Noscapine and Apoptosis in Breast and Other Cancers
Abstract
1. Introduction
2. Noscapine
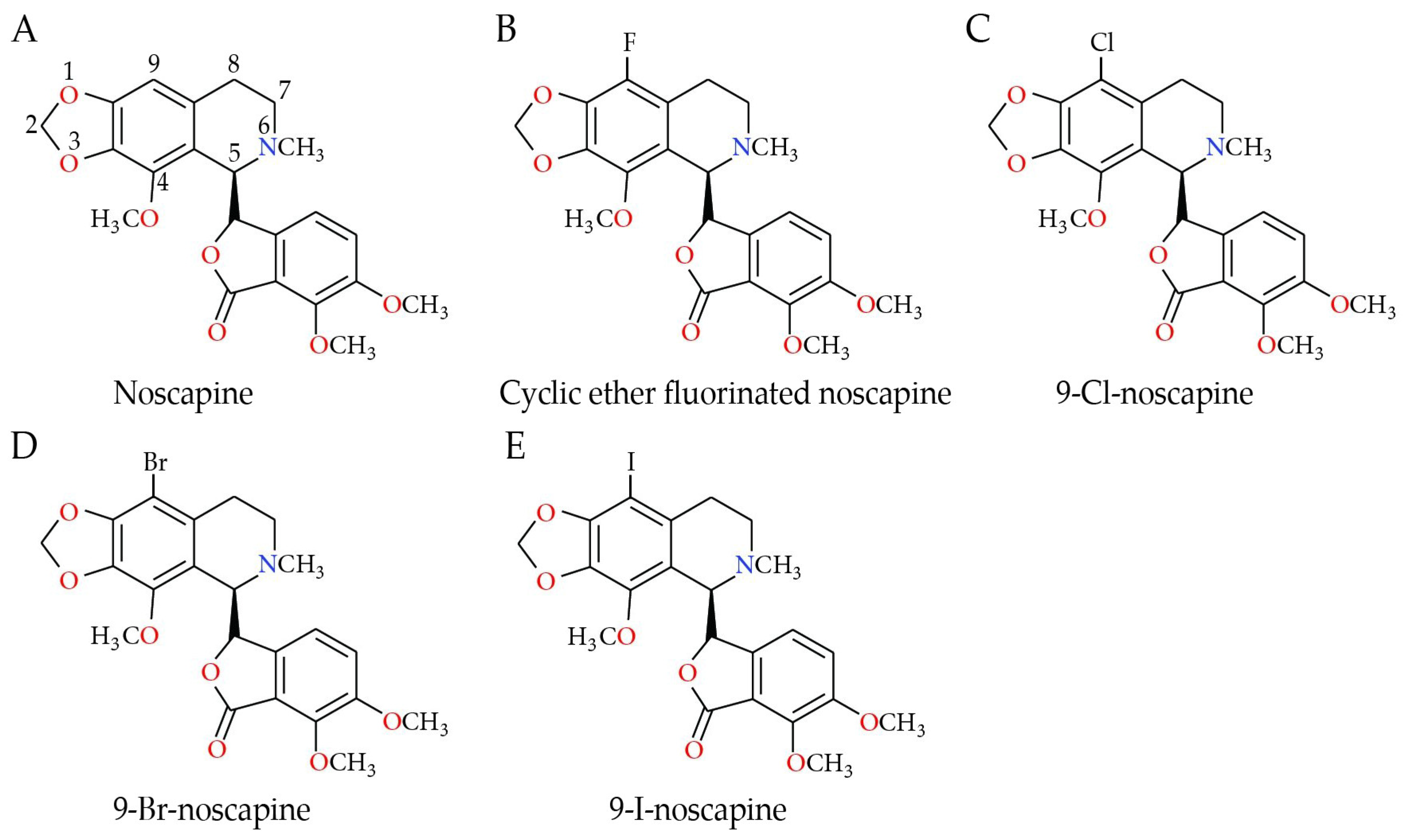
Analogs of Noscapine
3. Noscapine’s Effect in Several Types of Cancer
3.1. Breast Cancer
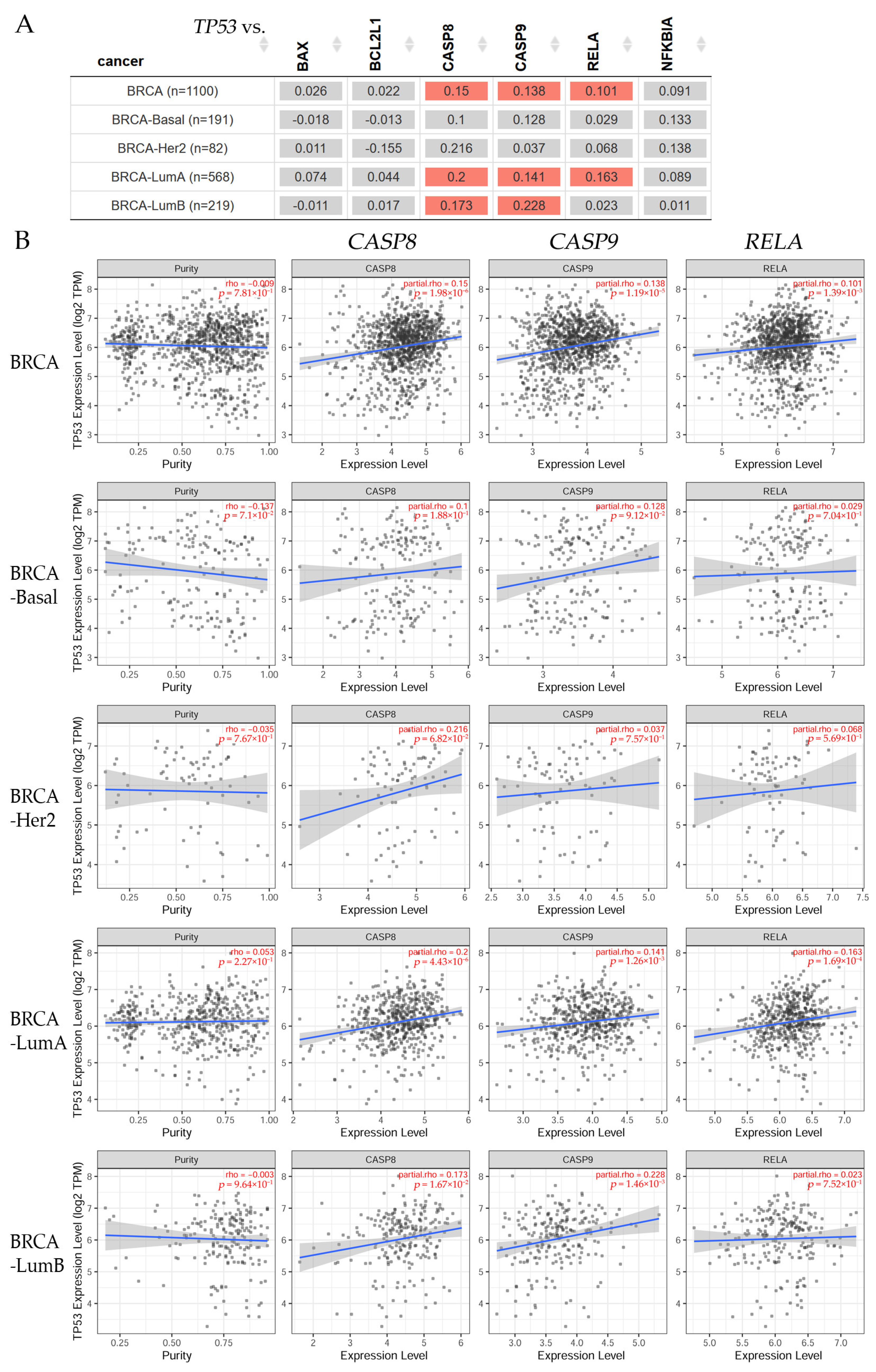
3.2. Clinical Relevance Analyzed by Bioinformatics in Breast Cancer Patients
3.2.1. Gene Correlation in Breast Cancer Patients
3.2.2. Differential Gene Expression Levels between Tumor and Normal Tissues across Various Breast Cancer Subtypes
3.2.3. Estrogen Receptor Status
3.2.4. Disease Stage Factor in Several Breast Cancer Subtypes
3.3. Lung Cancer
3.4. Ovarian Cancer
3.5. Endometrium
3.6. Colon Cancer
3.7. Stomach Cancer
3.8. Neuroblastoma
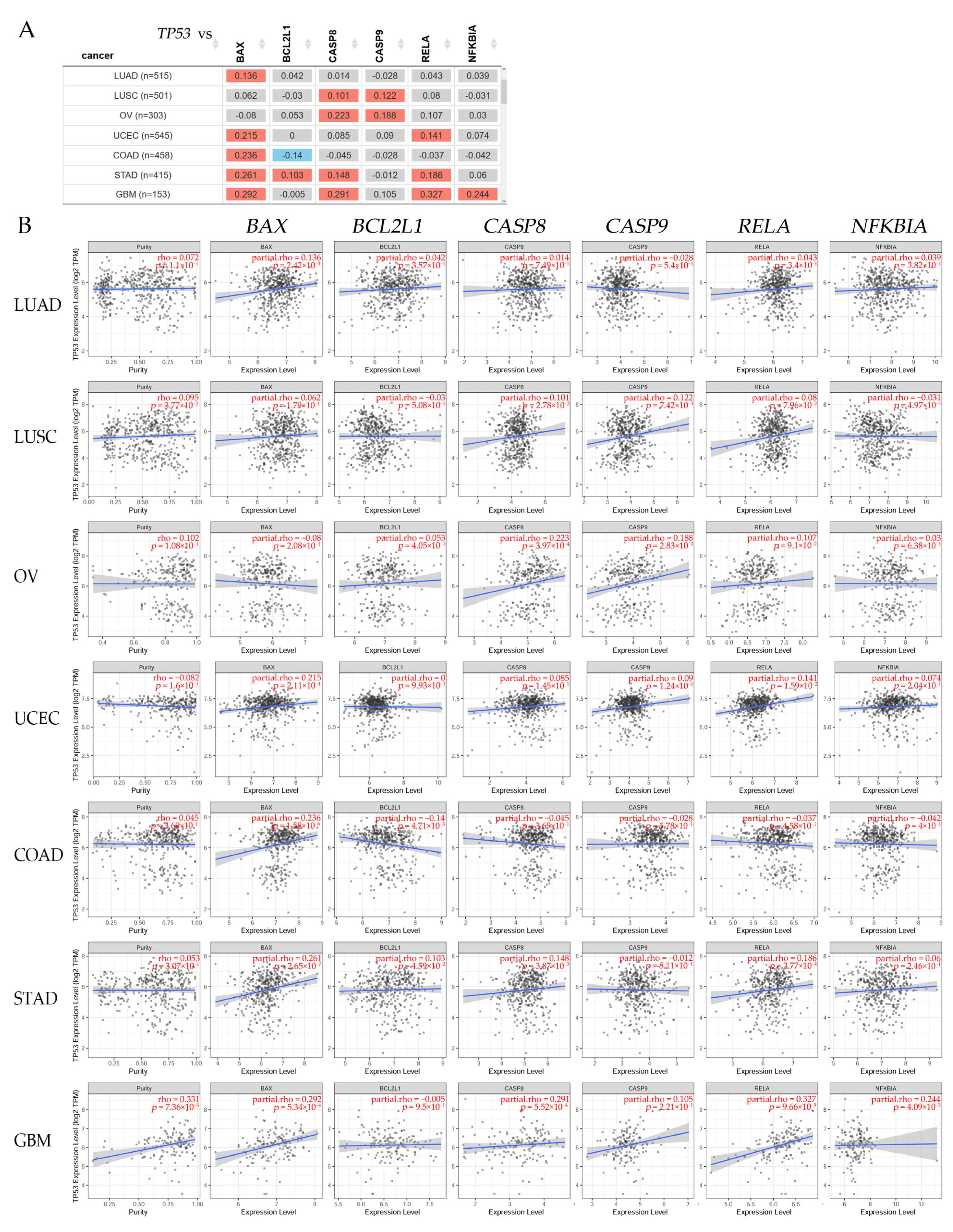
3.9. Clinical Relevance Analyzed by Bioinformatics in Several Types of Cancer
3.9.1. Gene Correlation in Different Cancer Patients
3.9.2. Differential Gene Expression Levels between Tumor and Normal Tissue across Different Types of Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Cancer Today: Estimated Age-Standardized Incidence and Mortality Rates (World) in 2020. International Agency for Research on Cancer 2023. Available online: https://gco.iarc.fr/today/ (accessed on 22 August 2023).
- Millimouno, F.M.; Dong, J.; Yang, L.; Li, J.; Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev. Res. 2014, 7, 1081–1107. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- NIH. Risk Factors for Cancer National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk (accessed on 6 February 2024).
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Li, M.X.; Dewson, G. Mitochondria and apoptosis: Emerging concepts. F1000Prime Rep. 2015, 7, 42. [Google Scholar] [CrossRef]
- Ionov, Y.; Yamamoto, H.; Krajewski, S.; Reed, J.C.; Perucho, M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc. Natl. Acad. Sci. USA 2000, 97, 10872–10877. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Yilmaz, S.G.; Yencilek, F.; Yildirim, A.; Yencilek, E.; Isbir, T. Effects of Caspase 9 Gene Polymorphism in Patients with Prostate Cancer. In Vivo 2017, 31, 205–208. [Google Scholar] [CrossRef][Green Version]
- Afzal, M.; Alarifi, A.; Abduh, N.A.Y.; Ayub, A.; Muddassir, M. Identification of anti-cancer organometallic compounds by inhibition of BCL-2/Bax interactions. Comput. Biol. Med. 2023, 167, 107657. [Google Scholar] [CrossRef]
- Shi, Z.E.; Zhang, M.Y.; Liu, J.Y.; Zhang, W.D.; Hu, D.M.; Wang, Q.X.; Ji, X.L.; Jiang, Y.Y.; Qu, Y.Q. Autophagy Induced by BCL2-Related ceRNA Network Participates in the Occurrence of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Barham, W.; Chen, L.; Tikhomirov, O.; Onishko, H.; Gleaves, L.; Stricker, T.P.; Blackwell, T.S.; Yull, F.E. Aberrant activation of NF-kappaB signaling in mammary epithelium leads to abnormal growth and ductal carcinoma in situ. BMC Cancer 2015, 15, 647. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-kappaB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Targeting nuclear factor-kappa B activation pathway by thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res. 2008, 6, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Ahn, K.S.; Aggarwal, B.B. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-kappaB signaling pathway. Cancer Res. 2010, 70, 3259–3268. [Google Scholar] [CrossRef]
- Khan, S.; Lopez-Dee, Z.; Kumar, R.; Ling, J. Activation of NFkB is a novel mechanism of pro-survival activity of glucocorticoids in breast cancer cells. Cancer Lett. 2013, 337, 90–95. [Google Scholar] [CrossRef]
- Cao, Y.; Karin, M. NF-kappaB in mammary gland development and breast cancer. J. Mammary Gland. Biol. Neoplasia 2003, 8, 215–223. [Google Scholar] [CrossRef]
- Cogswell, P.C.; Guttridge, D.C.; Funkhouser, W.K.; Baldwin, A.S., Jr. Selective activation of NF-kappa B subunits in human breast cancer: Potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 2000, 19, 1123–1131. [Google Scholar] [CrossRef]
- Wang, W.; Nag, S.A.; Zhang, R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015, 22, 264–289. [Google Scholar] [CrossRef]
- Wertz, I.E. TNFR1-activated NF-kappaB signal transduction: Regulation by the ubiquitin/proteasome system. Curr. Opin. Chem. Biol. 2014, 23, 71–77. [Google Scholar] [CrossRef]
- Croce, C.M. Oncogenes and cancer. N. Engl. J. Med. 2008, 358, 502–511. [Google Scholar] [CrossRef]
- Kassam, F.; Enright, K.; Dent, R.; Dranitsaris, G.; Myers, J.; Flynn, C.; Fralick, M.; Kumar, R.; Clemons, M. Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design. Clin. Breast Cancer 2009, 9, 29–33. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Afzali, M.; Ghaeli, P.; Khanavi, M.; Parsa, M.; Montazeri, H.; Ghahremani, M.H.; Ostad, S.N. Non-addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells. Daru 2015, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.M.; Mackenzie, A.J.; Spina, D.; Page, C.P. The pharmacology of cough. Trends Pharmacol. Sci. 2004, 25, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Takahama, K.; Shirasaki, T. Central and peripheral mechanisms of narcotic antitussives: Codeine-sensitive and -resistant coughs. Cough 2007, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gupta, K.; Yao, J.; Ye, K.; Panda, D.; Giannakakou, P.; Joshi, H.C. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J. Biol. Chem. 2002, 277, 39777–39785. [Google Scholar] [CrossRef] [PubMed]
- Aneja, R.; Lopus, M.; Zhou, J.; Vangapandu, S.N.; Ghaleb, A.; Yao, J.; Nettles, J.H.; Zhou, B.; Gupta, M.; Panda, D.; et al. Rational design of the microtubule-targeting anti-breast cancer drug EM015. Cancer Res. 2006, 66, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- DeBono, A.J.; Xie, J.H.; Ventura, S.; Pouton, C.W.; Capuano, B.; Scammells, P.J. Synthesis and biological evaluation of N-substituted noscapine analogues. ChemMedChem 2012, 7, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Manchukonda, N.K.; Naik, P.K.; Santoshi, S.; Lopus, M.; Joseph, S.; Sridhar, B.; Kantevari, S. Rational design, synthesis, and biological evaluation of third generation alpha-noscapine analogues as potent tubulin binding anti-cancer agents. PLoS ONE 2013, 8, e77970. [Google Scholar] [CrossRef]
- Aneja, R.; Zhou, J.; Zhou, B.; Chandra, R.; Joshi, H.C. Treatment of hormone-refractory breast cancer: Apoptosis and regression of human tumors implanted in mice. Mol. Cancer Ther. 2006, 5, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.O.; Dahlstrom, B.; Eckernas, S.A.; Johansson, M.; Alm, A.T. Pharmacokinetics of oral noscapine. Eur. J. Clin. Pharmacol. 1990, 39, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, B.; Mellstrand, T.; Lofdahl, C.G.; Johansson, M. Pharmacokinetic properties of noscapine. Eur. J. Clin. Pharmacol. 1982, 22, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Chougule, M.B.; Patel, A.R.; Jackson, T.; Singh, M. Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PLoS ONE 2011, 6, e17733. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, M.M.; Alisaraie, L.; Tuszynski, J.A. Peloruside, laulimalide, and noscapine interactions with beta-tubulin. Pharm. Res. 2012, 29, 2985–2993. [Google Scholar] [CrossRef]
- Zhou, J.; Panda, D.; Landen, J.W.; Wilson, L.; Joshi, H.C. Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J. Biol. Chem. 2002, 277, 17200–17208. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Hmadi, R.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Crown, J.; O’Leary, M. The taxanes: An update. Lancet 2000, 355, 1176–1178. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Jaafari, M.R.; Golmohammadzadeh, S.; Sanei-Far, Z.; Askari, V.R. Noscapine, an Emerging Medication for Different Diseases: A Mechanistic Review. Evid. Based Complement. Alternat. Med. 2021, 2021, 8402517. [Google Scholar] [CrossRef]
- Aneja, R.; Vangapandu, S.N.; Lopus, M.; Viswesarappa, V.G.; Dhiman, N.; Verma, A.; Chandra, R.; Panda, D.; Joshi, H.C. Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem. Pharmacol. 2006, 72, 415–426. [Google Scholar] [CrossRef]
- Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef]
- Ye, K.; Zhou, J.; Landen, J.W.; Bradbury, E.M.; Joshi, H.C. Sustained activation of p34(cdc2) is required for noscapine-induced apoptosis. J. Biol. Chem. 2001, 276, 46697–46700. [Google Scholar] [CrossRef]
- Saxton, W.M.; Stemple, D.L.; Leslie, R.J.; Salmon, E.D.; Zavortink, M.; McIntosh, J.R. Tubulin dynamics in cultured mammalian cells. J. Cell Biol. 1984, 99, 2175–2186. [Google Scholar] [CrossRef]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef]
- Lopus, M.; Naik, P.K. Taking aim at a dynamic target: Noscapinoids as microtubule-targeted cancer therapeutics. Pharmacol. Rep. 2015, 67, 56–62. [Google Scholar] [CrossRef]
- Sajadian, S.; Vatankhah, M.; Majdzadeh, M.; Kouhsari, S.M.; Ghahremani, M.H.; Ostad, S.N. Cell cycle arrest and apoptogenic properties of opium alkaloids noscapine and papaverine on breast cancer stem cells. Toxicol. Mech. Methods 2015, 25, 388–395. [Google Scholar] [CrossRef]
- Ye, K.; Ke, Y.; Keshava, N.; Shanks, J.; Kapp, J.A.; Tekmal, R.R.; Petros, J.; Joshi, H.C. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc. Natl. Acad. Sci. USA 1998, 95, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Alisaraie, L.; Tuszynski, J.A. Determination of noscapine’s localization and interaction with the tubulin-alpha/beta heterodimer. Chem. Biol. Drug Des. 2011, 78, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Aneja, R.; Vangapandu, S.N.; Joshi, H.C. Synthesis and biological evaluation of a cyclic ether fluorinated noscapine analog. Bioorg Med. Chem. 2006, 14, 8352–8358. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.; Rahimi-Moghaddam, P. The anti-cancer activity of noscapine: A review. Recent Pat. Anti-Cancer Drug Discov. 2009, 4, 92–97. [Google Scholar] [CrossRef]
- Quisbert-Valenzuela, E.O.; Calaf, G.M. Apoptotic effect of noscapine in breast cancer cell lines. Int. J. Oncol. 2016, 48, 2666–2674. [Google Scholar] [CrossRef]
- Kocak, C.; Kocak, F.E.; Ozturk, B.; Tekin, G.; Vatansev, H. Cytotoxic, anti-proliferative and apoptotic effects of noscapine on human estrogen receptor positive (MCF-7) and negative (MDA-MB-231) breast cancer cell lines. Bratisl. Lek. Listy 2020, 121, 43–50. [Google Scholar] [CrossRef]
- Dash, S.G.; Kantevari, S.; Pandey, S.K.; Naik, P.K. Synergistic interaction of N-3-Br-benzyl-noscapine and docetaxel abrogates oncogenic potential of breast cancer cells. Chem. Biol. Drug Des. 2021, 98, 466–479. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Patel, K.; Chowdhury, N.; Singh, M. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci. Rep. 2017, 7, 15824. [Google Scholar] [CrossRef]
- Toda, S.; Frankel, N.W.; Lim, W.A. Engineering cell-cell communication networks: Programming multicellular behaviors. Curr. Opin. Chem. Biol. 2019, 52, 31–38. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Baberjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Chougule, M.B.; Patel, A.; Sachdeva, P.; Jackson, T.; Singh, M. Enhanced anticancer activity of gemcitabine in combination with noscapine via antiangiogenic and apoptotic pathway against non-small cell lung cancer. PLoS ONE 2011, 6, e27394. [Google Scholar] [CrossRef]
- Shen, W.; Liang, B.; Yin, J.; Li, X.; Cheng, J. Noscapine Increases the Sensitivity of Drug-Resistant Ovarian Cancer Cell Line SKOV3/DDP to Cisplatin by Regulating Cell Cycle and Activating Apoptotic Pathways. Cell Biochem. Biophys. 2015, 72, 203–213. [Google Scholar] [CrossRef]
- Hsin, Y.H.; Chen, C.F.; Huang, S.; Shih, T.S.; Lai, P.S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef]
- Martin, L.T.P.; Nachtigal, M.W.; Selman, T.; Nguyen, E.; Salsman, J.; Dellaire, G.; Dupre, D.J. Bitter taste receptors are expressed in human epithelial ovarian and prostate cancers cells and noscapine stimulation impacts cell survival. Mol. Cell Biochem. 2019, 454, 203–214. [Google Scholar] [CrossRef]
- Khazaei, M.R.; Nasr-Esfahani, M.H.; Chobsaz, F.; Khazaei, M. Noscapine Inhibiting the Growth and Angiogenesis of Human Eutopic Endometrium of Endometriosis Patients through Expression of Apoptotic Genes and Nitric Oxide Reduction in Three-Dimensional Culture Model. Iran. J. Pharm. Res. 2019, 18, 836–845. [Google Scholar] [CrossRef]
- Yang, Z.R.; Liu, M.; Peng, X.L.; Lei, X.F.; Zhang, J.X.; Dong, W.G. Noscapine induces mitochondria-mediated apoptosis in human colon cancer cells in vivo and in vitro. Biochem. Biophys. Res. Commun. 2012, 421, 627–633. [Google Scholar] [CrossRef]
- Han, Z.; Meng, L.; Huang, X.; Tan, J.; Liu, W.; Chen, W.; Zou, Y.; Cai, Y.; Huang, S.; Chen, A.; et al. Inhibition of p38 MAPK increases the sensitivity of 5-fluorouracil-resistant SW480 human colon cancer cells to noscapine. Oncol. Lett. 2022, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Luo, X.J.; Liao, F.; Lei, X.F.; Dong, W.G. Noscapine induces mitochondria-mediated apoptosis in gastric cancer cells in vitro and in vivo. Cancer Chemother. Pharmacol. 2011, 67, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, J.; Li, S.; Cao, G.; Tang, S.; Tong, Q.; Joshi, H.C. Noscapine induced apoptosis via downregulation of survivin in human neuroblastoma cells having wild type or null p53. PLoS ONE 2012, 7, e40076. [Google Scholar] [CrossRef] [PubMed]
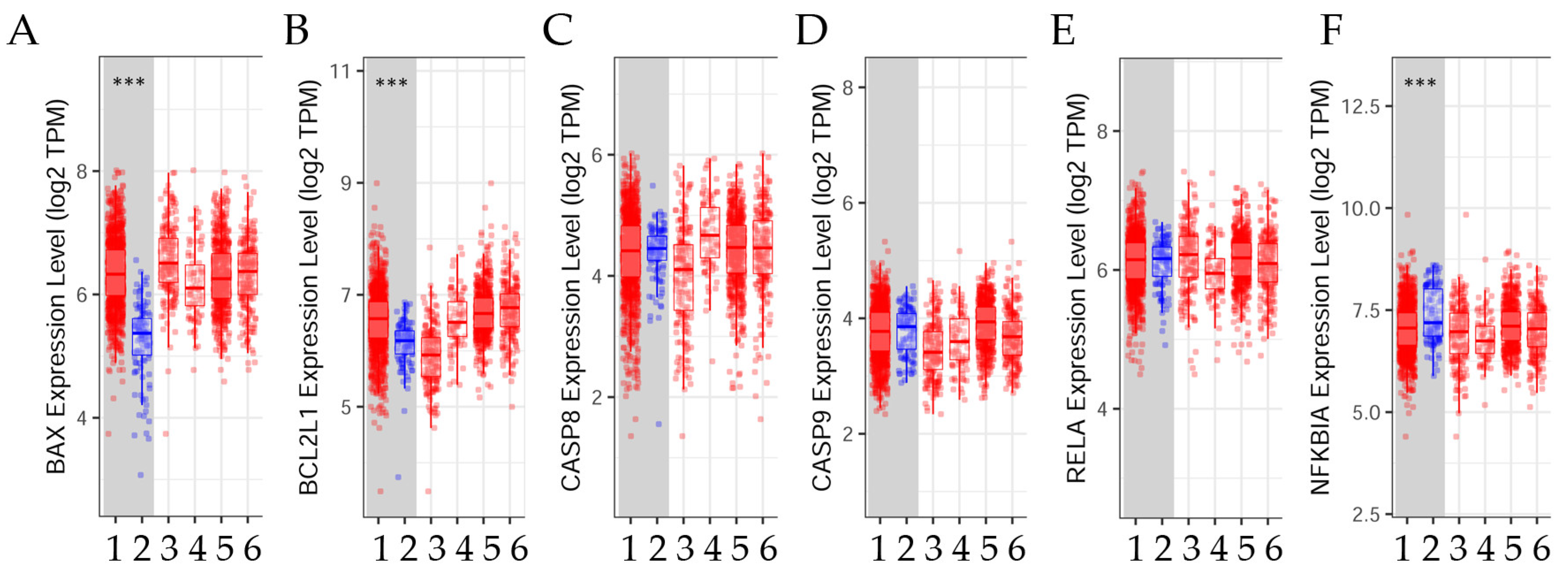

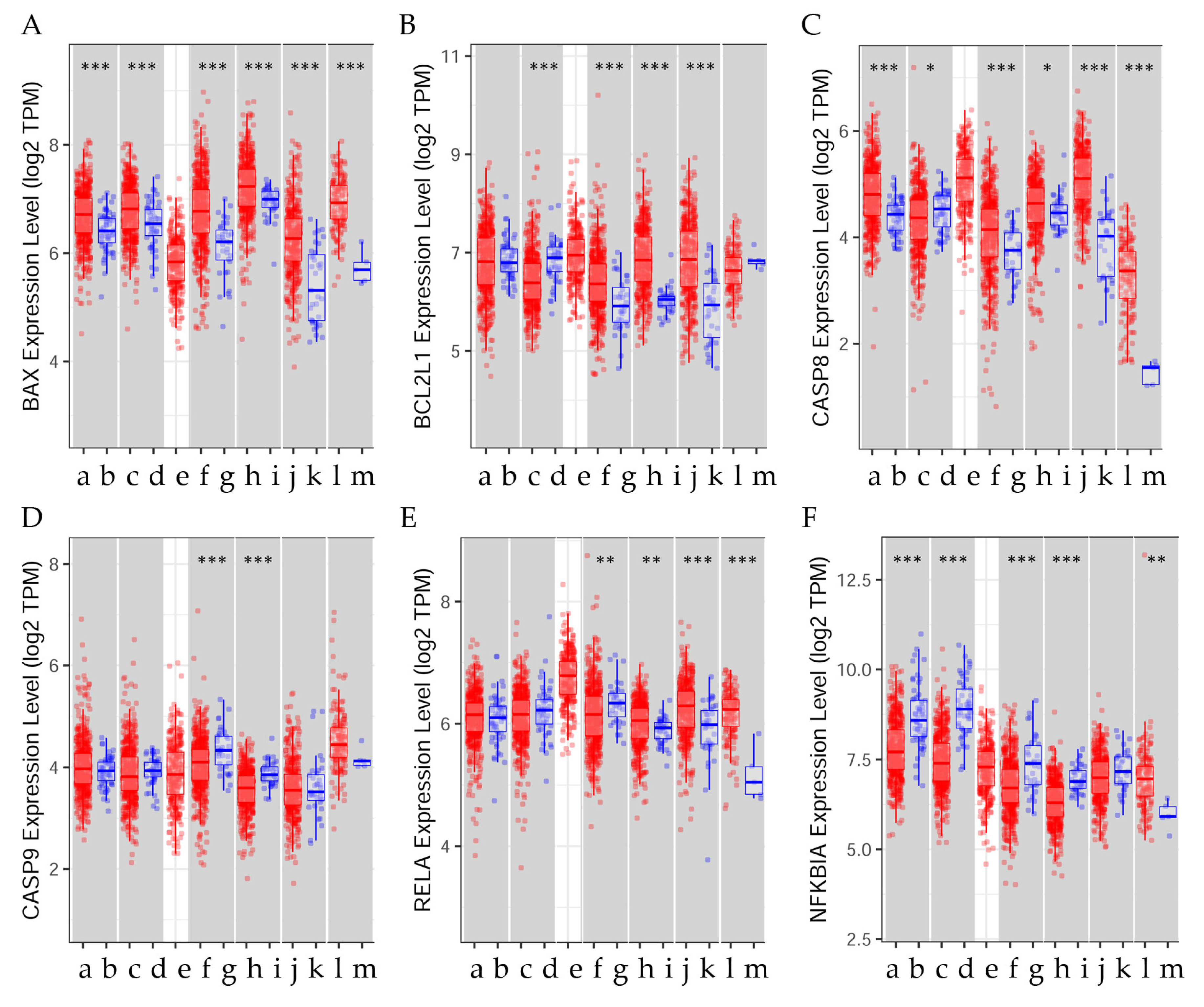

| Cancer | BAX | BCL2L1 | CASP8 | CASP9 | RELA | NFKBIA |
|---|---|---|---|---|---|---|
| BRCA (n = 1100) | 3, 4 *** | 3, 4 *** | 3, 4 *** | 3, 4 *** | 3, 4 *** | 3, 4 *** |
| BRCA-Basal (n = 191) | N. S. | N. S. | N. S. | N. S. | N. S. | N. S. |
| BRCA-Her2 (n = 82) | 4 * | 4 * | 4 * | 4 ** | 4 * | 4 * |
| BRCA-LumA (n = 568) | 4 *** | 4 *** | 4 *** | 4 *** | 4 *** | 4 *** |
| BRCA-LumB (n = 219) | 4 ** | 4 ** | 4 ** | 4 ** | 4 ** | 4 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calaf, G.M.; Crispin, L.A.; Quisbert-Valenzuela, E.O. Noscapine and Apoptosis in Breast and Other Cancers. Int. J. Mol. Sci. 2024, 25, 3536. https://doi.org/10.3390/ijms25063536
Calaf GM, Crispin LA, Quisbert-Valenzuela EO. Noscapine and Apoptosis in Breast and Other Cancers. International Journal of Molecular Sciences. 2024; 25(6):3536. https://doi.org/10.3390/ijms25063536
Chicago/Turabian StyleCalaf, Gloria M., Leodan A. Crispin, and Edwin O. Quisbert-Valenzuela. 2024. "Noscapine and Apoptosis in Breast and Other Cancers" International Journal of Molecular Sciences 25, no. 6: 3536. https://doi.org/10.3390/ijms25063536
APA StyleCalaf, G. M., Crispin, L. A., & Quisbert-Valenzuela, E. O. (2024). Noscapine and Apoptosis in Breast and Other Cancers. International Journal of Molecular Sciences, 25(6), 3536. https://doi.org/10.3390/ijms25063536




