) ↓ inflammatory markers (TNF-α, NF-κappaB, p65, COX-2, and iNOS) ↓ expression of cytochrome c and caspase-3 ↓ expression of poly(ADP-ribose) polymerase (PARP-1) | [215] |
| Rat | Control n = 6 Endometritis n = 6 Endometritis + marbofloxacin + PGF2α n = 6 Endometritis + marbofloxacin = 6 Endometritis + marbofloxacin + resveratrol n = 6 Resveratrol n = 6 | 30 mg/kg for 14 days | Endometritis: Better healing (macroscopic) of uterine and ovarian tissue ↓ serum cytokine levels CINC-3, CNTF, LIX, IL-4, IL-6, and CINC-1/CXCL-1 (resveratrol alone or in combination). Not significant: IL-10, CINC-2/CXCL-3, and TNF-α ↑ total antioxidant status ↓ oxidative stress index | [216] |
| Mouse | Control n = 9 Doxorubicin n = 9 Doxorubicin + resveratrol (7 mg/kg) n = 9 Doxorubicin + resveratrol (15 mg/kg) n = 9 | 7 and 15 mg/kg seven doses, one every 48 h | Doxorubicin (chemotherapeutic) toxicity: ↑ number of primary and antral follicles, preservation of primordial follicle number ↓ number of atretic follicles ↑ number of AMH-positive follicles ↓ DNA damage and apoptosis in preantral and early antral follicles ↑ proliferation index in follicular cells ↑ PCNA expression ↑ VEGF expression Restored architecture of the uterine tissue ↑ SOD expression (antioxidant maintenance) | [217] |
| Study Type | Number of Analyzed Patients | Dosage and Duration | Nationality | Age | Results | Study |
|---|---|---|---|---|---|---|
| Cross-sectional retrospective study | Control n = 2958 Resveratrol n = 102 | 200 mg daily Continuously (IVF embryo transfer cycles) | Japan | Control = 37.0 ± 3.78 Resveratrol 39.1 ± 3.01 | ↓ clinical pregnancy rate ↑ risk of miscarriage | [219] |
| Randomized, single-blind, controlled experimental study | Control n = 50 Resveratrol n = 40 | 150 mg daily for 3 months + folic acid (400 mcg), vitamin D (25 mcg), vitamin B12 (2.5 mcg), and vitamin B6 (1.4 mg) | Italy | Control = 36.6 ± 0.6 Resveratrol 36.1 ± 0.6 | ↑ number of oocytes and MII oocytes ↑ fertilization rate ↑ number of cleavage embryos/blastocytes and cryopreserved embryos per patient No significant differences between pregnancy rates and miscarriage and live birth rates | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgrajsek, R.; Ban Frangez, H.; Stimpfel, M. Molecular Mechanism of Resveratrol and Its Therapeutic Potential on Female Infertility. Int. J. Mol. Sci. 2024, 25, 3613. https://doi.org/10.3390/ijms25073613
Podgrajsek R, Ban Frangez H, Stimpfel M. Molecular Mechanism of Resveratrol and Its Therapeutic Potential on Female Infertility. International Journal of Molecular Sciences. 2024; 25(7):3613. https://doi.org/10.3390/ijms25073613
Chicago/Turabian StylePodgrajsek, Rebeka, Helena Ban Frangez, and Martin Stimpfel. 2024. "Molecular Mechanism of Resveratrol and Its Therapeutic Potential on Female Infertility" International Journal of Molecular Sciences 25, no. 7: 3613. https://doi.org/10.3390/ijms25073613
APA StylePodgrajsek, R., Ban Frangez, H., & Stimpfel, M. (2024). Molecular Mechanism of Resveratrol and Its Therapeutic Potential on Female Infertility. International Journal of Molecular Sciences, 25(7), 3613. https://doi.org/10.3390/ijms25073613






