Simultaneous Determination of One-Carbon Folate Metabolites and One-Carbon-Related Amino Acids in Biological Samples Using a UHPLC–MS/MS Method
Abstract
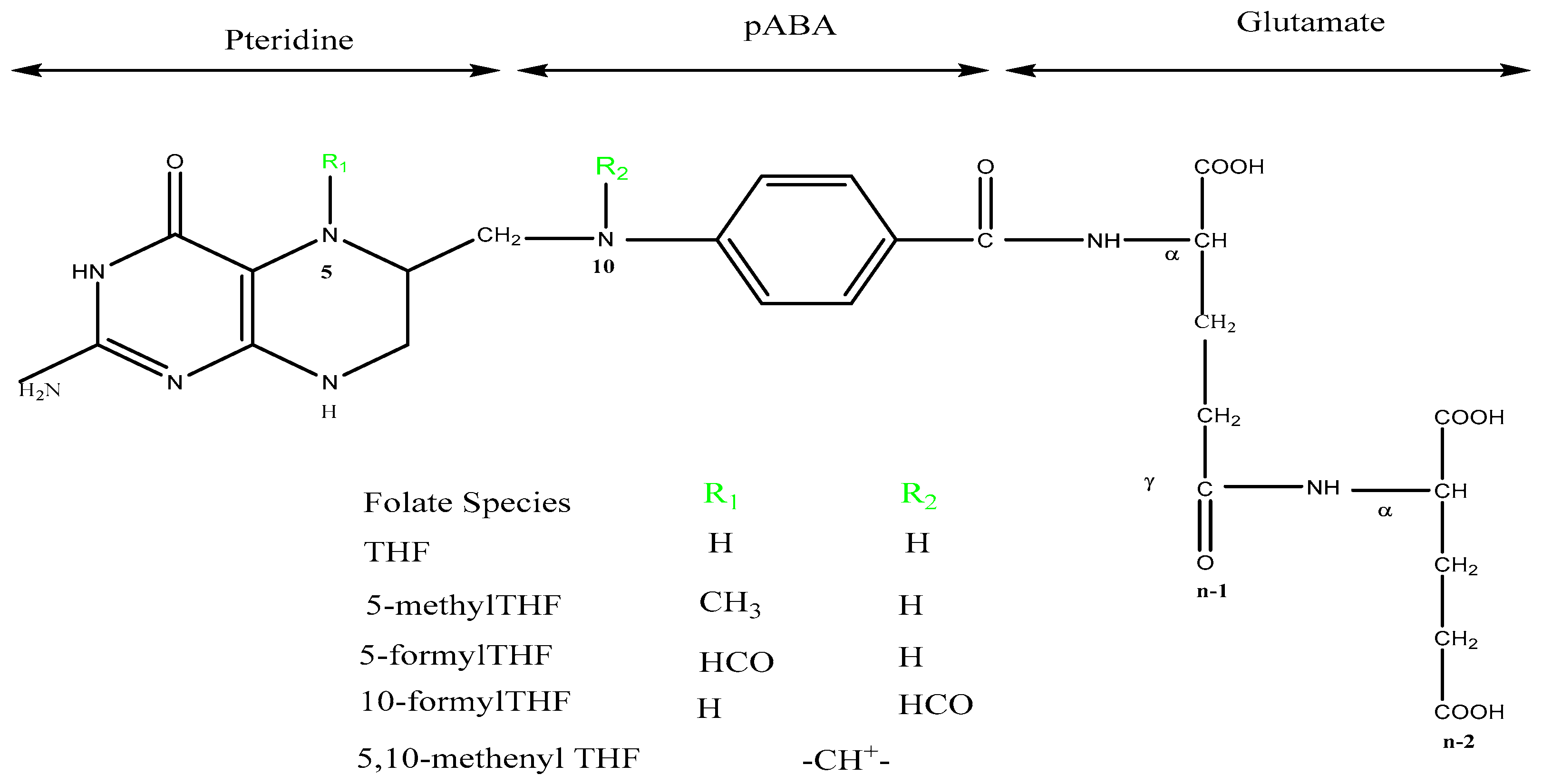
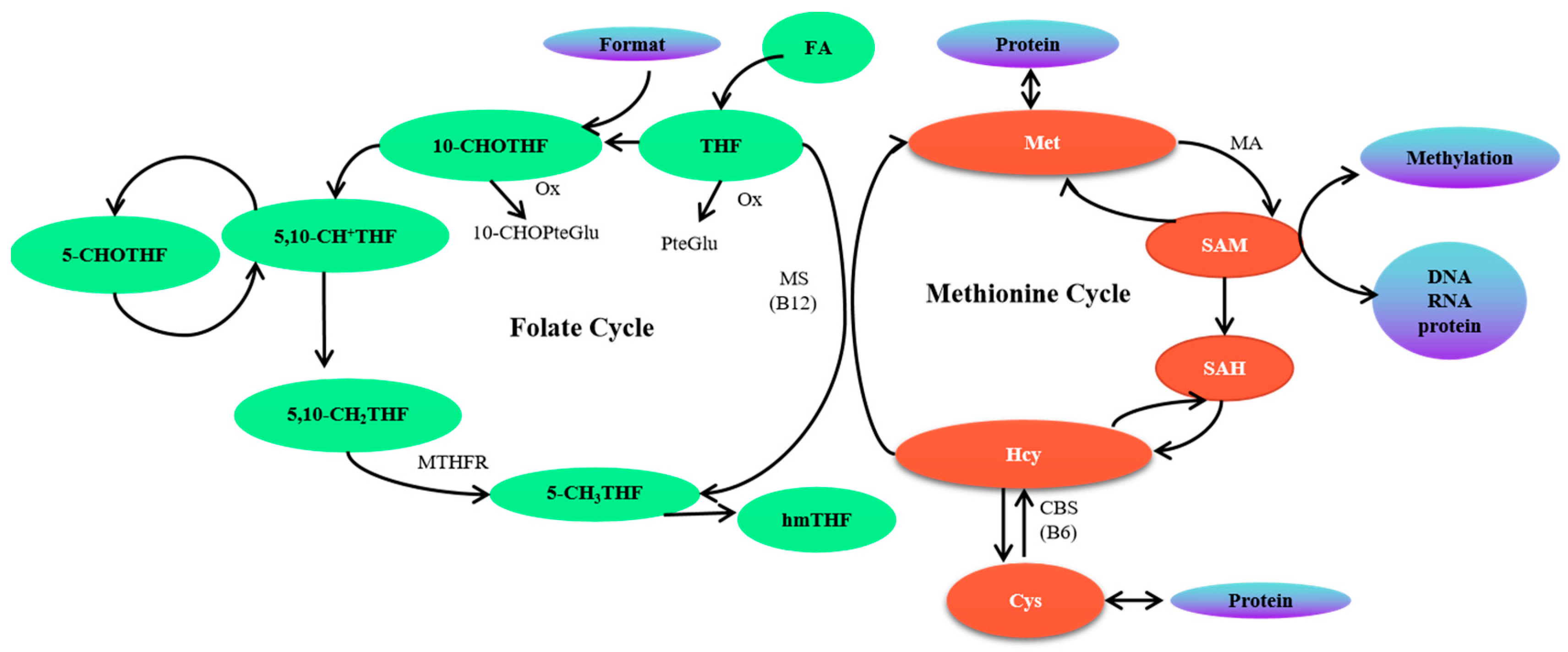
1. Introduction
2. Results
2.1. Method Optimization
2.2. Method Validation
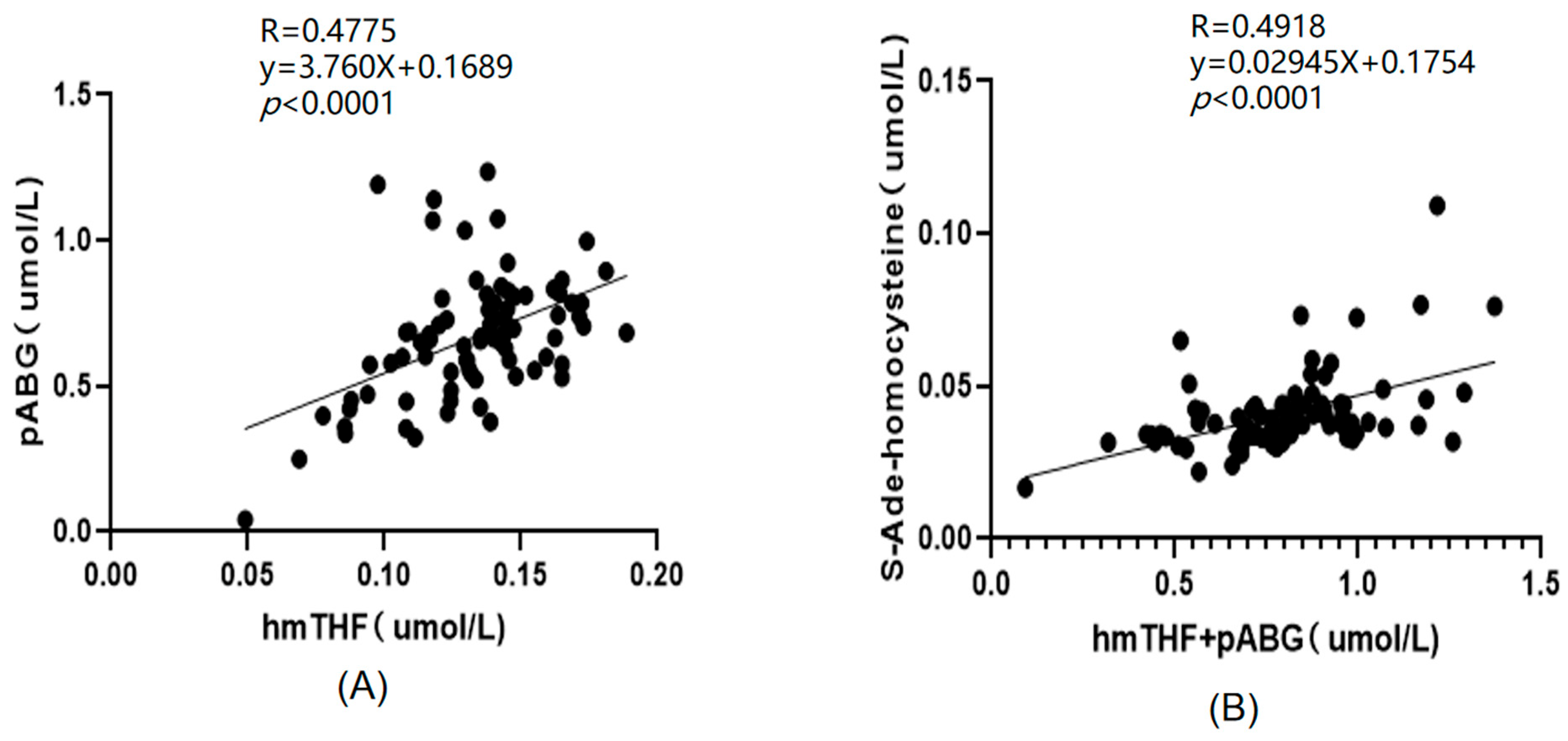
2.3. Application of the Method
Distribution of Folate Species in Different Biological Samples
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Standard Solutions
4.3. Sample Preparation
4.4. UHPLC–MS/MS Quantification
4.5. Method Validation
4.6. Calculation of Red Blood Cell (RBC) Folate Concentrations
4.7. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | ascorbic acid |
| CBS | cystathionine β-synthase |
| 5-CH3THF | methyltetrahydrofolic acid |
| 5-CH3THF-13C5 | 5-methyltetrahydrofolic acid-13C5 |
| 5-CHOTHF | 5-formyltetrahydrofolic acid |
| 10-CHOFA | 10-formylfolic acid |
| 5,10-CH+THF | 5,10-methenyl-5,6,7,8-tetrahydrofolic acid |
| FA | folic acid |
| FA-13C5 | folic acid-13C5 |
| FBP | Folate-binding proteins |
| Hcy | homocysteine |
| Ox | oxidation |
| 2-MCE | 2-mercaptoethanol |
| MA | methionine adenosyltransferase |
| Met | methionine |
| L-Met-13C5 | L-methionine-13C5 |
| MTHFR | 5,10-methenyl-5,6,7,8-tetrahydrofolic acid reductase |
| MS | methionine synthase |
| hmTHF | 4-α-hydroxy-5-methyltetrahydrofolate |
| pABG | p-aminobenzoyl-L-glutamic acid |
| SAH | S-ade-homocysteine |
| SAM | S-ade-methionine |
| THF | tetrahydrofolic acid |
| UHPLC–MS/MS | ultra-high-performance liquid chromatography–MS/MS |
| WB | whole blood |
References
- Wakeel, A.; Arif, S.; Bashir, M.A.; Ahmad, Z.; Rehman, H.U.; Kiran, A.; Ibrahim, S.; Khan, M.R. Perspectives of folate biofortification of cereal grains. J. Plant Nutr. 2018, 41, 2507–2524. [Google Scholar] [CrossRef]
- Hou, H.M.; Zhao, H.Y. Epigenetic factors in atherosclerosis: DNA methylation, folic acid metabolism, and intestinal microbiota. Clin. Chim. Acta 2021, 512, 7–11. [Google Scholar] [CrossRef]
- Basset, G.J.C.; Quinlivan, E.P.; Gregory, J.F.; Hanson, A.D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop Sci. 2005, 45, 449–453. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Herrmann, W.; Eckert, R.; Geisel, J.; Obeid, R. Factors affecting the distribution of folate forms in the serum of elderly German adults. Eur. J. Nutr. 2013, 52, 497–504. [Google Scholar] [CrossRef]
- Golja, M.V.; Trontelj, J.; Gersak, K.; Mlinaric-Rascan, I.; Smid, A. Simultaneous quantification of intracellular concentrations of clinically important metabolites of folate-homocysteine cycle by LC-MS/MS. Anal. Biochem. 2020, 605, 113830. [Google Scholar]
- Barua, S.; Kuizon, S.; Junaid, M.A. Folic acid supplementation in pregnancy and implications in health and disease. J. Biomed. 2014, 21, 77. [Google Scholar] [CrossRef]
- Gmelch, L.; Wirtz, D.; Witting, M.; Weber, N.; Striegel, L.; Schmitt-Kopplin, P.; Rychlik, M. Comprehensive Vitamer Profiling of Folate Mono- and Polyglutamates in Baker’s Yeast (Saccharomyces cerevisiae) as a Function of Different Sample Preparation Procedures. Metabolites 2020, 10, 301. [Google Scholar] [CrossRef]
- Kiyohara, C.; Horiuchi, T.; Takayama, K.; Nakanishi, Y. Methylenetetrahydrofolate reductase polymorphisms and interaction with smoking and alcohol consumption in lung cancer risk: A case-control study in a Japanese population. BMC Cancer 2011, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Berti, C.; Fekete, K.; Dullemeijer, C.; Trovato, M.; Souverein, O.W.; Cavelaars, A.; Dhonukshe-Rutten, R.; Massari, M.; Decsi, T.; Van’t Veer, P. Folate intake and markers of folate status in women of reproductive age, pregnant and lactating women: A meta-analysis. Nutr. Metab. 2012, 2012, 470656. [Google Scholar] [CrossRef] [PubMed]
- Prasodjo, A.; Pfeiffer, C.M.; Fazili, Z.; Xu, Y.Y.; Liddy, S.; Yolton, K.; Savitz, D.A.; Lanphear, B.P.; Braun, J.M. Serum cotinine and whole blood folate concentrations in pregnancy. Ann. Epidemiol. 2014, 24, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Belz, S.; Nau, H. Determination of folate patterns in mouse plasma, erythrocytes, and embryos by HPLC coupled with a microbiological assay. Anal. Biochem. 1998, 265, 157–166. [Google Scholar] [CrossRef]
- Leung, K.Y.; De Castro, S.C.P.; Cabreiro, F.; Gustavsson, P.; Copp, A.J.; Greene, N.D.E. Folate metabolite profiling of different cell types and embryos suggests variation in folate one-carbon metabolism, including developmental changes in human embryonic brain. Mol. Cell. Biochem. 2013, 378, 229–236. [Google Scholar] [CrossRef]
- Zou, Y.C.; Duan, H.Y.; Li, L.; Chen, X.J.; Wang, C. Quantification of polyglutamyl 5-methyltetrahydrofolate, monoglutamyl folate vitamers, and total folates in different berries and berry juice by UHPLC-MS/MS. Food Chem. 2019, 276, 1–8. [Google Scholar] [CrossRef]
- van Haandel, L.; Stobaugh, J.F. Folate determination in human health: UPLC-MS/MS is the emerging methodology of choice. Bioanalysis 2013, 5, 3023–3031. [Google Scholar] [CrossRef]
- Badiou, S.; Bariolet, S.; Laurens, C.; Bargnoux, A.; Mariano-Goulart, D.; Anne-Marie Cristol, J. Comparison of Isotopic and Immunoenzymatic Methods for Folate and Vitamin B12 Determination. J. Clin. Lab. Anal. 2010, 56, 547–552. [Google Scholar]
- Ivanov, A.V.; Luzyanin, B.P.; Virus, E.D.; Rotkina, A.S.; Kubatiev, A.A. Detection of S-adenosylhomocysteine and methylation index in blood by capillary electrophoresis. Electrophoresis 2014, 35, 2972–2977. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Fazili, Z.; McCoy, L.; Zhang, M.; Gunter, E.W. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin. Chem. 2004, 50, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.F.S.; Hsu, Y.C.; Lin, H.L.; Yang, F.L. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 2001, 131, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Zhang, T.; Zhao, X.; Guan, Z.; Wang, Z.; Zhu, Z.Q.; Xie, Q.; Wang, J.H.; Niu, B. Quantification of folate metabolites in serum using ultraperformance liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2014, 962, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.A.; Desiderio, D.M.; Groover, C.J.; Hartzes, A.; Yates, C.R.; Zucker-Levin, A.R.; Bloom, L.; Levin, M.C. LC-MS/MS identification of the one-carbon cycle metabolites in human plasma. Electrophoresis 2013, 34, 1710–1716. [Google Scholar] [CrossRef]
- Kopp, M.; Morisset, R.; Rychlik, M. Characterization and Interrelations of One-Carbon Metabolites in Tissues, Erythrocytes, and Plasma in Mice with Dietary Induced Folate Deficiency. Nutrients 2017, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. European Medicines Agency guideline on bioanalytical method validation: What more is there to say? Bioanalysis 2012, 4, 865–868. [Google Scholar] [CrossRef]
- Hannisdal, R.; Ueland, P.M.; Svardal, A. Liquid Chromatography-Tandem Mass Spectrometry Analysis of Folate and Folate Catabolites in Human Serum. Clin. Chem. 2009, 55, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Solvik, B.S.; Strand, T.A.; Kvestad, I.; Markhus, M.W.; Ueland, P.M.; McCann, A.; Oyen, J. Dietary Intake and Biomarkers of folate and Cobalamin Status in Norwegian Preschool Children: The FINS-KIDS Study. J. Nutr. 2020, 150, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Bustami, R.; Alabsi, W.; El-Elimat, T. Development and Validation of a Rapid High-Performance Liquid Chromatography-Tandem Mass Spectrometric Method for Determination of Folic Acid in Human Plasma. Pharmaceutics 2018, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Z.; Paladugula, N.; Zhang, M.; Pfeiffer, C.M. Folate Forms in RBC and Whole-Blood Lysates Appear Stable When Stored Frozen for 2 Years. J. Nutr. 2021, 151, 2852–2860. [Google Scholar] [CrossRef]
- Report on the Relationship between Analytical Results, Measurement Uncertainty, Recovery Factors and the Provisions of EU Food and Feed Legislation, with Particular Reference to Community Legislation Concerning. Available online: https://food.ec.europa.eu/system/files/2016-10/cs_contaminants_sampling_analysis-report_2004_en.pdf (accessed on 9 September 2023).
- Stamm, R.A.; Fazili, Z.; Pfeiffer, C.M. Addition of Exogenous gamma-Glutamyl Hydrolase Eliminates the Need for Lengthy Incubation of Whole-Blood Lysate for Quantitation of Folate Vitamers by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Curr. Dev. Nutr. 2018, 2, cdn002055. [Google Scholar] [CrossRef]
- Yang, L.; Sturgeon, R.E.; McSheehy, S.; Mester, Z. Comparison of extraction methods for quantitation of methionine and selenomethionine in yeast by species specific isotope dilution gas chromatography-mass spectrometry. J. Chromatogr. A 2004, 1055, 177–184. [Google Scholar] [CrossRef]
- Christensen, B.; Landaas, S.; Stensvold, I.; Djurovic, S.; Retterstol, L.; Ringstad, J.; Berg, K.; Thelle, D.S. Whole blood folate, homocysteine in serum, and risk of first acute myocardial infarction. Atherosclerosis 1999, 147, 317–326. [Google Scholar] [CrossRef]
- Kiekens, F.; Van Daele, J.; Blancquaert, D.; Van Der Straeten, D.; Lambert, W.E.; Stove, C.P. A validated ultra-high-performance liquid chromatography-tandem mass spectrometry method for the selective analysis of free and total folate in plasma and red blood cells. J. Chromatogr. A 2015, 1398, 20–28. [Google Scholar] [CrossRef]
- Kirsch, S.H.; Knapp, J.P.; Herrmann, W.; Obeid, R. Quantification of key folate forms in serum using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2010, 878, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Awwad, H.M.; Ohlmann, C.H.; Stoeckle, M.; Geisel, J.; Obeid, R. Serum concentrations of folate vitamers in patients with a newly diagnosed prostate cancer or hyperplasia. Clin. Biochem. 2018, 56, 41–46. [Google Scholar] [CrossRef]
- Hannisdal, R.; Ueland, P.M.; Eussen, S.; Svardal, A.; Hustad, S. Analytical Recovery of Folate Degradation Products Formed in Human Serum and Plasma at Room Temperature. J. Nutr. 2009, 139, 1415–1418. [Google Scholar] [CrossRef]
- Xu, J.N.; Xu, Z.K.; Ge, N.X.; Wang, C.; Hu, C.; Chen, Z.; Ouyang, J.; Pei, C.S. Association between folic acid, homocysteine, vitamin B12 and erectile dysfunction-A cross-sectional study. Andrologia 2021, 53, e14234. [Google Scholar] [CrossRef]
- Ji, Y.B.; Luo, H.J.; Li, H.; Lin, Z.X.; Luo, W.H. Determination of plasma homocysteine with a UHPLC-MS/MS method: Application to analyze the correlation between plasma homocysteine and whole blood 5-methyltetrahydrofolate in healthy volunteers. Biomed. Chromatogr. 2020, 34, e4845. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Zhang, Z.X.; Zhou, C.; Li, Q.Q.; He, P.P.; Zhang, Y.Y.; Li, H.; Liu, C.Z.; Liang, M.; Wang, X.B.; et al. Relationship of several serum folate forms with the risk of mortality: A prospective cohort study. Clin. Nutr. 2021, 40, 4255–4262. [Google Scholar] [CrossRef]
- Suh, J.R.; Herbig, A.K.; Stover, P.J. New perspectives on folate catabolism. Annu. Rev. Nutr. 2001, 21, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Striegel, L.; Brandl, B.; Kopp, M.; Sam, L.; Skurk, T.; Rychlik, M. Quantitation of 5-methyltetraydrofolic acid in plasma for determination of folate status and clinical studies by stable isotope dilution assays. PLoS ONE 2019, 14, e0212255. [Google Scholar] [CrossRef] [PubMed]
- Nandania, J.; Kokkonen, M.; Euro, L.; Velagapudi, V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2018, 1092, 168–178. [Google Scholar] [CrossRef]
- Ping, C.; Yun, T.; Qiangqiang, H.; Lishun, L.; Ziyi, Z.; Jie, J. A sensitive, robust and high-throughput isotope dilution LC-MS/MS method for quantifying three folate forms in serum. J. Pharm. Biomed. Anal. 2022, 219, 114944. [Google Scholar]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-Methyltetrahydrofolate and Folic Acid Levels in Maternal and Cord Blood Serum: Longitudinal Evaluation of Japanese Pregnant Women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef]
- Camara, J.E.; Pritchett, J.S.; Daniels, Y.C.; Bedner, M.; Nelson, M.A.; Stephen, A. Development of an improved standard reference material for folate vitamers in human serum. Anal. Bioanal. Chem. 2023, 415, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Jatin, N.; Meri, K.; Liliya, E.; Vidya, V. Simultaneous quantitation of folates, flavins and B6 metabolites in human plasma by LC–MS/MS assay: Applications in colorectal cancer. J. Pharm. Biomed. Anal. 2018, 158, 66–73. [Google Scholar]
- Guiraud, S.P.; Montoliu, I.; Da Silva, L.; Dayon, L.; Galindo, A.N.; Corthesy, J. High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and cofactors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.; Rychlik, M. Quantitation of 5-methyltetrahydrofolic acid in dried blood spots and dried plasma spots by stable isotope dilution assays. PLoS ONE 2015, 10, e0143639. [Google Scholar] [CrossRef] [PubMed]
- Lamers, Y.; Prinz-Langenohl, R.; Braemswig, S.; Pietrzik, K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am. J. Clin. Nutr. 2006, 84, 156–161. [Google Scholar] [CrossRef]

| S-30 1 | S-60 | S-90 | Protease–Amylase 2 | |
|---|---|---|---|---|
| 5,10-CH+THF | 103.8 ± 12.2 a | 112.1 ± 0.6 a | 120.4 ± 9.4 a | N.D. |
| pABG | 15.0 ± 5.2 a | 12.7 ± 1.2 a | 12.7 ± 2.9 a | 33.7 ± 3.5 b |
| 5-CH3THF | 45.5 ± 7.9 a | 24.0 ± 0.8 b | 12.5 ± 0.4 c | N.D. |
| Met | 7863.3 ± 179.0 a | 8194.5 ± 118.1 a | 8162.2 ± 266.2 a | (31.3 ± 2.9) × 103 b |
| SAH | 1629.2 ± 38.5 a | 1094.1 ± 19.8 b | 742.1 ± 91.1 c | 20.4 ± 1.0 d |
| SAM | 246.2 ± 26.7 a | 217.3 ± 10.7 ab | 204.1 ± 18.4 a | N.D. 2 |
| Experiment 1 1 | Experiment 2 2 | |
|---|---|---|
| pABG | 46.5 ± 2.7 b | 91.4 ± 4.8 a |
| 5-CH3THF | 25.3 ± 3.1 b | 48.2 ± 1.9 a |
| 10-CHOFA | 10.1 ± 1.4 b | 39.8 ± 2.9 a |
| FA | 74.9 ± 11.5 b | 132.2 ± 16.8 a |
| hmTHF | 10.9 ± 2.0 a | N.D. |
| Hcy | 12.8 ± 1.4 a | 11.3 ± 2.7 a |
| SAH | 59.7 ± 9.1 b | 80.5 ± 13.8 a |
| Met | 1199.3 ± 38.3 b | 2476.4 ± 164.0 a |
| Internal Standard | Type | Transition (m/z) | CE (V) | Fragmentor (V) |
|---|---|---|---|---|
| 5-CHOTHF | Quantifier | 474→327 | 19 | 140 |
| Qualifier | 474→299 | 45 | 140 | |
| pABG | Quantifier | 267→120 | 15 | 90 |
| Qualifier | 267→130 | 15 | 90 | |
| 5-CH3THF | Quantifier | 460→313 | 19 | 140 |
| Qualifier | 460→400 | 8 | 140 | |
| 10-CHOFA | Quantifier | 470→295 | 24 | 160 |
| Qualifier | 470→323 | 15 | 160 | |
| FA | Quantifier | 442→295 | 16 | 110 |
| Qualifier | 442→176 | 53 | 110 | |
| 510-CH+THF | Quantifier | 456→412 | 33 | 180 |
| Qualifier | 456→327 | 33 | 180 | |
| THF | Quantifier | 446→299 | 16 | 110 |
| Qualifier | 446→166 | 59 | 110 | |
| Met | Quantifier | 150→104 | 5 | 80 |
| Qualifier | 150→56 | 15 | 80 | |
| SAH | Quantifier | 385→136 | 25 | 70 |
| Qualifier | 385→134 | 35 | 70 | |
| SAM | Quantifier | 399→250 | 15 | 120 |
| Qualifier | 399→298 | 27 | 120 | |
| Hcy | Quantifier | 136→90 | 5 | 80 |
| Qualifier | 136→118 | 20 | 80 | |
| 5-CH3THF-13C5 | Quantifier | 465→313 | 19 | 140 |
| FA-13C5 | Quantifier | 447→295 | 16 | 110 |
| L-Met-13C5 | Quantifier | 155→104 | 5 | 80 |
| Compound | Linearity Range (nmol/L) | LOD (nmol/L) | LOQ (nmol/L) |
|---|---|---|---|
| 5-CHOTHF | 0.47–47.4 | 0.32 | 1.06 |
| pABG | 0.267–26.7 | 0.28 | 0.94 |
| 5-CH3THF | 0.69–46.0 | 0.33 | 1.09 |
| 10-CHOFA | 0.47–47.0 | 0.32 | 1.07 |
| FA | 0.44–44.2 | 0.31 | 1.02 |
| 510-CH+THF | 0.46–45.6 | 0.17 | 0.55 |
| THF | 0.89–44.6 | 0.68 | 2.25 |
| Met | 0.075–15.0 | 0.33 | 1.10 |
| SAH | 0.19–38.5 | 0.20 | 0.65 |
| SAM | 0.60–39.9 | 0.38 | 1.26 |
| Hcy | 0.14–13.6 | 0.56 | 1.85 |
| Compounds | Concentration (μmol/L) | Precision (%) | Accuracy (%) | ||
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| 5-CHOTHF | Low (0.02) | 3.76 | 7.11 | 107.88 | 100.82 |
| Medium (0.04) | 3.05 | 8.40 | 98.68 | 104.73 | |
| High (0.10) | 2.34 | 9.87 | 99.56 | 98.19 | |
| pABG | Low (0.04) | 5.55 | 6.32 | 103.86 | 110.59 |
| Medium (0.07) | 6.80 | 8.43 | 101.29 | 107.52 | |
| High (0.19) | 4.31 | 9.19 | 112.53 | 97.17 | |
| 5-CH3THF | Low (0.02) | 3.05 | 10.5 | 104.40 | 104.42 |
| Medium (0.04) | 5.57 | 6.70 | 103.19 | 108.99 | |
| High (0.10) | 3.41 | 4.83 | 107.73 | 103.62 | |
| 10-CHOFA | Low (0.02) | 4.33 | 12.04 | 104.34 | 92.85 |
| Medium (0.04) | 6.35 | 8.35 | 97.03 | 106.63 | |
| High (0.11) | 4.85 | 4.98 | 105.58 | 106.81 | |
| FA | Low (0.02) | 5.32 | 9.08 | 106.23 | 114.45 |
| Medium (0.05) | 2.51 | 8.53 | 103.18 | 97.13 | |
| High (0.11) | 6.48 | 10.9 | 96.40 | 99.64 | |
| 510-CH+THF | Low (0.02) | 1.37 | 5.07 | 96.08 | 108.23 |
| Medium (0.04) | 3.35 | 10.9 | 100.13 | 107.57 | |
| High (0.11) | 2.15 | 5.21 | 106.78 | 97.97 | |
| THF | Low (0.07) | 4.58 | 7.07 | 105.20 | 92.82 |
| Medium (0.13) | 3.74 | 9.42 | 98.84 | 92.36 | |
| High (0.20) | 5.34 | 6.81 | 100.09 | 102.34 | |
| Met | Low (0.2) | 7.55 | 9.62 | 95.88 | 115.29 |
| Medium (0.4) | 5.20 | 9.19 | 93.83 | 97.88 | |
| High (0.6) | 1.01 | 0.69 | 97.05 | 99.91 | |
| SAH | Low (0.05) | 1.43 | 7.33 | 95.64 | 105.87 |
| Medium (0.10) | 5.11 | 9.08 | 92.17 | 93.91 | |
| High (0.21) | 6.57 | 9.05 | 97.46 | 100.94 | |
| SAM | Low (0.05) | 3.37 | 1.07 | 100.21 | 101.25 |
| Medium (0.10) | 4.60 | 3.88 | 96.32 | 109.48 | |
| High (0.20) | 1.09 | 1.98 | 102.48 | 92.10 | |
| Hcy | Low (0.15) | 1.53 | 10.8 | 102.58 | 96.36 |
| Medium (0.29) | 3.64 | 4.92 | 98.99 | 104.45 | |
| High (0.59) | 3.36 | 4.35 | 97.55 | 97.03 | |
| Mouse WB (n = 3) | Mouse Serum (n = 3) | Mouse Plasma (n = 80) | Mouse Embryos (n = 24) | Human WB (n = 3) | Human Serum (n = 3) | Human Plasma (n = 480) | |
|---|---|---|---|---|---|---|---|
| 5,10-CH+THF | 123.4 ± 21.3 | N.D. | N.D. | 74.3 ± 14.4 | N.D. | N.D. | N.D. |
| pABG | 1051.1 ± 41.5 | 171.3 ± 45.3 | 864.0 ± 166.1 | 20.6 ± 10.4 | 92.7 ± 7.9 | 74.0 ± 10.6 | 1192.0 ± 202.4 |
| 5-CH3THF | 338.3 ± 68.7 | 70.1 ± 1.7 | 202.2 ± 165.5 | 185.4 ± 36.7 | 54.1 ± 10.2 | 38.8 ± 1.6 | 13.6 ± 2.4 |
| hmTHF | 200.1 ± 62.2 | 60.3 ± 10.3 | 122.2 ± 101.0 | N.D. | N.D. | 12.3 ± 1.1 | 24.6 ± 1.2 |
| 10-CHOFA | N.D. | N.D. | N.D. | N.D. | 44.1 ± 2.0 | N.D. | 50.4 ± 16.2 |
| FA | 35.3 ± 20.3 | N.D. | N.D. | N.D. | 131.1 ± 20.8 | N.D. | 23.8 ± 10.4 |
| Met | (196.2 ± 21.2) × 103 | 1880.3 ± 337.4 | (8.6 ± 1.4) × 103 | (16.5 ± 3.2) × 103 | 2482.3 ± 83.7 | 3429.4 ± 226.6 | (20.5 ± 17.0) × 103 |
| SAH | 1550.7 ± 133.2 | 31.4 ± 16.4 | 60.6 ± 10.4 | 1093.2 ± 216.1 | 81.4 ± 6.8 | 68.0 ± 10.4 | 39.2 ± 3.2 |
| Hcy | N.D. | 146.2 ± 18.3 | N.D. | N.D. | 12.8 ± 2.9 | 234.4 ± 23.4 | 643.3 ± 104.1 |
| SAM | 835.2 ± 9.2 | N.D. | N.D. | 132.8 ± 26.6 | N.D. | N.D. | 381.7 ± 52.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, Y.; Tan, M.; Wang, X.; Meng, Z.; Quan, X.; Ramaswamy, H.; Wang, C. Simultaneous Determination of One-Carbon Folate Metabolites and One-Carbon-Related Amino Acids in Biological Samples Using a UHPLC–MS/MS Method. Int. J. Mol. Sci. 2024, 25, 3458. https://doi.org/10.3390/ijms25063458
Ling Y, Tan M, Wang X, Meng Z, Quan X, Ramaswamy H, Wang C. Simultaneous Determination of One-Carbon Folate Metabolites and One-Carbon-Related Amino Acids in Biological Samples Using a UHPLC–MS/MS Method. International Journal of Molecular Sciences. 2024; 25(6):3458. https://doi.org/10.3390/ijms25063458
Chicago/Turabian StyleLing, Yi, Mei Tan, Xiaoyun Wang, Ziyi Meng, Xiaodong Quan, Hosahalli Ramaswamy, and Chao Wang. 2024. "Simultaneous Determination of One-Carbon Folate Metabolites and One-Carbon-Related Amino Acids in Biological Samples Using a UHPLC–MS/MS Method" International Journal of Molecular Sciences 25, no. 6: 3458. https://doi.org/10.3390/ijms25063458
APA StyleLing, Y., Tan, M., Wang, X., Meng, Z., Quan, X., Ramaswamy, H., & Wang, C. (2024). Simultaneous Determination of One-Carbon Folate Metabolites and One-Carbon-Related Amino Acids in Biological Samples Using a UHPLC–MS/MS Method. International Journal of Molecular Sciences, 25(6), 3458. https://doi.org/10.3390/ijms25063458








