Abstract
The few commercially available chemosensors and published probes for in vitro Zn2+ detection in two-photon microscopy are compromised by their flawed spectroscopic properties, causing issues in selectivity or challenging multistep syntheses. Herein, we present the development of an effective small molecular GFP chromophore-based fluorescent chemosensor with a 2,2′-bipyridine chelator moiety (GFZnP BIPY) for Zn2+ detection that has straightforward synthesis and uncompromised properties. Detailed experimental characterizations of the free and the zinc-bound compounds within the physiologically relevant pH range are presented. Excellent photophysical characteristics are reported, including a 53-fold fluorescence enhancement with excitation and emission maxima at 422 nm and 492 nm, respectively. A high two-photon cross section of 3.0 GM at 840 nm as well as excellent metal ion selectivity are reported. In vitro experiments on HEK 293 cell culture were carried out using two-photon microscopy to demonstrate the applicability of the novel sensor for zinc bioimaging.
1. Introduction
Metal ions are involved in many processes in living organisms, making their detection a principal task of modern analytical chemistry [1]. In particular, the detection of Zn2+ ions has attracted considerable research interest, as Zn2+ is the second most prevalent transition metal in the body, being endowed with significant roles in physiological processes [2]. Zn2+ is mainly present in a large variety of proteins, including enzymes. Moreover, biological processes such as cell division, metabolism, gene expression, and neurotransmission all require Zn2+ [3]. A reduced dietary intake of zinc correlates with several illnesses [4,5,6]. An imbalance of its homeostasis has also been associated with disorders such as Alzheimer’s and Parkinson’s disease, in addition to certain arthritic diseases [7,8,9,10]. The immunological relevance of Zn2+ has also been demonstrated recently, most notably in the connection between Zn2+ and SARS-CoV-2 infection [11].
The great importance of Zn2+ underlines the importance of having easily accessible analytical tools for Zn2+ detection, particularly in biological systems. Fluorescent methods provide a highly sensitive and accessible way to monitor Zn2+ both in space and time. Recently the use of fluorescence microscopy, and especially two-photon microscopy, has become widespread. The latter achieves excitation using high-intensity but lower-energy wavelengths, which enables the visualization of deeper tissues in 3D with localized excitation and less photodamage [12,13]. Fluorescence microscopy is the primary tool for studying metal ions in biological samples, and it requires fluorescent probes to detect the analyte. The availability of such probes can be a limiting factor in microscopy. Unfortunately, only a few fluorescent probes are available for two-photon imaging, and most of them are complex organic molecules with challenging multistep syntheses, making them hardly accessible for most users [14,15,16,17]. Typical Zn2+ sensors exhibit around a 10-fold fluorescence enhancement, the two-photon absorption values are in the 700–800 nm region, and most of them emit light at around 500 nm wavelength and have binding constants in the nanomolar concentration region. They have a few practical limitations, such as their solubility or selectivity [18]. In light of these aspects, as a continuation of our interest in novel fluorescent dyes and two-photon active compounds [19,20,21], we aimed to develop a novel family of two-photon sensors for Zn2+ by modifying the Green Fluorescent Protein (GFP) chromophore with a built-in 8-aminoquinoline motif, which made the probe Zn2+-sensitive [18]. The novel family, named GFZnP, exhibited excellent two-photon properties, its use was demonstrated in biological experiments, and it overcame the practical limitations of other sensors. However, due to the challenging synthesis of the 8-aminoquinalinde building blocks required for these sensors, they are still labor-intensive to access [18]. In this work, we aimed to prepare a similarly efficient sensor that is easily accessible in a single synthetic step.
The GFP chromophore exhibits intensive fluorescence inside the β-barrel of the GFP, where it is conformationally locked by hydrogen bonds (Figure 1A). However, in its bare form it loses the fluorescence due to non-emissive relaxation via intramolecular rotations [22]. Locking the conformation permanently or conditionally has been explored creatively to prepare various fluorescent dyes and sensor derivatives of the GFP chromophore [20,23,24,25].
The current work presents a usually applied zinc chelator, 2,2′-bipyridyl binding motif [26,27,28,29,30,31] attached to the GFP chromophore (Figure 1B), which is hypothesized to be nonfluorescent. However, we envision that Zn2+ binding to the 2,2′ bipyridine (BIPY) moiety would lock the conformation of the GFP chromophore and possibly disallow a photoinduced electron transfer (PeT) effect, resulting in a fluorescence turn-on. As (2,2′-bipyridine)-4-carbaldehyde is commercially available, the proposed probe would be accessible in a single Knoevenagel condensation, in line with the aim of this paper.
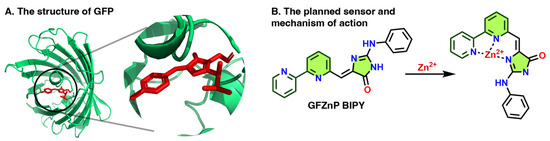
Figure 1.
(A) Illustrative structure of the Green Fluorescent Protein (GFP), where its chromophore is highlighted in red [32]. (B) The planned sensor and its mechanism of action. The rings filled with green show the parts of the sensors resembling the GFP chromophore; the dark green lines represent the ionophore part.
2. Results and Discussion
The synthesis of GFZnP BIPY was carried out based on [2,2′-bipyridine]-4-carbaldehyde and the respective imidazolinones (Scheme 1), which can be synthesized in a single step, as reported before [33]. After dissolving the two compounds in acetic acid, a catalytic amount of pyrrolidine was added, and the mixture was stirred at 110 °C for 1 h. After cooling to 5 °C, the product was precipitated from the reaction mixture, and the pure compound was isolated by simple filtration (>98%, HPLC). In the case of GFZnP BIPY, no further purification was needed, as opposed to the similar probes previously reported in the literature, which needed multiple synthetic steps and solvents and time-consuming purification with preparative HPLC.
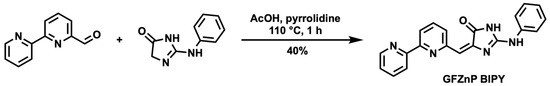
Scheme 1.
Simple synthesis of GFZnP BIPY chemosensor in Knoevenagel condensation.
The absorption and emission spectra were recorded in pH 7.4 HEPES buffer, which contained 2.75 µM of the prepared probe and either no Zn2+ (provided by the presence of 10 mM EGTA) or 1 mM Zn2+. The obtained spectroscopical properties of the prepared probe are summarized in Table 1. The prepared probe can be excited efficiently in the 400–450 nm range, which is compatible with the light sources of most one-photon microscope setups, and emits turquoise light (465–515 nm), with satisfactory brightness and an excellent fluorescence enhancement factor (FEF). The 52-fold FEF value is similar to the best measured FEF among our previously reported aminoquinoline-based sensor family. The turn-on of the sensor upon Zn2+ addition is instantaneous, and a full brightness is reached under a few seconds (Figure S13). Moreover, GFZnP BIPY has the same brightness and 1.5 times better FEF compared to GFZnP Pic, the brightest previously reported aminoquinoline, which had a four-step synthesis involving two chromatographies. Compared to these earlier probes, the absorption and emission wavelengths are ca. 20 nm blue shifted, which makes excitation possible using the blue channel light sources of microscopes instead of the green needed for previous sensors. Combined with the 70 nm Stokes shift, this feature enables the filtration of scattered excitation light without significant losses in the collection of fluorescence photons [34]. Any light source under 420 nm, including the very popular 405 nm laser, enables the complete detection of emitted light, as the emission spectrum does not overlap with this region. As shown in Figure 2A the absorption spectrum of GFZnP BIPY undergoes a 60 nm redshift during zinc binding, resulting in the final 422 nm absorption of the complex. This increases the contrast and fluorescence enhancement provided by the probe, as the free sensor does not undergo excitation at 422 nm since its highest wavelength absorption peak is centered around 362 nm in its free form. This spectral absorption shift is also visible to the naked eye, as the solution of GFZnP BIPY is colorless, but upon Zn2+ addition, it turns yellow. No such spectral redshift and no fluorescence signal was observed in a set of solvents with different polarity without Zn2+, confirming that the polarity of the environment does not interfere with the analyte sensing. However, a redshift and remarkable fluorescence was observed in glycerol, a highly viscous solvent. This is in agreement with our hypothesis that the conformational locking induces the fluorescence turn-on, since the conformational rotations are obstructed by the high viscosity, similarly to the ion binding (Figure S14). In summary, the probe presented herein offers comparable brightness and fluorescence enhancement to our best previously reported probes while also having a more optimal wavelength for most standard laser microscopes. It is accessible via a much simpler synthesis without the need for extensive purification methods, which makes it an excellent candidate for fluorescence microscopy and two-photon imaging [18].

Table 1.
The spectrophysical properties of a fluorescent GFZnP BIPY product and its zinc complex in HEPES (pH 7.4). FEF is for fluorescence enhancement factor. F stands for the area of the fluorescence peak. ε is the extinction coefficient; Φ marks the fluorescence quantum yield.
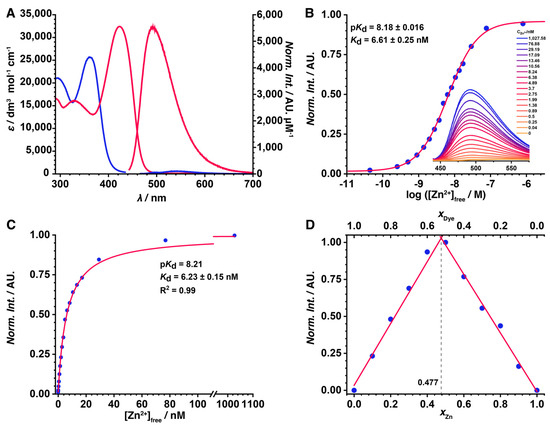
Figure 2.
(A) UV-VIS and fluorescence spectra of the reported probe without Zn2+ (blue) and in the presence of 1 mM Zn2+ (red) in HEPES (pH 7.4). (B) Fluorimetric titration of GFZnP BIPY with Zn2+. The fluorescence intensity (blue dots) is plotted against the free Zn2+ concentration on a logarithmic scale (red line: fitted logistic curve) and (C) on a linear scale (red line: fitted 1:1 complex model, Equation (5)). (D) Fluorescence Job’s plot of GFZnP BIPY with Zn2+.
After the promising initial results, the affinity of the probe towards Zn2+ was also determined via a competitive fluorescent titration experiment. The fluorescence intensities of different solutions containing EGTA and Zn2+ in varied ratios were plotted against the calculated free zinc ion concentration on a logarithmic scale (Figure 2B) to obtain a logistic plot. The midpoint of the fitted curve determined the apparent dissociation constant as K′d = 6.61 nM. Fitting a complex model that assumes a 1:1 complex stoichiometry to the intensity values visualized against the free zinc concentration (Figure 2C) provided a similar value, K′d = 6.23 nM. Both suggest that GFZnP BIPY forms a highly stable complex with zinc, similar to the best sensors reported, making it excellent for imaging [18,35]. The 1:1 stoichiometry of this complex was also confirmed by a fluorescence Job’s plot (Figure 2D). Similarly sized chelators with 2,2′-bipyridyl motifs also form 1:1 complexes, according to previous studies [26,27,30,31,36].
A molecular-level investigation through 1H
NMR spectroscopy also provided clear evidence of complexation between GFZnP
BIPY and Zn2+ ions. At room temperature, in DMSO-d6,
the probe exhibits broad signals, indicating a dynamically changing molecular structure
with conformational rates in the intermediate range (i.e., time constant on the
ms scale) relative to the NMR timescale (Figure S1).
The addition of Zn2+ ions (in the form of Zn(OTf)2)
caused substantial chemical shift changes and had a significant narrowing
effect on the formerly broad signals. This indicates that complex formation
blocks the free rotation of the ligand, giving rise to a rigid, non-fluxional
coordinative compound, as we hypothesized. At high Zn2+
concentrations (>1 equivalent, Figures S2–S9),
the well-resolved narrow peaks of the formed complex enabled comprehensive
characterization through 2D measurements (see Supplementary
Materials for details). In the initial titration region, we observe the
formation of a new peak series ( ), which are narrower compared
to those of the free probe (
), which are narrower compared
to those of the free probe ( )) but different from the peaks
of the final, stable complex (
)) but different from the peaks
of the final, stable complex ( )). We propose that at low Zn2+
concentrations, one Zn2+ ion can bind to more than one ligand,
resulting in a complex with a stoichiometry of M:L = 1:2, for example. This
complex has a lower stability than the final, 1:1 complex since, at 100%
titration, the ratio of the intermediate and final complex is 1:4, and the
final complex can only be observed by two equivalents (Figure 3A,B). Theoretical computations (see Schemes S1–4, Tables
S1–S4) also confirmed similar complex structure formation. Without Zn2+,
the free probe can undergo conformational rotations (Table S3). Zn2+ binding by the
sensor and the deprotonation of the formed complex—also observed in NMR
spectroscopy—are found to be energetically favorable.
)). We propose that at low Zn2+
concentrations, one Zn2+ ion can bind to more than one ligand,
resulting in a complex with a stoichiometry of M:L = 1:2, for example. This
complex has a lower stability than the final, 1:1 complex since, at 100%
titration, the ratio of the intermediate and final complex is 1:4, and the
final complex can only be observed by two equivalents (Figure 3A,B). Theoretical computations (see Schemes S1–4, Tables
S1–S4) also confirmed similar complex structure formation. Without Zn2+,
the free probe can undergo conformational rotations (Table S3). Zn2+ binding by the
sensor and the deprotonation of the formed complex—also observed in NMR
spectroscopy—are found to be energetically favorable.
 ), which are narrower compared
to those of the free probe (
), which are narrower compared
to those of the free probe ( )) but different from the peaks
of the final, stable complex (
)) but different from the peaks
of the final, stable complex ( )). We propose that at low Zn2+
concentrations, one Zn2+ ion can bind to more than one ligand,
resulting in a complex with a stoichiometry of M:L = 1:2, for example. This
complex has a lower stability than the final, 1:1 complex since, at 100%
titration, the ratio of the intermediate and final complex is 1:4, and the
final complex can only be observed by two equivalents (Figure 3A,B). Theoretical computations (see Schemes S1–4, Tables
S1–S4) also confirmed similar complex structure formation. Without Zn2+,
the free probe can undergo conformational rotations (Table S3). Zn2+ binding by the
sensor and the deprotonation of the formed complex—also observed in NMR
spectroscopy—are found to be energetically favorable.
)). We propose that at low Zn2+
concentrations, one Zn2+ ion can bind to more than one ligand,
resulting in a complex with a stoichiometry of M:L = 1:2, for example. This
complex has a lower stability than the final, 1:1 complex since, at 100%
titration, the ratio of the intermediate and final complex is 1:4, and the
final complex can only be observed by two equivalents (Figure 3A,B). Theoretical computations (see Schemes S1–4, Tables
S1–S4) also confirmed similar complex structure formation. Without Zn2+,
the free probe can undergo conformational rotations (Table S3). Zn2+ binding by the
sensor and the deprotonation of the formed complex—also observed in NMR
spectroscopy—are found to be energetically favorable.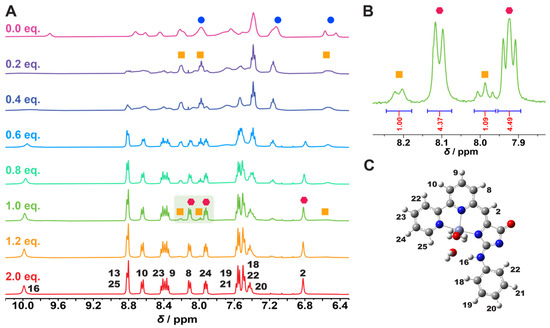
Figure 3.
(A) 1H NMR spectra of solutions of GFZnP BIPY in DMSO-d6 containing different amounts of Zn2+. (B) Zoomed-in region showing well-resolved peaks of the two formed complexes at 1 equiv. with added Zn2+. (C) Optimized structure of GFZnP BIPY–Zn2+ complex (M06-2X/6-311++G(2d,2p)//PCM(water)). Gray and white colors mark carbon and hydrogen respectively, while red, blue and light blue mark oxygen, nitrogen and zinc atoms, respectively.
Finally, after the detailed characterization presented above, the selectivity of the sensor was tested to confirm that the strong binding is only applicable to zinc. The GFZnP BIPY probe did not emit considerable fluorescence in the presence of other biologically relevant metal ions (Figure 4A). The strongest interference was in the cases of Ca2+ and Mg2+ under a 20% fluorescent signal for both compared to Zn2+ only when present in a very high, 1 mM concentration. Therefore, the possibility of an interference in a real-world application is unlikely, as a 1 mM Ca2+ concentration would give a similar signal to a 1 nM Zn2+ concentration. Cd2+ also had a similarly slight interference but one that is even less concerning for biological applications. Electron scavenger ions such as Fe3+ and Cu2+ quenched the fluorescence of the probe when present in equimolar quantities, which may lead to false-negative signals if they are present. However, in biological use cases, this is rarely a problem. No interference was observed in the anion screening experiments (Figure 4B). In general, the selectivity of the probe was excellent, further illustrated by the photograph shown in Figure 4A. The pH sensitivity of GFZnP BIPY was tested between pH 3 and 9. The probe does not give false positives for pH changes. However, very acidic pH levels (>pH 5) quench the fluorescence, which needs to be taken into account in the case of specific uses (e.g., acidic vesicles, lysosomes). Although the fluorescence is quenched by the protonation of the complex, the Zn2+ binding is still present even at low pH levels, as the UV-spectra of acidic solutions containing Zn2+ differ from zinc-free spectra at different pH levels (Figure S15). The fluorescence was relatively stable in a wide window around the physiological pH level (Figure 4C). The water solubility of the probe was found to be 73 µM (25 mg/L, Figures S16 and S17). The bench stability of the probe was studied using a 5 mM solution in DMSO/EtOH 1:1. No decomposition was observed after 4 months of storage at 5 °C. (Figure S12). The use of GFZnP BIPY for determining quantitative Zn2+ detection was demonstrated by recording a calibration line of total Zn2+ content using higher probe concentrations (Figure S16). The calibration was linear in the 0–20 µM range using a 33 µM probe concentration. The method exhibited good performance with a limit of detection of 129 µg/L.
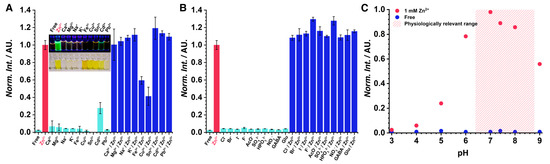
Figure 4.
(A) The cation and (B) anion selectivity of GFZnP BIPY. Normalized fluorescence intensity of the probe in the presence of 1 mM of different interfering ions (turquoise), Zn2+ (red), or both of them simultaneously (blue); inset: photographs of vials containing the same solutions under UV light (above) and ambient light (below). Error bars represent the standard deviation of triplicate measurements. (C) Normalized fluorescence intensity of the probe in buffers at different pH levels in the presence of Zn2+ (1mM, red) or EGTA (zinc-free, blue). The pink background shows the physiologically relevant pH range.
With the spectroscopic and Zn2+ binding properties in hand, the applicability of GFZnP BIPY in the proposed main application, two-photon fluorescence microscopy, was demonstrated. First, the two-photon action cross section of the probe was determined in the presence and absence of Zn2+. Almost no two-photon fluorescence could be detected in the solution that did not contain Zn2+, whereas a strong signal was emitted by the one that did (Figure 5A). The two-photon fluorescence enhancement (FEF2P) was 100-fold, while the peak action cross section was δ Φ = 3 GM, both similar to those of previously reported state-of-the-art probes. The two-photon spectrum coincides with the wavelength-doubled single-photon absorption spectrum with large precision (in the presence of Zn2+), which suggests that the same electronic transition is predominant during two-photon excitation (Figure 5B). This results in a two-photon excitation peak at 850 nm, which is ideal for the Ti/sapphire pulsed lasers most commonly used in two-photon setups. To demonstrate this, HEK 293 cells were incubated with GFZnP BIPY for 5 min in a 10 µM concentration, and two-photon images were recorded (Figure 5C). No colocalization (p < 0.1) with the nuclei, the membrane, or mitochondria were observed (Figures S20 and S21). Then, Zn2+ were added and imaging continued. An immediate strong increase in the fluorescence was observed (Figure 5D) at the parts of the image covered with cells. In randomly selected fields of view containing cells, the increase in fluorescence was 6.7-fold (Figure 5F), which was stable over the course of the 10 min imaging period. No cytotoxicity was observed in a standard cytotoxicity assay on HEK 293 cells (Figures S18 and S19). To prove that the sensing is reversible, a strong Zn2+ chelator (TPEN) was added to the cells to displace Zn2+ from its complex with GFZnP BIPY. Indeed, TPEN addition resulted in the quenching of the fluorescence (Figure 5E). This experiment is strong proof that GFZnP BIPY can be efficiently applied for the real-time monitoring of Zn2+ in biological samples.
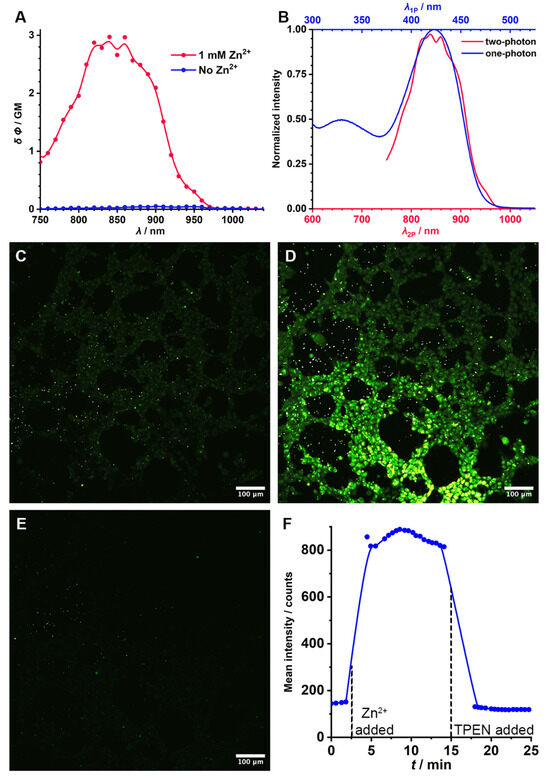
Figure 5.
(A) Two-photon action cross section spectrum of GFZnP BIPY in the presence of Zn2+ ions (red) and without Zn2+ (blue). (B) Comparison of the one-photon and two-photon absorption spectra of GFZnP BIPY in the presence of Zn2+. (C) Two-photon image of HEK 293 cells stained with GFZnP BIPY (D) after the addition of Zn2+ and (E) after the withdrawal of Zn2+. (F) Mean two-photon fluorescence intensity of whole images containing the shown cell samples plotted against the time from the start of the experiment. GM: Goeppert Mayer unit.
3. Materials and Methods
3.1. Materials
Reagents and solvents were purchased from Merck (Budapest, Hungary) and used as received. For spectroscopy, UVASol solvents (Supelco, Merck, Budapest, Hungary) were used. Deionized water was prepared in house using a Milli-Q RiOs-DI-3UV purifier, and resistivity was always kept >10 MΩ cm. Compound purity was confirmed by HPLC-MS using a Shimadzu LC-40D XR system equipped with a SIL-40C XR autosampler, SPD-M40 photodiode array detector, and an RF-20A XS fluorescent detector coupled to an LCMS-2020 DUIS Mass Spectrometer operated in alternating negative and positive modes. Separation was performed on an Ascentis Express C18, 2 μm UHPLC column (L × I.D. 5 cm × 2.1 mm) operated at 40 °C using a CTO-40s column oven. Analytical separation was achieved by a linear gradient elution in 5 min using 0.1% v/v TFA in water (A) and 0.1% v/v TFA in MeCN (B). NMR spectra were recorded on a Varian Unity INOVA spectrometer operating at an equivalent 1H frequency of 400 MHz. Spectra were acquired at room temperature unless noted otherwise. Notations for the 1H NMR spectral splitting patterns include singlet (s), doublet (d), triplet (t), broad (br), and multiplet/overlapping peaks (m). Chemical shifts are given as δ values in ppm, and coupling constants (J) are expressed in Hertz. Accurate mass measurements were carried out on a high-resolution Thermo Fisher Scientific Q-Exactive Focus hybrid quadrupole–orbitrap mass spectrometer used with a heated electrospray ionization source. Samples were dissolved in an acetonitrile/water 1:1 (v/v) solvent mixture containing 0.1% (v/v) formic acid. Flow injection analysis was performed using a 50 µL min−1 eluent flow provided by a Thermo Scientific UPLC. Spectroscopic studies were realized using a Shimadzu UV-1900i spectrophotometer and an RF-6000 spectrofluorometer using 1.0 cm path length quartz cell. Absorbances were measured with a 1 nm slit width in the high-speed mode of the instrument. The fluorescence spectra were recorded at 2000 nm min−1 scan speed, using 10 nm emission and excitation slit widths by excitation at the absorption maximum of the Zn2+ complex. Background correction of the solvent was applied to the UV-VIS spectra. Two-photon experiments were carried out with a Femtonics FemtoSMART-Dual microscope using a Nikon CFI75LWD 16× objective (NA = 1.0) and a tunable high-power Coherent Chameleon Discovery Ultra II Ti:Sapphire laser (λex = 700–1040 nm). The emitted light was detected using a Hamamatsu H11706-40 photomultiplier tube, recorded by Femtonics (Budapest, Hungary) MES 6.5.8966 software running on MATLAB 2017a, and analyzed in ImageJ (https://imagej.net/ij/).
3.2. Synthesis of GFZnP BIPY
A total of 100 mg (0.54 mmol) 2,2′-bipyridine-4-carbaldehyde was dissolved in 3 mL acetic acid, and 95 mg (0.54 mmol) 2-(phenylamino)-3,5-dihydro-4H-imidazol-4-one (prepared as previously reported [33]) was added to the solution. One drop of pyrrolidine was added, and the mixture was stirred at 110 °C for 1 h. The reaction mixture turned into a greenish brown color, and HPLC-MS analysis indicated a quantitative conversion. The mixture was cooled to room temperature, and the product was precipitated. After filtration and washing with ethanol and copious amounts of deionized water, an off-white powder was isolated. Yield: 70 mg, 40%. Due to the internal dynamics of the product, its characterization in NMR spectroscopy was challenging, as broad peaks were observed. Therefore, we characterized the NMR spectrum of the Zn2+-complex of GFZnP BIPY, which, as presented, has sharp peaks that are in accordance with the expected product (Figures S1–S12).
GFZnP BIPY: 1H NMR (400 MHz, DMSO-d6) δ 9.71 (brs, 1H), 8.68 (brd, 1H), 8.46 (brs, 1H), 8.23 (brs, 1H), 7.99 (brs, 2H), 7.52 (brd, 5H), 7.14 (brs, 1H), 6.52 (brd, 1H).
HRMS (ESI+) m/z calcd. for C20H15N5O+ [M+H]+: 342.1350. Found: 342.1342 δ = −2.28.
GFZnP BIPY Zn2+-complex: 1H NMR (400 MHz, DMSO-d6) δ 10.03 (s, 1H), 8.83–8.76 (m, 2H), 8.64 (d, J = 7.8 Hz, 1H), 8.40 (td, J = 7.8, 1.7 Hz, 1H), 8.36 (t, J = 8.0 Hz, 1H), 8.10 (d, J = 7.8 Hz, 1H), 7.96–7.88 (m, 1H), 7.55 (dd, J = 8.4, 7.2 Hz, 2H), 7.48–7.44 (m, 2H), 7.45–7.39 (m, 1H), 6.83 (s, 1H). 13C NMR (101 MHz, DMSO-d6) δ 169.23, 160.96, 153.56, 148.95, 148.76, 148.35, 142.12, 142.02, 140.24, 134.56, 130.61, 128.10, 127.82, 124.84, 123.84, 121.87, 107.66.
15N NMR (41 MHz, DMSO-d6) δ −127.74, −124.79, −229.28, −273.51.
3.3. Spectroscopical Characterization
The measurements were carried out in aqueous buffers by diluting a concentrated dye stock solution prepared as follows: 2.06 mg of GFZnP BIPY was dissolved in 10 mL DMSO to obtain a 600 μM stock solution. A total of 3 mL of the corresponding buffer solution was spiked with 20 μL of this stock solution to achieve a final dye concentration of 4 μM in each measurement and a DMSO concentration of 0.66%. To measure the fluorescence of the free probe and the Zn2+ complex buffer solutions were prepared by dissolving 720 mg EGTA (10 mM) or 72.7 mg Zn(OTf)2 (1 mM) in 200 mL deionized water containing 2.38 g HEPES and adjusting the pH to 7.4 by adding concentrated NaOH solution. The molar absorption coefficient was determined using the Lamber–Beer law, as shown in Equation (1):
ε/(dm3(mol cm)−1) = A c−1l−1
The fluorescence enhancement factor was calculated based on Equation (2), where F and F0 are the areas under the emission curve of the GFZnP BIPY–Zn2+ complex and free probe, respectively. The fluorescence quantum yield was determined using the standard relative method reported in the literature. Fluoresce in 0.1 M NaOH was used as a standard (Φref = 0.95), and Equation (3) was used to determine the quantum yield of GFZnP BIPY (Φ). The subscript s denotes the sample, ref denotes the reference values, F stands for the areas under the fluorescence emission spectra, A stands for the absorbance of the measured samples, and n marks the refractive index of the solvents used [37].
FEF = (F − F0)/F0
Direct titration of the presented probe showed only a saturation of the used 4 μM sensor concentration; therefore, a lower K′d was suspected, and a competitive titration was employed to determine the value. A 1 mM EGTA solution was prepared (72 mg EGTA and 2.38 g HEPES in 200 mL deionized water, pH adjustment to 7.4 by conc. NaOH). To 100 mL of this EGTA solution, 36.35 mg Zn(OTf)2 was added, yielding a solution containing 1 mM Zn2+ and 1 mM EGTA. Next, 16.5 μL dye stock solution was added to 2.5 mL of the 1 mM EGTA solution, and 133 μL dye stock was added to 20 mL of the 1 mM Zn2+-EGTA solution, resulting in a 6.6 μM dye concentration in both cases. Then, the spectrum of the only EGTA-containing solution was recorded, and small aliquots of the solution were exchanged with the Zn2+-EGTA solution while recording the spectrum in each step. As EGTA and Zn2+ are both in large excess concentrations compared to our dye, the free zinc concentration in the solution was determined based on the stability of the Zn2+-EGTA complex. Given, that the apparent stability of the Zn2+-EGTA complex is log(K′ZnEGTA) = −8.97, Equation (4) can be used to calculate the free zinc concentration.
The integrated intensities were plotted against log[Zn2+]free, and the midpoint of the logistic curve was taken as the K′d of GFZnP BIPY. Alternatively, a 1:1 complex model (Equation (5), where F is the integrated fluorescence intensity and Fmax and F0 are the integrated fluorescence intensities of the complex and free probe, respectively) was also fitted to the plot of the integrated fluorescence intensity against [Zn2+]free to confirm the initial value.
The binding ratio was determined using a fluorescence Job’s plot. Solutions containing the studied probe and Zn2+ in different molar ratios were prepared by the addition of small aliquots (<30 μL) of the dye stock solution and the 1 mM Zn2+-containing buffer to 3 mL of 50 mM HEPES pH 7.4 buffer. In each sample, the total concentration of GFZnP BIPY and Zn2+ was kept at cZn + cprobe = 10 μM.
The NMR titration experiment was carried out by sequentially adding a 33 mM solution of Zn(OTf)2 in DMSO-d6 to a 5 mM solution of GFZnP BIPY in DMSO-d6.
The pH sensitivity was characterized by measuring the fluorescence intensity and UV-VIS of GFZnP BIPY in 50 mM acetate (pH = 3.5–5.0) and HEPES (pH 6.0–9.0) buffers containing either 5 mM EGTA or 1 mM Zn(OTf)2. Selectivity was determined by recording the fluorescence intensity in 10 mM solutions of the following salts in pH 7.4 HEPES (50 mM): cations: CaCl2, MgCl2, NaCl, KCl, Fe(NO3)2, CuSO4, SnCl2, Cd(NO3)3, Pb(NO3)2, and Zn(OTf)2; anions: NaCl, KBr, KI, KF, NaOAc, K2SO4, Na2HPO3, KNO3, 4-aminobutyric acid, and L-glutamic acid. Intensity values were normalized to the Zn(OTf)2-containing solution, and the free sample contained only pH buffer. Interference measurements were repeated in similar solutions also containing 10 mM Zn(OTf)2.
The turn-on rate of the sensor was measured by recording the fluorescence of a 3 mL HEPES pH 7.4 solution containing 4 μM GFZnP BIPY and suddenly adding 30 μL of 100 mM Zn(OTf)2 solution to reach a final Zn2+ concentration of 1 mM. The excitation and emission wavelength was set to the respective maxima, and the slit widths were 3.0 nm. The instrument was set to a 500 ms accumulation time, and sampling was carried out at a 1 Hz rate.
The water solubility of GFZnP BIPY was determined by preparing a 100 mM solution in DMSO and adding 1 µL of the solution to 3 mL HEPES pH 7.4 buffer (0.03% DMSO content). The precipitated mixture was filtered through a 0.45 µM PES syringe filter to obtain a saturated aqueous solution. The absorbance of this solution was measured to determine the concentration of the saturated solution based on Equation (1).
To use GFZnP BIPY for the quantitative determination of Zn2+ in aqueous samples, a calibration line was recorded by adding known total aliquots of Zn2+ from a 2 mM aqueous stock solution to HEPES pH 7.4 buffered solution of the probe (33.3 µM). In total, 50 µL of Zn2+ stock was added in aliquots of 3 mL; dilution was not taken into account. A calibration line was fitted. The performance of the method was described by the LOD, which was calculated based on Equation (6), where σ is the standard deviation of the blank measurement, and s is the slope of the calibration curve:
LOD/µM = (3σ + Fblank)/s
The two-photon cross section (δ, in Goepper-Mayer units, noted as GM) was measured using a relative method similar to the one used for quantum yield determination [38]. Rhodamine 6G in MeOH (20 μM) was used as a standard. A 120 μM solution of GFZnP BIPY was prepared in zinc-free and 0.8 mM Zn2+-containing pH 7.4 HEPES buffers (50 mM, containing 10% DMSO). The samples were loaded into capillaries and placed into the field of view of the two-photon microscope. The incident light was focused into the capillary and the average intensity was recorded (F2P) while the laser light was kept at a constant 15 mW power and the excitation wavelength was increased in 10 nm steps between λex = 750 and 1040 nm. The emitted light was detected between 475 nm and 575 nm, which was corrected based on the one-photon spectrum as shown in Equation (7). Here, ref and s subscripts stand for sample and reference; c, n, and Φ mark the concentration, refractive index, and the one-photon quantum yield, respectively. The integral ratios are the relative amount of emitted light cut off by the filter based on the 1P emission spectra.
3.4. Biological Validation
HEK 293 cells (CRL-1573, ATCC) cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin were incubated (37 °C with 5% CO2) in a humidified incubator and subcultured every 2–3 days. The cultures were plated onto polylysinated glass coverslips at a density of approximately 26,300 cells/cm2 before further incubation for two more days. The cells were stained with a 10 μM GFZnP BIPY solution containing the following: 140 mM Na-gluconate, 5 mM K-gluconate, 3 mM CaCl2, 1 mM MgCl2, 5 mM D-glucose, and 10 mM HEPES adjusted to pH 7.4 using NaOH. The probe was added from a 5 mM DMSO-based stock solution, and staining for 5 min was carried out immediately before the measurements. After staining, the solution was changed to a fresh one and the coverslip was placed into the recording chamber. Baseline images were recorded; then, 10 μM Zn2+ pyrithione was added to the immersion from a concentrated stock solution and recording continued. Then, 100 mM N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) was added to the immersion and imaging continued. Images were recorded every ca. 30 s.
The cytotoxicity of GFZnP BIPY was studied with Hoechst 33342 and Propidium iodide stainings. The HEK293 cells were investigated in the wells of a 24-well plate at 10× magnification using an EVOS M5000 Imaging System (ThermoFisher). Four wells were dyed with GFZnP BIPY for 5 min in the same solution, as in the two-photon experiments, and four wells were left as controls. The solution was then changed, and the plates were incubated for 30 or 60 min. Images were taken again, and every well was stained with Hoechst 33342 (10 µg/mL) and Propidium iodide (1 µg/mL) in phosphate-buffered saline (PBS). The plates were incubated for 20 min, then imaged again. Propidium iodide staining was detected in the red channel (585/628 nm), and Hoechst staining was detected in the blue channel (357/447 nm). The composited images are shown in Figure S18. The ratio of propidium iodide-stained and Hoechst-stained cells gave the viability value, as propidium iodide stains only dead cells, whereas Hoechst stains all cells. Cell counting was performed with ImageJ. For each image, a threshold was set (55–255 for blue channel; 61–255 for red channel). For the blue channel images, watershed processing was applied to count conjoined cells more accurately. Then, objects with 100 px2 area and a roundness above 0.3 were automatically counted. The cell counts and viabilities are summarized in Figure S19. A colocalization study was carried out similarly using the same microscope setup and staining procedure with GFZnP BIPY and Hoechst. However, instead of propidium iodide, Mitotracker CMXRos (ThermoFisher) was used in a 100 nM concentration for labeling the mitochondria (detected in the red channel), and 100 nM MemBright-Cy5 was used for labeling the cell membranes detected in the magenta channel (635/692 nm).
3.5. Computational Methods
Theoretical calculations were carried out with Gaussian16, Revision C.01 software [39] using the standard convergence criteria given as default. Optimization and vibrational frequencies were calculated using the M06-2X method [40] using the 6-311++G(2d,2p) basis set and the IEFPCM method (ε = 78.3553 for water) [41,42]. Thermodynamic functions were computed at 298.15 K. For wavelength prediction, vertical excitation was modeled by the TD-B3LYP/6-311++G(2d,2p)//PCM(water) level of theory using the geometries optimized at B3LYP/6-311++G(2d,2p)//PCM(water). The emission wavelengths were calculated after optimization using the geometries provided by TD-B3LYP/6-311++G(2d,2p)//PCM(water).
4. Conclusions
In this work, we presented a novel two-photon fluorescent Zn2+ sensor for application in biology based on a modified GFP chromophore containing a 2,2′-bipyridyl binding motif. The novel probe presented herein showcases a 53-fold fluorescence increase, a 421 nm absorption wavelength with 492 nm emission, and a 3 GM two-photon cross section triggered by nanomolar concentrations of zinc ions. The Zn2+ binding results in a rigid 1:1 chelate complex with a locked conformation, which induces the fluorescence turn-on. The probe performs similarly to the state-of-the-art sensors; however, its synthesis is straightforward, making it accessible for zinc imaging on biological samples.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25063504/s1.
Author Contributions
Conceptualization, A.C. and E.K.; methodology, A.C., M.M., G.T., E.K. and Z.M.; software, G.K.; validation, A.C., M.M., G.T., E.K. and Z.M.; formal analysis, A.C., L.C., E.K. and Z.M.; investigation, A.C., M.M., G.T., E.K. and Z.M.; resources, G.K., B.R. and E.K.; data curation, A.C., M.M., G.T., L.C., E.K. and Z.M.; writing—original draft preparation, A.C., E.K., G.T. and Z.M.; writing—review and editing, A.C., L.C. and E.K.; visualization, A.C. and Z.M.; supervision, E.K. and Z.M.; project administration, E.K.; funding acquisition, G.K. and B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Innovation and Technology of Hungary from the National Research, Development, and Innovation Office, grant numbers 2018-1.3.1-VKE-2018-00032, TKP2021-EGA-42, TKP2021-NVA-14, 2020-1.1.5-GYORSÍTÓSÁV-2021-00004, KFI-18-2018-00097 and 2020-2.1.1.-ED-2022-00208 grants. This work was supported by the KDP-2021 program of the Ministry of Innovation and Technology from the Source of the National Research, Development, and Innovation Fund (NKFIH). Z.M. and L.C. are grateful for the Bolyai János Research Scholarship (BO/799/21/7, BO/00365/23/7, ÚNKP-23-ME4, ÚNKP-23-5-BME-460) provided by Hungarian Academy of Sciences and the National Research, Development and Innovation Fund. On behalf of the Development and mechanistic study of DNA dyes (PI: Ervin Kovács) project, we are grateful for the opportunity to use ELKH Cloud [43], which helped us achieve the results published in this paper.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper and the Supplementary Data. Additional raw data are available from the corresponding authors upon request.
Acknowledgments
The authors also acknowledge Áron Szepesi and Anett Matuscsák for their assistance in microscopy. Arnold Steckel and Gitta Schlosser are acknowledged for their generous help in MS analysis. The authors are thankful for Anna Fülöp for her help in the two-photon experiments.
Conflicts of Interest
Balázs Rózsa and Gergely Katona are founders of Femtonics and members of its scientific advisory board. Attila Csomos, Zoltán Mucsi and the other authors declare that no conflicts of interest exist.
References
- Gumienna-Kontecka, E.; Rowińska-Żyrek, M.; Łuczkowski, M. The Role of Trace Elements in Living Organisms. In Recent Advances in Trace Elements; Wiley Online Books: Hoboken, NJ, USA, 2018; pp. 177–206. ISBN 9781119133780. [Google Scholar]
- Kimura, E.; Koike, T. Recent Development of Zinc-Fluorophores. Chem. Soc. Rev. 1998, 27, 179–184. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The Neurobiology of Zinc in Health and Disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef]
- Karolczak, K.; Guligowska, A.; Sołtysik, B.K.; Kostanek, J.; Kostka, T.; Watala, C. Estimated Intake of Potassium, Phosphorus and Zinc with the Daily Diet Negatively Correlates with ADP-Dependent Whole Blood Platelet Aggregation in Older Subjects. Nutrients 2024, 16, 332. [Google Scholar] [CrossRef]
- Alcantara, E.H.; Kwon, J.-H.; Kang, M.-K.; Cho, Y.-E.; Kwun, I.-S. Zinc Deficiency Promotes Calcification in Vascular Smooth Muscle Cells Independent of Alkaline Phosphatase Action and Partly Impacted by Pit1 Upregulation. Nutrients 2024, 16, 291. [Google Scholar] [CrossRef]
- Patil, R.S.; Maloney, M.E.; Lucas, R.; Fulton, D.J.R.; Patel, V.; Bagi, Z.; Kovacs-Kasa, A.; Kovacs, L.; Su, Y.; Verin, A.D. Zinc-Dependent Histone Deacetylases in Lung Endothelial Pathobiology. Biomolecules 2024, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Doroszkiewicz, J.; Farhan, J.A.; Mroczko, J.; Winkel, I.; Perkowski, M.; Mroczko, B. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2023, 24, 15721. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lippard, S.J. Zinc Metalloneurochemistry: Physiology, Pathology, and Probes. In Metal Ions in Life Sciences; Sigel, A., Sigeland, H., Sigel, R.K.O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, pp. 321–370. ISBN 9780470028117. [Google Scholar]
- Czaplinska, B.; Spaczynska, E.; Musiol, R. Quinoline Fluorescent Probes for Zinc—From Diagnostic to Therapeutic Molecules in Treating Neurodegenerative Diseases. Med. Chem. 2018, 14, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ciaffaglione, V.; Rizzarelli, E. Carnosine, Zinc and Copper: A Menage a Trois in Bone and Cartilage Protection. Int. J. Mol. Sci. 2023, 24, 16209. [Google Scholar] [CrossRef] [PubMed]
- Asl, S.H.; Nikfarjam, S.; Majidi Zolbanin, N.; Nassiri, R.; Jafari, R. Immunopharmacological Perspective on Zinc in SARS-CoV-2 Infection. Int. Immunopharmacol. 2021, 96, 107630. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep Tissue Two-Photon Microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Katona, G.; Szalay, G.; Maák, P.; Kaszás, A.; Veress, M.; Hillier, D.; Chiovini, B.; Vizi, E.S.; Roska, B.; Rózsa, B. Fast Two-Photon in Vivo Imaging with Three-Dimensional Random-Access Scanning in Large Tissue Volumes. Nat. Methods 2012, 9, 201–208. [Google Scholar] [CrossRef]
- Sarkar, A.R.; Kang, D.E.; Kim, H.M.; Cho, B.R. Two-Photon Fluorescent Probes for Metal Ions in Live Tissues. Inorg. Chem. 2014, 53, 1794–1803. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-Photon Small-Molecule Fluorescence-Based Agents for Sensing, Imaging, and Therapy within Biological Systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Singh, H.; Lee, H.W.; Heo, C.H.; Byun, J.W.; Sarkar, A.R.; Kim, H.M. A Golgi-Localized Two-Photon Probe for Imaging Zinc Ions. Chem. Commun. 2015, 51, 12099–12102. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Seo, M.S.; An, M.J.; Hong, J.H.; Tian, Y.S.; Choi, J.H.; Kwon, O.; Lee, K.J.; Cho, B.R. Two-Photon Fluorescent Probes for Intracellular Free Zinc Ions in Living Tissue. Angew. Chem. Int. Ed. 2008, 47, 5167–5170. [Google Scholar] [CrossRef] [PubMed]
- Csomos, A.; Kovács, E.; Madarász, M.; Fedor, F.Z.; Fülöp, A.; Katona, G.; Rózsa, B.; Mucsi, Z. Two-Photon Fluorescent Chemosensors Based on the GFP-Chromophore for the Detection of Zn2+ in Biological Samples–From Design to Application. Sens. Actuators B Chem. 2024, 398, 134753. [Google Scholar] [CrossRef]
- Kovács, E.; Cseri, L.; Jancsó, A.; Terényi, F.; Fülöp, A.; Rózsa, B.; Galbács, G.; Mucsi, Z. Synthesis and Fluorescence Mechanism of the Aminoimidazolone Analogues of the Green Fluorescent Protein: Towards Advanced Dyes with Enhanced Stokes Shift, Quantum Yield and Two-Photon Absorption. Eur. J. Org. Chem. 2021, 2021, 5649–5660. [Google Scholar] [CrossRef]
- Chiovini, B.; Pálfi, D.; Majoros, M.; Juhász, G.; Szalay, G.; Katona, G.; Szőri, M.; Frigyesi, O.; Lukácsné Haveland, C.; Szabó, G.; et al. Theoretical Design, Synthesis, and In Vitro Neurobiological Applications of a Highly Efficient Two-Photon Caged GABA Validated on an Epileptic Case. ACS Omega 2021, 6, 15029–15045. [Google Scholar] [CrossRef] [PubMed]
- Szepesi Kovács, D.; Chiovini, B.; Müller, D.; Tóth, E.Z.; Fülöp, A.; Ábrányi-Balogh, P.; Wittner, L.; Várady, G.; Farkas, Ö.; Turczel, G.; et al. Synthesis and Application of Two-Photon Active Fluorescent Rhodol Dyes for Antibody Conjugation and In Vitro Cell Imaging. ACS Omega 2023, 8, 22836–22843. [Google Scholar] [CrossRef] [PubMed]
- Follenius-Wund, A.; Bourotte, M.; Schmitt, M.; Iyice, F.; Lami, H.; Bourguignon, J.J.; Haiech, J.; Pigault, C. Fluorescent Derivatives of the GFP Chromophore Give a New Insight into the GFP Fluorescence Process. Biophys. J. 2003, 85, 1839–1850. [Google Scholar] [CrossRef]
- Wu, L.; Burgess, K. Syntheses of Highly Fluorescent GFP-Chromophore Analogues. J. Am. Chem. Soc. 2008, 130, 4089–4096. [Google Scholar] [CrossRef]
- Baranov, M.S.; Lukyanov, K.A.; Borissova, A.O.; Shamir, J.; Kosenkov, D.; Slipchenko, L.V.; Tolbert, L.M.; Yampolsky, I.V.; Solntsev, K.M. Conformationally Locked Chromophores as Models of Excited-State Proton Transfer in Fluorescent Proteins. J. Am. Chem. Soc. 2012, 134, 6025–6032. [Google Scholar] [CrossRef]
- Ferreira, J.R.M.; Esteves, C.I.C.; Marques, M.M.B.; Guieu, S. Locking the GFP Fluorophore to Enhance Its Emission Intensity. Molecules 2023, 28, 234. [Google Scholar] [CrossRef]
- Mitchell, P.R. Hydrogen-1 Nuclear Magnetic Resonance Study of the Self-Association of 1,10-Phenanthroline, 2,2′-Bipyridyl, and Their Zinc(II) Complexes. J. Chem. Soc. Dalt. Trans. 1980, 7, 1079–1086. [Google Scholar] [CrossRef]
- Dianov, E.B.; Pervova, I.G.; Dvoskin, E.A.; Slepukhin, P.A. Synthesis and Structure of Zinc(II) Complexes with 2,2′-Bipyridine. Russ. J. Gen. Chem. 2018, 88, 843–845. [Google Scholar] [CrossRef]
- Yadav, Y.J.; Mastropietro, T.F.; Szerb, E.I.; Talarico, A.M.; Pirillo, S.; Pucci, D.; Crispini, A.; Ghedini, M. 2,2′-Bipyridine Zn(II) Complexes: Effect of the 4,4′ Substituents on the Crystalline Solid State Properties. New J. Chem. 2013, 37, 1486–1493. [Google Scholar] [CrossRef]
- Li, B.; Geoghegan, B.L.; Wölper, C.; Cutsail, G.E.; Schulz, S. Redox Activity of Noninnocent 2,2′-Bipyridine in Zinc Complexes: An Experimental and Theoretical Study. ACS Omega 2021, 6, 18325–18332. [Google Scholar] [CrossRef]
- Andrejević, T.; Ašanin, D.; Crochet, A.; Stevanović, N.; Vučenović, I.; Zobi, F.; Djuran, M.; Glišić, B. Structure and DNA/BSA Binding Study of Zinc(II) Complex with 4-Ethynyl-2,2’-Bipyridine: Scientific Paper. J. Serb. Chem. Soc. 2023, 88, 1293–1306. [Google Scholar] [CrossRef]
- Andrejević, T.P.; Aleksic, I.; Kljun, J.; Pantović, B.V.; Milivojevic, D.; Vojnovic, S.; Turel, I.; Djuran, M.I.; Glišić, B.Đ. Zinc(II) Complexes with Dimethyl 2,2’-Bipyridine-4,5-Dicarboxylate: Structure, Antimicrobial Activity and DNA/BSA Binding Study. Inorganics 2022, 10, 71. [Google Scholar] [CrossRef]
- 1EMA Structure from the Protein Data Bank (RCSB PDB). Available online: https://www.rcsb.org/structure/1ema (accessed on 2 February 2024). [CrossRef]
- Jancsó, A.; Kovács, E.; Cseri, L.; Rózsa, B.J.; Galbács, G.; Csizmadia, I.G.; Mucsi, Z. Synthesis and Spectroscopic Characterization of Novel GFP Chromophore Analogues Based on Aminoimidazolone Derivatives. Spectrochim. Acta A 2019, 218, 161–170. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, G.; Ke, G.; Ren, T.-B.; Yuan, L. Organic Fluorophores with Large Stokes Shift for Bioimaging and Biosensing. ChemPhotoChem 2024, e202300277. [Google Scholar] [CrossRef]
- Juvekar, V.; Park, S.J.; Yoon, J.; Kim, H.M. Recent Progress in the Two-Photon Fluorescent Probes for Metal Ions. Coord. Chem. Rev. 2021, 427, 213574. [Google Scholar] [CrossRef]
- Guo, Y.; Yao, L.; Luo, L.; Wang, H.-X.; Yang, Z.; Wang, Z.; Ai, S.-L.; Zhang, Y.; Zou, Q.-C.; Zhang, H.-L. Alkylaminomaleimide Fluorophores: Synthesis via Air Oxidation and Emission Modulation by Twisted Intramolecular Charge Transfer. Org. Chem. Front. 2021, 8, 239–248. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Masters, B.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2008; ISBN 0-387-31278-1. [Google Scholar]
- Makarov, N.S.; Drobizhev, M.; Rebane, A. Two-Photon Absorption Standards in the 550-1600 Nm Excitation Wavelength Range. Opt. Express 2008, 16, 4029. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Héder, M.; Rigó, E.; Medgyesi, D.; Lovas, R.; Tenczer, S.; Török, F.; Farkas, A.; Emődi, M.; Kadlecsik, J.; Mező, G.; et al. The Past, Present and Future of the ELKH Cloud. Információs Társadalom 2022, 22, 128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).