Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut
Abstract
1. Introduction
2. Results
3. Discussion
4. Methods
4.1. Participant Training and Sample Characteristics
4.2. Total RNA Sample/Library Preparation Sequencing
4.3. RNA-Seq Data Preprocessing and Microbial Bioinformatics Analysis
4.4. Intestinal Permeability Biomarkers Assayed Via ELISA
4.5. Data and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regasa, L.E.; Agimi, Y.; Stout, K.C. Traumatic brain injury following military deployment: Evaluation of diagnosis and cause of injury. J. Head Trauma Rehabil. 2019, 34, 21–29. [Google Scholar] [CrossRef]
- Belding, J.N.; Englert, R.M.; Fitzmaurice, S.; Jackson, J.R.; Koenig, H.G.; Hunter, M.A.; Thomsen, C.J.; da Silva, U.O. Potential health and performance effects of high-level and low-level blast: A scoping review of two decades of research. Front. Neurol. 2021, 12, 628782. [Google Scholar] [CrossRef]
- Owens, B.D.; Kragh Jr, J.F.; Wenke, J.C.; Macaitis, J.; Wade, C.E.; Holcomb, J.B. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J. Trauma Acute Care Surg. 2008, 64, 295–299. [Google Scholar] [CrossRef]
- Galarneau, M.R.; Woodruff, S.I.; Dye, J.L.; Mohrle, C.R.; Wade, A.L. Traumatic brain injury during operation Iraqi freedom: Findings from the United States Navy–marine corps combat trauma registry. J. Neurosurg. 2008, 108, 950–957. [Google Scholar] [CrossRef]
- Eierud, C.; Nathan, D.E.; Bonavia, G.H.; Ollinger, J.; Riedy, G. Cortical thinning in military blast compared to non-blast persistent mild traumatic brain injuries. Neuroimage Clin. 2019, 22, 101793. [Google Scholar] [CrossRef]
- Shively, S.B.; Horkayne-Szakaly, I.; Jones, R.V.; Kelly, J.P.; Armstrong, R.C.; Perl, D.P. Characterisation of interface astroglial scarring in the human brain after blast exposure: A post-mortem case series. Lancet Neurol. 2016, 15, 944–953. [Google Scholar] [CrossRef]
- Huber, B.R.; Meabon, J.S.; Martin, T.J.; Mourad, P.D.; Bennett, R.; Kraemer, B.C.; Cernak, I.; Petrie, E.C.; Emery, M.J.; Swenson, E.R. Blast exposure causes early and persistent aberrant phospho-and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimer’s Dis. 2013, 37, 309–323. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.-L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012, 4, 134ra160. [Google Scholar]
- Committee on Gulf War and Health. Long-Term Effects of Blast Exposures. In Board on the Health of Select Populations; National Academies Press (US): Washington, DC, USA, 2014; Volume 9. [Google Scholar]
- Bryden, D.W.; Tilghman, J.I.; Hinds, S.R. Blast-related traumatic brain injury: Current concepts and research considerations. J. Exp. Neurosci. 2019, 13, 1179069519872213. [Google Scholar] [CrossRef]
- Miller, M.R.; DiBattista, A.; Patel, M.A.; Daley, M.; Tenn, C.; Nakashima, A.; Rhind, S.G.; Vartanian, O.; Shiu, M.Y.; Caddy, N. A Distinct Metabolite Signature in Military Personnel Exposed to Repetitive Low-Level Blasts. Front. Neurol. 2022, 13, 831792. [Google Scholar]
- Junn, C.; Bell, K.R.; Shenouda, C.; Hoffman, J.M. Symptoms of concussion and comorbid disorders. Curr. Pain Headache Rep. 2015, 19, 46. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T. Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.S.; Grandhi, R.; Patterson, T.T.; Nicholson, S.E. A review of traumatic brain injury and the gut microbiome: Insights into novel mechanisms of secondary brain injury and promising targets for neuroprotection. Brain Sci. 2018, 8, 113. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Investig. 2021, 131, e143777. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar] [CrossRef]
- Tobias, P.S.; Soldau, K.; Ulevitch, R.J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J. Exp. Med. 1986, 164, 777–793. [Google Scholar] [CrossRef]
- Schumann, R.R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccharide binding protein. Science 1990, 249, 1429–1431. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.d.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef]
- Ryu, J.-K.; Kim, S.J.; Rah, S.-H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.-O.; Park, B.S.; Yoon, T.-Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Findley, M.K.; Koval, M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life 2009, 61, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Olde Loohuis, L.M.; Mangul, S.; Ori, A.P.; Jospin, G.; Koslicki, D.; Yang, H.T.; Wu, T.; Boks, M.P.; Lomen-Hoerth, C.; Wiedau-Pazos, M. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 2018, 8, 96. [Google Scholar] [CrossRef]
- Maheshwari, P.; Eslick, G.D. Bacterial infection and Alzheimer’s disease: A meta-analysis. J. Alzheimer’s Dis. 2015, 43, 957–966. [Google Scholar] [CrossRef]
- LaValle, C.; Carr, W.; Egnoto, M.; Misistia, A.; Salib, J.; Ramos, A.; Kamimori, G. Neurocognitive performance deficits related to immediate and acute blast overpressure exposure. Front. Neurol. 2019, 10, 949. [Google Scholar] [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with inflammatory bowel disease: A pilot study. Minerva Medica 2019, 110, 95–100. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Silva, C.; Greenfield, J.; Liu, W.-Q.; Metz, L.M.; Yong, V.W. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult. Scler. J. 2020, 26, 1340–1350. [Google Scholar] [CrossRef]
- Gokulakrishnan, K.; Nikhil, J.; Vs, S.; Holla, B.; Thirumoorthy, C.; Sandhya, N.; Nichenametla, S.; Pathak, H.; Shivakumar, V.; Debnath, M. Altered Intestinal Permeability Biomarkers in Schizophrenia: A Possible Link with Subclinical Inflammation. Ann. Neurosci. 2022, 29, 151–158. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.; Veerkamp, J. New insights into the structure and function of fatty acid-binding proteins. Cell. Mol. Life Sci. 2002, 59, 1096–1116. [Google Scholar] [CrossRef] [PubMed]
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): Function, structure and polymorphism. J. Appl. Genet. 2006, 47, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Thuijls, G.; Verdam, F.; Derikx, J.P.; Lenaerts, K.; Buurman, W.A. Non-invasive assessment of barrier integrity and function of the human gut. World J. Gastrointest. Surg. 2010, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Goswami, P.; Das, P.; Verma, A.K.; Prakash, S.; Das, T.; Nag, T.; Ahuja, V.; Gupta, S.D.; Makharia, G.K. Are alterations of tight junctions at molecular and ultrastructural level different in duodenal biopsies of patients with celiac disease and Crohn’s disease? Virchows Arch. 2014, 465, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; MacDonald, T.T.; Collins, J.E. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab. Investig. 2005, 85, 1139–1162. [Google Scholar] [CrossRef]
- Thuijls, G.; Derikx, J.P.; de Haan, J.-J.; Grootjans, J.; de Bruïne, A.; Masclee, A.A.; Heineman, E.; Buurman, W.A. Urine-based detection of intestinal tight junction loss. J. Clin. Gastroenterol. 2010, 44, e14–e19. [Google Scholar] [CrossRef]
- Lu, Z.; Ding, L.; Lu, Q.; Chen, Y.-H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue Barriers 2013, 1, e24978. [Google Scholar] [CrossRef]
- Zweigner, J.; Schumann, R.R.; Weber, J.R. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006, 8, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Latysheva, N.; Hindberg, K.; Ueland, T. Plasma lipopolysaccharide-binding protein is a biomarker for future venous thromboembolism: Results from discovery and validation studies. J. Intern. Med. 2022, 292, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.M.; Schürer-Maly, C.; Leturcq, D.; Moriarty, A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 1993, 90, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Masi, J.; Giménez, V.; Carpinelli, M.-M.; Laterza, O.; Hermoso, M.; Ortiz-Villalba, J.; Chamorro, M.-E.; Langjahr, P. Effect of gluten-free diet on levels of soluble CD14 and lipopolysaccharide-binding protein in adult patients with celiac disease. Cent. Eur. J. Immunol. 2021, 46, 225–230. [Google Scholar] [CrossRef]
- Myc, A.; Buck, J.; Gonin, J.; Reynolds, B.; Hammerling, U.; Emanuel, D. The level of lipopolysaccharide-binding protein is significantly increased in plasma in patients with the systemic inflammatory response syndrome. Clin. Diagn. Lab. Immunol. 1997, 4, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alonso, M.; Campos, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: The role of obesity. PLoS ONE 2013, 8, e54600. [Google Scholar] [CrossRef]
- Wang, Z.; Wilson, C.M.; Mendelev, N.; Ge, Y.; Galfalvy, H.; Elder, G.; Ahlers, S.; Yarnell, A.M.; LoPresti, M.L.; Kamimori, G.H. Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. J. Neurotrauma 2019, 37, 1221–1232. [Google Scholar] [CrossRef]
- Gill, J.; Motamedi, V.; Osier, N.; Dell, K.; Arcurio, L.; Carr, W.; Walker, P.; Ahlers, S.; LoPresti, M.; Yarnell, A. Moderate blast exposure results in increased IL-6 and TNFα in peripheral blood. Brain Behav. Immun. 2017, 65, 90–94. [Google Scholar] [CrossRef]
- Edwards, K.A.; Leete, J.J.; Smith, E.G.; Quick, A.; Modica, C.M.; Wassermann, E.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.; Walker, P. Elevations in tumor necrosis factor alpha and interleukin 6 from neuronal-derived extracellular vesicles in repeated low-level blast exposed personnel. Front. Neurol. 2022, 13, 723923. [Google Scholar] [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur. Neuropsychopharmacol. 2022, 56, 24–38. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation. Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.; Stovner, L.; Hagen, K.; Zwart, J. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia 2008, 28, 144–151. [Google Scholar] [PubMed]
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiol. Pain 2022, 11, 100090. [Google Scholar] [CrossRef] [PubMed]
- Sgro, M.; Ray, J.; Foster, E.; Mychasiuk, R. Making Migraine Easier to Stomach: The Role of the Gut Brain Immune Axis in Headache Disorders. Eur. J. Neurol. 2023, 30, 3605–3621. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Barber, J.; Andre, J.; Evans, N.; Panks, C.; Sun, S.; Zalewski, K.; Sanders, R.E.; Temkin, N. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clin. 2017, 14, 371–378. [Google Scholar] [CrossRef]
- Mac Donald, C.L.; Barber, J.; Patterson, J.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Association between 5-year clinical outcome in patients with nonmedically evacuated mild blast traumatic brain injury and clinical measures collected within 7 days postinjury in combat. JAMA Netw. Open 2019, 2, e186676. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Lucas, S.; Dikmen, S.; Braden, C.A.; Brown, A.W.; Brunner, R.; Diaz-Arrastia, R.; Walker, W.C.; Watanabe, T.K.; Bell, K.R. Natural history of headache after traumatic brain injury. J. Neurotrauma 2011, 28, 1719–1725. [Google Scholar] [CrossRef]
- Walker, W.C.; Marwitz, J.H.; Wilk, A.R.; Ketchum, J.M.; Hoffman, J.M.; Brown, A.W.; Lucas, S. Prediction of headache severity (density and functional impact) after traumatic brain injury: A longitudinal multicenter study. Cephalalgia 2013, 33, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Stacey, A.; Lucas, S.; Dikmen, S.; Temkin, N.; Bell, K.R.; Brown, A.; Brunner, R.; Diaz-Arrastia, R.; Watanabe, T.K.; Weintraub, A. Natural history of headache five years after traumatic brain injury. J. Neurotrauma 2017, 34, 1558–1564. [Google Scholar] [CrossRef]
- Mac Donald, C.L.; Barber, J.; Jordan, M.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. 2017, 74, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Carr, W.; Dell, K.; Yanagi, M.; Hassan, D.; LoPresti, M. Perspectives on repeated low-level blast and the measurement of neurotrauma in humans as an occupational exposure risk. Shock Waves 2017, 27, 829–836. [Google Scholar] [CrossRef]
- Carr, W.; Polejaeva, E.; Grome, A.; Crandall, B.; LaValle, C.; Eonta, S.E.; Young, L.A. Relation of repeated low-level blast exposure with symptomology similar to concussion. J. Head Trauma Rehabil. 2015, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, O.; Rhind, S.G.; Nakashima, A.; Tenn, C.; Lam, T.K.; Shiu, M.; Caddy, N.; King, K.; Natale, A.; Jetly, R. Blast effects on post-concussive and mental health outcomes: Data from Canadian Armed Forces breachers and snipers. J. Mil. Veteran Fam. Health 2022, 8, 82–96. [Google Scholar] [CrossRef]
- Brenner, L.A.; Forster, J.E.; Stearns-Yoder, K.A.; Stamper, C.E.; Hoisington, A.J.; Brostow, D.P.; Mealer, M.; Wortzel, H.S.; Postolache, T.T.; Lowry, C.A. Evaluation of an immunomodulatory probiotic intervention for veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: A pilot study. Front. Neurol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Kalmar, K. Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. J. Head Trauma Rehabil. 1995, 10, 1–17. [Google Scholar] [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Lab, H. FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 20 March 2024).
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Weber, N.S.; Gressitt, K.L.; Cowan, D.N.; Niebuhr, D.W.; Yolken, R.H.; Severance, E.G. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr. Res. 2018, 197, 465–469. [Google Scholar] [CrossRef]
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.-Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front. Immunol. 2021, 12, 686240. [Google Scholar] [CrossRef] [PubMed]
- McKenna, Z.; Houck, J.; Ducharme, J.; Li, Z.; Berkemeier, Q.; Fennel, Z.; Wells, A.; Mermier, C.; Deyhle, M.; Laitano, O. The effect of prolonged interval and continuous exercise in the heat on circulatory markers of intestinal barrier integrity. Eur. J. Appl. Physiol. 2022, 122, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, L.; Crane, A.; Heyne, H.; Tönjes, A.; Schleinitz, D.; Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front. Endocrinol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Xu, J.; Tanaka, H.; Shoyama, Y. One-step immunochromatographic separation and ELISA quantification of glycyrrhizin from traditional Chinese medicines. J. Chromatogr. B 2007, 850, 53–58. [Google Scholar] [CrossRef]
- Altamura, S.; Kopf, S.; Schmidt, J.; Müdder, K.; da Silva, A.R.; Nawroth, P.; Muckenthaler, M.U. Uncoupled iron homeostasis in type 2 diabetes mellitus. J. Mol. Med. 2017, 95, 1387–1398. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project (accessed on 20 March 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
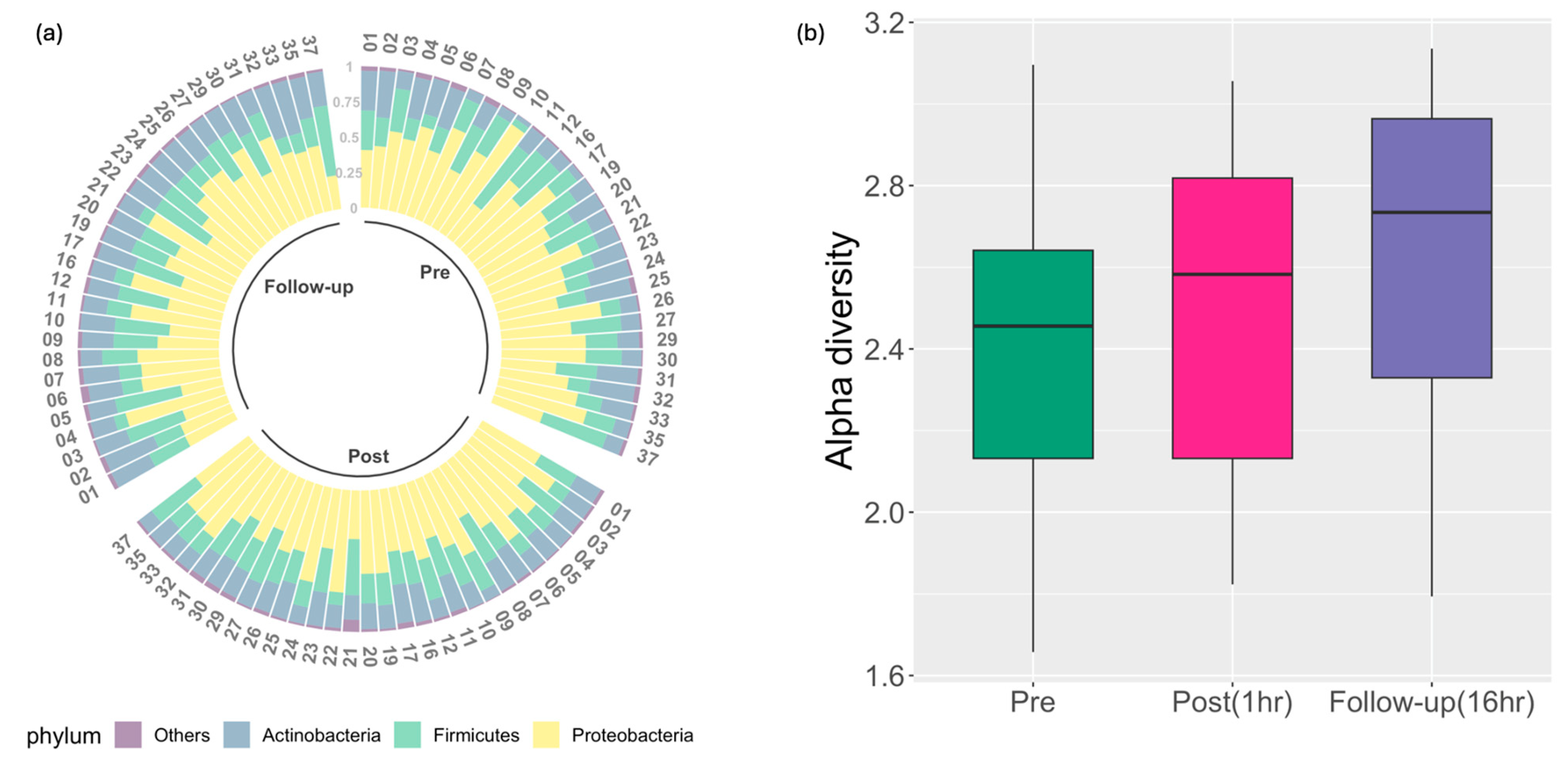
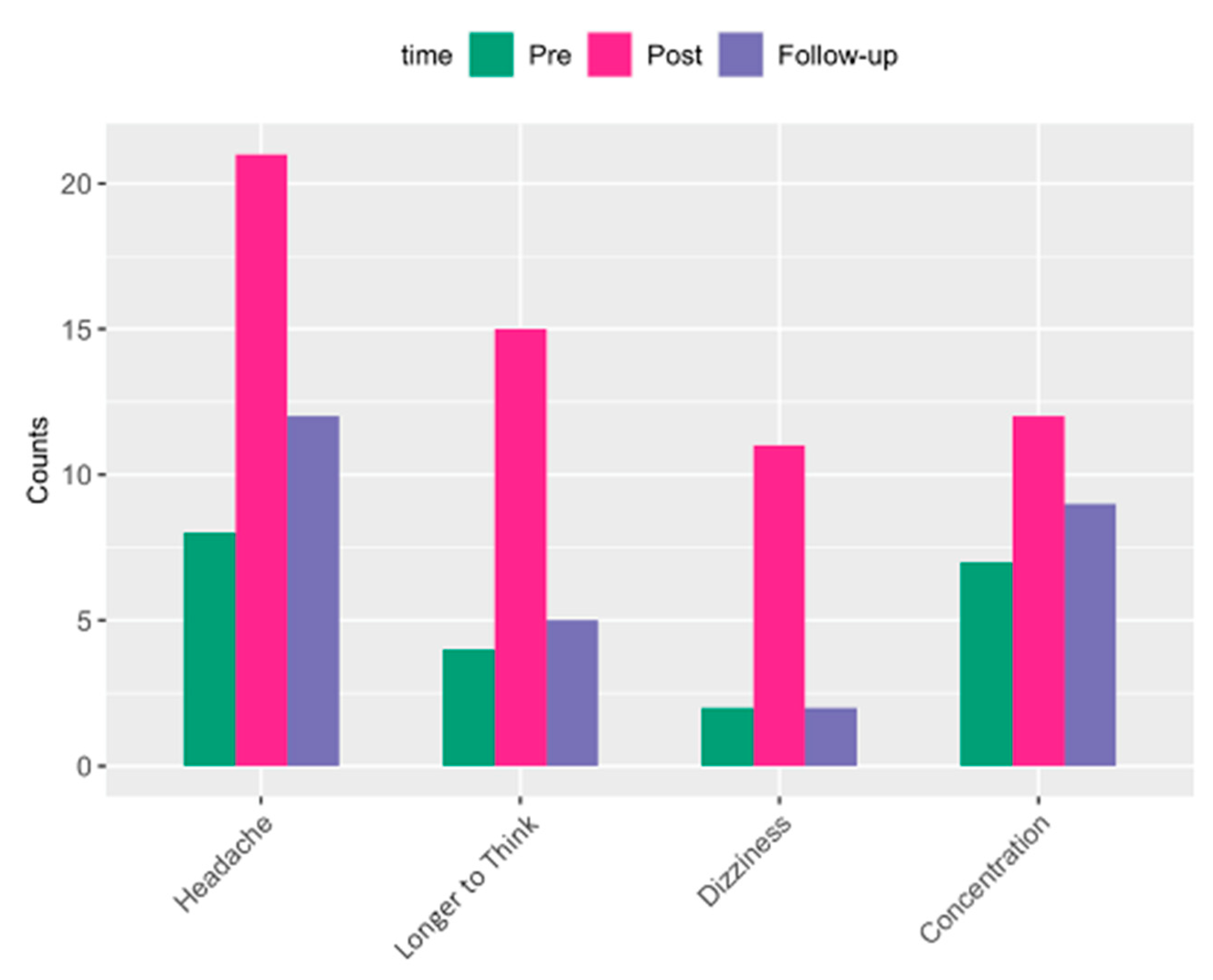

| Age in Years, Mean (SD) | 30 (6.25) |
|---|---|
| Duration of Service in years, mean (SD) | 8.90 (6.25) |
| MOS, No. (%) | |
| 12B/Combat Engineer | 25 (83%) |
| 31B/Military Police | 3 (10%) |
| 12H/Construction Engineer Supervisor | 1 (3%) |
| 19D/Calvary Scout 11B/Infantryman | 1 (3%) |
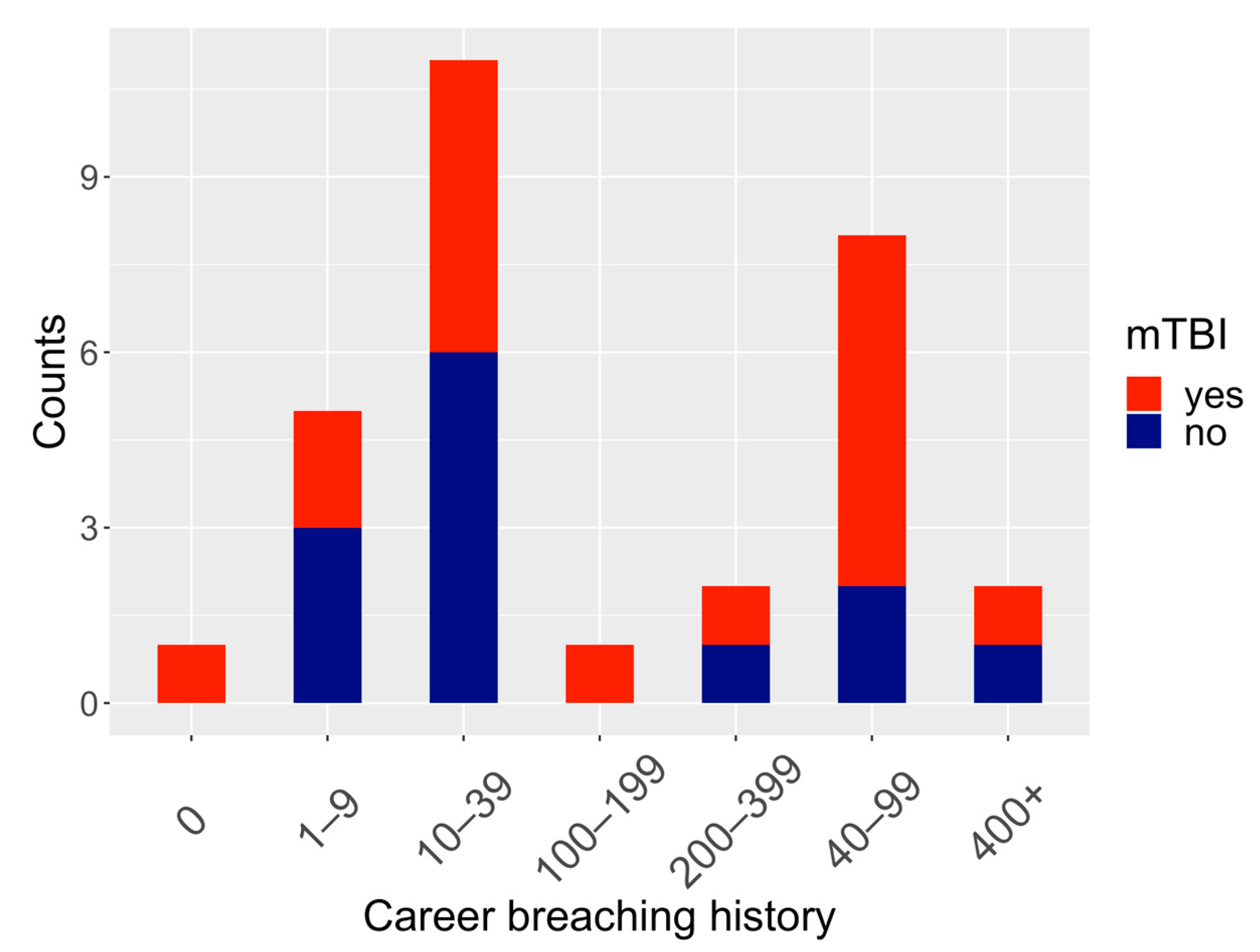
| History of mTBI, No. (%) | |
| Yes | 18 (60%) |
| No | 12 (40%) |
| Career breaching history, No. (%) | |
| 0 | 1 (3%) |
| 1–9 | 5 (17%) |
| 10–39 | 12 (40%) |
| 40–99 | 7 (23%) |
| 100–199 | 1 (3%) |
| 200–399 | 2 (7%) |
| 400+ | 2 (7%) |
| Time of last blast, No. (%) | |
| Past week | 29 (97%) |
| More than a year ago | 1 (3%) |
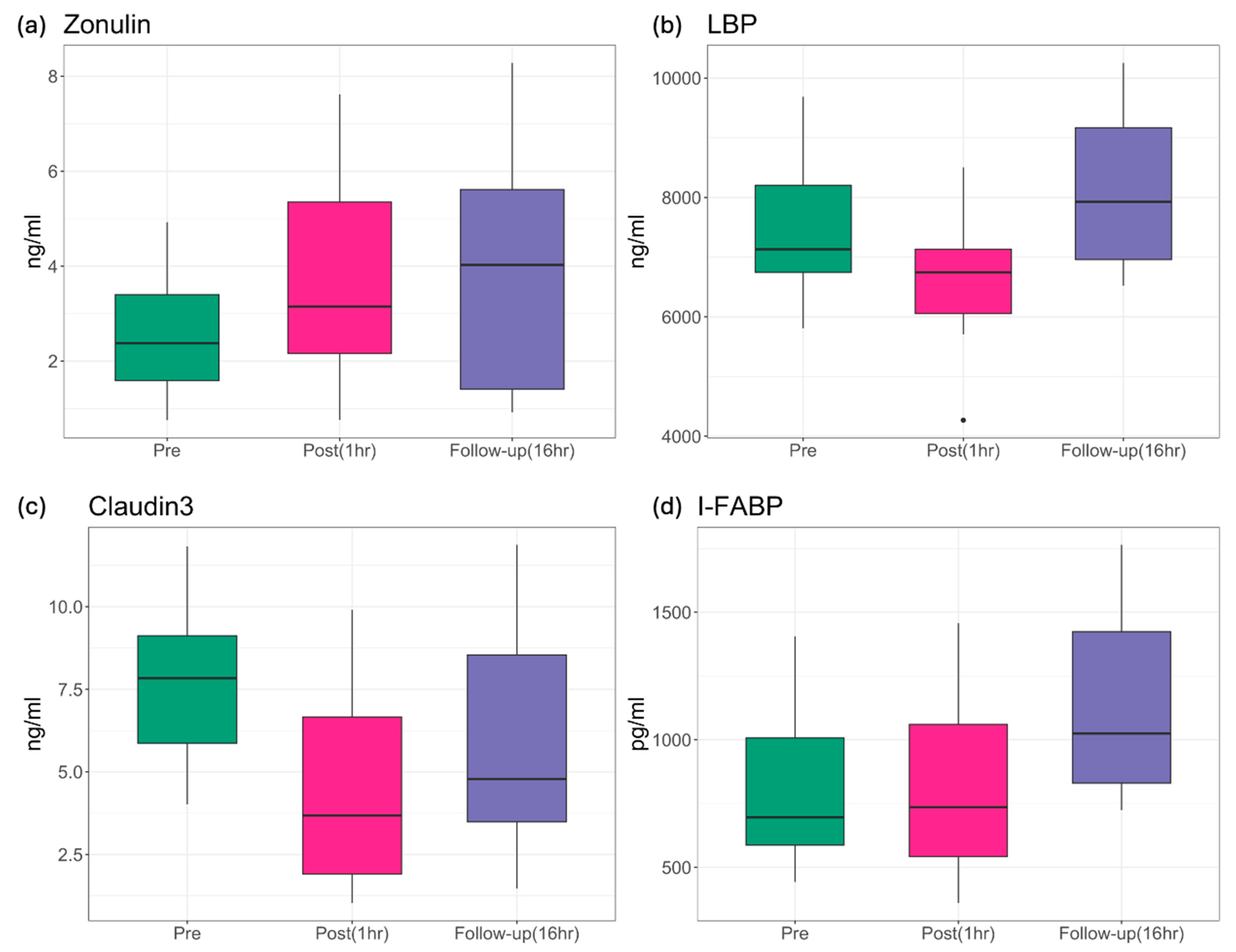
| p-Value | Zonulin | LBP | Claudin-3 | I-FABP |
|---|---|---|---|---|
| ANOVA | 0.0018 | 0.0002 | 6.24 × 10−5 | 8.34 × 10−5 |
| pre vs. post | 0.0029 | 0.0222 | 3.8 × 10−5 | 0.7870 |
| pre vs. follow-up | 0.0029 | 0.0513 | 0.0115 | 0.0003 |
| post vs. follow-up | 0.9680 | 0.0001 | 0.0412 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, Z.; Sun, S.; Nemes, J.; Brenner, L.A.; Hoisington, A.; Skotak, M.; LaValle, C.R.; Ge, Y.; Carr, W.; et al. Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut. Int. J. Mol. Sci. 2024, 25, 3549. https://doi.org/10.3390/ijms25063549
Liu Q, Wang Z, Sun S, Nemes J, Brenner LA, Hoisington A, Skotak M, LaValle CR, Ge Y, Carr W, et al. Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut. International Journal of Molecular Sciences. 2024; 25(6):3549. https://doi.org/10.3390/ijms25063549
Chicago/Turabian StyleLiu, Qingkun, Zhaoyu Wang, Shengnan Sun, Jeffrey Nemes, Lisa A. Brenner, Andrew Hoisington, Maciej Skotak, Christina R. LaValle, Yongchao Ge, Walter Carr, and et al. 2024. "Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut" International Journal of Molecular Sciences 25, no. 6: 3549. https://doi.org/10.3390/ijms25063549
APA StyleLiu, Q., Wang, Z., Sun, S., Nemes, J., Brenner, L. A., Hoisington, A., Skotak, M., LaValle, C. R., Ge, Y., Carr, W., & Haghighi, F. (2024). Association of Blast Exposure in Military Breaching with Intestinal Permeability Blood Biomarkers Associated with Leaky Gut. International Journal of Molecular Sciences, 25(6), 3549. https://doi.org/10.3390/ijms25063549






