Immunoregulatory Effects of Elemental Diet and Its Ingredient, Tryptophan, via Activation of the Aryl Hydrocarbon Receptor in Mice
Abstract
1. Introduction
2. Results
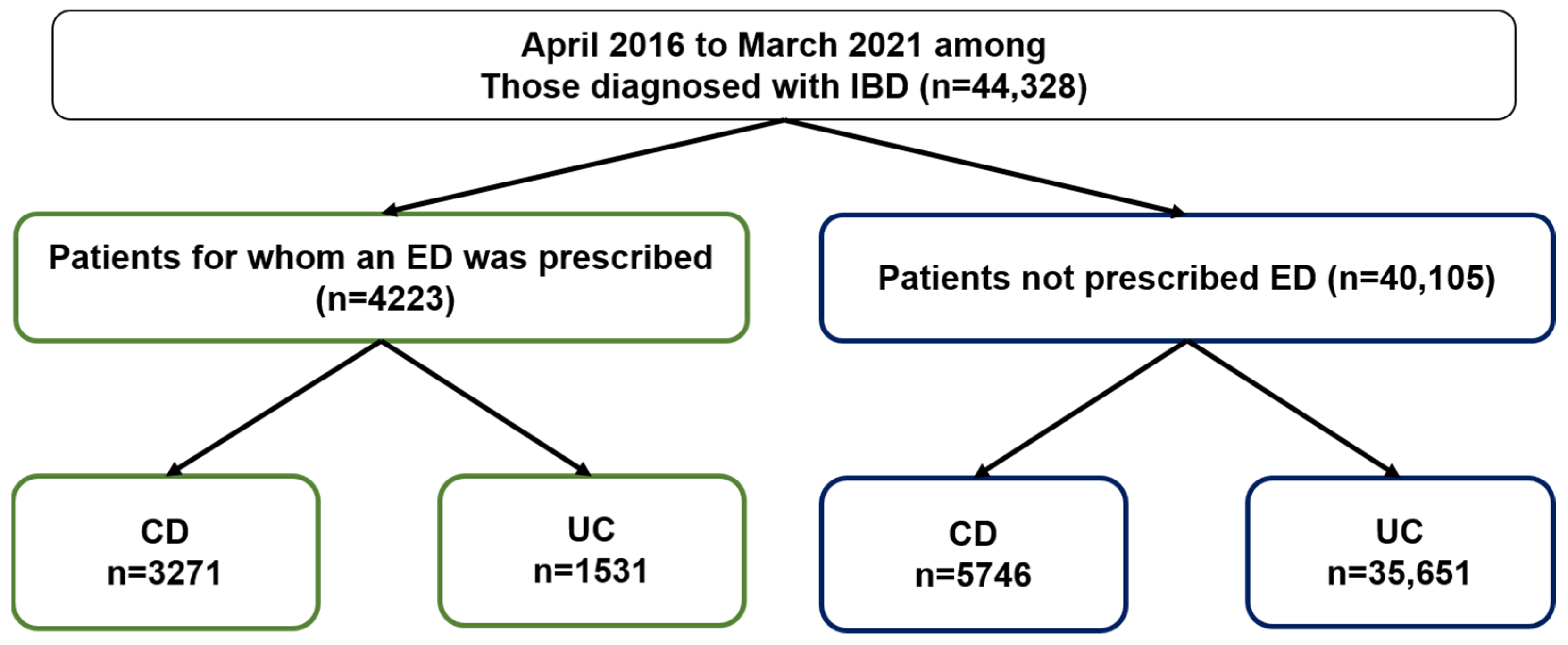
2.1. ED Prescribing Rates for IBD Patients Based on the JMDC Claims Database
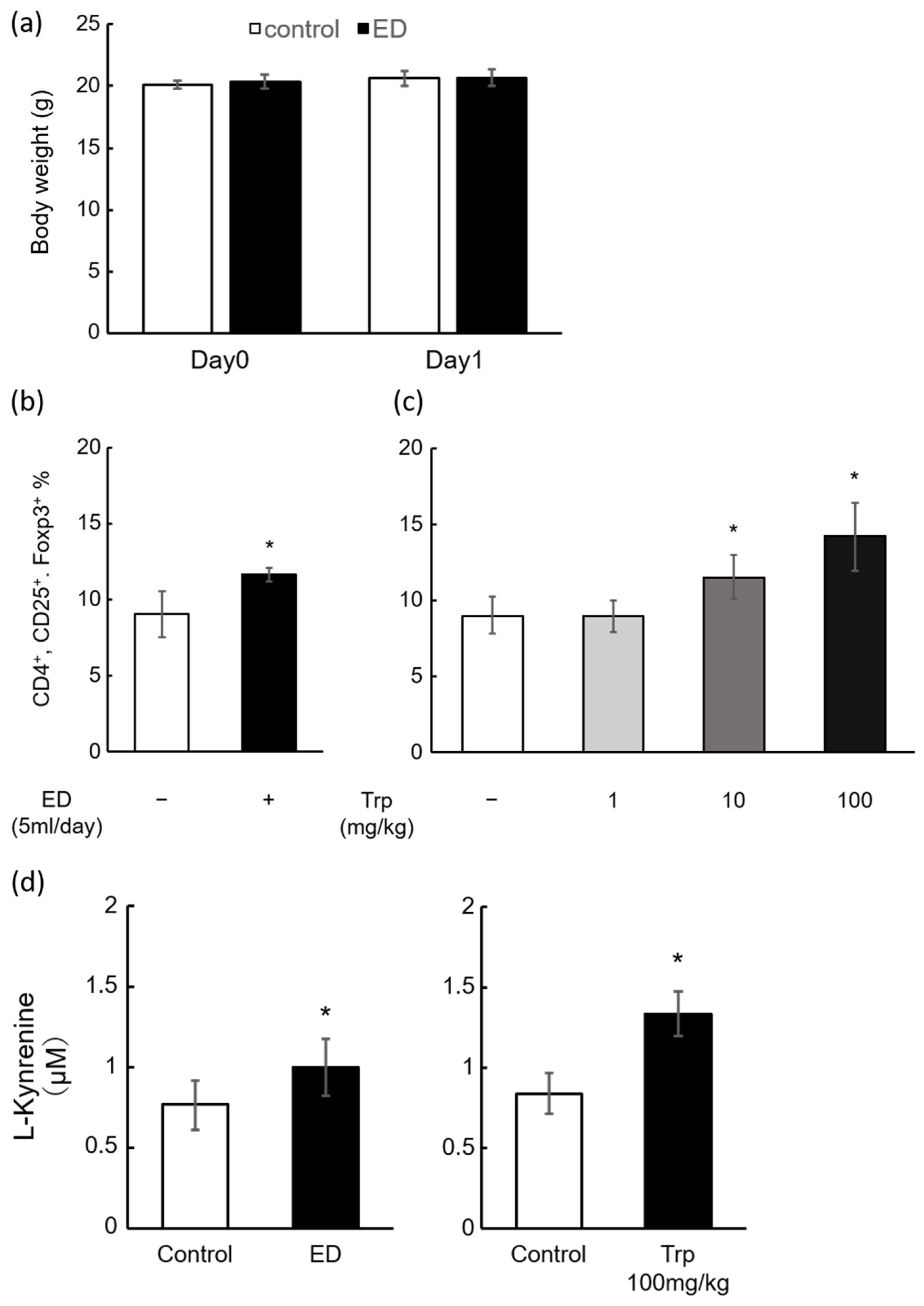
2.2. Induction of Mouse Splenic Tregs by ED and Trp
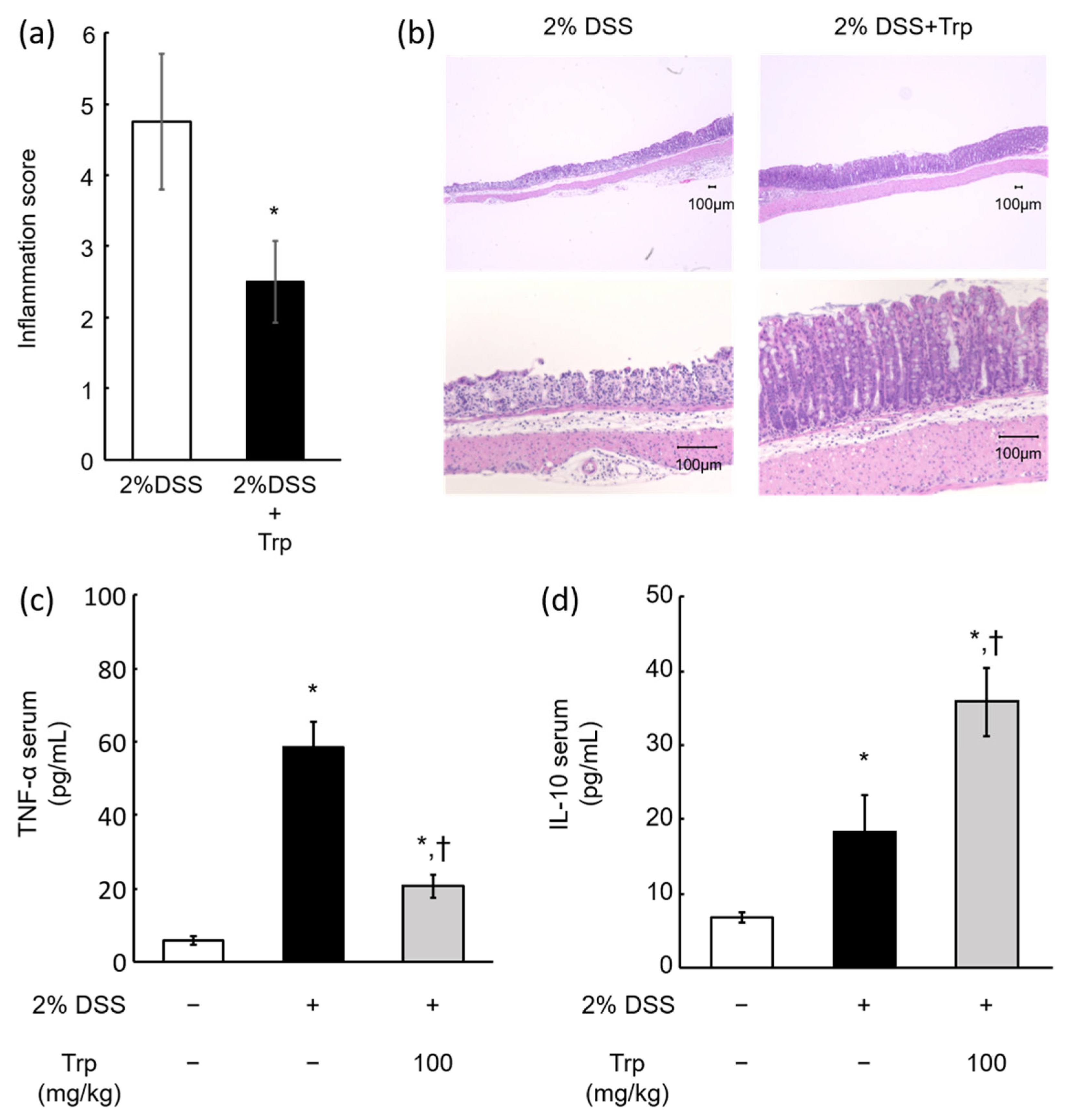
2.3. Effect of Oral Trp Administration on DSS-Induced Colitis Model Mice
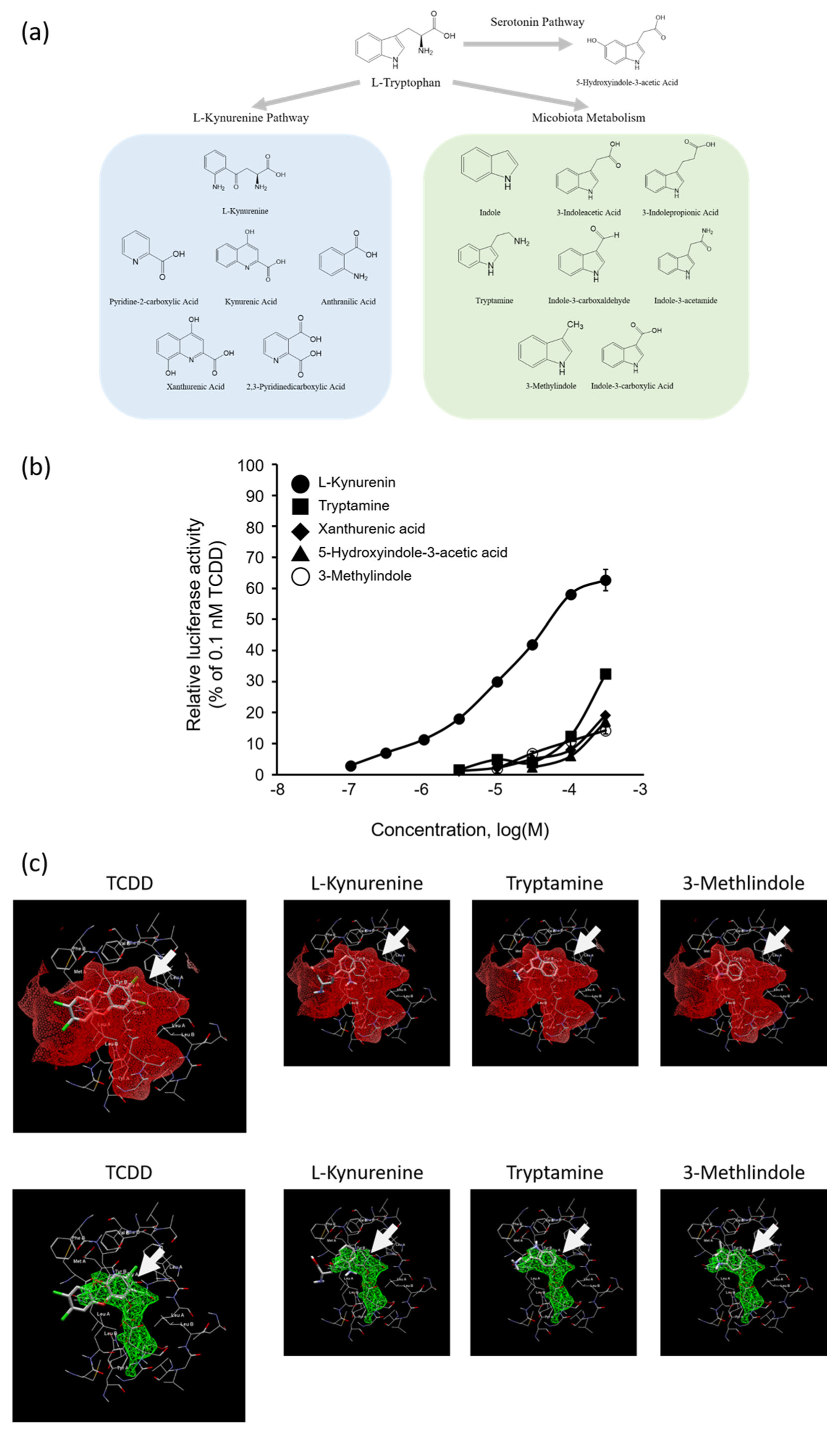
2.4. AhR Agonist Activity of Trp Metabolites and In Silico Docking Analysis of Kyn, Tryptamine, or 3-Methylindole with AhR
3. Discussion
4. Materials and Methods
4.1. Claims Database
4.2. Chemicals
4.3. Animals
4.4. Experimental Design
4.5. Flow Cytometry Analysis
4.6. Determination of L-Kyn in Mouse Serum
4.7. Quantification of TNF-α and IL-10 in Mouse Serum Using Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Histophathological Evaluation of the Intestines
4.9. AhR-Mediated Transactivation Assay Using DR-EcoScreen Cells
4.10. Cell Viability Test and Evaluation of AhR Agonistic Activity
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kornbluth, A.; Sachar, D.B. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Hanauer, S.B.; Sandborn, W.J. Practice Parameters Committee of American College of Gastroenterology. Management of Crohn’s disease in adults. Am. J. Gastroenterol. 2009, 104, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Cordeiro, G.; Dias, A.M.; Estevinho, M.M. Inflammatory Bowel Disease—Non-biological treatment. Pharmacol. Res. 2020, 106, 105075. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Masuda, S.; Nakase, H.; Matsuura, M.; Maruyama, S.; Hisamatsu, T.; Suzuki, Y.; Matsubara, K. Influence of pharmaceutical formulation on the mucosal concentration of 5-aminosalicylic acid and N-acetylmesalamine in Japanese patients with ulcerative colitis. Biol. Pharm. Bull. 2019, 42, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Masui, S.; Yonezawa, A.; Momo, K.; Nakagawa, S.; Itohara, K.; Imai, S.; Nakagawa, T.; Matsubara, K. Infliximab treatment persistence among Japanese patients with chronic inflammatory diseases: A retrospective Japanese Claims Data study. Biol. Pharm. Bull. 2022, 45, 323–332. [Google Scholar] [CrossRef]
- Kubota, A.; Terasaki, M.; Takai, R.; Kobayashi, M.; Muromoto, R.; Kojima, H. 5-Aminosalicylic acid, a weak agonist for aryl hydrocarbon receptor that induces splenic regulatory T cells. Pharmacology 2022, 107, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. 1995, 35, 307–340. [Google Scholar] [CrossRef]
- Mimura, J.; Fujii-Kuriyama, Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta 2003, 1619, 263–268. [Google Scholar] [CrossRef]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Singh, B.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE 2011, 6, e23522. [Google Scholar] [CrossRef]
- Oh-Oka, K.; Kojima, Y.; Uchida, K.; Yoda, K.; Ishimaru, K.; Nakajima, S.; Hemmi, J.; Kano, H.; Fujii-Kuriyama, Y.; Katoh, Y.; et al. Induction of colonic regulatory T cells by mesalamine by activating the aryl hydrocarbon receptor. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 135–151. [Google Scholar] [CrossRef]
- Asari, T.; Kikuchi, H.; Kawaguchi, S.; Sakuraba, H.; Yoshida, S.; Akemoto, Y.; Maeda, T.; Shinji, O.; Murai, Y.; Higuchi, N.; et al. Polygonum tinctorium leaves suppress sodium dextran sulfate-induced colitis through interleukin–10-related pathway. Biochem. Biophys. Rep. 2022, 30, 101272. [Google Scholar] [CrossRef]
- Naganuma, M.; Sugimoto, S.; Mitsuyama, K.; Kobayashi, T.; Yoshimura, N.; Ohi, H.; Tanaka, S.; Andoh, A.; Ohmiya, N.; Saigusa, K.; et al. Efficacy of Indigo Naturalis in a Multicenter Randomized Controlled Trial of Patients with Ulcerative Colitis. Gastroenterology 2018, 154, 935–947. [Google Scholar] [CrossRef]
- Kataoka, K.; Sakagami, J.; Hirota, M.; Masamune, A.; Shimosegawa, T. Effects of oral ingestion of the elemental diet in patients with painful chronic pancreatitis in the real-life setting in Japan. Pancreas 2014, 43, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Munkholm Larsen, P.; Rasmussen, D.; Rønn, B.; Munck, O.; Elmgreen, J.; Binder, V. Elemental diet: A therapeutic approach in chronic inflammatory bowel disease. J. Intern. Med. 1989, 225, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, M.; Pariano, M.; Costantini, C.; Giovagnoli, S.; Ricci, M. Pharmaceutically Active Microbial AhR Agonists as Innovative Biodrugs in Inflammation. Pharmaceuticals 2022, 15, 336. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Kunisaki, R.; Nakamura, S.; Tsujikawa, T.; Hirai, F.; Nakase, H.; Watanabe, K.; Yokoyama, K.; Nagahori, M.; Kanai, T.; et al. Effect of elemental diet combined with infliximab dose escalation in patients with Crohn’s disease with loss of response to infliximab: CERISIER trial. Intest. Res. 2018, 16, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, M.; Yokoyama, T.; Saigusa, N.; Sato, H.; Yazawa, K.; Tsurita, G.; Kurokawa, T.; Hata, K.; Yokoyama, Y. Elemental diet therapy plays a significant role in preventing surgical recurrence of Crohn’s disease in the era of biologics. Surg. Today 2021, 51, 250–257. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Safe, S.; Jin, U.H.; Park, H.; Chapkin, R.S.; Jayaraman, A. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int. J. Mol. Sci. 2020, 21, 6654. [Google Scholar] [CrossRef]
- Takeuchi, S.; Iida, M.; Yabushita, H.; Matsuda, T.; Kojima, H. In vitro screening for aryl hydrocarbon receptor agonistic activity in 200 pesticides using a highly sensitive reporter cell line, DR-EcoScreen cells, and in vivo mouse liver cytochrome P450-1A induction by propanil, diuron and linuron. Chemosphere 2008, 74, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Terasaki, M.; Sakuragi, Y.; Muromoto, R.; Ikeda-Araki, A.; Takada, H.; Kojima, H. Effects of benzotriazole UV stabilizers, UV-PS and UV-P, on the differentiation of splenic regulatory T cells via aryl hydrocarbon receptor. Ecotoxicol. Environ. Saf. 2022, 238, 113549. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Anezaki, K.; Kojima, H. Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors. Environ. Pollut. 2017, 227, 306–313. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Ono, N.; Imaizumi, A.; Mori, M.; Suzuki, H.; Uo, M.; Hashimoto, M.; Naganuma, M.; Matsuoka, K.; Mizuno, S.; et al. Decreased plasma histidine level predicts risk of relapse in patients with ulcerative colitis in remission. PLoS ONE 2015, 10, e0140716. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P.; et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE 2012, 7, e33546. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef]
- Segal, J.P.; Ding, N.S.; Worley, G.; Mclaughlin, S.; Preston, S.; Faiz, O.D.; Clark, S.K.; Hart, A.L. Systematic review with meta-analysis: The management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment. Pharmacol. Ther. 2017, 45, 581–592. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Islam, J.; Sato, S.; Watanabe, K.; Watanabe, T.; Ardiansyah; Hirahara, K.; Aoyama, Y.; Tomita, S.; Aso, H.; Komai, M.; et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J. Nutr. Biochem. 2017, 42, 43–50. [Google Scholar] [CrossRef]
- Kim, C.J.; Kovacs-Nolan, J.A.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. L-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, E.F.; Feriotti, C.; Galdino, N.A.L.; Preite, N.W.; Calich, V.L.G.; Loures, F.V. The IDO-AhR Axis Controls Th17/Treg Immunity in a Pulmonary Model of Fungal Infection. Front. Immunol. 2017, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef] [PubMed]
- Diani-Moore, S.; Labitzke, E.; Brown, R.; Garvin, A.; Wong, L.; Rifkind, A.B. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo. Toxicol. Sci. 2006, 90, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Ma, Y.M.; Huang, J.Y.; He, S.Q.; Li, S.P.; Lin, J.; Chen, R.; Lun, J.C.; Liu, J.; Guo, S.N. Polysaccharides derived from Shenling Baizhu San improve colitis via modulating tryptophan metabolism in mice. Int. J. Biol. Macromol. 2022, 222 Pt A, 1127–1136. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Ron, Y.; Kivity, S.; Ben-Horin, S.; Israeli, E.; Fraser, G.M.; Dotan, I.; Chowers, Y.; Confino-Cohen, R.; Weiss, B. Infliximab-Related Infusion Reactions: Systematic Review. J. Crohns. Colitis 2015, 9, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Carbonnel, F.; Laharie, D.; Stefanescu, C.; Hébuterne, X.; Abitbol, V.; Nachury, M.; Brixi, H.; Bourreille, A.; Picon, L.; et al. Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: A multicentre, prospective, observational cohort (CREOLE) study. Gut 2018, 67, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; Torloni, M.R.; Gueuvoghlanian-Silva, B.Y.; Alexandre, S.M.; Mattar, R.; Daher, S. Cytokine levels in gestational diabetes mellitus: A systematic review of the literature. Am. J. Reprod. Immunol. 2013, 69, 545–557. [Google Scholar] [CrossRef]
- Gouldthorpe, O.; Catto-Smith, A.G.; Alex, G.; Simpson, D. Loss of response to long-term infliximab therapy in children with Crohn’s disease. Pharmaceuticals 2013, 6, 1322–1334. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogeneous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Lee, S.O.; Sridharan, G.; Lee, K.; Davidson, L.A.; Jayaraman, A.; Chapkin, R.S.; Alaniz, R.; Safe, S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014, 85, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef]
- Hubbard, T.D.; Murray, I.A.; Bisson, W.H.; Lahoti, T.S.; Gowda, K.; Amin, S.G.; Patterson, A.D.; Perdew, G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015, 5, 12689. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Kubota, A.; Kobayashi, M.; Sarashina, S.; Takeno, R.; Yasuda, G.; Narumi, K.; Furugen, A.; Takahashi-Suzuki, N.; Iseki, K. Gamma-Aminobutyric Acid (GABA) Attenuates Ischemia Reperfusion-Induced Alterations in Intestinal Immunity. Biol. Pharm. Bull. 2018, 41, 1874–1878. [Google Scholar] [CrossRef]
| Composition | 80 g/Unit |
|---|---|
| L-Isoleucine | 642 mg |
| L-Leucine | 899 mg |
| L-Lysine hydrochloride | 888 mg |
| L-Methionine | 648 mg |
| L-Phenylalanine | 871 mg |
| L-Threonine | 523 mg |
| L-Tryptophan | 151 mg |
| L-Valine | 701 mg |
| L-Histidine hydrochloride hydrate | 501 mg |
| L-Arginine hydrochloride | 1125 mg |
| L-Alanine | 899 mg |
| L-magnesium and potassium aspartate | 1036 mg |
| L-Aspartate sodium monohydrate | 867 mg |
| L-Glutamine | 1932 mg |
| Glycine | 505 mg |
| L-Proline | 630 mg |
| L-Serine | 1159 mg |
| L-Tyrosine | 110 mg |
| Dextrin | 63.41 g |
| Sodium citrate hydrate | 616 mg |
| Potassium chloride | 150 mg |
| Calcium glycerophosphate | 825 mg |
| Ferrous gluconate dehydrate | 15.5 mg |
| Zing sulphate hydrate | 7.88 mg |
| Manganese sulphate pentahydrate | 1.30 mg |
| Copper sulphate | 0.82 mg |
| Potassium indide | 19.6 µg |
| Thiamine chloride hydrochloride | 194 µg |
| Riboflavin phosphate sodium | 256 µg |
| Pyridoxine hydrochloride | 267 µg |
| Cyanocabalamin | 0.7 µg |
| Calcium pantothenate | 1.19 mg |
| Nicotinic acid amide | 2.20 mg |
| Folic acid | 44 µg |
| Biotin | 39 µg |
| Choline bis (tartrate) | 17.93 mg |
| Ascorbic acid | 7.80 mg |
| Retinol acetate | 648 IU |
| Tocopherol acetate | 3.30 mg |
| Ergocalciferol | 1.3 µg |
| Phytonadione | 9 µg |
| Soybean oil | 509 mg |
| total calories | 300 kcal |
| Compound | REC10 (a) | Relative Potency (b) |
|---|---|---|
| TCDD | 1.0 × 10−12 | 1 |
| L-Tryptophan | N.E. (c) | <3.3 × 10−9 |
| L-Kynurenine | 7.0 × 10−7 | 1.4 × 10−6 |
| Tryptamine | 6.3 × 10−5 | 1.6 × 10−8 |
| 3-Methylindole | 7.8 × 10−5 | 1.3 × 10−8 |
| Xanthurenic acid | 1.2 × 10−4 | 8.3 × 10−9 |
| 5-Hydroxyindole-3-acetic acid | 1.5 × 10−4 | 6.7 × 10−9 |
| Indole-3-carboxylic acid | N.E. | <3.3 × 10−9 |
| Indole-3-carboxaldehyde | N.E. | <3.3 × 10−9 |
| Indole-3-acetamide | N.E. | <3.3 × 10−9 |
| 3-Indoleacetic acid | N.E. | <3.3 × 10−9 |
| 3-Indolepropionic acid | N.E. | <3.3 × 10−9 |
| Indole | N.E. | <3.3 × 10−9 |
| Kynurenic acid | N.E. | <3.3 × 10−9 |
| Anthranilic acid | N.E. | <3.3 × 10−9 |
| Pyridine-2-carboxylic acid | N.E. | <3.3 × 10−9 |
| 2,3-Pyridinedicarboxylic acid | N.E. | <3.3 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, A.; Imai, S.; Aoyagi, R.; Murase, W.; Terasaki, M.; Sugawara, M.; Takekuma, Y.; Kojima, H. Immunoregulatory Effects of Elemental Diet and Its Ingredient, Tryptophan, via Activation of the Aryl Hydrocarbon Receptor in Mice. Int. J. Mol. Sci. 2024, 25, 3448. https://doi.org/10.3390/ijms25063448
Kubota A, Imai S, Aoyagi R, Murase W, Terasaki M, Sugawara M, Takekuma Y, Kojima H. Immunoregulatory Effects of Elemental Diet and Its Ingredient, Tryptophan, via Activation of the Aryl Hydrocarbon Receptor in Mice. International Journal of Molecular Sciences. 2024; 25(6):3448. https://doi.org/10.3390/ijms25063448
Chicago/Turabian StyleKubota, Atsuhito, Shungo Imai, Ryoichi Aoyagi, Wataru Murase, Masaru Terasaki, Mitsuru Sugawara, Yoh Takekuma, and Hiroyuki Kojima. 2024. "Immunoregulatory Effects of Elemental Diet and Its Ingredient, Tryptophan, via Activation of the Aryl Hydrocarbon Receptor in Mice" International Journal of Molecular Sciences 25, no. 6: 3448. https://doi.org/10.3390/ijms25063448
APA StyleKubota, A., Imai, S., Aoyagi, R., Murase, W., Terasaki, M., Sugawara, M., Takekuma, Y., & Kojima, H. (2024). Immunoregulatory Effects of Elemental Diet and Its Ingredient, Tryptophan, via Activation of the Aryl Hydrocarbon Receptor in Mice. International Journal of Molecular Sciences, 25(6), 3448. https://doi.org/10.3390/ijms25063448






