Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond
Abstract
1. Introduction
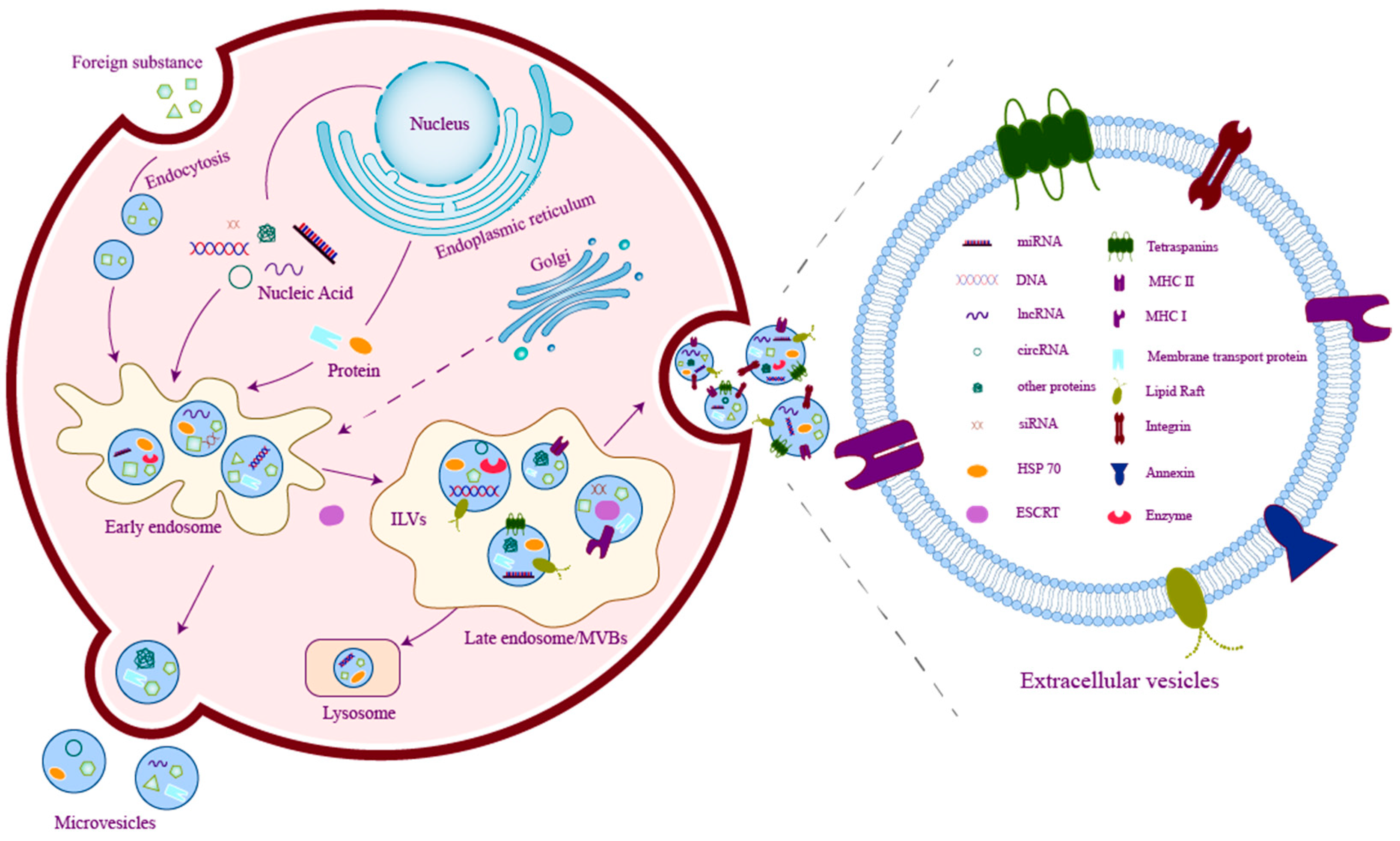
2. Basics of Extracellular Vesicles
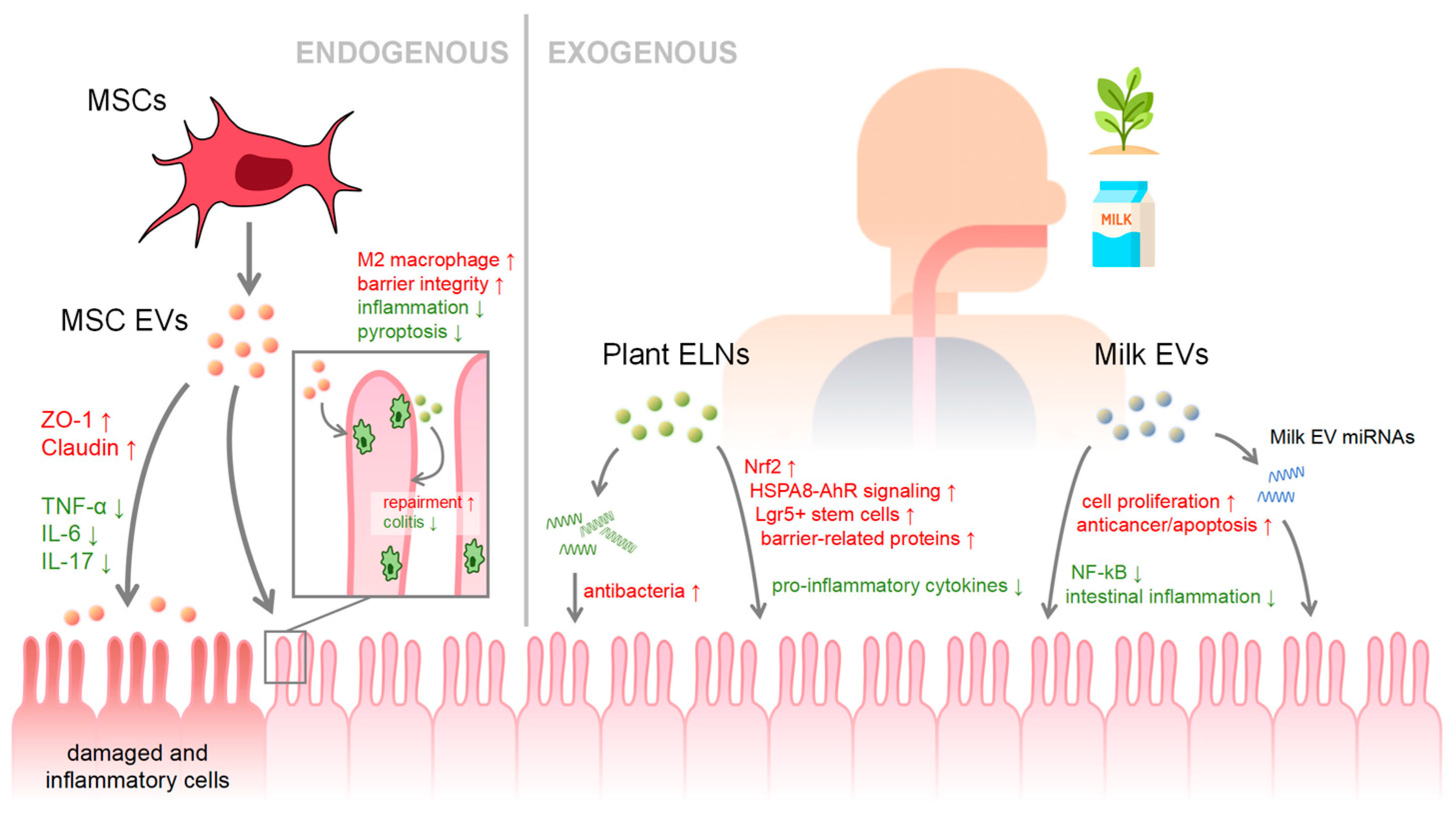
3. EVs in Health and Disease
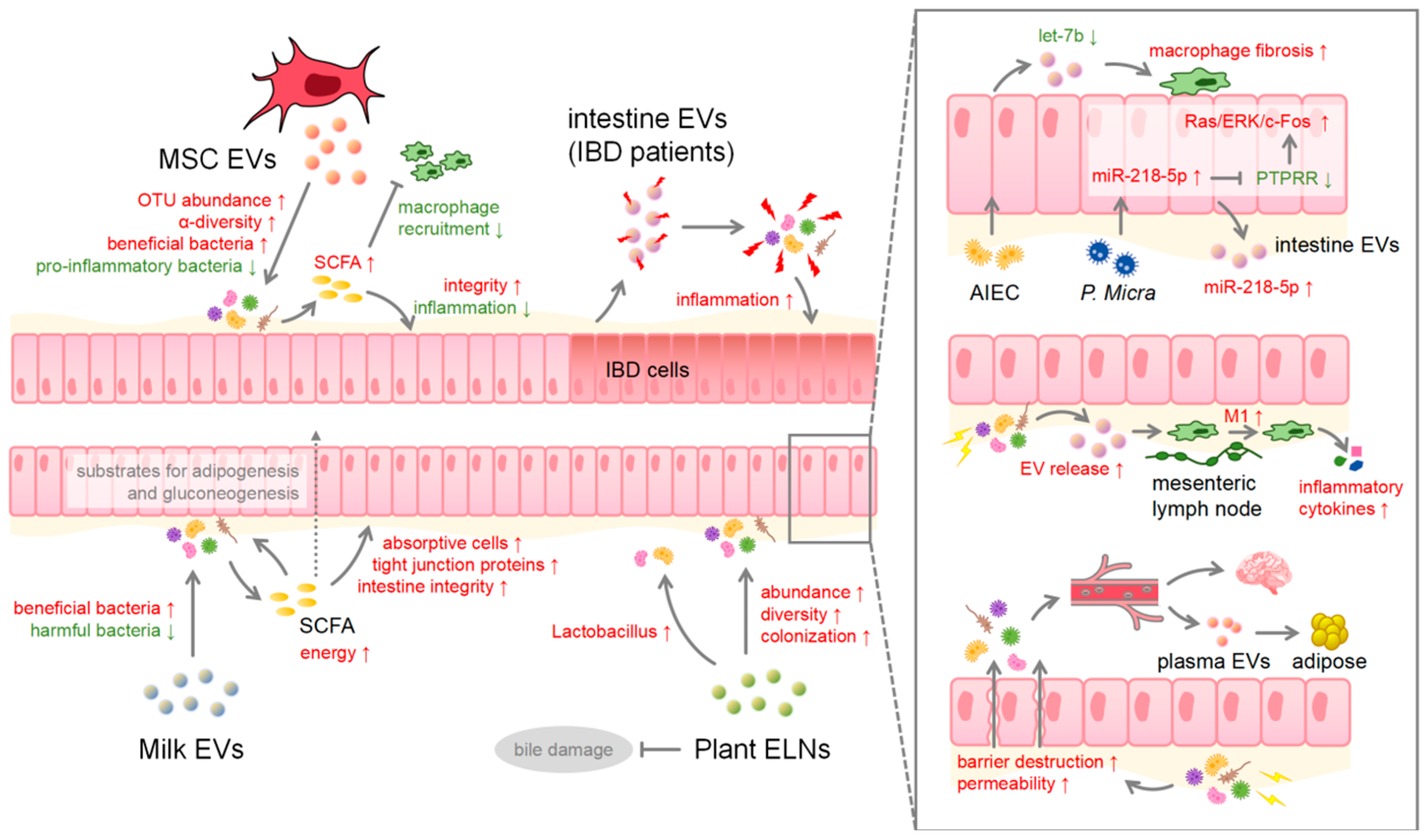
4. Microbiota Involved in and beyond the Intestine
5. Microbiome–Gut–Brain Axis
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jan, A.T.; Rahman, S.; Sarko, D.K.; Redwan, E.M. Editorial: Exploring the role of exosomes in disease progression and therapeutics in neurodegeneration. Front. Aging Neurosci. 2023, 15, 1177063. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Rahman, S.; Badierah, R.; Lee, E.J.; Mattar, E.H.; Redwan, E.M.; Choi, I. Expedition into Exosome Biology: A Perspective of Progress from Discovery to Therapeutic Development. Cancers 2021, 13, 1157. [Google Scholar] [CrossRef]
- Maghraby, M.K.; Li, B.; Chi, L.; Ling, C.; Benmoussa, A.; Provost, P.; Postmus, A.C.; Abdi, A.; Pierro, A.; Bourdon, C.; et al. Extracellular vesicles isolated from milk can improve gut barrier dysfunction induced by malnutrition. Sci. Rep. 2021, 11, 7635. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Barry, M.; Trivedi, A.; Pathipati, P.; Miyazawa, B.Y.; Vivona, L.R.; Togarrati, P.P.; Khakoo, M.; Tanner, H.; Norris, P.; Pati, S. Mesenchymal stem cell extracellular vesicles mitigate vascular permeability and injury in the small intestine and lung in a mouse model of hemorrhagic shock and trauma. J. Trauma Acute Care Surg. 2022, 92, 489–498. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Taitz, J.J.; Tan, J.K.; Potier-Villette, C.; Ni, D.; King, N.J.; Nanan, R.; Macia, L. Diet, commensal microbiota-derived extracellular vesicles, and host immunity. Eur. J. Immunol. 2023, 53, e2250163. [Google Scholar] [CrossRef]
- Gershon, M.D. The enteric nervous system: A second brain. Hosp. Pract. 1999, 34, 31–52. [Google Scholar] [CrossRef]
- Baghdadi, M.B.; Kim, T.H. The multiple roles of enteric glial cells in intestinal homeostasis and regeneration. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2023; Volume 150–151, pp. 43–49. [Google Scholar]
- Rosenberg, H.J.; Rao, M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience 2021, 24, 102863. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Birjandi, A.A.; Ren, F.; Sun, T.; Sharpe, P.T.; Sun, H.; An, Z. Advances in oral mesenchymal stem cell-derived extracellular vesicles in health and disease. Genes Dis. 2024, 11, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Cordero, C.; Olivo-Martinez, Y.; Badia, J.; Baldomà, L. Cell-to-Cell Communication by Host-Released Extracellular Vesicles in the Gut: Implications in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2213. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, T.; Okada, T.; Miya, S.; Zhang, L.F.; Nakamura, S.I. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat. Commun. 2013, 4, 2712. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Ahmad, S.; Srivastava, R.K.; Singh, P.; Naik, U.P.; Srivastava, A.K. Role of Extracellular Vesicles in Glia-Neuron Intercellular Communication. Front. Mol. Neurosci. 2022, 15, 844194. [Google Scholar] [CrossRef]
- Hanley, S.; Chen, Y.Y.; Hazeldine, J.; Lord, J.M. Senescent cell-derived extracellular vesicles as potential mediators of innate immunosenescence and inflammaging. Exp. Gerontol. 2024, 187, 112365. [Google Scholar] [CrossRef]
- Kumari, S.; Bandyopadhyay, B.; Singh, A.; Aggarwal, S.; Yadav, A.K.; Vikram, N.K.; Guchhait, P.; Banerjee, A. Extracellular vesicles recovered from plasma of severe dengue patients induce CD4+ T cell suppression through PD-L1/PD-1 interaction. mBio 2023, 14, e0182323. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yan, J.; Wang, C.; Qin, W.; Han, X.; Qin, Z.; Wei, Y.; Xu, H.; Gao, J.; Gao, C.; et al. Interorgan communication in neurogenic heterotopic ossification: The role of brain-derived extracellular vesicles. Bone Res. 2024, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Saman, S.; Kim, W.; Raya, M.; Visnick, Y.; Miro, S.; Saman, S.; Jackson, B.; McKee, A.C.; Alvarez, V.E.; Lee, N.C.; et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 2012, 287, 3842–3849. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef]
- Van Niel, G.; Raposo, G.; Candalh, C.; Boussac, M.; Hershberg, R.; Cerf-Bensussan, N.; Heyman, M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 2001, 121, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Elashiry, M.; Morandini, A.C.; Cornelius Timothius, C.J.; Ghaly, M.; Cutler, C.W. Selective Antimicrobial Therapies for Periodontitis: Win the “Battle and the War”. Int. J. Mol. Sci. 2021, 22, 6459. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, K.; Jiang, J.; Jiang, L.; Ma, X.; Ai, F.; Qiu, S.; Si, W. Perinatal tissue-derived exosomes ameliorate colitis in mice by regulating the Foxp3 + Treg cells and gut microbiota. Stem Cell Res. Ther. 2023, 14, 43. [Google Scholar] [CrossRef]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021, 19, 302. [Google Scholar] [CrossRef]
- Yang, S.; Liang, X.; Song, J.; Li, C.; Liu, A.; Luo, Y.; Ma, H.; Tan, Y.; Zhang, X. A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res. Ther. 2021, 12, 315. [Google Scholar] [CrossRef]
- Gu, L.; Ren, F.; Fang, X.R.; Yuan, L.W.; Liu, G.L.; Wang, S.L. Exosomal MicroRNA-181a Derived From Mesenchymal Stem Cells Improves Gut Microbiota Composition, Barrier Function, and Inflammatory Status in an Experimental Colitis Model. Front. Med. 2021, 8, 660614. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, e131273. [Google Scholar] [CrossRef]
- Deng, C.; Hu, Y.; Conceição, M.; Wood, M.J.A.; Zhong, H.; Wang, Y.; Shao, P.; Chen, J.; Qiu, L. Oral delivery of layer-by-layer coated exosomes for colitis therapy. J. Control Release 2023, 354, 635–650. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, X.; Fang, A.; Yan, J.; Kofi Wiredu Ocansey, D.; Zhang, X.; Mao, F. HucMSC-Ex carrying miR-203a-3p.2 ameliorates colitis through the suppression of caspase11/4-induced macrophage pyroptosis. Int. Immunopharmacol. 2022, 110, 108925. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, J.; Liu, H.; Li, S.; Wang, Q. The Role of Tissue-Resident Macrophages in the Development and Treatment of Inflammatory Bowel Disease. Front. Cell Dev. Biol. 2022, 10, 896591. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liao, L.; Gao, M.; Liu, Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-κB pathway and gut microbiota modulation. Food Funct. 2023, 14, 7520–7534. [Google Scholar] [CrossRef]
- Babaker, M.A.; Aljoud, F.A.; Alkhilaiwi, F.; Algarni, A.; Ahmed, A.; Khan, M.I.; Saadeldin, I.M.; Alzahrani, F.A. The Therapeutic Potential of Milk Extracellular Vesicles on Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6812. [Google Scholar] [CrossRef] [PubMed]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Xie, M.Y.; Chen, T.; Xi, Q.Y.; Hou, L.J.; Luo, J.Y.; Zeng, B.; Li, M.; Sun, J.J.; Zhang, Y.L. Porcine milk exosome miRNAs protect intestinal epithelial cells against deoxynivalenol-induced damage. Biochem. Pharmacol. 2020, 175, 113898. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Stremmel, W.; Weiskirchen, R.; John, S.M.; Schmitz, G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules 2021, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Vahkal, B.; Altosaar, I.; Tremblay, E.; Gagné, D.; Hüttman, N.; Minic, Z.; Côté, M.; Blais, A.; Beaulieu, J.F.; Ferretti, E. Gestational age at birth influences protein and RNA content in human milk extracellular vesicles. J. Extracell. Biol. 2024, 3, e128. [Google Scholar] [CrossRef]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef]
- Xie, M.Y.; Hou, L.J.; Sun, J.J.; Zeng, B.; Xi, Q.Y.; Luo, J.Y.; Chen, T.; Zhang, Y.L. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-kappa B and p53 Pathways in Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 9477–9491. [Google Scholar] [CrossRef]
- Gao, H.N.; Ren, F.Z.; Wen, P.C.; Xie, L.X.; Wang, R.; Yang, Z.N.; Li, Y.X. Yak milk-derived exosomal microRNAs regulate intestinal epithelial cells on proliferation in hypoxic environment. J. Dairy Sci. 2021, 104, 1291–1303. [Google Scholar] [CrossRef]
- Gao, H.N.; Hu, H.; Wen, P.C.; Lian, S.; Xie, X.L.; Song, H.L.; Yang, Z.N.; Ren, F.Z. Yak milk-derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation. J. Dairy Sci. 2021, 104, 8411–8424. [Google Scholar] [CrossRef]
- Gao, H.N.; Guo, H.Y.; Zhang, H.; Xie, X.L.; Wen, P.C.; Ren, F.Z. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J. Dairy Sci. 2019, 102, 985–996. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and Peptidomic Profiling of Exosomes in Preterm Human Milk: Insights Into Necrotizing Enterocolitis Prevention. Mol. Nutr. Food Res. 2019, 63, e1801247. [Google Scholar] [CrossRef]
- El-Kattawy, A.M.; Algezawy, O.; Alfaifi, M.Y.; Noseer, E.A.; Hawsawi, Y.M.; Alzahrani, O.R.; Algarni, A.; Kahilo, K.A.; El-Magd, M.A. Therapeutic potential of camel milk exosomes against HepaRG cells with potent apoptotic, anti-inflammatory, and anti-angiogenesis effects for colostrum exosomes. Biomed. Pharmacother. 2021, 143, 112220. [Google Scholar] [CrossRef]
- Stephen, B.J.; Pareek, N.; Saeed, M.; Kausar, M.A.; Rahman, S.; Datta, M. Xeno-miRNA in Maternal-Infant Immune Crosstalk: An Aid to Disease Alleviation. Front. Immunol. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.G.; Pink, R.C.; Erdbrügger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part II: Pathology. J. Extracell. Vesicles. 2022, 11, e12190. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, J.; De Wever, O.; Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 2020, 15, 40–67. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.Q.; Chen, W.D.; Wang, Y.D. Gut Microbiota: An Integral Moderator in Health and Disease. Front. Microbiol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Chen, S.; Xiao, M.; Liu, Z.; Li, J.; Cheng, Y. Mechanism and therapeutic strategies of depression after myocardial infarction. Psychopharmacology 2021, 238, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Díez-Sainz, E.; Milagro, F.I.; Riezu-Boj, J.I.; Lorente-Cebrián, S. Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J. Physiol. Biochem. 2022, 78, 485–499. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Zuo, M.; Ding, S.; Li, J.; Feng, S.; Xiao, Y.; Tao, S. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ. Int. 2024, 185, 108525. [Google Scholar] [CrossRef]
- Díaz-Garrido, N.; Badia, J.; Baldomà, L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J. Extracell. Vesicles 2021, 10, e12161. [Google Scholar] [CrossRef]
- Zhou, F.; Paz, H.A.; Sadri, M.; Cui, J.; Kachman, S.D.; Fernando, S.C.; Zempleni, J. Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G618–G624. [Google Scholar] [CrossRef]
- Du, C.; Wang, K.; Zhao, Y.; Nan, X.; Chen, R.; Quan, S.; Xiong, B. Supplementation with Milk-Derived Extracellular Vesicles Shapes the Gut Microbiota and Regulates the Transcriptomic Landscape in Experimental Colitis. Nutrients 2022, 14, 1808. [Google Scholar] [CrossRef]
- Manzaneque-López, M.C.; Sánchez-López, C.M.; Pérez-Bermúdez, P.; Soler, C.; Marcilla, A. Dietary-Derived Exosome-like Nanoparticles as Bacterial Modulators: Beyond MicroRNAs. Nutrients 2023, 15, 1265. [Google Scholar] [CrossRef]
- Anusha, R.; Priya, S. Dietary Exosome-Like Nanoparticles: An Updated Review on Their Pharmacological and Drug Delivery Applications. Mol. Nutr. Food Res. 2022, 66, e2200142. [Google Scholar] [CrossRef]
- Yang, L.; Wang, T.; Zhang, X.; Zhang, H.; Yan, N.; Zhang, G.; Yan, R.; Li, Y.; Yu, J.; He, J.; et al. Exosomes derived from human placental mesenchymal stem cells ameliorate myocardial infarction via anti-inflammation and restoring gut dysbiosis. BMC Cardiovasc. Disord. 2022, 22, 61. [Google Scholar] [CrossRef]
- Cheung, K.C.P.; Ma, J.; Chen, X.; Jia, W. Extracellular vesicles derived from host and gut microbiota as promising nanocarriers for targeted therapy in osteoporosis and osteoarthritis. Front. Pharmacol. 2022, 13, 1051134. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Teng, Y.; He, L.; Sayed, M.; Mu, J.; Xu, F.; Zhang, X.; Kumar, A.; Sundaram, K.; Sriwastva, M.K.; et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience 2021, 24, 102511. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Gao, Y.; Shen, L.; Li, S.; Chen, S. miRNA-Based Potential Biomarkers and New Molecular Insights in Ulcerative Colitis. Front. Pharmacol. 2021, 12, 707776. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; López de Las Hazas, M.C.; Margollés, A.; Dávalos, A.; García-Ruiz, A. Eating microRNAs: Pharmacological opportunities for cross-kingdom regulation and implications in host gene and gut microbiota modulation. Br. J. Pharmacol. 2021, 178, 2218–2245. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mikhailova, M.V.; Rasoolzadegan, S.; Falaki, M.; Akhavanfar, R.; Gonzáles, J.L.A.; Rigi, A.; Kiasari, B.A. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur. J. Med. Res. 2023, 28, 47. [Google Scholar] [CrossRef]
- Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Hamelin, R.; Demurtas, D.; Alibashe Ahmed, M.; Piemonti, L.; Hirosue, S.; Swartz, M.A.; De Palma, M.; et al. Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes 2017, 66, 460–473. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, Y.D.; Qi, X.Y.; Liao, Z.Z.; Mai, Y.N.; Xiao, X.H. Organokines and Exosomes: Integrators of Adipose Tissue Macrophage Polarization and Recruitment in Obesity. Front. Endocrinol. 2022, 13, 839849. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhou, Y.; Chen, Z.; Li, H.; Xiao, Y.; Hao, W.; Zhu, Y.; Vong, C.T.; Farag, M.A.; Wang, Y.; et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics 2022, 12, 5596–5614. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Ericsson, A.; Qiao, Z.; Almendros, I.; Farré, R.; Gozal, D. Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: Effects of physical activity. EBioMedicine 2021, 64, 103208. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, Z.; Hou, F.; Liu, Y.; Wang, L.; Wang, Z.; Sun, Y.; Pan, Z.; Tan, Y.; Ding, L.; et al. Parvimonas micra activates the Ras/ERK/c-Fos pathway by upregulating miR-218-5p to promote colorectal cancer progression. J. Exp. Clin. Cancer Res. 2023, 42, 13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, W.; Huang, L.; Wen, W.; Li, Y.; Guo, F.; Zhu, Z.; Li, Z.; Gong, J.; Yu, Z.; et al. Crohn’s disease-associated AIEC inhibiting intestinal epithelial cell-derived exosomal let-7b expression regulates macrophage polarization to exacerbate intestinal fibrosis. Gut Microbes 2023, 15, 2193115. [Google Scholar] [CrossRef]
- Xi, S.; Wang, Y.; Wu, C.; Peng, W.; Zhu, Y.; Hu, W. Intestinal Epithelial Cell Exosome Launches IL-1β-Mediated Neuron Injury in Sepsis-Associated Encephalopathy. Front. Cell Infect. Microbiol. 2021, 11, 783049. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Liu, K.; Zhang, H.L. Microbiota-gut-brain axis and Alzheimer’s disease: Implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev. 2021, 199, 111560. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, B.; Meng, W.; Hu, J. Elucidating a fresh perspective on the interplay between exosomes and rheumatoid arthritis. Front. Cell Dev. Biol. 2023, 11, 1177303. [Google Scholar] [CrossRef] [PubMed]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, Y.; Gu, L.; Jian, Z.; Stary, C.M.; Xiong, X. Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J. Transl. Med. 2021, 19, 202. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Quan, X.; Chuang, Y.; Liang, Q.; Li, Y.; Yuan, Z.; Bian, Y.; Wei, L.; Wang, J.; Zhao, Y. A multi-omics analysis for the prediction of neurocognitive disorders risk among the elderly in Macao. Clin. Transl. Med. 2022, 12, e909. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef]
- Yates, A.G.; Pink, R.C.; Erdbrügger, U.; Siljander, P.R.; Dellar, E.R.; Pantazi, P.; Akbar, N.; Cooke, W.R.; Vatish, M.; Dias-Neto, E.; et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology. J. Extracell. Vesicles 2022, 11, e12151. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Jiang, M.; Peng, D.; Dou, X.; Song, Y.; Shi, J. The Potential Role of Gut Microbial-Derived Exosomes in Metabolic-Associated Fatty Liver Disease: Implications for Treatment. Front. Immunol. 2022, 13, 893617. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, L.; Mao, Z.; Wang, Z.; Zhang, Z.; Li, M. Bidirectional Communication Between the Brain and Other Organs: The Role of Extracellular Vesicles. Cell Mol. Neurobiol. 2023, 43, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Choi, Y.Y.; Mo, S.J.; Kim, T.H.; Ha, J.H.; Hong, D.K.; Lee, H.; Park, S.D.; Shim, J.J.; Lee, J.L.; et al. Effect of gut microbiome-derived metabolites and extracellular vesicles on hepatocyte functions in a gut-liver axis chip. Nano Converg. 2023, 10, 5. [Google Scholar] [CrossRef]
- Diaz-Garrido, N.; Badia, J.; Baldomà, L. Modulation of Dendritic Cells by Microbiota Extracellular Vesicles Influences the Cytokine Profile and Exosome Cargo. Nutrients 2022, 14, 344. [Google Scholar] [CrossRef]
- Jones, E.J.; Booth, C.; Fonseca, S.; Parker, A.; Cross, K.; Miquel-Clopés, A.; Hautefort, I.; Mayer, U.; Wileman, T.; Stentz, R.; et al. The Uptake, Trafficking, and Biodistribution of Bacteroides thetaiotaomicron Generated Outer Membrane Vesicles. Front. Microbiol. 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, E.J.; Sirisaengtaksin, N.; Browning, D.F.; Bielska, E.; Hadis, M.; Fernandez-Trillo, F.; Alderwick, L.; Jabbari, S.; Krachler, A.M. Lipopolysaccharide structure impacts the entry kinetics of bacterial outer membrane vesicles into host cells. PLoS Pathog. 2017, 13, e1006760. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.; Nygård Skalman, L.; Obi, I.; Lundmark, R.; Arnqvist, A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. mBio 2014, 5, e00979-14. [Google Scholar] [CrossRef]
- Sultan, S.; Mottawea, W.; Yeo, J.; Hammami, R. Gut Microbiota Extracellular Vesicles as Signaling Molecules Mediating Host-Microbiota Communications. Int. J. Mol. Sci. 2021, 22, 13166. [Google Scholar] [CrossRef]
- Yaghoubfar, R.; Behrouzi, A.; Ashrafian, F.; Shahryari, A.; Moradi, H.R.; Choopani, S.; Hadifar, S.; Vaziri, F.; Nojoumi, S.A.; Fateh, A.; et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020, 10, 22119. [Google Scholar] [CrossRef]
- Sun, J.; Xu, G. Mesenchymal Stem Cell-Derived Exosomal miR-150-3p Affects Intracerebral Hemorrhage By Regulating TRAF6/NF-κB Axis, Gut Microbiota and Metabolism. Stem Cell Rev. Rep. 2023, 19, 1907–1921. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H.; Liu, T.; Yan, J.; Zhang, F.; Zhang, X.; Zhao, J.; Mu, X.; Jiang, J. Depletion of gut microbiota resistance in 5×FAD mice enhances the therapeutic effect of mesenchymal stem cell-derived exosomes. Biomed. Pharmacother. 2023, 161, 114455. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Izco, M.; Schleef, M.; Schmeer, M.; Carlos, E.; Verona, G.; Alvarez-Erviti, L. Targeted Extracellular Vesicle Gene Therapy for Modulating Alpha-Synuclein Expression in Gut and Spinal Cord. Pharmaceutics 2023, 15, 1230. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Zhou, J.; Su, C. Extracellular Vesicles for Drug Delivery in Cancer Treatment. Biol. Proced. Online 2023, 25, 28. [Google Scholar] [CrossRef]
- Huang, X.; Li, A.; Xu, P.; Yu, Y.; Li, S.; Hu, L.; Feng, S. Current and prospective strategies for advancing the targeted delivery of CRISPR/Cas system via extracellular vesicles. J. Nanobiotechnol. 2023, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. A new route for EV biogenesis. Cell Res. 2023, 33, 87–88. [Google Scholar] [CrossRef] [PubMed]
| Microbiota Type | Role in the Intestine |
|---|---|
| Bifidobacterium | Decompose SCFAs produced by dietary fiber, improve the intestinal environment, help the absorption of nutrients, interact with intestinal immune cells, and regulate immune responses. |
| Dubosiella | Maintain the integrity of the intestinal mucosal barrier and regulate the diversity of the intestinal microbial community |
| Lachnoclostridium | Break down dietary fiber and other non-digestible polysaccharides, maintain intestinal health, regulate the immune system, provide an energy source. |
| Lachnospiraceae | Decompose a variety of plant fibers and polysaccharides, produce beneficial metabolites. |
| Lactobacillus rhamnosus | Enhance immune function, improve gut health. |
| Lactobacillus rhamnosus GG | Regulate immune response, enhance intestinal barrier function, and alleviate intestinal diseases. |
| Firmicutes | Extract energy and maintain intestinal health. |
| Bacteroidetes | Break down fiber and maintain immune regulation |
| Akkermansia muciniphila | Break down mucus, produce SCFAs, strengthen the intestinal barrier, and reduce inflammation. |
| Escherichia coli | Certain strains of E. coli can produce vitamin K and B group vitamins, participate in the nutritional intake, help to decompose nutrients |
| Parvimonas micra | Considered one of the main causative agents of periodontitis, and infections at other sites. |
| Adherent-invasive Escherichia coli (AIEC) | Adhesion and invasion of intestinal epithelial cells, leading to impairment of intestinal barrier function and triggering inflammatory response. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Luo, J.; Wang, H.; Chen, T.; Sun, J.; Xi, Q.; Zhang, Y. Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond. Int. J. Mol. Sci. 2024, 25, 3478. https://doi.org/10.3390/ijms25063478
Wang S, Luo J, Wang H, Chen T, Sun J, Xi Q, Zhang Y. Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond. International Journal of Molecular Sciences. 2024; 25(6):3478. https://doi.org/10.3390/ijms25063478
Chicago/Turabian StyleWang, Shumeng, Junyi Luo, Hailong Wang, Ting Chen, Jiajie Sun, Qianyun Xi, and Yongliang Zhang. 2024. "Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond" International Journal of Molecular Sciences 25, no. 6: 3478. https://doi.org/10.3390/ijms25063478
APA StyleWang, S., Luo, J., Wang, H., Chen, T., Sun, J., Xi, Q., & Zhang, Y. (2024). Extracellular Vesicles: A Crucial Player in the Intestinal Microenvironment and Beyond. International Journal of Molecular Sciences, 25(6), 3478. https://doi.org/10.3390/ijms25063478





