Abstract
Zoonoses are diseases and infections naturally transmitted between humans and vertebrate animals. They form the dominant group of diseases among emerging infectious diseases and represent critical threats to global health security. This dilemma is largely attributed to our insufficient knowledge of the pathogenesis regarding zoonotic spillover. Long non-coding RNAs (lncRNAs) are transcripts with limited coding capacity. Recent technological advancements have enabled the identification of numerous lncRNAs in humans, animals, and even pathogens. An increasing body of literature suggests that lncRNAs function as key regulators in zoonotic infection. They regulate immune-related epigenetic, transcriptional, and post-transcriptional events across a broad range of organisms. In this review, we discuss the recent research progress on the roles of lncRNAs in zoonoses. We address the classification and regulatory mechanisms of lncRNAs in the interaction between host and zoonotic pathogens. Additionally, we explore the surprising function of pathogen-derived lncRNAs in mediating the pathogenicity and life cycle of zoonotic bacteria, viruses, and parasites. Understanding how these lncRNAs influence the zoonotic pathogenesis will provide important therapeutic insights to the prevention and control of zoonoses.
1. Introduction
Zoonoses constitute a class of diseases naturally transmitted between humans and vertebrate animals [1]. They are typically classified based on their epidemiological characteristics into three categories: endemic zoonotic diseases, prevalent in numerous regions and impacting both human and animal health (e.g., brucellosis, rabies); epidemic zoonoses, which occur sporadically over time and space (e.g., H1N1 influenza); and emerging or re-emerging zoonotic diseases (e.g., Ebola hemorrhagic fever, Nipah virus encephalitis, severe acute respiratory syndrome) [2], the latter two of which pose a greater threat due to recent outbreaks. Given the inseparable connection among humans, animals, and their respective environments, it is inevitable for pathogens to spread between humans and animals. Approximately 60% of known infectious diseases and up to 75% of emerging infectious diseases are zoonotic in origin [3,4], making them a significant global public health concern with serious implications for human health and socio-economic well-being. The spillover of zoonotic pathogens is determined by a series of processes, and the probability of spillover is determined by the interactions among the barriers and the associated bottlenecks that might prevent cross-species transmission. These include: (1) pathogen dynamics in reservoir hosts, (2) pathogen release from reservoir hosts, (3) pathogen survival or dispersal outside of reservoir hosts, (4) exposure of the recipient host to the pathogen, (5) structural and physical barriers within the recipient host, (6) innate immune response and molecular compatibility, and (7) the replication and life cycle of the pathogen within the recipient host [5,6]. Failure to overcome any of these obstacles prevents pathogen spillover. Despite an extensive literature on emerging zoonotic diseases, there remains a gap of systematic understanding of the mechanisms underlying cross-species transmission of pathogens, especially the molecular mechanisms behind the pathogen’s ability to overcome the last three critical barriers. Therefore, comprehending how pathogens breach host barriers, evade the host immune system, and replicate during the process, along with identifying key regulatory molecules, is crucial for elucidating the cross-species transmission of zoonotic diseases. This knowledge is vital for developing preventive and control strategies for these zoonoses.
The advancement of high-throughput sequencing technologies has illuminated the extensive presence of non-coding RNAs (ncRNAs) in the transcriptomes of humans, animals and even pathogens. In humans, for instance, less than 3% of transcripts are responsible for encoding proteins [7], indicating that the majority of the transcriptional output of the human genome is non-coding. Among these ncRNA entities, a significant type is the long non-coding RNAs (lncRNA), which are characterized by transcripts exceeding 200 nucleotides in length and generally do not encode proteins [8]. Initially dismissed as “transcriptional noise”, subsequent research has established lncRNAs as crucial regulatory elements in numerous biological processes [9]. To date, over 90,000 long non-coding transcripts have been annotated in humans alone, with this figure undergoing continuous refinement [10,11]. These functional lncRNAs play key roles in uncovering the complexities of biological systems, and understanding the mechanisms of action of functional lncRNAs contributes to a deeper understanding of the hidden secrets within specific biological processes. According to the rules established by lncipedia, lncRNAs in mammals can be classified into five categories based on their genomic proximity to protein-coding genes: (1) sense overlapping lncRNA (also termed intragenic lncRNA), transcribed from the sense strand with complete or partial overlapping with coding genes; (2) antisense lncRNA, transcribed from the antisense strand of coding genes; (3) intronic lncRNA, transcribed entirely from introns of coding genes; (4) bidirectional lncRNA, transcribed from a promoter of a coding gene, yet in the opposite direction; and (5) intergenic lncRNA, transcribed from the intergenic regions between protein-coding genes [10,12,13]. The regulatory mechanisms of these lncRNAs are diverse, with most being closely associated with their subcellular localization [14,15]. Typically, in the nucleus, lncRNAs are involved in epigenetic and transcriptional regulation [16,17], including chromatin modifications, and transcriptional modulation by recruiting, binding or antagonizing transcription factors [18,19]. Conversely, in the cytoplasm, lncRNAs are primarily influenced by post-transcriptional events, including maintaining the stability of mRNA, sponging microRNAs to influence gene silencing, and regulating the integrity and activity of protein complexes [20,21]. Additionally, certain cytoplasmic lncRNAs can indirectly impact transcription by interacting with transcription factors [22]. Besides conventional regulatory mechanisms, recent studies have identified open reading frames (ORFs) within some cytoplasmic lncRNAs, suggesting they can encode functional peptides, and perform significant roles in various pathological processes [23,24,25]. Studies have indicated that lncRNAs are present not only in nonspecific barriers, such as endothelial cells and epithelial cells, but also in immune cells, such as macrophages, T cells, monocytes, neutrophils, dendritic cells, and B cells [26,27,28]. Some lncRNAs exhibit significant differential expression in response to pathogen infection [29,30], highlighting their potential association with infectious diseases, particularly zoonoses. The objective of this review is to provide a comprehensive overview of the roles played by lncRNAs in zoonotic diseases, a group of infectious diseases of considerable global importance, and to offer insights into emerging connections between lncRNAs and the transmission of zoonotic pathogens, thereby providing a new perspective for exploring preventive and therapeutic interventions against zoonotic diseases.
2. LncRNAs in Bacterial Zoonoses
Statistics reveal that bacterial infections represent the largest proportion of zoonotic diseases. Taking bovine-origin zoonotic pathogens as an example, bacterial agents account for 42%, compared to 22% viral, 29% parasitic, 7% fungal, and others [31]. Bacteria in this context encompasses both Gram-negative and Gram-positive bacteria, and their transmission routes include foodborne/fecal-oral transmission, occupational exposure, transmission through animal bites/scratches, transmission through contaminated environments, and vector-borne transmission [32]. The widespread adoption of antibiotics has been effective in controlling bacterial diseases for an extended period, minimizing the occurrence of public health crises. However, the increasing prevalence of antibiotic-resistant strains, especially multidrug-resistant organisms, in recent years [33] highlights the underestimated harm of bacterial zoonotic diseases. Therefore, research of the underlying transmission and pathogenesis mechanisms is urgently required. There is compelling evidence that lncRNAs exhibit significant differential expression in response to bacterial infections. Pathogens such as Mycobacterium tuberculosis, Escherichia coli, Brucella, Salmonella enterica, Pseudomonas aeruginosa, Listeria monocytogenes, and Staphylococcus aureus have been shown to induce changes in lncRNA expression, suggesting their vital regulatory roles in bacterial zoonoses (Table 1) [29]. These findings indicate the potential of lncRNAs as targets for understanding and managing zoonotic bacterial infections.
2.1. Tuberculosis
Tuberculosis, caused by the facultative intracellular bacterium Mycobacterium tuberculosis (Mtb), triggers a cellular immune response, and is predominantly mediated by CD4+ T cells, which in turn activate macrophage effector functions. Mtb is adept at persisting and multiplying within macrophages, leading to severe infections in hosts [34]. Moreover, CD8+ T cells are also recognized as critical defenders against Mtb, contributing to the host’s immune defense [35]. Studies have shed light on the significant roles of lncRNAs in regulating host responses to Mtb infection and facilitating the pathogen’s intracellular survival. Notably, lncRNAs such as lncRNA-CD244, nuclear paraspeckle assembly transcript 1 (NEAT1), XLOC_012582, PCED1B-AS1, MIR3954HG, lincRNA-EPS, lincRNA-Cox2 and lnc-EST12 are implicated in the processes of Mtb invasion and the initiation of immune responses [29,36,37,38,39,40]. Mechanically, for example, a lncRNA-CD244 induced by CD244 during tuberculosis infection recruits enhancer of zeste homolog 2 (EZH2), an inducer of H3K27 methylation, to the infg/tnfa promoter, promoting H3K27 trimethylation, suppressing the expression of IFN-γ/TNF-α in CD8+ T cells, and exacerbating the infection [36]. Furthermore, lncRNAs, such as differentiation antagonizing non-protein coding RNA (DANCR), MIR99AHG, X inactive specific transcript (XIST), and myocardial infarction associated transcript (MIAT), have been identified as facilitators of Mtb intracellular survival, among which MIR99AHG is seen to promote Mtb intracellular persistence within macrophages by interacting with hnRNPA2/B1 and regulating host inflammatory response [41,42,43,44]. Other lncRNAs, such as LINC00870, colon cancer associated transcript 1 (CCAT1), and LOC152742, are emerging as potential novel biomarkers for the diagnosis of tuberculosis [45,46]. Their differential expression in response to Mtb highlights their potential utility in improving diagnostic accuracy and contributing to improved understanding of the disease’s pathogenesis.
2.2. Colibacillosis
Escherichia coli (E. coli), among the most prevalent bacteria in nature, poses an urgent threat to human and animal health, with infections affecting the intestines, urinary tract, blood, and brain. In intestinal infections, research has highlighted that Shiga toxin-producing E. coli infections result in the differential expression of 702 lncRNAs within human intestinal epithelial cells [47,48]. Specifically, the F18 E. coli strain, known for causing intestinal infections and diarrhea, is influenced by lncRNA FUT3-AS1. FUT3-AS1 regulates the expression of FUT3 through H4K16ac modification or the miR-212/FUT3 pathway, and FUT3 in turn controls the invasion of E. coli into intestinal epithelial cells, ultimately leading to an enhancement of E. coli infection in the host. [49]. In extraintestinal infections, such as those affecting the brain, significant alterations in lncRNA expression patterns have been observed. Astrocytes and human brain microvascular endothelial cells show differential transcription of 74 and 289 lncRNAs, respectively, during E. coli infections [50,51]. Among these, lncRSPH9-4 functions as a regulatory sponge, maintaining blood–brain barrier integrity by competitively interacting with miR-17-5p and matrix metallopeptidase 3 (MMP3) [52]; while lncC11orf54-1 and DDIT-AS1 mediate central nervous system inflammatory responses by interacting with interleukin 1 receptor associated kinase 1 (IRAK1) and DNA damage inducible transcript 4 (DDIT4) mRNA, respectively [51,53]. In mammary infections, XIST plays a protective role against damage from excessive inflammatory responses via the NF-κB/NLRP3 inflammasome pathway [54]. Additionally, lipopolysaccharide (LPS), the major virulence factor of E. coli, is also reported to alter lncRNA expression patterns, and detailed studies suggest that the best characterized lncRNAs, including HOX transcript antisense RNA (HOTAIR), SOX2 overlapping transcript (SOX2OT) and metastasis associated lung adenocarcinoma transcript 1 (MALAT1), are considered the essential regulator of LPS-related inflammation, as they all work as endogenous miRNA sponge and eventually affect the level of effector molecules [55,56,57]. This underscores the essential regulatory roles of lncRNAs in both intestinal and extraintestinal E. coli infections, as well as in the broader inflammatory response.
2.3. Brucellosis
Brucella, a prevalent zoonotic pathogen in veterinary medicine, also poses occasional but significant risks to humans. Similar to Mtb, Brucella is also a facultative intracellular bacterium capable of causing systemic infections in the host, leading to a range of symptoms, including undulant fever, endocarditis, arthritis, osteomyelitis, and reproductive disorders [58]. Evidence suggests that the pathogenesis of Brucella is attributed to the bacterial surviving intracellularly within both the phagocytic and non-phagocytic cells of its hosts [59]. Macrophages, which serve as the primary target cells for Brucella, are infected with the Brucella in studies involving RAW264.7 cells. Subsequent analyses reveal that 8, 6, 130, and 94 lncRNAs are differentially expressed at 4, 8, 24 and 48 h post-infection, respectively. Among them, lnc_000428 promotes the intracellular replication of Brucella within macrophages, leading to stealthy and sustained spread [60]. Conversely, the infection leads to a decreased expression of another lncRNA, Gm28309, which activates inflammatory pathways and ultimately enhances bacterial clearance in macrophages. This regulatory process is initiated by activating NF-κB signaling through the modulation of the Gm28309/miR-3068-5p/κB-Ras2 axis [61]. Additionally, studies have identified potential biomarkers for Brucella infection, including linc-MAF-4, IFNG-AS1, and others [62,63].
2.4. Salmonellosis
Salmonella is recognized as one of the major food-borne zoonotic pathogens, with Salmonella typhimurium (S. typhimurium) being the most common cause of human infections. With increasing demands for food consumption, concentrated farming of livestock and poultry, and the rise of antibiotic-resistant strains, the incidence of S. typhimurium infections in humans is steadily increasing. S. typhimurium is an enteroinvasive pathogen causing gastrointestinal symptoms such as diarrhea and vomiting; in severe cases, it can escalate to systemic infections via the lymphatic and bloodstream, affecting multiple organs [64]. NEAT1, a lncRNA, emerges as a biomarker for S. typhimurium infection, and is significantly upregulated during infection, serving as a differential marker from other Salmonella strains or heat-inactivated S. typhimurium [65]. Another lncRNA, LNCGM1082, induced by S. typhimurium in macrophages serves as a molecular scaffold, mediating the binding of protein kinase Cδ with the inflammasome NLRC4 to induce the phosphorylation and activation of NLRC4, thereby promoting the host immune defense against infection [66]. The T cell-derived enhancer-like lncRNA termed NeST (also known as IFNG-AS1) can alter the host susceptibility to S. typhimurium by regulating the epigenetic marking of IFNγ-encoding chromatin, affecting the expression of related genes. Hosts deficient in IFNG-AS1 exhibit increased vulnerability to fatal infection with Salmonella enteritidis, highlighting the crucial role of IFNG-AS1 in the host defense against Salmonella infections [67]. In addition to its gastrointestinal impact, S. typhimurium can also affect the central nervous system, with studies, like those by Zou et al., indicating that lncRNA TVX1 can mitigate S. typhimurium-induced microglial inflammation [68].
2.5. Pseudomonas aeruginosa Infection
Pseudomonas aeruginosa (P. aeruginosa), an opportunistic pathogen, is notorious for causing severe infections in immunocompromised individuals, particularly leading to hospital-acquired pneumonia and respiratory failure [69]. The escalating resistance of P. aeruginosa to diverse antibiotics serves to underscore its deleterious impact. Recent studies have illuminated the role of lncRNAs in the host response to P. aeruginosa infection, revealing intricate mechanisms by which these pathogens evade immune defenses and proliferate within the host. Infection with P. aeruginosa has been shown to suppress the expression of lncRNAs maternally expressed 9 (MEG9) and bladder cancer-associated transcript 1 (BLACAT1) in bronchial epithelial cells [70]. Additionally, maternally expressed 3 (MEG3), another lncRNA, is downregulated in P. aeruginosa-infected lungs through a TLR4/NF-κB-dependent pathway. MEG3 normally acts by competitively binding to miR-138 alongside IL-1β mRNA. The suppression of MEG3 leads to decreased levels of IL-1β, disturbing the immune balance during infection and affecting the proliferation of P. aeruginosa within the host [71]. Moreover, a small bacterial signaling molecule, termed N-3-(oxododecanoyl)-L-homoserine lactone (3-O-C12-HSL), which is secreted by P. aeruginosa, has been observed to enhance the expression of the lncRNA negative regulator of interferon response (NRIR). This, in turn, hinders the maturation of monocyte-derived dendritic cells and suppresses host immune responses [72].
2.6. Listeriosis
Listeria monocytogenes (L. monocytogenes) stands out as the only species of Listeria that is pathogenic to both humans and other vertebrates [73], and this environmental stress-tolerant pathogen is associated with serious public health and economic implications. It ranks among the most lethal foodborne pathogens, causing severe infections in immunocompromised individuals, such as septicemia, miscarriage, meningitis, and encephalitis [74,75]; in healthy individuals, meanwhile, it establishes latent infections, causing gastroenteritis [76]. Being a facultative intracellular pathogen, its pathogenicity is evident in its ability to survive and proliferate within host cells post-phagocytosis, as well as its immune-evasion capacity against cellular immune responses [77,78]. Specifically, in macrophages and dendritic cells, a lncRNA termed lincRNA-EPS binds to chromatin and interacts with heterogeneous nuclear ribonucleoprotein L (hnRNPL), a member of a large family of heterogeneous ribonucleoproteins, to alter nucleosome positioning and repress the transcription of immune-related genes (IRGs). The substantial reduction of lincRNA-EPS during L. monocytogenes infection leads to an enhanced inflammation, oxidative stress and lethality in hosts, highlighting its pivotal function in bolstering host defense responses against infections [79,80]. Similarly, lincRNA-Cox2 is also characterized as regulating the inflammatory response and the macrophages’ function [81]. Furthermore, L. monocytogenes induces the production of lncRNA AS-IL-1α, which recruits RNAPII to the IL-1α promoter, thus resulting in heightened host inflammatory levels [82]. Additionally, L. monocytogenes-induced miR-1 targets non-coding RNA suppressor of Stat1 (Sros1) for degradation, relieving the inhibitory effect of Sros1 on CAPRIN1/STAT1/IFN-γ axis and facilitating the bacterial clearance by the host [83]. Intriguingly, L. monocytogenes itself harbors lncRNAs, including a series of long antisense non-coding RNAs (lasRNAs), such as las0333, las0936, las0996, las1136, and las2677, which potentially affect the bacterium’s intracellular survival within eukaryotic hosts [84]. This highlights the complexity of lncRNA-mediated regulatory mechanisms in Listeriosis.
2.7. Staphylococcosis
In veterinary clinical settings, the Staphylococcus genus, characterized by its grape-like cluster appearance, comprises a diverse array of opportunistic pathogenic bacteria. Among these, the coagulase-positive staphylococci constitute the most pathogenic species Staphylococcus aureus (S. aureus). This historically emerging zoonotic pathogen poses substantial public health and veterinary challenges [85]. A critical concern with S. aureus is its antibiotic resistance—this is especially noted in methicillin-resistant S. aureus (MRSA), which is associated with a variety of severe infections ranging from food poisoning to more severe conditions, like endocarditis, pneumonia, otitis media, osteomyelitis, and skin or soft tissue infections [86]. Alpha-hemolysin, a critical virulence factor of S. aureus, induces hemolysis, cell lysis, and apoptosis, and its regulation is mediated by the two-component system [87,88]. Within this context, a prokaryotic lncRNA named SSR42, regulated by the global regulator Rsp, has been identified in S. aureus. The upregulation of SSR42 positively regulates the two-component system SaeRS, thereby promoting alpha-hemolysin expression, enhancing S. aureus pathogenicity, and potentially influencing cross-species transmission [89,90]. From the host’s perspective, the host-derived bovine mastitis-related long non-coding RNA (BMNCR) triggers an inflammatory response in bovine mammary glands through the miR-145/CBFB axis, bolstering the autoprotective mechanism against S. aureus infection [91]. In addition, in bovine mammary epithelial cells, the antisense lncRNA LRRC75A-AS protects leucine rich repeat containing 75A (LRRC75A) mRNA from degradation by binding its coding sequence (CDS) region. During S. aureus infection, downregulation of LRRC75A-AS1 acts as a protective mechanism, preserving tight junction proteins and impeding bacterial invasion [92].

Table 1.
LncRNAs in the regulation of bacterial zoonoses.
Table 1.
LncRNAs in the regulation of bacterial zoonoses.
| Pathogen | LncRNA | Category | Function or Mechanism | Reference |
|---|---|---|---|---|
| Mtb | lncRNA-CD244 | Host antisense lncRNA | Regulate T-cell responses against TB infection | [36] |
| NEAT1 | Host intergenic lncRNA | Regulate the inflammatory responses in macrophages | [37,93] | |
| XLOC_012582 | Host intergenic lncRNA | Regulate the expression of SOCS3 | [38] | |
| PCED1B-AS1 | Host antisense lncRNA | Modulate macrophage apoptosis and autophagy by targeting miR-155 | [39] | |
| lincRNA-EPS | Host intergenic lncRNA | Regulate apoptosis and autophagy of macrophages via JNK/MAPK signaling | [94] | |
| lincRNA-Cox2 | Host intergenic lncRNA | Regulate macrophage apoptosis | [95] | |
| lnc-EST12 | Host intergenic lncRNA | Regulate anti-Mtb innate immunity through FUBP3 | [40] | |
| DANCR | Host intergenic lncRNA | Restrain intracellular survival of Mtb via miR-1301-3p and miR-5194 | [41] | |
| XIST | Host intergenic lncRNA | Promote the polarization of macrophages to the M1 phenotype via miR-125b-5p/A20/NF-κB axis | [42] | |
| MIAT | Host intergenic lncRNA | Regulate autophagy and antimicrobial responses | [43] | |
| MIR99AHG | Host intergenic lncRNA | Promote Mtb growth by regulating inflammation and macrophage polarization | [44] | |
| LINC00870 | Host intergenic lncRNA | Biomarker | [45] | |
| CCAT1 | Host intergenic lncRNA | Biomarker | [45] | |
| LOC152742 | Host intergenic lncRNA | Biomarker | [45] | |
| MIR3945HG | Host intergenic lncRNA | Biomarker | [46] | |
| E. coli | FUT3-AS1 | Host antisense lncRNA | Modulates E. coli susceptibility via histone H4 modifications | [49] |
| lncRSPH9-4 | Host sense overlapping lncRNA | Disrupt endothelial barrier via miR-17-5p/MMP3 axis | [52] | |
| lncC11orf54-1 | Host intronic lncRNA | Modulate neuroinflammation responses | [51] | |
| DDIT-AS1 | Host antisense lncRNA | Modulate DDIT4 expression and promote neuroinflammation responses | [53] | |
| XIST | Host intergenic lncRNA | Regulate NF-κB/NLRP3 inflammasome pathway | [54] | |
| HOTAIR | Host antisense lncRNA | Promote kidney injury in sepsis | [55] | |
| SOX2OT | Host sense overlapping lncRNA | Mitigate LPS-induced injuries in cardiomyocytes | [56] | |
| MALAT1 | Host intergenic lncRNA | Regulate macrophage polarization | [57] | |
| Brucella | lnc_000428 | Host antisense lncRNA | Regulate Brucella intracellular replication | [60] |
| Gm28309 | Host intronic lncRNA | Regulate inflammatory and anti-Brucella responses via NF-κB/NLRP3 signaling | [61] | |
| linc-MAF-4 | Host intergenic lncRNA | Biomarker | [62] | |
| IFNG-AS1 | Host intergenic lncRNA | Biomarker | [63] | |
| S. typhimurium | LNCGM1082 | Host intergenic lncRNA | Activate NLRC4 and induce resistance to S. typhimurium | [66] |
| NeST (IFNG-AS1) | Host intergenic lncRNA | Modulate host susceptibility to pathogens by altering epigenetic marking of IFNγ-encoding chromatin | [67] | |
| TVX1 | Host intergenic lncRNA | Attenuated S. typhimurium-induced microglial inflammation | [68] | |
| NEAT1 | Host intergenic lncRNA | Biomarker | [65] | |
| P. aeruginosa | MEG3 | Host intergenic lncRNA | Influence the proliferation of P. aeruginosa by miR-138/IL-1β axis | [71] |
| NRIR | Host intergenic lncRNA | Affect the maturation of dendritic cell and the activation of T cell | [72] | |
| MEG9 | Host intergenic lncRNA | Biomarker | [70] | |
| BLACAT1 | Host intronic lncRNA | Biomarker | [70] | |
| L. monocytogenes | lincRNA-EPS | Host intergenic lncRNA | Impair the host defense against L. monocytogenes infection | [79,80] |
| lincRNA-Cox2 | Host intergenic lncRNA | Regulate migration and phagocytosis of macrophages | [81] | |
| AS-IL-1α | Host antisense lncRNA | A regulator of innate immune response by regulating IL-1α transcription | [82] | |
| SROS1 | Host intergenic lncRNA | Promote IFN-γ-STAT1-mediated innate immunity | [83] | |
| lasRNAs | Pathogen-derived lncRNA | Represent a regulatory pattern that connect adjacent genes with opposing functions | [84] | |
| S. aureus | BMNCR | Host intronic lncRNA | Influence the proliferation and apoptosis of epithelial cells | [91] |
| LRRC75A-AS | Host antisense lncRNA | Regulate the expression of tight junctions and affect inflammation | [92] | |
| SSR42 | Pathogen-derived lncRNA | Modulate the expression of several virulence factors | [89,90] |
3. LncRNAs in Viral Zoonoses
Although viruses do not constitute the majority of zoonotic diseases, they are often responsible for explosive outbreaks in humans due to their high variability and the scarcity of specific treatments. This leads to a series of public health events, adversely affecting health, socio-economic, and political landscapes. The last four pandemics were all attributed to viruses, making them a globally prioritized zoonotic disease. In the realm of molecular biology, lncRNAs have emerged as crucial players in the spillover and pathogenic mechanisms of zoonotic viruses. Extensive research has documented the involvement of lncRNAs across a variety of zoonotic viral infections, spanning several families such as Rhabdoviridae (e.g., rabies virus), Filoviridae (e.g., Ebola virus), Flaviviridae (e.g., Japanese encephalitis and Dengue viruses), Poxviridae (e.g., Monkeypox virus), Retroviridae (e.g., HIV), and various influenza viruses, coronaviruses and herpesviruses (Table 2) [96,97].
3.1. Rabies
Rabies, an ancient and deadly zoonotic disease caused by the Rabies virus (RABV), results in over 59,000 deaths annually worldwide [98]. Despite being vaccinepreventable, rabies progresses rapidly and is almost invariably fatal once clinical symptoms manifest [99]. RABV is a non-segmented negative-stranded RNA virus belonging to the Lyssavirus genus of the Rhabdoviridae family in the order Mononegavirales. Most RABV infections initiate from a dermal or muscular wound, which means RABV replicates locally in muscle tissue and then enters peripheral neurons at axon termini, requiring long distance axonal transport and trans-synaptic spread between neurons for the infection of the central nervous system [100,101]. Studies have identified a RABV-inducible lncRNA in neuronal cells, known as EDAL, which is short for EZH2 Degradation-Associated lncRNA. EDAL interacts with the T309 region of the EZH2 gene, diminishing EZH2 levels and its enzymatic output H3K27me3 via the lysosomal pathway. This ultimately hinders the replication of the RABV by regulating the transcription of corresponding peptides, highlighting the pivotal role of EDAL as a prominent restriction factor in the cross-species spillover of RABV [102]. Moreover, the introduction of EDAL expression in engineered RABV substantially reduces its pathogenicity following nasal infection [103].
3.2. Ebola Virus Disease
Ebola virus disease (EVD), triggered by EBOV, is an acute and often fatal illness. An epidemic occurring in West Africa stemmed from a single zoonotic transmission event to a two-year-old boy in Meliandou, Guinea, and led to subsequent human-to-human transmission [104]. EVD is characterized by hemorrhagic fever, gastrointestinal symptoms, and multiple organ dysfunction syndrome with high fatality rates [105]. Despite the extensive research of EVD, the role of lncRNAs in its pathogenesis had remained unexplored until a recent single-cell sequencing study shed light on this area. This study identified 3979 unannotated novel lncRNAs in EBOV-infected rhesus monkeys, with a significant number showing differential expression in response to EBOV infection, including the upregulation of lncRNAs small nucleolar RNA host gene 6 (SNHG6) and LINC00861, and the downregulation of lncRNA NEAT1 [106]. This investigation further elucidates the mechanisms underlying the tissue-specific characteristics of lncRNAs, based on single-cell analyses. Fundamentally, lncRNAs are present exclusively within certain specific cell types, which explains their apparent predilection for particular tissues. This indicates that the tissue specificity of lncRNAs is not due to their low-level expression across all cell types within certain tissues but rather because they are expressed in a limited number of cell types. Detailed mechanisms showed that lncRNAs harbor fewer transcription factor binding sites and higher chromatin repressive marks in their promoter regions, thereby decreasing the probability of transcription rather than the strength of transcription. This study not only identifies potential lncRNA markers in the context of EBOV infection that underlie the involvement of lncRNAs in immune regulations but also addresses the question of how lncRNAs differentially respond to viral infection at single-cell resolution.
3.3. Flavivirus Infection
Both Dengue fever and Japanese encephalitis are mosquito-borne acute encephalitis syndromes caused by DENV and JEV, respectively. These viruses, belonging to the Flaviviridae family, are single-stranded positive-sense RNA-enveloped viruses with zoonotic characteristics [107,108]. DENV transmission occurs through mosquito bites, proliferating throughout the body with white blood cells and triggering signaling protein production, resulting in the manifestation of symptoms such as pain and fever. This process also increases vascular permeability, causing hemorrhage and multiorgan involvement [109]. JEV, on the other hand, followed by mosquito bites, enters the mononuclear-phagocyte system and undergoes replication [110]. It subsequently leads to a robust viremia in individuals due to weakened immune systems, allows the penetration of the blood–brain barrier, and causes extensive meningoencephalitis [111,112]. Research has linked the lncRNA NEAT1 with DENV proliferation. Knocking down NEAT1 enhances the expression of interferon alpha-inducible protein 27 (IFI27) through the RIG-I pathway, thereby inhibiting the DENV replication [113]. Another DENV-induced lncRNA, ERG-Associated lncRNA (ERGAL) competitively binds to miR-183-5p, mitigating the inhibitory effect of miR-183-5p on VE-cadherin, and claudin-5, which are important markers of blood–brain barrier (BBB) function, thereby enhancing the integrity of the BBB. In addition, ERGAL reduces early apoptosis of endothelial cells and facilitates cytoskeleton remodeling, thereby improving the blood–brain barrier stability and restricting DENV brain invasion [114]. As for JEV infection, an increased expression of lncRNA-SUSAJ1 is observed as being regulated by the neuroinflammatory inducer CCR1/SP. LncRNA-SUSAJ1, in turn hampers JEV replication and interrupts the transmission of JEV [115,116]. In addition, several broad-spectrum regulatory lncRNAs, such as JINR1, ZAP-IT1, MALAT1, and Gm20559, are characterized in flaviviral diseases [117,118,119,120]. For instance, the flavivirus-induced lncRNA JINR1, mediated by NF-κB, is considered to be the facilitator of EV/DENV/WNV replication. It functions by interacting with RNA-binding protein RBM10, and manipulating the expression of NF-κB target genes, such as glucose-regulated protein 78 (GRP78) [117]. Another lncRNA ZAP-IT1 is however induced by type I IFN, and is recognized as a negative regulator for flavivirus infection, and as inhibiting the replication of ZIKV, DENV, JEV and vesicular stomatitis virus (VSV) in a type I IFN signaling independent manner [118]. Interestingly, from the perspective of pathogens, all members of the Flaviviridae are likely to produce lncRNAs in their infected cells, these lncRNAs are generated by the stalling and degrading of host exonuclease Xrn1 on viral RNA structures, which impacts viral replication, cytopathology as well as pathogenesis, opening up the door to new therapeutic targets for the development of broad-spectrum antiflaviviral therapeutics [121].
3.4. AIDS
Acquired Immunodeficiency Syndrome (AIDS), caused by HIV, emerged as a global pandemic since being initially reported by the U.S. CDC in the early 1980s. To date, it has infected over 80 million individuals worldwide, resulting in approximately 40 million deaths [122,123]. It is currently widely believed that HIV may be of multiple origins instead of a single one, having evolved from various simian immunodeficiency viruses (SIVs), with HIV-1 being the predominant type responsible for human transmission. Traceability analyses propose the hypothesis that HIV-1 originated from recurrent SIV spillover events that can be traced back to the early 20th century [124,125]. In the context of HIV infection, lncRNAs play pivotal roles in regulating immune responses. In HIV-infected individuals, lncRNA RUNX1 overlapping RNA (RUNXOR) promotes the proliferation of myeloid-derived suppressor cells (MDSCs) and regulates the expression of various immune inhibitory signaling molecules by targeting the transcription factor runt-related transcription factor-1 (RUNX1), leading to an immune suppression [126]. Similarly, HOXA transcript antisense RNA myeloid-specific 1 (HOTAIRM1) exhibits comparable functionality in inhibitory immune regulation [127]. Another lncRNA, termed growth arrest specific 5 (GAS5), is shown to control HIV replication through interaction with miR-873 [128]. Additionally, GAS5 also controls miR-21 expression and regulates signaling molecules involved in DNA damage and cellular responses following T cell receptor stimulation, reversing T cell dysfunction and improving CD4+ T cell exhaustion incurred during HIV infection [129]. NF-kappaB interacting lncRNA (NKILA) inhibits HIV-1 replication and reactivation by suppressing HIV-1-long-terminal-repeat-driven transcription initiation in an NF-κB-dependent manner, which holds potential significance for elucidating the mechanisms underlying HIV transmission and latent infection [130].
3.5. Influenza
Influenza is a contagious respiratory disease caused by influenza viruses, which are classified into four genera: influenza A viruses (IAV), influenza B viruses (IBV), influenza C viruses (ICV), and influenza D viruses (IDV). These viruses, along with other arthropods or fish-associated genera, such as Thogotovirus, Quaranja virus, Sardinevirus, Mykissvirus, and Isavirus, collectively form the family Orthomyxoviridae. [131]. Only three, IAV, IBV and ICV, have so far been described in humans, while only Influenza A is commonly transmitted from animals to human and vice-versa [132,133,134]. Zoonotic influenza viruses occasionally infect humans, leading to various outcomes, ranging from mild conjunctivitis to severe pneumonia and even death [135]. Over the past few decades, there have been several spillover events involving influenza viruses, such as outbreaks of H5N1, H9N2, H1N1, H3N2, H7N9, and H9N2 [136]. In the context of lncRNA research, a database termed VirhostlncR has been developed by Rajesh Raju et al. [137], which compiles differential expression profiles of lncRNAs in viral infections, incorporating data on six lncRNAs relevant to influenza. Analysis of this database indicates that lncRNAs, like LINC01191, DANCR, breast cancer anti-estrogen resistance 4 (BCAR4), and PSMB8-AS1, are pivotal in modulating influenza replication and pathogenesis [138,139,140]. For instance, influenza viruses, such as H1N1, H5N1, H7N9, induce the expression of LncRNA#61, which disrupts viral invasion, RNA synthesis, and release through its four long arms, effectively curbing viral replication and enhancing host immune defense [141]. Subsequent investigations have provided additional evidence supporting the broad-spectrum antiviral properties of LncRNA#61, as well as the similar functionality observed in LncRNA#45 [142]. Other lncRNAs, like cholesterol induced regulator of metabolism RNA (CHROMR), lncNSPL, and RIG-I-dependent antiviral response regulator RNA (RDUR), contribute to the host anti-influenza virus response in an IFN-dependent manner [143,144,145].
3.6. Herpesvirus Infection
Herpesviruses, belonging to the family Orthoherpesviridae, are double-stranded DNA viruses comprising 17 genera [146]. Among them, certain ones, including pseudorabies virus (PRV), monkey B virus, and Epstein–Barr virus (EBV) are recognized as zoonotic. Additionally, some other herpesviruses, such as avian Marek’s disease virus (MDV), human herpes simplex virus type-1 (HSV-1), and equine herpesvirus type 1 (EHV), are considered to possess cross-species transmission potential between humans and animals in specific cases, although the available evidence regarding the zoonotic capabilities of these viruses remains insufficient [147,148]. During PRV infection, the host-derived lncRNA, lnc_000641, is identified to modulate viral replication by inhibiting the JAK-STAT1 signaling pathway, thereby influencing the expression of type I IFN [149]. Similarly, lncRNA lncA02830 also influences PRV multiplication through akin mechanisms [150]. In the context of EBV, associated primarily with laryngeal cancer, the high expression of lncRNA H19 in EBV-positive individuals is significantly correlated with the occurrence of laryngeal cancer, likely via the transcriptional repressor EZH2 regulation [151]. Remarkably, herpes viruses themselves harbor lncRNAs within their genomes. In PRV, lncRNAs NOIR1 and NOIR2 are located in the inverted repeat (IR) region, while lncRNAs PTO and PTO-US1 are located in the vicinity of the viral replication origin sequence oriS. Additionally, lncRNAs CTO-S and CTO-L are positioned in the vicinity of the oriL sequence, and lncRNA AZURE is situated at the boundary of the US-IR region [152]. Subsequently, studies have established a transcriptional interference network with the involvement of these viral lncRNAs and their neighboring genes, thereby exerting an intriguing epigenetic regulatory function. This suggests the pivotal involvement of viral-derived lncRNAs in the regulation of herpesvirus pathogenesis and potentially their spillover mechanisms.
3.7. Coronavirus Disease
Since the outbreaks of SARS and COVID-19, coronavirus diseases have garnered significant global attention. Belonging to the Orthocoronavirinae subfamily, coronaviruses encompass four genera: α coronavirus, β coronavirus, γ coronavirus, and δ coronavirus. These single-stranded positive-sense RNA viruses were first identified in cases of infectious bronchitis in chickens [153,154]. Throughout millennia of evolution, coronaviruses have continually crossed species boundaries, causing profound infections in a diverse range of species, including humans, mammals, and birds [155]. The spike (S) protein of coronaviruses is widely acknowledged as the primary determinant of tissue tropism and the cross-species transmission capacities [156]. To date, human-infective coronaviruses have been identified with α-CoV, such as HCoV-NL63, HCoV-229E, CCoV-HuPn-2018 of, as well as β-CoV such as HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2. Among these, SARS-CoV, MERS-CoV, and SARS-CoV-2 exhibit the highest pathogenicity, and are capable of causing severe respiratory distress syndrome and extrapulmonary manifestations [157,158]. Research indicates that the angiotensin converting enzyme 2 (ACE2) receptor for the S protein is closely associated with coronavirus invasion, with lncRNA GAS5 regulating the expression of ACE2 through the GAS5/miRNA-200/ACE2 axis, thereby affecting the invasion of SARS-CoV-2 into the host [159]. Another lncRNA, SNHG15, modulates the hostinvasion of SARS-CoV-2 in an ACE2 independent manner through interacting with RABL2A, an essential regulator of vesicular trafficking in human cells [160]. During SARS-CoV-2 infection, changes in lncRNA expression, such as the downregulation of PU.1-induced regulator of alarmin transcription (PIRAT) and upregulation of lung cancer associated transcript 1 (LUCAT1), have been found to alter the production of immune mediators, impacting the host systemic antiviral responses [161]. In CD8+ T cells, the SARS-CoV-2-induced lncRNA small nucleolar RNA host gene 15 (SNHG15) interacts with the vesicle transport protein Vps13D and regulates the IL-7 signaling pathway, promoting the generation of memory CD8+ T cells. Furthermore, a series of lncRNAs, such as MALAT1, MEG3, XIST, ZFY-AS1, and TTTY14, serve as noteworthy biomarkers for SARS-CoV-2 infection [162,163]. These lncRNAs exhibit some pathological impacts and present novel targets for the advancement of diagnostic and therapeutic approaches.

Table 2.
LncRNAs in the regulation of viral zoonoses.
Table 2.
LncRNAs in the regulation of viral zoonoses.
| Pathogen | LncRNA | Category | Function or Mechanism | Reference |
|---|---|---|---|---|
| RABV | EDAL | Host intergenic lncRNA | Inhibit the replication of neurotropic virus | [102,103] |
| DENV (Flaviviridae) | NEAT1 | Host intergenic lncRNA | Affect antiviral response and viral replication in dengue infection | [113] |
| ERGAL | Host intergenic lncRNA | Promote stability and integrity of vascular endothelial barrier during DENV infection | [114] | |
| JEV (Flaviviridae) | SUSAJ1 | Host sense overlapping lncRNA | Inhibit JEV proliferation and replication | [115,116] |
| Flaviviridae | JINR1 | Host intergenic lncRNA | Regulate viral replication and cell death | [117] |
| ZAP-IT1 | Host intronic lncRNA | Exert antiviral effect in an IFN-independent manner | [118] | |
| MALAT1 | Host intergenic lncRNA | Potential antiviral function | [119] | |
| Gm20559 | Host intergenic lncRNA | Modulate the expression of various pro-inflammatory cytokines during flavivirus infection | [120] | |
| sfRNAs/ xrRNAs | Pathogen-derived lncRNA | Impact viral replication | [121] | |
| HIV | RUNXOR | Host sense overlapping lncRNA | Regulate multiple immunosuppressive signaling molecules | [126,164] |
| HOTAIRM1 | Host intergenic lncRNA | Increase levels of immunosuppressive molecules | [127] | |
| GAS5 | Host antisense lncRNA | Control HIV replication, regulate the activity and longevity of CD4 T cells | [128,129] | |
| NKILA | Host antisense lncRNA | Inhibit HIV-1 replication by suppressing HIV-1 LTR promoter activity | [130] | |
| Influenza viruses | PSMB8-AS1 | Host antisense lncRNA | Promotes influenza virus replication | [137,138] |
| LINC01191 (VIN) | Host intergenic lncRNA | Regulate viral protein synthesis | [137,140] | |
| DANCR | Host intergenic lncRNA | Involved in respiratory infections and regulate inflammation | [137,139] | |
| BCAR4 | Host intergenic lncRNA | Biomarker | [137] | |
| LncRNA#61 | Host sense overlapping lncRNA | Suppress viral replication, mediate host immune responses | [141] | |
| LncRNA#45 | Host intronic lncRNA | Function as a broad-spectrum antiviral factor | [142] | |
| CHROMR | Host antisense lncRNA | Restrict influenza virus replication by sequestering IRF2/IRF2BP2 complex | [143] | |
| lncNSPL | Host intergenic lncRNA | Influence influenza immune escape by modulating IFN-I expression | [144] | |
| RDUR | Host intergenic lncRNA | Regulate innate immunity against virus by controlling IFN-β and ISGs | [145] | |
| PRV (Orthoherpesviridae) | lnc_000641 | Host intergenic lncRNA | Influence PRV replication through JAK-STAT1 pathway | [149] |
| lncA02830 | Host intronic lncRNA | Affect PRV replication in a IFN-dependent manner | [150] | |
| NOIR1/NOIR2 | Pathogen-derived lncRNA | Locate in the IR region of the PRV | [152] | |
| PTO/PTO-US1 | Pathogen-derived lncRNA | Overlap with the oriS region of the PRV | [152] | |
| CTO-S/CTO-L | Pathogen-derived lncRNA | Function as TATA boxes in herpesviruses | [152] | |
| AZURE | Pathogen-derived lncRNA | Locate in the IR-US overlapping region of the PRV | [152] | |
| EBV (Orthoherpesviridae) | H19 | Host intergenic lncRNA | Biomarker | [151] |
| SARS-CoV-2 | GAS5 | Host antisense lncRNA | Affect SARS-CoV-2 invasion via GAS5/miRNA-200/ACE2 axis | [159] |
| SNHG15 | Host intergenic lncRNA | Aid SARS-CoV-2 entry through RABL2A, facilitate memory CD8+ T cell production | [160] | |
| PIRAT | Host intergenic lncRNA | Modulate systemic antiviral responses to SARS-CoV-2 | [161] | |
| LUCAT1 | Host intergenic lncRNA | Modulate systemic antiviral responses to SARS-CoV-2 | [161] | |
| XIST | Host intergenic lncRNA | Biomarker | [162] | |
| ZFY-AS1 | Host antisense lncRNA | Biomarker | [162] | |
| TTTY14 | Host intergenic lncRNA | Biomarker | [162] | |
| MALAT1 | Host intergenic lncRNA | Biomarker | [163] | |
| MEG3 | Host intergenic lncRNA | Biomarker | [163] |
4. LncRNAs in Parasitic Zoonoses
Zoonotic parasites are significant pathogens found in both animals and humans, and continue to cause substantial morbidity and mortality worldwide, indicating that the efforts of drug administration and parasite eradication campaigns have not yet effectively addressed all parasites that are of significance to public health and veterinary medicine [165,166]. Recent studies have demonstrated that lncRNAs are of major importance in both parasites and hosts, exerting diverse functions throughout the parasitic infection process [167,168]. Herewith, we compiled and summarized the current knowledge concerning the role of lncRNA in parasitic diseases, such as malaria, Echinococcosis, schistosomiasis, cryptosporidiosis, and toxoplasmosis (Table 3).
4.1. Malaria
Malaria is a mosquito-borne infectious disease caused by a protozoan parasite that belongs to the genus Plasmodium [169]. It is interesting to note that, as a eukaryote, lncRNAs are widespread in Plasmodium itself. For example, more than 2500 lncRNA transcripts have been found in Plasmodium falciparum (P. falciparum) [170]. Among these, lncRNAs, a Pfgdv1 gene-derived antisense lncRNA named gdv1, regulates the expression of Pfgdv1, which functions as an inhibitor of sexual differentiation and ultimately modulates the sexual development of Plasmodium [171,172]. Two lncRNAs transcribed from the telomere-associated repetitive elements (TARE), TARE-3-lncRNA and TARE-6-lncRNA, are able to influence the intra-erythrocytic developmental cycle of Plasmodium. Due to the enrichment of binding sites for various transcription factors, it is therefore postulated that TARE-lncRNAs function by regulating neighboring genes [172,173,174]. Another class of var gen-specific lncRNAs can be incorporated into chromatin, and play a key role in the activation of var gene, which is able to encode variable antigens and enhance the virulence of P. falciparum. In addition, interfering with these var-specific lncRNAs leads to the down-regulation of the var gene and alters its epigenetic imprint, which results in a switching of expression to different var genes [175]. Recently, Gayani Batugedara et al., identified 1768 intergenic lncRNAs in P. falciparum, using deep sequencing and nascent RNA expression. They also demonstrated that a nuclear lncRNA, lncRNA-ch14, plays an important role in gametocyte development and in the infectivity of these gametocytes for mosquitoes, by recruiting histone demethylase and histone acetyl transferase to change the epigenetic state of the chromatin and activate the expression of these genes during sexual differentiation [170]. Thus, it will be important to elucidate the function Plasmodium-derived lncRNA to understand the pathogenicity and pathogenesis of Plasmodium. On the other hand, host-derived lncRNA plays a significant role in regulating the interaction between parasites and their host. Previous studies have reported that 291 lncRNAs were differentially expressed in Plasmodium-infected mice; ENMSUSG00000111521.1, XLOC_038009, XLOC_058629, and XLOC_065676 are considered to be involved in malaria infection. These four lncRNAs function as regulators of host immunity by activating TGF-β/Smad2/3 signaling pathway [176]. Moreover, an abundant, ubiquitously expressed lncRNA MALAT1 is found to modulate Maf/IL-10 axis in CD4+ T cells by functioning as a negative regulator of cellular immune response upon Plasmodium infection. Knockout of the MALAT leads to an activated macrophage and reduced parasite loads [177].
4.2. Schistosomiasis
Schistosomiasis is one of the most serious zoonoses caused by schistosomes, which frequently causes intestinal symptoms, urogenital symptoms, and other systemic symptoms such as fever [178]. Similar to Malaria, lncRNAs are present both in the pathogen level and the host level during Schistosomiasis. Silveira et al., have identified 16583 lncRNAs in Schistosoma mansoni (S. mansoni). Among these, SmLINC101519, SmLINC175062, and SmLINC110998 are correlated with the motility of adult worms, which further influences worm burden and egg hatching [179]. Another study reported 5-azacytidine, a potential antiparasitic agent, was considered to affect lncRNA levels in S. mansoni and be involved in S. mansoni reproductive biology [180]. Moreover, a total of 3033 potential lncRNAs are identified in Schistosoma japonicum (S. japonicum) [181]. Overall, these studies suggest lncRNAs may play an essential role in Schistosoma itself. On the host side, 759 and 789 differentially expressed lncRNAs are observed in liver and spleen of S. japonicum parasitized mice, respectively [182]. LncRNA Gm16685 is upregulated during S. japonicum infection, and participates in the pathogenesis throughout schistosomiasis by regulating miR-205-5p [183]. The involvement of lncRNA H19 in S. japonica has also been corroborated, as it governs the hepatic reaction to Praziquantel therapy against S. japonicum infection through the H19/miR-130b-3p/Cyp4a14 axis [184].
4.3. Cryptosporidiosis
Cryptosporidium is the causative agent of cryptosporidiosis, zoonotic cryptosporidiosis in humans, which usually causes diarrhea in immunocompromised individuals, and especially children [185]. The intestinal epithelial cells provide the first line of defense against Cryptosporidium infection. A study finds that a lncRNA, U90926, is induced by Cryptosporidium parvum (C. parvum) within the intestinal epithelial cells.This lncRNA targets the transcription of host defense genes and suppresses the epithelial antiparasitic response. Intriguingly, the U90926 appears to be triggered by an RNA virus present in Cryptosporidium [186]. The pathogen-associated molecular patterns (PAMPs) related to signaling pathways, like NF-κB and IFN signaling pathways, are also implicated in lncRNA-dependent cryptosporidiosis pathogenesis. For instance, the NF-κB signaling-dependent lncRNAs Nostrill, NR_045064 and XR_001779380 regulate intestinal epithelial anti-Cryptosporidium defense through modulating downstream molecules NF-κB p65, NOS2/CSF2, or IFN-γ [187,188,189]; an up-regulated lncRNA NR_033736, in response to cryptosporidial infection, provides negative feedback regulation of type I IFN signaling through suppressing the transcript of type I IFN-controlled genes, thus influencing the epithelial innate defense against C. parvum [190].
4.4. Toxoplasmosis
Toxoplasmosis is a zoonotic parasitic disease caused by Toxoplasma gondii (T. gondii), an obligate intracellular apcomplexan parasite that is common in dogs and cats. Toxoplasmic encephalitis and ocular toxoplasmosis are two important manifestations of toxoplasmosis [191]. Studies report that 1522 lncRNAs are differentially regulated during infection with the high-virulence Type I T. gondii strain, versus 528 with the lessvirulent Type II T. gondii strain in mice; among these, host lncRNAs Csf1-lnc and Socs2-lnc are manipulated by toxoplasma rhoptry kinase ROP16, suggesting the strong influence of Toxoplasma on lncRNA expression patterns [192]. T. gondii is neurotropic and affects the function of nerve cells in the mouse brain, and researchers have found that differentially expressed lncRNA147410.3 and lncRNA-11496 elicited by T. gondii infection are involved in the processes of microglial apoptosis; among which lncRNA147410.3 induces microglial apoptosis by positively regulating its target gene Hoxb3, while lncRNA-11496 influences the biological processes of microglia by regulating the expression of the MEF2C/HDAC2 axis [193,194]. Moreover, in human foreskin fibroblast (HFF) cells, a total of 996 lncRNAs are identified as the differential expression candidates in response to T. gondii infection, of which one lncRNA, named NONHSAT022487, is able to stimulate the secretion of cytokines by suppressing the expression of UNC93B1, representing a novel mechanism by which Toxoplasma regulates lncRNA-mediated host immune signaling [195].
4.5. Echinococcosis
Echinococcosis is a serious zoonotic disease caused by the infections of Echinococcus multilocularis (Em) and Echinococcus granulosus (Eg) larvae, causing alveolar echinococcosis (AE) and cystic echinococcosis (CE), respectively [196]. In Em-infection models, 218 lncRNAs were differentially regulated in mice hepatocytes [197]. In Eg-infection models, a total of 649 differentially expressed lncRNAs were identified in splenic monocytic myeloid-derived suppressor cells, and 234 differentially expressed lncRNAs were found in human serum exosomes, which typically contain abundant DNA, mRNA, non-coding RNA, and proteins [198,199]. Functionally, lncRNA028466 was considered the regulator of recombinant Eg antigen P29 (rEg.P29) vaccination-mediated Th1 protective immunity, with reduced lncRNA028466 caused by rEg.P29 leading to the increased Th1 immune response and the lower IL-4 and IL-10 expression, suggesting the participation of lncRNAs in host–parasite interaction and CD4+ T cell differentiation [200].

Table 3.
LncRNAs in the regulation of parasitic zoonoses.
Table 3.
LncRNAs in the regulation of parasitic zoonoses.
| Pathogen | LncRNA | Category | Function or Mechanism | Reference |
|---|---|---|---|---|
| Plasmodium | GDV1 | Pathogen-derived lncRNA | Regulate sexual development | [171,172] |
| TARE-3-lncRNA/TARE-6-lncRNA | Pathogen-derived lncRNA | Affect the intra-erythrocytic developmental cycle of Plasmodium | [172,173,174] | |
| Var-specific lncRNA | Pathogen-derived lncRNA | Enhance the virulence of P. falciparum by modulating var | [175] | |
| LncRNA-ch14 | Pathogen-derived lncRNA | Regulate gametocyte development | [170] | |
| ENMSUSG00000111521.1 | Host antisense lncRNA | Regulate host immunity by TGF-β/Smad2/3 signaling | [176] | |
| XLOC_038009 | Host intergenic lncRNA | Regulate host immunity by TGF-β/Smad2/3 signaling | [176] | |
| XLOC_058629 | Host intergenic lncRNA | Regulate host immunity by TGF-β/Smad2/3 signaling | [176] | |
| XLOC_065676 | Host intergenic lncRNA | Regulate host immunity by TGF-β/Smad2/3 signaling | [176] | |
| MALAT1 | Host intergenic lncRNA | Function as a negative regulator of cellular immune response | [177] | |
| Schistosoma | SmLINC101519 | Pathogen-derived lncRNA | Regulate the motility of adult worms | [179] |
| SmLINC175062 | Pathogen-derived lncRNA | Regulate the motility of adult worms | [179] | |
| SmLINC110998 | Pathogen-derived lncRNA | Regulate the motility of adult worms | [179] | |
| Gm16685 | Host antisense lncRNA | Promote M1 macrophage polarization by regulating miR-205-5p | [183] | |
| H19 | Host intergenic lncRNA | Influence S. japonica infection via H19/miR-130b-3p/Cyp4a14 axis | [184] | |
| Cryptosporidium | U90926 | Host intergenic lncRNA (peptide coding) | Regulate cell autonomous antiparasitic defense in a pro-parasitic manner | [186] |
| Nostrill | Host intergenic lncRNA | Promote antiparasitic defense through regulating NF-κB p65 | [187] | |
| NR_045064 | Host intergenic lncRNA | Promote host defense against Cryptosporidium by modulating NOS2/CSF2 | [188] | |
| XR_001779380 | Host intergenic lncRNA | Relevant to anti-Cryptosporidium defense in a IFN- dependent manner | [189] | |
| NR_033736 | Host intergenic lncRNA | Contribute to host innate defense against Cryptosporidium | [190] | |
| Toxoplasma | Csf1-lnc | Host sense overlapping lncRNA | Controlled by secretory kinase ROP16 | [192] |
| Socs2-lnc | Host sense overlapping lncRNA | Controlled by secretory kinase ROP16 | [192] | |
| lncRNA147410.3 | Host antisense lncRNA | Affect microglial proliferation, differentiation and apoptosis by targeting Hoxb3 | [193] | |
| lncRNA-11496 | Host sense overlapping lncRNA | Affect microglial proliferation, differentiation and apoptosis by targeting Mef2c | [194] | |
| NONSHAT022487 | Host antisense lncRNA | Suppress the expression of the immune-related molecule UNC93B1 | [195] | |
| Echinococcus | lncRNA028466 | Host intergenic lncRNA | Be involved in cytokine expression of Th1 and Th2 | [200] |
5. Conclusions
Microorganisms are ubiquitous. Zoonotic microorganisms, in particular, continue to cause communicable diseases with high incidence rates and result in considerable mortality. Mechanically, the spillover of these microorganisms typically involves breaching host barriers, inducing and evading innate immune responses, and surviving and proliferating within the host [6,201,202]. To our knowledge, these necessary processes to achieve spillover have not been systemically connected or elaborated for zoonotic pathogens. This review focuses on the roles of lncRNAs in the pathogenesis of zoonotic diseases. It also addresses the question of if lncRNAs are employed by pathogens to manipulate host immune status to their own advantage (Figure 1).
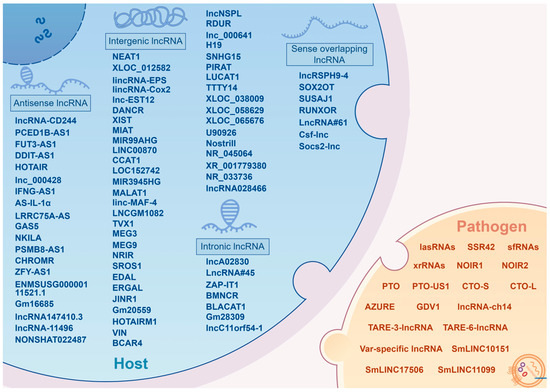
Figure 1.
A brief summary of lncRNAs as a regulatory factor affecting zoonotic diseases (By Figdraw version 2.0, www.figdraw.com).
It is well known that lncRNAs are implicated in pathogen–host interaction by modulating host innate immune pathways. Several functional lncRNAs have been characterized in zoonotic infections, with the revelation of their involvement in pathogen invasion collectively contributing to the creation of a multidimensional regulatory network of lncRNAs in zoonoses (Figure 2). Among these, several broad-spectrum regulatory lncRNAs, such as NEAT1, MALAT1, XIST, IFNG-AS1, lincRNA-EPS, DANCR, GAS5, and H19, are identified as being implicated in a variety of zoonotic diseases [41,42,54,63,67,79,80,113,119,128,139,159,177]. Notably, these widely recognized functional lncRNAs exhibit a degree of conservation, holding potential as neoteric diagnostic markers and therapeutic targets. A prominent example is the NEAT1, which exhibits upregulation in most infections and assumes crucial anti-viral functions, thereby potentially serving as a therapeutic target for antisense and small molecule RNA inhibitor approaches. Conversely, the specific depletion of NEAT1 has been associated with severe hemorrhagic fevers, suggesting its potential utility as a diagnostic biomarker [106].
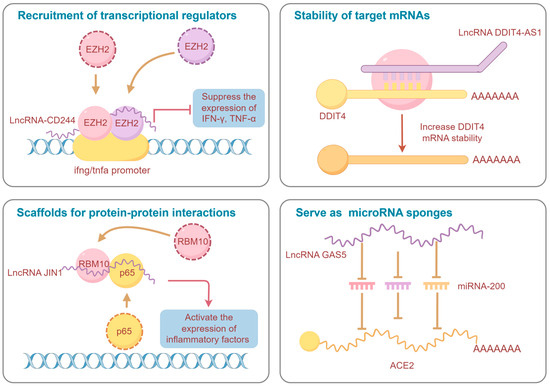
Figure 2.
Schematic diagram of the regulatory mechanisms of representative lncRNAs in zoonoses (By Figdraw version 2.0, www.figdraw.com).
Furthermore, this review also synthesizes the findings on pathogen-derived lncRNAs that display distinct characteristics in bacteria, viruses, and parasites. Specifically, lncRNAs are identified in zoonotic bacteria (e.g., L. monocytogenes, P. aeruginosa), viruses (e.g., EBV, Flaviviridae family viruses), and parasites (e.g., Plasmodium, Schistosoma) [84,89,121,152,170,179]. These pathogen-derived lncRNAs typically regulate both pathogenicity and the life cycle of the pathogens, leading to altered invasive behavior and potentially influencing pathogen spillover events. However, the current exploration and comprehension of lncRNAs within pathogens remains inadequate. Developing a more comprehensive lncRNAs map at the pathogen level is imperative to enhancing the identification of diagnostic and therapeutic targets, heralding promising future directions for lncRNA research of zoonotic diseases and even infectious diseases in general.
Author Contributions
Conceptualization, X.W. and B.X.; manuscript writing, B.X.; visualization, B.X. and Y.H.; supervision, J.L. and R.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LQ23C180004), National Natural Science Foundation of China (No. 32302954), Ningbo Non-profit Technology Research Program (No. 2023S021, No. 2021S135), and Natural Science Foundation of Ningbo (No. 2023J074).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tomori, O.; Oluwayelu, D.O. Domestic Animals as Potential Reservoirs of Zoonotic Viral Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 33–55. [Google Scholar] [CrossRef]
- Kalawat, U.; Mohan, A. Endemic, Emerging and Re-Emerging Zoonotic Diseases: The Way Forward! J. Clin. Sci. Res. 2023, 12, 79–80. [Google Scholar] [CrossRef]
- Head, J.R.; Bumburidi, Y.; Mirzabekova, G.; Rakhimov, K.; Dzhumankulov, M.; Salyer, S.J.; Knust, B.; Berezovskiy, D.; Kulatayeva, M.; Zhetibaev, S.; et al. Risk Factors for and Seroprevalence of Tickborne Zoonotic Diseases among Livestock Owners, Kazakhstan. Emerg. Infect. Dis. 2020, 26, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to Zoonotic Spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.R.; Holmes, E.C.; Morens, D.M.; Park, E.-C.; Burke, D.S.; Calisher, C.H.; Laughlin, C.A.; Saif, L.J.; Daszak, P. Cross-Species Virus Transmission and the Emergence of New Epidemic Diseases. Microbiol. Mol. Biol. Rev. 2008, 72, 457–470. [Google Scholar] [CrossRef] [PubMed]
- The ENCODE Project Consortium. An Integrated Encyclopedia of DNA Elements in the Human Genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Ponting, C.P.; Haerty, W. Genome-Wide Analysis of Human Long Noncoding RNAs: A Provocative Review. Annu. Rev. Genom. Hum. Genet. 2022, 23, 153–172. [Google Scholar] [CrossRef]
- Volders, P.-J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a Reference Set of Human Long Non-Coding RNAs. Nucleic Acids Res. 2019, 47, D135–D139. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Feng, C.; Qin, Y.; Xiao, J.; Zhang, Z.; Ma, L. LncBook 2.0: Integrating Human Long Non-Coding RNAs with Multi-Omics Annotations. Nucleic Acids Res. 2022, 51, D186–D191. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Wu, M. New Insights into Long Noncoding RNAs and Their Roles in Glioma. Mol. Cancer 2018, 17, 61. [Google Scholar] [CrossRef]
- St Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of Long Non-Coding RNA Classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and Abundance Analysis of Human lncRNAs at Single-Cell and Single-Molecule Resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA Localization and Function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Guh, C.-Y.; Hsieh, Y.-H.; Chu, H.-P. Functions and Properties of Nuclear lncRNAs-from Systematically Mapping the Interactomes of lncRNAs. J. Biomed. Sci. 2020, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Vance, K.W.; Ponting, C.P. Transcriptional Regulatory Functions of Nuclear Long Noncoding RNAs. Trends Genet. 2014, 30, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yan, Z.; Fu, C.; Wen, X.; Jia, L.; Zhou, L.; Du, Z.; Wang, C.; Wang, Y.; Chen, J.; et al. LncRNA Osilr9 Coordinates Promoter DNA Demethylation and the Intrachromosomal Loop Structure Required for Maintaining Stem Cell Pluripotency. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Xu, J.; Sun, R.; Mumbach, M.R.; Carter, A.C.; Chen, Y.G.; Yost, K.E.; Kim, J.; He, J.; Nevins, S.A.; et al. Promoter of lncRNA Gene PVT1 Is a Tumor-Suppressor DNA Boundary Element. Cell 2018, 173, 1398–1412.e22. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Sirey, T.M.; Roberts, K.; Haerty, W.; Bedoya-Reina, O.; Rogatti-Granados, S.; Tan, J.Y.; Li, N.; Heather, L.C.; Carter, R.N.; Cooper, S.; et al. The Long Non-Coding RNA Cerox1 Is a Post Transcriptional Regulator of Mitochondrial Complex I Catalytic Activity. eLife 2019, 8, e45051. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Zhang, Y.; Yang, J.; Zhang, M.; Li, S.; Xue, G.; Li, X.; Zhang, X.; Yang, J.; et al. Cytoplasmic Sequestration of P53 by lncRNA-CIRPILalleviates Myocardial Ischemia/Reperfusion Injury. Commun. Biol. 2022, 5, 716. [Google Scholar] [CrossRef]
- Slavoff, S.A.; Mitchell, A.J.; Schwaid, A.G.; Cabili, M.N.; Ma, J.; Levin, J.Z.; Karger, A.D.; Budnik, B.A.; Rinn, J.L.; Saghatelian, A. Peptidomic Discovery of Short Open Reading Frame-Encoded Peptides in Human Cells. Nat. Chem. Biol. 2013, 9, 59–64. [Google Scholar] [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and Muscle Regeneration Are Regulated by the LINC00961-Encoded SPAR Polypeptide. Nature 2017, 541, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Z.; Chen, M.; Chen, D.; Gao, X.-C.; Zhu, S.; Huang, H.; Hu, M.; Zhu, H.; Yan, G.-R. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell 2017, 68, 171–184.e6. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, C.; Ji, Y.; Ge, M.-K.; Yu, Y.; Zhang, N.; Yang, S.; Yu, J.-X.; Shen, S.-M.; Chen, G.-Q. Epithelial Cells-Enriched lncRNA SNHG8 Regulates Chromatin Condensation by Binding to Histone H1s. Cell. Death. Differ. 2022, 29, 1569–1581. [Google Scholar] [CrossRef]
- Qin, W.; Qi, X.; Xie, Y.; Wang, H.; Wu, S.; Sun, M.; Bao, W. LncRNA446 Regulates Tight Junctions by Inhibiting the Ubiquitinated Degradation of Alix after Porcine Epidemic Diarrhea Virus Infection. J. Virol. 2023, 97, e01884-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, M.; Zhang, L.; Wang, W.; Hua, S.; Zhou, C.; Sun, X. Long Non-Coding RNAs and Immune Cells: Unveiling the Role in Viral Infections. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 170, 115978. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Luo, F.; Zhou, H.; Li, Z. Roles of Long Noncoding RNAs in Bacterial Infection. Life Sci. 2020, 263, 118579. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.; Li, H. LncRNA, miRNA and lncRNA-miRNA Interaction in Viral Infection. Virus Res. 2018, 257, 25–32. [Google Scholar] [CrossRef]
- McDaniel, C.J.; Cardwell, D.M.; Moeller, R.B.; Gray, G.C. Humans and Cattle: A Review of Bovine Zoonoses. Vector Borne Zoonotic Dis. 2014, 14, 1–19. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; Zowalaty, M.E.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Olaru, I.D.; Walther, B.; Schaumburg, F. Zoonotic Sources and the Spread of Antimicrobial Resistance from the Perspective of Low and Middle-Income Countries. Infect. Dis. Poverty 2023, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Howard, N.C.; Khader, S.A. Immunometabolism during Mycobacterium tuberculosis Infection. Trends Microbiol. 2020, 28, 832–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Mott, D.; Sutiwisesak, R.; Lu, Y.-J.; Raso, F.; Stowell, B.; Babunovic, G.H.; Lee, J.; Carpenter, S.M.; Way, S.S.; et al. Mycobacterium tuberculosis-Specific CD4+ and CD8+ T Cells Differ in Their Capacity to Recognize Infected Macrophages. PLoS Pathog. 2018, 14, e1007060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, H.; Xie, X.; Chen, C.Y.; Huang, D.; Shen, L.; Zhang, H.; Chen, Z.W.; Zeng, G. Long Noncoding RNA Derived from CD244 Signaling Epigenetically Controls CD8+ T-Cell Immune Responses in Tuberculosis Infection. Proc. Natl. Acad. Sci. USA 2015, 112, E3883–E3892. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, Y.; Zhang, X.; Cheng, M.; Hu, J.; Liu, J.; Chen, X.; Zhu, J. Comprehensive Identification of Immuno-Related Transcriptional Signature for Active Pulmonary Tuberculosis by Integrated Analysis of Array and Single Cell RNA-Seq. J. Infect. 2022, 85, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, X.; Xue, J.; Duan, W.; Yi, Z. Deregulated lncRNAs in B Cells from Patients with Active Tuberculosis. PLoS ONE 2017, 12, e0170712. [Google Scholar] [CrossRef]
- Li, M.; Cui, J.; Niu, W.; Huang, J.; Feng, T.; Sun, B.; Yao, H. Long Non-Coding PCED1B-AS1 Regulates Macrophage Apoptosis and Autophagy by Sponging miR-155 in Active Tuberculosis. Biochem. Biophys. Res. Commun. 2019, 509, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Xie, Y.; Xu, D.; Qu, Z.; Wu, J.; Zhou, Y.; Wei, Y.; Xiong, H.; Zhang, X.-L. Lnc-EST12, Which Is Negatively Regulated by Mycobacterial EST12, Suppresses Antimycobacterial Innate Immunity through Its Interaction with FUBP3. Cell. Mol. Immunol. 2022, 19, 883–897. [Google Scholar] [CrossRef]
- Qu, Y.; Jiang, D.; Liu, M.; Wang, H.; Xu, T.; Zhou, H.; Huang, M.; Shu, W.; Xu, G. LncRNA DANCR Restrained the Survival of Mycobacterium tuberculosis H37Ra by Sponging miR-1301-3p/miR-5194. Front. Microbiol. 2023, 14, 1119629. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-B.; Li, L.-T.; Xi, J.-C.; Liu, H.-T.; Liu, Z.; Yu, L.; Tang, P.-F. Negative Pressure Promotes Macrophage M1 Polarization after Mycobacterium tuberculosis Infection via the lncRNA XIST/microRNA-125b-5p/A20/NF-κB Axis. Ann. N. Y. Acad. Sci. 2022, 1514, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Lou, J.; Zheng, X.-M.; Yang, X.-Y. LncRNA MIAT Regulates Autophagy and Apoptosis of Macrophage Infected by Mycobacterium tuberculosis through the miR-665/ULK1 Signaling Axis. Mol. Immunol. 2021, 139, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gcanga, L.; Tamgue, O.; Ozturk, M.; Pillay, S.; Jacobs, R.; Chia, J.E.; Mbandi, S.K.; Davids, M.; Dheda, K.; Schmeier, S.; et al. Host-Directed Targeting of LincRNA-MIR99AHG Suppresses Intracellular Growth of Mycobacterium tuberculosis. Nucleic Acid Ther. 2022, 32, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, Y.; Ma, Y.; Yang, F.; Ruan, Y.; Xu, J.-F.; Pi, J. Advances of Long Non-Coding RNAs as Potential Biomarkers for Tuberculosis: New Hope for Diagnosis? Pharmaceutics 2023, 15, 2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Wang, J.; Wen, Q.; Wang, H.; He, J.; Hu, S.; He, W.; Du, X.; Liu, S.; et al. Microarray Analysis of Long Noncoding RNA and mRNA Expression Profiles in Human Macrophages Infected with Mycobacterium tuberculosis. Sci. Rep. 2016, 6, 38963. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Tong, P.; Zheng, B.; Zhu, M.; Peng, B.; Wang, J.; Liu, Y. RNA-Seq Analysis of Long Non-Coding RNA in Human Intestinal Epithelial Cells Infected by Shiga Toxin-Producing Escherichia coli. Cytokine 2024, 173, 156421. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Liu, H.; Chen, J.; Tan, C.; Chen, H.; Wang, X. Egr-1 Is a Key Regulator of the Blood-Brain Barrier Damage Induced by Meningitic Escherichia coli. Cell Commun. Signal. 2024, 22, 44. [Google Scholar] [CrossRef]
- Wu, Z.; Fan, H.; Jin, J.; Gao, S.; Huang, R.; Wu, S.; Bao, W. Insight into Mechanisms of Pig lncRNA FUT3-AS1 Regulating E. coli F18-Bacterial Diarrhea. PLoS Pathog. 2022, 18, e1010584. [Google Scholar] [CrossRef]
- Yang, B.; Yin, P.; Yang, R.; Xu, B.; Fu, J.; Zhi, S.; Dai, M.; Tan, C.; Chen, H.; Wang, X. Holistic Insights into Meningitic Escherichia coli Infection of Astrocytes Based on Whole Transcriptome Profiling. Epigenomics 2020, 12, 1611–1632. [Google Scholar] [CrossRef]
- Xu, B.; Yang, R.; Yang, B.; Li, L.; Chen, J.; Fu, J.; Qu, X.; Huo, D.; Tan, C.; Chen, H.; et al. Long Non-Coding RNA lncC11orf54-1 Modulates Neuroinflammatory Responses by Activating NF-κB Signaling during Meningitic Escherichia Coli Infection. Mol. Brain 2022, 15, 4. [Google Scholar] [CrossRef]
- Xu, B.; Yang, R.; Fu, J.; Yang, B.; Chen, J.; Tan, C.; Chen, H.; Wang, X. LncRSPH9-4 Facilitates Meningitic Escherichia Coli-Caused Blood-Brain Barrier Disruption via miR-17-5p/MMP3 Axis. Int. J. Mol. Sci. 2021, 22, 6343. [Google Scholar] [CrossRef]
- Yang, B.; Xu, B.; Yang, R.; Fu, J.; Li, L.; Huo, D.; Chen, J.; Yang, X.; Tan, C.; Chen, H.; et al. Long Non-Coding Antisense RNA DDIT4-AS1 Regulates Meningitic Escherichia Coli-Induced Neuroinflammation by Promoting DDIT4 mRNA Stability. Mol. Neurobiol. 2022, 59, 1351–1365. [Google Scholar] [CrossRef]
- Ma, M.; Pei, Y.; Wang, X.; Feng, J.; Zhang, Y.; Gao, M.-Q. LncRNA XIST Mediates Bovine Mammary Epithelial Cell Inflammatory Response via NF-κB/NLRP3 Inflammasome Pathway. Cell Prolif. 2019, 52, e12525. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Jiang, X.; Wang, H.; Pan, G. LncRNA HOX Transcript Antisense RNA Accelerated Kidney Injury Induced by Urine-Derived Sepsis through the miR-22/High Mobility Group Box 1 Pathway. Life Sci. 2018, 210, 185–191. [Google Scholar] [CrossRef]
- Zhu, W.; Peng, F.; Cui, X.; Li, J.; Sun, C. LncRNA SOX2OT Facilitates LPS-Induced Inflammatory Injury by Regulating Intercellular Adhesion Molecule 1 (ICAM1) via Sponging miR-215-5p. Clin. Immunol. 2022, 238, 109006. [Google Scholar] [CrossRef]
- Ahmad, I.; Naqvi, R.A.; Valverde, A.; Naqvi, A.R. LncRNA MALAT1/microRNA-30b Axis Regulates Macrophage Polarization and Function. Front. Immunol. 2023, 14, 1214810. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.B.; Lee, W.J.; Shin, M.K.; Jung, M.H.; Shin, S.W.; Yoo, A.N.; Kim, J.W.; Yoo, H.S. Early Transcriptional Responses of Internalization Defective Brucella Abortus Mutants in Professional Phagocytes, RAW 264.7. BMC Genom. 2013, 14, 426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- von Bargen, K.; Gorvel, J.P.; Salcedo, S.P. Internal Affairs: Investigating the Brucella Intracellular Lifestyle. FEMS Microbiol. Rev. 2012, 36, 533–562. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hu, H.; Tian, M.; Zhuang, H.; Ding, C.; Yu, S. Differentially Expressed Long Noncoding RNAs in RAW264.7 Macrophages during Brucella Infection and Functional Analysis on the Bacterial Intracellular Replication. Sci. Rep. 2022, 12, 21320. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Guo, J.; Sun, Z.; Liu, L.; Zhao, T.; Li, J.; Tang, G.; Zhu, D.; Tao, T.; Zhang, H. Brucella-Induced Downregulation of lncRNA Gm28309 Triggers Macrophages Inflammatory Response Through the miR-3068-5p/NF-κB Pathway. Front. Immunol. 2020, 11, 805275. [Google Scholar] [CrossRef] [PubMed]
- Gheitasi, R.; Keramat, F.; Solgi, G.; Hajilooi, M. Investigation of Linc-MAF-4 Expression as an Effective Marker in Brucellosis. Mol. Immunol. 2020, 123, 60–63. [Google Scholar] [CrossRef]
- Gheitasi, R.; Jourghasemi, S.; Pakzad, I.; Hosseinpour Sarmadi, V.; Samieipour, Y.; Sekawi, Z.; Azizi Jalilian, F. A Potential Marker in Brucellosis, Long Non Coding RNA IFNG-AS1. Mol. Biol. Rep. 2019, 46, 6495–6500. [Google Scholar] [CrossRef] [PubMed]
- Poppe, C.; Smart, N.; Khakhria, R.; Johnson, W.; Spika, J.; Prescott, J. Salmonella typhimurium DT104: A Virulent and Drug-Resistant Pathogen. Can. Vet. J. 1998, 39, 559–565. [Google Scholar] [PubMed]
- Imamura, K.; Takaya, A.; Ishida, Y.; Fukuoka, Y.; Taya, T.; Nakaki, R.; Kakeda, M.; Imamachi, N.; Sato, A.; Yamada, T.; et al. Diminished Nuclear RNA Decay upon Salmonella Infection Upregulates Antibacterial Noncoding RNAs. EMBO J. 2018, 37, e97723. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, Y.; Wei, J.; Yue, J.; Wang, Y.; Zhang, Q.; Jin, M.; Wang, R.; Yang, X.; Zhang, J.; et al. LNCGM1082-Mediated NLRC4 Activation Drives Resistance to Bacterial Infection. Cell. Mol. Immunol. 2023, 20, 475–488. [Google Scholar] [CrossRef]
- Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. NeST, a Long Noncoding RNA, Controls Microbial Susceptibility and Epigenetic Activation of the Ifng Locus. Cell 2013, 152, 743. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, J.; Zhang, K.; Peng, Z.; Xin, R.; Wang, L.; Li, J. Asiatic Acid Attenuates Inflammation Induced by Salmonella via Upregulating LncRNA TVX1 in Microglia. Int. J. Mol. Sci. 2022, 23, 10978. [Google Scholar] [CrossRef]
- Saleh, L.A.; Boyd, A.; Aragon, I.V.; Koloteva, A.; Spadafora, D.; Mneimneh, W.; Barrington, R.A.; Richter, W. Ablation of PDE4B Protects from Pseudomonas aeruginosa-Induced Acute Lung Injury in Mice by Ameliorating the Cytostorm and Associated Hypothermia. FASEB J. 2021, 35, e21797. [Google Scholar] [CrossRef]
- Balloy, V.; Koshy, R.; Perra, L.; Corvol, H.; Chignard, M.; Guillot, L.; Scaria, V. Bronchial Epithelial Cells from Cystic Fibrosis Patients Express a Specific Long Non-Coding RNA Signature upon Pseudomonas aeruginosa Infection. Front. Cell. Infect. Microbiol. 2017, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, L.; Pu, Q.; Bu, H.; Zhu, P.; Chen, Z.; Yu, M.; Li, X.; Weiland, T.; Bansal, A.; et al. MEG3-4 Is a miRNA Decoy That Regulates IL-1β Abundance to Initiate and Then Limit Inflammation to Prevent Sepsis during Lung Infection. Sci. Signal. 2018, 11, eaao2387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Lu, Y.; Li, S.; Liu, J.; Zhang, Y.; Wang, L.; Li, M.; Luo, Y.; Zhang, W.; et al. N-3-(Oxododecanoyl)-L-Homoserine Lactone Suppresses Dendritic Cell Maturation by Upregulating the Long Noncoding RNA NRIR. J. Biosci. 2021, 46, 65. [Google Scholar] [CrossRef]
- Mogotu, M.W.; Abong, G.O.; Mburu, J.; Ndambi, O.A. Assessment of Hygiene Practices and Microbial Safety of Milk Supplied by Smallholder Farmers to Processors in Selected Counties in Kenya. Trop. Anim. Health Prod. 2022, 54, 220. [Google Scholar] [CrossRef] [PubMed]
- Indramohan, M.; Sieve, A.N.; Break, T.J.; Berg, R.E. Inflammatory Monocyte Recruitment Is Regulated by Interleukin-23 during Systemic Bacterial Infection. Infect. Immun. 2012, 80, 4099. [Google Scholar] [CrossRef] [PubMed]
- Dussurget, O.; Bierne, H.; Cossart, P. The Bacterial Pathogen Listeria monocytogenes and the Interferon Family: Type I, Type II and Type III Interferons. Front. Cell. Infect. Microbiol. 2014, 4, 50. [Google Scholar] [CrossRef]
- Quereda, J.J.; Dussurget, O.; Nahori, M.-A.; Ghozlane, A.; Volant, S.; Dillies, M.-A.; Regnault, B.; Kennedy, S.; Mondot, S.; Villoing, B.; et al. Bacteriocin from Epidemic Listeria Strains Alters the Host Intestinal Microbiota to Favor Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 5706–5711. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.Y.; Cemma, M.; Muise, A.M.; Higgins, D.E.; Brumell, J.H. Host and Bacterial Factors That Regulate LC3 Recruitment to Listeria monocytogenes during the Early Stages of Macrophage Infection. Autophagy 2013, 9, 985–995. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, D.; Chen, G.; Li, G.; Dou, S.; Wang, R.; Xiao, H.; Hou, C.; Li, Y.; Feng, J.; et al. Tim-3 Inhibits Macrophage Control of Listeria Monocytogenes by Inhibiting Nrf2. Sci. Rep. 2017, 7, 42095. [Google Scholar] [CrossRef]
- Atianand, M.K.; Hu, W.; Satpathy, A.T.; Shen, Y.; Ricci, E.P.; Alvarez-Dominguez, J.R.; Bhatta, A.; Schattgen, S.A.; McGowan, J.D.; Blin, J.; et al. A Long Noncoding RNA lincRNA-EPS Acts as a Transcriptional Brake to Restrain Inflammation. Cell 2016, 165, 1672–1685. [Google Scholar] [CrossRef]
- Agliano, F.; Fitzgerald, K.A.; Vella, A.T.; Rathinam, V.A.; Medvedev, A.E. Long Non-Coding RNA LincRNA-EPS Inhibits Host Defense Against Listeria monocytogenes Infection. Front. Cell. Infect. Microbiol. 2019, 9, 481. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Y.; Yuan, L.; Ling, W.; Jiang, X.; Chen, S.; Hu, B. LincRNA-Cox2 Regulates IL6/JAK3/STAT3 and NF-κB P65 Pathway Activation in Listeria monocytogenes-Infected RAW264.7 Cells. Int. J. Med. Microbiol. 2021, 311, 151515. [Google Scholar] [CrossRef]
- Chan, J.; Atianand, M.; Jiang, Z.; Carpenter, S.; Aiello, D.; Elling, R.; Fitzgerald, K.A.; Caffrey, D.R. Cutting Edge: A Natural Antisense Transcript, AS-IL1α, Controls Inducible Transcription of the Proinflammatory Cytokine IL-1α. J. Immunol. 2015, 195, 1359–1363. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Xu, X.; Su, X.; Liu, Y.; Ma, Y.; Zhao, Y.; Shen, Z.; Huang, B.; Cao, X. Inducible Degradation of lncRNA Sros1 Promotes IFN-γ-Mediated Activation of Innate Immune Responses by Stabilizing Stat1 mRNA. Nat. Immunol. 2019, 20, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, O.; Sesto, N.; Mellin, J.R.; Karunker, I.; Edelheit, S.; Bécavin, C.; Archambaud, C.; Cossart, P.; Sorek, R. Comparative Transcriptomics of Pathogenic and Non-Pathogenic Listeria Species. Mol. Syst. Biol. 2012, 8, 583. [Google Scholar] [CrossRef]
- Foster, T. Staphylococcus. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Wilke, G.A.; Wardenburg, J.B. Role of a Disintegrin and Metalloprotease 10 in Staphylococcus Aureus α-Hemolysin–Mediated Cellular Injury. Proc. Natl. Acad. Sci. USA 2010, 107, 13473–13478. [Google Scholar] [CrossRef]
- Duan, J.; Li, M.; Hao, Z.; Shen, X.; Liu, L.; Jin, Y.; Wang, S.; Guo, Y.; Yang, L.; Wang, L.; et al. Subinhibitory Concentrations of Resveratrol Reduce Alpha-Hemolysin Production in Staphylococcus Aureus Isolates by Downregulating saeRS. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Klepsch, M.; Manger, M.; Wolz, C.; Rudel, T.; Fraunholz, M. Long Noncoding RNA SSR42 Controls Staphylococcus Aureus Alpha-Toxin Transcription in Response to Environmental Stimuli. J. Bacteriol. 2018, 200, e00252-18. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.M.; Miller, E.W.; Benson, M.A.; Alonzo, F.; Yoong, P.; Torres, V.J.; Hinrichs, S.H.; Dunman, P.M. Characterization of SSR42, a Novel Virulence Factor Regulatory RNA That Contributes to the Pathogenesis of a Staphylococcus Aureus USA300 Representative. J. Bacteriol. 2012, 194, 2924–2938. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, T.; Ma, Y.; Wu, X.; Chu, S.; Yang, Z. Multiple Roles of LncRNA-BMNCR on Cell Proliferation and Apoptosis by Targeting miR-145/CBFB Axis in BMECs. Vet. Q. 2023, 43, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, R.; Li, D.; Gao, M.-Q. LRRC75A Antisense lncRNA1 Knockout Attenuates Inflammatory Responses of Bovine Mammary Epithelial Cells. Int. J. Biol. Sci. 2020, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Syed, R.; Ahmed, M.; Xu, T. Modulation of lncRNA NEAT1 Overturns the Macrophages Based Immune Response in M. tuberculosis Infected Patients via miR-373 Regulation. J. Appl. Genet. 2023. [Google Scholar] [CrossRef]
- Ke, Z.; Lu, J.; Zhu, J.; Yang, Z.; Jin, Z.; Yuan, L. Down-Regulation of lincRNA-EPS Regulates Apoptosis and Autophagy in BCG-Infected RAW264.7 Macrophages via JNK/MAPK Signaling Pathway. Infect. Genet. Evol. 2020, 77, 104077. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, J.; Ma, C.; Gong, Z.; Wu, X.; Deng, G. Impact of Knockdown LincRNA-Cox2 on Apoptosis of Macrophage Infected with Bacillus Calmette-Guérin. Mol. Immunol. 2021, 130, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Heeney, J.L. Zoonotic Viral Diseases and the Frontier of Early Diagnosis, Control and Prevention. J. Intern. Med. 2006, 260, 399–408. [Google Scholar] [CrossRef]
- Reed, K.D. Viral Zoonoses. In Reference Module in Biomedical Sciences; Elsevier: Madison, WI, USA, 2018; ISBN 978-0-12-801238-3. [Google Scholar]
- Gan, H.; Hou, X.; Wang, Y.; Xu, G.; Huang, Z.; Zhang, T.; Lin, R.; Xue, M.; Hu, H.; Liu, M.; et al. Global Burden of Rabies in 204 Countries and Territories, from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Int. J. Infect. Dis. 2023, 126, 136–144. [Google Scholar] [CrossRef]
- Diallo, M.K.; Diallo, A.O.; Dicko, A.; Richard, V.; Espié, E. Human Rabies Post Exposure Prophylaxis at the Pasteur Institute of Dakar, Senegal: Trends and Risk Factors. BMC Infect. Dis. 2019, 19, 321. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, S.; Zhong, Z.; Yang, S.; Xia, X. Quantitative Characterization of the B Cell Receptor Repertoires of Human Immunized with Commercial Rabies Virus Vaccine. Hum. Vaccines Immunother. 2021, 17, 2538–2546. [Google Scholar] [CrossRef]
- Potratz, M.; Zaeck, L.M.; Weigel, C.; Klein, A.; Freuling, C.M.; Müller, T.; Finke, S. Neuroglia Infection by Rabies Virus after Anterograde Virus Spread in Peripheral Neurons. Acta Neuropathol. Commun. 2020, 8, 199. [Google Scholar] [CrossRef]
- Sui, B.; Chen, D.; Liu, W.; Wu, Q.; Tian, B.; Li, Y.; Hou, J.; Liu, S.; Xie, J.; Jiang, H.; et al. A Novel Antiviral lncRNA, EDAL, Shields a T309 O-GlcNAcylation Site to Promote EZH2 Lysosomal Degradation. Genome Biol. 2020, 21, 228. [Google Scholar] [CrossRef]
- Sui, B.; Zhao, J.; Zheng, J.; Zhou, M.; Chen, H.; Fu, Z.F.; Zhao, L. lncRNA EDAL Restricts Rabies Lyssavirus Replication in a Cell-Specific and Infection Route-Dependent Manner. J. Gen. Virol. 2022, 103, 001725. [Google Scholar] [CrossRef]
- Marí Saéz, A.; Weiss, S.; Nowak, K.; Lapeyre, V.; Zimmermann, F.; Düx, A.; Kühl, H.S.; Kaba, M.; Regnaut, S.; Merkel, K.; et al. Investigating the Zoonotic Origin of the West African Ebola Epidemic. EMBO Mol. Med. 2015, 7, 17–23. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; Vega, M.A.D.L.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola Virus Disease. Nat. Rev. Dis. Primer 2020, 6, 13. [Google Scholar] [CrossRef]
- Santus, L.; Sopena-Rios, M.; García-Pérez, R.; Lin, A.E.; Adams, G.C.; Barnes, K.G.; Siddle, K.J.; Wohl, S.; Reverter, F.; Rinn, J.L.; et al. Single-Cell Profiling of lncRNA Expression during Ebola Virus Infection in Rhesus Macaques. Nat. Commun. 2023, 14, 3866. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Brinton, M.A.; Basu, M. Functions of the 3′ and 5′ Genome RNA Regions of Members of the Genus Flavivirus. Virus Res. 2015, 206, 108–119. [Google Scholar] [CrossRef]
- Murugesan, A.; Manoharan, M. Dengue Virus. Emerg. Reemerging Viral Pathog. 2020, 1, 281–359. [Google Scholar] [CrossRef]
- Kumar, S.; Nyodu, R.; Maurya, V.K.; Saxena, S.K.; Kumar, S.; Nyodu, R.; Maurya, V.K.; Saxena, S.K. Pathogenesis and Host Immune Response during Japanese Encephalitis Virus Infection. In Innate Immunity in Health and Disease; IntechOpen: London, UK, 2021; ISBN 978-1-83880-766-5. [Google Scholar]
- Hsieh, J.T.; St. John, A.L. Japanese Encephalitis Virus and Its Mechanisms of Neuroinvasion. PLoS Pathog. 2020, 16, e1008260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qi, L.; Li, T.; Li, X.; Yang, D.; Cao, S.; Ye, J.; Wei, B. PD1+CCR2+CD8+ T Cells Infiltrate the Central Nervous System during Acute Japanese Encephalitis Virus Infection. Virol. Sin. 2019, 34, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Thapa, U.; Bandyopadhyay, B.; Vrati, S.; Banerjee, A. Knockdown of NEAT1 Restricts Dengue Virus Replication by Augmenting Interferon Alpha-Inducible Protein 27 via the RIG-I Pathway. J. Gen. Virol. 2023, 104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, H.; Cui, G.; Guo, Q.; Si, L.; Yan, H.; Fang, D.; Jiang, L.; Jiang, Z.; Zhou, J. ERG-Associated lncRNA (ERGAL) Promotes the Stability and Integrity of Vascular Endothelial Barrier During Dengue Viral Infection via Interaction With miR-183-5p. Front. Cell. Infect. Microbiol. 2020, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yuan, Q.; Zhang, C.; Dai, Z.; Du, C.; Wang, H.; Li, X.; Yang, S.; Zhao, A. Inhibition of Japanese Encephalitis Virus Proliferation by Long Non-Coding RNA SUSAJ1 in PK-15 Cells. Virol. J. 2021, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Fan, J.; Wang, H.; Li, X.; Yang, S.; Zhao, A.; Zhou, X. LncRNA-SUSAJ1 Activates the ER Stress Pathway Inhibiting JEV Proliferation by Promoting PK15 Cells Apoptosis. Front. Biosci. Landmark Ed. 2022, 27, 260. [Google Scholar] [CrossRef]
- Tripathi, S.; Sengar, S.; Shree, B.; Mohapatra, S.; Basu, A.; Sharma, V. An RBM10 and NF-κB Interacting Host lncRNA Promotes JEV Replication and Neuronal Cell Death. J. Virol. 2023, 97, e0118323. [Google Scholar] [CrossRef]
- Huang, Y.; Su, Y.; Shen, L.; Huo, Z.; Chen, C.; Sun, T.; Tian, X.; Li, N.; Yang, C. A Novel IFNbeta-Induced Long Non-Coding RNA ZAP-IT1 Interrupts Zika Virus Replication in A549 Cells. Virol. Sin. 2022, 37, 904. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Vrati, S. The Malat1 Long Non-Coding RNA Is Upregulated by Signalling through the PERK Axis of Unfolded Protein Response during Flavivirus Infection. Sci. Rep. 2015, 5, 17794. [Google Scholar] [CrossRef]
- Mohapatra, S.; Tripathi, S.; Sharma, V.; Basu, A. Regulation of Microglia-Mediated Inflammation by Host lncRNA Gm20559 upon Flaviviral Infection. Cytokine 2023, 172, 156383. [Google Scholar] [CrossRef]
- Charley, P.A.; Wilusz, J. Standing Your Ground to Exoribonucleases: Function of Flavivirus Long Non-Coding RNAs. Virus Res. 2016, 212, 70–77. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Trends in Sexual Risk Behavior among High School Students—United States, 1990, 1991, and 1993. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 124–125, 131–132. [Google Scholar]
- van Schalkwyk, C.; Mahy, M.; Johnson, L.F.; Imai-Eaton, J.W. Updated Data and Methods for the 2023 UNAIDS HIV Estimates. J. Acquir. Immune Defic. Syndr. 2024, 95, e1–e4. [Google Scholar] [CrossRef]
- Sharp, P.M.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841. [Google Scholar] [CrossRef] [PubMed]
- Le Goaster, J.; Bouree, P.; El Sissy, F.N.; Phuong Bui, F.; Pokossy Epee, J.; Rollin, P.; Tangy, F.; Haenni, A.-L. HSV-1/HSV-2 Infection-Related Cancers in Bantu Populations Driving HIV-1 Prevalence in Africa: Tracking the Origin of AIDS at the Onset of the 20th Century. Case Rep. Oncol. 2016, 9, 815–825. [Google Scholar] [CrossRef]
- Zhang, J.; Thakuri, B.K.C.; Zhao, J.; Nguyen, L.N.; Nguyen, L.N.T.; Khanal, S.; Cao, D.; Dang, X.; Schank, M.; Lu, Z.; et al. Long Noncoding RNA RUNXOR Promotes Myeloid-Derived Suppressor Cell Expansion and Functions via Enhancing Immunosuppressive Molecule Expressions during Latent HIV Infection. J. Immunol. 2021, 206, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Thakuri, B.K.C.; Zhao, J.; Nguyen, L.N.; Nguyen, L.N.T.; Cao, D.; Dang, X.; Khanal, S.; Schank, M.; Lu, Z.; et al. Long Noncoding RNA HOTAIRM1 Promotes Myeloid-Derived Suppressor Cell Expansion and Suppressive Functions through up-Regulating HOXA1 Expression during Latent HIV Infection. AIDS Lond. Engl. 2020, 34, 2211–2221. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Zuo, L.; Gao, Z.; Shi, Y.; Yuan, P.; Han, S.; Yin, J.; Peng, B.; He, X.; et al. Short Communication: Long Noncoding RNA GAS5 Inhibits HIV-1 Replication Through Interaction with miR-873. AIDS Res. Hum. Retroviruses 2018, 34, 544–549. [Google Scholar] [CrossRef]
- Nguyen, L.N.T.; Nguyen, L.N.; Zhao, J.; Schank, M.; Dang, X.; Cao, D.; Khanal, S.; Chand Thakuri, B.K.; Lu, Z.; Zhang, J.; et al. Long Non-Coding RNA GAS5 Regulates T Cell Functions via miR21-Mediated Signaling in People Living With HIV. Front. Immunol. 2021, 12, 601298. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Huan, C.; Yang, J.; Li, Z.; Zheng, B.; Wang, Y.; Zhang, W. NF-κB-Interacting Long Noncoding RNA Regulates HIV-1 Replication and Latency by Repressing NF-κB Signaling. J. Virol. 2020, 94, e01057-20. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Abe, J.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Baranwal, V.K.; et al. Annual (2023) Taxonomic Update of RNA-Directed RNA Polymerase-Encoding Negative-Sense RNA Viruses (Realm Riboviria: Kingdom Orthornavirae: Phylum Negarnaviricota). J. Gen. Virol. 2023, 104, 001864. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef]
- Goneau, L.W.; Mehta, K.; Wong, J.; L’Huillier, A.G.; Gubbay, J.B. Zoonotic Influenza and Human Health-Part 1: Virology and Epidemiology of Zoonotic Influenzas. Curr. Infect. Dis. Rep. 2018, 20, 37. [Google Scholar] [CrossRef]
- Janke, B.H. Influenza A Virus Infections in Swine: Pathogenesis and Diagnosis. Vet. Pathol. 2014, 51, 410–426. [Google Scholar] [CrossRef]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef]
- Richard, M.; Fouchier, R.A.M. Influenza A Virus Transmission via Respiratory Aerosols or Droplets as It Relates to Pandemic Potential. FEMS Microbiol. Rev. 2016, 40, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Thatai, A.K.S.; Ammankallu, S.; Devasahayam Arokia Balaya, R.; Soman, S.P.; Nisar, M.; Babu, S.; John, L.; George, A.; Anto, C.K.; Sanjeev, D.; et al. VirhostlncR: A Comprehensive Database to Explore lncRNAs and Their Targets in Viral Infections. Comput. Biol. Med. 2023, 164, 107279. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Zhu, Z.; Lin, K.; Huang, C.; Pushparaj, S.; Liang, Y.; Sathiaseelan, R.; Yang, X.; Liu, L. Long Non-Coding RNA PSMB8-AS1 Regulates Influenza Virus Replication. RNA Biol. 2019, 16, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Meydan, C.; Madrer, N.; Soreq, H. The Neat Dance of COVID-19: NEAT1, DANCR, and Co-Modulated Cholinergic RNAs Link to Inflammation. Front. Immunol. 2020, 11, 590870. [Google Scholar] [CrossRef] [PubMed]
- Winterling, C.; Koch, M.; Koeppel, M.; Garcia-Alcalde, F.; Karlas, A.; Meyer, T.F. Evidence for a Crucial Role of a Host Non-Coding RNA in Influenza A Virus Replication. RNA Biol. 2014, 11, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Zheng, X.; Wang, G.; Chen, X.; Hu, Z.; Chen, Y.; Wang, X.; Gu, M.; Hu, S.; et al. Long Noncoding RNA #61 Exerts a Broad Anti-Influenza a Virus Effect by Its Long Arm Rings. Antivir. Res. 2023, 215, 105637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zheng, X.; Li, J.; Wang, G.; Hu, Z.; Chen, Y.; Wang, X.; Gu, M.; Gao, R.; Hu, S.; et al. Long Noncoding RNA#45 Exerts Broad Inhibitory Effect on Influenza a Virus Replication via Its Stem Ring Arms. Virulence 2021, 12, 2443–2460. [Google Scholar] [CrossRef] [PubMed]
- van Solingen, C.; Cyr, Y.; Scacalossi, K.R.; de Vries, M.; Barrett, T.J.; de Jong, A.; Gourvest, M.; Zhang, T.; Peled, D.; Kher, R.; et al. Long Noncoding RNA CHROMR Regulates Antiviral Immunity in Humans. Proc. Natl. Acad. Sci. USA 2022, 119, e2210321119. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Sun, Z.; Gong, L.; Li, X.; Shi, F.; Yao, J.; Meng, Y.; Meng, X.; Zhang, Q.; et al. LncNSPL Facilitates Influenza A Viral Immune Escape by Restricting TRIM25-Mediated K63-Linked RIG-I Ubiquitination. iScience 2022, 25, 104607. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Liu, S.; Chen, B.; Xiao, M.; Li, Y.; Liao, Y.; Rai, K.R.; Zhao, Z.; Ouyang, J.; et al. RDUR, a lncRNA, Promotes Innate Antiviral Responses and Provides Feedback Control of NF-κB Activation. Front. Immunol. 2021, 12, 672165. [Google Scholar] [CrossRef]
- Gatherer, D.; Depledge, D.P.; Hartley, C.A.; Szpara, M.L.; Vaz, P.K.; Benkő, M.; Brandt, C.R.; Bryant, N.A.; Dastjerdi, A.; Doszpoly, A.; et al. ICTV Virus Taxonomy Profile: Herpesviridae 2021. J. Gen. Virol. 2021, 102, 001673. [Google Scholar] [CrossRef]
- Woźniakowski, G.; Samorek-Salamonowicz, E. Animal Herpesviruses and Their Zoonotic Potential for Cross-Species Infection. Ann. Agric. Environ. Med. AAEM 2015, 22, 191–194. [Google Scholar] [CrossRef]
- Yang, L.; Maruo, S.; Takada, K. CD21-Mediated Entry and Stable Infection by Epstein-Barr Virus in Canine and Rat Cells. J. Virol. 2000, 74, 10745–10751. [Google Scholar] [CrossRef][Green Version]
- Fang, L.; Gao, Y.; Liu, X.; Bai, J.; Jiang, P.; Wang, X. Long Non-Coding RNA LNC_000641 Regulates Pseudorabies Virus Replication. Vet. Res. 2021, 52, 52. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, K.; Huang, W.; Tang, W.; Li, H.; Dong, W.; Gu, J.; Zhou, J. Identification of Functional lncRNAs in Pseudorabies Virus Type II Infected Cells. Vet. Microbiol. 2020, 242, 108564. [Google Scholar] [CrossRef] [PubMed]
- Hotoboc, I.E.; Fudulu, A.; Grigore, R.; Bertesteanu, S.; Huica, I.; Iancu, I.V.; Botezatu, A.; Bleotu, C.; Anton, G. The Association between lncRNA H19 and EZH2 Expression in Patients with EBV-Positive Laryngeal Carcinoma. Acta Otorhinolaryngol. Ital. 2021, 41, 537–543. [Google Scholar] [CrossRef]
- Tombácz, D.; Csabai, Z.; Oláh, P.; Balázs, Z.; Likó, I.; Zsigmond, L.; Sharon, D.; Snyder, M.; Boldogkői, Z. Full-Length Isoform Sequencing Reveals Novel Transcripts and Substantial Transcriptional Overlaps in a Herpesvirus. PLoS ONE 2016, 11, e0162868. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; de Groot, R.J.; Haagmans, B.; Lau, S.K.P.; Neuman, B.W.; Perlman, S.; Sola, I.; van der Hoek, L.; Wong, A.C.P.; Yeh, S.-H. ICTV Virus Taxonomy Profile: Coronaviridae 2023. J. Gen. Virol. 2023, 104, 001843. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Zhao, J.; Li, L.; Huang, X.; Yang, H.; Cheng, J.; Liu, C.; Zhang, G. Pathogenic Characteristics of a QX-like Infectious Bronchitis Virus Strain SD in Chickens Exposed at Different Ages and Protective Efficacy of Combining Live Homologous and Heterologous Vaccination. Vet. Res. 2020, 51, 86. [Google Scholar] [CrossRef]
- Alluwaimi, A.M.; Alshubaith, I.H.; Al-Ali, A.M.; Abohelaika, S. The Coronaviruses of Animals and Birds: Their Zoonosis, Vaccines, and Models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020, 7, 582287. [Google Scholar] [CrossRef]
- Graham, R.L.; Baric, R.S. Recombination, Reservoirs, and the Modular Spike: Mechanisms of Coronavirus Cross-Species Transmission. J. Virol. 2010, 84, 3134–3146. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic Origins of Human Coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Walls, A.C.; Joshi, A.; Park, Y.-J.; Eguia, R.T.; Miranda, M.C.; Kepl, E.; Dosey, A.; Stevens-Ayers, T.; Boeckh, M.J.; et al. Structure, Receptor Recognition, and Antigenicity of the Human Coronavirus CCoV-HuPn-2018 Spike Glycoprotein. Cell 2022, 185, 2279–2291.e17. [Google Scholar] [CrossRef] [PubMed]
- Ayeldeen, G.; Shaker, O.G.; Amer, E.; Zaafan, M.A.; Herzalla, M.R.; Keshk, M.A.; Abdelhamid, A.M. The Impact of lncRNA-GAS5/miRNA-200/ACE2 Molecular Pathway on the Severity of COVID-19. Curr. Med. Chem. 2024, 31, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Pushparaj, S.; Gandikota, C.; Vaddadi, K.; Liang, Y.; Liu, L. SNHG15 Aids SARS-CoV-2 Entry via RABL2A. RNA Biol. 2023, 20, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Aznaourova, M.; Schmerer, N.; Janga, H.; Zhang, Z.; Pauck, K.; Bushe, J.; Volkers, S.M.; Wendisch, D.; Georg, P.; Ntini, E.; et al. Single-Cell RNA Sequencing Uncovers the Nuclear Decoy lincRNA PIRAT as a Regulator of Systemic Monocyte Immunity during COVID-19. Proc. Natl. Acad. Sci. USA 2022, 119, e2120680119. [Google Scholar] [CrossRef] [PubMed]
- Askari, N.; Hadizadeh, M.; Rashidifar, M. A New Insight into Sex-Specific Non-Coding RNAs and Networks in Response to SARS-CoV-2. Infect. Genet. Evol. 2022, 97, 105195. [Google Scholar] [CrossRef] [PubMed]
- Genena, S.E.S.R.; Fadhil, M.M.; Mansour, M.M.; Attwa, A.H.M.; Khalil, M.M.I.M. Expression Pattern of Long Non-Coding RNAs MALAT1 and MEG3 in COVID-19 Patients. J. Gene Med. 2023, 25, e3532. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhou, L.; Wang, H.; Chen, N.; Jia, L.; Wang, C.; Wang, Y.; Chen, J.; Wen, X.; Niu, C.; et al. Profiling the Epigenetic Interplay of lncRNA RUNXOR and Oncogenic RUNX1 in Breast Cancer Cells by Gene in Situ Cis-Activation. Am. J. Cancer Res. 2019, 9, 1635–1649. [Google Scholar] [PubMed]
- Mathison, B.A.; Pritt, B.S. The Landscape of Parasitic Infections in the United States. Mod. Pathol. 2023, 36, 100217. [Google Scholar] [CrossRef] [PubMed]
- Alonso, P.L. The Role of Mass Drug Administration of Antimalarials. Am. J. Trop. Med. Hyg. 2020, 103, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Olajide, J.S.; Olopade, B.; Cai, J. Functional Intricacy and Symmetry of Long Non-Coding RNAs in Parasitic Infections. Front. Cell. Infect. Microbiol. 2021, 11, 751523. [Google Scholar] [CrossRef] [PubMed]
- Leija-Montoya, A.G.; González-Ramírez, J.; Martínez-Coronilla, G.; Mejía-León, M.E.; Isiordia-Espinoza, M.; Sánchez-Muñoz, F.; Chávez-Cortez, E.G.; Pitones-Rubio, V.; Serafín-Higuera, N. Roles of microRNAs and Long Non-Coding RNAs Encoded by Parasitic Helminths in Human Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8173. [Google Scholar] [CrossRef] [PubMed]
- Garrigós, M.; Ylla, G.; Martínez-de la Puente, J.; Figuerola, J.; Ruiz-López, M.J. Two Avian Plasmodium Species Trigger Different Transcriptional Responses on Their Vector Culex Pipiens. Mol. Ecol. 2023, 00, e17240. [Google Scholar] [CrossRef]
- Batugedara, G.; Lu, X.M.; Hristov, B.; Abel, S.; Chahine, Z.; Hollin, T.; Williams, D.; Wang, T.; Cort, A.; Lenz, T.; et al. Novel Insights into the Role of Long Non-Coding RNA in the Human Malaria Parasite, Plasmodium falciparum. Nat. Commun. 2023, 14, 5086. [Google Scholar] [CrossRef]
- Filarsky, M.; Fraschka, S.A.; Niederwieser, I.; Brancucci, N.M.B.; Carrington, E.; Carrió, E.; Moes, S.; Jenoe, P.; Bártfai, R.; Voss, T.S. GDV1 Induces Sexual Commitment of Malaria Parasites by Antagonizing HP1-Dependent Gene Silencing. Science 2018, 359, 1259–1263. [Google Scholar] [CrossRef]
- Simantov, K.; Goyal, M.; Dzikowski, R. Emerging Biology of Noncoding RNAs in Malaria Parasites. PLoS Pathog. 2022, 18, e1010600. [Google Scholar] [CrossRef]
- Broadbent, K.M.; Park, D.; Wolf, A.R.; Van Tyne, D.; Sims, J.S.; Ribacke, U.; Volkman, S.; Duraisingh, M.; Wirth, D.; Sabeti, P.C.; et al. A Global Transcriptional Analysis of Plasmodium falciparum Malaria Reveals a Novel Family of Telomere-Associated lncRNAs. Genome Biol. 2011, 12, R56. [Google Scholar] [CrossRef]
- Thompson, T.A.; Chahine, Z.; Roch, K.G.L. The Role of Long Noncoding RNAs in Malaria Parasites. Trends Parasitol. 2023, 39, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Amit-Avraham, I.; Pozner, G.; Eshar, S.; Fastman, Y.; Kolevzon, N.; Yavin, E.; Dzikowski, R. Antisense Long Noncoding RNAs Regulate Var Gene Activation in the Malaria Parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2015, 112, E982–E991. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, S.-C.; Fan, X.-Y.; Jin, Y.-L.; Li, X.; Du, Y.-T. Plasmodium Manipulates the Expression of Host Long Non-Coding RNA during Red Blood Cell Intracellular Infection. Parasit. Vectors 2022, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; West, K.A.; James, K.R.; Rani, G.F.; Dey, N.; Romano, A.; Brown, N.; Teichmann, S.A.; Kaye, P.M.; Lagos, D. Malat1 Suppresses Immunity to Infection through Promoting Expression of Maf and IL-10 in Th Cells. J. Immunol. 2020, 204, 2949–2960. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primer 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Silveira, G.O.; Coelho, H.S.; Pereira, A.S.A.; Miyasato, P.A.; Santos, D.W.; Maciel, L.F.; Olberg, G.G.G.; Tahira, A.C.; Nakano, E.; Oliveira, M.L.S.; et al. Long Non-Coding RNAs Are Essential for Schistosoma mansoni Pairing-Dependent Adult Worm Homeostasis and Fertility. PLoS Pathog. 2023, 19, e1011369. [Google Scholar] [CrossRef]
- Amaral, M.S.; Maciel, L.F.; Silveira, G.O.; Olberg, G.G.O.; Leite, J.V.P.; Imamura, L.K.; Pereira, A.S.A.; Miyasato, P.A.; Nakano, E.; Verjovski-Almeida, S. Long Non-Coding RNA Levels Can Be Modulated by 5-Azacytidine in Schistosoma mansoni. Sci. Rep. 2020, 10, 21565. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; Zhu, Y.; Chen, J.; Dong, C.; Tao, Y.; He, A.; Liu, J.; Wu, Z. Identification of Long Noncoding RNAs in Schistosoma mansoni and Schistosoma japonicum. Exp. Parasitol. 2018, 191, 82–87. [Google Scholar] [CrossRef]
- Xia, T.; Giri, B.R.; Liu, J.; Du, P.; Li, X.; Li, X.; Li, S.; Cheng, G. RNA Sequencing Analysis of Altered Expression of Long Noncoding RNAs Associated with Schistosoma japonicum Infection in the Murine Liver and Spleen. Parasit. Vectors 2020, 13, 601. [Google Scholar] [CrossRef]
- Zhao, R.; Tang, X.; Lin, H.; Xing, C.; Xu, N.; Dai, B.; Wang, P.; Shao, W.; Liu, M.; Shen, J.; et al. Knocking Down Gm16685 Decreases Liver Granuloma in Murine Schistosomiasis japonica. Microorganisms 2023, 11, 796. [Google Scholar] [CrossRef]
- Ma, R.; Liu, Q.; Liu, Z.; Sun, X.; Jiang, X.; Hou, J.; Zhang, Y.; Wu, Y.; Cheng, M.; Dong, Z. H19/Mir-130b-3p/Cyp4a14 Potentiate the Effect of Praziquantel on Liver in the Treatment of Schistosoma japonicum Infection. Acta Trop. 2023, 247, 107012. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Dwintasari, S.W.; Connelly, L.; Nichols, R.A.B.; Yunihastuti, E.; Karyadi, T.; Djauzi, S. Cryptosporidium Species from Human Immunodeficiency-Infected Patients with Chronic Diarrhea in Jakarta, Indonesia. Ann. Epidemiol. 2013, 23, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L.; Li, M.; Gong, A.-Y.; Deng, S.; Jin, K.; Wang, S.; Chen, X.-M. Cryptosporidium parvum Hijacks a Host’s Long Noncoding RNA U90926 to Evade Intestinal Epithelial Cell-Autonomous Antiparasitic Defense. Front. Immunol. 2023, 14, 1205468. [Google Scholar] [CrossRef] [PubMed]
- Mathy, N.W.; Deng, S.; Gong, A.-Y.; Li, M.; Wang, Y.; Burleigh, O.; Kochvar, A.; Whiteford, E.R.; Shibata, A.; Chen, X.-M. The Long Non-Coding RNA Nostrill Regulates Transcription of Irf7 Through Interaction With NF-κB P65 to Enhance Intestinal Epithelial Defense against Cryptosporidium parvum. Front. Immunol. 2022, 13, 863957. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gong, A.-Y.; Zhang, X.-T.; Wang, Y.; Mathy, N.W.; Martins, G.A.; Strauss-Soukup, J.K.; Chen, X.-M. Induction of a Long Noncoding RNA Transcript, NR_045064, Promotes Defense Gene Transcription and Facilitates Intestinal Epithelial Cell Responses against Cryptosporidium Infection. J. Immunol. 2018, 201, 3630–3640. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.-Y.; Wang, Y.; Li, M.; Zhang, X.-T.; Deng, S.; Chen, J.M.; Lu, E.; Mathy, N.W.; Martins, G.A.; Strauss-Soukup, J.K.; et al. LncRNA XR_001779380 Primes Epithelial Cells for IFN-γ-Mediated Gene Transcription and Facilitates Age-Dependent Intestinal Antimicrobial Defense. mBio 2021, 12, e0212721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, K.; Li, M.; Mathy, N.W.; Gong, A.-Y.; Deng, S.; Martins, G.A.; Sun, M.; Strauss-Soukup, J.K.; Chen, X.-M. A Host Cell Long Noncoding RNA NR_033736 Regulates Type I Interferon-Mediated Gene Transcription and Modulates Intestinal Epithelial Anti-Cryptosporidium Defense. PLoS Pathog. 2021, 17, e1009241. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R&D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- Menard, K.L.; Haskins, B.E.; Colombo, A.P.; Denkers, E.Y. Toxoplasma gondii Manipulates Expression of Host Long Noncoding RNA during Intracellular Infection. Sci. Rep. 2018, 8, 15017. [Google Scholar] [CrossRef]
- Wang, Y.; Han, R.; Xu, Z.; Sun, X.; Zhou, C.; Han, B.; He, S.; Cong, H. Upregulation of lncRNA147410.3 in the Brain of Mice with Chronic Toxoplasma Infection Promoted Microglia Apoptosis by Regulating Hoxb3. Front. Cell. Neurosci. 2021, 15, 648047. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Wang, Y.; Ai, K.; Pan, G.; Li, Y.; Zhou, C.; He, S.; Cong, H. Downregulation of lncRNA-11496 in the Brain Contributes to Microglia Apoptosis via Regulation of Mef2c in Chronic T. gondii Infection Mice. Front. Mol. Neurosci. 2020, 13, 77. [Google Scholar] [CrossRef]
- Liu, W.; Huang, L.; Wei, Q.; Zhang, Y.; Zhang, S.; Zhang, W.; Cai, L.; Liang, S. Microarray Analysis of Long Non-Coding RNA Expression Profiles Uncovers a Toxoplasma-Induced Negative Regulation of Host Immune Signaling. Parasit. Vectors 2018, 11, 174. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, e00075-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, H.; Li, Y.; Wang, L.; Chen, G.; Pu, G.; Guo, X.; Cho, W.C.; Fasihi Harandi, M.; Zheng, Y.; et al. Integrative Analysis of RNA Expression and Regulatory Networks in Mice Liver Infected by Echinococcus multilocularis. Front. Cell Dev. Biol. 2022, 10, 798551. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Wang, Y.; Yin, J.; Zhang, J.; Cao, S.; Cao, J.; Shen, Y. Microarray Analysis of Long Non-Coding RNA Expression Profiles in Monocytic Myeloid-Derived Suppressor Cells in Echinococcus granulosus-Infected Mice. Parasit. Vectors 2018, 11, 327. [Google Scholar] [CrossRef]
- Yang, R.; Qu, X.; Zhi, S.; Wang, J.; Fu, J.; Tan, C.; Chen, H.; Wang, X. Exosomes Derived from Meningitic Escherichia Coli-Infected Brain Microvascular Endothelial Cells Facilitate Astrocyte Activation. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.-H.; Niu, N.; Tao, J.; Du, X.-C.; Yang, J.-H.; Zhu, M.-X.; Wang, Y.-N.; Zhao, W. lncRNA028466 Regulates Th1/Th2 Cytokine Expression and Associates with Echinococcus granulosus Antigen P29 Immunity. Parasit. Vectors 2021, 14, 295. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Wang, F.; Zhang, H.; Tan, C.; Chen, H.; Wang, X. Blood-Brain Barrier Integrity Damage in Bacterial Meningitis: The Underlying Link, Mechanisms, and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 2852. [Google Scholar] [CrossRef]
- Letko, M.; Seifert, S.N.; Olival, K.J.; Plowright, R.K.; Munster, V.J. Bat-Borne Virus Diversity, Spillover and Emergence. Nat. Rev. Microbiol. 2020, 18, 461–471. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).