Impact of TRP Channels on Extracellular Matrix Remodeling: Focus on TRPV4 and Collagen
Abstract
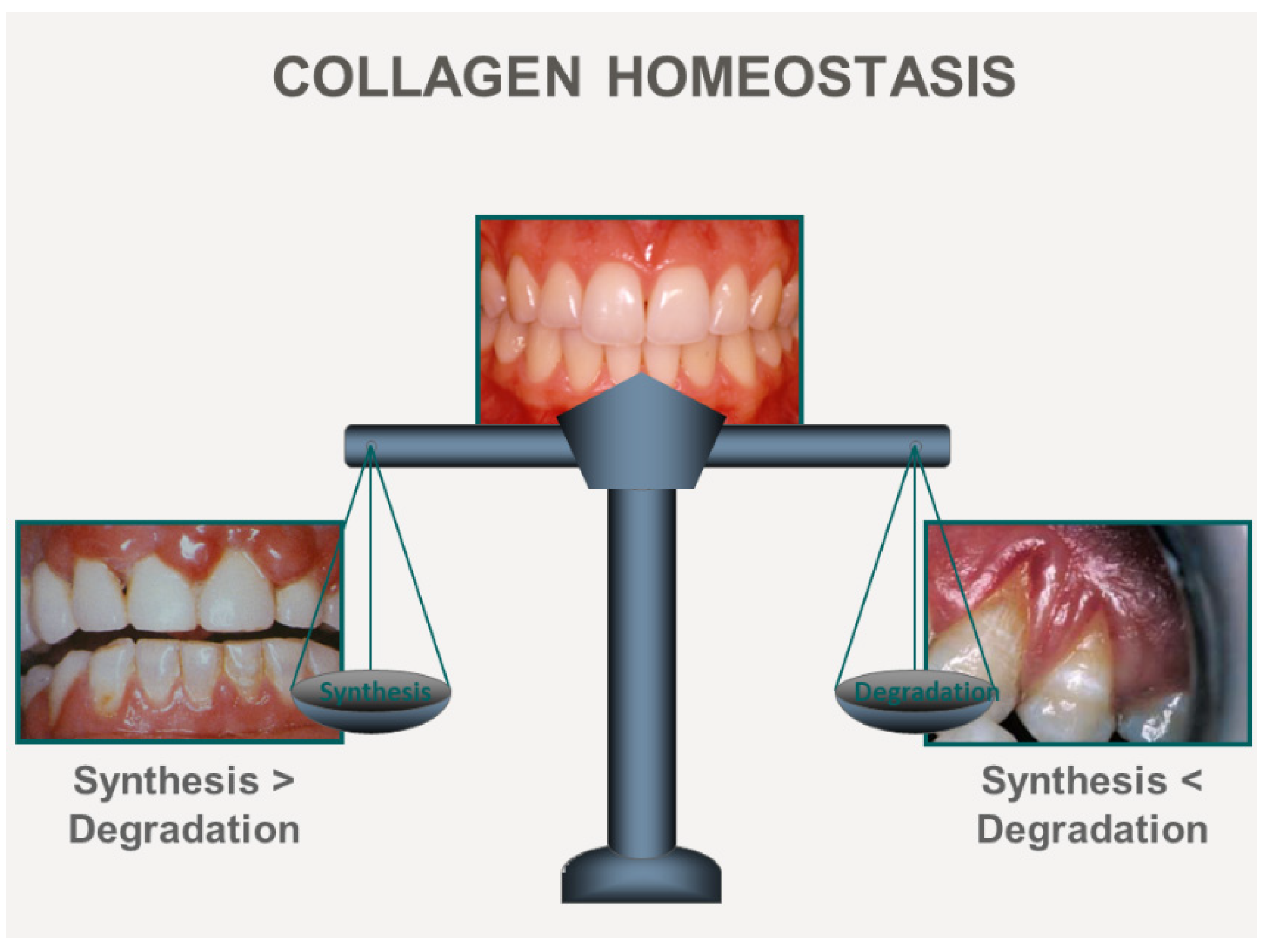
1. Why Examine Regulation of ECM Remodeling?
2. What Are Connective Tissues?
3. ECM Remodeling Mechanisms
4. Control of ECM Synthesis by Ca2+
5. Ca2+ Control of Collagen Degradation
6. Ca2+ Control of Post-Translational Modifications of Collagen
7. Ca2+ Control of Collagen Remodeling by Contraction
8. Polymodal TRP Channels Conduct Ca2+ and Regulate ECM Remodeling
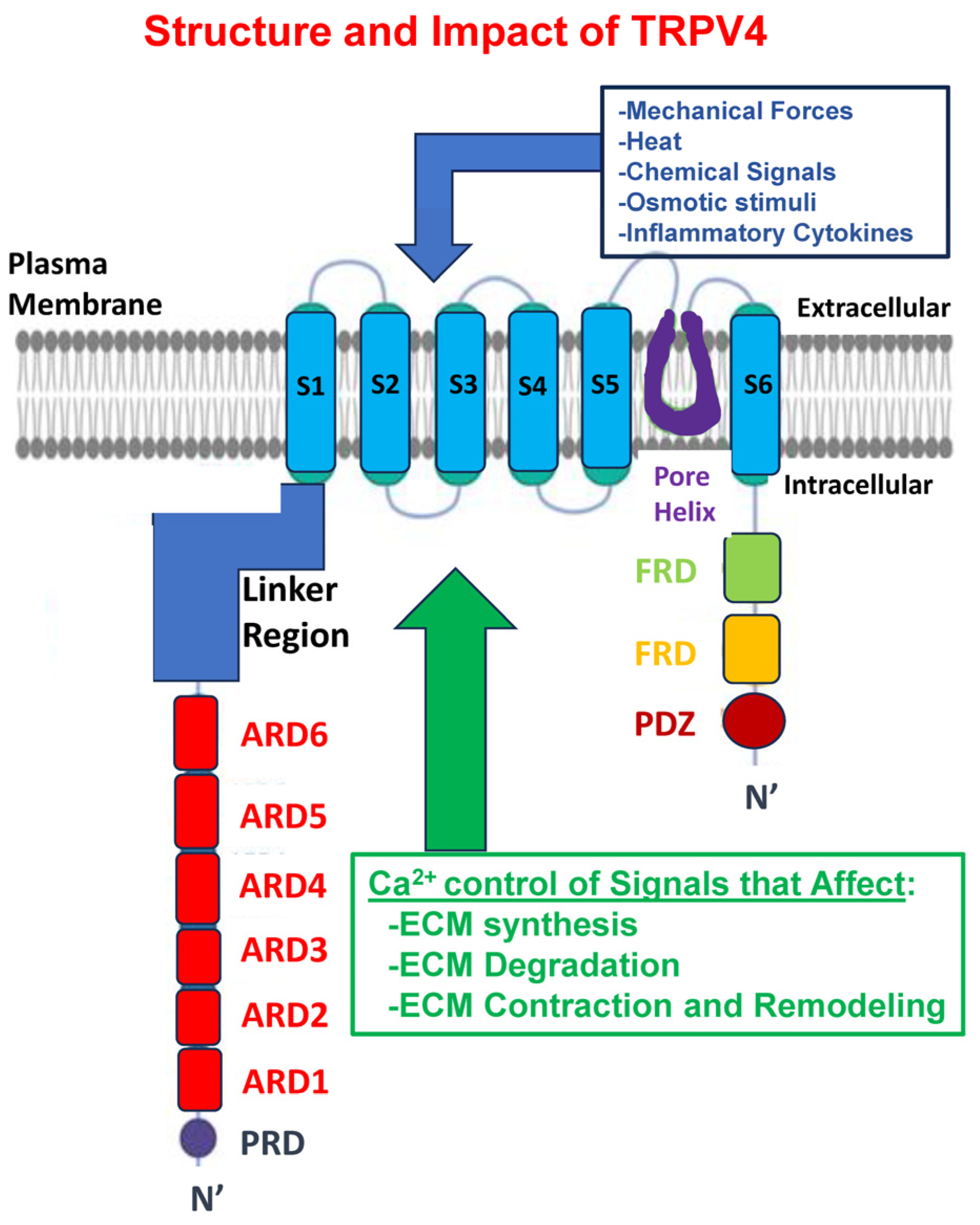
9. Why Focus on TRPV4?
10. Regulation of TRVP4 Expression and Activity
11. TRPV4 Expression and Function Are Associated with ECM Remodeling
12. TRPV4 Is Sensitive to Tissue Mechanics in Diverse Processes
13. Relationship between Cell Adhesion Receptors, ECM Remodeling, and TRPV4
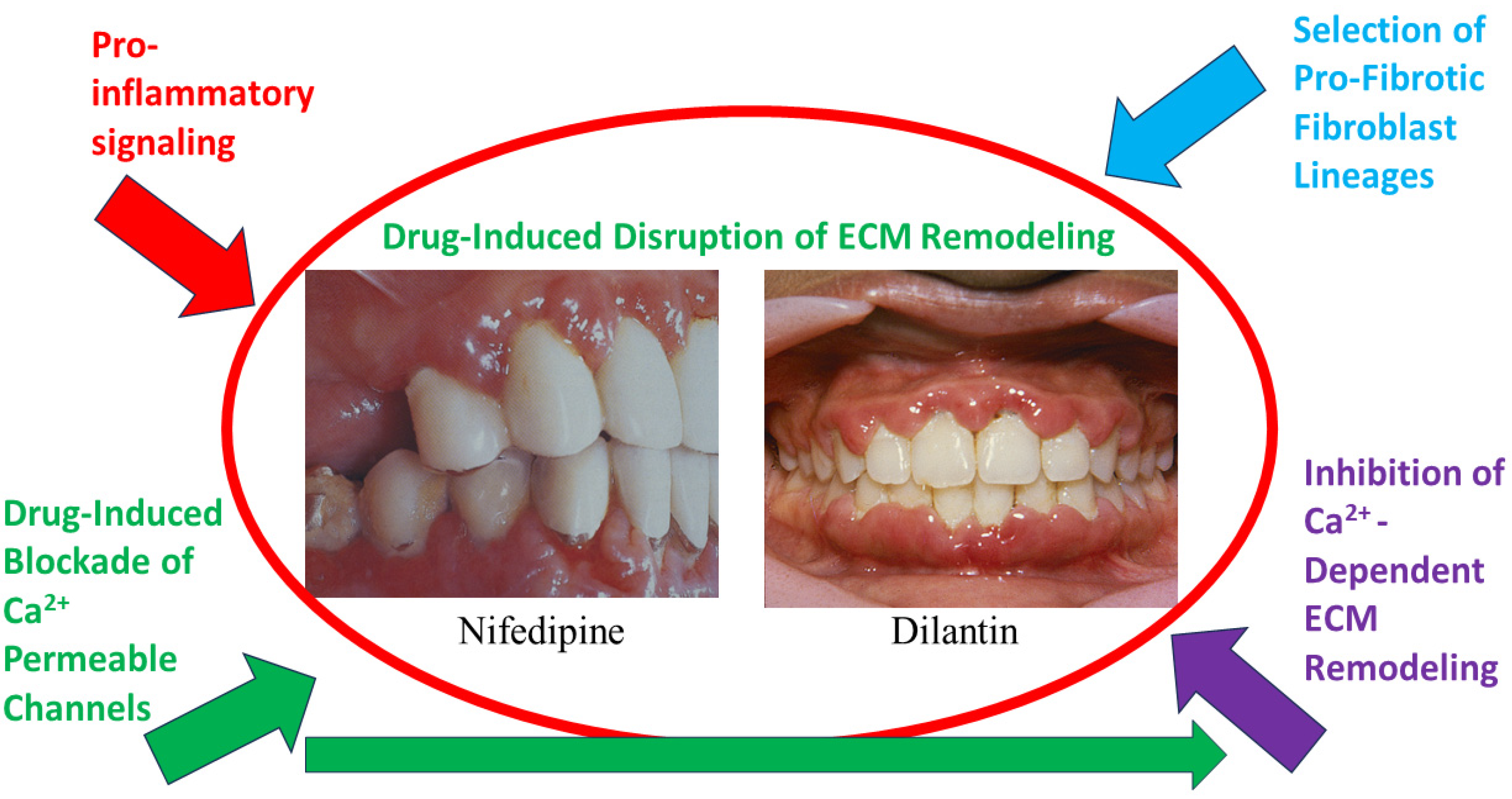
14. TRPV4 Signaling and the Fibrotic ECM
15. TRPV4 Mutations Manifest as Connective Tissue Abnormalities in Human Disease
16. Conclusions: Roles of TRPV4 in ECM Remodeling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perez-Tamayo, R. Pathology of collagen degradation. A review. Am. J. Pathol. 1978, 92, 508–566. [Google Scholar]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef]
- Sodek, J.; Brunette, D.M.; Feng, J.; Heersche, J.N.; Limeback, H.F.; Melcher, A.H.; Ng, B. Collagen synthesis is a major component of protein synthesis in the periodontal ligament in various species. Arch. Oral Biol. 1977, 22, 647–653. [Google Scholar] [CrossRef]
- Drozdzik, A.; Drozdzik, M. Drug-Induced Gingival Overgrowth-Molecular Aspects of Drug Actions. Int. J. Mol. Sci. 2023, 24, 5448. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- McHedlidze, T.; Waldner, M.; Zopf, S.; Walker, J.; Rankin, A.L.; Schuchmann, M.; Voehringer, D.; McKenzie, A.N.; Neurath, M.F.; Pflanz, S.; et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013, 39, 357–371. [Google Scholar] [CrossRef]
- Yuan, P.; Xue, H.; Zhou, L.; Qu, L.; Li, C.; Wang, Z.; Ni, J.; Yu, C.; Yao, T.; Huang, Y.; et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol. Dial. Transplant. 2011, 26, 2119–2126. [Google Scholar] [CrossRef]
- Buechler, M.B.; Pradhan, R.N.; Krishnamurty, A.T.; Cox, C.; Calviello, A.K.; Wang, A.W.; Yang, Y.A.; Tam, L.; Caothien, R.; Roose-Girma, M.; et al. Cross-tissue organization of the fibroblast lineage. Nature 2021, 593, 575–579. [Google Scholar] [CrossRef]
- Stefanovic, B.; Stefanovic, L.; Schnabl, B.; Bataller, R.; Brenner, D.A. TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis. Mol. Cell. Biol. 2004, 24, 1758–1768. [Google Scholar] [CrossRef]
- Janssen, L.J.; Farkas, L.; Rahman, T.; Kolb, M.R. ATP stimulates Ca2+-waves and gene expression in cultured human pulmonary fibroblasts. Int. J. Biochem. Cell Biol. 2009, 41, 2477–2484. [Google Scholar] [CrossRef]
- Bardou, O.; Menou, A.; Francois, C.; Duitman, J.W.; von der Thusen, J.H.; Borie, R.; Sales, K.U.; Mutze, K.; Castier, Y.; Sage, E.; et al. Membrane-anchored Serine Protease Matriptase Is a Trigger of Pulmonary Fibrogenesis. Am. J. Respir. Crit. Care Med. 2016, 193, 847–860. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Seltzer, J.L.; Welgus, H.G.; Jeffrey, J.J.; Eisen, A.Z. The function of Ca+ in the action of mammalian collagenases. Arch. Biochem. Biophys. 1976, 173, 355–361. [Google Scholar] [CrossRef]
- Unemori, E.N.; Werb, Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J. Biol. Chem. 1988, 263, 16252–16259. [Google Scholar] [CrossRef]
- Navarro-Requena, C.; Perez-Amodio, S.; Castano, O.; Engel, E. Wound healing-promoting effects stimulated by extracellular calcium and calcium-releasing nanoparticles on dermal fibroblasts. Nanotechnology 2018, 29, 395102. [Google Scholar] [CrossRef]
- Kohn, E.C.; Jacobs, W.; Kim, Y.S.; Alessandro, R.; Stetler-Stevenson, W.G.; Liotta, L.A. Calcium influx modulates expression of matrix metalloproteinase-2 (72-kDa type IV collagenase, gelatinase A). J. Biol. Chem. 1994, 269, 21505–21511. [Google Scholar] [CrossRef]
- Villalta, P.C.; Rocic, P.; Townsley, M.I. Role of MMP2 and MMP9 in TRPV4-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L652–L659. [Google Scholar] [CrossRef]
- Lee, H.; Overall, C.M.; McCulloch, C.A.; Sodek, J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol. Biol. Cell 2006, 17, 4812–4826. [Google Scholar] [CrossRef]
- Arora, P.D.; Manolson, M.F.; Downey, G.P.; Sodek, J.; McCulloch, C.A. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J. Biol. Chem. 2000, 275, 35432–35441. [Google Scholar] [CrossRef]
- Penumatsa, K.C.; Toksoz, D.; Warburton, R.R.; Kharnaf, M.; Preston, I.R.; Kapur, N.K.; Khosla, C.; Hill, N.S.; Fanburg, B.L. Transglutaminase 2 in pulmonary and cardiac tissue remodeling in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L752–L762. [Google Scholar] [CrossRef]
- Aumiller, V.; Strobel, B.; Romeike, M.; Schuler, M.; Stierstorfer, B.E.; Kreuz, S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci. Rep. 2017, 7, 149. [Google Scholar] [CrossRef]
- Hinz, B.; Mastrangelo, D.; Iselin, C.E.; Chaponnier, C.; Gabbiani, G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 2001, 159, 1009–1020. [Google Scholar] [CrossRef]
- Abbonante, V.; Di Buduo, C.A.; Gruppi, C.; De Maria, C.; Spedden, E.; De Acutis, A.; Staii, C.; Raspanti, M.; Vozzi, G.; Kaplan, D.L.; et al. A new path to platelet production through matrix sensing. Haematologica 2017, 102, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, G. 50 Years of Myofibroblasts: How the Myofibroblast Concept Evolved; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2299, pp. 1–5. [Google Scholar]
- Castella, L.F.; Buscemi, L.; Godbout, C.; Meister, J.J.; Hinz, B. A new lock-step mechanism of matrix remodelling based on subcellular contractile events. J. Cell Sci. 2010, 123 Pt 10, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Valenzi, E.; Bulik, M.; Tabib, T.; Morse, C.; Sembrat, J.; Trejo Bittar, H.; Rojas, M.; Lafyatis, R. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann. Rheum. Dis. 2019, 78, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; McCulloch, C.A. TRPV4 integrates matrix mechanosensing with Ca2+ signaling to regulate extracellular matrix remodeling. FEBS J. 2021, 288, 5867–5887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Q.; Zhang, Y.; Geng, L.; Wang, W.; Zhang, H.; He, X.; Li, Q. Multimodal roles of transient receptor potential channel activation in inducing pathological tissue scarification. Front. Immunol. 2023, 14, 1237992. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, R.; Dai, R.; Knoedler, S.; Tao, J.; Machens, H.G.; Rinkevich, Y. The Multifaceted Functions of TRPV4 and Calcium Oscillations in Tissue Repair. Int. J. Mol. Sci. 2024, 25, 1179. [Google Scholar] [CrossRef]
- Peng, S.; Poole, D.P.; Veldhuis, N.A. Mini-review: Dissecting receptor-mediated stimulation of TRPV4 in nociceptive and inflammatory pathways. Neurosci. Lett. 2022, 770, 136377. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.K.; Katari, V.; Teegala, L.R.; Thodeti, S.; Paruchuri, S.; Thodeti, C.K. TRPV4 Mechanotransduction in Fibrosis. Cells 2021, 10, 3053. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Kurahara, L.H.; Hiraishi, K. TRP channels in cardiac and intestinal fibrosis. Semin. Cell Dev. Biol. 2019, 94, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.H.; Clemons, N.J.; Miyashita, T.; Dupuy, A.J.; Zhang, W.; Szczepny, A.; Corcoran-Schwartz, I.M.; Wilburn, D.L.; Montgomery, E.A.; Wang, J.S.; et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology 2010, 138, 1810–1822. [Google Scholar] [CrossRef]
- Iwata, Y.; Ohtake, H.; Suzuki, O.; Matsuda, J.; Komamura, K.; Wakabayashi, S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc. Res. 2013, 99, 760–768. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef]
- Liedtke, W.; Choe, Y.; Marti-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Di Gregorio, M.; He, P.; McCulloch, C.A. TRPV4 mediates the Ca2+ influx required for the interaction between flightless-1 and non-muscle myosin, and collagen remodeling. J. Cell Sci. 2017, 130, 2196–2208. [Google Scholar]
- Goswami, C.; Kuhn, J.; Heppenstall, P.A.; Hucho, T. Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS ONE 2010, 5, e11654. [Google Scholar] [CrossRef]
- Fan, H.C.; Zhang, X.; McNaughton, P.A. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J. Biol. Chem. 2009, 284, 27884–27891. [Google Scholar] [CrossRef]
- Kwon, D.H.; Zhang, F.; McCray, B.A.; Feng, S.; Kumar, M.; Sullivan, J.M.; Im, W.; Sumner, C.J.; Lee, S.Y. TRPV4-Rho GTPase complex structures reveal mechanisms of gating and disease. Nat. Commun. 2023, 14, 3732. [Google Scholar] [CrossRef]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef]
- Matthews, B.D.; Thodeti, C.K.; Tytell, J.D.; Mammoto, A.; Overby, D.R.; Ingber, D.E. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr. Biol. 2010, 2, 435–442. [Google Scholar] [CrossRef]
- Adapala, R.K.; Thoppil, R.J.; Ghosh, K.; Cappelli, H.C.; Dudley, A.C.; Paruchuri, S.; Keshamouni, V.; Klagsbrun, M.; Meszaros, J.G.; Chilian, W.M.; et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 2016, 35, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Cohen, J.; Sharma, S.; Zhang, D.X.; Lafyatis, R.; Bhawan, J.; Rahaman, S.O. TRPV4 ION Channel Is Associated with Scleroderma. J. Investig. Dermatol. 2017, 137, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Grove, L.M.; Paruchuri, S.; Southern, B.D.; Abraham, S.; Niese, K.A.; Scheraga, R.G.; Ghosh, S.; Thodeti, C.K.; Zhang, D.X.; et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014, 124, 5225–5238. [Google Scholar] [CrossRef]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef]
- Taivanbat, B.; Yamazaki, S.; Nasanbat, B.; Uchiyama, A.; Amalia, S.N.; Nasan-Ochir, M.; Inoue, Y.; Ishikawa, M.; Kosaka, K.; Sekiguchi, A.; et al. Transient receptor potential vanilloid 4 promotes cutaneous wound healing by regulating keratinocytes and fibroblasts migration and collagen production in fibroblasts in a mouse model. J. Dermatol. Sci. 2023, 112, 54–62. [Google Scholar] [CrossRef]
- Goswami, R.; Arya, R.K.; Sharma, S.; Dutta, B.; Stamov, D.R.; Zhu, X.; Rahaman, S.O. Mechanosensing by TRPV4 mediates stiffness-induced foreign body response and giant cell formation. Sci. Signal. 2021, 14, eabd4077. [Google Scholar] [CrossRef] [PubMed]
- Cussac, L.A.; Cardouat, G.; Tiruchellvam Pillai, N.; Campagnac, M.; Robillard, P.; Montillaud, A.; Guibert, C.; Gailly, P.; Marthan, R.; Quignard, J.F.; et al. TRPV4 channel mediates adventitial fibroblast activation and adventitial remodeling in pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L135–L146. [Google Scholar] [CrossRef]
- Swain, S.M.; Romac, J.M.; Vigna, S.R.; Liddle, R.A. Piezo1-mediated stellate cell activation causes pressure-induced pancreatic fibrosis in mice. JCI Insight 2022, 7, e158288. [Google Scholar] [CrossRef] [PubMed]
- Cambria, E.; Heusser, S.; Scheuren, A.C.; Tam, W.K.; Karol, A.A.; Hitzl, W.; Leung, V.Y.; Muller, R.; Ferguson, S.J.; Wuertz-Kozak, K. TRPV4 mediates cell damage induced by hyperphysiological compression and regulates COX2/PGE2 in intervertebral discs. JOR Spine 2021, 4, e1149. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.M.; Burns, M.J.; Serjeant, M.E.; Seguin, C.A. The mechano-response of murine annulus fibrosus cells to cyclic tensile strain is frequency dependent. JOR Spine 2020, 3, e21114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, X.; Du, G.; Chen, W.; Zhang, Q. Substrate stiffness-dependent regulatory volume decrease and calcium signaling in chondrocytes. Acta Biochim. Biophys. Sin. 2022, 54, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goswami, R.; Rahaman, S.O. The TRPV4-TAZ mechanotransduction signaling axis in matrix stiffness- and TGFbeta1-induced epithelial-mesenchymal transition. Cell. Mol. Bioeng. 2019, 12, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Leddy, H.A.; Kaye, L.; Case, N.D.; Rothenberg, K.E.; Little, D.; Liedtke, W.; Hoffman, B.D.; Guilak, F. TRPV4-mediated calcium signaling in mesenchymal stem cells regulates aligned collagen matrix formation and vinculin tension. Proc. Natl. Acad. Sci. USA 2019, 116, 1992–1997. [Google Scholar] [CrossRef]
- Sainio, A.; Jarvelainen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell. Signal. 2020, 66, 109487. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y.; Wang, Q.; Wang, A.; Ali, A.; McCulloch, C.A. TRPV4 regulates beta1 integrin-mediated cell-matrix adhesions and collagen remodeling. FASEB J. 2023, 37, e22946. [Google Scholar] [CrossRef]
- Horton, E.R.; Byron, A.; Askari, J.A.; Ng, D.H.J.; Millon-Fremillon, A.; Robertson, J.; Koper, E.J.; Paul, N.R.; Warwood, S.; Knight, D.; et al. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 2015, 17, 1577–1587. [Google Scholar] [CrossRef]
- Wang, A.Y.; Coelho, N.M.; Arora, P.D.; Wang, Y.; Eymael, D.; Ji, C.; Wang, Q.; Lee, W.; Xu, J.; Kapus, A.; et al. DDR1 associates with TRPV4 in cell-matrix adhesions to enable calcium-regulated myosin activity and collagen compaction. J. Cell. Physiol. 2022, 237, 2451–2468. [Google Scholar] [CrossRef]
- Gonzalez-Molina, J.; Kirchhof, K.M.; Rathod, B.; Moyano-Galceran, L.; Calvo-Noriega, M.; Kokaraki, G.; Bjorkoy, A.; Ehnman, M.; Carlson, J.W.; Lehti, K. Mechanical Confinement and DDR1 Signaling Synergize to Regulate Collagen-Induced Apoptosis in Rhabdomyosarcoma Cells. Adv. Sci. 2022, 9, e2202552. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, K.V.; Armitage, G.C.; Shiboski, C.H. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J. Periodontol. 2008, 79, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Trackman, P.C.; Kantarci, A. Molecular and clinical aspects of drug-induced gingival overgrowth. J. Dent. Res. 2015, 94, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Uzel, M.I.; Kantarci, A.; Hong, H.H.; Uygur, C.; Sheff, M.C.; Firatli, E.; Trackman, P.C. Connective tissue growth factor in drug-induced gingival overgrowth. J. Periodontol. 2001, 72, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.D.; Silvestri, L.; Ganss, B.; Sodek, J.; McCulloch, C.A. Mechanism of cyclosporin-induced inhibition of intracellular collagen degradation. J. Biol. Chem. 2001, 276, 14100–14109. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Nilius, B.; Voets, T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013, 14, 152–163. [Google Scholar] [CrossRef]
- Cho, T.J.; Matsumoto, K.; Fano, V.; Dai, J.; Kim, O.H.; Chae, J.H.; Yoo, W.J.; Tanaka, Y.; Matsui, Y.; Takigami, I.; et al. TRPV4-pathy manifesting both skeletal dysplasia and peripheral neuropathy: A report of three patients. Am. J. Med. Genet. A 2012, 158A, 795–802. [Google Scholar] [CrossRef]
- Masuyama, R.; Mizuno, A.; Komori, H.; Kajiya, H.; Uekawa, A.; Kitaura, H.; Okabe, K.; Ohyama, K.; Komori, T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. 2012, 27, 1708–1721. [Google Scholar] [CrossRef]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lamande, S.R.; Yuan, Y.; Gresshoff, I.L.; Rowley, L.; Belluoccio, D.; Kaluarachchi, K.; Little, C.B.; Botzenhart, E.; Zerres, K.; Amor, D.J.; et al. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 2011, 43, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ji, C.; Smith, P.; McCulloch, C.A. Impact of TRP Channels on Extracellular Matrix Remodeling: Focus on TRPV4 and Collagen. Int. J. Mol. Sci. 2024, 25, 3566. https://doi.org/10.3390/ijms25073566
Wang Q, Ji C, Smith P, McCulloch CA. Impact of TRP Channels on Extracellular Matrix Remodeling: Focus on TRPV4 and Collagen. International Journal of Molecular Sciences. 2024; 25(7):3566. https://doi.org/10.3390/ijms25073566
Chicago/Turabian StyleWang, Qin, Chenfan Ji, Patricio Smith, and Christopher A. McCulloch. 2024. "Impact of TRP Channels on Extracellular Matrix Remodeling: Focus on TRPV4 and Collagen" International Journal of Molecular Sciences 25, no. 7: 3566. https://doi.org/10.3390/ijms25073566
APA StyleWang, Q., Ji, C., Smith, P., & McCulloch, C. A. (2024). Impact of TRP Channels on Extracellular Matrix Remodeling: Focus on TRPV4 and Collagen. International Journal of Molecular Sciences, 25(7), 3566. https://doi.org/10.3390/ijms25073566







