Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial
Abstract
1. Introduction
2. Results
2.1. Study Participants and Normotensive Controls
2.2. Variation in Vitamin D and Free Vitamin D
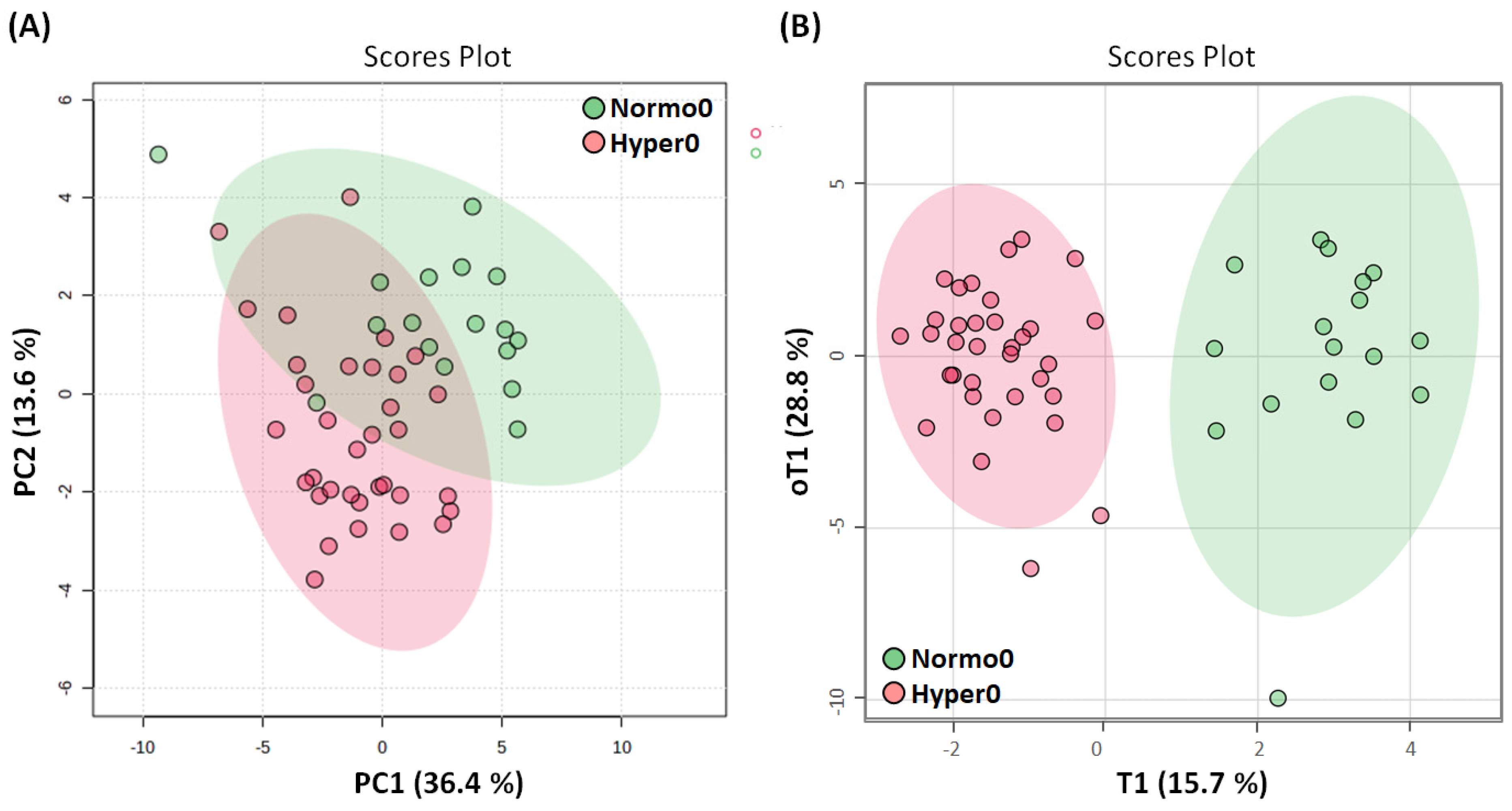
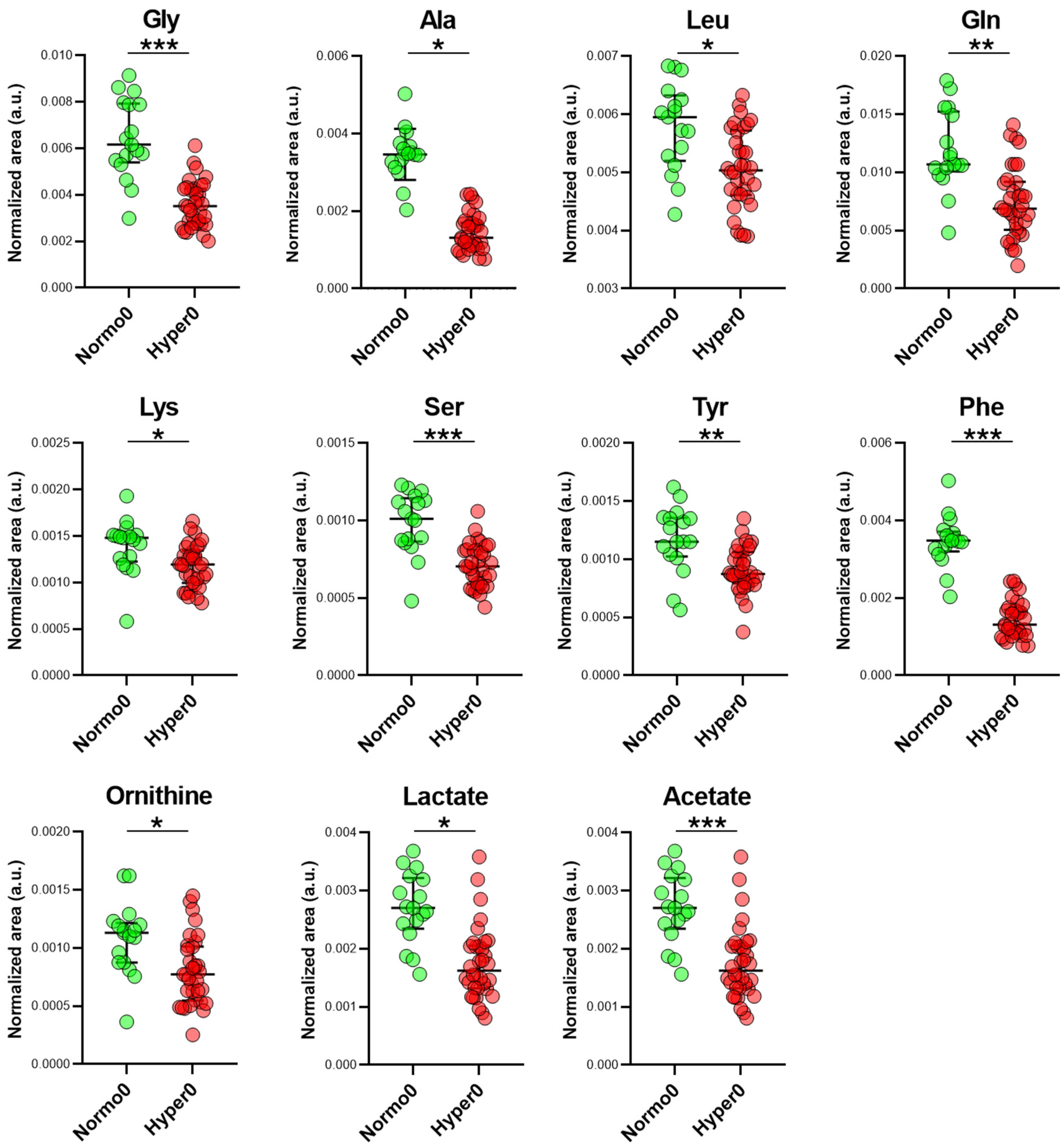
2.3. Serum Metabolome of Normotensive Controls and Hypertensive Trial Participants
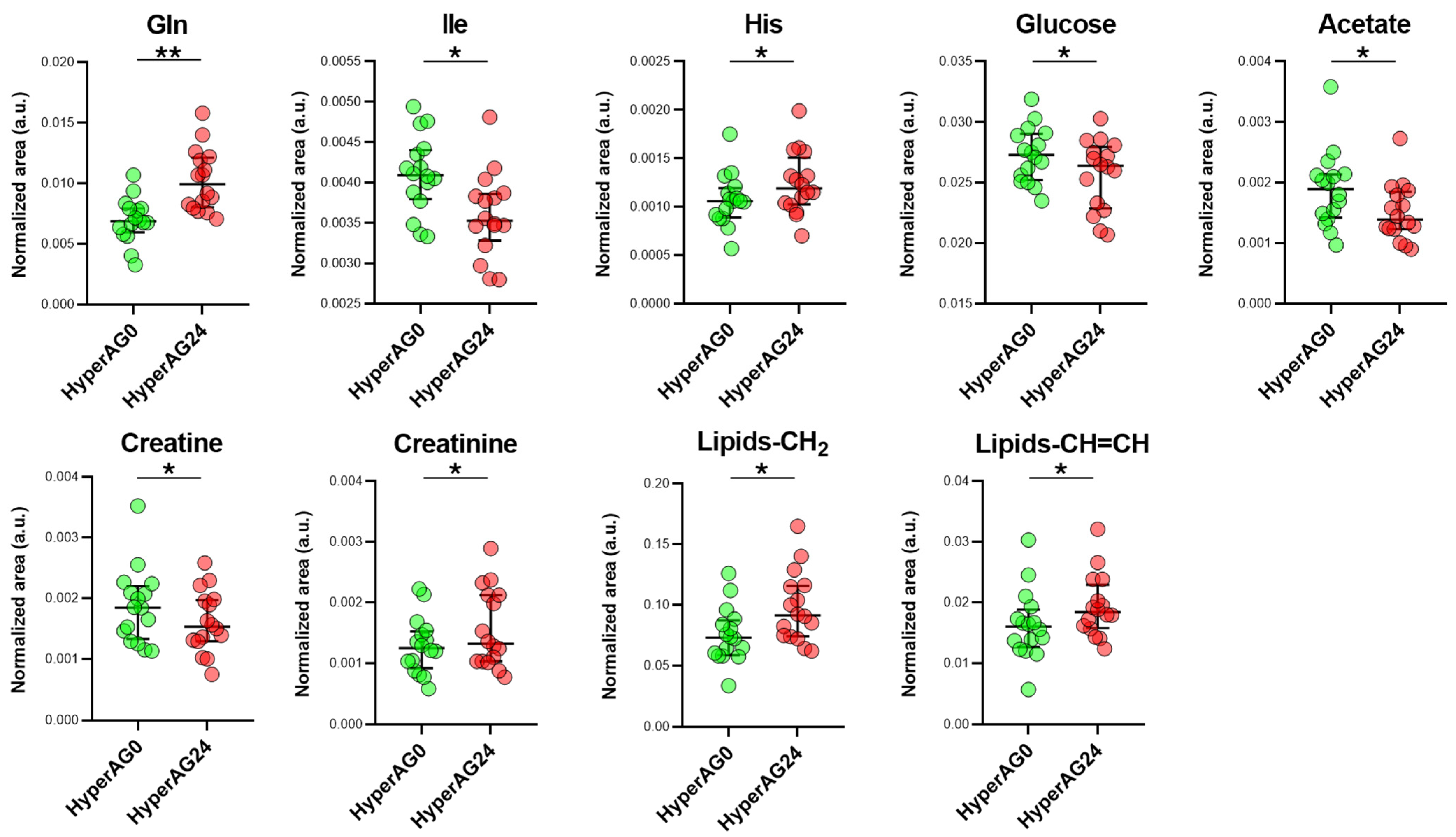
2.4. Serum Metabolome after Cholecalciferol Supplementation
3. Discussion
4. Materials and Methods
4.1. Trial Design and Patient Population
4.2. Cholecalciferol Prescription and Vitamin D Monitoring
4.3. Endpoint Assessment
4.4. Nuclear Magnetic Resonance-Based Metabolomics
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bomfim, G.F.; Cau, S.B.A.; Bruno, A.S.; Fedoce, A.G.; Carneiro, F.S. Hypertension: A new treatment for an old disease? Targeting the immune system. Br. J. Pharmacol. 2019, 176, 2028–2048. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An Update on the Effects of Vitamin D on the Immune System and Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Halim, S.A.; Santos, J.M.; Khan, F.; Khan, A.; Rahman, M.; Hussain, F.; Al-Harassi, A.; Gollahon, L.S.; Rahman, S.M. A High Dose of Calcitriol Inhibits Glycolysis and M2 Macrophage Polarization in the Tumor Microenvironment by Repressing mTOR Activation: In vitro and Molecular Docking Studies. Cell Physiol. Biochem. 2023, 57, 105–122. [Google Scholar]
- Jie, L.; Hengyue, W.; Ting, H. Calcitriol suppresses gastric cancer progression and cisplatin resistance by inhibiting glycolysis and M2 macrophage polarization through inhibition of mTOR activation. Environ. Toxicol. 2024, 39, 830–839. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Qu, H.; Wei, H.; Ji, B.; Yang, Z.; Wu, J.; He, Q.; Luo, Y.; Liu, D.; et al. In vitro and in vivo inhibition of mTOR by 1,25-dihydroxyvitamin D(3) to improve early diabetic nephropathy via the DDIT4/TSC2/mTOR pathway. Endocrine 2016, 54, 348–359. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, F.; Qu, H.; Wang, H.; Xiao, X.; Deng, H. 1, 25(OH)2D3 protects β cell against high glucose-induced apoptosis through mTOR suppressing. Mol. Cell Endocrinol. 2015, 414, 111–119. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, C.; Wang, Y.; Sun, H.; Yu, S.; Xue, Y.; Liu, Y.; Li, W.; Li, X. Effect of Vitamin D on Blood Pressure and Hypertension in the General Population: An Update Meta-Analysis of Cohort Studies and Randomized Controlled Trials. Prev. Chronic Dis. 2020, 17, 190307. [Google Scholar] [CrossRef]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- Bhargava, P.; Fitzgerald, K.C.; Calabresi, P.A.; Mowry, E.M. Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation. JCI Insight 2017, 2, e95302. [Google Scholar] [CrossRef]
- Chailurkit, L.; Nimitphong, H.; Saetung, S.; Ongphiphadhanakul, B. Urinary metabolic profiles after vitamin D(2) versus vitamin D(3) supplementation in prediabetes. J. Clin. Transl. Endocrinol. 2019, 16, 100194. [Google Scholar] [CrossRef]
- Zhuang, Z.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Millwood, I.Y.; Walters, R.G.; Chen, Y.; Xu, Q.; Zou, M.; et al. Metabolic Signatures of Genetically Elevated Vitamin D Among Chinese: Observational and Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e3249–e3260. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Hernández-Aguilera, A.; De Vries, M.A.; Burggraaf, B.; Van Der Zwan, E.; Pouw, N.; Joven, J.; Castro Cabezas, M. Effect of Vitamin D3 on the Postprandial Lipid Profile in Obese Patients: A Non-Targeted Lipidomics Study. Nutrients 2019, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Lasky-Su, J.A.; Dobnig, H.; Christopher, K.B. Metabolomic basis for response to high dose vitamin D in critical illness. Clin. Nutr. 2021, 40, 2053–2060. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Chong, E.Y.; Walker, D.I.; Chandler, J.D.; Michalski, E.S.; Grossmann, R.E.; Uppal, K.; Li, S.; Frediani, J.K.; Tirouvanizam, R.; et al. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: A pilot randomized study of high-dose vitamin D 3 administration. Metabolism 2017, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Santos, C.; Monteiro, A.; Rodrigues, R.; Ferreira, C.; Coutinho, J.; Filipe, R.; Ramos, S.; Castelo Branco, M.; Fonseca, M. In obese hypertensives cholecalciferol inhibits circulating TH17 cells but not macrophage infiltration on adipose tissue. Clin. Immunol. 2023, 247, 109244. [Google Scholar] [CrossRef]
- Taylor, A.L.; Davis, D.E.; Codreanu, S.G., Jr.; Harrison, F.E.; Sherrod, S.D.; McLean, J.A. Targeted and Untargeted Mass Spectrometry Reveals the Impact of High-Fat Diet on Peripheral Amino Acid Regulation in a Mouse Model of Alzheimer’s Disease. J. Proteome Res. 2021, 20, 4405–4414. [Google Scholar] [CrossRef]
- Shu, J.; Matarese, A.; Santulli, G. Diabetes, body fat, skeletal muscle, and hypertension: The ominous chiasmus? J. Clin. Hypertens. 2018, 21, 239. [Google Scholar] [CrossRef]
- Kovarik, J.J.; Morisawa, N.; Wild, J.; Marton, A.; Takase-Minegishi, K.; Minegishi, S.; Daub, S.; Sands, J.M.; Klein, J.D.; Bailey, J.L.; et al. Adaptive physiological water conservation explains hypertension and muscle catabolism in experimental chronic renal failure. Acta Physiol. 2021, 232, e13629. [Google Scholar] [CrossRef]
- Liang, Y.J.; Chiang, K.M.; Xiu, L.L.; Chung, C.M.; Lo, C.J.; Shiao, M.S.; Cheng, M.-L.; Kuo, C.-C.; Yang, H.-C.; Pan, W.-H. Pharmacometabolomic study of drug response to antihypertensive medications for hypertension marker identification in Han Chinese individuals in Taiwan. Comput. Struct. Biotechnol. J. 2022, 20, 6458–6466. [Google Scholar] [CrossRef]
- Mehanna, M.; McDonough, C.W.; Smith, S.M.; Gong, Y.; Gums, J.G.; Chapman, A.B.; Jonhson, J.A.; McIntyre, L.; Cooper-DeHoff, R.M. Metabolomics Signature of Plasma Renin Activity and Linkage with Blood Pressure Response to Beta Blockers and Thiazide Diuretics in Hypertensive European American Patients. Metabolites 2021, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Rizos, C.V. Antihypertensive drugs and glucose metabolism. World J. Cardiol. 2014, 6, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, R.; Sun, M.; Guo, X.; Yang, J.; Bian, W.; Xie, C.; Miao, D.; Mao, L. Sirt1 Mediates Vitamin D Deficiency-Driven Gluconeogenesis in the Liver via mTorc2/Akt Signaling. J. Diabetes Res. 2022, 2022, 1755563. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Drzewoski, J.; Śliwińska, A. The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders. Int. J. Mol. Sci. 2020, 21, 6644. [Google Scholar] [CrossRef] [PubMed]
- Valente, V.; Izzo, R.; Manzi, M.V.; De Luca, M.R.; Barbato, E.; Morisco, C. Modulation of insulin resistance by renin angiotensin system inhibitors: Implications for cardiovascular prevention. Monaldi Arch. Chest Dis. 2021, 91, 1602. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Gibney, M.J.; Connor, A.O.; Mion, B.; Kaluskar, S.; Cashman, K.D.; Flynn, A.; Shanahan, F.; Brennan, L. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome. Mol. Nutr. Food Res. 2011, 55, 679–690. [Google Scholar] [CrossRef]
- Miyajima, M. Amino acids: Key sources for immunometabolites and immunotransmitters. Int. Immunol. 2020, 32, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Tarditi, A.M.; Klipfel, M.W.; Rodriguez, A.M.; Suvire, F.D.; Chasse, G.A.; Farkas, O.; Perczel, A.; Enriz, R.D. An ab initio exploratory study of side chain conformations for selected backbone conformations of N-acetyl-l-glutamine-N-methylamide. J. Mol. Struct. THEOCHEM 2001, 545, 29–47. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef]
- Beaudin, S.; Welsh, J. 1,25-Dihydroxyvitamin D Regulation of Glutamine Synthetase and Glutamine Metabolism in Human Mammary Epithelial Cells. Endocrinology 2017, 158, 4174–4188. [Google Scholar] [CrossRef]
- Papadopoulou, G.; Xanthou, G. Metabolic rewiring: A new master of Th17 cell plasticity and heterogeneity. FEBS J. 2022, 289, 2448–2466. [Google Scholar] [CrossRef]
- Bishop, E.L.; Gudgeon, N.H.; Mackie, G.M.; Chauss, D.; Roberts, J.; Tennant, D.A.; Maslowski, K.M.; Afzali, B.; Hewison, M.; Dimeloe, S. 1,25-Dihydroxyvitamin D3 suppresses CD4+ T-cell effector functionality by inhibition of glycolysis. Immunology 2022, 166, 299–309. [Google Scholar] [CrossRef]
- Ferreira, G.B.; Kleijwegt, F.S.; Waelkens, E.; Lage, K.; Nikolic, T.; Hansen, D.A.; Workman, C.T.; Roep, B.O.; Overbergh, L.; Mathieu, C. Differential Protein Pathways in 1,25-Dihydroxyvitamin D3 and Dexamethasone Modulated Tolerogenic Human Dendritic Cells. J. Proteome Res. 2012, 11, 941–971. [Google Scholar] [CrossRef]
- Jozi, F.; Kheiripour, N.; Taheri, M.A.; Ardjmand, A.; Ghavipanjeh, G.; Nasehi, Z.; Shahaboddin, M.E. L-Lysine Ameliorates Diabetic Nephropathy in Rats with Streptozotocin-Induced Diabetes Mellitus. BioMed Res. Int. 2022, 2022, 4547312. [Google Scholar] [CrossRef]
- Bloomgarden, Z. Diabetes and branched-chain amino acids: What is the link? J. Diabetes 2018, 10, 350–352. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA Metabolism and Insulin Sensitivity–Dysregulated by Metabolic Status? Mol. Nutr. Food Res. 2018, 62, 1700756. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef]
- Alfaqih, M.A.; Melhem, N.Y.; F. Khabour, O.; Al-Dwairi, A.; Elsalem, L.; Alsaqer, T.G.; Allouh, M.Z. Normalization of Vitamin D Serum Levels in Patients with Type Two Diabetes Mellitus Reduces Levels of Branched Chain Amino Acids. Medicina 2022, 58, 1267. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-regulated Gene Expression Profiles: Species-specificity and Cell-specific Effects on Metabolism and Immunity. Endocrinology 2021, 162, bqaa218. [Google Scholar] [CrossRef]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1α,25(OH) 2D3. Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength. JAMA 2019, 322, 736. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-N.; Tain, Y.-L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]


| Week 0 | |||
|---|---|---|---|
| Non-Cholecalciferol Group | Cholecalciferol Group | p-Value | |
| Gender (men/women) | 9/9 | 9/9 | -- |
| Vitamin D (ng/mL) | 17.2 ± 5.6 | 14.4 ± 4.3 | 0.100 |
| Free vitamin D (pg/mL) | 6 ± 2.4 | 5.2 ± 1.2 | 0.210 |
| Visceral adiposity index | 5.7 ± 4.1 | 5.6 ± 3.4 | 0.957 |
| HbA1c (%) | 5.8 ± 0.7 | 6.1 ± 1.1 | 0.416 |
| BMI (Kg/m2) | 35.1 ± 5.9 | 34 ± 4.3 | 0.502 |
| Waist circumference (cm) | 117.7 ± 8.7 | 110 ± 11.3 * | 0.026 |
| Systolic BP (mmHg) | 138.8 ± 19.8 | 131.3 ± 15 | 0.210 |
| Diastolic BP (mmHg) | 84.2 ± 9.1 | 79 ± 10.9 | 0.133 |
| Number of diabetic patients | 5 | 6 | 0.727 |
| Total cholesterol (mg/dL) | 164.2 ± 34.8 | 164.6 ± 27.5 | 0.966 |
| HDL cholesterol (mg/dL) | 47.4 ± 12.5 | 48.2 ± 12.3 | 0.842 |
| Triglycerides (mg/dL) | 137.7 ± 73.7 | 147.1 ± 63.1 | 0.684 |
| Patients on statin therapy | 11 | 17 * | 0.015 |
| Number of AH drugs | 2.8 ± 1 | 2.3 ± 0.9 | 0.146 |
| ACEI/ARA | 16 | 16 | 0.992 |
| CCB | 4 | 8 | 0.747 |
| Beta-blockers | 9 | 5 | 0.087 |
| Diuretic | 16 | 14 | 0.218 |
| α-blockers | 1 | 0 | 0.324 |
| Estimated GFR | 100.4 ± 13.1 | 103.3 ± 11.7 | 0.572 |
| Week 0 | |||
|---|---|---|---|
| Normotensive Controls (N = 17) | Trial Participants (N = 36) | p-Value | |
| BMI (Kg/m2) | 27.8 ± 4.1 | 34.5 ± 5.1 ** | 0.0001 |
| Systolic BP (mmHg) | 117.8 ± 5.6 | 135.6 ± 18.2 ** | 0.0003 |
| Diastolic BP (mmHg) | 75.8 ± 5.1 | 81.9 ± 10.2 * | 0.024 |
| HbA1c (%) | -- | 5.9 ± 0.89 | |
| Total cholesterol (mg(dL) | 183.5 ± 36.4 | 164.4 ± 30.9 | 0.053 |
| HDL cholesterol (mg/dL) | 47.4 ± 12.5 | 48.2 ± 12.3 | 0.842 |
| Triglycerides (mg/dL) | 113.8 ± 41.2 | 142.4 ± 67.8 | 0.115 |
| Patients on statins (%) | 58.8% | 77.7% | 0.621 |
| Estimated GFR (mL/min/1.73 m2) | 115.2 ± 34.5 | 101.8 ± 35.6 | 0.202 |
| Week 24 | |||
|---|---|---|---|
| Non-Cholecalciferol Group | Cholecalciferol Group | p-Value | |
| Vitamin D (ng/mL) | 15.5 ± 3.4 | 26.9 ± 5.6 ** | <0.001 |
| Free vitamin D (pg/mL) | 4.6 ± 2.3 | 6.2 ± 2.3 * | 0.044 |
| Visceral adiposity index | 5.4 ± 4.1 | 5.2 ± 3.8 | 0.869 |
| HbA1c (%) | 5.6 ± 0.5 | 6.1 ± 1.1 | 0.347 |
| BMI (Kg/m2) | 34.9 ± 6.1 | 34.5 ± 4.7 | 0.763 |
| Waist circumference (cm) | 114 ± 11 | 107 ± 9.9 | 0.049 |
| Systolic BP (mmHg) | 137 ± 17 | 133.4 ± 15.3 | 0.356 |
| Diastolic BP (mmHg) | 84.5 ± 8.8 | 81 ± 9.8 | 0.176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.; Carvalho, R.; Fonseca, A.M.; Castelo Branco, M.; Alves, M.; Jarak, I. Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial. Int. J. Mol. Sci. 2024, 25, 3416. https://doi.org/10.3390/ijms25063416
Santos C, Carvalho R, Fonseca AM, Castelo Branco M, Alves M, Jarak I. Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial. International Journal of Molecular Sciences. 2024; 25(6):3416. https://doi.org/10.3390/ijms25063416
Chicago/Turabian StyleSantos, Catarina, Rui Carvalho, Ana Mafalda Fonseca, Miguel Castelo Branco, Marco Alves, and Ivana Jarak. 2024. "Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial" International Journal of Molecular Sciences 25, no. 6: 3416. https://doi.org/10.3390/ijms25063416
APA StyleSantos, C., Carvalho, R., Fonseca, A. M., Castelo Branco, M., Alves, M., & Jarak, I. (2024). Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial. International Journal of Molecular Sciences, 25(6), 3416. https://doi.org/10.3390/ijms25063416










