Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology
Abstract
1. Introduction
2. Results
2.1. Effects of TNF Inhibition on Disease Activity and Systemic Inflammation
2.2. Effects of TNF Inhibition on Vascular Pathophysiology
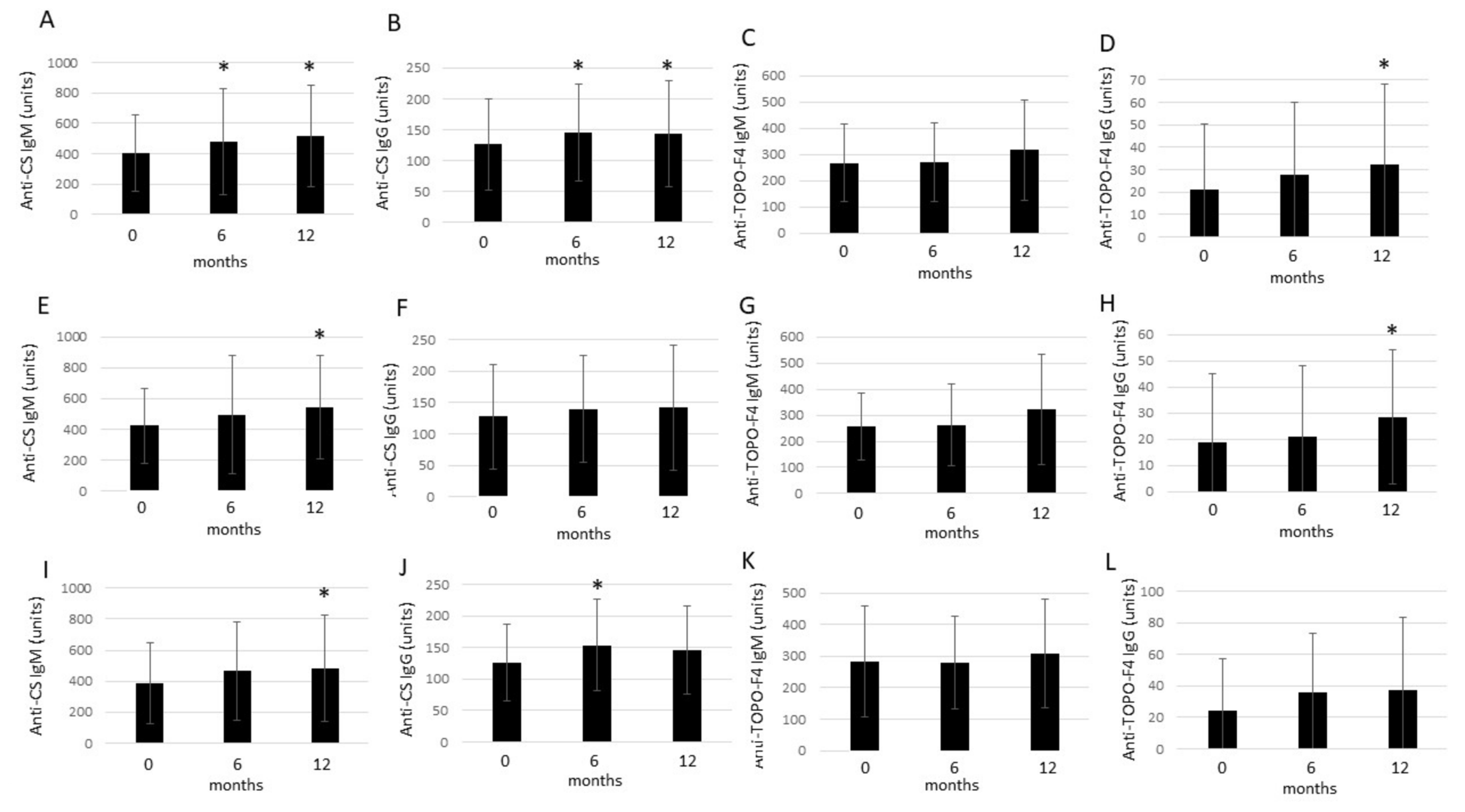
2.3. Effects of Anti-TNF Therapy on Circulating nAAb Levels
2.4. Correlations of nAAb Levels with Other Parameters
3. Patients and Methods
3.1. Patients
3.2. Laboratory Measurements
3.3. Determination of nAAbs
3.4. Assessment of Vascular Physiology by Ultrasound
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerekes, G.; Szekanecz, Z.; Der, H.; Sandor, Z.; Lakos, G.; Muszbek, L.; Csipo, I.; Sipka, S.; Seres, I.; Paragh, G.; et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: A multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J. Rheumatol. 2008, 35, 398–406. [Google Scholar]
- Nurmohamed, M.T.; Heslinga, M.; Kitas, G.D. Cardiovascular comorbidity in rheumatic diseases. Nat. Rev. Rheumatol. 2015, 11, 693–704. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- Borocz, K.; Simon, D.; Erdo-Bonyar, S.; Kovacs, K.T.; Tuba, E.; Czirjak, L.; Nemeth, P.; Berki, T. Relationship between natural and infection-induced antibodies in systemic autoimmune diseases (SAD): SLE, SSc and RA. Clin. Exp. Immunol. 2021, 203, 32–40. [Google Scholar] [CrossRef]
- Simon, D.; Czompoly, T.; Berki, T.; Minier, T.; Peti, A.; Toth, E.; Czirjak, L.; Nemeth, P. Naturally occurring and disease-associated auto-antibodies against topoisomerase I: A fine epitope mapping study in systemic sclerosis and systemic lupus erythematosus. Int. Immunol. 2009, 21, 415–422. [Google Scholar] [CrossRef][Green Version]
- Horvath, L.; Czirjak, L.; Fekete, B.; Jakab, L.; Prohaszka, Z.; Cervenak, L.; Romics, L.; Singh, M.; Daha, M.R.; Fust, G. Levels of antibodies against C1q and 60 kDa family of heat shock proteins in the sera of patients with various autoimmune diseases. Immunol. Lett. 2001, 75, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Joyal, F.; Fritzler, M.J.; Roussin, A.; Abrahamowicz, M.; Boire, G.; Goulet, J.R.; Rich, E.; Grodzicky, T.; Raymond, Y.; et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008, 58, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Rosato, E.; Gigante, A.; Barbano, B.; Cianci, R.; Molinaro, I.; Pisarri, S.; Salsano, F. In systemic sclerosis macrovascular damage of hands digital arteries correlates with microvascular damage. Microvasc. Res. 2011, 82, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Hamar, A.; Horvath, A.; Gulyas, K.; Vegh, E.; Bodnar, N.; Kerekes, G.; Czokolyova, M.; Bodoki, L.; Hodosi, K.; et al. Soluble vascular biomarkers in rheumatoid arthritis and ankylosing spondylitis: Effects of one-year anti-TNF-alpha therapy. J. Rheumatol. 2021, 48, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Afek, A.; George, J.; Gilburd, B.; Rauova, L.; Goldberg, I.; Kopolovic, J.; Harats, D.; Shoenfeld, Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 2000, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ghayour-Mobarhan, M.; Lamb, D.J.; Tavallaie, S.; Ferns, G.A. Relationship between plasma cholesterol, von Willebrand factor concentrations, extent of atherosclerosis and antibody titres to heat shock proteins-60, -65 and -70 in cholesterol-fed rabbits. Int. J. Exp. Pathol. 2007, 88, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Mantej, J.; Polasik, K.; Piotrowska, E.; Tukaj, S. Autoantibodies to heat shock proteins 60, 70, and 90 in patients with rheumatoid arthritis. Cell Stress. Chaperones 2019, 24, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.; Metzler, B.; Kiechl, S.; Willeit, J.; Schett, G.; Xu, Q.; Wick, G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: Immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 1999, 99, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Sherer, Y.; Cerinic, M.M.; Bartoli, F.; Blagojevic, J.; Conforti, M.L.; Gilburd, B.; Ehrenfeld, M.; Shoenfeld, Y. Early atherosclerosis and autoantibodies to heat-shock proteins and oxidized LDL in systemic sclerosis. Ann. N. Y. Acad. Sci. 2007, 1108, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sherer, Y.; Gerli, R.; Bocci, E.B.; Gilburd, B.; Vaudo, G.; Bistoni, O.; Shoenfeld, Y. Heat-shock protein 65 autoantibodies are differently associated with early atherosclerosis in rheumatoid arthritis and in healthy subjects. Ann. N. Y. Acad. Sci. 2007, 1108, 408–413. [Google Scholar] [CrossRef]
- Tishler, M.; Shoenfeld, Y. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin. Arthritis Rheum. 1996, 26, 558–563. [Google Scholar] [CrossRef]
- Tsoulfa, G.; Rook, G.A.; Bahr, G.M.; Sattar, M.A.; Behbehani, K.; Young, D.B.; Mehlert, A.; Van-Embden, J.D.; Hay, F.C.; Isenberg, D.A.; et al. Elevated IgG antibody levels to the mycobacterial 65-kDa heat shock protein are characteristic of patients with rheumatoid arthritis. Scand. J. Immunol. 1989, 30, 519–527. [Google Scholar] [CrossRef]
- Van Halm, V.P.; Slot, M.C.; Nurmohamed, M.T.; Cohen Tervaert, J.W.; Dijkmans, B.A.; Voskuyl, A.E. Antibodies against human 60 kDa heat shock protein are not associated with cardiovascular disease in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 590–594. [Google Scholar] [CrossRef]
- Vegh, E.; Kerekes, G.; Pusztai, A.; Hamar, A.; Szamosi, S.; Vancsa, A.; Bodoki, L.; Pogacsas, L.; Balazs, F.; Hodosi, K.; et al. Effects of 1-year anti-TNF-alpha therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol. Int. 2020, 40, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Baulmann, J.; Schillings, U.; Rickert, S.; Uen, S.; Dusing, R.; Illyes, M.; Cziraki, A.; Nickering, G.; Mengden, T. A new oscillometric method for assessment of arterial stiffness: Comparison with tonometric and piezo-electronic methods. J. Hypertens. 2008, 26, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Boussiotis, V.A.; Nadler, L.M.; Strominger, J.L.; Goldfeld, A.E. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc. Natl. Acad. Sci. USA 1994, 91, 7007–7011. [Google Scholar] [CrossRef] [PubMed]

| Full (RA + AS) Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent Variable | Independent Variable | Univariable Analysis | Multivariable Analysis | ||||||
| β | CI 95% | B | p | β | CI 95% | B | p | ||
| RF-6 | TOPO-F4 IgM-0 | 0.477 | 0.215–4.658 | 2.437 | 0.033 | ||||
| RF-12 | TOPO-F4 IgM-12 | 0.511 | 0.499–5.458 | 2.979 | 0.021 | ||||
| CEP-6 | CS IgG-0 | 0.586 | 0.037–0.275 | 0.156 | 0.013 | ||||
| FMD-12 | TOPO-F4 IgM-0 | 0.394 | 0.013–0.026 | 0.070 | 0.017 | ||||
| TOPO-F4 IgM-6 | 0.343 | 0.003–0.016 | −0.054 | 0.040 | |||||
| HSP60-0 | TOPO-F4 IgG-0 | 0.458 | 0.139–0.719 | 0.429 | 0.005 | ||||
| HSP60-6 | TOPO-F4 IgG-0 | 0.485 | 0.167–0.731 | 0.449 | 0.003 | 0.492 | 0.188–0.785 | 0.456 | 0.001 |
| HSP60-12 | TOPO-F4 IgG-0 | 0.543 | 0.240–0.799 | 0.519 | <0.001 | 0.549 | 0.265–0.811 | 0.522 | <0.001 |
| RA subset | |||||||||
| Dependent variable | Independent variable | Univariable analysis | Multivariable analysis | ||||||
| β | CI 95% | B | p | β | CI 95% | B | p | ||
| RF-0 | TOPO-F4 IgM-0 | 0.464 | 0.134–4.713 | 2.423 | 0.039 | ||||
| RF-6 | TOPO-F4 IgM-0 | 0.477 | 0.215–4.658 | 2.437 | 0.033 | ||||
| RF-12 | TOPO-F4 IgM-12 | 0.511 | 0.499–5.458 | 2.979 | 0.021 | ||||
| CEP-6 | CS IgG-0 | 0.586 | 0.037–0.275 | 0.156 | 0.013 | ||||
| FMD-12 | CS IgM-0 | 0.455 | 0–0.019 | 0.010 | 0.044 | ||||
| PWV-6 | TOPO-F4 IgG-0 | −0.456 | −0.011–0 | −0.006 | 0.043 | ||||
| TOPO-F4 IgG-6 | −0.465 | −0.009–0 | −0.005 | 0.039 | |||||
| HSP60-0 | TOPO-F4 IgG-0 | 0.469 | 0.029–0.831 | 0.430 | 0.037 | ||||
| HSP60-6 | TOPO-F4 IgG-0 | 0.624 | 0.276–1.173 | 0.724 | 0.003 | ||||
| HSP60-12 | TOPO-F4 IgG-0 | 0.646 | 0.332–1.266 | 0.799 | 0.002 | ||||
| AS subset | |||||||||
| Dependent variable | Independent variable | Univariable analysis | Multivariable analysis | ||||||
| β | CI 95% | B | p | β | CI 95% | B | p | ||
| BASDAI-12 | CS IgM-0 | 0.666 | 0.001–0.004 | 0.002 | 0.005 | ||||
| CS IgM-6 | 0.517 | 0–0.003 | 0.002 | 0.040 | |||||
| CS IgM-12 | 0.520 | 0–0.003 | 0.001 | 0.039 | |||||
| FMD-12 | TOPO-F4 IgM-0 | 0.578 | 0.017–0.157 | 0.087 | 0.019 | 0.515 | 0.027–0.127 | 0.077 | 0.005 |
| TOPO-F4 IgM-6 | 0.514 | 0.003–0.133 | 0.068 | 0.041 | |||||
| TOPO-F4 IgG-6 | 0.548 | 0.002–0.035 | 0.018 | 0.028 | |||||
| TOPO-F4 IgG-12 | 0.655 | 0.006–0.032 | 0.019 | 0.006 | 0.601 | 0.008–0.027 | 0.017 | 0.002 | |
| Full (RA + AS) Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RM-ANOVA | Two-Way ANOVA | ||||||||
| Dependent Variable | Effect | F | Partial η2 | p | Dependent Variable | Effect | F | Partial η2 | p |
| CRP (0–12) | CS IgM-0 | 4.885 | 0.126 | 0.010 | CRP (0–12) | CS IgM (0–12) | 20.980 | 0.375 | <0.001 |
| FMD (0–12) | TOPO-F4 IgG-0 | 3.617 | 0.096 | 0.032 | CS IgG (0–12) | 10.186 | 0.225 | 0.003 | |
| TOPO-F4 IgM (0–12) | 16.208 | 0.317 | <0.001 | ||||||
| DAS28-CRP/ BASDAI (0–12) | CS IgM (0–12) | 2.013 | 0.364 | <0.001 | |||||
| CS IgG (0–12) | 6.458 | 0.156 | 0.016 | ||||||
| TOPO-F4 IgM (0–12) | 7.227 | 0.171 | 0.011 | ||||||
| FMD (0–12) | CS IgM (0–12) | 18.315 | 0.344 | <0.001 | |||||
| PWV (0–12) | CS IgM (0–12) | 19.252 | 0.355 | <0.001 | |||||
| CS IgG (0–12) | 5.119 | 0.128 | 0.030 | ||||||
| TOPO-F4 IgM (0–12) | 5.120 | 0.128 | 0.030 | ||||||
| RA subset | |||||||||
| RM-ANOVA | Two-way ANOVA | ||||||||
| Dependent variable | Effect | F | partial η2 | p | Dependent variable | Effect | F | partial η2 | p |
| CRP (0–12) | CS IgM (0–12) | 12.277 | 0.393 | 0.002 | |||||
| CS IgG (0–12) | 4.673 | 0.197 | 0.044 | ||||||
| TOPO-F4 IgM (0–12) | 6.903 | 0.267 | 0.017 | ||||||
| DAS28-CRP (0–12) | CS IgM (0–12) | 11.941 | 0.986 | 0.003 | |||||
| AS subset | |||||||||
| RM-ANOVA | Two-way ANOVA | ||||||||
| Dependent variable | Effect | F | partial η2 | p | Dependent variable | Effect | F | partial η2 | p |
| BASDAI (0–12) | CS IgM-0 | 3.911 | 0.376 | 0.047 | CRP (0–12) | CS IgM (0–12) | 8.263 | 0.355 | 0.012 |
| BASDAI (0–12) | CS IgM (0–12) | 7.640 | 0.337 | 0.014 | |||||
| ccIMT (0–12) | CS IgM (0–12) | 15.000 | 0.318 | 0.018 | |||||
| PWV (0–12) | CS IgM (0–12) | 7.074 | 0.320 | 0.018 | |||||
| RA | AS | Total | |
|---|---|---|---|
| n | 36 | 17 | 53 |
| female:male | 31:5 | 3:14 | 34:19 |
| age (mean ± SD) (range), years | 55.9 ± 9.8 (35–83) | 43.6 ± 12.4 (24–72) | 52.0 ± 12.1 (24–83) |
| disease duration (mean ± SEM) (range), years | 9.1 ± 8.3 (1–44) | 7.2 ± 7.0 (1–26) | 8.5 ± 7.9 (1–44) |
| age at diagnosis | 47.0 ± 8.7 (28–62) | 36.4 ± 11.6 (23–50) | 43.5 ± 12.1 (23–62) |
| smoking (current) | 7 | 7 | 14 |
| positive CV history | 8 | 1 | 9 |
| BMI (mean ± SD), kg/m2 | 29.3 ± 3.6 | 31.1 ± 3.8 | 29.9 ± 3.7 |
| obesity (BMI > 30 kg/m2) | 17 | 11 | 28 |
| diabetes mellitus history | 3 | 1 | 4 |
| hypertension history | 17 | 4 | 21 |
| RF positivity, n (%) | 26 (72) | - | - |
| Anti-CCP positivity, n (%) | 21 (58) | - | - |
| DAS28-CRP (baseline) (mean ± SD) | 5.00 ± 0.86 | - | - |
| BASDAI (baseline) (mean ± SD) | - | 5.79 ± 1.19 | - |
| Treatment (ETN, CZP) | 20 ETN, 16 CZP | 17 ETN | 37 ETN, 16 CZP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, D.; Kacsándi, D.; Pusztai, A.; Soós, B.; Végh, E.; Kerekes, G.; Bodoki, M.; Szamosi, S.; Szűcs, G.; Prohászka, Z.; et al. Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology. Int. J. Mol. Sci. 2024, 25, 3429. https://doi.org/10.3390/ijms25063429
Simon D, Kacsándi D, Pusztai A, Soós B, Végh E, Kerekes G, Bodoki M, Szamosi S, Szűcs G, Prohászka Z, et al. Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology. International Journal of Molecular Sciences. 2024; 25(6):3429. https://doi.org/10.3390/ijms25063429
Chicago/Turabian StyleSimon, Diána, Dorottya Kacsándi, Anita Pusztai, Boglárka Soós, Edit Végh, György Kerekes, Monika Bodoki, Szilvia Szamosi, Gabriella Szűcs, Zoltán Prohászka, and et al. 2024. "Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology" International Journal of Molecular Sciences 25, no. 6: 3429. https://doi.org/10.3390/ijms25063429
APA StyleSimon, D., Kacsándi, D., Pusztai, A., Soós, B., Végh, E., Kerekes, G., Bodoki, M., Szamosi, S., Szűcs, G., Prohászka, Z., Németh, P., Berki, T., & Szekanecz, Z. (2024). Natural Autoantibodies in Biologic-Treated Rheumatoid Arthritis and Ankylosing Spondylitis Patients: Associations with Vascular Pathophysiology. International Journal of Molecular Sciences, 25(6), 3429. https://doi.org/10.3390/ijms25063429






