Human–Fungal Pathogen Interactions from the Perspective of Immunoproteomics Analyses
Abstract
1. Human Mycotic Diseases: The Need for Better Therapeutic Interventions
2. Antibodies Mediate the Host Defense against Fungi
3. Discovery of Antigenic Proteins in Fungal Pathogens: The First Major Step Forward in Developing Antibody-Based Therapeutic Agents
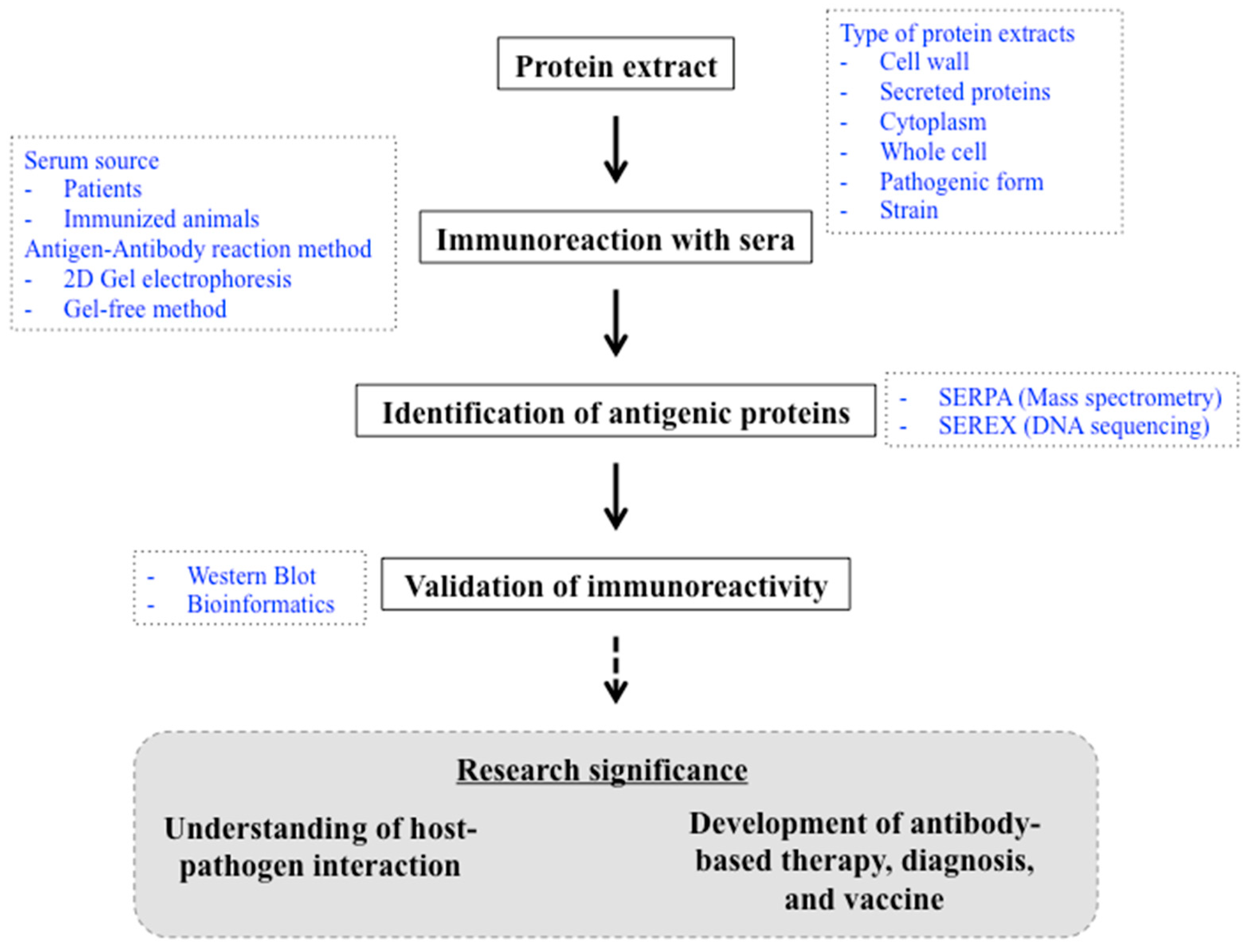
The Methods and the Challenges
4. Current Progress in the Identification of Fungal Antigenic Proteins
4.1. Heat-Shock Proteins
4.2. Carbon Metabolism
4.3. Translation
4.4. Antioxidant Systems
5. Mechanisms That Render Intracellular Proteins “Antigenic” during Infection: A Perspective
6. Applications of Fungal Antigenic Proteins: Current Progress and Challenges
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Huy, N.T. Rising incidence of mucormycosis in patients with COVID-19: Another challenge for India amidst the second wave? Lancet Respir. Med. 2021, 9, e77. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Carter, D.A. Climate change and the emergence of fungal pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef]
- Denning, D.W.; Perlin, D.S.; Muldoon, E.G.; Colombo, A.L.; Chakrabarti, A.; Richardson, M.D.; Sorrell, T.C. Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg. Infect. Dis. 2017, 23, 177–183. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal drug resistance: An emergent health threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Antifungal drug resistance: An update. Eur. J. Hosp. Pharm. 2022, 29, 109–112. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and ggriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef]
- Casadevall, A. Immunity to invasive fungal diseases. Annu. Rev. Immunol. 2022, 40, 121–141. [Google Scholar] [CrossRef]
- Höft, M.A.; Hoving, J.C.; Brown, G.D. Signaling C-type lectin receptors in antifungal immunity. Curr. Top. Microbiol. Immunol. 2020, 429, 63–101. [Google Scholar] [CrossRef]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. C-type lectin receptors in antifungal immunity: Current knowledge and future developments. Parasite Immunol. 2023, 45, e12951. [Google Scholar] [CrossRef]
- Drummond, R.A.; Gaffen, S.L.; Hise, A.G.; Brown, G.D. Innate defense against fungal pathogens. Cold Spring Harb. Perspect. Med. 2014, 5, a019620. [Google Scholar] [CrossRef]
- Burgess, T.B.; Condliffe, A.M.; Elks, P.M. A fun-guide to innate immune responses to fungal infections. J. Fungi 2022, 8, 805. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Wüthrich, M.; Deepe, G.; Klein, B. Adaptive immunity to fungi. Cold Spring Harb. Perspect. Med. 2014, 5, a019612. [Google Scholar] [CrossRef] [PubMed]
- Scheffold, A.; Bacher, P.; LeibundGut-Landmann, S. T cell immunity to commensal fungi. Curr. Opin. Microbiol. 2020, 58, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Drummond, R.A.; Hohl, T.M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 2023, 23, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 2012, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Winkelstein, J.A.; Marino, M.C.; Ochs, H.; Fuleihan, R.; Scholl, P.R.; Geha, R.; Stiehm, E.R.; Conley, M.E. The X-linked hyper-IgM syndrome: Clinical and immunologic features of 79 patients. Medicine 2003, 82, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.; Lao-Araya, M.; Yang, J.; Chan, K.W.; Ma, H.; Pei, L.C.; Kui, L.; Mao, H.; Yang, W.; Zhao, X.; et al. Application of flow cytometry in the diagnostics pipeline of primary immunodeficiencies underlying disseminated Talaromyces marneffei infection in HIV-negative children. Front. Immunol. 2019, 10, 2189. [Google Scholar] [CrossRef]
- Leven, E.A.; Maffucci, P.; Ochs, H.D.; Scholl, P.R.; Buckley, R.H.; Fuleihan, R.L.; Geha, R.S.; Cunningham, C.K.; Bonilla, F.A.; Conley, M.E.; et al. Hyper IgM Syndrome: A Report from the USIDNET Registry. J. Clin. Immunol. 2016, 36, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.M.L.; Morelli, F.; Negri, M.; Bonfim-Mendonça, P.; Kioshima, É.S.; Salci, T.; Voidaleski, M.F.; Vicente, V.A.; Svidzinski, T. FATAL cryptococcal meningitis in a child with hyper-immunoglobulin M syndrome, with an emphasis on the agent. J. Mycol. Med. 2019, 29, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Williamson, P.R.; Di Cesare, S.; Di Matteo, G.; De Luca, M.; Carsetti, R.; Figà-Talamanca, L.; Cancrini, C.; Rossi, P.; Finocchi, A. Cryptococcal meningitis and post-infectious inflammatory response syndrome in a patient with X-Linked hyper IgM syndrome: A case report and review of the literature. Front. Immunol. 2021, 12, 708837. [Google Scholar] [CrossRef] [PubMed]
- Françoise, U.; Lafont, E.; Suarez, F.; Lanternier, F.; Lortholary, O. Disseminated cryptococcosis in a patient with CD40 ligand deficiency. J. Clin. Immunol. 2022, 42, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shin, J.A.; Han, S.B.; Chung, N.G.; Jeong, D.C. Pneumocystis jirovecii pneumonia as an initial manifestation of hyper-IgM syndrome in an infant: A case report. Medicine 2019, 98, e14559. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.B.; Tian, M.Q.; Bai, Y.H.; Ran, X.; Li, L.; Chen, Y. CD40LG-associated X-linked Hyper-IgM Syndrome (XHIGM) with pulmonary alveolar proteinosis: A case report. BMC Pediatr. 2023, 23, 239. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, L.A.; Guerrero, N.; Stray-Pedersen, A.; Tafur, C.; Macias, R.; Muñoz, G.; Akdemir, Z.C.; Jhangiani, S.N.; Watkin, L.B.; Chinn, I.K.; et al. First case of CD40LG deficiency in Ecuador, diagnosed after whole exome sequencing in a patient with severe cutaneous histoplasmosis. Front. Pediatr. 2017, 5, 17. [Google Scholar] [CrossRef][Green Version]
- Cabral-Marques, O.; Arslanian, C.; Ramos, R.N.; Morato, M.; Schimke, L.; Soeiro Pereira, P.V.; Jancar, S.; Ferreira, J.F.; Weber, C.W.; Kuntze, G.; et al. Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J. Allergy Clin. Immunol. 2012, 129, 778–786. [Google Scholar] [CrossRef]
- Glocker, E.O.; Hennigs, A.; Nabavi, M.; Schäffer, A.A.; Woellner, C.; Salzer, U.; Pfeifer, D.; Veelken, H.; Warnatz, K.; Tahami, F.; et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 2009, 361, 1727–1735. [Google Scholar] [CrossRef]
- Doron, I.; Leonardi, I.; Li, X.V.; Fiers, W.D.; Semon, A.; Bialt-DeCelie, M.; Migaud, M.; Gao, I.H.; Lin, W.Y.; Kusakabe, T.; et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 2021, 184, 1017–1031.e14. [Google Scholar] [CrossRef]
- Freeman, A.F.; Holland, S.M. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr. Res. 2009, 65, 32r–37r. [Google Scholar] [CrossRef]
- Frede, N.; Rojas-Restrepo, J.; Caballero Garcia de Oteyza, A.; Buchta, M.; Hübscher, K.; Gámez-Díaz, L.; Proietti, M.; Saghafi, S.; Chavoshzadeh, Z.; Soler-Palacin, P.; et al. Genetic analysis of a cohort of 275 patients with hyper-IgE syndromes and/or chronic mucocutaneous candidiasis. J. Clin. Immunol. 2021, 41, 1804–1838. [Google Scholar] [CrossRef]
- Cobos, G.; Rubin, A.I.; Gober, L.M.; Treat, J.R. A case of exuberant candidal onychomycosis in a child with hyper IgE syndrome. J. Allergy Clin. Immunol. Pract. 2014, 2, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Vinh, D.C.; Sugui, J.A.; Hsu, A.P.; Freeman, A.F.; Holland, S.M. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J. Allergy Clin. Immunol. 2010, 125, 1389–1390. [Google Scholar] [CrossRef]
- Kasuga, K.; Nakamoto, K.; Doi, K.; Kurokawa, N.; Saraya, T.; Ishii, H. Chronic pulmonary aspergillosis in a patient with hyper-IgE syndrome. Respirol. Case Rep. 2022, 10, e0887. [Google Scholar] [CrossRef] [PubMed]
- Danion, F.; Aimanianda, V.; Bayry, J.; Duréault, A.; Wong, S.S.W.; Bougnoux, M.E.; Tcherakian, C.; Alyanakian, M.A.; Guegan, H.; Puel, A.; et al. Aspergillus fumigatus infection in humans with STAT3-Deficiency is associated with defective interferon-gamma and Th17 responses. Front. Immunol. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Odio, C.D.; Milligan, K.L.; McGowan, K.; Rudman Spergel, A.K.; Bishop, R.; Boris, L.; Urban, A.; Welch, P.; Heller, T.; Kleiner, D.; et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J. Allergy Clin. Immunol. 2015, 136, 1411–1412.e2. [Google Scholar] [CrossRef] [PubMed]
- Abbara, S.; Freeman, A.F.; Cohen, J.F.; Leclerc-Mercier, S.; Sanchez, L.; Schlatter, J.; Cisternino, S.; Parker, R.; Cowen, E.W.; Rouzaud, C.; et al. Primary invasive cutaneous fusariosis in patients with STAT3 hyper-IgE syndrome. J. Clin. Immunol. 2023, 43, 647–652. [Google Scholar] [CrossRef]
- Corvilain, E.; Casanova, J.L.; Puel, A. Inherited CARD9 deficiency: Invasive disease caused by Ascomycete fungi in previously healthy children and adults. J. Clin. Immunol. 2018, 38, 656–693. [Google Scholar] [CrossRef] [PubMed]
- Millet, N.; Solis, N.V.; Swidergall, M. Mucosal IgA prevents commensal Candida albicans dysbiosis in the oral cavity. Front. Immunol. 2020, 11, 555363. [Google Scholar] [CrossRef]
- Ost, K.S.; O’Meara, T.R.; Stephens, W.Z.; Chiaro, T.; Zhou, H.; Penman, J.; Bell, R.; Catanzaro, J.R.; Song, D.; Singh, S.; et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 2021, 596, 114–118. [Google Scholar] [CrossRef]
- Wich, M.; Greim, S.; Ferreira-Gomes, M.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A.; Jacobsen, I.D.; Hube, B.; Jungnickel, B. Functionality of the human antibody response to Candida albicans. Virulence 2021, 12, 3137–3148. [Google Scholar] [CrossRef]
- Tsilifis, C.; Freeman, A.F.; Gennery, A.R. STAT3 Hyper-IgE syndrome-an update and unanswered questions. J. Clin. Immunol. 2021, 41, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.M.; DeLeo, F.R.; Elloumi, H.Z.; Hsu, A.P.; Uzel, G.; Brodsky, N.; Freeman, A.F.; Demidowich, A.; Davis, J.; Turner, M.L.; et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 2007, 357, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Loomis, C.A. STAT3 signaling and the hyper-IgE syndrome. N. Engl. J. Med. 2007, 357, 1655–1658. [Google Scholar] [CrossRef]
- Olbrich, P.; Vinh, D.C. Inborn errors of immunity causing pediatric susceptibility to fungal diseases. J. Fungi 2023, 9, 149. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Fulton, K.M.; Baltat, I.; Twine, S.M. Immunoproteomics methods and techniques. Methods Mol. Biol. 2019, 2024, 25–58. [Google Scholar] [CrossRef]
- Pongpom, P.; Cooper, C.R., Jr.; Vanittanakom, N. Isolation and characterization of a catalase-peroxidase gene from the pathogenic fungus, Penicillium marneffei. Med. Mycol. 2005, 43, 403–411. [Google Scholar] [CrossRef]
- Virginio, E.D.; Kubitschek-Barreira, P.H.; Batista, M.V.; Schirmer, M.R.; Abdelhay, E.; Shikanai-Yasuda, M.A.; Lopes-Bezerra, L.M. Immunoproteome of Aspergillus fumigatus using sera of patients with invasive aspergillosis. Int. J. Mol. Sci. 2014, 15, 14505–14530. [Google Scholar] [CrossRef]
- Shi, L.N.; Li, F.Q.; Huang, M.; Lu, J.F.; Kong, X.X.; Wang, S.Q.; Shao, H.F. Immunoproteomics based identification of thioredoxin reductase GliT and novel Aspergillus fumigatus antigens for serologic diagnosis of invasive aspergillosis. BMC Microbiol. 2012, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.; Pitarch, A.; Gómez-Molero, E.; Amador-García, A.; Weig, M.; Bader, O.; Monteoliva, L.; Gil, C. Mass Spectrometry-based proteomic and immunoproteomic analyses of the Candida albicans hyphal secretome reveal diagnostic biomarker candidates for invasive candidiasis. J. Fungi 2021, 7, 501. [Google Scholar] [CrossRef]
- Kamli, M.R.; Sabir, J.S.M.; Malik, M.A.; Ahmad, A. Characterization of the secretome of pathogenic Candida glabrata and their effectiveness against systemic candidiasis in BALB/c mice for vaccine development. Pharmaceutics 2022, 14, 1989. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Gam, L.H.; Yong, V.C.; Rosli, R.; Ng, K.P.; Chong, P.P. Identification of immunogenic proteins of Candida parapsilosis by serological proteome analysis. J. Appl. Microbiol. 2014, 116, 999–1009. [Google Scholar] [CrossRef]
- Martins, L.M.; de Andrade, H.M.; Vainstein, M.H.; Wanke, B.; Schrank, A.; Balaguez, C.B.; dos Santos, P.R.; Santi, L.; Pires Sda, F.; da Silva, A.S.; et al. Immunoproteomics and immunoinformatics analysis of Cryptococcus gattii: Novel candidate antigens for diagnosis. Future Microbiol. 2013, 8, 549–563. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Weintraub, S.T.; Lopez-Ribot, J.L.; Wormley, F.L., Jr. Identification and characterization of Cryptococcus neoformans protein fractions that induce protective immune responses. Proteomics 2013, 13, 3429–3441. [Google Scholar] [CrossRef] [PubMed]
- Gressler, A.E.; Volke, D.; Firacative, C.; Schnabel, C.L.; Müller, U.; Krizsan, A.; Schulze-Richter, B.; Brock, M.; Brombacher, F.; Escandón, P.; et al. Identification of disease-associated cryptococcal proteins reactive with serum IgG from cryptococcal meningitis patients. Front. Immunol. 2021, 12, 709695. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Basrur, V.; Hung, C.Y.; Gardner, M.J.; Cole, G.T. A recombinant aspartyl protease of Coccidioides posadasii induces protection against pulmonary coccidioidomycosis in mice. Infect. Immun. 2006, 74, 516–527. [Google Scholar] [CrossRef]
- Almeida, M.A.; Almeida-Paes, R.; Guimarães, A.J.; Valente, R.H.; Soares, C.M.A.; Zancopé-Oliveira, R.M. Immunoproteomics reveals pathogen’s antigens involved in Homo sapiens-Histoplasma capsulatum interaction and specific linear B-cell epitopes in histoplasmosis. Front. Cell Infect. Microbiol. 2020, 10, 591121. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.E.; Oliveira, M.A.P.; Silva, L.O.S.; Inácio, M.M.; Bailão, A.M.; Parente-Rocha, J.A.; Cruz-Leite, V.R.M.; Paccez, J.D.; de Almeida Soares, C.M.; Weber, S.S.; et al. Immunoproteomic approach of extracellular antigens from Paracoccidioides species reveals exclusive B-cell epitopes. Front. Microbiol. 2019, 10, 2968. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Fernandes, G.F.; de Almeida, S.R.; Lopes-Bezerra, L.M.; de Camargo, Z.P. Immunoproteomic analysis reveals a convergent humoral response signature in the Sporothrix schenckii complex. J. Proteomics 2015, 115, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Wangsanut, T.; Amsri, A.; Pongpom, M. Antibody screening reveals antigenic proteins involved in Talaromyces marneffei and human interaction. Front. Cell Infect. Microbiol. 2023, 13, 1118979. [Google Scholar] [CrossRef] [PubMed]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef]
- Pongpom, M.; Vanittanakom, N. Characterization of an MPLP6, a gene coding for a yeast phase specific, antigenic mannoprotein in Penicillium marneffei. Med. Mycol. 2011, 49, 32–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, L.; Chan, C.M.; Lee, C.; Wong, S.S.; Yuen, K.Y. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 1998, 66, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Antibody-mediated protection through cross-reactivity introduces a fungal heresy into immunological dogma. Infect. Immun. 2007, 75, 5074–5078. [Google Scholar] [CrossRef][Green Version]
- Pitarch, A.; Nombela, C.; Gil, C. Seroprofiling at the Candida albicans protein species level unveils an accurate molecular discriminator for candidemia. J. Proteomics 2016, 134, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Pinheiro, B.G.; Teixeira-Ferreira, A.; Hahn, R.C.; de Camargo, Z.P. Immunoproteomic Analysis reveals novel candidate antigens for the diagnosis of paracoccidioidomycosis due to Paracoccidioides lutzii. J. Fungi 2020, 6, 357. [Google Scholar] [CrossRef]
- da Fonseca, C.A.; Jesuino, R.S.; Felipe, M.S.; Cunha, D.A.; Brito, W.A.; Soares, C.M. Two-dimensional electrophoresis and characterization of antigens from Paracoccidioides brasiliensis. Microbes Infect. 2001, 3, 535–542. [Google Scholar] [CrossRef]
- López-Medrano, R.; Ovejero, M.C.; Calera, J.A.; Puente, P.; Leal, F. An immunodominant 90-kilodalton Aspergillus fumigatus antigen is the subunit of a catalase. Infect. Immun. 1995, 63, 4774–4780. [Google Scholar] [CrossRef]
- Tiwari, S.; Thakur, R.; Shankar, J. Role of heat-shock proteins in cellular function and in the biology of fungi. Biotechnol. Res. Int. 2015, 2015, 132635. [Google Scholar] [CrossRef]
- Horianopoulos, L.C.; Kronstad, J.W. Chaperone networks in fungal pathogens of humans. J. Fungi 2021, 7, 209. [Google Scholar] [CrossRef]
- Ene, I.V.; Brunke, S.; Brown, A.J.; Hube, B. Metabolism in fungal pathogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a019695. [Google Scholar] [CrossRef]
- Dolan, S.K.; Welch, M. The Glyoxylate Shunt, 60 Years On. Annu. Rev. Microbiol. 2018, 72, 309–330. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Z.; He, L.; Xiao, X.; Chen, C.; Chu, J.; Mylonakis, E.; Xi, L. AcuD Gene knockout attenuates the virulence of Talaromyces marneffei in a zebrafish model. Mycobiology 2019, 47, 207–216. [Google Scholar] [CrossRef]
- Shibasaki, S.; Aoki, W.; Nomura, T.; Karasaki, M.; Sewaki, T.; Ueda, M. Evaluation of Mdh1 protein as an antigenic candidate for a vaccine against candidiasis. Biocontrol Sci. 2014, 19, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Shishkova, E.; Hose, J.; Coon, J.J.; Gasch, A.P. Decoupling yeast cell division and stress defense implicates mRNA repression in translational reallocation during Stress. Curr. Biol. 2018, 28, 2673–2680.e2674. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.M.; McIntyre, K.M.; Panepinto, J.C. Tools for assessing translation in Cryptococcus neoformans. J. Fungi 2021, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Mundodi, V.; Choudhary, S.; Smith, A.D.; Kadosh, D. Global translational landscape of the Candida albicans morphological transition. G3 2021, 11, jkaa043. [Google Scholar] [CrossRef]
- Gilmore, S.A.; Voorhies, M.; Gebhart, D.; Sil, A. Correction: Genome-wide reprogramming of transcript architecture by temperature specifies the developmental states of the human pathogen Histoplasma. PLoS Genet. 2021, 17, e1009509. [Google Scholar] [CrossRef]
- Leipheimer, J.; Bloom, A.L.M.; Campomizzi, C.S.; Salei, Y.; Panepinto, J.C. Translational regulation promotes oxidative stress resistance in the human fungal pathogen Cryptococcus neoformans. mBio 2019, 10, e02143-19. [Google Scholar] [CrossRef]
- Bloom, A.L.; Solomons, J.T.; Havel, V.E.; Panepinto, J.C. Uncoupling of mRNA synthesis and degradation impairs adaptation to host temperature in Cryptococcus neoformans. Mol. Microbiol. 2013, 89, 65–83. [Google Scholar] [CrossRef]
- Panepinto, J.C.; Komperda, K.W.; Hacham, M.; Shin, S.; Liu, X.; Williamson, P.R. Binding of serum mannan binding lectin to a cell integrity-defective Cryptococcus neoformans ccr4Delta mutant. Infect. Immun. 2007, 75, 4769–4779. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Whitesell, L.; Porco, J.A., Jr.; Henkel, T.; Brown, L.E.; Robbins, N.; Cowen, L.E. Translation inhibition by rocaglates activates a species-specific cell death program in the emerging fungal pathogen Candida auris. mBio 2020, 11, e03329-19. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Ballou, E.R. Oxidative responses and fungal infection biology. Semin. Cell Dev. Biol. 2019, 89, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wangsanut, T.; Sukantamala, P.; Pongpom, M. Identification of glutathione metabolic genes from a dimorphic fungus Talaromyces marneffei and their gene expression patterns under different environmental conditions. Sci. Rep. 2023, 13, 13888. [Google Scholar] [CrossRef]
- Heung, L.J. Monocytes and the host response to fungal pathogens. Front. Cell Infect. Microbiol. 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.; Rodrigues, M.L.; Janbon, G. Extracellular vesicles in fungi: Past, present, and future perspectives. Front. Cell Infect. Microbiol. 2020, 10, 346. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.D.; Chen, S.H.; Camacho, E.; Casadevall, A.; Williamson, P.R. Role of the ESCRT pathway in laccase trafficking and virulence of Cryptococcus neoformans. Infect. Immun. 2020, 88, e00954-19. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, J.; Jiang, H.; Lin, H.; Ou, Z.; Ullah, A.; Hua, Y.; Chen, J.; Lin, X.; Hu, X.; et al. Extracellular vesicles derived from Talaromyces marneffei yeasts mediate inflammatory response in macrophage cells by bioactive protein components. Front. Microbiol. 2020, 11, 603183. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.C.; Rigg, G.; Hodgetts, S.; Carter, T.; Chapman, C.; Gregory, C.; Illidge, C.; Burnie, J. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 2003, 47, 2208–2216. [Google Scholar] [CrossRef]
- Matthews, R.; Hodgetts, S.; Burnie, J. Preliminary assessment of a human recombinant antibody fragment to hsp90 in murine invasive candidiasis. J. Infect. Dis. 1995, 171, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Zhang, X.; Ge, Y.; Zhao, S.; Hu, Y.; Ashman, R.B. Immunisation with the glycolytic enzyme enolase confers effective protection against Candida albicans infection in mice. Vaccine 2011, 29, 5526–5533. [Google Scholar] [CrossRef]
- Pongpom, M.; Sawatdeechaikul, P.; Kummasook, A.; Khanthawong, S.; Vanittanakom, N. Antioxidative and immunogenic properties of catalase-peroxidase protein in Penicillium marneffei. Med. Mycol. 2013, 51, 835–842. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lau, C.C.; Tung, E.T.; Chong, K.T.; Yang, F.; Zhang, H.; Lo, R.K.; Cai, J.P.; Au-Yeung, R.K.; et al. Mp1p is a virulence factor in Talaromyces (Penicillium) marneffei. PLoS Negl. Trop. Dis. 2016, 10, e0004907. [Google Scholar] [CrossRef]
- Sze, K.H.; Lam, W.H.; Zhang, H.; Ke, Y.H.; Tse, M.K.; Woo, P.C.Y.; Lau, S.K.P.; Lau, C.C.Y.; Cai, J.P.; Tung, E.T.K.; et al. Talaromyces marneffei Mp1p is a virulence factor that binds and sequesters a key proinflammatory lipid to dampen host innate immune response. Cell Chem. Biol. 2017, 24, 182–194. [Google Scholar] [CrossRef]
- Ly, V.T.; Thanh, N.T.; Thu, N.T.M.; Chan, J.; Day, J.N.; Perfect, J.; Nga, C.N.; Vinh Chau, N.V.; Le, T. Occult Talaromyces marneffei infection unveiled by the novel Mp1p antigen detection assay. Open Forum Infect. Dis. 2020, 7, ofaa502. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ou, X.; Wang, H.; Li, L.; Guo, P.; Chen, X.; Cai, W.; Tang, X.; Li, L. Talaromyces marneffei Mp1p antigen detection may play an important role in the early diagnosis of talaromycosis in patients with acquired immunodeficiency syndrome. Mycopathologia 2022, 187, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pachl, J.; Svoboda, P.; Jacobs, F.; Vandewoude, K.; van der Hoven, B.; Spronk, P.; Masterson, G.; Malbrain, M.; Aoun, M.; Garbino, J.; et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 2006, 42, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Bugli, F.; Cacaci, M.; Martini, C.; Torelli, R.; Posteraro, B.; Sanguinetti, M.; Paroni Sterbini, F. Human monoclonal antibody-based therapy in the treatment of invasive candidiasis. Clin. Dev. Immunol. 2013, 2013, 403121. [Google Scholar] [CrossRef]
- Moura, Á.N.D.; Oliveira, D.S.L.; Paredes, V.; Rocha, L.B.; Oliveira, F.F.M.; Lessa, G.M.; Riasco-Palacios, J.F.; Casadevall, A.; Albuquerque, P.; Felipe, M.S.S.; et al. Paracoccidioides HSP90 can be found in the cell surface and is a target for antibodies with therapeutic potential. J. Fungi 2020, 6, 193. [Google Scholar] [CrossRef]
- Guimarães, A.J.; Frases, S.; Gomez, F.J.; Zancopé-Oliveira, R.M.; Nosanchuk, J.D. Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of Histoplasma capsulatum. Infect. Immun. 2009, 77, 1357–1367. [Google Scholar] [CrossRef]
- Yang, F.; Yuen, K.-Y.; Lau, S.K.; Woo, P.C. Vaccine mediates protection against penicilliosis in BALB/c mice (MPF2P. 810). J. Immunol. 2014, 192, 67–69. [Google Scholar] [CrossRef]
- Gomez, F.J.; Allendoerfer, R.; Deepe, G.S., Jr. Vaccination with recombinant heat shock protein 60 from Histoplasma capsulatum protects mice against pulmonary histoplasmosis. Infect. Immun. 1995, 63, 2587–2595. [Google Scholar] [CrossRef]
- de Bastos Ascenço Soares, R.; Gomez, F.J.; de Almeida Soares, C.M.; Deepe, G.S., Jr. Vaccination with heat shock protein 60 induces a protective immune response against experimental Paracoccidioides brasiliensis pulmonary infection. Infect. Immun. 2008, 76, 4214–4221. [Google Scholar] [CrossRef]
- Oliveira, L.V.N.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: Close but still a long way to go. NPJ Vaccines 2021, 6, 33. [Google Scholar] [CrossRef] [PubMed]
| Condition | Relevant Effect | Associated Fungal Disease | References |
|---|---|---|---|
| X-linked hyper IgM syndrome | Defective B-cells and elevated IgM | Cryptococcosis | [20,23,25] |
| Pneumocystic pneumonia | [22,26,27] | ||
| Candidiasis | [22] | ||
| Histoplasmosis | [22,28] | ||
| Paracoccidioidomycosis | [29] | ||
| Talaromycosis | [21] | ||
| CARD9 deficiency | Decreased IgG levels | Candidiasis | [30,31] |
| Hyper IgE syndrome | Elevated IgE levels | Candidiasis | [32,33,34,35] |
| Aspergillosis | [35,36,37] | ||
| Cryptococcosis | [35,38] | ||
| Histoplasmosis | [35,38] | ||
| Coccidioidomycosis | [38] | ||
| Pseudallescheriasis | [38] | ||
| Fusariosis | [35,39] |
| Species | Method | Key Antigens | Function | References |
|---|---|---|---|---|
| Aspergillus fumigatus (True mold) | - 2D gel analysis of fungal cell wall extracts (germlings) with patient sera + mass spectrometry - 2D gel analysis of fungal extracellular proteins with patient sera + mass spectrometry | - Cytochrome P450 D - eEF3 D - Thioredoxin reductase Glit (TR) D | - Xenobiotic detoxification - Translation - Secretory protein | [52,53] |
| Candida albicans (Polymorphic yeast) | - 2D gel analysis of hyphal secretome with patient sera + mass spectrometry | - Bgl2, Eno1, Pgk1, Glx3 D - Sap5, Pra1, Tdh3 D | [54] | |
| Candida glabrata (Nakaseomyces glabrata, Yeast) | - Mass spectrometry analysis of secretory proteins + bioinformatics prediction of antigenic proteins - Mice vaccinated with secreted proteins | - Secretome V | - Vaccination with secretome provided protection against infection in mice | [55] |
| Candida parapsilosis (Yeast) | - 2D gel analysis of fungal cell wall extracts with sera from infected mice + mass spectrometry | - Idh2 B | - Isocitrate dehydrogenase (TCA cycle) | [56] |
| Cryptococcus gattii (Yeast) Cryptococcus neoformans (Yeast) | - 2D gel analysis of protein extracts from four species with sera from patients + mass spectrometry - 2D gel analysis of cell wall and cytoplasmic proteins with sera from infected mice + mass spectrometry - 2D gel analysis of cell wall and cytoplasmic proteins with sera from patients + mass spectrometry | - Hsp70 D - GrpE D - Tpx1 D - Cell wall and cytoplasmic protein extracts V - Mpr1 V - CNAG_02943 V - HP_06113 V - UreG V | - Heat-shock protein - Heat-shock protein - Thiol peroxidase - Vaccination with protein fractions provided protection against infection in mice - Extracellular elastolytic metalloprotease - Cytoplasmic protein - Hypothetical protein - Urease accessory protein | [57] [58,59] |
| Coccidioides posadasii (Thermal dimorphic fungi) | - 2D gel analysis of parasitic cell wall extract (spherule) + mass spectrometry | - Pep1 V | Aspartyl protease | [60] |
| Histoplasma capsulatum (Thermal dimorphic fungi) | - Co-immunoprecipitation assay of pathogenic yeast protein extracts with patient sera + mass spectrometry | - YPS3 D - M antigen D - Catalase P D | - Yeast-specific cell wall and secreted protein - M antigen - Oxidative stress | [61] |
| Paracocidioides spp. (Thermal dimorphic fungi) | - Immunoprecipitation of exoantigens (secreted antigens) from Paracoccidioides spp. with polyclonal antibodies derived from animals immunized with the secretome + mass spectrometry | - PAAG_05807 and PAAG_06925 from P. lutzii P | - Hypothetical proteins | [62] |
| Sporothrix schenckii (Thermal dimorphic fungi) | - 2D gel analysis of yeast cell extracts with patient sera + mass spectrometry | - 3-Carboxymuconate cyclase (gp70) P | - Secreted protein | [63] |
| Talaromyces marneffei (Thermal dimorphic fungi) | - Antibody screen using pathogenic yeast recombinant cDNA expression libraries with patient sera + DNA sequencing of positive clones | - P26 P - Nuo21.3 P - Nbr1 P | [64] |
| Antigenic Proteins | ID | Description | References |
|---|---|---|---|
| Molecular chaperones Aspergillus fumigatus - Hsp88 - Hsp90/Asp f 12 - Hsp70 - Hsp70 (HscA) - Mitochondrial Hsp70 (Ssc70) - Mitochondrial Hsp60 | Q6MYM4 P40292 Q4WJ30 Q4WCM2 Q4X1H5 Q4X1P0 | - Heat-shock protein - Heat-shock protein - Heat-shock protein - Heat-shock protein - Heat-shock protein - Heat-shock protein | [52] |
| Candida albicans - Hsp12 - Ssa2 - Hsp90 - Hsp70/Ssa4 - Ssb1 - Ssc1 - Msi3/Sse1 | - Hsp12 - Hsp70 Family chaperone - 90-kDa Heat-shock protein - Hsp70 family - Hsp70 family - Hsp70 family - Hsp70 family | [54,69] | |
| Candida parapsilosis - Hsp70 | P87222 | - Heat-shock protein 70 (Ssb1) | [56] |
| Coccidioides posadasii - Hsp70 - Hsp60 | DQ674543 | - 70-kDa Heat-shock protein - 60-kDa Heat-shock protein | [60] |
| Cryptococcus gattii - Hsp70 - Sks2 | CNBG_4912 CNBG_0239 | - Heat-shock protein - Heat-shock protein sks2 | [57] |
| Cryptococcus neoformans - Heat-shock protein - Hsp90 - Hsp70 | AFR94464 AAN76525 AFR97119 | - 72-kDa Heat-shock protein - Heat-shock protein 90 - Heat-shock protein 70 | [58] |
Paracoccidioides brasiliensis - Hsp72-like protein - Hsp75-like protein - Hsp60 - Hsp7-like protein Paracoccidioides lutzii - Hsp72-like protein - Hsp60 Paracoccidioides spp. - Hsp70-like protein - Hsp 60-like protein | PADG_08118 PADG_02761 PADG_08369 PADG_00430 PADG_08118 PADG_08369 PAAG_08003 PABG_05342 PADG_08118 A0A1D2J907 PAAG_08059 PABG_07300 A0A1D2J9F0 | - Hsp72-like protein - Hsp75-like protein - Hsp60, Mitochondrial - Hsp7-like protein - Hsp72-like protein - Hsp60, Mitochondrial - 70-kDa Heat-shock protein - 60-kDa Heat-shock protein | [62,70] |
| Talaromyces marneffei - Hsp30 | PMAA_014600 | - Heat-shock protein 30 | [64] |
Carbon metabolism Aspergillus fumigatus - Fructose-bisphosphate aldolase - Enolase (Asp F22) - Aconitase (aconitate hydratase) | Q4WY39 Q96X30 Q4WLN1 | - Fructose-bisphosphate aldolase (glycolysis) - Enolase (glycolysis and gluconeogenesis) - Aconitase (TCA cycle) | [52] |
| Candida albicans - Fba1 - Eno1 - TDH3/GAP1 - Tpi1 - Pgk1 - Aco1 - Mdh1 | - Fructose-bisphosphate aldolase (glycolysis) - Enolase (glycolysis and gluconeogenesis) - Glyceraldehyde-3-phosphate dehydrogenase (glycolysis) - Triose phosphate isomerase (glycolysis) - Phosphoglycerate kinase (glycolysis) - Aconitase (TCA cycle) - Malate dehydrogenase (TCA cycle) | [54,56,69] | |
Candida parapsilosis - Fba1 - Eno1 - GAP1 - Pgk1 - Idh2 | CPAR2_401230 CPAR2_207210 CPAR2_808670 P46273 CPAR2_211610 | - Fructose-bisphosphate aldolase (glycolysis) - Enolase (glycolysis and gluconeogenesis) - Glyceraldehyde-3-phosphate dehydrogenase (glycolysis) - Phosphoglycerate kinase (glycolysis) - Isocitrate dehydrogenase (TCA cycle) | [56] |
| Coccidioides posadasii - Aldolase - Enolase - Aconitase - Malate dehydrogenase - NADH-ubiquinone oxidoreductase unit | DQ674539 DQ674538 DQ674544 DQ674541 DQ674550 | - Fructose-bisphosphate aldolase (glycolysis) - Enolase (glycolysis and gluconeogenesis) - Aconitase (TCA cycle) - Malate dehydrogenase (TCA cycle) - Electron transport chain (ETC) | [60] |
Cryptococcus gattii - Enolase (phosphopyruvate hydratase) - Aconitase - GAPDH Cryptococcus neoformans - Enolase (phosphopyruvate hydratase) | CNBG_3703 CNBG_0705 CNBG_1866 | - Enolase (glycolysis and gluconeogenesis) - Aconitase (TCA cycle) - Glyceraldehyde-3-phosphate dehydrogenase (glycolysis) - Enolase (glycolysis and gluconeogenesis) | [57] |
Histoplasma capsulatum - Aconitase (aconitate hydratase) - NADH-ubiquinone oxidoreductase 21 kDa unit | C6H4P0 C6HMG0 A6QUB6 | - Aconitase (TCA cycle) - Electron transport chain (ETC) | [61] |
| Paracoccidioides brasiliensis - Aldolase - Aconitase - GAPDH - TPI Paracoccidioides lutzii - Aldolase - Enolase - GAPDH - TPI - ICL Paracoccidioides spp. - MDH - ICL | PADG_00668 PADG_11845 PADG_02411 PADG_06906 PADG_00668 PADG_04059 PADG_02411 PADG_06906 PADG_01483 PAAG_08449 PADG_08054 A0A1E2Y1Z7 PAAG_06951 A0A1E2YBS1 | - Fructose-bisphosphate aldolase (glycolysis) - Aconitase (TCA cycle) - Glyceraldehyde-3-phosphate dehydrogenase (glycolysis) - Triose phosphate isomerase (glycolysis) - Fructose-bisphosphate aldolase (glycolysis) - Enolase (glycolysis and gluconeogenesis) - Glyceraldehyde-3-phosphate dehydrogenase (glycolysis) - Triose phosphate isomerase (glycolysis) - Isocitrate lyase (glyoxylate cycle) - Malate dehydrogenase (TCA cycle) - Isocitrate lyase (glyoxylate cycle) | [62,70,71] |
| Talaromyces marneffei - Fbp1 - Nuo21.3 | PMAA_041280 PMAA_028280 | - Fructose-1,6-bisphosphatase (glycolysis/gluconeogenesis) - NADH-ubiquinone oxidoreductase (ETC) | [64] |
| Protein synthesis Aspergillus fumigatus - 60S ribosome biogenesis protein Sqt1 - RL3_NEUCR 60S ribosomal protein L3 - Translation elongation factor eEF-1 subunit gamma - Translation elongation factor eEF-3 - Elongation factor Tu | Q4WU69 Q5AZS8 Q4WDF5 Q4WGN6 Q8TGG6 | - Ribosome biogenesis - Ribosomal protein - Protein synthesis - Protein synthesis - Protein synthesis | [52] |
| Candida albicans - Asc1 - Tif | - Ribosomal protein - Translation initiation factor (protein synthesis) | [54] | |
| Candida glabrata (Nakaseomyces glabrata) - Rps8A - Rps29B - Asc1 - Rpl12B - Rpl26A - Rpl32 - Rps24A - Rps21A - Rpl10 - Rpl33A | CAGL0A04521g CAGL0D00858g CAGL0D02090g CAGL0F02937g CAGL0G01078g CAGL0H04521g CAGL0J03234g CAGL0K08382g CAGL0K12826g CAGL0M02497g | - Ribosomal protein - Ribosomal protein - 40S Ribosomal subunit - Ribosomal protein - Ribosomal protein - Ribosomal protein - Ribosomal protein - Ribosomal protein - Ribosomal protein - Ribosomal protein | [55] |
Candida parapsilosis - Tif1 | P87206 | - Eukaryotic initiation factor 4A (translation apparatus) | [56] |
| Cryptococcus gattii - 40S ribosomal protein S0 - 40S ribosomal protein S7 - Initiation factor 5a - Elongation factor 1-beta - Translation elongation factor EF1-alpha | CNBG_2923 CNBG_2617 CNBG_5941 CNBG-3378 CNBG_4834 | - Ribosomal protein - Ribosomal protein - Protein synthesis - Protein synthesis - Protein synthesis | [57] |
| Histoplasma capsulatum - Large subunit ribosomal protein l3 - Ribosomal l10 protein - Ribosomal protein l14 - Ribosomal protein l15 - Ribosomal protein l22 - Ribosomal protein l31e - Ribosomal protein l34 protein - Ribosomal protein l37 - Ribosomal protein l37a - Ribosomal protein s13 - Ribosomal protein s16 - Ribosomal protein s2 - Ribosomal protein s5 - Ribosomal protein s9 - Ribosomal protein srp1 - Ribosomal protein yml20 | C0NDC6 C0NCP4 C0NHN4 A6R1V3 A6R1J7 A6R6D4 C6H9U0 F0U7R8 F0UB50 A6R4V4 A6R4L7 F0URZ8 A6RE96 A6REK8 C0NLR4 C6HP82 | - Translation - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis - Protein synthesis | [61] See the references for the full list |
| Paracoccidioides spp. - 40S ribosomal protein S15 | PAAG_04690 A0A1E2YDH8 | - Ribosomal protein | [62] |
| Talaromyces marneffei - RPL20A | PMAA_054240 | - 60S Ribosomal protein L20A | [64] |
| Antioxidative system Catalase Aspergillus fumigatus - Catalase | - Catalase-peroxidase | [72] | |
| Histoplasma capsulatum - Catalase | C0NVF6 Q9Y7C2 | - Catalase | [61] |
| Paracoccidioides brasiliensis - Catalase | [71] | ||
| Sporothrix mexicana - Catalase/peroxidase | SS08703/SB01256 | - Catalase-peroxidase | [63] |
| Talaromyces marneffei - CpeA | Q8NJN2.1 | - Catalase-peroxidase | [51] |
| Glutathione system Candida albicans - Prx1 | - Thioredoxin peroxidase | [54] | |
| Cryptococcus gattii - Glutathione transferase - Tpx1 - Grx5 | CNBG_6043 CNBG_2132 CNBG_5485 | - Xenobiotic detoxification - Thioredoxin peroxidase - Glutaredoxin Grx5-prov protein | [57] |
| Talaromyces marneffei - Gpx1 | PMAA_007230 | - Glutathione peroxidase | [64] |
| Cryptococcus neoformans - Superoxide dismutase | AFR97119 | - Mitochondrial superoxide dismutase | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangsanut, T.; Pongpom, M. Human–Fungal Pathogen Interactions from the Perspective of Immunoproteomics Analyses. Int. J. Mol. Sci. 2024, 25, 3531. https://doi.org/10.3390/ijms25063531
Wangsanut T, Pongpom M. Human–Fungal Pathogen Interactions from the Perspective of Immunoproteomics Analyses. International Journal of Molecular Sciences. 2024; 25(6):3531. https://doi.org/10.3390/ijms25063531
Chicago/Turabian StyleWangsanut, Tanaporn, and Monsicha Pongpom. 2024. "Human–Fungal Pathogen Interactions from the Perspective of Immunoproteomics Analyses" International Journal of Molecular Sciences 25, no. 6: 3531. https://doi.org/10.3390/ijms25063531
APA StyleWangsanut, T., & Pongpom, M. (2024). Human–Fungal Pathogen Interactions from the Perspective of Immunoproteomics Analyses. International Journal of Molecular Sciences, 25(6), 3531. https://doi.org/10.3390/ijms25063531






