Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells
Abstract
1. Introduction
2. Results
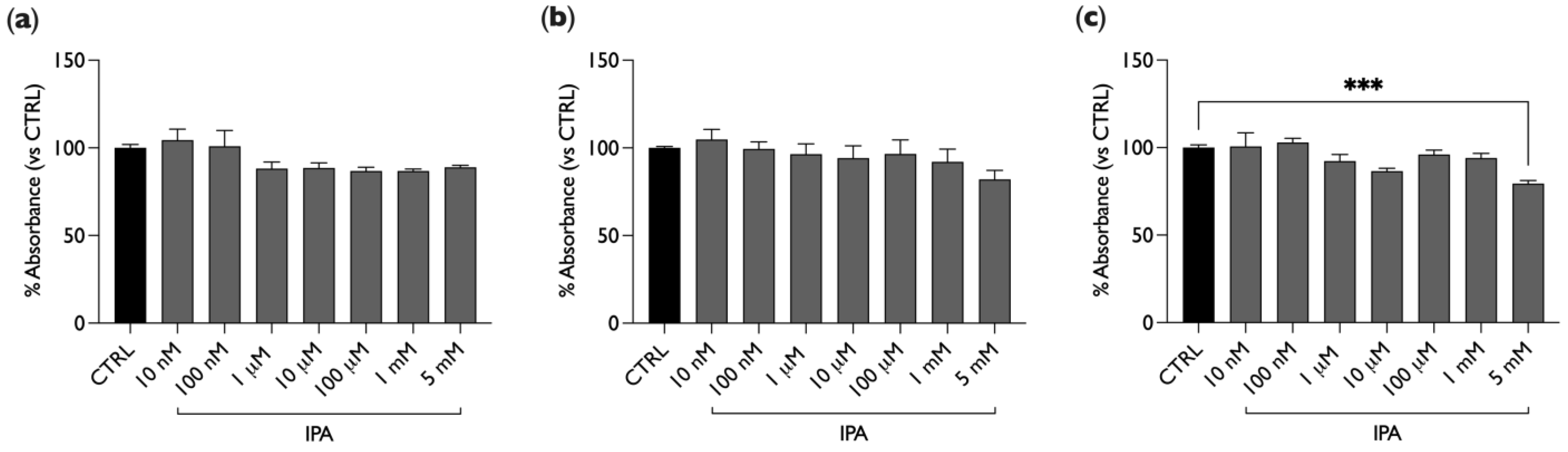
2.1. IPA Does Not Affect BAE-1 Cells Viability
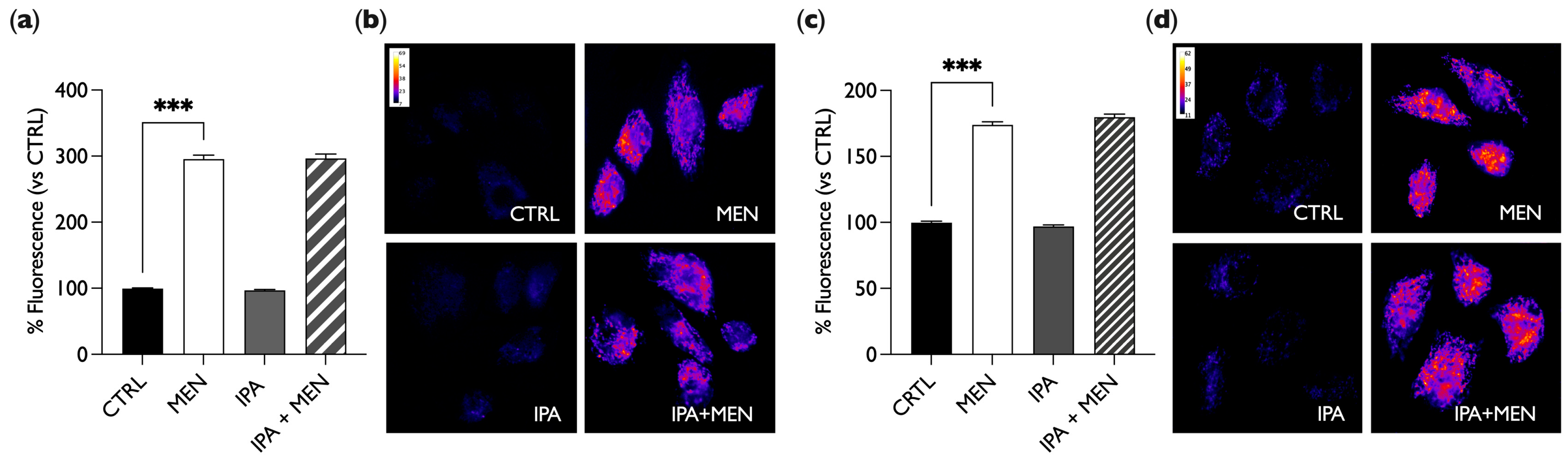
2.2. IPA Does Not Show Oxidant or Antioxidant Activity
2.3. IPA Reduces NO Release Induced by ATP
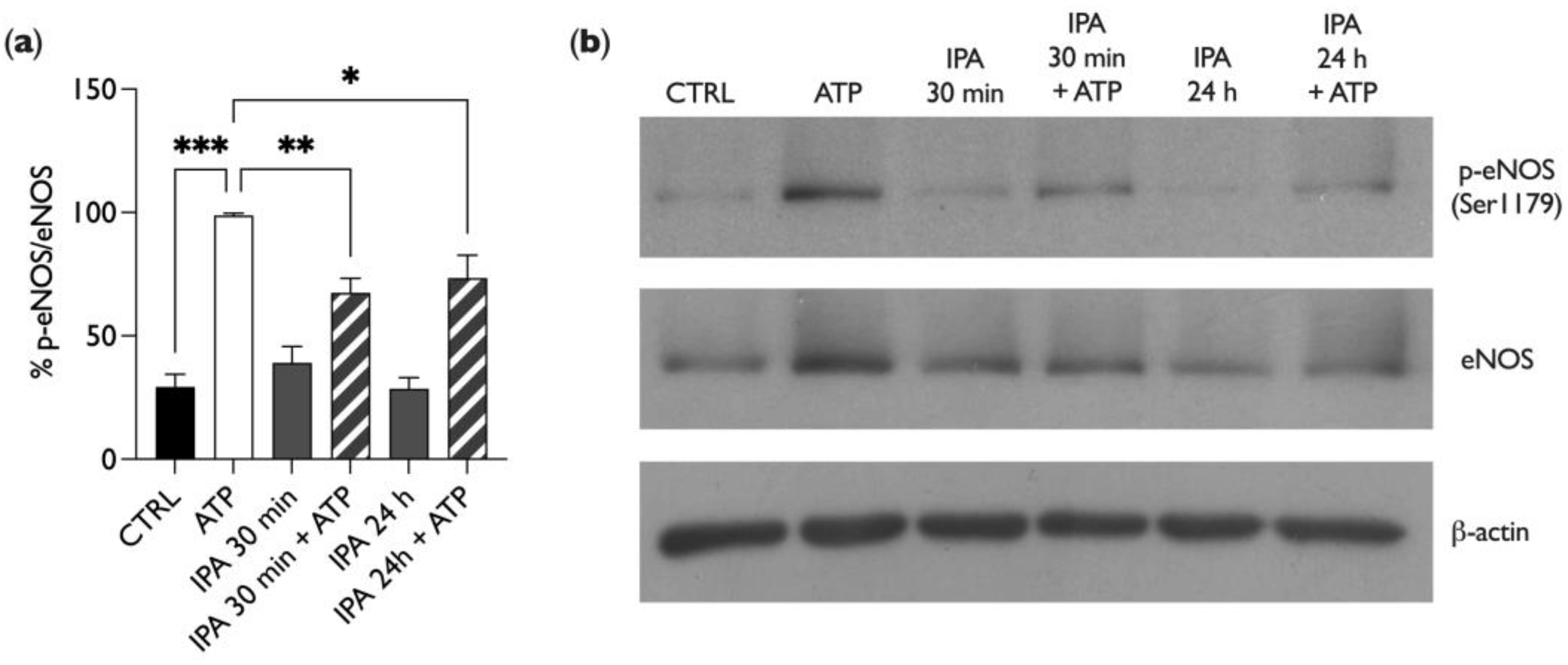
2.4. IPA Affects Endothelial Nitric Oxide Synthase (eNOS) Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Reactive Oxygen Species (ROS): Microplate Reader
4.5. Reactive Oxygen Species (ROS): Fluorescence Microscopy
4.6. NO Release
4.7. Immunoblotting
4.8. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adak, A.; Khan, M.R. An Insight into Gut Microbiota and Its Functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.V.A.; Hwangbo, H.; Lai, Y.; Hong, S.B.; Choi, Y.-J.; Park, H.-J.; Ban, K. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ. J. 2023, 53, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-Chain Fatty Acid Metabolism and Multiple Effects on Cardiovascular Diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Chen, H.-C.; Liu, Y.-W.; Chang, K.-C.; Wu, Y.-W.; Chen, Y.-M.; Chao, Y.-K.; You, M.-Y.; Lundy, D.J.; Lin, C.-J.; Hsieh, M.L.; et al. Gut Butyrate-Producers Confer Post-Infarction Cardiac Protection. Nat. Commun. 2023, 14, 7249. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ke, B.; Du, J. TMAO: How Gut Microbiota Contributes to Heart Failure. Transl. Res. 2021, 228, 109–125. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Geddo, F.; Levi, R.; Gallo, M.P. Trimethylamine N-Oxide (TMAO) Impairs Purinergic Induced Intracellular Calcium Increase and Nitric Oxide Release in Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 3982. [Google Scholar] [CrossRef]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Cheng, X.; Qiu, X.; Liu, Y.; Yuan, C.; Yang, X. Trimethylamine N-Oxide Promotes Tissue Factor Expression and Activity in Vascular Endothelial Cells: A New Link between Trimethylamine N-Oxide and Atherosclerotic Thrombosis. Thromb. Res. 2019, 177, 110–116. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. [Google Scholar] [CrossRef]
- Poeggeler, B.; Pappolla, M.A.; Hardeland, R.; Rassoulpour, A.; Hodgkins, P.S.; Guidetti, P.; Schwarcz, R. Indole-3-Propionate: A Potent Hydroxyl Radical Scavenger in Rat Brain. Brain Res. 1999, 815, 382–388. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, X.; Han, J.; Zhang, Z.; Feng, Z.; Ouyang, Q.; Hua, S.; Liu, Z.; Li, C.; Cai, Y.; et al. Indole-3-Propionic Acid Alleviates Ischemic Brain Injury in a Mouse Middle Cerebral Artery Occlusion Model. Exp. Neurol. 2022, 353, 114081. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-Propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef]
- Serger, E.; Luengo-Gutierrez, L.; Chadwick, J.S.; Kong, G.; Zhou, L.; Crawford, G.; Danzi, M.C.; Myridakis, A.; Brandis, A.; Bello, A.T.; et al. The Gut Metabolite Indole-3 Propionate Promotes Nerve Regeneration and Repair. Nature 2022, 607, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ghiboub, M.; Verburgt, C.M.; Sovran, B.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy to Modulate Tryptophan Metabolism and Aryl Hydrocarbon-Receptor Signaling Activation in Human Diseases. Nutrients 2020, 12, 2846. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Hu, Z.; Lu, C.-X.; Luo, S.-D.; Chen, Y.; Zhou, Z.-P.; Hu, J.-J.; Zhang, F.-L.; Deng, F.; Liu, K.-X. Gut Microbiota-Derived Indole 3-Propionic Acid Partially Activates Aryl Hydrocarbon Receptor to Promote Macrophage Phagocytosis and Attenuate Septic Injury. Front. Cell Infect. Microbiol. 2022, 12, 1015386. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Lim, J.J.; Cui, J.Y. Pregnane X Receptor and the Gut-Liver Axis: A Recent Update. Drug Metab. Dispos. 2022, 50, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Chen, X.; Yu, C.; Deng, Y.; Zhang, Y.; Chen, S.; Chen, X.; Chen, K.; Yang, Y.; Ling, W. Gut Microbially Produced Indole-3-Propionic Acid Inhibits Atherosclerosis by Promoting Reverse Cholesterol Transport and Its Deficiency Is Causally Related to Atherosclerotic Cardiovascular Disease. Circ. Res. 2022, 131, 404–420. [Google Scholar] [CrossRef]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma Microbiome-Modulated Indole- and Phenyl-Derived Metabolites Associate with Advanced Atherosclerosis and Postoperative Outcomes. J. Vasc. Surg. 2018, 68, 1552–1562.e7. [Google Scholar] [CrossRef] [PubMed]
- Konopelski, P.; Chabowski, D.; Aleksandrowicz, M.; Kozniewska, E.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-Propionic Acid, a Tryptophan-Derived Bacterial Metabolite, Increases Blood Pressure via Cardiac and Vascular Mechanisms in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 321, R969–R981. [Google Scholar] [CrossRef]
- Gesper, M.; Nonnast, A.B.H.; Kumowski, N.; Stoehr, R.; Schuett, K.; Marx, N.; Kappel, B.A. Gut-Derived Metabolite Indole-3-Propionic Acid Modulates Mitochondrial Function in Cardiomyocytes and Alters Cardiac Function. Front. Med. 2021, 8, 648259. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-Derived Tryptophan Metabolites in Vascular Inflammation and Cardiovascular Disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arter. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Mount, P.F.; Kemp, B.E.; Power, D.A. Regulation of Endothelial and Myocardial NO Synthesis by Multi-Site eNOS Phosphorylation. J. Mol. Cell. Cardiol. 2007, 42, 271–279. [Google Scholar] [CrossRef]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of Serum Indolepropionic Acid, a Gut Microbiota Metabolite, with Type 2 Diabetes and Low-Grade Inflammation in High-Risk Individuals. Nutr. Diabetes 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Cabou, C.; Martinez, L.O. The Interplay of Endothelial P2Y Receptors in Cardiovascular Health: From Vascular Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 5883. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Kashanipoor, S.; Mazaheri, P.; Alibabaei, F.; Babaeizad, A.; Asli, S.; Mohammadi, S.; Gorgin, A.H.; Ghods, K.; Yousefi, B.; et al. Importance of Gut Microbiota Metabolites in the Development of Cardiovascular Diseases (CVD). Life Sci. 2023, 329, 121947. [Google Scholar] [CrossRef] [PubMed]
- Florea, C.M.; Baldea, I.; Rosu, R.; Moldovan, R.; Decea, N.; Filip, G.A. The Acute Effect of Trimethylamine-N-Oxide on Vascular Function, Oxidative Stress, and Inflammation in Rat Aortic Rings. Cardiovasc. Toxicol. 2023, 23, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Sun, W.; Ding, L.; Yan, M.; Sun, C.; Qiu, C.; Wang, D.; Wu, L. Short-Chain Fatty Acids Weaken Ox-LDL-Induced Cell Inflammatory Injury by Inhibiting the NLRP3/Caspase-1 Pathway and Affecting Cellular Metabolism in THP-1 Cells. Molecules 2022, 27, 8801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Qi, R.; Wang, J.; Liu, Z.; Wu, Z. Indole-3-Propionic Acid, a Functional Metabolite of Clostridium Sporogenes, Promotes Muscle Tissue Development and Reduces Muscle Cell Inflammation. Int. J. Mol. Sci. 2021, 22, 12435. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.J.; Ramirez, J.L.; Kulkarni, R.; Harris, K.G.; Helenowski, I.; Xiong, L.; Ozaki, C.K.; Grenon, S.M. Plasma Gut Microbe-Derived Metabolites Associated with Peripheral Artery Disease and Major Adverse Cardiac Events. Microorganisms 2022, 10, 2065. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.Y.; Chung, S.M.; Chung, J.H. Menadione-Induced Vascular Endothelial Dysfunction and Its Possible Significance. Toxicol. Appl. Pharmacol. 1999, 161, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bae, O.N.; Chung, S.M.; Lee, M.Y.; Chung, J.H. Menadione Induces Endothelial Dysfunction Mediated by Oxidative Stress and Arylation. Chem. Biol. Interact. 2001, 137, 169–183. [Google Scholar] [CrossRef]
- Bik, E.; Mateuszuk, L.; Stojak, M.; Chlopicki, S.; Baranska, M.; Majzner, K. Menadione-Induced Endothelial Inflammation Detected by Raman Spectroscopy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118911. [Google Scholar] [CrossRef]
- Rynkowska, A.; Stępniak, J.; Karbownik-Lewińska, M. Melatonin and Indole-3-Propionic Acid Reduce Oxidative Damage to Membrane Lipids Induced by High Iron Concentrations in Porcine Skin. Membranes 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Gitto, E.; Lewiñski, A.; Reiter, R.J. Relative Efficacies of Indole Antioxidants in Reducing Autoxidation and Iron-Induced Lipid Peroxidation in Hamster Testes. J. Cell. Biochem. 2001, 81, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Karbownik, M.; Reiter, R.J.; Garcia, J.J.; Cabrera, J.; Burkhardt, S.; Osuna, C.; Lewiński, A. Indole-3-Propionic Acid, a Melatonin-Related Molecule, Protects Hepatic Microsomal Membranes from Iron-Induced Oxidative Damage: Relevance to Cancer Reduction. J. Cell. Biochem. 2001, 81, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sári, Z.; Mikó, E.; Kovács, T.; Jankó, L.; Csonka, T.; Lente, G.; Sebő, É.; Tóth, J.; Tóth, D.; Árkosy, P.; et al. Indolepropionic Acid, a Metabolite of the Microbiome, Has Cytostatic Properties in Breast Cancer by Activating AHR and PXR Receptors and Inducing Oxidative Stress. Cancers 2020, 12, 2411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular Nitric Oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.G.; Specht, A.; Wegiel, B.; Ferran, C.; Kaczmarek, E. Mechanism of Purinergic Activation of Endothelial Nitric Oxide Synthase in Endothelial Cells. Circulation 2009, 119, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Pulakazhi Venu, V.K.; Saifeddine, M.; Mihara, K.; Tsai, Y.-C.; Nieves, K.; Alston, L.; Mani, S.; McCoy, K.D.; Hollenberg, M.D.; Hirota, S.A. The Pregnane X Receptor and Its Microbiota-Derived Ligand Indole 3-Propionic Acid Regulate Endothelium-Dependent Vasodilation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E350–E361. [Google Scholar] [CrossRef] [PubMed]
- Geddo, F.; Querio, G.; Asteggiano, A.; Antoniotti, S.; Porcu, A.; Occhipinti, A.; Medana, C.; Gallo, M.P. Improving Endothelial Health with Food-Derived H2S Donors: An in Vitro Study with S-Allyl Cysteine and with a Black-Garlic Extract Enriched in Sulfur-Containing Compounds. Food Funct. 2023, 14, 4163–4172. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddo, F.; Antoniotti, S.; Gallo, M.P.; Querio, G. Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 3389. https://doi.org/10.3390/ijms25063389
Geddo F, Antoniotti S, Gallo MP, Querio G. Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(6):3389. https://doi.org/10.3390/ijms25063389
Chicago/Turabian StyleGeddo, Federica, Susanna Antoniotti, Maria Pia Gallo, and Giulia Querio. 2024. "Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells" International Journal of Molecular Sciences 25, no. 6: 3389. https://doi.org/10.3390/ijms25063389
APA StyleGeddo, F., Antoniotti, S., Gallo, M. P., & Querio, G. (2024). Indole-3-Propionic Acid, a Gut Microbiota-Derived Tryptophan Metabolite, Promotes Endothelial Dysfunction Impairing Purinergic-Induced Nitric Oxide Release in Endothelial Cells. International Journal of Molecular Sciences, 25(6), 3389. https://doi.org/10.3390/ijms25063389




