Plasma and Platelet Brain-Derived Neurotrophic Factor (BDNF) Levels in Bipolar Disorder Patients with Post-Traumatic Stress Disorder (PTSD) or in a Major Depressive Episode Compared to Healthy Controls
Abstract
:1. Introduction
2. Results
2.1. Subjects Characteristics
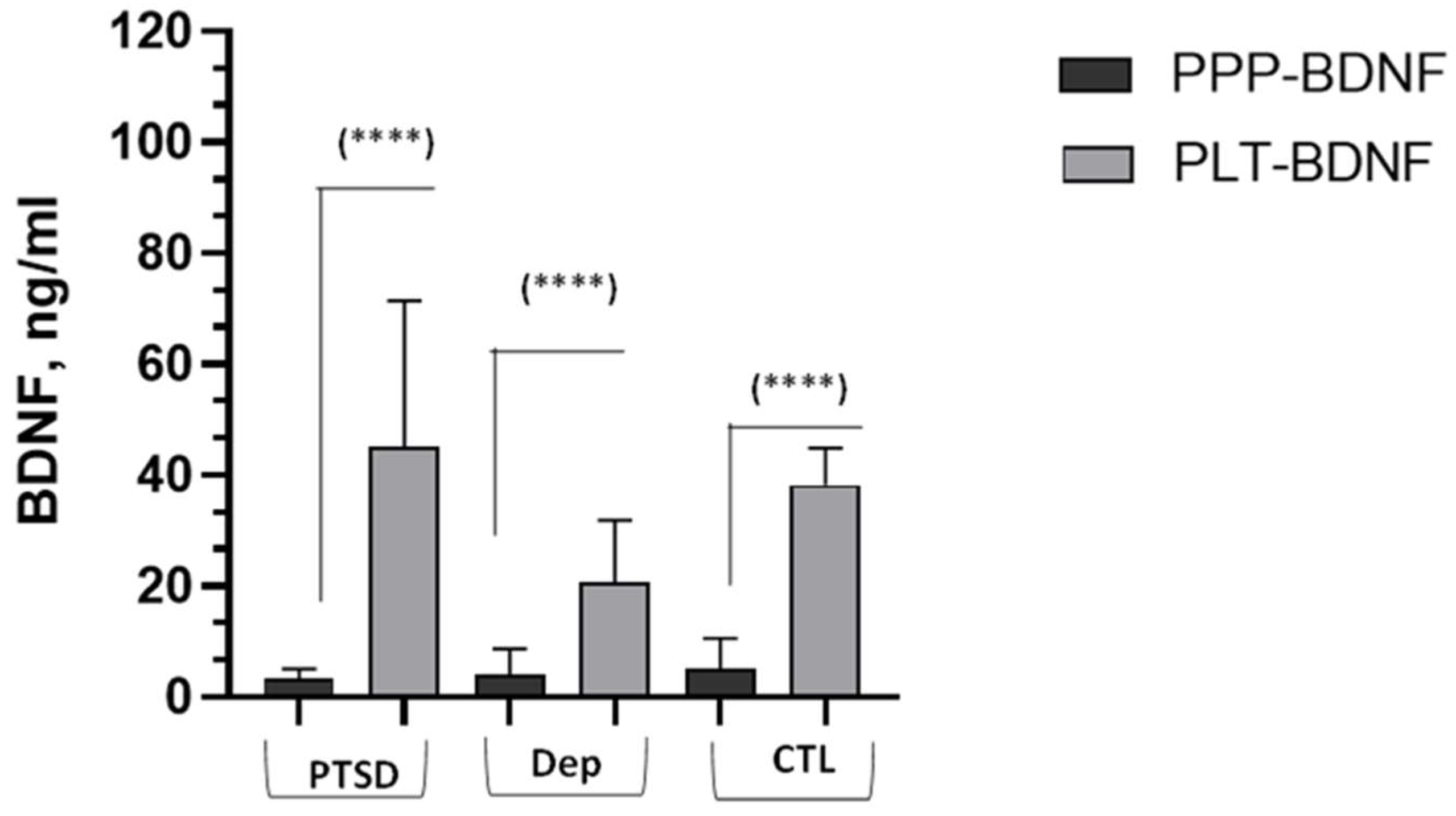
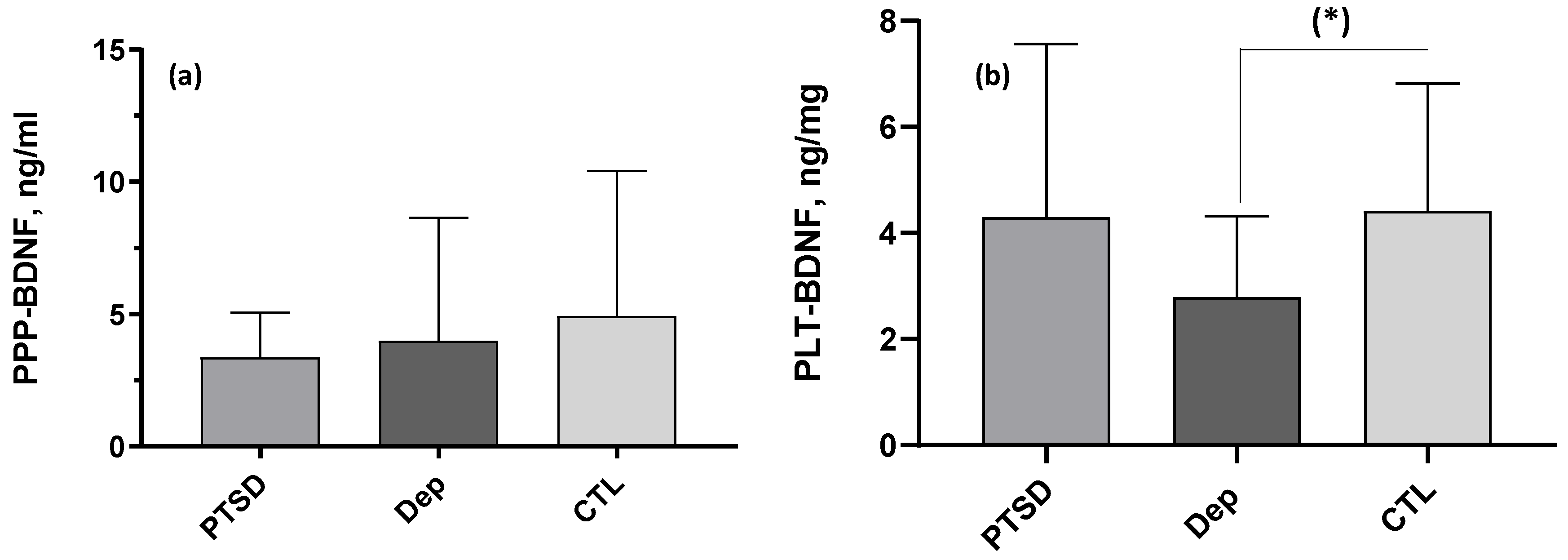
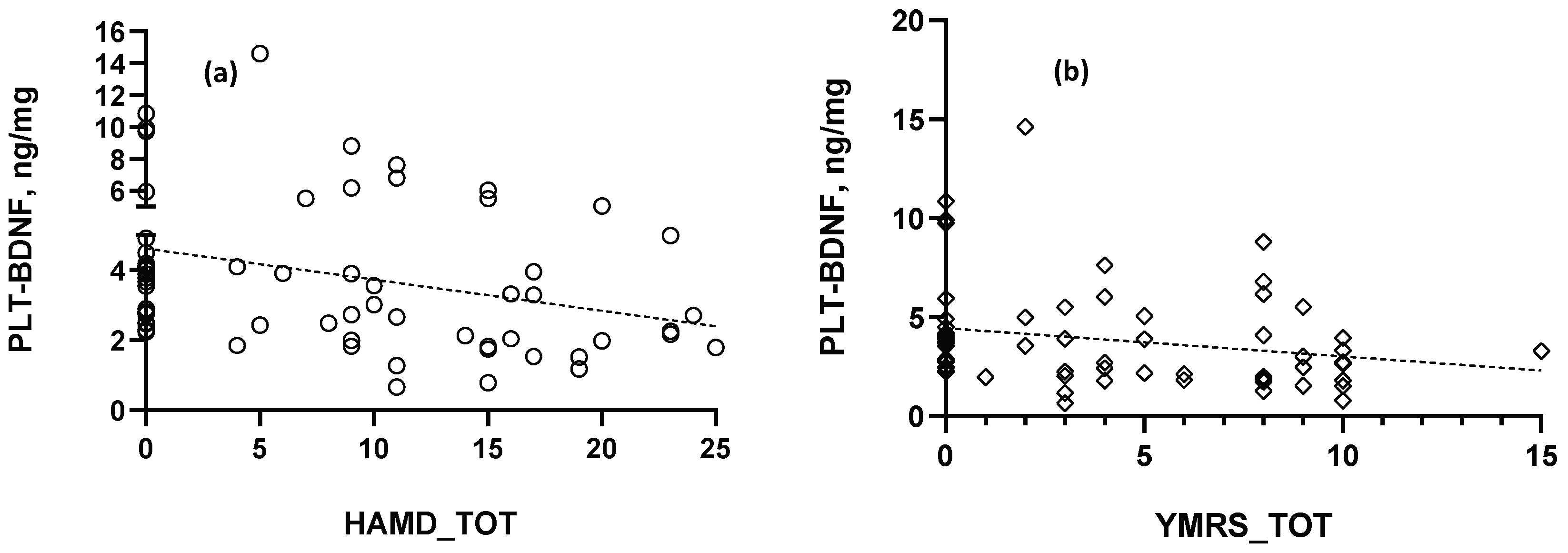
2.2. BDNF Comparisons and Correlations
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Psychometric Instruments
4.3. BDNF Measurements
4.3.1. Instruments, Chemicals and Reagents
4.3.2. Blood Sampling, Preparation and Storage Procedures
4.3.3. ELISA Assays for PPP-BDNF and PLT-BDNF Measurements
4.4. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dell’Osso, L.; Carmassi, C.; Consoli, G.; Conversano, C.; Ramacciotti, C.E.; Musetti, L.; Massimetti, E.; Pergentini, I.; Corsi, M.; Ciapparelli, A.; et al. Lifetime post-traumatic stress symptoms are related to the health-related quality of life and severity of pain/fatigue in patients with fibromyalgia. Clin. Exp. Rheumatol. 2011, 29 (Suppl. 69), S73–S78. [Google Scholar]
- Carmassi, C.; Stratta, P.; Massimetti, G.; Bertelloni, C.A.; Conversano, C.; Cremone, I.M.; Miccoli, M.; Baggiani, A.; Rossi, A.; Dell’Osso, L. New DSM-5 maladaptive symptoms in PTSD: Gender differences and correlations with mood spectrum symptoms in a sample of high school students following survival of an earthquake. Ann. Gen. Psychiatry 2014, 13, 28. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Amidfar, M.; Won, E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 91, 103–112. [Google Scholar] [CrossRef]
- Dell’oste, V.; Fantasia, S.; Gravina, D.; Palego, L.; Betti, L.; Dell’osso, L.; Giannaccini, G.; Carmassi, C. Metabolic and Inflammatory Response in Post-Traumatic Stress Disorder (PTSD): A Systematic Review on Peripheral Neuroimmune Biomarkers. Int. J. Environ. Res. Public Health 2023, 20, 2937. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Poo, M.-M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef]
- Lindsay, R.M. Trophic protection of motor neurons: Clinical potential in motor neuron diseases. J. Neurol. 1994, 242, S8–S11. [Google Scholar] [CrossRef]
- Snider, W.D. Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell 1994, 77, 627–638. [Google Scholar] [CrossRef]
- Hultman, R.; Kumari, U.; Michel, N.; Casey, P.J. Gαz regulates BDNF-induction of axon growth in cortical neurons. Mol. Cell. Neurosci. 2013, 58, 53–61. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Yang, S.; Wang, C.; Sun, X.; Lu, J.; Yin, H.; Jiang, W.; Meng, H.; Rao, F.; et al. Bioactive self-assembling peptide hydrogels functionalized with brain-derived neurotrophic factor and nerve growth factor mimicking peptides synergistically promote peripheral nerve regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2994–3005. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Kafitz, K.W.; Rose, C.R.; Thoenen, H.; Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999, 401, 918–921. [Google Scholar] [CrossRef]
- Hartmann, M.; Heumann, R.; Lessmann, V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001, 20, 5887–5897. [Google Scholar] [CrossRef]
- Kang, H.; A Welcher, A.; Shelton, D.; Schuman, E.M. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 1997, 19, 653–664. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhang, Y.; Lester, H.A.; Schuman, E.M.; Davidson, N. Enhancement of Neurotransmitter Release Induced by Brain-Derived Neurotrophic Factor in Cultured Hippocampal Neurons. J. Neurosci. 1998, 18, 10231–10240. [Google Scholar] [CrossRef]
- Carter, A.R.; Chen, C.; Schwartz, P.M.; Segal, R.A. Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J. Neurosci. 2002, 22, 1316–1327. [Google Scholar] [CrossRef]
- Schinder, A.F.; Berninger, B.; Poo, M.-M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron 2000, 25, 151–163. [Google Scholar] [CrossRef]
- Murakami, S.; Hiroki, I.; Yoshihiro, M.; Chiharu, K.; Emiko, S. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005, 53, 129–139. [Google Scholar] [CrossRef]
- Roth, T.L.; Zoladz, P.R.; Sweatt, J.D.; Diamond, D.M. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J. Psychiatr. Res. 2011, 45, 919–926. [Google Scholar] [CrossRef]
- Andero, R.; Ressler, K. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012, 11, 503–512. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Gold, P.W.; Chrousos, G.P. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol. Psychiatry 2002, 7, 254–275. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Duman, R.S.; Deyama, S.; Fogaça, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2019, 53, 126–139. [Google Scholar] [CrossRef]
- Kozlovsky, N.; Matar, M.A.; Kaplan, Z.; Kotler, M.; Zohar, J.; Cohen, H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int. J. Neuropsychopharmacol. 2007, 10, 741–758. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2020, 71, 2008–2021. [Google Scholar] [CrossRef]
- Fujimura, H.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-I.; Sun, B.; Altar, C.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Arthritis Res. Ther. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Rosenfeld, R.; Zeni, L.; Haniu, N.; Talvenheimo, J.; Radka, S.; Bennett, L.; Miller, J.; Welcher, A. Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr. Purif. 1995, 6, 465–471. [Google Scholar] [CrossRef]
- Radka, S.F.; Hoist, P.A.; Fritsche, M.; Altar, C.A. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996, 709, 122–130. [Google Scholar] [CrossRef]
- Pliego-Rivero, F.; Bayatti, N.; Giannakoulopoulos, X.; Glover, V.; Bradford, H.F.; Stern, G.; Sandier, M. Brain-derived neurotrophic factor in human platelets. Biochem. Pharmacol. 1997, 54, 207–209. [Google Scholar] [CrossRef]
- Bus, B.; Molendijk, M.; Penninx, B.; Buitelaar, J.; Kenis, G.; Prickaerts, J.; Elzinga, B.; Voshaar, R.O. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 2011, 36, 228–239. [Google Scholar] [CrossRef]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.-M.; Bertschy, G. Low Brain-Derived Neurotrophic Factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068–1072. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, L.H.; Yang, W.; Cui, R.J.; Xu, S.B. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front. Neurol. 2019, 10, 515. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Y.; Sun, Z. The Relationships Between Stress, Mental Disorders, and Epigenetic Regulation of BDNF. Int. J. Mol. Sci. 2020, 21, 1375. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Fasold, M.B.; Bluth, J.; Kastin, A.J. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 1998, 37, 1553–1561. [Google Scholar] [CrossRef]
- Karege, F.; Schwald, M.; Cisse, M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002, 328, 261–264. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Curran, G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier drug targeting enables neuroprotection in brain ischemia following delayed intravenous administration of neurotrophins. Adv. Exp. Med. Biol. 2002, 513, 397–430. [Google Scholar]
- Colle, R.; Trabado, S.; David, D.J.; Brailly-Tabard, S.; Hardy, P.; Falissard, B.; Fève, B.; Becquemont, L.; Verstuyft, C.; Corruble, E. Plasma BDNF Level in Major Depression: Biomarker of the Val66Met BDNF Polymorphism and of the Clinical Course in Met Carrier Patients. Neuropsychobiology 2017, 75, 39–45. [Google Scholar] [CrossRef]
- Serra-Millàs, M. Are the changes in the peripheral brain-derived neurotrophic factor levels due to platelet activation? World J. Psychiatry 2016, 6, 84–101. [Google Scholar] [CrossRef]
- Tamura, S.; Suzuki, H.; Hirowatari, Y.; Hatase, M.; Nagasawa, A.; Matsuno, K.; Kobayashi, S.; Moriyama, T. Release reaction of brain-derived neurotrophic factor (BDNF) through PAR1 activation and its two distinct pools in human platelets. Thromb. Res. 2011, 128, e55–e61. [Google Scholar] [CrossRef]
- Chacón-Fernández, P.; Säuberli, K.; Colzani, M.; Moreau, T.; Ghevaert, C.; Barde, Y.-A. Brain-derived Neurotrophic Factor in Megakaryocytes. J. Biol. Chem. 2016, 291, 9872–9881. [Google Scholar] [CrossRef]
- Betti, L.; Palego, L.; Unti, E.; Mazzucchi, S.; Kiferle, L.; Palermo, G.; Bonuccelli, U.; Giannaccini, G.; Ceravolo, R. Brain-Derived Neurotrophic Factor (BDNF) and Serotonin Transporter (SERT) in Platelets of Patients with Mild Huntington’s Disease: Relationships with Social Cognition Symptoms. Arch. Ital. Biol. 2018, 156, 27–39. [Google Scholar] [CrossRef]
- Le Blanc, J.; Fleury, S.; Boukhatem, I.; Bélanger, J.-C.; Welman, M.; Lordkipanidzé, M. Platelets Selectively Regulate the Release of BDNF, But Not That of Its Precursor Protein, proBDNF. Front. Immunol. 2020, 11, 575607. [Google Scholar] [CrossRef]
- Mitoma, M.; Yoshimura, R.; Sugita, A.; Umene, W.; Hori, H.; Nakano, H.; Ueda, N.; Nakamura, J. Stress at work alters serum brain-derived neurotrophic factor (BDNF) levels and plasma 3-methoxy-4-hydroxyphenylglycol (MHPG) levels in healthy volunteers: BDNF and MHPG as possible biological markers of mental stress? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 679–685. [Google Scholar] [CrossRef]
- Kauer-Sant’Anna, M.; Tramontina, J.; Andreazza, A.C.; Cereser, K.; da Costa, S.; Santin, A.; Yatham, L.N.; Kapczinski, F. Traumatic life events in bipolar disorder: Impact on BDNF levels and psychopathology. Bipolar Disord. 2007, 9, 128–135. [Google Scholar] [CrossRef]
- Grassi-Oliveira, R.; Stein, L.M.; Lopes, R.P.; Teixeira, A.L.; Bauer, M.E. Low plasma brain-derived neurotrophic factor and childhood physical neglect are associated with verbal memory impairment in major depression--a preliminary report. Biol. Psychiatry 2008, 64, 281–285. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Del Debbio, A.; Dell’Osso, M.C.; Bianchi, C.; da Pozzo, E.; Origlia, N.; Domenici, L.; Massimetti, G.; Marazziti, D.; et al. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 899–902. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Molendijk, M.L.; Voshaar, R.C.O.; Bus, B.A.A.; Prickaerts, J.; Spinhoven, P.; Penninx, B.J.W.H. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacol. 2010, 214, 319–328. [Google Scholar] [CrossRef]
- Stratta, P.; Bonanni, R.L.; Sanità, P.; de Cataldo, S.; Angelucci, A.; Origlia, N.; Domenici, L.; Carmassi, C.; Piccinni, A.; Dell’osso, L.; et al. Plasma brain-derived neurotrophic factor in earthquake survivors with full and partial post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2013, 67, 363–364. [Google Scholar] [CrossRef]
- Berger, W.; Mehra, A.; Lenoci, M.; Metzler, T.J.; Otte, C.; Tarasovsky, G.; Mellon, S.H.; Wolkowitz, O.M.; Marmar, C.R.; Neylan, T.C. Serum brain-derived neurotrophic factor predicts responses to escitalopram in chronic posttraumatic stress disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1279–1284. [Google Scholar] [CrossRef]
- Dotta-Panichi, R.M.; Bins, H.D.; Tramontina, J.F.; Ceresér, K.M.; de Aguiar, B.W.; Paz, A.C.; Taborda, J.G. Serum concentrations of brain-derived neurotrophic factor and mental disorders in imprisoned women. Braz. J. Psychiatry 2015, 37, 113–120. [Google Scholar] [CrossRef]
- Martinotti, G.; Sepede, G.; Brunetti, M.; Ricci, V.; Gambi, F.; Chillemi, E.; Vellante, F.; Signorelli, M.; Pettorruso, M.; De Risio, L.; et al. BDNF concentration and impulsiveness level in post-traumatic stress disorder. Psychiatry Res. 2015, 229, 814–818. [Google Scholar] [CrossRef]
- van den Heuvel, L.; Suliman, S.; Malan-Müller, S.; Hemmings, S.; Seedat, S. Brain-derived neurotrophic factor Val66met polymorphism and plasma levels in road traffic accident survivors. Anxiety Stress Coping 2016, 29, 616–629. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; Van den Heuvel, L.; Bucker, J.; Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241928. [Google Scholar] [CrossRef]
- Bajor, L.A.; Lai, Z.; Goodrich, D.E.; Miller, C.J.; Penfold, R.B.; Kim, H.M.; Bauer, M.S.; Kilbourne, A.M. Posttraumatic stress disorder, depression, and health-related quality of life in patients with bipolar disorder: Review and new data from a multi-site community clinic sample. J. Affect. Disord. 2013, 145, 232–239. [Google Scholar] [CrossRef]
- Rytwinski, N.K.; Scur, M.D.; Feeny, N.C.; Youngstrom, E.A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J. Trauma. Stress 2013, 26, 299–309. [Google Scholar] [CrossRef]
- Flory, J.D.; Yehuda, R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialog-Clin. Neurosci. 2015, 17, 141–150. [Google Scholar] [CrossRef]
- Longo, M.S.d.C.; Vilete, L.M.P.; Figueira, I.; Quintana, M.I.; Mello, M.F.; Bressan, R.A.; Mari, J.d.J.; Ribeiro, W.S.; Andreoli, S.B.; Coutinho, E.S.F. Comorbidity in post-traumatic stress disorder: A population-based study from the two largest cities in Brazil. J. Affect. Disord. 2020, 263, 715–721. [Google Scholar] [CrossRef]
- Hernandez, J.M.; Cordova, M.J.; Ruzek, J.; Reiser, R.; Gwizdowski, I.S.; Suppes, T.; Ostacher, M.J. Presentation and prevalence of PTSD in a bipolar disorder population: A STEP-BD examination. J. Affect. Disord. 2013, 150, 450–455. [Google Scholar] [CrossRef]
- Passos, I.C.; Mwangi, B.; Cao, B.; Hamilton, J.E.; Wu, M.-J.; Zhang, X.Y.; Zunta-Soares, G.B.; Quevedo, J.; Kauer-Sant’anna, M.; Kapczinski, F.; et al. Identifying a clinical signature of suicidality among patients with mood disorders: A pilot study using a machine learning approach. J. Affect. Disord. 2016, 193, 109–116. [Google Scholar] [CrossRef]
- Carmassi, C.; Bertelloni, C.A.; Dell’Oste, V.; Foghi, C.; Diadema, E.; Cordone, A.; Pedrinelli, V.; Dell’Osso, L. Post-traumatic stress burden in a sample of hospitalized patients with Bipolar Disorder: Which impact on clinical correlates and suicidal risk? J. Affect. Dis. 2020, 262, 267–272. [Google Scholar] [CrossRef]
- Buselli, R.; Veltri, A.; Baldanzi, S.; Marino, R.; Bonotti, A.; Chiumiento, M.; Girardi, M.; Pellegrini, L.; Guglielmi, G.; Dell’Osso, L.; et al. Plasma Brain-Derived Neurotrophic Factor (BDNF) and serum cortisol levels in a sample of workers exposed to occupational stress and suffering from Adjustment Disorders. Brain Behav. 2019, 9, e01298. [Google Scholar] [CrossRef]
- Castillo-Navarrete, J.-L.; Guzmán-Castillo, A.; Bustos, C.; Rojas, R. Peripheral brain-derived neurotrophic factor (BDNF) and salivary cortisol levels in college students with different levels of academic stress. Study protocol. PLoS ONE 2023, 18, e0282007. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a biomarker for successful treatment of mood disorders: A systematic & quantitative meta-analysis. J. Affect. Disord. 2015, 174, 432–440. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 2010, 14, 347–353. [Google Scholar] [CrossRef]
- Dell’osso, L.; Del Debbio, A.; Veltri, A.; Bianchi, C.; Roncaglia, I.; Carlini, M.; Massimetti, G.; Dell’osso, M.C.; Vizzaccaro, C.; Marazziti, D.; et al. Associations between brain-derived neurotrophic factor plasma levels and severity of the illness, recurrence and symptoms in depressed patients. Neuropsychobiology 2010, 62, 207–212. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol. Psychiatry 2013, 19, 791–800. [Google Scholar] [CrossRef]
- Primo de Carvalho Alves, L.; Sica da Rocha, N. Lower levels of brain-derived neurotrophic factor are associated with melancholic psychomotor retardation among depressed inpatients. Bipolar Dis. 2018, 20, 746–752. [Google Scholar] [CrossRef]
- Monteiro, B.C.; Monteiro, S.; Candida, M.; Adler, N.; Paes, F.; Rocha, N.; Nardi, A.E.; Murillo-Rodriguez, E.; Machado, S. Relationship Between Brain-Derived Neurotrofic Factor (Bdnf) and Sleep on Depression: A Critical Review. Clin. Pract. Epidemiol. Ment. Health 2017, 13, 213–219. [Google Scholar] [CrossRef]
- Kotan, Z.; Sarandöl, E.; Kırhan, E.; Özkaya, G.; Kırlı, S. Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Ther. Adv. Psychopharmacol. 2012, 2, 65–74. [Google Scholar] [CrossRef]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin. Med. Ins. Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Allen, A.; Naughton, M.; Dowling, J.; Walsh, A.; Ismail, F.; Shorten, G.; Scott, L.; McLoughlin, D.; Cryan, J.; Dinan, T.; et al. Serum BDNF as a peripheral biomarker of treatment-resistant depression and the rapid antidepressant response: A comparison of ketamine and ECT. J. Affect. Disord. 2015, 186, 306–311. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, F.; Yang, S.; Fang, P.; Deng, Z.F.; Xiao, J.L.; Hu, Z.L.; Chen, J.G. SKF83959 produces antidepressant effects in a chronic social defeat stress model of depression through BDNF-TrkB pathway. Int. J. Neuropsychopharmacol. 2014, 18, pyu096. [Google Scholar]
- Piccinni, A.; Veltri, A.; Costanzo, D.; Vanelli, F.; Franceschini, C.; Moroni, I.; Domenici, L.; Origlia, N.; Marazziti, D.; Akiskal, H.S.; et al. Decreased plasma levels of brain-derived neurotrophic factor (BDNF) during mixed episodes of bipolar disorder. J. Affect. Disord. 2015, 171, 167–170. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, H.; Park, S.-H.; Kim, Y.-K. Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 2007, 101, 239–244. [Google Scholar] [CrossRef]
- Serra-Millàs, M.; López-Vílchez, I.; Navarro, V.; Galán, A.-M.; Escolar, G.; Penadés, R.; Catalán, R.; Fañanás, L.; Arias, B.; Gastó, C. Changes in plasma and platelet BDNF levels induced by S-citalopram in major depression. Psychopharmacol 2011, 216, 1–8. [Google Scholar] [CrossRef]
- Piccinni, A.; Marazziti, D.; Catena, M.; Domenici, L.; Del Debbio, A.; Bianchi, C.; Mannari, C.; Martini, C.; Da Pozzo, E.; Schiavi, E.; et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J. Affect. Disord. 2008, 105, 279–283. [Google Scholar] [CrossRef]
- Haas, L.; Portela, L.V.C.; Böhmer, A.E.; Oses, J.P.; Lara, D.R. Increased Plasma Levels of Brain Derived Neurotrophic Factor (BDNF) in Patients with Fibromyalgia. Neurochem. Res. 2010, 35, 830–834. [Google Scholar] [CrossRef]
- Aldoghachi, A.F.; Tor, Y.S.; Redzun, S.Z.; Bin Lokman, K.A.; Razaq, N.A.A.; Shahbudin, A.F.; Badamasi, I.M.; Cheah, P.-S.; Stanslas, J.; Veerakumarasivam, A.; et al. Screening of brain-derived neurotrophic factor (BDNF) single nucleotide polymorphisms and plasma BDNF levels among Malaysian major depressive disorder patients. PLoS ONE 2019, 14, e0211241. [Google Scholar] [CrossRef]
- Falaschi, V.; Palego, L.; Marazziti, D.; Betti, L.; Musetti, L.; Maglio, A.; Dell’oste, V.; Sagona, S.; Felicioli, A.; Carpita, B.; et al. Variation of Circulating Brain-Derived Neurotrophic Factor (BDNF) in Depression: Relationships with Inflammatory Indices, Metabolic Status and Patients’ Clinical Features. Life 2023, 13, 1555. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef]
- Tsuchimine, S.; Sugawara, N.; Ishioka, M.; Yasui-Furukori, N. Preanalysis storage conditions influence the measurement of brain-derived neurotrophic factor levels in peripheral blood. Neuropsychobiology 2014, 69, 83–88. [Google Scholar] [CrossRef]
- Okragly, A.J.; Haak-Frendscho, M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp. Neurol. 1997, 145, 592–596. [Google Scholar] [CrossRef]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef]
- Bonnín, C.; Sánchez-Moreno, J.; Martínez-Arán, A.; Solé, B.; Reinares, M.; Rosa, A.; Goikolea, J.; Benabarre, A.; Ayuso-Mateos, J.; Ferrer, M.; et al. Subthreshold symptoms in bipolar disorder: Impact on neurocognition, quality of life and disability. J. Affect. Disord. 2011, 136, 650–659. [Google Scholar] [CrossRef]
- Bazzichi, L.; Da Valle, Y.; Rossi, A.; Giacomelli, C.; Sernissi, F.; Giannaccini, G.; Betti, L.; Ciregia, F.; Giusti, L.; Scarpellini, P.; et al. A multidisciplinary approach to study the effects of balneotherapy and mud-bath therapy treatments on fibromyalgia. Clin. Exp. Rheumatol. 2013, 31, S111–S120. [Google Scholar]
- Wang, T.-Y.; Lee, S.-Y.; Chen, S.-L.; Chang, Y.-H.; Wang, L.-J.; Chen, P.S.; Chen, S.-H.; Chu, C.-H.; Huang, S.-Y.; Tzeng, N.-S.; et al. Comparing clinical responses and the biomarkers of BDNF and cytokines between subthreshold bipolar disorder and bipolar II disorder. Sci. Rep. 2016, 6, 27431. [Google Scholar] [CrossRef]
- Uint, L.; Bastos, G.M.; Thurow, H.S.; Borges, J.B.; Hirata, T.D.C.; França, J.I.D.; Hirata, M.H.; Sousa, A.G.d.M.R. Increased levels of plasma IL-1b and BDNF can predict resistant depression patients. Front. Public Health 2019, 65, 361–369. [Google Scholar] [CrossRef]
- Rakofsky, J.J.; Ressler, K.J.; Dunlop, B.W. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Mol. Psychiatry 2012, 17, 22–35. [Google Scholar] [CrossRef]
- Hauck, S.; Kapczinski, F.; Roesler, R.; de Moura Silveira, E., Jr.; Magalhães, P.V.; Kruel, L.R.; Schestatsky, S.S.; Ceitlin, L.H. Serum brain-derived neurotrophic factor in patients with trauma psychopathology. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 459–462. [Google Scholar] [CrossRef]
- Blandini, F.; Rinaldi, L.; Tassorelli, C.; Sances, G.; Motta, M.; Samuele, A.; Fancellu, R.; Nappi, G.; Leon, A. Peripheral Levels of BDNF and NGF in Primary Headaches. Cephalalgia 2006, 26, 136–142. [Google Scholar] [CrossRef]
- Lee, B.-H.; Kim, Y.-K. Reduced platelet BDNF level in patients with major depression. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2009, 33, 849–853. [Google Scholar] [CrossRef]
- Storey, R.F.; Thomas, M.R. The role of platelets in inflammation. Thromb. Haemost. 2015, 114, 449–458. [Google Scholar] [CrossRef]
- First, M.B.; Williams, J.B.; Karg, R.S.; Spitzer, R.L. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders; Research Version; American Psychiatric Association Publishing: Arlington, VA, USA, 2015. [Google Scholar]
- Hamilton, M.A. Rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Weiss, D.S.; Marmar, C.R. The Impact of Event Scale-Revised. In Assessing Psychological Trauma and PTSD; Wilson, J.P., Keane, T.M., Eds.; Guilford Press: New York, NY, USA, 1997; pp. 399–411. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
| PTSD (n = 20) | DEP (n = 20) | CTL (n = 24) | ||||
|---|---|---|---|---|---|---|
| IES-R | Z | p | post hoc | |||
| Intrusion | 2.39 ± 0.90, 29.63 | 0.51 ± 0.36, 10.85 | - | −5.156 | <0.001 | PTSD>DEP |
| Avoidance | 2.14 ± 0.69, 29.61 | 0.53 ± 0.40, 10.88 | - | −5.135 | <0.001 | PTSD>DEP |
| Hyperarousal | 2.37 ± 0.75, 29.42 | 0.54 ± 0.37, 11.05 | - | −5.047 | <0.001 | PTSD>DEP |
| Total score | 50.42 ± 11.18, 30.00 | 11.55 ± 6.99, 10.50 | - | −5.344 | <0.001 | PTSD>DEP |
| HAM-D | - | Z | p | post hoc | ||
| Total score | 8.40 ± 2.41, 10.50 | 18.40 ± 3.55, 30.50 | - | −5.433 | <0.001 | DEP>PTSD |
| YMRS | - | Z | p | post hoc | ||
| Total score | 6.60 ± 2.70, 20.93 | 6.25 ± 3.73, 20.08 | - | −0.232 | 0.736 | - |
| BDNF | H | p | post hoc | |||
| PPP-BDNF, ng/mL | 3.37 ± 1.70, 33.40 | 3.98 ± 4.67, 27.05 | 4.93 ± 5.49, 36.29 | 2.76 | 0.252 | - |
| PLT-BDNF, ng/mg prot. | 4.30 ± 3.27, 33.70 | 2.79 ± 1.54, 22.90 | 4.42 ± 2.40, 39.50 | 8.79 | 0.012 | DEP<CTL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Oste, V.; Palego, L.; Betti, L.; Fantasia, S.; Gravina, D.; Bordacchini, A.; Pedrinelli, V.; Giannaccini, G.; Carmassi, C. Plasma and Platelet Brain-Derived Neurotrophic Factor (BDNF) Levels in Bipolar Disorder Patients with Post-Traumatic Stress Disorder (PTSD) or in a Major Depressive Episode Compared to Healthy Controls. Int. J. Mol. Sci. 2024, 25, 3529. https://doi.org/10.3390/ijms25063529
Dell’Oste V, Palego L, Betti L, Fantasia S, Gravina D, Bordacchini A, Pedrinelli V, Giannaccini G, Carmassi C. Plasma and Platelet Brain-Derived Neurotrophic Factor (BDNF) Levels in Bipolar Disorder Patients with Post-Traumatic Stress Disorder (PTSD) or in a Major Depressive Episode Compared to Healthy Controls. International Journal of Molecular Sciences. 2024; 25(6):3529. https://doi.org/10.3390/ijms25063529
Chicago/Turabian StyleDell’Oste, Valerio, Lionella Palego, Laura Betti, Sara Fantasia, Davide Gravina, Andrea Bordacchini, Virginia Pedrinelli, Gino Giannaccini, and Claudia Carmassi. 2024. "Plasma and Platelet Brain-Derived Neurotrophic Factor (BDNF) Levels in Bipolar Disorder Patients with Post-Traumatic Stress Disorder (PTSD) or in a Major Depressive Episode Compared to Healthy Controls" International Journal of Molecular Sciences 25, no. 6: 3529. https://doi.org/10.3390/ijms25063529





