Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis gigantea to Heat, Cold, and Osmotic Shocks
Abstract
1. Introduction
2. Results
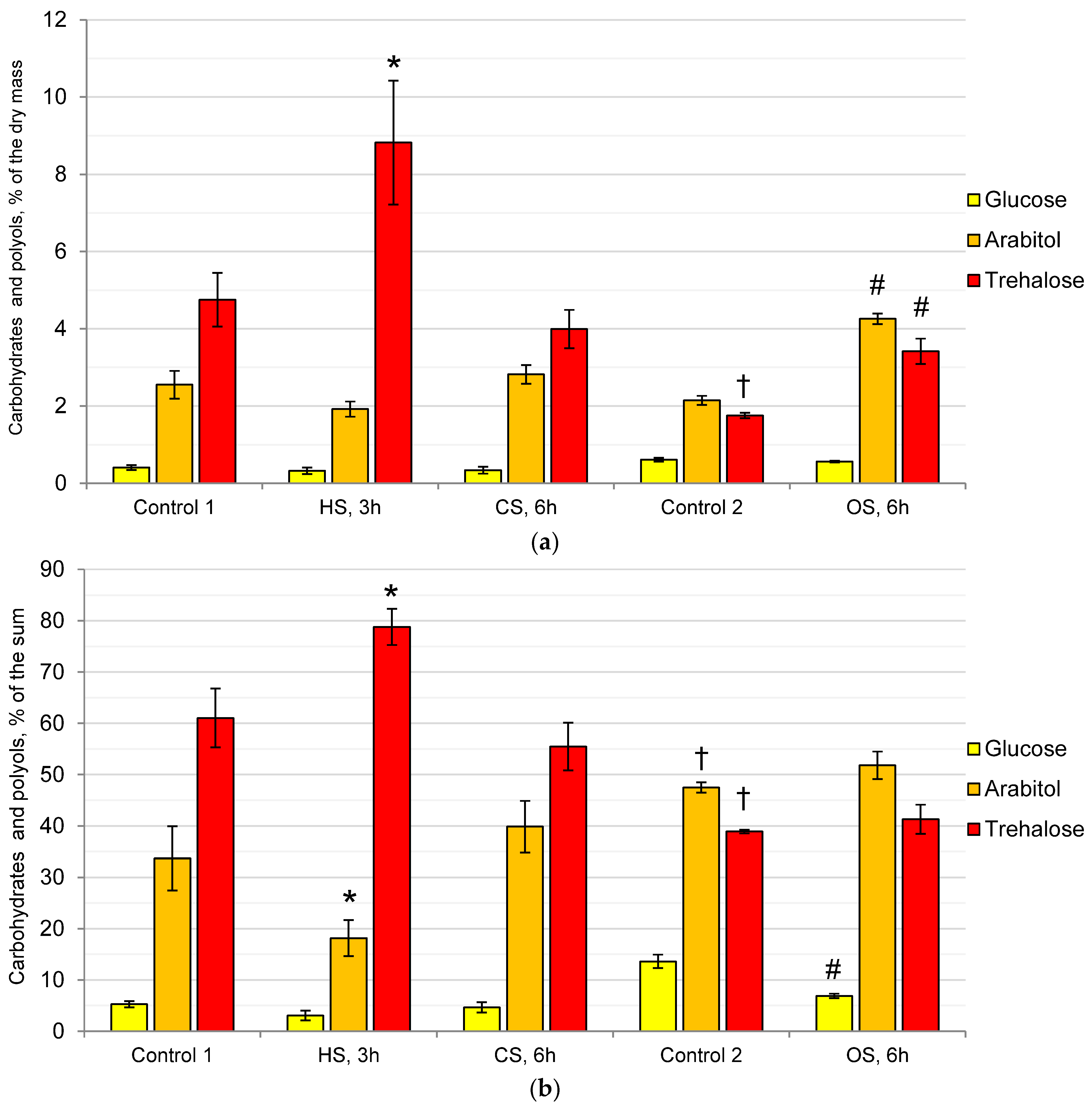
2.1. Composition of the Cytosol Carbohydrates and Polyols in the Mycelium of the Fungus under the Action of Heat, Cold, and Osmotic Shocks
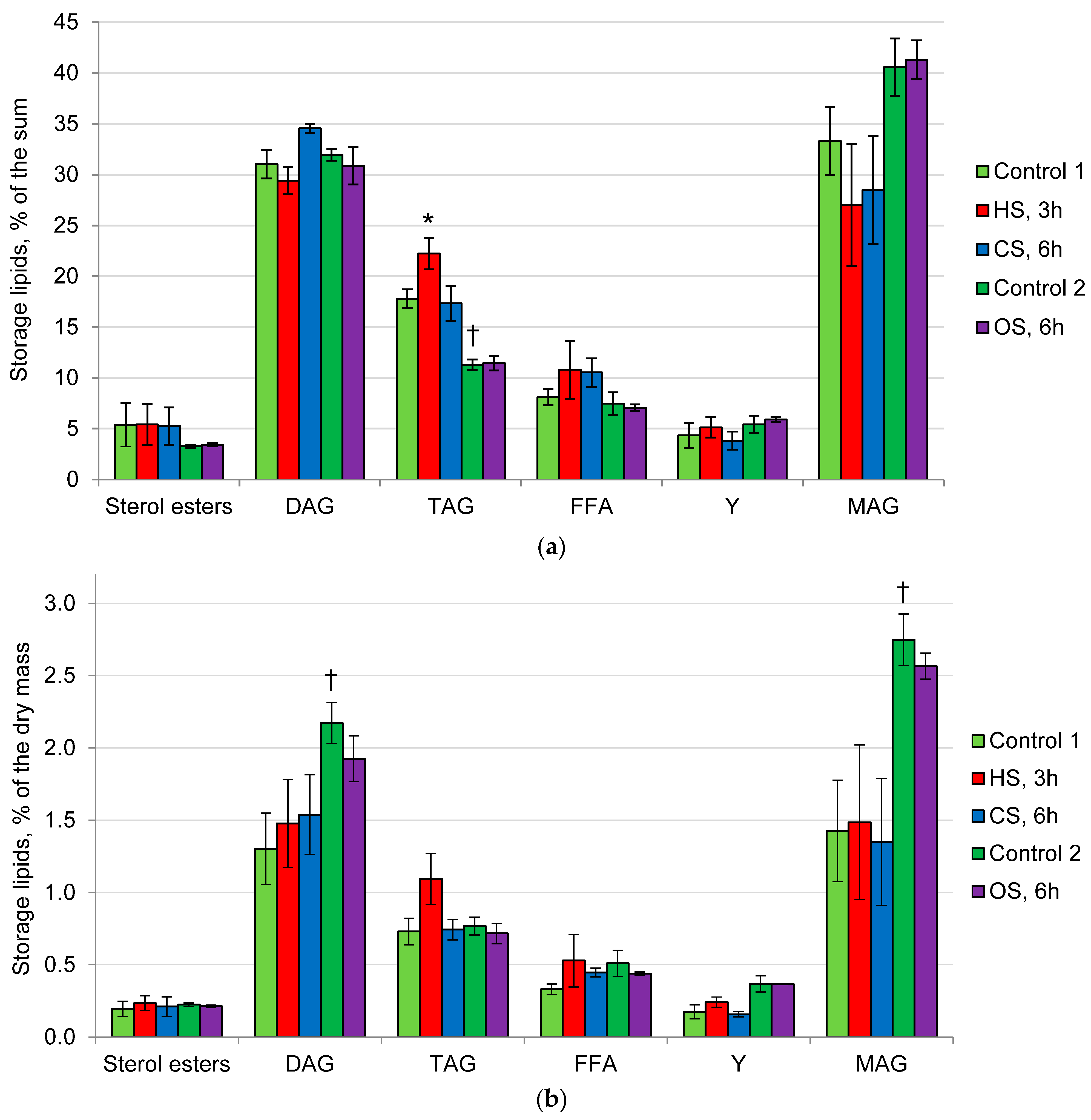
2.2. Composition of Membrane and Storage Lipids in the Mycelium of the Fungus under the Action of Heat, Cold, and Osmotic Shocks
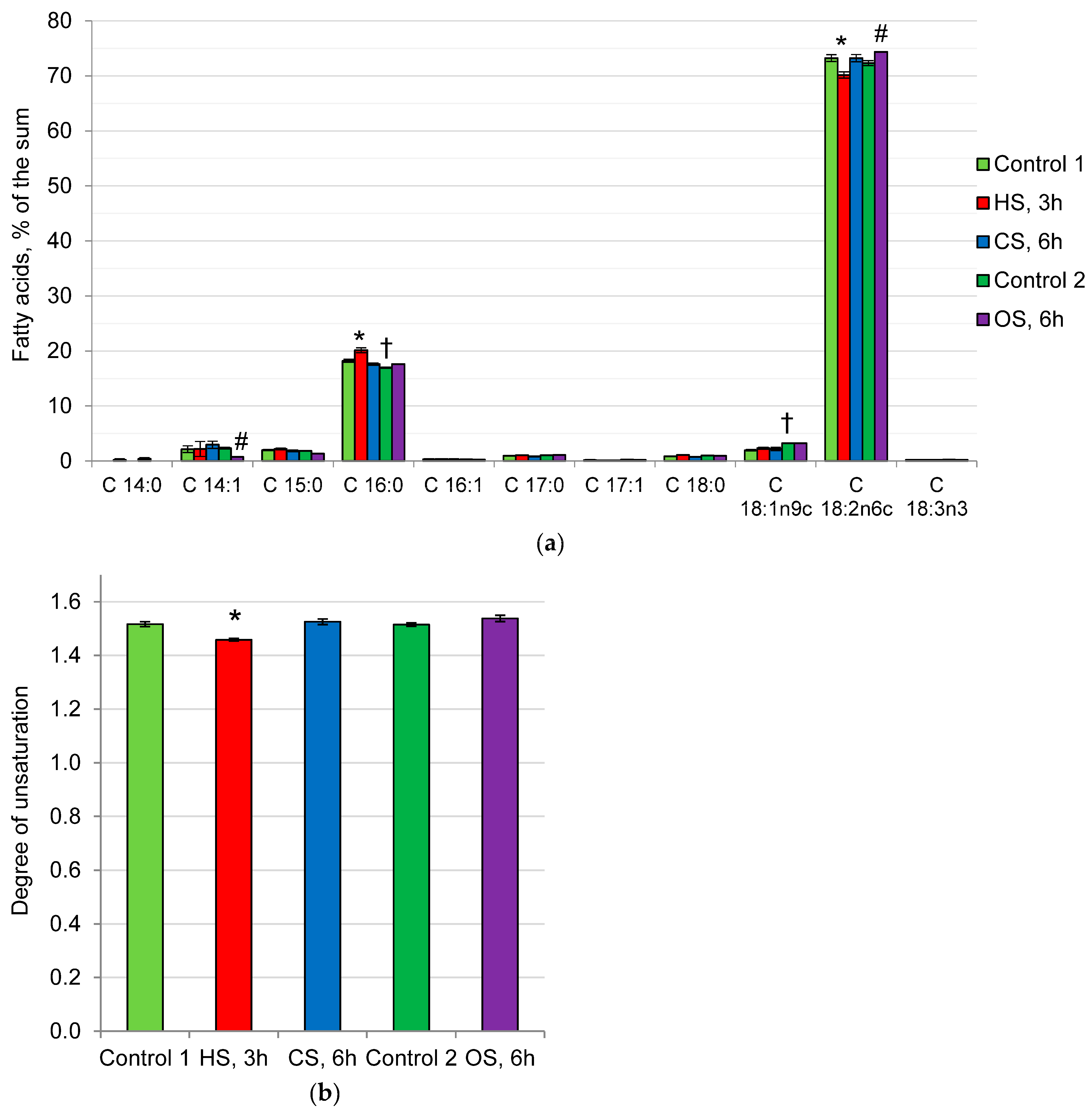
2.3. Fatty Acid Composition of Phospholipids in the Mycelium of the Fungus under Heat, Cold, and Osmotic Shocks
3. Discussion
4. Materials and Methods
4.1. Objects of Study and Cultivation Protocol
4.2. Lipids, Sugars and Polyols Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coleine, C.; Stajich, J.E.; Selbmann, L. Fungi Are Key Players in Extreme Ecosystems. Trends Ecol. Evol. 2022, 37, 517–528. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Mancinelli, R.L.; Conley, C.A.; Dallas, T.D.; Rinaldi, T.; Davila, A.F.; Benison, K.C.; Rapoport, A.; Cavalazzi, B.; Selbmann, L.; et al. Astrobiology of Life on Earth. Environ. Microbiol. 2021, 23, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Tiquia-Arashiro, S.M.; Grube, M. Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S.M., Grube, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-19029-3. [Google Scholar]
- Gao, H.; Wang, Y.; Luo, Q.; Yang, L.; He, X.; Wu, J.; Kachanuban, K.; Wilaipun, P.; Zhu, W.; Wang, Y. Bioactive Metabolites from Acid-Tolerant Fungi in a Thai Mangrove Sediment. Front. Microbiol. 2021, 11, 609952. [Google Scholar] [CrossRef] [PubMed]
- Hujslová, M.; Bystrianský, L.; Benada, O.; Gryndler, M. Fungi, a Neglected Component of Acidophilic Biofilms: Do They Have a Potential for Biotechnology? Extremophiles 2019, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Robbins, E.I. Acidophilic and Acid-Tolerant Fungi and Yeasts. Hydrobiologia 2000, 433, 91–109. [Google Scholar] [CrossRef]
- Baker, B.J.; Tyson, G.W.; Goosherst, L.; Banfield, J.F. Insights into the Diversity of Eukaryotes in Acid Mine Drainage Biofilm Communities. Appl. Environ. Microbiol. 2009, 75, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; González-Toril, E. Eukaryotic Life in Extreme Environments: Acidophilic Fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–38. [Google Scholar]
- Amaral-Zettler, L.A. Eukaryotic Diversity at pH Extremes. Front. Microbiol. 2012, 3, 441. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Aguilera, A. Extremophiles and Acidic Environments. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019; pp. 206–227. ISBN 9780128117378. [Google Scholar]
- Segal-Kischinevzky, C.; Romero-Aguilar, L.; Alcaraz, L.D.; López-Ortiz, G.; Martínez-Castillo, B.; Torres-Ramírez, N.; Sandoval, G.; González, J. Yeasts Inhabiting Extreme Environments and Their Biotechnological Applications. Microorganisms 2022, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
- Coker, J.A. Recent Advances in Understanding Extremophiles. F1000Research 2019, 8, 1917. [Google Scholar] [CrossRef]
- Kane, P.M. Proton Transport and pH Control in Fungi. In Yeast Membrane Transport; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 892, pp. 33–68. ISBN 978-3-319-25302-2. [Google Scholar]
- Ou, S.; Liang, J.-L.; Jiang, X.; Liao, B.; Jia, P.; Shu, W.; Li, J. Physiological, Genomic and Transcriptomic Analyses Reveal the Adaptation Mechanisms of Acidiella Bohemica to Extreme Acid Mine Drainage Environments. Front. Microbiol. 2021, 12, 705839. [Google Scholar] [CrossRef]
- Michaux, C.; Pouyez, J.; Mayard, A.; Vandurm, P.; Housen, I.; Wouters, J. Structural Insights into the Acidophilic pH Adaptation of a Novel Endo-1,4-β-Xylanase from Scytalidium acidophilum. Biochimie 2010, 92, 1407–1415. [Google Scholar] [CrossRef]
- Sánchez-Arreguin, J.A.; Ruiz-Herrera, J.; Mares-Rodriguez, F.d.J.; León-Ramírez, C.G.; Sánchez-Segura, L.; Zapata-Morín, P.A.; Coronado-Gallegos, J.; Aréchiga-Carvajal, E.T. Acid pH Strategy Adaptation through NRG1 in Ustilago Maydis. J. Fungi 2021, 7, 91. [Google Scholar] [CrossRef]
- Piper, P.W. Molecular Events Associated with Acquisition of Heat Tolerance by the Yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 1993, 11, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Vigh, L.; Escribá, P.V.; Sonnleitner, A.; Sonnleitner, M.; Piotto, S.; Maresca, B.; Horváth, I.; Harwood, J.L. The Significance of Lipid Composition for Membrane Activity: New Concepts and Ways of Assessing Function. Prog. Lipid Res. 2005, 44, 303–344. [Google Scholar] [CrossRef]
- Péter, M.; Gudmann, P.; Kóta, Z.; Török, Z.; Vígh, L.; Glatz, A.; Balogh, G. Lipids and Trehalose Actively Cooperate in Heat Stress Management of Schizosaccharomyces pombe. Int. J. Mol. Sci. 2021, 22, 13272. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Organic Osmolytes as Compatible, Metabolic and Counteracting Cytoprotectants in High Osmolarity and Other Stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Siebenaller, J.F. Co-Evolution of Proteins and Solutions: Protein Adaptation versus Cytoprotective Micromolecules and Their Roles in Marine Organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef]
- Brown, A.D.; Simpson, J.R. Water Relations of Sugar-Tolerant Yeasts: The Role of Intracellular Polyols. J. Gen. Microbiol. 1972, 72, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimailo, O.A.; Groza, N.V.; Tereshina, V.M. Adaptation of The Acidophilic Fungus Sistotrema Brinkmannii to The pH Factor. Microbiology 2023, 92, 370–378. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, O.A.; Tereshina, V.M. The Role of Osmolytes and Membrane Lipids in the Adaptation of Acidophilic Fungi. Microorganisms 2023, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.H. Polyol Metabolism in Fungi. In Advances in Microbial Physiology; Rose, A.H., Tempest, D.W., Eds.; Academic Press: London, UK, 1985; Volume 25, pp. 149–193. ISBN 0-12027725-4. [Google Scholar]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New Insights on Trehalose: A Multifunctional Molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose Metabolism: From Osmoprotection to Signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Tapia, H.; Koshland, D.E. Trehalose Is a Versatile and Long-Lived Chaperone for Desiccation Tolerance. Curr. Biol. 2014, 24, 2758–2766. [Google Scholar] [CrossRef]
- Mattoon, E.R.; Casadevall, A.; Cordero, R.J. Beat the Heat: Correlates, Compounds, and Mechanisms Involved in Fungal Thermotolerance. Fungal Biol. Rev. 2021, 36, 60–75. [Google Scholar] [CrossRef]
- Cabrera, E.; Welch, L.C.; Robinson, M.R.; Sturgeon, C.M.; Crow, M.M.; Segarra, V.A. Cryopreservation and the Freeze–Thaw Stress Response in Yeast. Genes 2020, 11, 835. [Google Scholar] [CrossRef]
- Koide, R.T.; Shumway, D.L.; Stevens, C.M. Soluble Carbohydrates of Red Pine (Pinus resinosa) Mycorrhizas and Mycorrhizal Fungi. Mycol. Res. 2000, 104, 834–840. [Google Scholar] [CrossRef]
- Costenoble, R.; Valadi, H.; Gustafsson, L.; Niklasson, C.; Johan Franzen, C. Microaerobic Glycerol Formation in Saccharomyces cerevisiae. Yeast 2000, 16, 1483–1495. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, S.; Wu, J.; Sun, X.; Ma, A. Heat Stress in Macrofungi: Effects and Response Mechanisms. Appl. Microbiol. Biotechnol. 2021, 105, 7567–7576. [Google Scholar] [CrossRef]
- Święciło, A. Cross-Stress Resistance in Saccharomyces Cerevisiae Yeast—New Insight into an Old Phenomenon. Cell Stress. Chaperones 2016, 21, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Gasch, A.P. The Environmental Stress Response: A Common Yeast Response to Diverse Environmental Stresses. Top. Curr. Genet. 2002, 1, 11–70. [Google Scholar] [CrossRef]
- Brown, A.J.P.; Larcombe, D.E.; Pradhan, A. Thoughts on the Evolution of Core Environmental Responses in Yeasts. Fungal Biol. 2020, 124, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Harish, E.; Osherov, N. Fungal Priming: Prepare or Perish. J. Fungi 2022, 8, 448. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Groza, N.V.; Tereshina, V.M. Membrane Lipids and Cytosol Carbohydrates in Aspergillus niger under Osmotic, Oxidative, and Cold Impact. Microbiology 2016, 85, 302–310. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Bondarenko, S.A.; Tereshina, V.M. Membrane Lipid and Osmolyte Readjustment in the Alkaliphilic Micromycete Sodiomyces tronii under Cold, Heat and Osmotic Shocks. Microbiology 2021, 167, 001112. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Dopson, M. Life in Acid: pH Homeostasis in Acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef]
- Inouye, M.; Phadtare, S. Cold-Shock Response and Adaptation to Near-Freezing Temperature in Cold-Adapted Yeasts. In Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 243–257. ISBN 978-3-642-39680-9. [Google Scholar]
- Fedoseeva, E.V.; Danilova, O.A.; Ianutsevich, E.A.; Terekhova, V.A.; Tereshina, V.M. Micromycete Lipids and Stress. Microbiology 2021, 90, 37–55. [Google Scholar] [CrossRef]
- Weete, J.D. Introduction to Fungal Lipids. In Fungal Lipid Biochemistry; Kritchevsky, D., Ed.; Springer US: Boston, MA, USA, 1974; Volume 1, pp. 3–36. ISBN 9781468428315. [Google Scholar]
- Ianutsevich, E.A.; Danilova, O.A.; Groza, N.V.; Kotlova, E.R.; Tereshina, V.M. Heat Shock Response of Thermophilic Fungi: Membrane Lipids and Soluble Carbohydrates under Elevated Temperatures. Microbiology 2016, 162, 989–999. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, A.A.; Kotlova, E.R.; Kamzolkina, O.V.; Bilanenko, E.N.; Tereshina, V.M. Membrane Lipids and Soluble Sugars Dynamics of the Alkaliphilic Fungus Sodiomyces tronii in Response to Ambient pH. Extremophiles 2017, 21, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Danilova, O.A.; Ianutsevich, E.A.; Bondarenko, S.A.; Antropova, A.B.; Tereshina, V.M. Membrane Lipids and Osmolytes Composition of Xerohalophilic Fungus Aspergillus penicillioides during Growth on High NaCl and Glycerol Media. Microbiology 2022, 91, 503–513. [Google Scholar] [CrossRef]
- Zhou, H.; Huo, Y.; Yang, N.; Wei, T. Phosphatidic Acid: From Biophysical Properties to Diverse Functions. FEBS J. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic Acid in Membrane Rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Grum-Grzhimaylo, O.A.; Debets, A.J.M.; Bilanenko, E.N. The Diversity of Microfungi in Peatlands Originated from the White Sea. Mycologia 2016, 108, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.W. Separation of the Lipids of Photosynthetic Tissues: Improvements in Analysis by Thin-Layer Chromatography. Biochim. Biophys. Acta 1963, 70, 417–422. [Google Scholar] [CrossRef]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a Novel Glycolipid and a Betaine Lipid in Cells of Rhodobacter sphaeroides Grown under Phosphate Limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef]
- Somogyi, M. Determination of Blood Sugar. J. Biol. Chem. 1945, 160, 69–73. [Google Scholar] [CrossRef]
- Brobst, K.M. Gas–Liquid Chromatography of Trimethylsilyl Derivatives: Analysis of Corn Syrup. In General Carbohydrate Method; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: New York, NY, USA; London, UK, 1972; pp. 3–8. ISBN 9780127462066. [Google Scholar]

| Control 1 | HS, 3 h | CS, 6 h | Control 2 | OS, 6 h | |
|---|---|---|---|---|---|
| Membrane lipids, % of the dry mass | 4.23 ± 0.18 | 3.66 ± 0.38 | 3.95 ± 0.29 | 6.08 ± 0.42 | 6.01 ± 0.44 |
| Storage lipids, % of the dry mass | 4.16 ± 0.69 | 5.06 ± 1.06 | 4.45 ± 0.79 | 6.79 ± 0.32 (†) | 6.23 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, O.A.; Tereshina, V.M. Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis gigantea to Heat, Cold, and Osmotic Shocks. Int. J. Mol. Sci. 2024, 25, 3380. https://doi.org/10.3390/ijms25063380
Ianutsevich EA, Danilova OA, Grum-Grzhimaylo OA, Tereshina VM. Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis gigantea to Heat, Cold, and Osmotic Shocks. International Journal of Molecular Sciences. 2024; 25(6):3380. https://doi.org/10.3390/ijms25063380
Chicago/Turabian StyleIanutsevich, Elena A., Olga A. Danilova, Olga A. Grum-Grzhimaylo, and Vera M. Tereshina. 2024. "Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis gigantea to Heat, Cold, and Osmotic Shocks" International Journal of Molecular Sciences 25, no. 6: 3380. https://doi.org/10.3390/ijms25063380
APA StyleIanutsevich, E. A., Danilova, O. A., Grum-Grzhimaylo, O. A., & Tereshina, V. M. (2024). Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis gigantea to Heat, Cold, and Osmotic Shocks. International Journal of Molecular Sciences, 25(6), 3380. https://doi.org/10.3390/ijms25063380






