Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review
Abstract
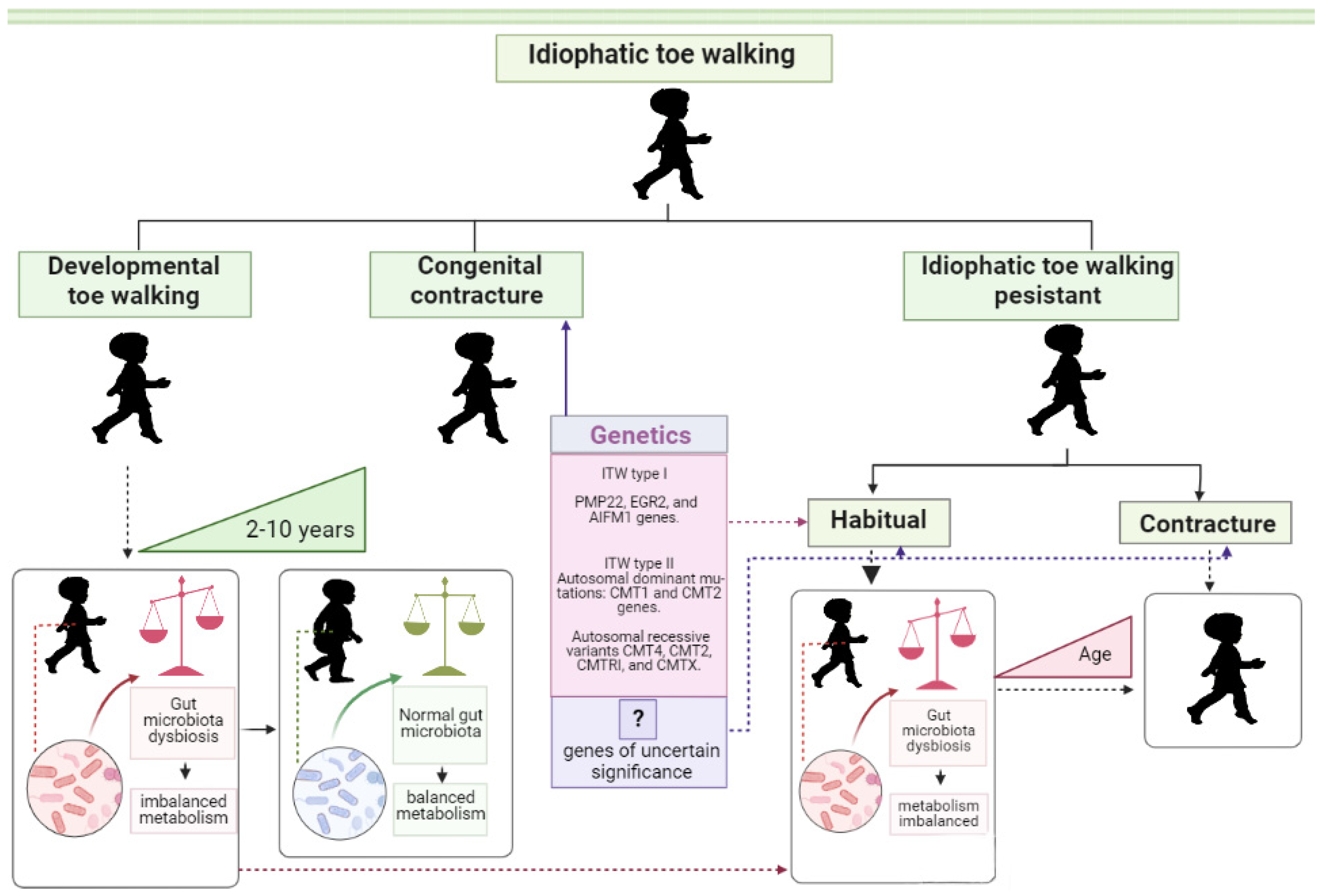
1. Introduction
2. Mechanics of Walking
3. Brain Development
4. Visual–Motor Learning and Brain Control of Movement
5. Dopamine Signaling
6. Microbiome and Motor Development
6.1. Colonization of the Neonatal Intestine and Development of Gut Microbiota in Children
6.2. Control of Brain Development by Microbiota
6.3. Impact Factors on Colonization of the Neonatal Intestine
6.4. Link between Infant Nervous System Development and Gut Microbiota Content
6.5. Microbiota–Gut–Brain Axis
7. Metabolites of the Intestinal Microbiota
7.1. Neurotransmitters
7.2. Group B Vitamins
7.3. SCFAs
8. Gut Microbiota, Diet and Exercise
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bremer, E.; Cairney, J. Fundamental Movement Skills and Health-Related Outcomes: A Narrative Review of Longitudinal and Intervention Studies Targeting Typically Developing Children. Am. J. Lifestyle Med. 2016, 12, 148–159. [Google Scholar] [CrossRef]
- Gvirts Probolovski, H.Z.; Dahan, A. The Potential Role of Dopamine in Mediating Motor Function and Interpersonal Synchrony. Biomedicines 2021, 9, 382. [Google Scholar] [CrossRef]
- Viholanen, H.; Ahonen, T.; Cantell, M.; Tolvanen, A.; Lyytinen, H. The early motor milestones in infancy and later motor skills in toddlers: A structural equation model of motor development. Phys. Occup. Ther. Pediatr. 2006, 26, 91–113. [Google Scholar] [CrossRef]
- Bach, M.M.; Daffertshofer, A.; Dominici, N. The development of mature gait patterns in children during walking and running. Eur. J. Appl. Physiol. 2021, 121, 1073–1085. [Google Scholar] [CrossRef]
- Adolph, K.E.; Cole, W.G.; Komati, M.; Garciaguirre, J.S.; Badaly, D.; Lingeman, J.M.; Chan, G.L.; Sotsky, R.B. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol. Sci. 2012, 23, 1387–1394. [Google Scholar] [CrossRef]
- Williams, C.M.; Tinley, P.; Rawicki, B. Idiopathic toe-walking: Have we progressed in our knowledge of the causality and treatment of this gait type? J. Am. Podiatr. Med. Assoc. 2014, 104, 253–262. [Google Scholar] [CrossRef]
- Engström, P.; Tedroff, K. The prevalence and course of idiopathic toe-walking in 5-year-old children. Pediatrics 2012, 130, 279–284. [Google Scholar] [CrossRef]
- Engström, P.; Tedroff, K. Idiopathic Toe-Walking: Prevalence and Natural History from Birth to Ten Years of Age. J. Bone Jt. Surg. Am. 2018, 100, 640–647. [Google Scholar] [CrossRef]
- Johnston, L.; Eastwood, D.; Jacobs, B. Variations in normal gait development. Paediatr. Child Health 2014, 24, 204–207. [Google Scholar] [CrossRef]
- Engelbert, R.; Gorter, J.W.; Uiterwaal, C.; van de Putte, E.; Helders, P. Idiopathic toe-walking in children, adolescents and young adults: A matter of local or generalised stiffness? BMC Musculoskelet. Disord. 2011, 12, 61. [Google Scholar] [CrossRef]
- Caserta, A.J.; Pacey, V.; Fahey, M.; Gray, K.; Engelbert, R.H.; Williams, C.M. Interventions for idiopathic toe walking. Cochrane Database Syst. Rev. 2019, 10, CD012363. [Google Scholar] [CrossRef] [PubMed]
- Freiman, H.D.; Mensah, C.; Codrington, J.; Frick, S.L. Idiopathic Toe-Walking in Children and Adolescents: Diagnosis, Natural History, and Treatment Options. JBJS Rev. 2022, 10, e21.00193. [Google Scholar] [CrossRef] [PubMed]
- Furrer, F.; Deonna, T. Persistent toe-walking in children. A comprehensive clinical study of 28 cases. Helv. Paediatr. Acta 1982, 37, 301–316. [Google Scholar] [PubMed]
- Sala, D.A. Idiopathic Toe-Walking: A Review from 1967 to 2021. J. Pediatr. Neurol. 2022, 20, 237–251. [Google Scholar] [CrossRef]
- Bauer, J.P.; Sienko, S.; Davids, J.R. Idiopathic Toe Walking: An Update on Natural History, Diagnosis, and Treatment. J. Am. Acad. Orthop. Surg. 2022, 30, e1419–e1430. [Google Scholar] [CrossRef]
- Radtke, K.; Karch, N.; Goede, F.; Vaske, B.; von Lewinski, G.; Noll, Y.; Thren, A. Outcomes of Noninvasively Treated Idiopathic Toe Walkers. Foot Ankle Spec. 2019, 12, 54–61. [Google Scholar] [CrossRef]
- Davies, K.; Black, A.; Hunt, M.; Holsti, L. Long-term gait outcomes following conservative management of idiopathic toe walking. Gait Posture 2018, 62, 214–219. [Google Scholar] [CrossRef]
- Bartoletta, J.; Tsao, E.; Bouchard, M. A Retrospective Analysis of Nonoperative Treatment Techniques for Idiopathic Toe Walking in Children: Outcomes and Predictors of Success. PM R 2021, 13, 1127–1135. [Google Scholar] [CrossRef]
- McMulkin, M.L.; Gordon, A.B.; Genung, S.; Huck, M.; Caskey, P.M.; Baird, G.O. Prevalence of associated diagnoses in children who present with toe walking. Phys. Med. Rehabil. Res. 2016, 2, 1–3. [Google Scholar] [CrossRef][Green Version]
- Stricker, S.J.; Angulo, J.C. Idiopathic toe walking: A comparison of treatment methods. J. Pediatr. Orthop. 1998, 18, 289–293. [Google Scholar] [CrossRef]
- Pomarino, D.; Ramírez Llamas, J.; Pomarino, A. Idiopathic Toe Walking: Family Predisposition and Gender Distribution. Foot Ankle Spec. 2016, 9, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Pomarino, D.; Thren, J.R.; Thren, A.; Rostasy, K.; Schoenfeldt, J. Toe Walking as the Initial Symptom of a Spinocerebellar Ataxia 13 in a Patient Presenting with a Mutation in the KCNC3 Gene. Glob. Med. Genet. 2021, 9, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Pomarino, D.; Emelina, A.; Heidrich, J.; Rostásy, K.; Schirmer, S.; Schönfeldt, J.O.; Thren, A.; Wagner, F.; Thren, J.R.; Berger, N. NGS-Panel Diagnosis Developed for the Differential Diagnosis of Idiopathic Toe Walking and Its Application for the Investigation of Possible Genetic Causes for the Gait Anomaly. Glob. Med. Genet. 2023, 10, 63–71. [Google Scholar] [CrossRef]
- Clark, E.; Sweeney, J.K.; Yocum, A.; McCoy, S.W. Effects of motor control intervention for children with idiopathic toe walking: A 5-case series. Pediatr. Phys. Ther. 2010, 22, 417–426. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.; Arrebola, L.; De Oliveira, P.; Yi, L. Investigation of Muscle Strength, Motor Coordination and Balance in Children with Idiopathic Toe Walking: A Case-control Study. Dev. Neurorehabil. 2021, 24, 540–546. [Google Scholar] [CrossRef]
- Williams, C.M.; Tinley, P.; Curtin, M. Idiopathic toe walking and sensory processing dysfunction. J. Foot Ankle Res. 2010, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Donne, J.H.; Powell, J.A.; Fahey, M.C.; Beare, R.; Kolic, J.; Williams, C.M. Some children with idiopathic toe walking display sensory processing difficulties but not all: A systematic review. Acta Paediatr. 2023, 112, 1620–1632. [Google Scholar] [CrossRef]
- Williams, C.M.; Tinley, P.; Curtin, M.; Wakefield, S.; Nielsen, S. Is idiopathic toe walking really idiopathic? The motor skills and sensory processing abilities associated with idiopathic toe walking gait. J. Child Neurol. 2014, 29, 71–78. [Google Scholar] [CrossRef]
- Chu, V.; Girolami, G.L.; Grant-Beuttler, M. Assessing sensory processing differences in children with idiopathic toe walking: A pilot study. Physiother. Theory Pract. 2022, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caserta, A.; Morgan, P.; Williams, C. Exploration of physiotherapists’ use of motor control strategies for the treatment of idiopathic toe walking in children: A qualitative study. BMJ Open 2022, 12, e062704. [Google Scholar] [CrossRef]
- Donne, J.; Farrell, M.J.; Kolic, J.; Powell, J.; Fahey, M.; Williams, C. Two-point discrimination responses in children with idiopathic toe walking: A feasibility fMRI study. Sci. Prog. 2022, 105, 368504221132141. [Google Scholar] [CrossRef]
- Engström, P.; Van’t Hooft, I.; Tedroff, K. Neuropsychiatric symptoms and problems among children with idiopathic toe-walking. J. Pediatr. Orthop. 2012, 32, 848–852. [Google Scholar] [CrossRef]
- Shulman, L.H.; Sala, D.A.; Chu, M.L.; McCaul, P.R.; Sandler, B.J. Developmental implications of idiopathic toe walking. J. Pediatr. 1997, 130, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Martín-Casas, P.; Ballestero-Pérez, R.; Meneses-Monroy, A.; Beneit-Montesinos, J.V.; Atín-Arratibel, M.A.; Portellano-Pérez, J.A. Neurodevelopment in preschool idiopathic toe-walkers. Neurologia 2017, 32, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Baber, S.; Michalitsis, J.; Fahey, M.; Rawicki, B.; Haines, T.; Williams, C. A Comparison of the Birth Characteristics of Idiopathic Toe Walking and Toe Walking Gait Due to Medical Reasons. J. Pediatr. 2016, 171, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Afif, I.Y.; Manik, A.R.; Munthe, K.; Maula, M.I.; Ammarullah, M.I.; Jamari, J.; Winarni, T.I. Physiological Effect of Deep Pressure in Reducing Anxiety of Children with ASD during Traveling: A Public Transportation Setting. Bioengineering 2022, 9, 157. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-related Changes in Bile Acid and Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Saurman, V.; Margolis, K.G.; Luna, R.A. Autism Spectrum Disorder as a Brain-Gut-Microbiome Axis Disorder. Dig. Dis. Sci. 2020, 65, 818–828. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.A.; Szabo, K.; Teleky, B.E.; Vodnar, D.C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Szopinska-Tokov, J.; Dam, S.; Naaijen, J.; Konstanti, P.; Rommelse, N.; Belzer, C.; Buitelaar, J.; Franke, B.; Aarts, E.; Arias Vasquez, A. Investigating the Gut Microbiota Composition of Individuals with Attention-Deficit/Hyperactivity Disorder and Association with Symptoms. Microorganisms 2020, 8, 406, Erratum in Microorganisms 2021, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Xu, F.; Fu, Y.; Sun, T.Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.W.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef]
- Hillman, C.H.; McDonald, K.M.; Logan, N.E. A Review of the Effects of Physical Activity on Cognition and Brain Health across Children and Adolescence. Nestle Nutr. Inst. Workshop Ser. 2020, 95, 116–126. [Google Scholar] [CrossRef]
- Liu, W.; Mei, Q.; Yu, P.; Gao, Z.; Hu, Q.; Fekete, G.; István, B.; Gu, Y. Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review. Children 2022, 9, 406. [Google Scholar] [CrossRef]
- Zimmerman, E.; Carnaby, G.; Lazarus, C.L.; Malandraki, G.A. Motor Learning, Neuroplasticity, and Strength and Skill Training: Moving From Compensation to Retraining in Behavioral Management of Dysphagia. Am. J. Speech Lang. Pathol. 2020, 29, 1065–1077. [Google Scholar] [CrossRef]
- Hallemans, A.; Aerts, P. Effects of visual deprivation on intra-limb coordination during walking in children and adults. Exp. Brain Res. 2009, 198, 95–106. [Google Scholar] [CrossRef]
- Gimunová, M.; Sebera, M.; Bozděch, M.; Kolářová, K.; Vodička, T.; Zvonař, M. The Impact of Different Periods of Walking Experience on Kinematic Gait Parameters in Toddlers. Int. J. Environ. Res. Public Health 2021, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Adolph, K.E.; Franchak, J.M. The development of motor behavior. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, e1430. [Google Scholar] [CrossRef] [PubMed]
- Kornafel, T.; Paremski, A.C.; Prosser, L.A. Unweighting infants reveals hidden motor skills. Dev. Sci. 2022, 26, e13279. [Google Scholar] [CrossRef]
- Polleux, F.; Dehay, C.; Goffinet, A.; Kennedy, H. Pre- and post-mitotic events contribute to the progressive acquisition of area-specific connectional fate in the neocortex. Cereb. Cortex. 2001, 11, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Minakova, E.; Warner, B.B. Maternal immune activation, central nervous system development and behavioral phenotypes. Birth Defects Res. 2018, 110, 1539–1550. [Google Scholar] [CrossRef]
- Sefiani, A.; Geoffroy, C.G. The Potential Role of Inflammation in Modulating Endogenous Hippocampal Neurogenesis After Spinal Cord Injury. Front. Neurosci. 2021, 15, 682259. [Google Scholar] [CrossRef]
- Cao, M.; He, Y.; Dai, Z.; Liao, X.; Jeon, T.; Ouyang, M.; Chalak, L.; Bi, Y.; Rollins, N.; Dong, Q.; et al. Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb. Cortex. 2017, 27, 1949–1963. [Google Scholar] [CrossRef]
- Bisi, M.C.; Fabbri, M.; Cordelli, D.M.; Stagni, R. Gait performance in toddlers born preterm: A sensor based quantitative characterization. Comput. Methods Programs Biomed. 2022, 220, 106808. [Google Scholar] [CrossRef]
- Barth, A.L.; Ray, A. Progressive Circuit Changes during Learning and Disease. Neuron 2019, 104, 37–46. [Google Scholar] [CrossRef]
- Diaz Heijtz, R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin. Fetal Neonatal. Med. 2016, 21, 410–417. [Google Scholar] [CrossRef]
- Marrus, N.; Eggebrecht, A.T.; Todorov, A.; Elison, J.T.; Wolff, J.J.; Cole, L.; Gao, W.; Pandey, J.; Shen, M.D.; Swanson, M.R.; et al. Walking, Gross Motor Development, and Brain Functional Connectivity in Infants and Toddlers. Cereb. Cortex 2018, 28, 750–763. [Google Scholar] [CrossRef]
- van Polanen, V.; Davare, M. Interactions between dorsal and ventral streams for controlling skilled grasp. Neuropsychologia 2015, 79 Pt B, 186–191. [Google Scholar] [CrossRef]
- Chakraborty, A.; Anstice, N.S.; Jacobs, R.J.; Paudel, N.; LaGasse, L.L.; Lester, B.M.; McKinlay, C.J.D.; Harding, J.E.; Wouldes, T.A.; Thompson, B.; et al. Global motion perception is related to motor function in 4.5-year-old children born at risk of abnormal development. Vis. Res. 2017, 135, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ionta, S. Visual Neuropsychology in Development: Anatomo-Functional Brain Mechanisms of Action/Perception Binding in Health and Disease. Front. Hum. Neurosci. 2021, 15, 689912. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; McKinlay, C.J.D.; Chakraborty, A.; Anstice, N.S.; Jacobs, R.J.; Paudel, N.; Yu, T.Y.; Ansell, J.M.; Wouldes, T.A.; Harding, J.E.; et al. Global motion perception is associated with motor function in 2-year-old children. Neurosci. Lett. 2017, 658, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Delval, A.; Braquet, A.; Dirhoussi, N.; Bayot, M.; Derambure, P.; Defebvre, L.; Tard, C.; Dujardin, K. Motor Preparation of Step Initiation: Error-related Cortical Oscillations. Neuroscience 2018, 393, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cheron, G.; Bouillot, E.; Dan, B.; Bengoetxea, A.; Draye, J.P.; Lacquaniti, F. Development of a kinematic coordination pattern in toddler locomotion: Planar covariation. Exp. Brain Res. 2001, 137, 455–466. [Google Scholar] [CrossRef]
- Krajenbrink, H.; Lust, J.; Wilson, P.; Steenbergen, B. Development of motor planning in children: Disentangling elements of the planning process. J. Exp. Child Psychol. 2020, 199, 104945. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tejada, A.; Neisewander, J.; Katsanos, C.S. Regulation of Voluntary Physical Activity Behavior: A Review of Evidence Involving Dopaminergic Pathways in the Brain. Brain Sci. 2022, 12, 333. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Seppälä, K.; Putkinen, V. Molecular Imaging of the Human Emotion Circuit. In Social and Affective Neuroscience of Everyday Human Interaction; Boggio, P.S., Wingenbach, T.S.H., da Silveira Coêlho, M.L., Comfort, W.E., Murrins Marques, L., Alves, M.V.C., Eds.; Springer: Cham, Switzerland, 2023; pp. 3–21. [Google Scholar] [CrossRef]
- Korchounov, A.; Meyer, M.F.; Krasnianski, M. Postsynaptic nigrostriatal dopamine receptors and their role in movement regulation. J. Neural. Transm. 2010, 117, 1359–1369. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Williams, O.O.F.; Coppolino, M.; George, S.R.; Perreault, M.L. Sex Differences in Dopamine Receptors and Relevance to Neuropsychiatric Disorders. Brain Sci. 2021, 11, 1199. [Google Scholar] [CrossRef] [PubMed]
- Dib, T.; Martínez-Pinto, J.; Reyes-Parada, M.; Torres, G.E.; Sotomayor-Zárate, R. Neonatal programming with testosterone propionate reduces dopamine transporter expression in nucleus accumbens and methylphenidate-induced locomotor activity in adult female rats. Behav. Brain Res. 2018, 346, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Krock, R.M.; Shepard, S.; Moore, T. Dopamine Receptor Expression Among Local and Visual Cortex-Projecting Frontal Eye Field Neurons. Cereb. Cortex 2020, 30, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Andressoo, J.O.; Saarma, M. Signalling mechanisms underlying development and maintenance of dopamine neurons. Curr. Opin. Neurobiol. 2008, 18, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ge, W.R.; Zhang, S.; Sun, Y.L.; Wang, B.; Yang, G. Case-Control Study of the Effects of Gut Microbiota Composition on Neurotransmitter Metabolic Pathways in Children With Attention Deficit Hyperactivity Disorder. Front. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef]
- Shaw, W. Elevated Urinary Glyphosate and Clostridia Metabolites With Altered Dopamine Metabolism in Triplets With Autistic Spectrum Disorder or Suspected Seizure Disorder: A Case Study. Integr. Med. 2017, 16, 50–57. [Google Scholar]
- Vicini, J.L.; Jensen, P.K.; Young, B.M.; Swarthout, J.T. Residues of glyphosate in food and dietary exposure. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5226–5257. [Google Scholar] [CrossRef]
- Barnett, J.A.; Bandy, M.L.; Gibson, D.L. Is the Use of Glyphosate in Modern Agriculture Resulting in Increased Neuropsychiatric Conditions Through Modulation of the Gut-brain-microbiome Axis? Front. Nutr. 2022, 9, 827384. [Google Scholar] [CrossRef]
- Gallegos, C.E.; Bartos, M.; Bras, C.; Gumilar, F.; Antonelli, M.C.; Minetti, A. Exposure to a glyphosate-based herbicide during pregnancy and lactation induces neurobehavioral alterations in rat offspring. Neurotoxicology 2016, 53, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Roswall, J.; Olsson, L.M.; Kovatcheva-Datchary, P.; Nilsson, S.; Tremaroli, V.; Simon, M.C.; Kiilerich, P.; Akrami, R.; Krämer, M.; Uhlén, M.; et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe 2021, 29, 765–776.e3. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Chen, H.; Zhang, S.; Zhuang, J.; Li, Q.; Feng, Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019, 17, 13–25. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Schwab, C. The development of human gut microbiota fermentation capacity during the first year of life. Microb. Biotechnol. 2022, 15, 2865–2874. [Google Scholar] [CrossRef]
- Kononova, S.; Litvinova, E.; Vakhitov, T.; Skalinskaya, M.; Sitkin, S. Acceptive Immunity: The Role of Fucosylated Glycans in Human Host-Microbiome Interactions. Int. J. Mol. Sci. 2021, 22, 3854. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef]
- Laursen, M.F.; Pekmez, C.T.; Larsson, M.W.; Lind, M.V.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Bode, L.; Dragsted, L.O.; Michaelsen, K.F.; et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021, 1, 21. [Google Scholar] [CrossRef]
- Hourihane, J.; Murray, D.; Fuligni, F.; Gueimonde, M.; Margolles, A.; De Bellis, G.; O’Toole, P.W.; van Sinderen, D.; Marchesi, J.R.; Ventura, M. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Laue, H.E.; Coker, M.O.; Madan, J.C. The Developing Microbiome From Birth to 3 Years: The Gut-Brain Axis and Neurodevelopmental Outcomes. Front. Pediatr. 2022, 10, 815885. [Google Scholar] [CrossRef] [PubMed]
- Thion, M.S.; Low, D.; Silvin, A.; Chen, J.; Grisel, P.; Schulte-Schrepping, J.; Blecher, R.; Ulas, T.; Squarzoni, P.; Hoeffel, G.; et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 2018, 172, 500–516.e16. [Google Scholar] [CrossRef]
- Scott, G.A.; Terstege, D.J.; Vu, A.P.; Law, S.; Evans, A.; Epp, J.R. Disrupted Neurogenesis in Germ-Free Mice: Effects of Age and Sex. Front. Cell Dev. Biol. 2020, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Budavari, A.C.; Rodriguez, K.M.; Zisman, C.R.; Windheim, G.; Fallin, M.D. Sex Differences in the Gut-Brain Axis: Implications for Mental Health. Curr. Psychiatry Rep. 2020, 22, 83. [Google Scholar] [CrossRef]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocrinol. 2020, 56, 100815. [Google Scholar] [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Lüningschrör, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF Expression in Layer II/III and Layer V Neurons of the Motor Cortex Is Essential for Motor Learning. J. Neurosci. 2020, 40, 6289–6308. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, N.; Mohajeri, M.H. Involvement of Intestinal Microbiota in Adult Neurogenesis and the Expression of Brain-Derived Neurotrophic Factor. Int. J. Mol. Sci. 2022, 23, 15934. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Triantafyllou, A.R.; Tsaousi, M.; Pouliakis, A.; Petropoulou, C.; Sokou, R.; Volaki, P.; Boutsikou, T.; Iacovidou, N. Gut Microbiome and Neurodevelopmental Disorders: A Link Yet to Be Disclosed. Microorganisms 2023, 11, 487. [Google Scholar] [CrossRef]
- Browne, H.P.; Shao, Y.; Lawley, T.D. Mother-infant transmission of human microbiota. Curr. Opin. Microbiol. 2022, 69, 102173. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wan, W.; Zhu, C. Breastfeeding after a cesarean section: A literature review. Midwifery 2021, 103, 103117. [Google Scholar] [CrossRef]
- The Lancet. Stemming the global caesarean section epidemic. Lancet 2018, 392, 1279. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Aktar, R.; Parkar, N.; Stentz, R.; Baumard, L.; Parker, A.; Goldson, A.; Brion, A.; Carding, S.; Blackshaw, A.; Peiris, M. Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microbes 2020, 11, 1745–1757. [Google Scholar] [CrossRef]
- Moser, V.C.; Morris-Schaffer, K.; Richardson, J.R.; Li, A.A. Glyphosate and neurological outcomes: A systematic literature review of animal studies. J. Toxicol. Environ. Health B Crit. Rev. 2022, 25, 162–209. [Google Scholar] [CrossRef]
- Leino, L.; Tall, T.; Helander, M.; Saloniemi, I.; Saikkonen, K.; Ruuskanen, S.; Puigbò, P. Classification of the glyphosate target enzyme (5-enolpyruvylshikimate-3-phosphate synthase) for assessing sensitivity of organisms to the herbicide. J. Hazard. Mater. 2021, 408, 124556. [Google Scholar] [CrossRef]
- Hoban, A.E.; Moloney, R.D.; Golubeva, A.V.; McVey Neufeld, K.A.; O’Sullivan, O.; Patterson, E.; Stanton, C.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016, 339, 463–477, Erratum in Neuroscience 2017, 344, 418. [Google Scholar] [CrossRef]
- Russell, J.T.; Lauren Ruoss, J.; de la Cruz, D.; Li, N.; Bazacliu, C.; Patton, L.; McKinley, K.L.; Garrett, T.J.; Polin, R.A.; Triplett, E.W.; et al. Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci. Rep. 2021, 11, 1943. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Pärtty, A.; Kalliomäki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant Gut Microbiome Associated With Cognitive Development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Acuña, I.; Cerdó, T.; Ruiz, A.; Torres-Espínola, F.J.; López-Moreno, A.; Aguilera, M.; Suárez, A.; Campoy, C. Infant Gut Microbiota Associated with Fine Motor Skills. Nutrients 2021, 13, 1673. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Y.; Li, Q.; Xu, X.; Li, X.; Wang, C.; Cai, H.; Zhu, J.; Yu, Y. Metabolic and Neural Mechanisms Underlying the Associations Between Gut Bacteroides and Cognition: A Large-Scale Functional Network Connectivity Study. Front. Neurosci. 2021, 15, 750704. [Google Scholar] [CrossRef]
- Brown, E.M.; Ke, X.; Hitchcock, D.; Jeanfavre, S.; Avila-Pacheco, J.; Nakata, T.; Arthur, T.D.; Fornelos, N.; Heim, C.; Franzosa, E.A.; et al. Bacteroides-Derived Sphingolipids Are Critical for Maintaining Intestinal Homeostasis and Symbiosis. Cell Host Microbe 2019, 25, 668–680.e7. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Huang, Y.; Yin, A.; Zhang, L.; Han, J.; Lyu, Y.; Xu, X.; Zhai, Y.; Sun, H.; et al. Gut metagenomic characteristics of ADHD reveal low Bacteroides ovatus-associated host cognitive impairment. Gut Microbes 2022, 14, 2125747. [Google Scholar] [CrossRef] [PubMed]
- Shirvani-Rad, S.; Ejtahed, H.S.; Ettehad Marvasti, F.; Taghavi, M.; Sharifi, F.; Arzaghi, S.M.; Larijani, B. The Role of Gut Microbiota-Brain Axis in Pathophysiology of ADHD: A Systematic Review. J. Atten. Disord. 2022, 26, 1698–1710. [Google Scholar] [CrossRef]
- Wang, L.J.; Yang, C.Y.; Chou, W.J.; Lee, M.J.; Chou, M.C.; Kuo, H.C.; Yeh, Y.M.; Lee, S.Y.; Huang, L.H.; Li, S.C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Blanke, E.N.; Holmes, G.M.; Besecker, E.M. Altered physiology of gastrointestinal vagal afferents following neurotrauma. Neural. Regen. Res. 2021, 16, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.W.; Pandya, J.D.; Shear, D.A. Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Front. Neurol. 2019, 10, 875. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Hamamah, S.; Hajnal, A.; Covasa, M. Impact of Nutrition, Microbiota Transplant and Weight Loss Surgery on Dopaminergic Alterations in Parkinson’s Disease and Obesity. Int. J. Mol. Sci. 2022, 23, 7503. [Google Scholar] [CrossRef]
- Martínez-Guardado, I.; Arboleya, S.; Grijota, F.J.; Kaliszewska, A.; Gueimonde, M.; Arias, N. The Therapeutic Role of Exercise and Probiotics in Stressful Brain Conditions. Int. J. Mol. Sci. 2022, 23, 3610. [Google Scholar] [CrossRef] [PubMed]
- Evrensel, A.; Ünsalver, B.Ö.; Ceylan, M.E. Psychobiotics. Adv. Exp. Med. Biol. 2019, 1192, 565–581. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef] [PubMed]
- Abg Abd Wahab, D.Y.; Gau, C.H.; Zakaria, R.; Muthu Karuppan, M.K.; A-Rahbi, B.S.; Abdullah, Z.; Alrafiah, A.; Abdullah, J.M.; Muthuraju, S. Review on Cross Talk between Neurotransmitters and Neuroinflammation in Striatum and Cerebellum in the Mediation of Motor Behaviour. Biomed. Res. Int. 2019, 2019, 1767203. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Z.M.; Fan, X.Y.; Jin, Y.L.; Li, X.; Wu, S.R.; Ge, W.W.; Lv, C.H.; Wang, Y.K.; Chen, J.G. Gut-Brain-Skin Axis in Psoriasis: A Review. Dermatol. Ther. 2021, 11, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Northoff, G.; Hirjak, D.; Wolf, R.C.; Magioncalda, P.; Martino, M. All roads lead to the motor cortex: Psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol. Psychiatry 2021, 26, 92–102. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins—underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef]
- Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 12, 580001. [Google Scholar] [CrossRef]
- Grüter, T.; Mohamad, N.; Rilke, N.; Blusch, A.; Sgodzai, M.; Demir, S.; Pedreiturria, X.; Lemhoefer, K.; Gisevius, B.; Haghikia, A.; et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2023, 120, e2216941120. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Frøland, A.S.; Pettersen, M.N.; Storm-Mathisen, J.; Gundersen, V.; Rise, F.; Hassel, B. Propionate enters GABAergic neurons, inhibits GABA transaminase, causes GABA accumulation and lethargy in a model of propionic acidemia. Biochem. J. 2018, 475, 749–758. [Google Scholar] [CrossRef]
- Hao, C.; Gao, Z.; Liu, X.; Rong, Z.; Jia, J.; Kang, K.; Guo, W.; Li, J. Intravenous administration of sodium propionate induces antidepressant or prodepressant effect in a dose dependent manner. Sci. Rep. 2020, 10, 19917. [Google Scholar] [CrossRef] [PubMed]
- Spichak, S.; Donoso, F.; Moloney, G.M.; Gunnigle, E.; Brown, J.M.; Codagnone, M.; Dinan, T.G.; Cryan, J.F. Microbially-derived short-chain fatty acids impact astrocyte gene expression in a sex-specific manner. Brain Behav. Immun. Health 2021, 16, 100318. [Google Scholar] [CrossRef] [PubMed]
- Song, K.Y. Preliminary Data on the Ratio of D (−)-Lactate and L (+)-Lactate Levels in Various Lactic Acid Bacteria as Evaluated using an Enzymatic Method. J. Dairy Sci. Biotechnol. 2022, 40, 15–22. [Google Scholar] [CrossRef]
- Beller, L.; Deboutte, W.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Valles-Colomer, M.; Rymenans, L.; Jansen, D.; Van Espen, L.; Papadaki, M.I.; et al. Successional Stages in Infant Gut Microbiota Maturation. mBio 2021, 12, e0185721. [Google Scholar] [CrossRef] [PubMed]
- Haschke-Becher, E.; Baumgartner, M.; Bachmann, C. Assay of D-lactate in urine of infants and children with reference values taking into account data below detection limit. Clin. Chim. Acta. 2000, 298, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Salvador, A.C.; Barrington, W.T.; Gacasan, C.A.; D’Souza, E.M.; Deus Ramirez, L.; Threadgill, D.W.; Bennett, B.J. Gut microbiota and host genetics modulate the effect of diverse diet patterns on metabolic health. Front. Nutr. 2022, 9, 896348. [Google Scholar] [CrossRef]
- Cheng, M.; Ning, K. Stereotypes About Enterotype: The Old and New Ideas. Genom. Proteom. Bioinform. 2019, 17, 4–12. [Google Scholar] [CrossRef]
- Qin, Y.; Havulinna, A.S.; Liu, Y.; Jousilahti, P.; Ritchie, S.C.; Tokolyi, A.; Sanders, J.G.; Valsta, L.; Brożyńska, M.; Zhu, Q.; et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022, 54, 134–142. [Google Scholar] [CrossRef]
- Xu, J.; Lawley, B.; Wong, G.; Otal, A.; Chen, L.; Ying, T.J.; Lin, X.; Pang, W.W.; Yap, F.; Chong, Y.S.; et al. Ethnic diversity in infant gut microbiota is apparent before the introduction of complementary diets. Gut Microbes 2020, 11, 1362–1373. [Google Scholar] [CrossRef]
- Naneix, F.; Peters, K.Z.; Young, A.M.J.; McCutcheon, J.E. Age-dependent effects of protein restriction on dopamine release. Neuropsychopharmacology 2021, 46, 394–403. [Google Scholar] [CrossRef]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef]
- Bondi, C.O.; Taha, A.Y.; Tock, J.L.; Totah, N.K.; Cheon, Y.; Torres, G.E.; Rapoport, S.I.; Moghaddam, B. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol. Psychiatry 2014, 75, 38–46. [Google Scholar] [CrossRef]
- Cheng, C.C.; Duar, R.M.; Lin, X.; Perez-Munoz, M.E.; Tollenaar, S.; Oh, J.H.; van Pijkeren, J.P.; Li, F.; van Sinderen, D.; Gänzle, M.G.; et al. Ecological Importance of Cross-Feeding of the Intermediate Metabolite 1,2-Propanediol between Bacterial Gut Symbionts. Appl. Environ. Microbiol. 2020, 86, e00190-20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, T.L. Dietary advanced glycation end-products elicit toxicological effects by disrupting gut microbiome and immune homeostasis. J. Immunotoxicol. 2021, 18, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Veres, S.P. Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J. Appl. Physiol. 2019, 126, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, D.; Svensson, R.B.; Scheijen, J.; Eliasson, P.; Mogensen, P.; Hag, A.M.; Kjær, M.; Schalkwijk, C.G.; Schjerling, P.; Magnusson, S.P.; et al. An advanced glycation endproduct (AGE)-rich diet promotes accumulation of AGEs in Achilles tendon. Physiol. Rep. 2017, 5, e13215. [Google Scholar] [CrossRef]
- Grant, W.P.; Sullivan, R.; Sonenshine, D.E.; Adam, M.; Slusser, J.H.; Carson, K.A.; Vinik, A.I. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J. Foot Ankle Surg. 1997, 36, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Semin. Arthritis Rheum. 2019, 49, 474–484. [Google Scholar] [CrossRef]
- Cheng, M.; Zhao, Y.; Cui, Y.; Zhong, C.; Zha, Y.; Li, S.; Cao, G.; Li, M.; Zhang, L.; Ning, K.; et al. Stage-specific roles of microbial dysbiosis and metabolic disorders in rheumatoid arthritis. Ann. Rheum. Dis. 2022, 81, 1669–1677. [Google Scholar] [CrossRef]
- Lim, J.G.; Lee, J.J.; Park, S.H.; Park, J.H.; Kim, S.J.; Cho, H.C.; Baek, W.K.; Kim, D.K.; Song, D.K. Glucagon-like peptide-1 protects NSC-34 motor neurons against glucosamine through Epac-mediated glucose uptake enhancement. Neurosci. Lett. 2010, 479, 13–17. [Google Scholar] [CrossRef]
- Dietrich, F.; Hammerman, M.; Blomgran, P.; Tätting, L.; Bampi, V.F.; Silva, J.B.; Aspenberg, P. Effect of platelet-rich plasma on rat Achilles tendon healing is related to microbiota. Acta Orthop. 2017, 88, 416–421, Erratum in Acta Orthop. 2017, 88, 463. [Google Scholar] [CrossRef]
- Dietrich-Zagonel, F.; Hammerman, M.; Eliasson, P.; Aspenberg, P. Response to mechanical loading in rat Achilles tendon healing is influenced by the microbiome. PLoS ONE 2020, 15, e0229908. [Google Scholar] [CrossRef]
- Sobel, E.; Caselli, M.A.; Velez, Z. Effect of persistent toe walking on ankle equinus. Analysis of 60 idiopathic toe walkers. J. Am. Podiatr. Med. Assoc. 1997, 87, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef] [PubMed]
- Harkness-Armstrong, C.; Maganaris, C.; Walton, R.; Wright, D.M.; Bass, A.; Baltzopoulos, V.; O’Brien, T.D. Muscle architecture and passive lengthening properties of the gastrocnemius medialis and Achilles tendon in children who idiopathically toe-walk. J. Anat. 2021, 239, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Caserta, A.; Reedman, S.; Morgan, P.; Williams, C.M. Physical activity and quality of life in children with idiopathic toe walking: A cross sectional study. BMC Pediatr. 2022, 22, 544. [Google Scholar] [CrossRef]
- Knopf, A. Idiopathic toe walking can be reduced by more physical activity and less screen time. Brown Univ. Child Adolesc. Psychopharm. Update 2022, 24, 6. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef]
- Welly, R.J.; Liu, T.W.; Zidon, T.M.; Rowles, J.L., 3rd; Park, Y.M.; Smith, T.N.; Swanson, K.S.; Padilla, J.; Vieira-Potter, V.J. Comparison of Diet versus Exercise on Metabolic Function and Gut Microbiota in Obese Rats. Med. Sci. Sports Exerc. 2016, 48, 1688–1698. [Google Scholar] [CrossRef]
- Sinclair, D.; Oranje, B.; Razak, K.A.; Siegel, S.J.; Schmid, S. Sensory processing in autism spectrum disorders and Fragile X syndrome-From the clinic to animal models. Neurosci. Biobehav. Rev. 2017, 76 Pt B, 235–253. [Google Scholar] [CrossRef]
- Valagussa, G.; Purpura, G.; Nale, A.; Pirovano, R.; Mazzucchelli, M.; Grossi, E.; Perin, C. Sensory Profile of Children and Adolescents with Autism Spectrum Disorder and Tip-Toe Behavior: Results of an Observational Pilot Study. Children 2022, 9, 1336. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Radar, A.M.; Baig, A.T.; Leyba, M.F.; Costabel, M.M.; Zavala-Crichton, J.P.; Sanchez-Martinez, J.; MacKenzie, A.E.; Solis-Urra, P. Physical Activity, Gut Microbiota, and Genetic Background for Children and Adolescents with Autism Spectrum Disorder. Children 2022, 9, 1834. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononova, S.; Kashparov, M.; Xue, W.; Bobkova, N.; Leonov, S.; Zagorodny, N. Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review. Int. J. Mol. Sci. 2023, 24, 13204. https://doi.org/10.3390/ijms241713204
Kononova S, Kashparov M, Xue W, Bobkova N, Leonov S, Zagorodny N. Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review. International Journal of Molecular Sciences. 2023; 24(17):13204. https://doi.org/10.3390/ijms241713204
Chicago/Turabian StyleKononova, Svetlana, Mikhail Kashparov, Wenyu Xue, Natalia Bobkova, Sergey Leonov, and Nikolaj Zagorodny. 2023. "Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review" International Journal of Molecular Sciences 24, no. 17: 13204. https://doi.org/10.3390/ijms241713204
APA StyleKononova, S., Kashparov, M., Xue, W., Bobkova, N., Leonov, S., & Zagorodny, N. (2023). Gut Microbiome Dysbiosis as a Potential Risk Factor for Idiopathic Toe-Walking in Children: A Review. International Journal of Molecular Sciences, 24(17), 13204. https://doi.org/10.3390/ijms241713204




