Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy
Abstract
1. Introduction
2. Results
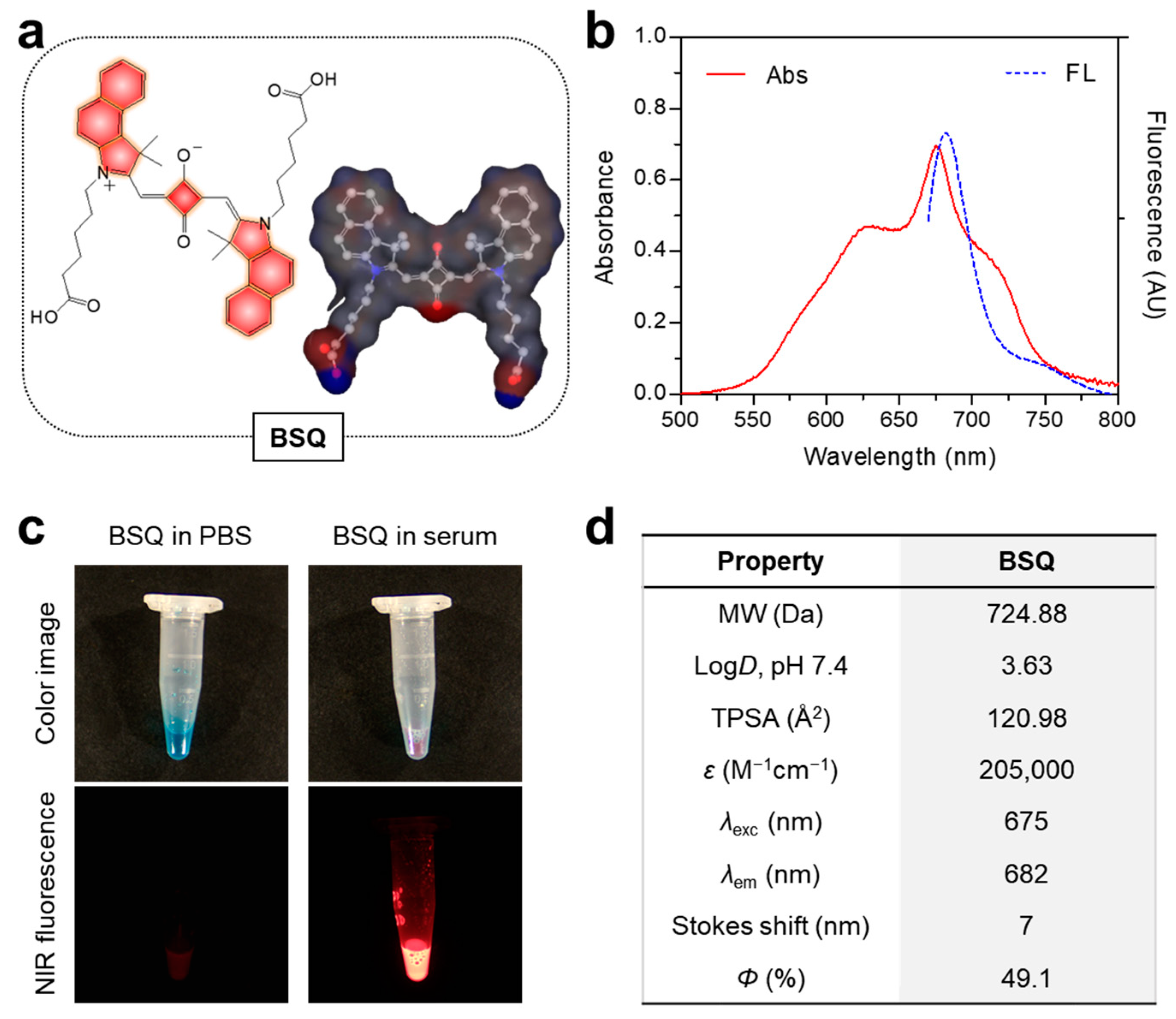
2.1. Optical Characterization and In Vitro ROS Assay
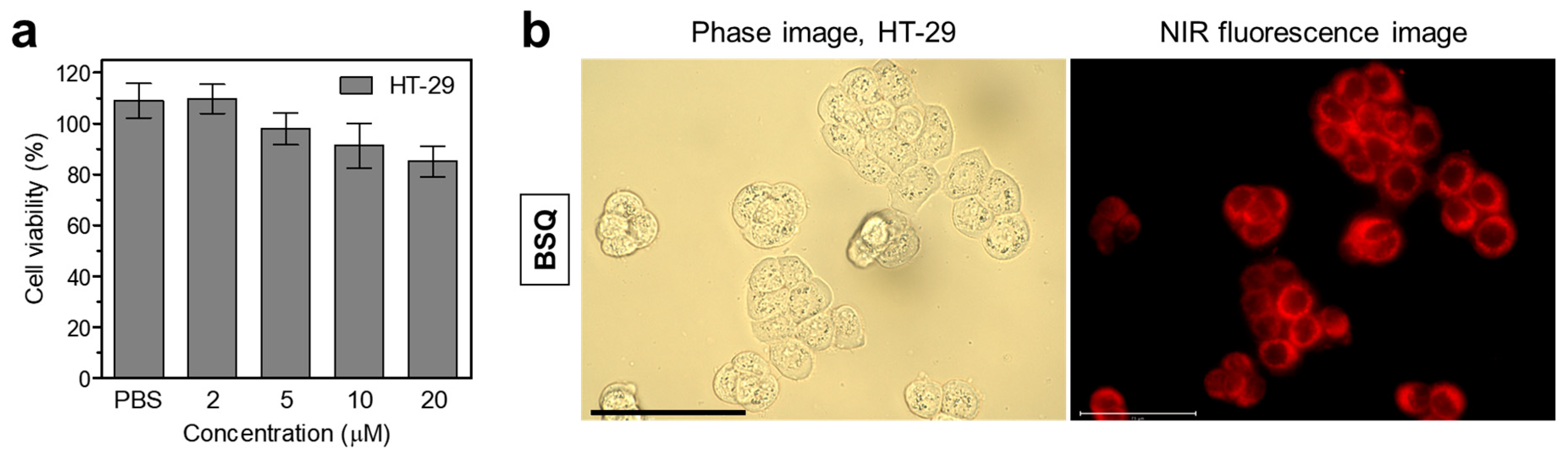
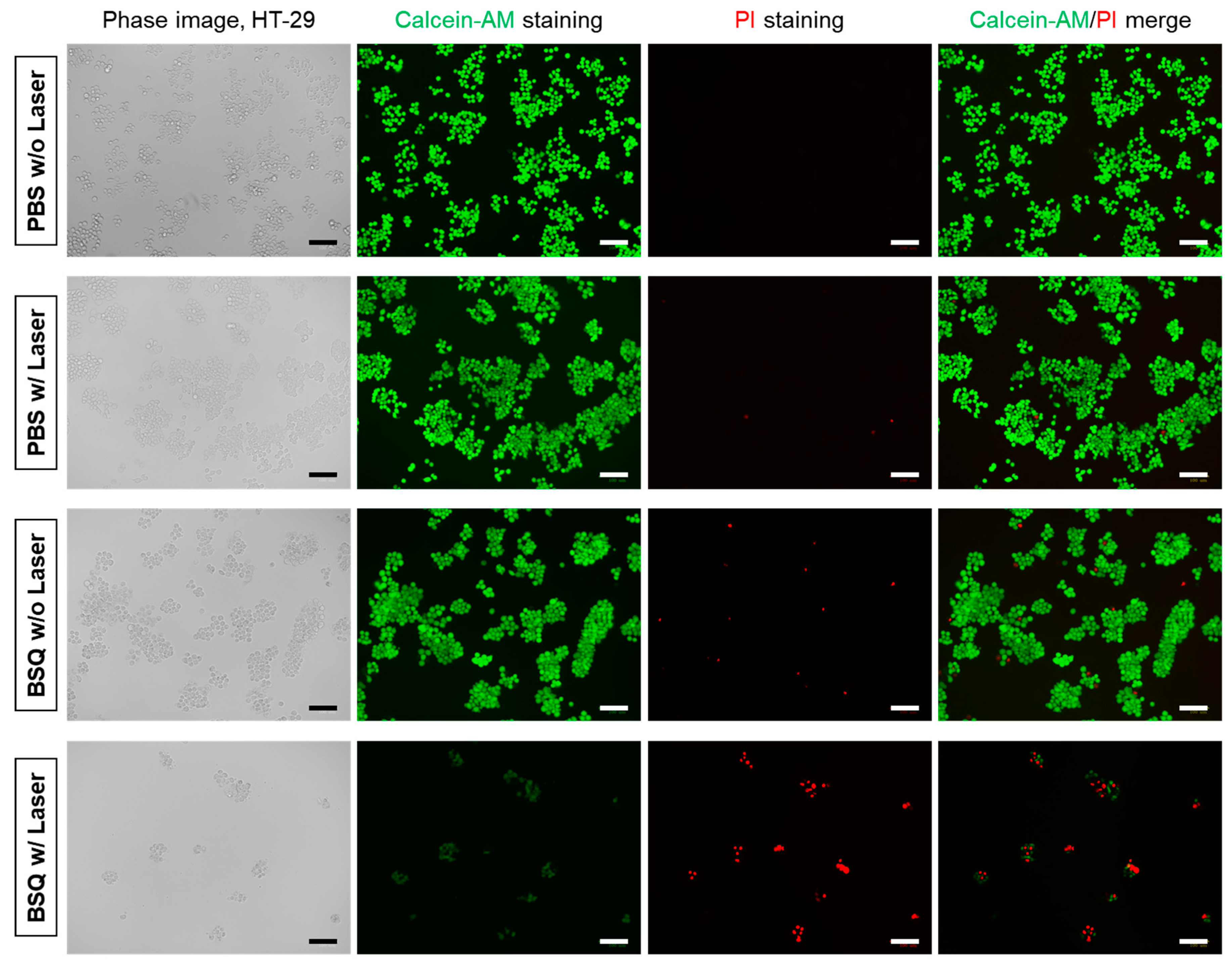
2.2. In Vitro Cytotoxicity and Intracellular ROS Generation
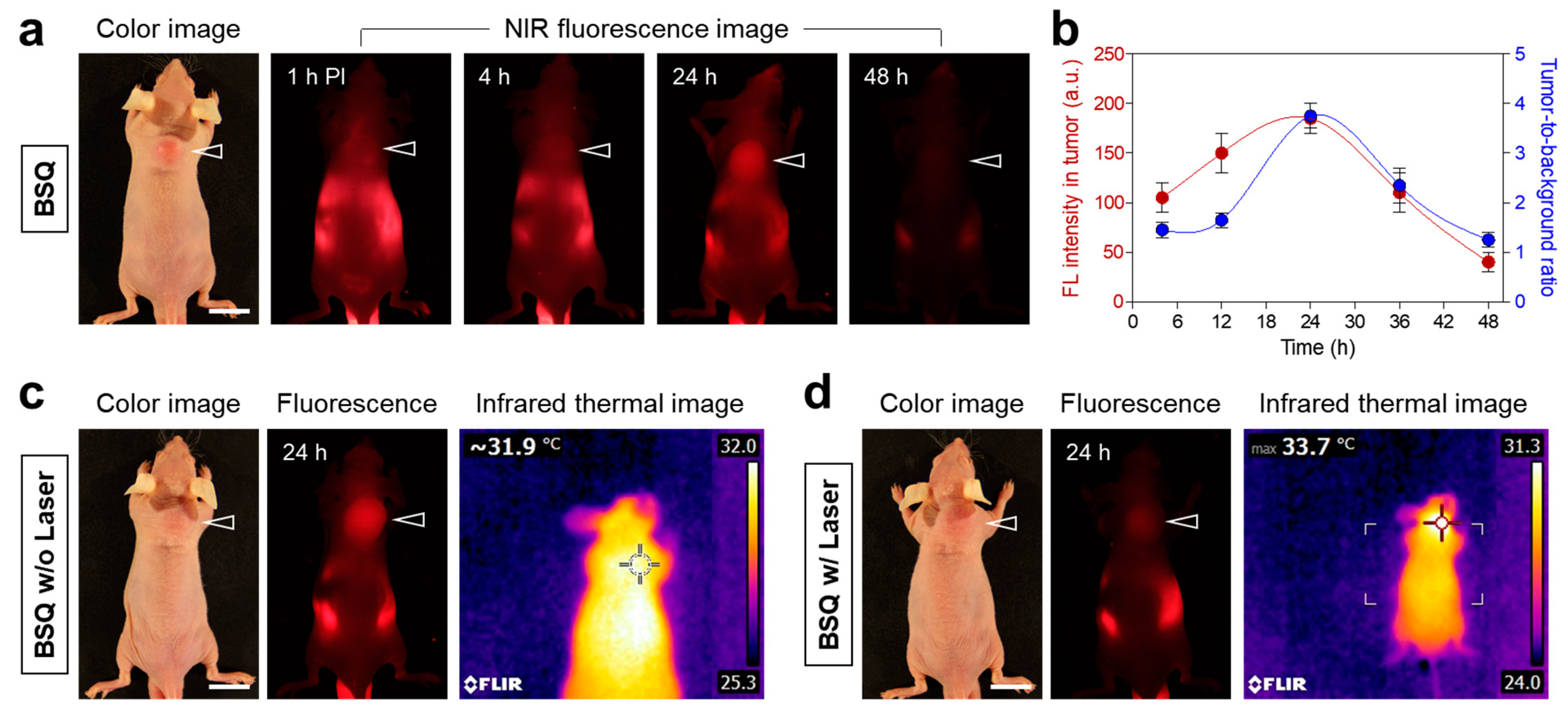
2.3. Time-Dependent In Vivo Tumor Retention and Photothermal Effect Test
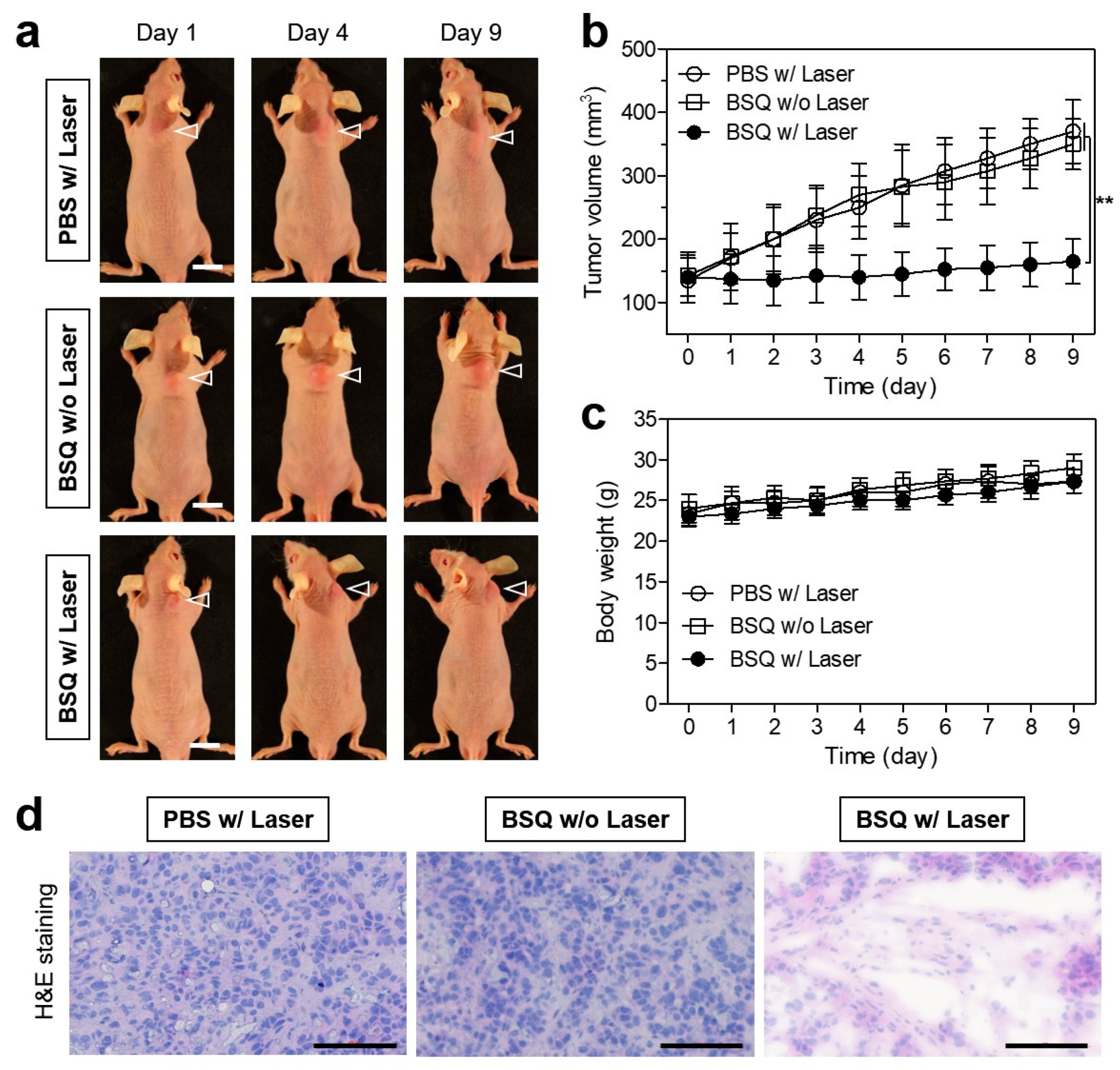
2.4. In Vivo Photodynamic Therapeutic Efficacy
3. Discussion
4. Materials and Methods
4.1. Optical and Physicochemical Property Analyses
4.2. In Vitro Live-Cell Imaging
4.3. In Vitro Cytotoxicity Assay
4.4. In Vitro Cellular ROS Assay and Photodynamic Cytotoxicity
4.5. HT-29 Xenograft Mouse Model
4.6. In Vivo Time-Dependent Tumor Imaging
4.7. In Vivo Photodynamic Therapeutic Efficacy
4.8. Histological Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Zou, Y.; Long, S.; Xiong, T.; Zhao, X.; Sun, W.; Du, J.; Fan, J.; Peng, X. Single-Molecule Förster Resonance Energy Transfer-Based Photosensitizer for Synergistic Photodynamic/Photothermal Therapy. ACS Cent. Sci. 2021, 7, 327–334. [Google Scholar] [CrossRef]
- Pucelik, B.; Sułek, A.; Barzowska, A.; Dąbrowski, J.M. Recent Advances in Strategies for Overcoming Hypoxia in Photodynamic Therapy of Cancer. Cancer Lett. 2020, 492, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheung, Y.K.; Ma, C.; Zhao, S.; Gao, D.; Lo, P.C.; Fong, W.P.; Wong, K.S.; Ng, D.K. Endoplasmic reticulum-localized two-photon-absorbing boron dipyrromethenes as advanced photosensitizers for photodynamic therapy. J. Med. Chem. 2018, 61, 3952–3961. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Liu, B.; Tan, Y.N. Emerging Strategies in Enhancing Singlet Oxygen Generation of Nano-Photosensitizers toward Advanced Phototherapy. Nano-Micro Lett. 2022, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jo, G.; Kim, E.J.; Jung, J.S.; Hyun, H. Tumor-targeted near-infrared fluorophore for fluorescence-guided phototherapy. Chem. Commun. 2020, 56, 4180–4183. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly efficient and photostable photosensitizer based on BODIPY chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; Van Den Bergh, H.; Wagnieres, G.; Burgess, K.; Lee, H.B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. [Google Scholar] [CrossRef]
- Karges, J. Clinical Development of Metal Complexes as Photosensitizers for Photodynamic Therapy of Cancer. Angew. Chem. Int. Ed. 2022, 61, e202112236. [Google Scholar] [CrossRef]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Potential Application of Photosensitizers with High-Z Elements for Synergic Cancer Therapy. Front. Pharmacol. 2022, 13, 921729. [Google Scholar] [CrossRef]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Oliveira, A.S.; Vieira Ferreira, L.F. Singlet oxygen generation ability of squarylium cyanine dyes. J. Photochem. Photobiol. A 2003, 160, 159–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, X.; Kim, B.; Yao, S.; Bondar, M.V.; Belfield, K.D. Bovine serum albumin nanoparticles with fluorogenic near-IR-emitting squaraine dyes. ACS Appl. Mater. Interfaces. 2013, 5, 8710–8717. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine dyes in PDT: From basic design to in vivo demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Alex, S.; Basheer, M.C.; Arun, K.T.; Ramaiah, D.; Das, S. Aggregation properties of heavy atom substituted squaraine dyes: Evidence for the formation of J-type dimer aggregates in aprotic solvents. J. Phys. Chem. A 2007, 111, 3226–3230. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Sudeep, P.K.; Kamat, P.V.; Noll, B.C.; Smith, B.D. Singlet oxygen generation using iodinated squaraine and squaraine-rotaxane dyes. New J. Chem. 2007, 31, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.T.; Epe, B.; Ramaiah, D. Aggregation Behavior of Halogenated Squaraine Dyes in Buffer, Electrolytes, Organized Media, and DNA. J. Phys. Chem. B 2002, 106, 11622–11627. [Google Scholar] [CrossRef]
- Park, Y.D.; Park, J.E.; Kim, H.S.; Choi, S.H.; Park, J.E.; Jeon, J.; Park, S.H. Development of a Squaraine-Based Molecular Probe for Dual-Modal in Vivo Fluorescence and Photoacoustic Imaging. Bioconjug. Chem. 2020, 31, 2607–2617. [Google Scholar] [CrossRef]
- Jisha, V.S.; Arun, K.T.; Hariharan, M.; Ramaiah, D. Site-selective interactions: Squaraine dyeserum albumin complexes with enhanced fluorescence and triplet yields. J. Phys. Chem. B 2010, 114, 5912–5919. [Google Scholar] [CrossRef]
- Ilina, K.; MacCuaig, W.M.; Laramie, M.; Jeouty, J.N.; McNally, L.R.; Henary, M. Squaraine Dyes: Molecular Design for Different Applications and Remaining Challenges. Bioconjug. Chem. 2020, 31, 194–213. [Google Scholar] [CrossRef]
- Gao, F.P.; Lin, Y.X.; Li, L.L.; Liu, Y.; Mayerhöffer, U.; Spenst, P.; Su, J.G.; Li, J.Y.; Würthner, F.; Wang, H. Supramolecular adducts of squaraine and protein for noninvasive tumor imaging and photothermal therapy in vivo. Biomaterials 2014, 35, 1004–1014. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Dereje, D.M.; Pontremoli, C.; Plata, M.J.M.; Visentin, S.; Barbero, N. Polymethine dyes for PDT: Recent advances and perspectives to drive future applications. Photochem. Photobiol. Sci. 2022, 21, 397–419. [Google Scholar] [CrossRef]
- Kauscher, U.; Ravoo, B.J. A self-assembled cyclodextrin nanocarrier for photoreactive squaraine. Beilstein J. Org. Chem. 2016, 12, 2535–2542. [Google Scholar] [CrossRef]
- Ramaiah, D.; Eckert, I.; Arun, K.T.; Weidenfeller, L.; Epe, B. Squaraine Dyes for Photodynamic Therapy: Study of Their Cytotoxicity and Genotoxicity in Bacteria and Mammalian Cells. Photochem. Photobiol. 2002, 76, 672–677. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, G.; Wang, J.; Wang, M.; Guo, W.; Tan, M.; Si, L.; Yang, Y.; Wang, H.; Wang, H. A synergetic strategy of NIR-II squaraine dyes with ultrahigh photothermal conversion efficiency for photothermal therapy. Sci. China Chem. 2024, 67, 612–621. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, G.; Tan, M.; Wang, M.; Li, Y.; Wang, H. H-Dimeric Nanospheres of Amphipathic Squaraine Dye with an 81.2% Photothermal Conversion Efficiency for Photothermal Therapy. Adv. Funct. Mater. 2022, 32, 2113098. [Google Scholar] [CrossRef]
- Sens, R.; Drexhage, K.H. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Luminesc. 1981, 24–25, 709–712. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Park, M.H.; Hyun, H. Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 3428. https://doi.org/10.3390/ijms25063428
Park Y, Park MH, Hyun H. Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy. International Journal of Molecular Sciences. 2024; 25(6):3428. https://doi.org/10.3390/ijms25063428
Chicago/Turabian StylePark, Yoonbin, Min Ho Park, and Hoon Hyun. 2024. "Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy" International Journal of Molecular Sciences 25, no. 6: 3428. https://doi.org/10.3390/ijms25063428
APA StylePark, Y., Park, M. H., & Hyun, H. (2024). Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy. International Journal of Molecular Sciences, 25(6), 3428. https://doi.org/10.3390/ijms25063428






