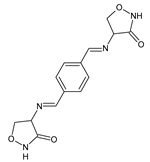
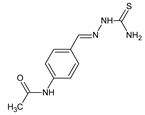
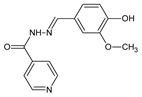
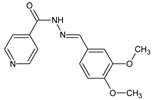
2. Fluorinated Aldimine-Type Schiff Bases
Raache et al. [
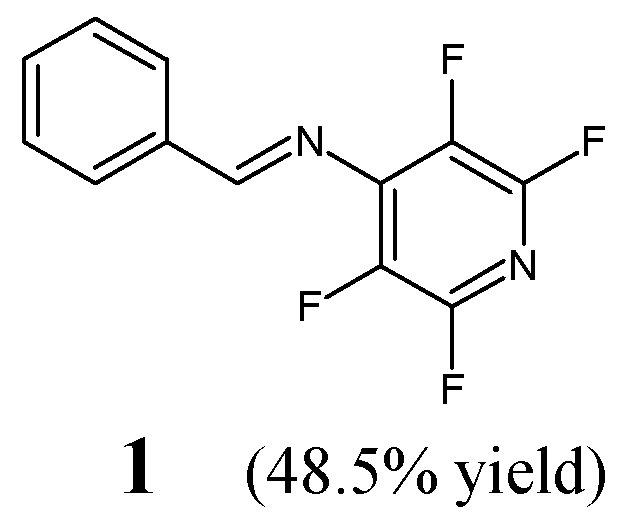
15] have reported the synthesis, structural investigations and preliminary antibacterial activity studies of (
E)-1-phenyl-
N-(2,3,5,6-tetrafluoropyridin-4-yl)methanimine (
1) (
Figure 1). The synthesis of this aldimine was achieved successfully by reacting equimolar ratios of 4-amino-2,3,5,6-tetrafluoropyridine and benzaldehyde in tetrahydrofuran containing an ethanolic solution of potassium hydroxide for 72 h at ambient temperature.
The antibacterial activity of aldimine
1 has been evaluated in the disc diffusion assay using both Gram-positive (
S. aureus ATCC 6538,
E. faecium ATCC 19434,
S. agalactiae) and Gram-negative (
E. coli ATCC 8739,
S. typhimurium ATCC 14028) bacterial strains. Ampicillin—a broad-spectrum aminopenicillin—was used as a standard antibiotic. Tetrafluorinated aldimine
1 at the highest concentration tested (983.5 µM) was shown to reveal moderate activity against both Gram-negative bacteria of clinical interest, considering its zone inhibition sizes in relation to that of ampicillin [
15].
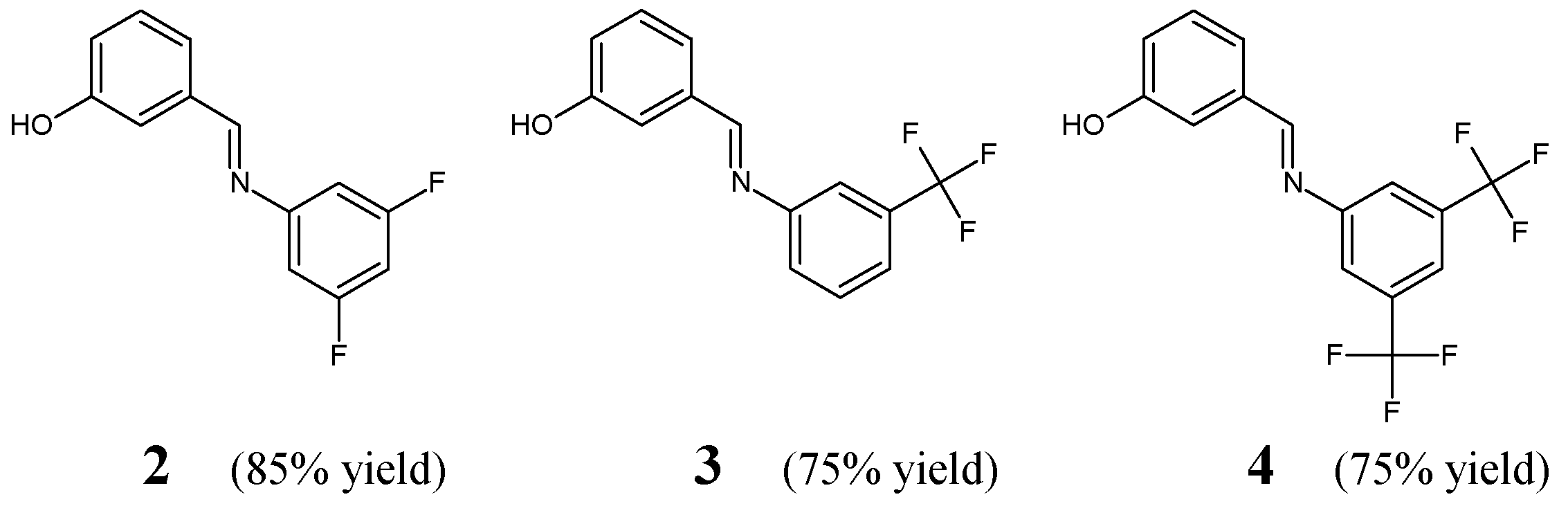
Avila-Sorrosa et al. [
16] have described a straightforward synthesis route, determination of the structure (including that in the solid state) and preliminary antibacterial evaluation of fluorinated aldimine-type Schiff bases
2–
4 (
Figure 2). The synthesis of these aldimines was performed by reacting almost equimolar amounts of 3,5-difluoroaniline, 3-(trifluoromethyl)aniline or 3,5-
bis(trifluoromethyl)aniline with 3-hydroxybenzaldehyde. The condensation process was carried out in dichloromethane at ambient temperature for 48 h, and the removal of water from an intermediate hemiaminal was facilitated by the use of activated molecular sieves.
Avila-Sorrosa research group has employed
S. aureus ATCC 25922 and
B. subtilis ATCC 9372 as Gram-positive bacilli, whereas
E. coli ATCC 25923 and
K. pneumoniae ATCC 700603 as Gram-negative bacilli of clinical interest to assess the antibacterial activity of fluorinated aldimines
2–
4 in the disc diffusion assay. Ampicillin was used as a standard antibiotic to confirm the susceptibility of all pathogenic strains of bacteria. Results of this antimicrobial test revealed that both Gram-positive and Gram-negative bacterial strains are susceptible to all the aldimines fluorinated in the
meta positions/position (
2–
4), whose activities are comparable to that of ampicillin. The conducted studies gave convincing proof that the substitution at
meta positions/position of the phenyl moiety by two fluorine atoms, two trifluoromethyl groups or one trifluoromethyl group is preferred for the antibacterial activity in this class of small molecules [
16].
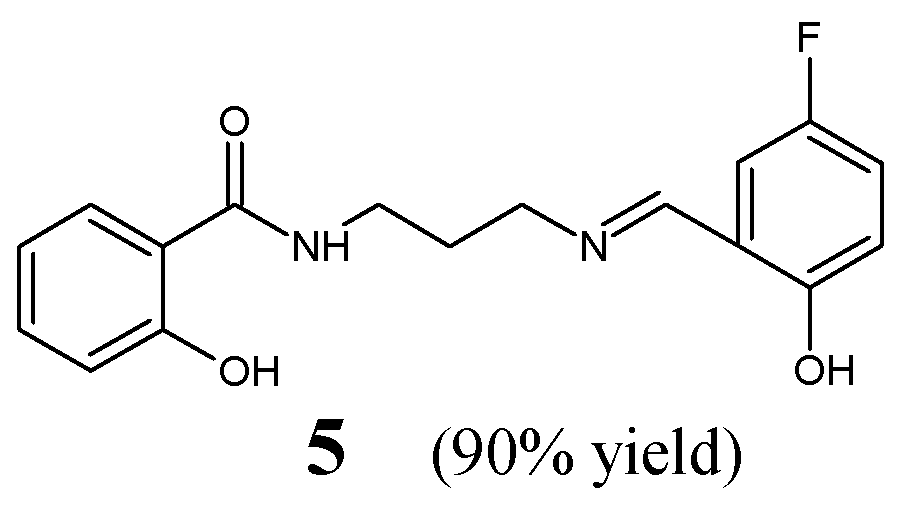
Cheng et al. [
17] have reported the synthesis and results of in vitro antibacterial studies for fluorinated aldimine-type Schiff base
5, i.e.,
N-{3-[(
E)-(5-fluoro-2-hydroxybenzylidene)amino]propyl}-2-hydroxybenzamide (
Figure 3). The synthesis of this aldimine was carried out by condensing
N-(3-aminopropyl)-2-hydroxybenzamide and 5-fluorosalicylaldehyde in methanol—as the reaction medium—at 50 °C for 3 h.
The authors have employed clinical isolates of two Gram-positive (
S. aureus ATCC 6538,
B. subtilis ATCC 6633) and two Gram-negative (
P. aeruginosa ATCC 13525,
E. coli ATCC 35218) bacterial strains to determine MIC values of Schiff base
5 in the assay MTT-based. An aminoglycoside antibiotic—kanamycin B—was used as a positive control, to confirm the susceptibility of all bacterial strains recruited. Fluorinated aldimine
5 was found to be antibacterially active, revealing significant potencies against
P. aeruginosa,
S. aureus,
E. coli and moderate activity against
B. subtilis (
Table 2). Simultaneously, its MIC value against
P. aeruginosa proved to be 1.3-fold lower than that of kanamycin B. In addition, this molecule was disclosed as a highly potent inhibitor (having the half-maximal inhibition constant (IC
50) of 5.6 µM) of the ecKAS III [
17]. This suggests that the enzymatic inhibition mechanism is responsible for its potent antibacterial activity.
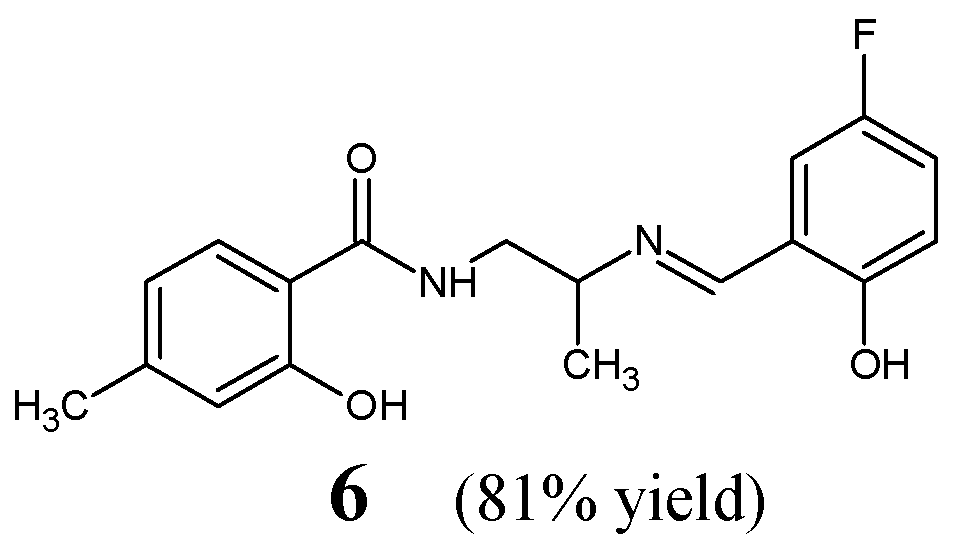
Employing a similar concept, Cheng et al. [
18] have synthesized, confirmed the structure and then antibacterially studied fluorinated Schiff base
6, i.e.,
N-{2-[(
E)-(5-fluoro-2-hydroxybenzylidene)amino]propyl}-2-hydroxy-4-methylbenzamide (
Figure 4). This aldimine was afforded as the final product of the reaction between
N-(2-aminopropyl)-2-hydroxy-4-methylbenzamide and 5-fluorosalicylaldehyde. The synthesis of this molecule was performed successfully by boiling the stoichiometric ratios of the functionalized reactants in methanol at 50 °C for 3 h.
Cheng et al. have screened fluorinated Schiff base
6 against two Gram-positive (
S. aureus ATCC 6538,
B. subtilis ATCC 6633) and two Gram-negative (
P. aeruginosa ATCC 13525,
E. coli ATCC 35218) bacteria of clinical interest in the assay MTT-based. As a positive control, kanamycin B was used. Fluorinated aldimine
6 has been shown to possess remarkable activities against
B. subtilis,
E. coli,
S. aureus and moderate activity against
P. aeruginosa (
Table 2). Furthermore, this compound was reported to reveal the IC
50 of 17.1 µM when tested in the target enzymatic assay for its inhibitory activity against the ecKAS III [
18].
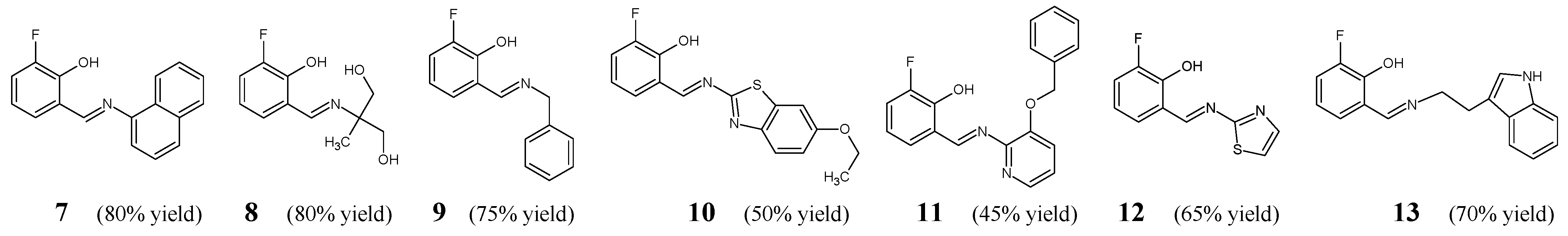
Shanmugam et al. [
19] have designed, synthesized and confirmed the structure (with the use of spectroscopic techniques) of a number of aldimine-type Schiff bases (
7–
13) (
Figure 5). The synthesis of these fluorinated aldimines was carried out by stirring (at an ambient temperature for 1 h) and then refluxing (at 50 °C for 4–6 h) the stoichiometric ratios of primary (aromatic, aliphatic, aromatic-aliphatic, heterocyclic or heterocyclic-aliphatic) amines with
meta-fluorosalicylaldehyde, in methanol, under assistance of an efficient catalyst (i.e., 1-butyl-3-methylimidazolium
bis(trifluoromethylsulfonyl)imide and potassium hydroxide).
All the obtained aldimine-type Schiff bases (
7–
13) have been subjected to the assay based on two-fold serial dilutions for estimating their MIC values on Gram-positive (
S. aureus ATCC 25930,
B. subtilis ATCC 530) as well as Gram-negative (
K. pneumoniae ATCC 700603,
S. typhi ATCC 25021,
P. aeruginosa ATCC 27853,
E. coli ATCC 26032) bacteria of clinical interest. As a positive control, an aminoglycoside antibiotic—streptomycin—was employed to confirm the susceptibility of all bacterial strains recruited. The vast majority of fluorinated aldimines have been reported to reveal moderate antibacterial activities in these studies (
Table 3). Schiff base
10 (containing the benzo[
d]thiazol-2-yl moiety with an electron-donating ethoxy group) was disclosed to be more potent against most of the selected bacteria than remaining fluorinated molecules, revealing strong antibacterial effects against
B. subtilis,
K. pneumoniae,
P. aeruginosa,
S. typhi and
S. aureus (MIC values superior to that of streptomycin). Simultaneously, aldimine
13 (bearing the 1
H-indol-3-ylethyl formation) proved to be 1.9-fold more active against
S. aureus than a standard drug. Schiff base
9 (containing the benzyl moiety) exhibited the highest activities against
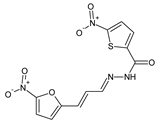
E. coli (comparable to that of streptomycin) and
B. subtilis. Aldimine
12 (bearing the 2-thiazolyl moiety) revealed comparable MIC values against
E. coli and
P. aeruginosa to that of streptomycin. However, Schiff base
7 (containing the naphthyl moiety) was reported to possess the best activity against
S. typhi (similar to that of a standard drug) and
E. coli. In turn, two aldimines
8 (bearing the 1,3-dihydroxy-2-methylpropan-2-yl moiety) and
11 (containing the benzyloxypyridin-2-yl moiety) were disclosed to be the least antibacterially active molecules [
19].
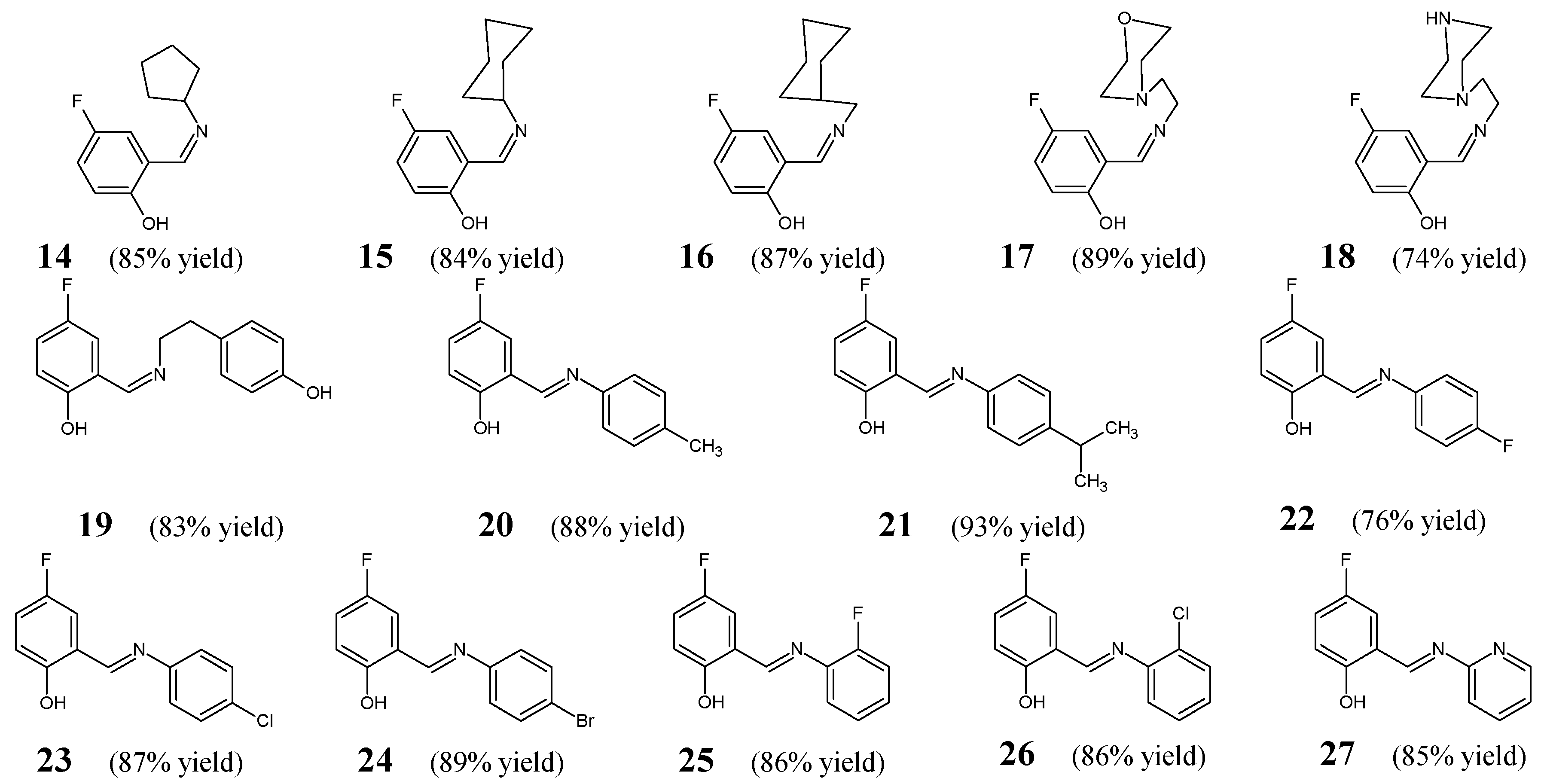
Shi et al. [
20] have reported the general synthesis route and results of the in vitro antibacterial examination for a series of fluorinated aldimine-type Schiff bases (
14–
27) (
Figure 6). The synthesis of the above-mentioned fluorinated aldimines was performed successfully by condensing the stoichiometric ratios of differently substituted primary amines and 5-fluorosalicylaldehyde, in a methanolic medium, at ambient temperature, without any catalyst assistance.
All the synthesized aldimines
14–
27 were subjected to the two-fold serial dilution assay in order to determine their in vitro abilities to inhibit the growth of four clinical isolates of bacterial Gram-positive (
B. subtilis,
S. aureus) and Gram-negative (
E. coli,
P. fluorescence) strains. As a positive control, the antibiotic kanamycin—possessing a broad-spectrum of antibacterial activity—was employed to confirm the susceptibility of all bacterial strains recruited. MIC values of Schiff bases
14–
27 (
Table 4) were established in the assay MTT-based. Aldimine
19 (bearing the 4-fluorophenol moiety linked via an azomethine linkage in
ortho position to the 4-hydroxyphenylethyl moiety) was reported to be the most potent, revealing significant activities against
E. coli,
S. aureus,
P. fluorescence and
B. subtilis. Noteworthy is that its activity against
E. coli was comparable to that of kanamycin, while against
S. aureus and
P. fluorescence it was 1.9-fold lower than that of a standard drug. Shi et al. have found that the replacement of a hydrophilic hydroxy group of the phenyl moiety in the most active structure (
19) by electron-donating alkyl groups results in a remarkable decrease in antibacterial activity which can be seen in the case of two structures containing the methyl (
20) or isopropyl (
21) group (with MIC values ranging from 218.1 to >436.2 µM). It has been reported that among all fluorinated aldimines with additional hydrophobic electron-withdrawing halogen substituents attached to the phenyl moiety (
22–
26), two structures:
ortho-fluoro- and
ortho-chlorosubstituted (
25 and
26)—revealing MIC values ranging from 25.0 to 53.6 µM—are more active than their
para-fluoro- and
para-chlorosubstituted counterparts (
22 and
23)—revealing MIC values ranging from 50.1 to 200.3 µM. Furthermore, the antibacterial effects of all the compounds bearing a fluoro substitution (
22 and
25) were found to be superior to those containing a chloro substitution (
23 and
26). On the other hand, it has been proved that the substitution by cyclopentyl, cyclohexyl and cyclohexylmethyl did not affect the activity since it resulted in synthetic fluorinated aldimines
14,
15 and
16 possessing comparable antibacterial activities (MIC values ranging from 26.6 to 120.6 µM). In turn, the substitution by morpholinoethyl or piperazinoethyl moiety was not favorable for the antibacterial effect as is clearly seen for fluorinated aldimines
17 and
18 with MIC values ranging from 49.7 to 199.0 µM. The most antibacterially active molecule—aldimine
19—when tested by Shi and co-workers in the target enzymatic assay, was identified to be a potent inhibitor (IC
50 = 2.7 µM) of the ecKAS III, playing a significant role in fatty acid synthesis pathway in bacteria. Therefore, their results have proved that the presence of an electron-withdrawing and lipophilic fluorine atom at the phenol moiety attached to an azomethine bridge is preferred for the antibacterial activity as well as inhibitory potency towards the ecKAS III. The authors have carried out ligand-docking studies and have shown the most likely binding conformation of compound
19 at the active site of the crystal structure of ecKAS III, suggesting that the enzymatic inhibition mechanism is responsible for its potent antibacterial activity [
20].
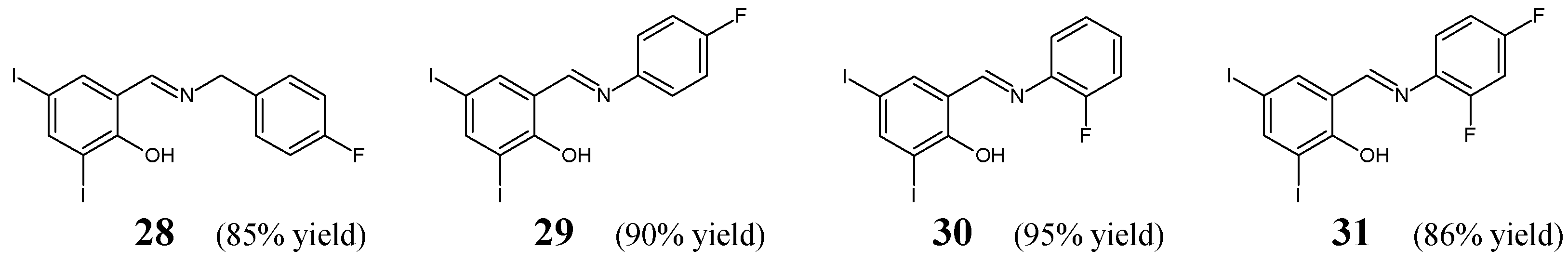
Xu et al. [
21] have synthesized and conducted antimicrobial studies on fluorinated aldimine-type Schiff bases (
28–
31) (
Figure 7). The synthesis of these aldimines was performed by condensing the stoichiometric ratios of various primary amines (i.e., 4-fluorobenzylamine, 4-fluoroaniline, 2-fluoroaniline or 2,4-difluoroaniline) with 2-hydroxy-3,5-diiodobenzaldehyde, in ethanol as the reaction medium without any catalytic assistance.
The authors have used clinical isolates of three Gram-positive (
B. subtilis,
S. aureus,
S. faecalis) and three Gram-negative (
P. aeruginosa,
E. coli,
E. cloacae) bacterial strains as well as penicillin (benzylpenicillin) and kanamycin as antibacterial agents for comparison purposes. MIC values of Schiff bases
28–
31 were established in the assay MTT-based. The majority of aldimines revealed strong antibacterial activities against all recruited bacteria (
Table 5). MIC values of molecules
29–
31 against
E. cloacae,
28–
29,
31 against
E. coli,
28 against
S. faecalis and
31 against
B. subtilis proved to be lower or comparable to that of standard drugs. Schiff base
31—bearing the 2,4-difluorophenyl moiety–has been disclosed to be the most potent among the screened compounds [
21].
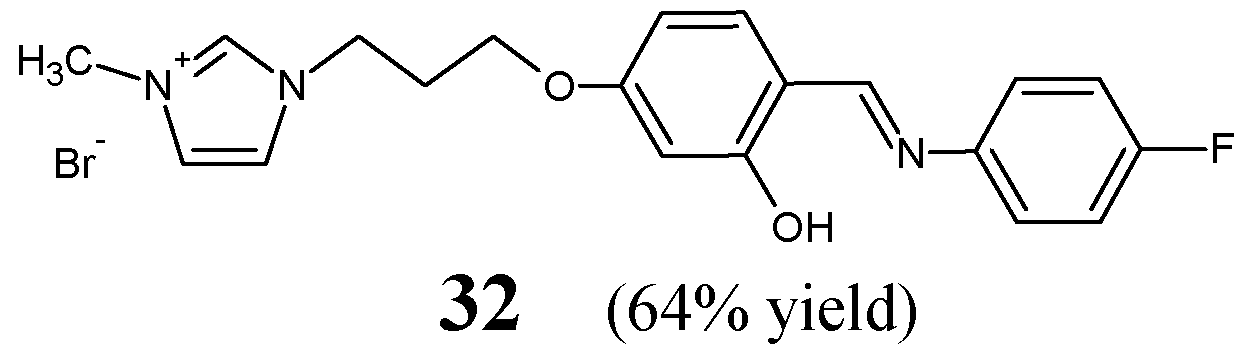
Khungar et al. [
22] have synthesized and confirmed the structure of fluorinated Schiff base
32, i.e., 1-[3-(4-{(
E)-[(4-fluorophenyl)imino]methyl}-3-hydroxyphenoxy)propyl]-3-methyl-1
H-imidazol-3-ium bromide (
Figure 8). This aldimine was synthesized by reacting 4-fluoroaniline with the suitable ionic liquid salicylaldehyde derivative (i.e., 1-[3-(4-formyl-3-hydroxyphenoxy)propyl]-3-methyl-1
H-imidazol-3-ium bromide) at a molar ratio of 4:3, in boiling ethanol for 4 h.
Schiff base
32 was screened for the in vitro ability to inhibit the growth of six bacterial strains in the assay based on two-fold serial dilutions. For this, two Gram-positive (
B. cereus MTCC 430,
S. aureus MTCC 96) and four Gram-negative (
E. coli MTCC 1652,
K. pneumoniae MTCC 432,
S. typhimurium MTCC 98,
P. putida MTCC 102) bacteria were selected. Unfortunately, this fluorinated aldimine was proven to be weak antibacterially active (revealing no inhibitory effects against all pathogenic bacterial strains recruited—MIC values above 294.7 µM) [
22].
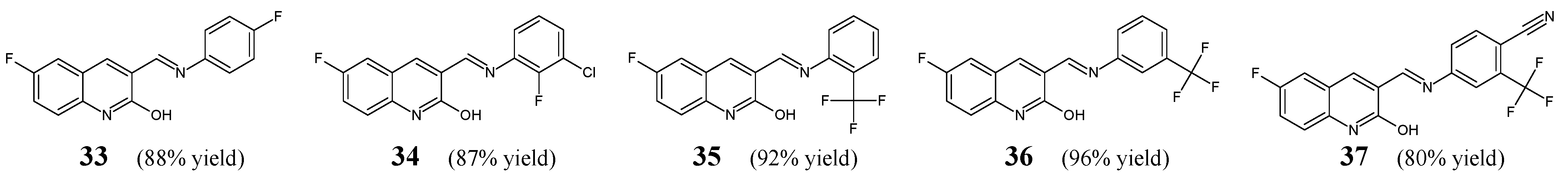
Mandewale et al. [
23] have reported the procedure for synthesis, and they have carried out the antimycobacterial evaluation of aldimine-type Schiff bases
33–
37 (
Figure 9). These molecules were obtained by reacting equimolar quantities of 4-fluoroaniline, 2-fluoro-3-chloroaniline, 2-(trifluoromethyl)aniline, 3-(trifluoromethyl)aniline or 4-amino-2-(trifluoromethyl)benzonitrile with 6-fluoro-2-hydroxyquinoline-3-carboxaldehyde, under reflux for 0.5 h in ethanol without any catalyst assistance.
Activities of Schiff bases
33–
37 against
M. tuberculosis H
37Rv strain were established in the in vitro microplate Alamar Blue assay. Pyrazinamide (a pyrazine derivative), ciprofloxacin (a fluoroquinolone) and streptomycin (an aminoglycoside antibiotic) were used as reference drugs for comparison purposes. The designed aldimines
33–
37 can be considered promising antimycobacterial agents as they revealed MIC values lower or comparable to that of recruited antimycobacterial agents (
Table 6). The most active against
M. tuberculosis H37Rv proved to be three molecules: 4-{(
E)-[(6-fluoro-2-hydroxyquinolin-3-yl)methylidene]amino}-2-(trifluoromethyl)benzonitrile (
37), 6-fluoro-3-{(
E)-[(4-fluorophenyl)imino]methyl}quinolin-2-ol (
33) and 6-fluoro-3-[(
E)-{[2-(trifluoromethyl)phenyl]imino}methyl]quinolin-2-ol (
35) [
23].
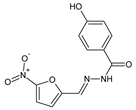
İskeleli et al. [
24] have reported the synthesis and structural characterization of 4-[(3-fluoro-4-hydroxy-5-methoxybenzylidene)amino]-1,5-dimethyl-2-phenyl-1,2-dihydro-3
H-pyrazol-3-one (
38) (
Figure 10). This fluorinated aldimine was obtained by refluxing equimolar ratios of 4-amino-1,5-dimethyl-2-phenyl-1,2-dihydro-3
H-pyrazol-3-one and 3-fluoro-4-hydroxy-5-methoxybenzaldehyde for 3 h in ethanol as the reaction medium.
The aldimine-type Schiff base
38 was subjected to the bioassay based on two-fold serial dilutions to determine its MIC values against some strains of bacteria: methicillin-sensitive
S. aureus ATCC 25923, methicillin-resistant
S. aureus ATCC 43300,
S. pneumoniae ATTC 49619,
E. faecalis ATTC 29212,
E. coli ATCC 25922,
K. pneumoniae ATCC 700603,
P. aeruginosa ATCC 27853,
S. maltophiliae ATCC 17666,
H. influenzae ATCC 40247,
E. casseliflavus ATCC 700327 and
Salmonella spp. An aminoglycoside antibiotic—amikacin—was recruited as a standard antibacterial agent. Fluorinated aldimine
38 proved to be antibacterially active against three (
S. pneumoniae,
H. influenzae and
E. faecalis) out of all eleven bacteria selected. In addition, its activity against
S. pneumoniae and
H. influenzae was higher than that of amikacin (
Table 7) [
24].
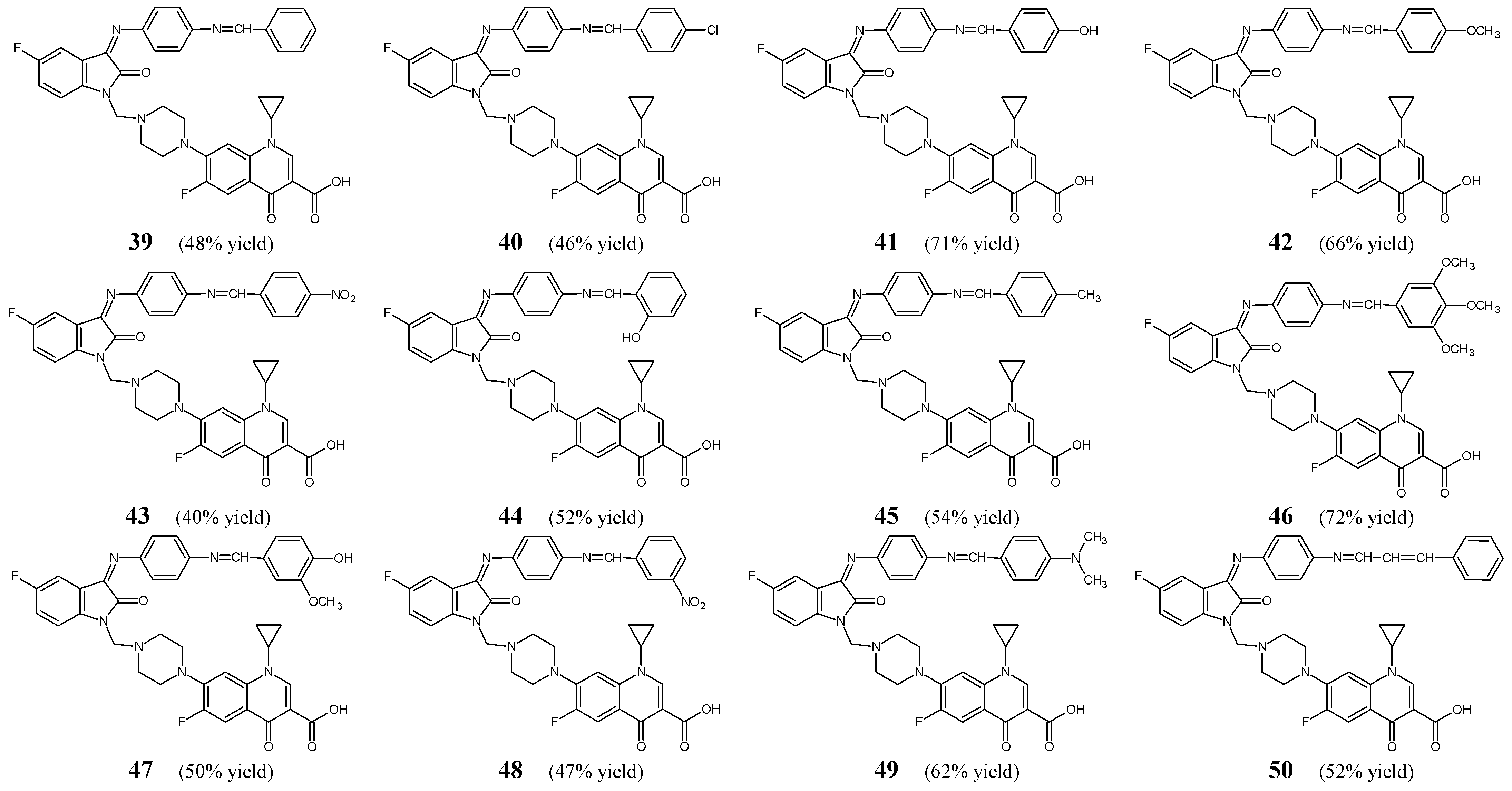
Prakash and Raja [
25] have synthesized and confirmed the structure and then conducted antimicrobial studies on fluorinated aldimine-type Schiff bases
39–
50 (
Figure 11) which may be regarded as novel hybrids with fluorinated quinolone ciprofloxacin. All these fluorinated structures may also be considered important Mannich bases due to the presence of the piperazin-1-ylmethyl moiety at the
N1 of the indolin-2-one template. The synthesis of these aldimines was carried out by reacting equimolar ratios of 7-{4-(3-[4-aminophenylimino]-5-fluoro-2-oxoindolin-1-yl)methyl)piperazin-1-yl)}-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid with benzaldehyde, variously substituted benzaldehydes or cinnamylaldehyde, in refluxing ethanol for 8 h, containing a small amount of glacial acetic acid as an efficient catalyst.
To screen the antibacterial activities of particular fluorinated hybrids
39–
50, that differ in electron-donating and electron-withdrawing substituent/substituents on the phenyl moiety attached to an azomethine function, three Gram-positive (
S. aureus ATCC 9144,
S. epidermidis ATCC 155,
M. luteus ATCC 4698) and three Gram-negative (
E. coli ATCC 25922,
P. aeruginosa ATCC 2853,
K. pneumoniae ATCC 11298) bacterial strains of clinical interest were recruited. To confirm the susceptibility of all bacterial strains a broad-spectrum antibacterial agent—ciprofloxacin—from the class of fluoroquinolones was used. Prakash and Raja have determined the MICs of all the synthesized fluorinated aldimines in the agar streak dilution assay. The majority of them revealed strong to moderate antibacterial activities (
Table 8). Compounds containing electron-donating groups (
41,
42,
44–
47,
49) were found to be more active than those bearing electron-withdrawing groups (
40,
43,
48). Therefore, the authors have suggested that electron-donating groups are preferred for the antibacterial activities of fluorinated aldimines. Schiff base
47—containing the 3-methoxy-4-hydroxyphenyl moiety—has been disclosed to be the most potent against
K. pneumoniae. Its activity against this bacterial strain was 2.2-fold superior to that of ciprofloxacin. Additionally, its MIC values for
S. epidermidis,
M. luteus,
S. aureus and
E. coli were comparable to those of a standard drug. Aldimine
41—with the
para-hydroxyphenyl moiety—has been reported to demonstrate the highest activity towards
S. aureus,
P. aeruginosa and
E. coli with MIC values 4.2-, 2.1- and 2.1-fold lower, respectively, than those of ciprofloxacin. In addition, its activity against
S. epidermidis and
M. luteus proved to be similar to that of a standard drug. Schiff base
46—bearing the 3,4,5-trimethoxyphenyl moiety—has been identified to be the most active against
S. epidermidis and
M. luteus with MIC values 2.4-fold superior than those of ciprofloxacin. Moreover, its activity against
S. aureus,
P. aeruginosa and
E. coli was higher or comparable to that of a standard drug. Aldimine
49—containing the
para-dimethylaminophenyl moiety—has been reported to be the most active against
P. aeruginosa and
M. luteus. Its activity against these two bacterial strains was 2-fold superior to that of ciprofloxacin. Additionally, its MIC values for
S. aureus,
E. coli and
S. epidermidis were lower or comparable to those of a standard drug. However, Schiff base
42—bearing the
para-methoxyphenyl moiety—has been disclosed to exhibit 2.2-fold higher activity against
S. aureus and
E. coli when compared to ciprofloxacin [
25].
Durmuş et al. [
26] have disclosed a synthesis scheme and preliminary results of the antibacterial evaluation of fluorinated dimeric disulfide Schiff base (
51), i.e., (
Z,
Z)-
N,
N′-(disulfanediyldibenzene-2,1-diyl)
bis[1-(2-fluorophenyl)methanimine] (
Figure 12). The synthesis of this molecule was prepared by condensing 2,2′-disulfanediyldianiline with
ortho-fluorobenzaldehyde (in molar ratios 1:2), in ethanol containing cerium oxide nanoparticles as an efficient catalyst, according to the general procedure reported earlier [
27].
Durmuş research group have employed three Gram-negative bacilli such as
K. pneumoniae,
E. coli and
A. baumannii, and one Gram-positive strain of
S. aureus (all bacteria isolated from the hospitalized patients) to assess the antibacterial activities of fluorinated aldimine
51 in the disc diffusion assay. A third-generation cephalosporin, i.e., cefotaxime, and a broad-spectrum aminopenicillin, i.e., amoxicillin (in combination with an irreversible β-lactamase inhibitor—clavulanic acid), were used as antibacterial agents for comparison purposes. This fluorinated Schiff base was reported to reveal superior—to that of cefotaxime—activities against all human pathogenic bacterial strains, and comparable—to that of amoxicillin/clavulanic acid—effects against
S. aureus and
K. pneumoniae. The authors suggested that the attendance of two electron-withdrawing fluoro groups in an
ortho position of both phenyl moieties as well as a very important structural feature, i.e., the reductive disulfide bridge, are necessary for the antibacterial activity of this fluorinated aldimine [
26].
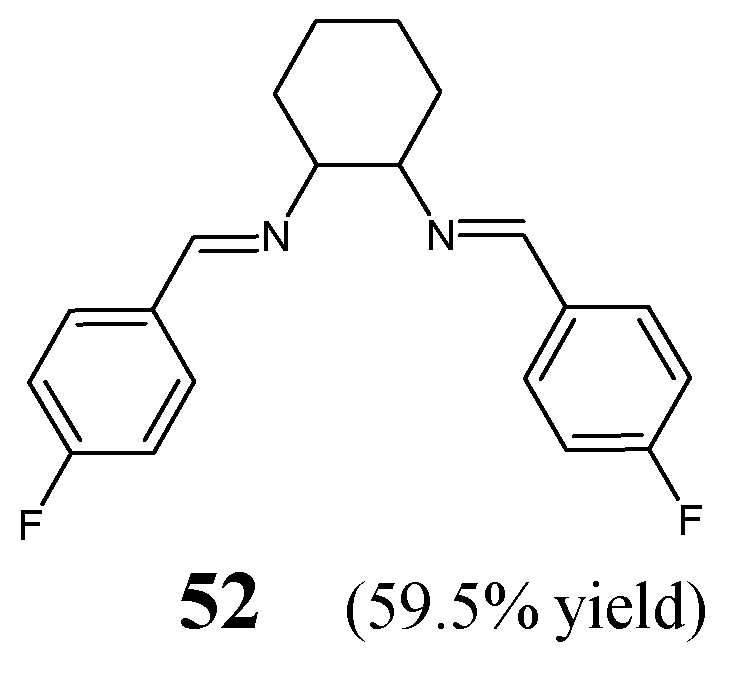
Oboňová et al. [
28] have designed and synthesized (
E,
E)-
N,
N′-cyclohexane-1,2-diylbis[1-(4-fluorophenyl)methanimine (
52) (
Figure 13) by reacting 1,2-cyclohexanediamine with
para-fluorobenzaldehyde (in molar ratios 1:2) in methanol at room temperature for 2 h and allowing the reaction mixture to successful crystallization for several days. This synthesis was relatively straightforward and proceeded without any catalyst assistance. The structure of this fluorinated aldimine-type
bis-Schiff base was determined on the basis of spectroscopic and X-ray diffraction data [
28].
The aldimine-type Schiff base
52 has been tested against a Gram-negative bacterial strain of
E. coli CNCTC 377/79 and a Gram-positive bacterial strain of
S. aureus CNCTC Mau 29/58 in the broth dilution assay. The most potent antibacterial agent among fluoroquinolones—ciprofloxacin—was chosen as a positive control. Fluorinated aldimine
52 revealed the same MIC value of 5706.3 µM against both bacteria. The authors suggested that the weak antibacterial activity of this compound is due to its rigid scaffold. Such a rigid structure containing two double CH=N bonds was most likely not flexible enough to enable interactions with the active sites of enzymes [
28].
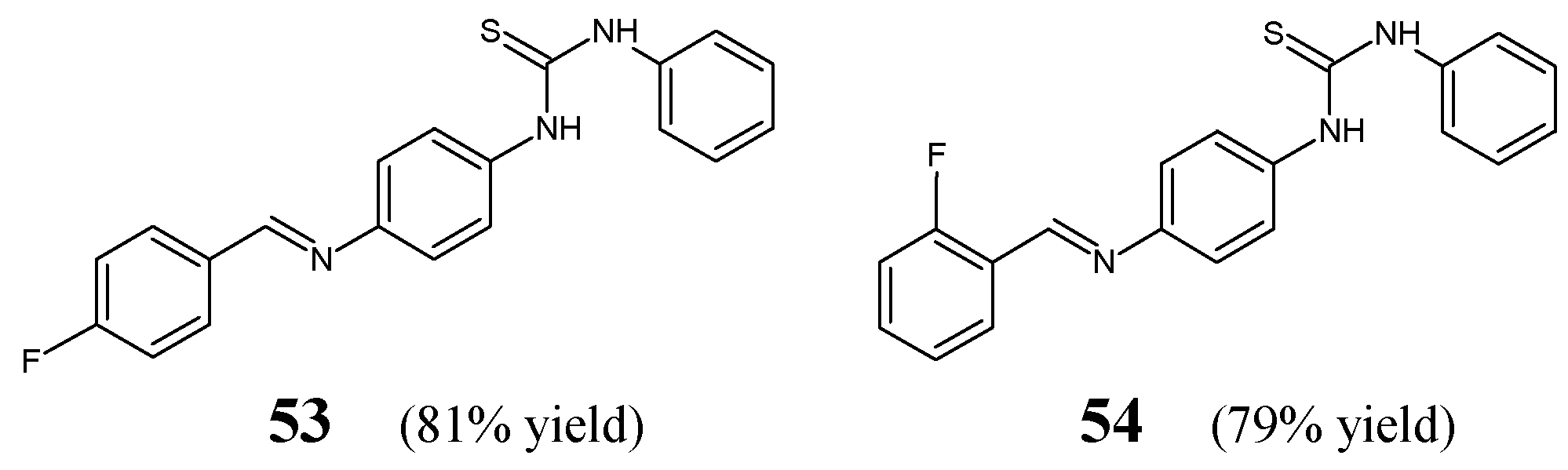
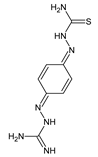
Zhang et al. [
29] have reported the synthesis scheme, structure determination and results of in vitro antibacterial studies for two fluorinated aldimine-type Schiff bases—
53 and
54 (
Figure 14)—containing in their structures the moiety of 1-phenyl-3-phenylthiourea linked via an azomethine bridge to the 4-fluorophenyl or 2-fluorophenyl moiety, respectively. The synthesis of these aldimines was achieved successfully by condensing the stoichiometric ratios of 1-(4-aminophenyl)-3-phenylthiourea and 4-fluorobenzaldehyde or 2-fluorobenzaldehyde for 3–4 h at 80 °C in toluene containing PTSA as an efficient catalyst.
Zhang and co-workers have employed
S. aureus ATCC 6538 and
B. subtilis ATCC 6633 as Gram-positive bacterial strains, whereas
E. coli ATCC 35218 and
P. aeruginosa ATCC 13525 as Gram-negative bacterial strains to assess the antibacterial activities of fluorinated aldimines
53 and
54 in the two-fold serial dilution assay. As a positive control, two antibiotics such as penicillin G (benzylpenicillin) and kanamycin B (an aminoglycoside antibiotic), were employed in order to confirm the susceptibility of all bacterial strains recruited. The aldimine-type Schiff base
53, having the fluoro group at
para position of the phenyl moiety, was reported to be moderately active against all pathogens selected (
Table 9). This molecule revealed the highest activity against
S. aureus, although its potency was found to be 3.8- and 11.2-fold lower than that of penicillin G and kanamycin B. However, the
ortho-fluoro substituted aldimine
54 proved to be antibacterially inactive even at a concentration of 286.2 µM. Thus, the results of these antibacterial studies clearly indicated that the substitution by a fluorine atom at the
para position of the phenyl moiety is more favorable for the antibacterial effect [
29].
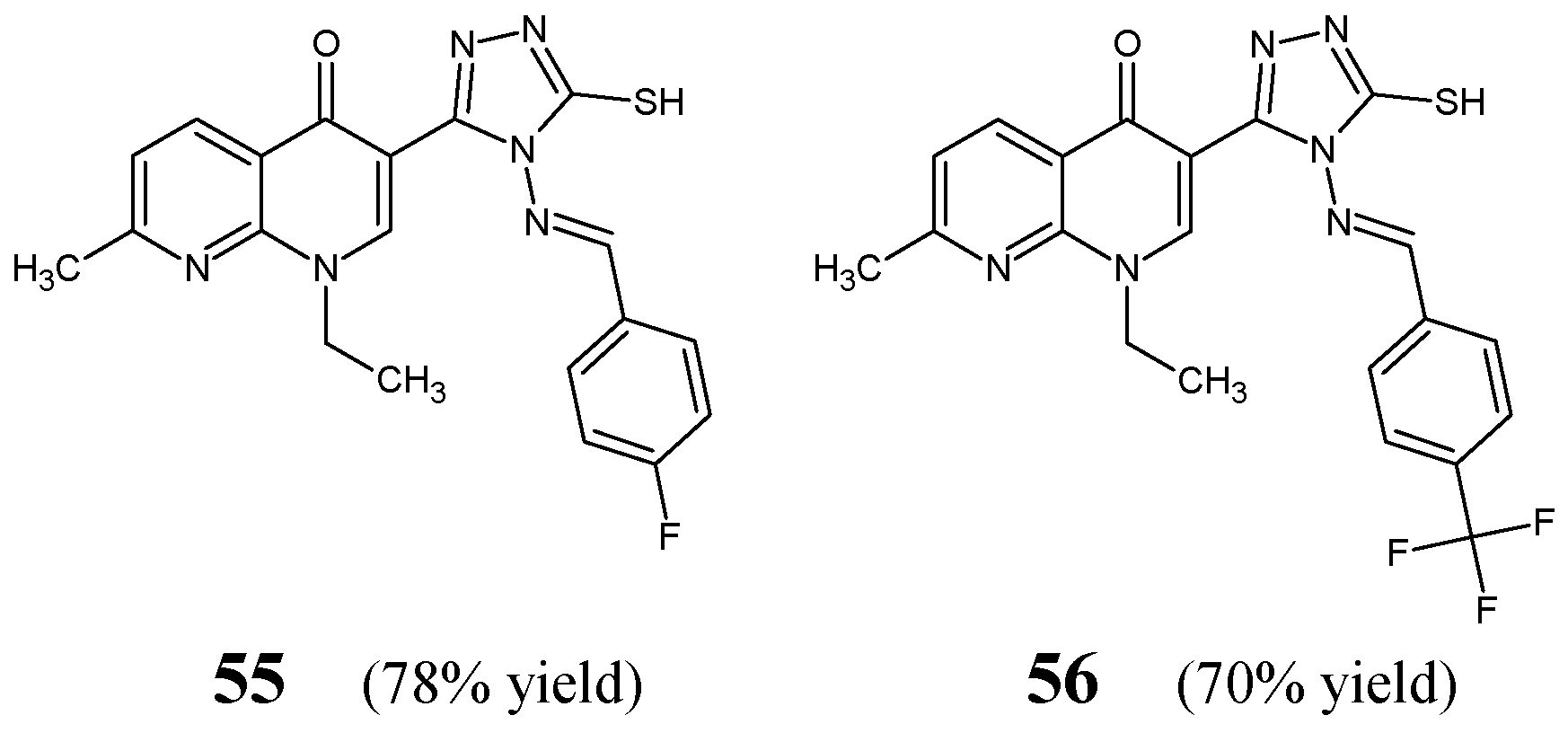
Aggarwal et al. [
30] have synthesized, confirmed the structure and then performed antimicrobial examination on two fluorinated aldimine-type Schiff bases—structures
55 and
56 (
Figure 15)—which have in their molecular framework the privileged 4
H-1,2,4-triazole-5-thiol scaffold linked via an azomethine bridge to the aromatic benzene ring bearing the 4-fluoro or 4-trifluoromethyl group, respectively. Molecules
55 and
56 were prepared by reacting 3-(4-amino-5-sulfanyl-4
H-1,2,4-triazol-3-yl)-1-ethyl-7-methyl-1,8-naphthyridin-4(1
H)-one with a molar excess of 4-fluorobenzaldehyde or 4-trifluoromethylbenzaldehyde, respectively, in boiling dioxane, with efficient catalytic assistance of a small amount of concentrated sulfuric acid.
Aldimine-type Schiff bases
55 and
56 were tested in the assay based on two-fold serial dilutions to estimate their in vitro abilities to inhibit the growth of two Gram-positive (
S. aureus ATCC 2937,
B. subtilis ATCC 12711) and three Gram-negative (
E. coli ATCC 8739,
K. pneumoniae ATCC 31488,
P. aeruginosa ATCC 9027) bacterial strains of clinical interest. As a positive control, an aminoglycoside antibiotic streptomycin and a fluoroquinolone ciprofloxacin were employed to confirm the susceptibility of all bacterial strains used. The relatively high MIC values of fluorinated aldimines
55 and
56 against the majority of bacterial strains were reported in the studies of Aggarwal and co-workers (
Table 10), confirming a low susceptibility of most pathogenic bacteria to both compounds. Notwithstanding, aldimine
55, with the fluoro substitution in
para position of the phenyl moiety, proved to be 3.5-fold more active against
P. aeruginosa than that containing the
para-trifluoromethyl substitution (
56), revealing a MIC value of 39.2 µM. Simultaneously, its activity was found to be 5.7- and 10.3-fold lower than that of streptomycin and ciprofloxacin, respectively. In turn, the fluorinated aldimine
56 was found to be 2.2-fold more active than
55 against
K. pneumoniae [
30].
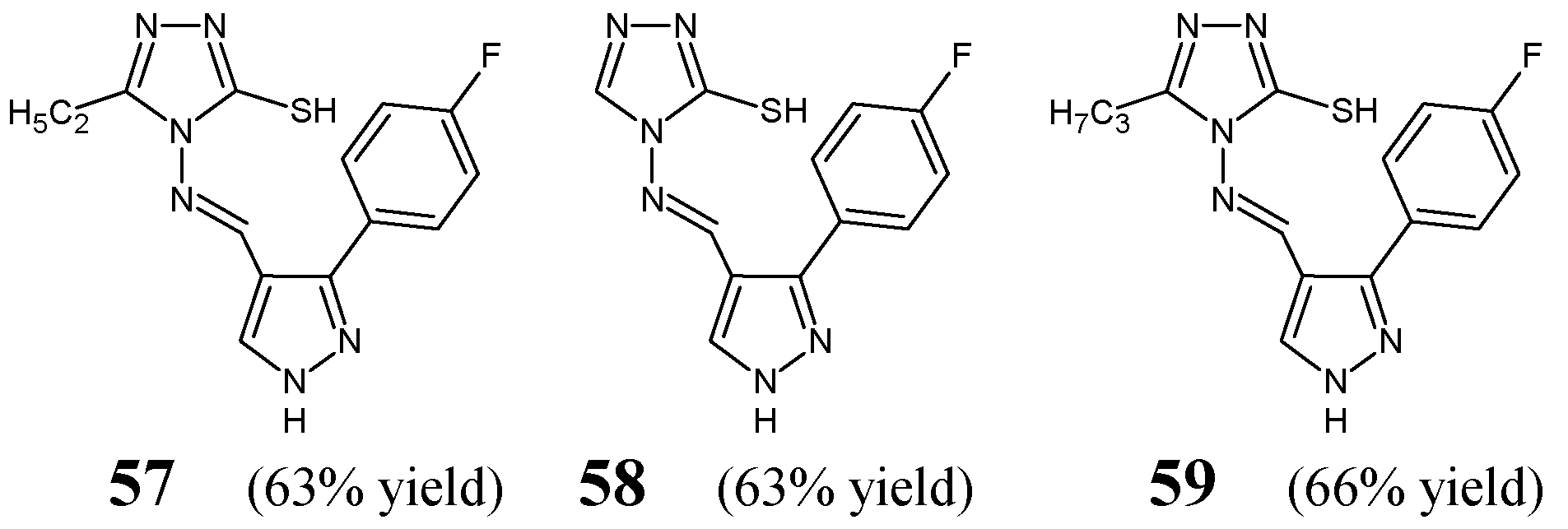
Malladi et al. [
31] have synthesized, confirmed the structure and investigated the antibacterial activities of fluorinated aldimine-type Schiff bases
57–
59 (
Figure 16). These hybrid molecules contain in their molecular framework the privileged 4
H-1,2,4-triazole-3-thiol template linked via an azomethine bridge to the pyrrazole scaffold bearing the 4-fluorophenyl at
C3. The synthesis of these fluorinated aldimines was achieved successfully by condensing equimolar ratios of 4-amino-4
H-1,2,4-triazole-3-thiol (unsubstituted or substituted by one alkyl group such as the ethyl or propyl at position 5) with 3-(4-fluorophenyl)-1
H-pyrrazole-4-carboxaldehyde, in a two-component solvent medium (containing ethanol and dioxane), under reflux with the catalytic assistance of a small amount of concentrated sulfuric acid.
The authors have used clinical isolates of two Gram-positive (
S. aureus,
B. subtilis) and two Gram-negative (
E. coli,
P. aeruginosa) bacterial strains as well as antibiotic ceftriaxone from third-generation cephalosporins as a positive control to assess the antibacterial activities of aldimines
57–
59. All fluorinated Schiff bases were reported to reveal significant antibacterial potencies against bacterial strains of
S. aureus,
B. subtilis,
E. coli and
P. aeruginosa with MIC values ranging from 5.1 to 43.4 µM (
Table 11) when tested in the assay based on two-fold serial dilutions. Fluorinated aldimine
57 (with the ethyl substitution at position 5 of 1,2,4-triazole ring) has been identified as the most effective against all the selected bacteria. Nevertheless, its MIC values against
S. aureus,
B. subtilis,
E. coli and
P. aeruginosa proved to be about 1.8-fold higher than that of ceftriaxone [
31].
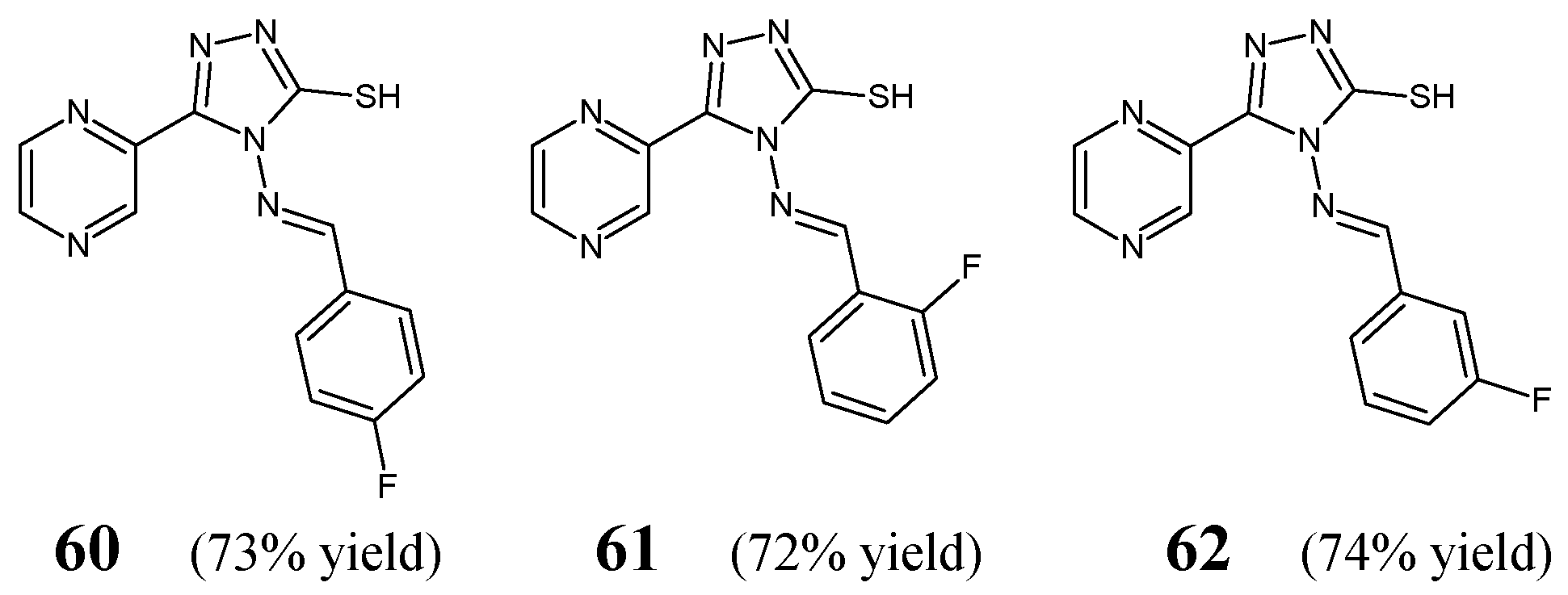
Zhang et al. [
32] have designed and synthesized three fluorinated aldimine-type Schiff bases (structures
60–
62) (
Figure 17) bearing the 5-(2-pyrazinyl)-4
H-1,2,4-triazole-3-thiol template linked via an azomethine bridge to the benzene ring containing a fluorine atom in various positions. The synthesis of compounds
60–
62 was performed by reacting the starting 4-amino-5-(pyrazin-2-yl)-4
H-1,2,4-triazole-3-thiol with
para-fluorobenzaldehyde,
ortho-fluorobenzaldehyde or
meta-fluorobenzaldehyde, respectively, in an ethanolic medium containing a small amount of acetic acid as an efficient catalyst.
To evaluate antibacterial activities of Schiff bases
60–
62, the authors used three Gram-positive (
S. aureus,
B. subtilis,
B. amyloliquefaciens) and two Gram-negative (
E. coli,
P. aeruginosa) bacterial strains of clinical interest as well as an antibiotic kanamycin B. MIC values of three fluorinated aldimines—established in the assay based on two-fold serial dilutions—ranged from 83.2 to above 166.5 µM (
Table 12). All Schiff bases revealed the highest—although lower than that of kanamycin B—activity against
E. coli. Zhang and co-workers have disclosed that the
ortho-fluorophenyl group in this class of aldimines is a preferred substituent. Schiff base
61 with that substituent proved to be more active against
P. aeruginosa than its
para- and
meta-fluorinated counterparts (
60 and
62). The
para-and
ortho-fluorinated aldimine structures (
60 and
61) were also reported to be more active against
B. subtilis than their
meta-fluorinated congener (
62). In turn, all fluorinated Schiff bases (
60–
62) were found to be equally effective against
S. aureus and
B. amyloliquefaciens [
32].
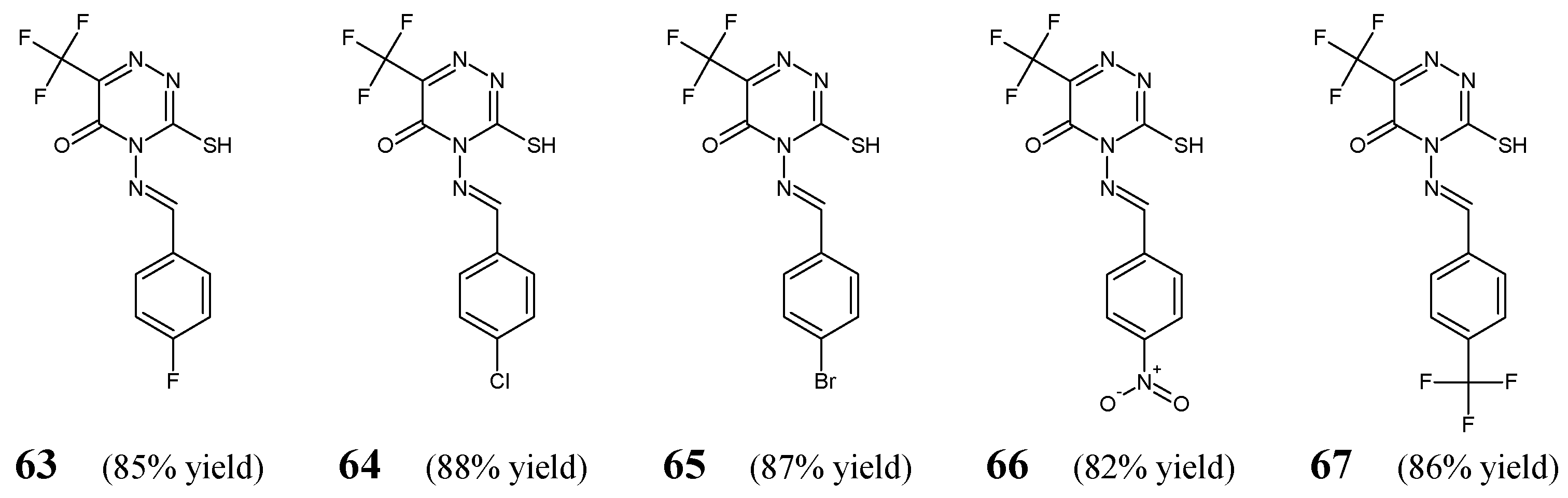
Alshammari et al. [
33] have synthesized and antibacterially screened fluorinated aldimine-type Schiff bases
63–
67 (
Figure 18). These compounds were obtained by condensing equimolar ratios of 4-amino-3-sulfanyl-6-(trifluoromethyl)-1,2,4-triazin-5(4
H)-one with various aromatic aldehydes (i.e., 4-fluorobenzaldehyde, 4-chlorobenzaldehyde, 4-bromobenzaldehyde, 4-nitrobenzaldehyde or 4-(trifluoromethyl)benzaldehyde) in boiling ethanol containing a catalytic amount of sulfuric acid.
Aldimines
63–
67 have been evaluated for their antibacterial activity against two Gram-negative (
E. coli ATCC 25955,
S. typhi) and two Gram-positive (
S. aureus NRRL B-767,
B. subtilis ATCC 6633) bacterial strains. Ciprofloxacin (a fluoroquinolone) was used as a standard drug. Among all fluorinated Schiff bases, the
para-fluorophenyl-substituted aldimine (
63) was found to be the most potent against
E.coli,
S. aureus and
B. subtilis (
Table 13) [
33].
3. Fluorinated Ketimine-Type Schiff Bases
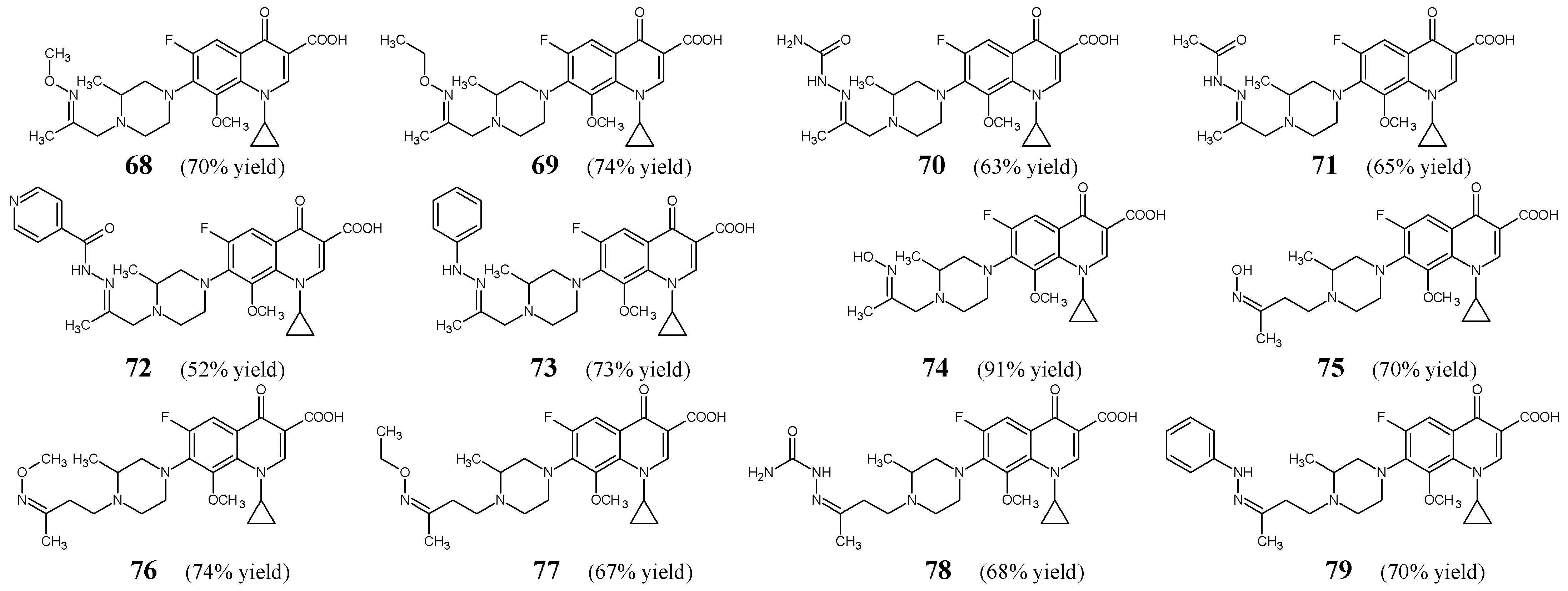
Chai et al. [
34] have synthesized, confirmed the structure and then carried out antimicrobial studies on a series of ketimine-type Schiff bases (
68–
79) (
Figure 19) related to gatifloxacin—a drug that belongs to the family of fluorinated quinolones. They may be regarded as novel imine hybrids with gatifloxacin. Ketimines
68–
75 and
76–
79 were obtained by refluxing for 3–4 h variable substituted amine hydrochlorides (in a molar excess) with 1-cyclopropyl-6-fluoro-8-methoxy-7-[4-(2-oxopropyl)-3-methylpiperazin-1-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid or 1-cyclopropyl-6-fluoro-8-methoxy-7-[4-(3-oxobutyl)-3-methylpiperazin-1-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid, respectively, in methanol containing an aqueous solution of sodium bicarbonate.
Chai et al. have selected Gram-positive (
S. aureus ATCC 25923, methicillin-resistant
S. aureus 08-1, methicillin-sensitive
S. aureus 08-1, methicillin-resistant
S. epidermidis 09-4, methicillin-sensitive
S. epidermidis 09-3, methicillin-sensitive
S. epidermidis 09-6,
S. pneumoniae 08-2,
S. pneumoniae 08-4,
E. faecium 08-2,
E. faecium 08-7,
E. faecalis 08-10,
E. faecalis 08-12) and Gram-negative (
E. coli ATCC 25922,
E. coli 08-21,
E. coli 08-22,
K. pneumoniae 09-22,
K. pneumoniae 09-23,
P. aeruginosa ATCC 27853,
P. aeruginosa 09-32,
P. aeruginosa 09-33,
P. aeruginosa 09-34) bacterial strains of clinical interest that are susceptible or resistant to commonly used antibacterial agents to study antibacterial activities of all the synthesized fluorinated ketimines (
68–
79) in the assay based on two-fold serial dilutions. Gatifloxacin and levofloxacin (belonging to fluorinated quinolones) were employed as drugs for comparison purposes. It has been disclosed that the substitution at the
C7 is a decisive factor for antibacterial activity in this class of compounds and that the vast majority of fluorinated ketimine structures exhibit high antibacterial potencies (
Table 14). Fluorinated ketimine-type Schiff base
79 was identified as a possible antibacterial agent with a broad spectrum of activity. This molecule proved to be the most potent among all fluorinated ketimines, revealing MIC values ranging from 0.1 µM to 1.9 µM. In addition, its antibacterial activity against all the recruited strains was found to be superior to that of gatifloxacin and/or levofloxacin. Schiff base
73 also proved to be more active against the vast majority of bacteria than standard drugs.
S. aureus,
S. epidermidis and their methicillin-resistant strains were reported to be the most susceptible to fluorinated ketimines
69–
71,
73,
74 and
79, revealing MIC values ranging from 0.1 µM to 0.6 µM. In turn, some Gram-negative strains of bacteria were found to be the most susceptible to compounds
71–
73,
78 and
79. In addition, in cytotoxicity studies reported by the authors, gatifloxacin-derived Schiff base
71 was found to be the least toxic (IC
50 = 1450.4 µM) among all other ketimines (having IC
50 values ranging from 21.2 to 933.5 µM) towards mammalian Vero cells of the epithelial origin, indicating its high selectivity for bacterial cells [
34].
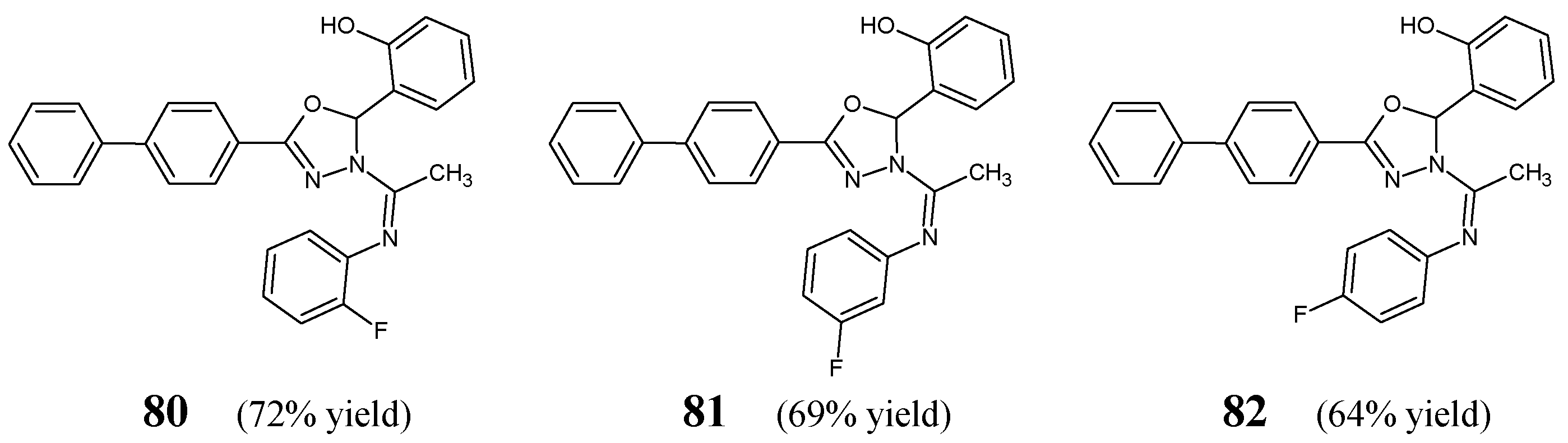
Malhotra et al. [
35] have reported the synthesis and results of the antimicrobial examination for a series of fluorinated ketimine-type Schiff bases
80–
82 (
Figure 20) bearing the privileged scaffold of 2,3-dihydro-1,3,4-oxadiazole. The synthesis of the above ketimines was carried out by reacting the stoichiometric ratios of the primary aromatic amine, such as
ortho-fluoroaniline,
meta-fluoroaniline or
para-fluoroaniline, and ketone, i.e., 1-[5-(biphenyl-4-yl)-2-(2-hydroxyphenyl)-1,3,4-oxadiazol-3(2
H)-yl]ethanone, for 9–11 h in refluxing anhydrous ethanol, containing a small amount of glacial acetic acid as an efficient catalyst.
The authors have recruited two Gram-positive (
B. subtilis MTCC 96,
S. aureus MTCC 121) and two Gram-negative (
P. aeruginosa MTCC 2453,
E. coli MTCC 40) bacterial strains of clinical interest to investigate antibacterial activities of ketimines
80–
82 in the assay based on two-fold serial dilutions. Ciprofloxacin was used as an antibacterial agent. The MIC and MBC (minimum bactericidal concentration) values of Schiff bases
80–
82—which differ in the position of fluorine substitution (
ortho,
meta and
para) at the phenyl moiety—against all the recruited bacterial strains are listed in
Table 15. Results revealed that fluorinated ketimines possess MIC values ranging from 27.7 to 110.7 µM and MBC values ranging from 55.4 to 221.4 µM. It has been shown that structure
80, bearing the
ortho-fluorophenyl moiety, reveals the highest antibacterial activities, although lower than those of ciprofloxacin. Furthermore, it has been established that molecule with the
para-fluorophenyl moiety (
82) is more antibacterially active than that with the
meta-fluorophenyl moiety (
81) [
35].
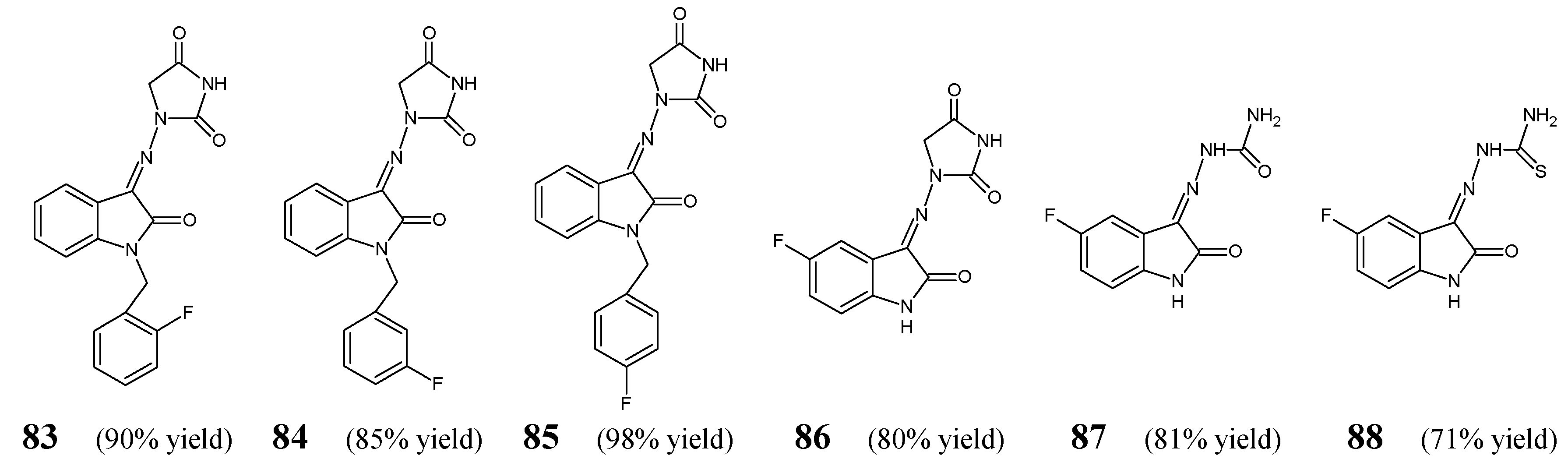
Haj Mohammad Ebrahim Tehrani et al. [
36] have described the antibacterial evaluation of fluorinated ketimine-type Schiff bases
83–
88 (
Figure 21) obtained by a condensation/dehydration reaction of 1-aminohydantoin, semicarbazide or thiosemicarbazide and variously substituted isatines (stoichiometric amounts). The synthetic process has been carried out in refluxing ethanol for 5 h, with the assistance of small amount of glacial acetic acid as an efficient catalyst. The obtained fluorinated compounds possess in their molecular framework the privileged 2-oxo-1,2-dihydro-3
H-indole template linked via an azomethine bridge to the important pharmacophoric moieties.
The authors have recruited three Gram-positive (
S. aureus ATCC 25923, methicillin-resistant
S. aureus ATCC 43300,
E. faecalis ATCC 29212) and two Gram-negative (
P. aeruginosa ATCC 27853,
E. coli ATCC 25922) bacterial strains of clinical interest to investigate antibacterial activities of fluorinated ketimines
83–
88 in the assay based on two-fold serial dilutions. As a positive control, a broad-spectrum antibiotic—amikacin—was included to confirm the susceptibility of all bacterial strains used. Haj Mohammad Ebrahim Tehrani et al. have reported that the antibacterial activity of the synthesized small molecules is dependent on their lipophilicity, and the most active compounds reveal Clog
p values ranging from 0.72 to 2.27. It has been disclosed that enhanced antibacterial activity is achieved by introducing at
N1 the phenylmethyl moiety fluorinated in
ortho, meta or
para position. Three 1,2-dihydro-3
H-indolin-2-one-hydantoin hybrids (
83,
84 and
85) have been identified to be the most promising molecules that might find utility in the future as possible antibacterial agents after further lead optimization studies. Their MIC values against
E. coli,
S. aureus and methicillin-resistant
S. aureus were 1.5 or 3.0-fold lower than those of amikacin (
Table 16). In turn, it has been proved that replacing the fluorinated phenylmethyl moiety with a hydrogen atom and introducing a fluorine atom into the position
C5 of 1,2-dihydro-3
H-indolin-2-one template is disadvantageous, leading to a less antibacterially active fluorinated molecule
86. The authors have also disclosed that fluorinated isatin-based thiosemicarbazone (
88) is more active than fluorinated isatin-based semicarbazone (
87) [
36].
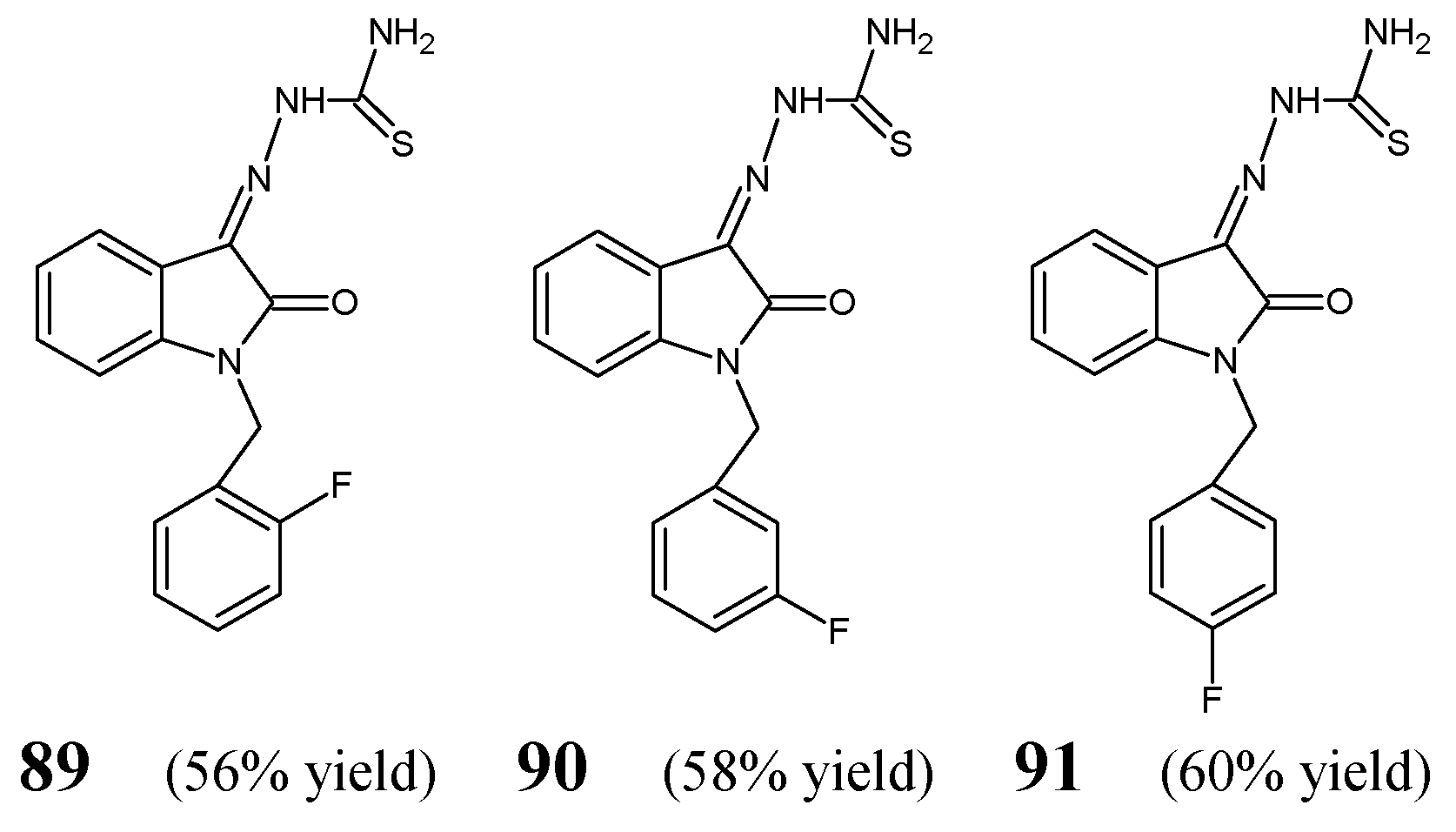
Hassan et al. [
37] have described the general synthetic approach leading to thiosemicarbazones with the privileged template of 2-oxo-1,2-dihydro-3
H-indole. The synthesis of these fluorinated ketimine-type Schiff bases (
89–
91) (
Figure 22) was carried out by condensing equimolar ratios of thiosemicarbazide with 1-(2-fluorobenzyl)-1
H-indole-2,3-dione, 1-(3-fluorobenzyl)-1
H-indole-2,3-dione or 1-(4-fluorobenzyl)-1
H-indole-2,3-dione, respectively, for 6 h in refluxing ethanol containing a catalytic amount of glacial acetic acid.
A number of bacterial strains of clinical interest (
S. aureus PTCC 1337,
S. epidermidis PTCC 1435,
B. cereus PTCC 1015,
E. coli PTCC 1330,
P. aeruginosa PTCC 1310,
E. faecalis PTCC 13294, methicillin-resistant
S. aureus,
Salmonella spp.) were used in the microbroth assay based on two-fold serial dilutions to investigate antibacterial activities (expressed as MICs and MBCs—
Table 17) of ketimines
89–
91. As positive controls, two broad-spectrum antibiotics, such as amikacin (a semisynthetic kanamycin derivative) and teicoplanin (a bactericidal glycopeptide), were employed to confirm the susceptibility of all bacterial strains recruited. Hassan et al. have reported that all fluorinated ketimine-type Schiff bases (
89–
91), bearing an electron-withdrawing and lipophilic fluoro group in
ortho, meta or
para position of the phenylmethyl moiety, are able to inhibit the growth of all recruited bacterial strains (including opportunistic strains of
Salmonella spp. and
P. aeruginosa) at MICs ranging from 152.3 µM to 243.6 µM. In addition, these fluorinated thiosemicarbazones have been shown to be bactericidal against most bacterial strains at a concentration of 243.6 µM. Interestingly, all the compounds—although different in having the fluorine atom in
ortho, meta or
para position at the benzyl moiety—showed the same antibacterial activities against Gram-positive and Gram-negative bacterial strains, suggesting that this activity is due to electron-withdrawing properties of the fluorine atom, and not its position [
37].
4. Fluorinated Hydrazine-Hydrazones
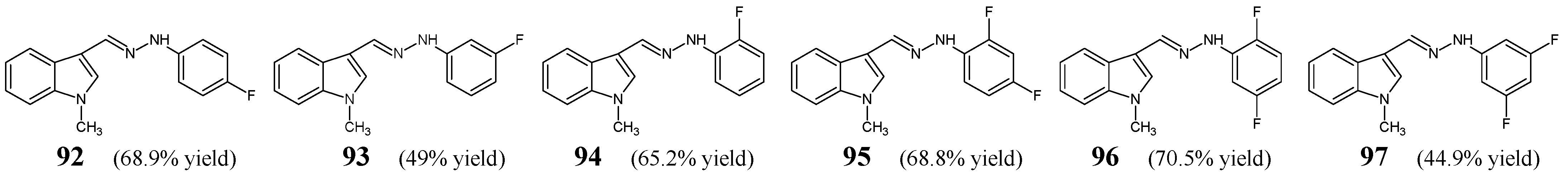
Shirinzadeh et al. [
38] have published a report in which they performed the antibacterial evaluation of six fluorinated hydrazine-hydrazones with the indole scaffold (
92–
97) (
Figure 23). These compounds were obtained by condensing 1-methylindole-3-carboxaldehyde with a small molar excess of
para-fluorophenylhydrazine,
meta-fluorophenylhydrazine,
ortho-fluorophenylhydrazine, 2,4-difluorophenylhydrazine, 2,5-difluorophenylhydrazine or 3,5-difluorophenylhydrazine, in ethanol containing sodium acetate as a catalyst [
39].
The authors have used four Gram-positive (
S. aureus ATCC 25923, methicillin-resistant
S. aureus ATCC 43300, methicillin-resistant
S. aureus isolate,
B. subtilis ATCC 6633) and one Gram-negative (
E. coli 23556) bacterial strains to determine antibacterial activities of the designed hydrazones
92–
97. For this, the biological assay based on two-fold serial dilutions was performed, in which sultamicillin, ampicillin (aminopenicillins) and ciprofloxacin (a fluoroquinolone) were used as positive controls. Among all the molecules tested only two fluorinated hydrazones: 3-{(
E)-[2-(2,4-difluorophenyl)hydrazinylidene]methyl}-1-methyl-1
H-indole (
95) and 3-{(
E)-[2-(4-fluorophenyl)hydrazinylidene]methyl}-1-methyl-1
H-indole (
92) proved to be more active than ampicillin against
B. subtilis (
Table 18) [
38].
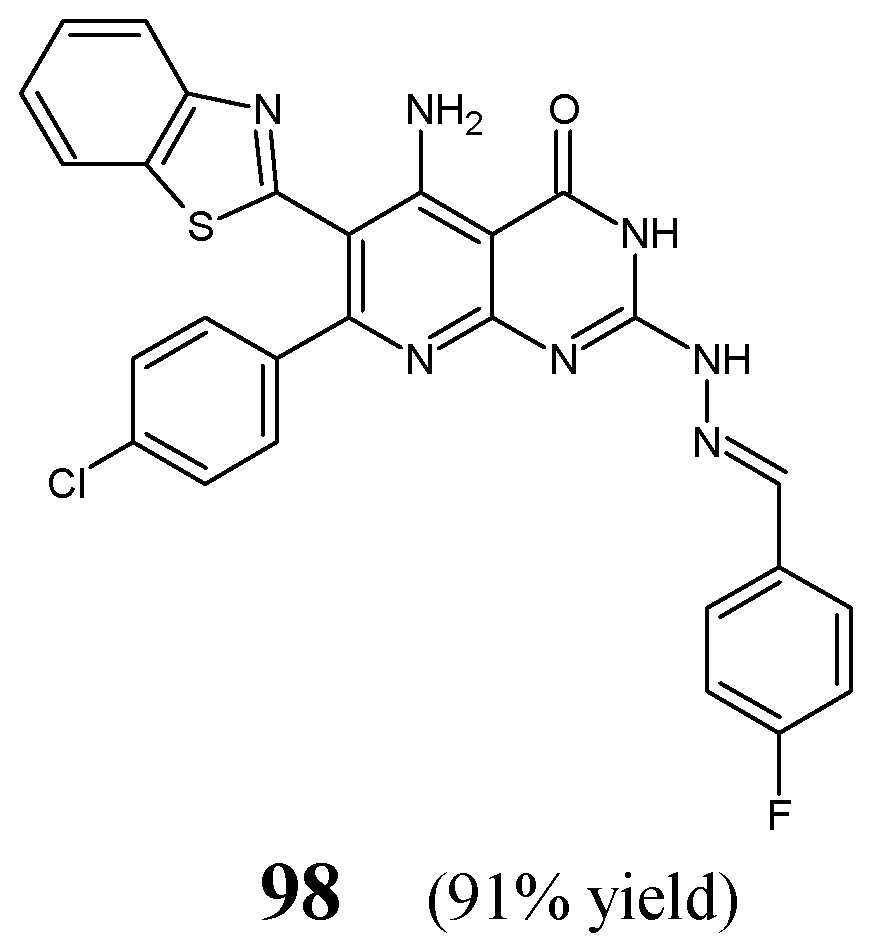
Maddila et al. [
40] have synthesized and antibacterially investigated fluorinated hydrazine-hydrazone
98 (
Figure 24) bearing the privileged pyrido[2,3-
d]pyrimidin-4(3
H)-one template. The synthesis of this hydrazone was carried out in a straightforward manner, by reacting heterocyclic hydrazine (i.e., 5-amino-6-(1,3-benzothiazol-2-yl)-7-(4-chlorophenyl)-2-hydrazinylpyrido[2,3-
d]pyrimidin-4(3
H)-one) with 4-fluorobenzaldehyde in molar ratios 1:3, at ambient temperature for 10 h without any catalyst assistance, employing
N,
N-dimethylformamide as the reaction medium.
The authors have recruited two Gram-positive (
S. aureus,
S. pyogenes) and three Gram-negative (
E. coli,
K. pneumoniae,
P. aeruginosa) bacterial strains of clinical interest to determine the antibacterial activities of hydrazone
98. For this, the biological assay based on two-fold serial dilutions was carried out. In turn, ciprofloxacin was used as a positive control. Fluorinated hydrazone
98 was reported to reveal remarkable potencies against all Gram-positive as well as Gram-negative bacteria (
Table 19). Its activity against
S. aureus and
K. pneumoniae was 3.3-fold superior to that of ciprofloxacin, while against
S. pyogenes,
E. coli and
P. aeruginosa it was 1.6-fold better when compared to this standard drug. Therefore, this molecule was proposed as a possible antibacterial agent [
40].
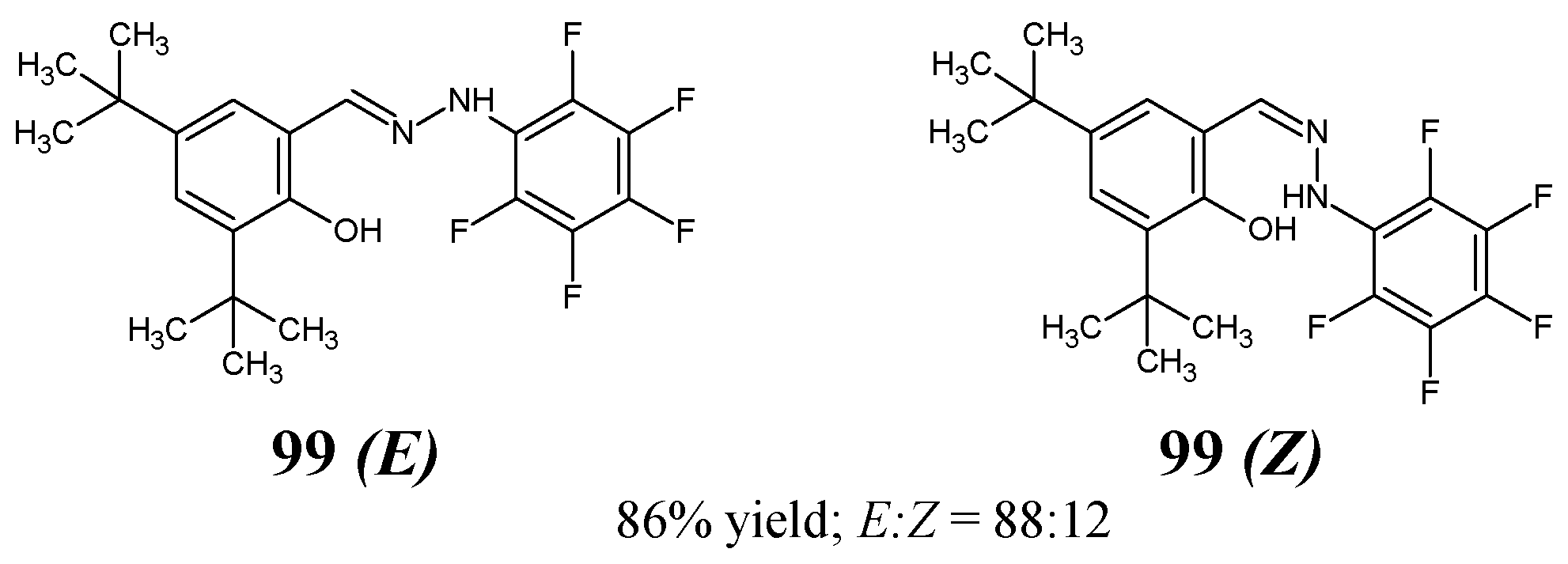
Hamurcu et al. [
41]—by reacting equimolar ratios of 3,5-di-
tert-butyl-2-hydroxybenzaldehyde and (pentafluorophenyl)hydrazine in ethanol at room temperature in the presence of catalytic amount of magnesium sulfate—have synthesized new fluorinated hydrazine-hydrazone, i.e., 3,5-di-
tert-butyl-6-[2-(pentafluorophenyl)hydrazinylidene]metyl}phenol as a mixture of
E:
Z enantiomers (
99 E and
99 Z) (
Figure 25). Based on integral intensities of signals corresponding to the proton of the OH group in 500 MHz PMR spectrum, the
E:Z enantiomer ratios in solution were established as 88:12. Additionally, the synthesized molecule was characterized by FTIR,
13C NMR,
19F NMR and X-ray diffraction data [
41].
The authors’ original paper has included information that this molecule was tested against pathogenic bacterial strains of
E. coli and
S. aureus in the broth microdilution method. However, there is a lack of a full description of these bacterial strains. Compound
99 revealed only weak antibacterial activity towards
E. coli and
S. aureus as its determined MIC values against these bacterial strains were found to be higher than 603.3 µM [
41].
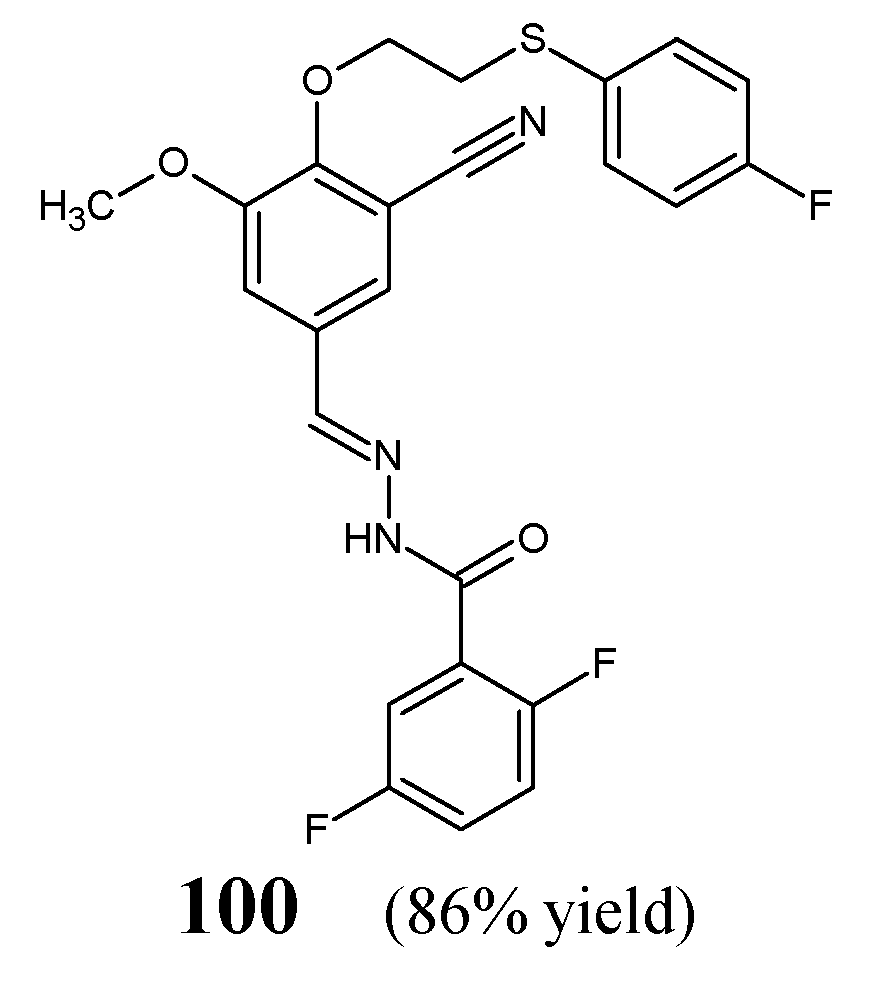
Dommati et al. [
42] have synthesized fluorinated hydrazine-hydrazone
100 (
Figure 26), by condensing equimolar ratios of 2-{2-[(4-fluorophenyl)sulfanyl]ethoxy}-5-[(
E)-hydrazinylidenemethyl]-3-methoxybenzonitrile and 2,5-difluorobenzaldehyde in ethanol under reflux for 1 h without any catalyst assistance. The authors have recorded duplication of signals in the 400 MHz
1H NMR spectrum for this compound, giving proof that this hydrazone exists as a mixture of
anti- and
syn-periplanar conformers.
The antibacterial activity of hydrazone
100 towards Gram-negative (
E. coli MTCC 2692,
P. aeruginosa MTCC 2453) and Gram-positive (
S. aureus MTCC 902,
B. subtilis MTCC 441) bacteria has been determined in the disc-diffusion method, employing streptomycin (an aminoglycoside antibiotic) as a standard drug. Unfortunately, this fluorinated hydrazone was capable of revealing only moderate antibacterial activity against bacterial strains recruited when compared to that of streptomycin [
42].
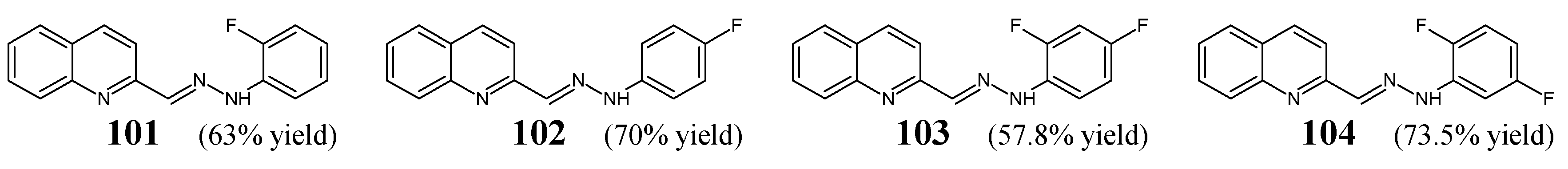
Celik et al. [
43] have reported the antibacterial studies of four fluorinated hydrazine-hydrazones (
101–
104) (
Figure 27) that have been previously synthesized. Compounds
101 and
102 were obtained by condensing quinoline-2-carbaldehyde with a small molar excess of 2-fluorophenylhydrazine or 4-fluorophenylhydrazine in boiling ethanol for 8 h [
44], whereas molecules
103 and
104 were synthesized by reacting quinoline-2-carbaldehyde with a molar excess of 2,4-difluorophenylhydrazine or 2,5-difluorophenylhydrazine in refluxing ethanol containing sodium acetate as an efficient catalyst [
45].
The authors have employed
S. aureus ATCC 29213,
S. aureus isolate,
E. faecalis ATCC 29212 and
E. faecalis isolate as Gram-positive bacteria and
E. coli ATCC 25922,
E. coli isolate,
P. aeruginosa ATCC 27853 and
P. aeruginosa isolate as Gram-negative bacteria to investigate the antibacterial activity of fluorinated hydrazones
101–
104 in the microbroth assay based on two-fold serial dilutions. Ampicillin (an aminopenicillin), vancomycin (a glycopeptide), gentamycin (an aminoglycoside), ciprofloxacin (a fluoroquinolone) and cefotaxime (a second-generation cephalosporin) were used as positive controls to confirm the susceptibility of all bacterial strains recruited. Among all the screened hydrazones, compound
104—bearing the 2,5-difluorophenyl substitution—was found to be the most active, revealing a MIC value against
E. faecalis ATCC 29212 that was superior or comparable to most antibiotics recruited (
Table 20) [
43].
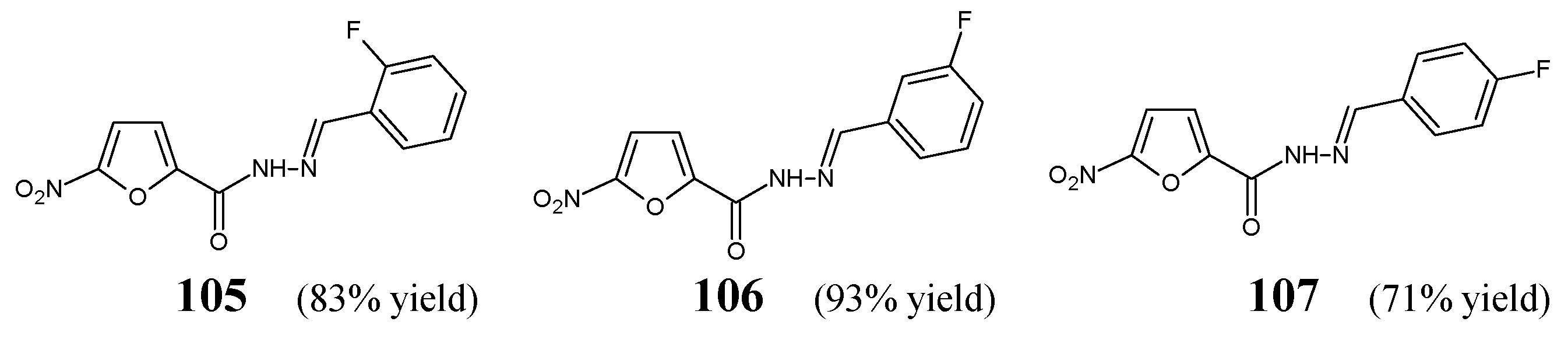
5. Fluorinated Hydrazide-Hydrazones
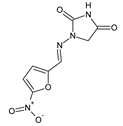
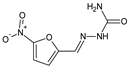
Popiołek et al. [
46] have synthesized fluorinated hydrazide-hydrazones
105–
107 (
Figure 28) by reacting 5-nitrofuran-2-carboxylic acid hydrazide with a small molar excess of
ortho-fluorobenzaldehyde,
meta-fluorobenzaldehyde or
para-fluorobenzaldehyde, respectively, in ethanol for 2 h without any catalyst assistance.
Seven Gram-positive (
S. aureus ATCC 25923,
S. aureus ATCC 6538,
S. aureus ATCC 43300,
S. epidermidis ATCC 12228,
M. luteus ATCC 10240,
B. subtilis ATCC 6633,
B. cereus ATCC 10876) and six Gram-negative (
B. bronchiseptica ATCC 4617,
K. pneumoniae ATCC 13883,
P. mirabilis ATCC 12453,
S. typhimurium ATCC 14028,
E. coli ATCC 25922,
P. aeruginosa ATCC 9027) bacterial strains have been recruited by the authors to determine—in the assay based on two-fold serial dilutions—antibacterial activities of the synthesized hydrazones
105–
107. Four antimicrobial agents—such as ciprofloxacin (a fluoroquinolone), cefuroxime (a second-generation cephalosporin), ampicillin (an aminopenicillin) and nitrofurantoin (a derivative of 5-nitrofurfural)—were used as standard drugs. All the designed fluorinated hydrazones proved to be more active against Gram-positive
S. aureus ATCC 6538,
S. epidermidis ATCC 12228 and
B. subtilis ATCC 6633 than nitrofurantoin, while against
B. subtilis ATCC 6633 also than cefuroxime and ampicillin. Additionally, compounds
105 and
106 revealed better activity against
S. aureus ATCC 43300 than that of nitrofurantoin (
Table 21) [
46].
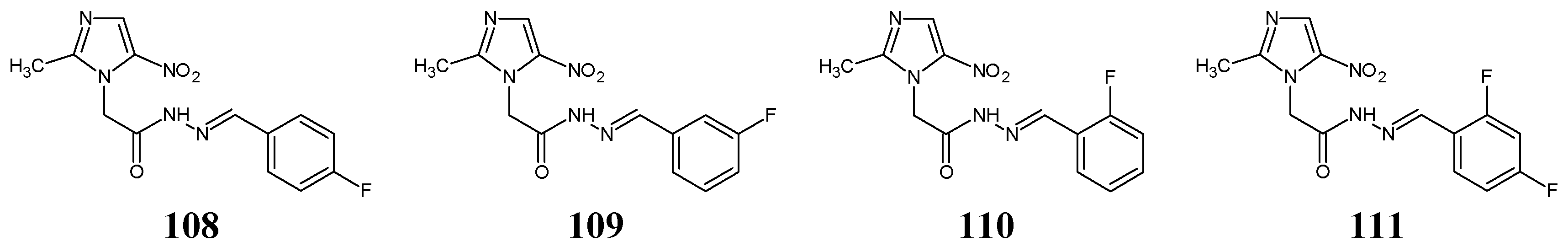
Li et al. [
47] have reported the design, synthesis scheme and results of in vitro antibacterial studies for a series of fluorinated hydrazide-hydrazones (structures
108–
111) (
Figure 29). They may also be regarded as derivatives of the drug secnidazole, revealing antibacterial and antiprotozoal activities and belonging to the common family of 5-nitroimidazoles. The synthesis of these fluorinated small molecules was performed by reacting stoichiometric ratios of the starting 2-(2-methyl-5-nitro-1
H-imidazol-1-yl)acetohydrazide with benzaldehyde fluorinated in different position/positions, in methanol (as the reaction medium) without any catalytic assistance, on ice bath for 3–6 h.
Two Gram-positive (
S. aureus ATCC 6538,
B. subtilis ATCC 530) and two Gram-negative (
E. coli ATCC 25922,
P. aeruginosa ATCC 27853) bacterial strains of clinical interest have been selected in order to determine—in the assay based on two-fold serial dilutions—antibacterial activities of all fluorinated hydrazones (
108–
111). An aminoglycoside antibiotic, kanamycin B, was employed as a positive control to confirm the susceptibility of all bacterial strains. Hydrazone
111, containing the 2,4-difluoro substitution at the phenyl moiety, was reported to reveal the highest activity against
S. aureus. However, comparing the results of preliminary antibacterial screenings carried out on particular hydrazones (
108,
109 and
110) with the substitution by one fluoro group at the phenyl moiety in
para,
meta and
ortho positions, respectively, it is clearly seen that a
meta-fluoro substitution in this class of molecules is necessary for the antibacterial activity against all bacterial strains. It has been confirmed for fluorinated hydrazone
109, which was reported to be the most active against
S. aureus and
B. subtilis (
Table 22). Even the least active fluorinated hydrazones (
108 and
110) were reported by Li and co-workers to reveal the IC
50 values of 58.3 and 47.5 µM, respectively, when tested as ligands in the target enzymatic assay for their inhibitory activities against the ecKAS III [
47].
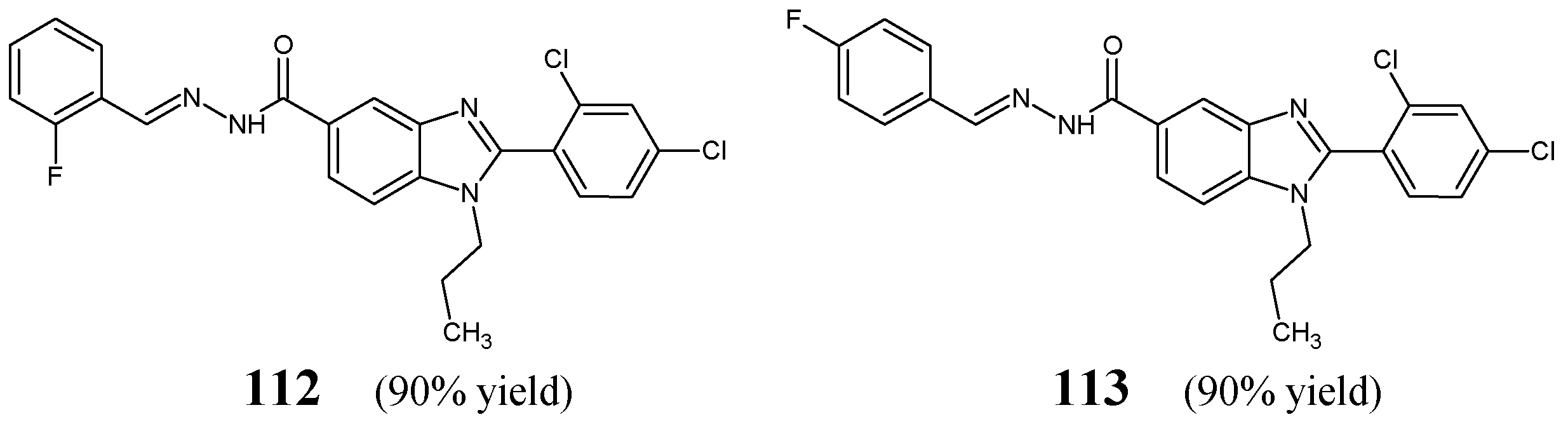
Kumar et al. [
48] have prepared fluorinated hydrazide-hydrazones
112 and
113 (
Figure 30) containing the privileged scaffold of 1
H-benzo[
d]imidazole via the condensation reaction of 1-propyl-2-(2,4-dichlorophenyl)-1
H-benzo[
d]imidazol-5-ylcarbohydrazide with 2-fluorobenzaldehyde or 4-fluorobenzaldehyde, respectively, in ethanol, in the presence of small amount of glacial acetic acid as an efficient catalyst.
Two Gram-positive (
S. aureus MTCC 3160,
B. subtilis MTCC 441) and two Gram-negative (
E. coli MTCC 4351,
K. pneumoniae MTCC 3384) bacterial strains of clinical interest have been recruited to determine antibacterial activities of hydrazones
112 and
113 in the bioassay based on two-fold serial dilutions. Ampicillin has been used as a standard antibacterial agent. Both fluorinated compounds were capable of revealing lower activities against
S. aureus,
B. subtilis,
E. coli and
K. pneumoniae than ampicillin (
Table 23). The most antibacterially active was found to be hydrazone
113 with a fluorine atom at position 4 of the phenyl moiety which proved to be two-fold more active against
S. aureus and
K. pneumoniae than hydrazone
112 with a fluorine atom at position 2, suggesting that in this case fluorine substitution in the
para position of the phenyl is preferred [
48].
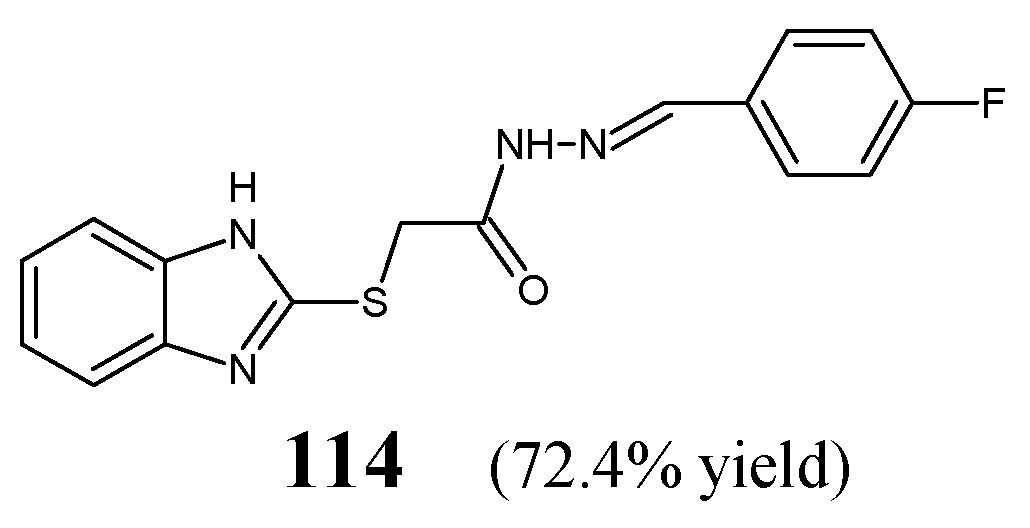
Yadav et al. [
49] have designed and synthesized fluorinated hydrazone
114, i.e., 2-(1
H-benzimidazol-2-ylsulfanyl)-
N′-[(
E)-(4-fluorophenyl)methylidene]acetohydrazide (
Figure 31), by refluxing equimolar ratios of 2-(1
H-benzimidazol-2-ylsulfanyl)acetohydrazide with 4-fluorobenzaldehyde in ethanol, in the presence of small amount of glacial acetic acid as a catalyst.
The authors have recruited three reference bacterial strains (
E. coli MTCC 1652,
B. subtilis MTCC 2063 and
S. aureus MTCC 2901) and one reference strain of
M. tuberculosis H
37Rv to determine antibacterial activities of fluorinated hydrazone
114. Standard drugs—cefadroxil (a first-generation cephalosporin) and streptomycin (an aminoglycoside antibiotic)—have been used. This compound proved to be 9-fold more potent than cefadroxil against
E. coli,
B. subtilis and
S. aureus. On the other hand, hydrazone
114 was capable of revealing 2.1-fold lower antitubercular activity than streptomycin against
M. tuberculosis (
Table 24) [
49].
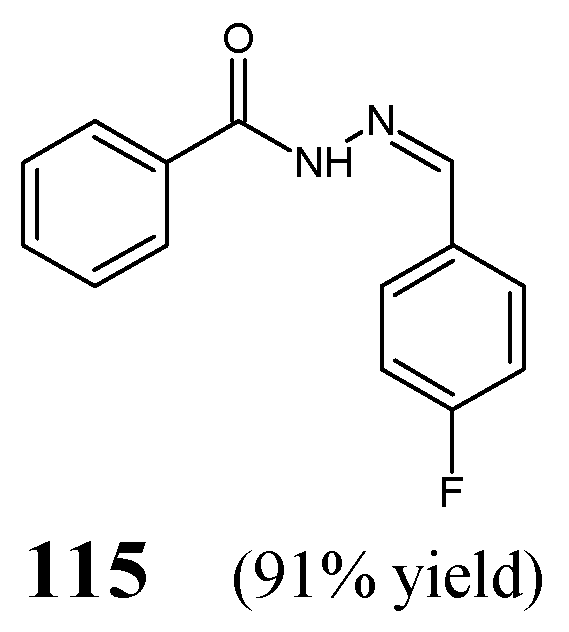
Manikandan et al. [
50] have synthesized
N′-[(
Z)-(4-fluorophenyl)methylidene]benzohydrazide
115 (
Figure 32), by stirring equimolar ratios of benzohydrazide with 4-fluorophenylbenzaldehyde, at ambient temperature for 0.5 h, in anhydrous ethanol, in the presence of sodium hydroxide.
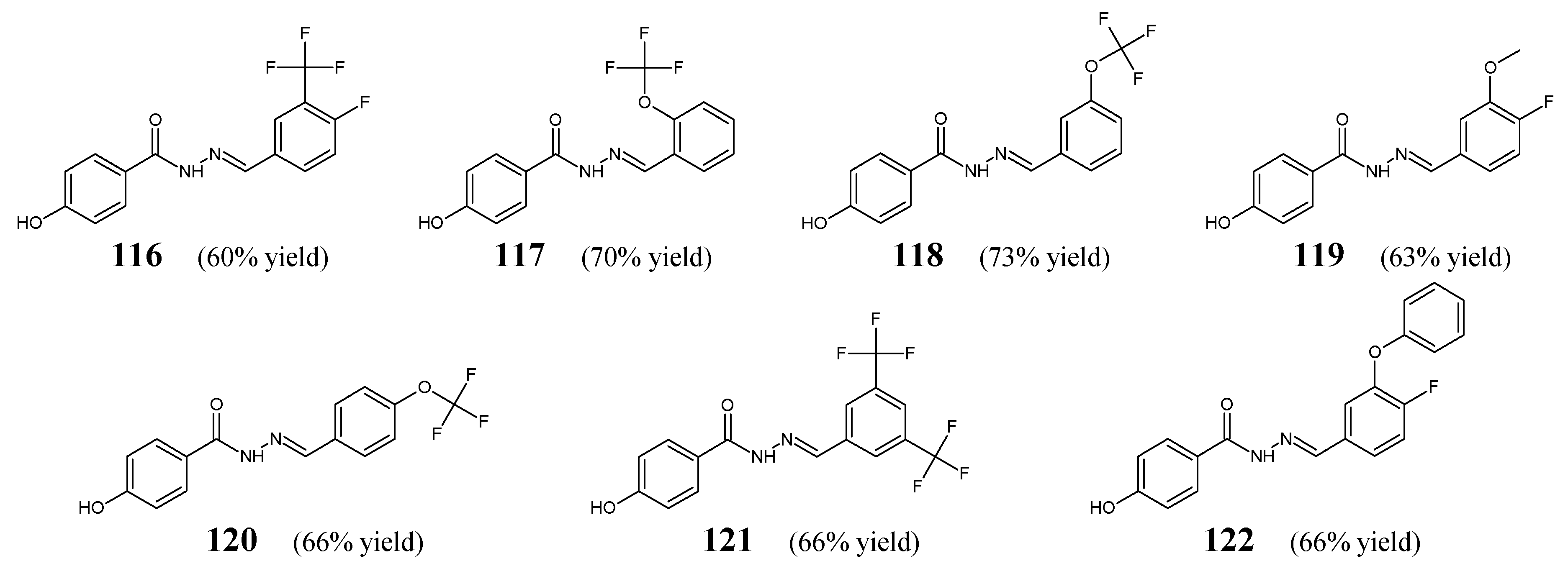
Ince et al. [
51] have conducted antibacterial studies on fluorinated hydrazide-hydrazones
116–
122 derived from
para-hydroxybenzoic acid hydrazide (
Figure 33). These compounds were synthesized by condensing equimolar quantities of substituted aromatic aldehydes (i.e., 4-fluoro-3-(trifluoromethyl)benzaldehyde, 2-(trifluoromethoxy)benzaldehyde, 3-(trifluoromethoxy)benzaldehyde, 4-fluoro-3-methoxybenzaldehyde, 4-(trifluoromethoxy)benzaldehyde, 3,5-
bis(trifluoromethoxy)benzaldehyde or 4-fluoro-3-phenoxybenzaldehyde) with this hydrazide in ethanol containing a catalytic amount of glacial acetic acid [
52].
All fluorinated hydrazones (
116–
122) have been screened in the assay MTT-based for their antibacterial activity against
S. aureus (ATCC 29213, as well as the clinical isolate) and
E. coli (ATCC 25922, as well as the clinical isolate). For comparison purposes, ampicillin, gentamicin and vancomycin were used as standard antibiotics. Among the studied molecules, only hydrazone
121 revealed significant inhibition of
S. aureus ATCC 29213 strain, and its activity was comparable (MIC = 5.3 µM) to that of ampicillin (MIC = 5.1 µM). MIC values of the remaining compounds against bacterial strains recruited ranged from 197.4 to 888.1 µM [
51]. Based on this, it can be assumed that the substitution of the phenyl moiety with two
meta-trifluoromethyl groups in
121 was responsible for the potent activity of this promising antibacterial molecule candidate.
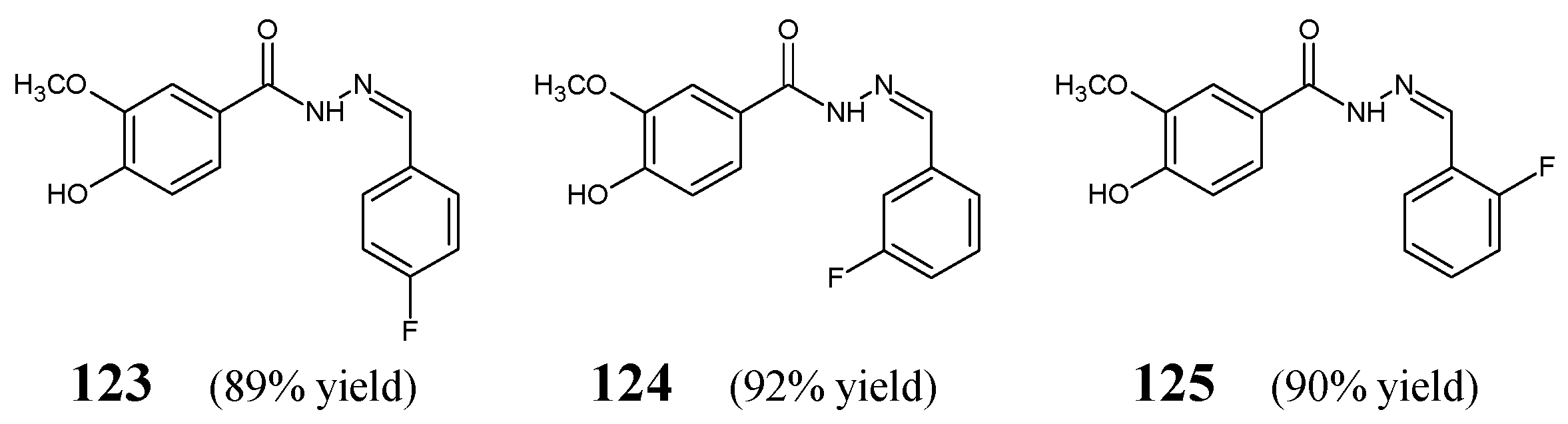
Wang et al. [
53] have designed, synthesized and investigated fluorinated hydrazide-hydrazones
123–
125 (
Figure 34) derived from vanillic acid carbohydrazide. The synthesis of the above-mentioned hydrazones was successfully performed by condensing equimolar ratios of the starting 4-hydroxy-3-methoxybenzohydrazide with
para-fluorobenzaldehyde,
meta-fluorobenzaldehyde or
ortho-fluorobenzaldehyde, in ethanol containing a small amount of glacial acetic acid as a catalyst.
Wang et al. have recruited two Gram-positive (
S. aureus ATTC 6538,
B. subtilis ATCC 530) and two Gram-negative (
E. coli ATCC 25922,
P. aeruginosa ATCC 27853) strains of bacteria to study antibacterial activities of fluorinated hydrazones
123–
125 in the assay based on two-fold serial dilutions. Kanamycin B has been mentioned to be a positive control. Results of these studies revealed that the substitution by a fluorine atom in the
meta position of the phenyl moiety in this class of compounds is the most profitable for the antibacterial activity. Therefore, fluorinated hydrazone
124, revealing MIC values ranging from 43.4 to 86.7 µM (
Table 25), proved to be distinctly more active than its
para and
ortho counterparts. Furthermore, it was confirmed that the substitution by a fluorine atom in the
para position (structure
123) is more profitable than the substitution by a fluorine atom in the
ortho position (molecule
125). Two bacterial strains such as
P. aeruginosa and
B. subtilis were found to be more susceptible to compound
123 and less susceptible to molecule
125 [
53].
Rambabu et al. [
54] have synthesized fluorinated hydrazide-hydrazones
126 and
127 (
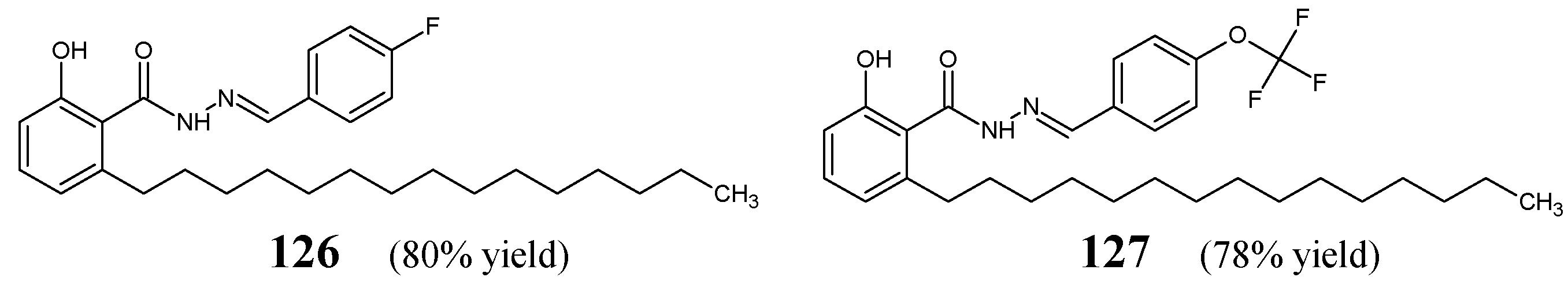
Figure 35) by condensing equimolar quantities of 2-hydroxy-6-pentadecylbenzoylhydrazide with 4-fluorobenzaldehyde or 4-(trifluoromethoxy)benzaldehyde, respectively, in ethanol under reflux for 0.5 h.
The antibacterial activity of hydrazones
126 and
127 towards Gram-negative (
P. aeruginosa MTCC 424,
E. coli MTCC 443) and Gram-positive (
S. aureus MTCC 96,
S. pyogenes MTCC 442) bacterial strains has been established at concentrations of 53.4, 106.7, 213.4 and 533.5 μM for
126 and at concentrations of 46.8, 93.5, 187.0 and 467.6 μM for
127, in the disc-diffusion method, employing ampicillin as a standard antibiotic. Hydrazone
127—with the
para-(trifluoromethoxy)phenyl group—was found to be more antibacterially active than
126—with the
para-fluorophenyl moiety. The activity of both compounds at the highest concentration proved to be similar to that of ampicillin at a concentration of 715.5 μM [
54].
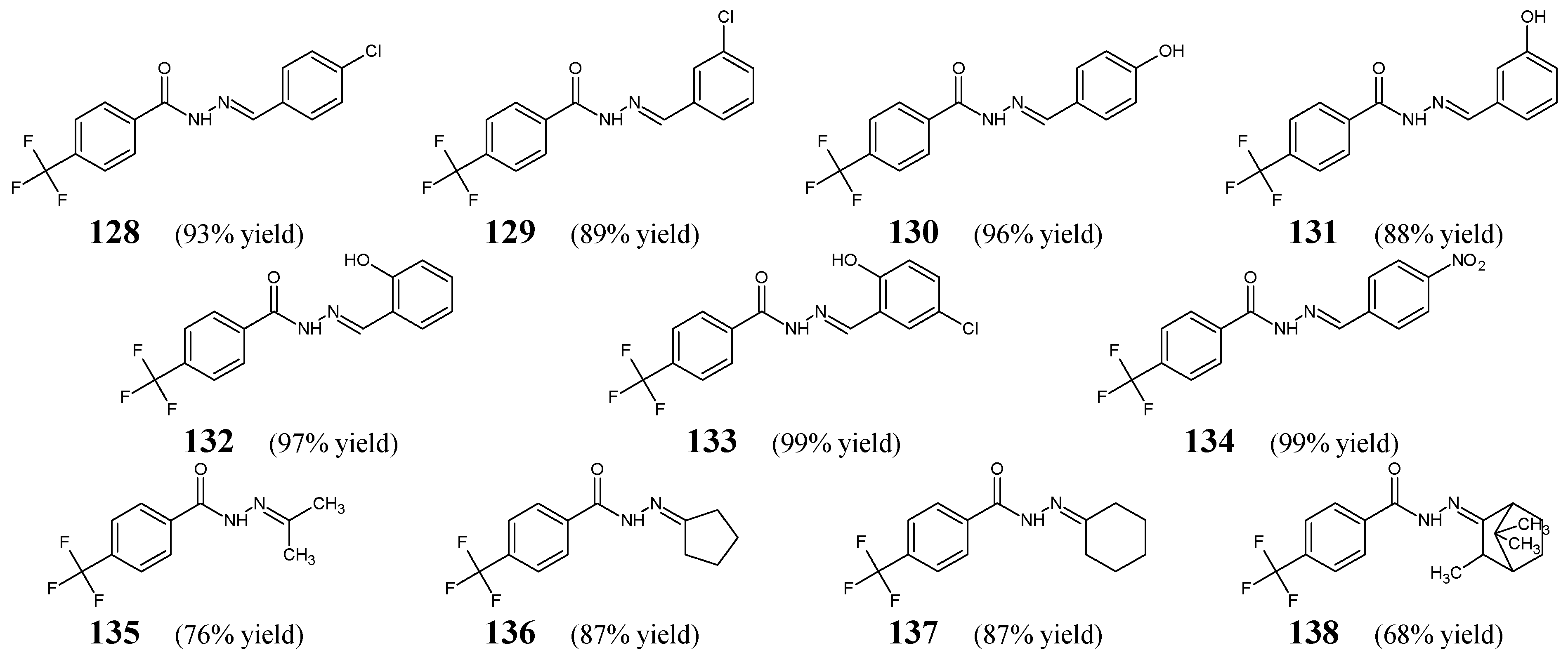
Kratky et al. [
55] have designed and synthesized fluorinated hydrazide-hydrazones
128–
134 (
Figure 36), by condensing 4-(trifluoromethyl)benzohydrazide with 4-chlorobenzaldehyde, 3-chlorobenzaldehyde, 4-hydroxybenzaldehyde, 3-hydroxybenzaldehyde, 2-hydroxybenzaldehyde, 2-hydroxy-5-chlorobenzaldehyde or 4-nitrobenzaldehyde, respectively. Moreover, the authors have obtained fluorinated hydrazide-hydrazones
135–
138 (
Figure 36) by reacting 4-(trifluoromethyl)benzohydrazide in a slight molar excess with propan-2-one, cyclopentanone, cyclohexanone or camphor, respectively, in methanol under reflux for 2 h, using a catalytic amount of concentrated sulfuric acid.
All these hydrazones (
128–
138) have been screened for their antimycobacterial activity against clinical isolates of
M. tuberculosis 331/88,
M. avium 330/88,
M. kansasii 235/80 and
M. kansasii 6509/96. Additionally, hydrazones
128–
134 have been tested for their antibacterial activity against some Gram-positive (
S. aureus CCM 4516/08, methicillin-resistant
S. aureus H 5996/08,
S. epidermidis H 6966/08,
E. faecalis J 14365/08) and Gram-negative (
E. coli CCM 4517,
K. pneumoniae D 11750/08,
K. pneumoniae J 14368/08,
P. aeruginosa CCM 1961) strains. Isoniazid (an antimycobacterial agent) and bacitracin (a cyclic peptide antibiotic) were used as standard drugs. The majority of fluorinated hydrazones were found to be more active against
M. kansasii 235/80 (
128–
134) and
M. avium 330/88 (
128,
129,
131–
133) than isoniazid, and also against
E. coli (
128,
130,
132,
133) than bacitracin (
Table 26 and
Table 27). Exclusively hydrazone
138 (i.e., 4-(trifluoromethyl)-
N′-[(2
E)-3,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]benzohydrazide) showed significant activity against
M. tuberculosis 331/88, although its potency was found to be 8-fold (after 14 days) and 4-fold (after 21 days) lower than that of isoniazid (
Table 26). In turn, compound
133, containing the 2-hydroxy-5-chlorophenyl moiety, proved to be the most active molecule against all the recruited Gram-positive bacteria, showing clearly better MIC values (2–3.9 μM) than those of bacitracin (7.8–62.5 μM) (
Table 27) [
55]. Therefore, special attention should be paid to this molecule as a possible antibacterial agent.
Coelho et al. [
56] have synthesized three fluorinated hydrazide-hydrazones, i.e.,
N′-[(
E)-(2-fluorophenyl)methylidene]pyridine-4-carbohydrazide (
139),
N′-[(
E)-(3-fluorophenyl)methylidene]pyridine-4-carbohydrazide (
140) and
N′-[(
E)-(4-fluorophenyl)methylidene]pyridine-4-carbohydrazide (
141) (
Figure 37), by reacting pyridine-4-carbohydrazide (i.e., isoniazid) with 2-fluorobenzaldehyde, 3-fluorobenzaldehyde or 4-fluorobenzaldehyde, respectively.
Fluorinated hydrazones
139–
141 have been screened for their activity against clinical isolates of
M. tuberculosis, such as isoniazid-susceptible
M. tuberculosis RG500 and isoniazid-resistant
M. tuberculosis RGH102,
M. tuberculosis RGH103 and
M. tuberculosis RGH113. All the compounds were capable of revealing significant activity—although lower than that of isoniazid—against
M. tuberculosis RG500. Hydrazone
140, bearing the
meta-fluorophenyl moiety, proved to be the most active among these compounds against
M. tuberculosis RGH103 and
M. tuberculosis RGH113 (
Table 28) [
56].
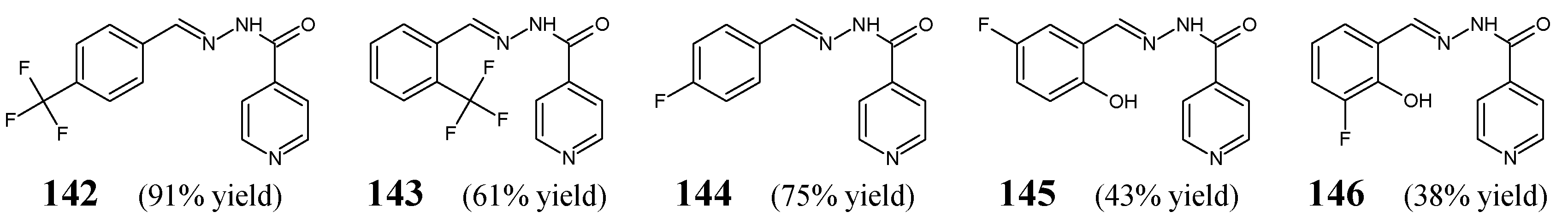
Habala et al. [
57] have synthesized and confirmed the structure (both in the solution and solid state) and studied antibacterial activities of fluorinated hydrazide-hydrazones
142–
146 (
Figure 38) derived from an antitubercular agent isoniazid (i.e., pyridine-4-carbohydrazide). The synthesis of these hydrazones was accomplished by refluxing for 80 min in a two-component methanol-chloroform solution with equimolar ratios of pyridine-4-carbohydrazide and the suitable fluorinated benzaldehyde, i.e., 4-(trifluoromethyl)benzaldehyde, 2-(trifluoromethyl)benzaldehyde, 4-fluorobenzaldehyde, 5-fluoro-2-hydroxybenzaldehyde or 3-fluoro-2-hydroxybenzaldehyde.
The authors have used Gram-positive
S. aureus CNCTC Mau 82/78 and Gram-negative
E. coli CNCTC 327/73 to study their vulnerability to fluorinated hydrazones
142–
146, to which antibacterial activities were determined in the assay based on two-fold serial dilutions. Ciprofloxacin was employed as an antibacterial agent. Results have revealed that the synthesized compounds possess very weak activities against
S. aureus and
E. coli (
Table 29). In addition, their activities against the above bacterial strains were found to be distinctly lower than that of ciprofloxacin. Hydrazone
142, containing a
para-trifluoromethyl group at the phenyl moiety, was reported to be 2-fold more active against
E. coli than its counterpart
143, bearing an
ortho-trifluoromethyl group at the phenyl moiety. In turn, hydrazone
145, containing 5-fluoro-2-hydroxy substitutions at the phenyl moiety, was disclosed to be 16-fold more active against
E. coli than its counterpart
146, bearing 3-fluoro-2-hydroxy substitutions in the same moiety. In addition, all hydrazones were found to the distinctly less active (ICs
50 > 500 µM) than acetohydroxamic acid (IC
50 = 185 µM) when tested as urease inhibitors [
57]. Considering the fact that all these fluorinated hydrazones (
142–
146) were obtained from isoniazid effective against human tuberculosis, further studies with the use of
Mycobacterium tuberculosis H
37Rv and its resistant strains to antitubercular agents are needed to confirm or rule out their anticipated antituberculosis activity.
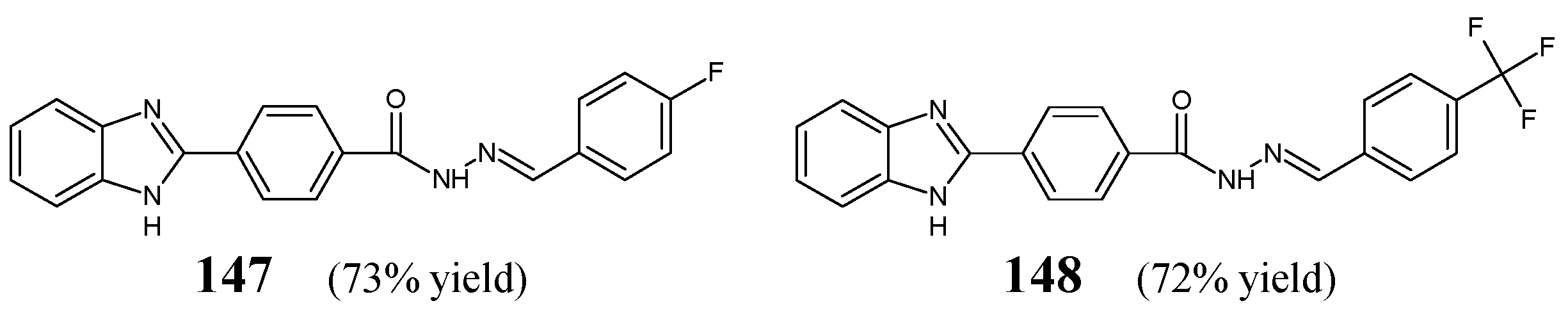
Ozkay et al. [
58] have obtained 4-(1
H-benzimidazol-2-yl)-
N′-[(
E)-(4-fluorophenyl)methylidene]benzohydrazide (
147) and 4-(1
H-benzimidazol-2-yl)-
N′-{(
E)-[4-(trifluoromethyl)phenyl]methylidene}benzohydrazide (
148) (
Figure 39) by condensing equimolar ratios of 4-(1
H-benzimidazole-2-yl)benzoic acid hydrazide with 4-fluorobenzaldehyde or 4-(trifluoromethyl)benzaldehyde, respectively, in
n-butanol under reflux for 3 h, with a small amount of glacial acetic acid as an efficient catalyst.
Fluorinated hydrazones
147 and
148 have been evaluated for their activity against four Gram-positive strains of bacteria, such as
L. monocytogenes,
S. aureus ATCC 25923,
E. faecalis ATCC 29212,
B. subtilis and six Gram-negative strains of bacteria, such as
E. coli ATCC 35218,
E. coli ATCC 25922,
P. vulgaris NRRL B-123,
S. typhimurium NRRL B-4420,
K. pneumoniae ATCC 13883,
P. aeruginosa ATCC 27853. Chloramphenicol (a derivative of propandiol) has been used as a standard antibiotic. Both fluorinated compounds revealed better activities against Gram-negative
P. vulgaris,
S. typhimurium and
P. aeruginosa than those of chloramphenicol. Additionally, hydrazone
148, containing the
para-trifluoromethyl moiety, proved to be more active against Gram-positive
E. faecalis than this standard drug (
Table 30) [
58].
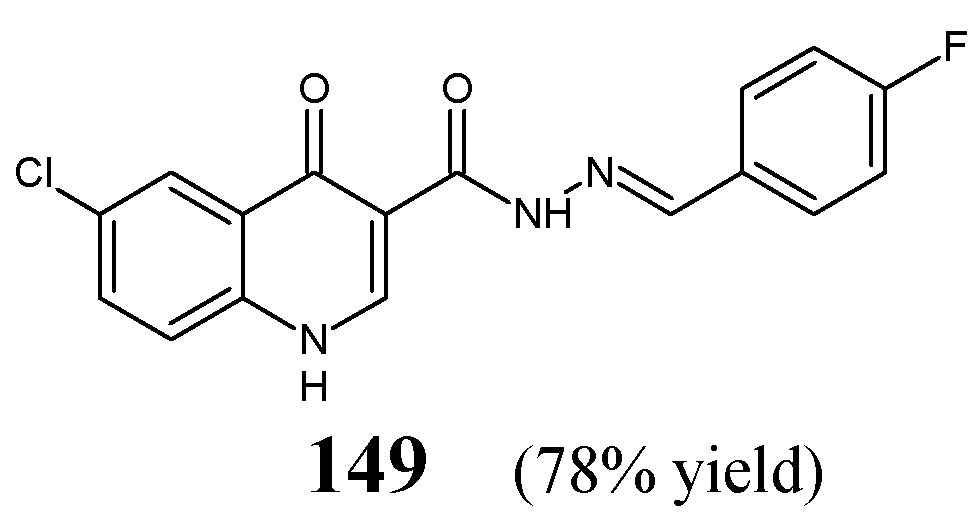
Abdelrahman et al. [
59] have synthesized heterocyclic hydrazide-hydrazone, i.e., 6-chloro-
N′-[(
E)-(4-fluorophenyl)methylidene]-4-oxo-1,4-dihydroquinoline-3-carbohydrazide (
149) (
Figure 40) by condensing equimolar ratios of 6-chloro-4-oxo-1,4-dihydroquinoline-3-carbohydrazide and 4-fluorobenzaldehyde in
N,
N-dimethylformamide under reflux for 4 h.
The fluorinated hydrazone
149 has been tested for its activity against
S. pneumoniae RCMB 010010,
S. aureus RCMB 010028,
P. aeruginosa RCMB 010043 and
E. coli RCMB 010052 in the two-fold dilution assay. Ampicillin and ciprofloxacin have been employed as standard drugs. Hydrazone
149 was able to reveal higher activity against Gram-positive
S. pneumoniae and
S. aureus. Nevertheless, its activity towards these bacterial strains was unfortunately about 16- and 32-fold lower than that of ampicillin (
Table 31) [
59].
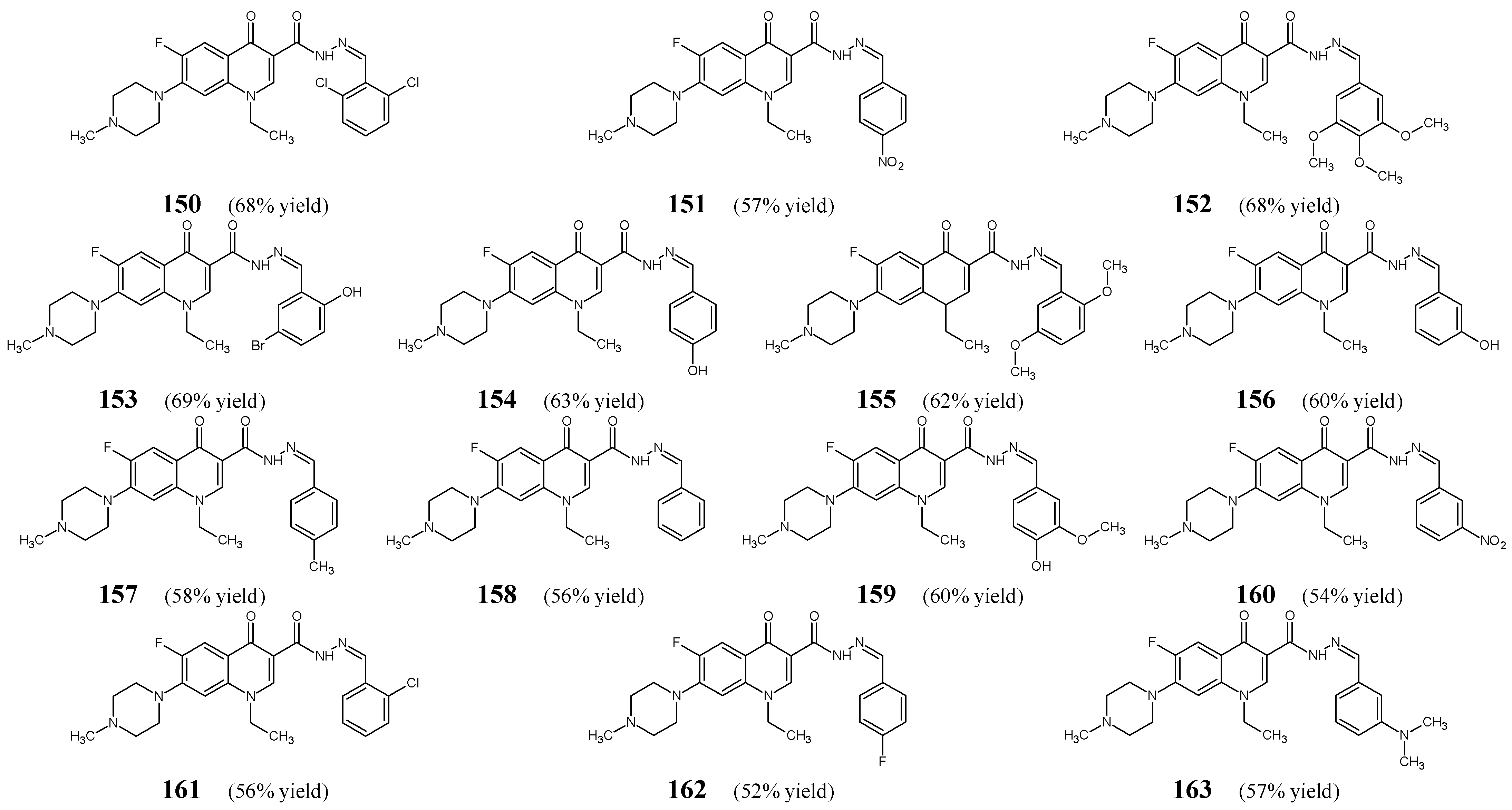
Allaka et al. [
60] have designed and synthesized fourteen fluorinated hydrazide-hydrazones
150–
163 (
Figure 41), by stirring for 2.5–4 h in ethanol at ambient temperature 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carbohydrazide with a small molar excess of 2,6-dichlorobenzaldehyde, 4-nitrobenzaldehyde, 3,4,5-trimethoxybenzaldehyde, 5-bromo-2-hydroxybenzadehyde, 4-hydroxybenzaldehyde, 2,5-dimethoxybenzaldehyde, 3-hydroxybenzaldehyde, 4-methylbenzaldehyde, benzaldehyde, 3-methoxy-4-hydroxybenzaldehyde, 3-nitrobenzaldehyde, 2-chlorobenzaldehyde, 4-fluorobenzaldehyde or 3-(
N,N-dimethylamino)benzaldehyde, respectively. Moreover, the same compounds have been prepared under microwave irradiation for 1–3 min.
All these hydrazones (
150–
163) have been initially tested for their ability to inhibit the growth of
M. smegmatis. Exclusively active compounds—that were capable of showing at least 30% inhibition of the growth of this bacterium—have been subjected to further evaluation of their MICs. Finally, the MIC values of molecules
150,
152,
156, and
157 have been determined and compared to those of commonly used antitubercular agents such as rifampicin and isoniazid. The activity of the most potent compounds bearing the 2,6-dichlorophenyl or
meta-hydroxyphenyl moiety (
150 and
156) proved to be more than twice less active than isoniazid (
Table 32) [
60].
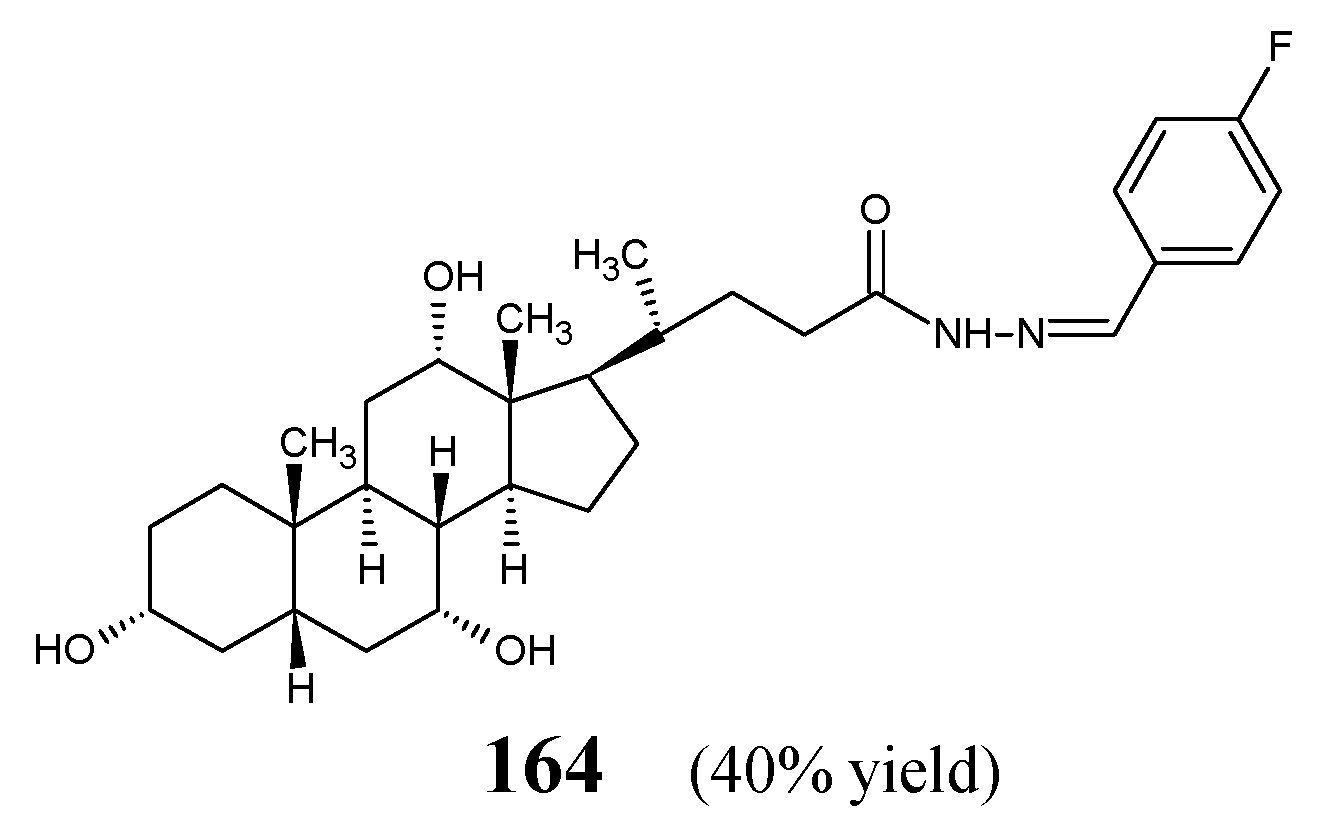
Rasras et al. [
61] have obtained steroidal hydrazide-hydrazone, i.e., (3α,5β,7α,12α)-3,7,12-trihydroxy-
N-[(1
E)-4-fluorophenylmethylene]cholan-24-hydrazide (
164) (
Figure 42) by heating cholinic acid hydrazide with 4-fluorobenzaldehyde in anhydrous ethanol for 10 h.
The activity of fluorinated hydrazone
164, expressed as MIC values, has been evaluated against
E. coli,
P. aeruginosa,
E. aerogenes (Gram-negative bacteria) and
S. aureus,
E. faecalis,
B. megaterium (Gram-positive bacteria) in the two-fold serial dilution assay and compared to that of cefaclor and cefixime from a second- and third-generation cephalosporins, respectively. This hydrazone was active against Gram-positive
S. aureus,
E. faecalis and
B. megaterium. Simultaneously, this molecule was capable of revealing higher activity against
S. aureus than cefaclor and cefixime as well as against
B. megaterium than cefaclor. In turn, hydrazone
164 was found to be inactive against all the recruited Gram-negative bacterial strains (
Table 33) [
61].
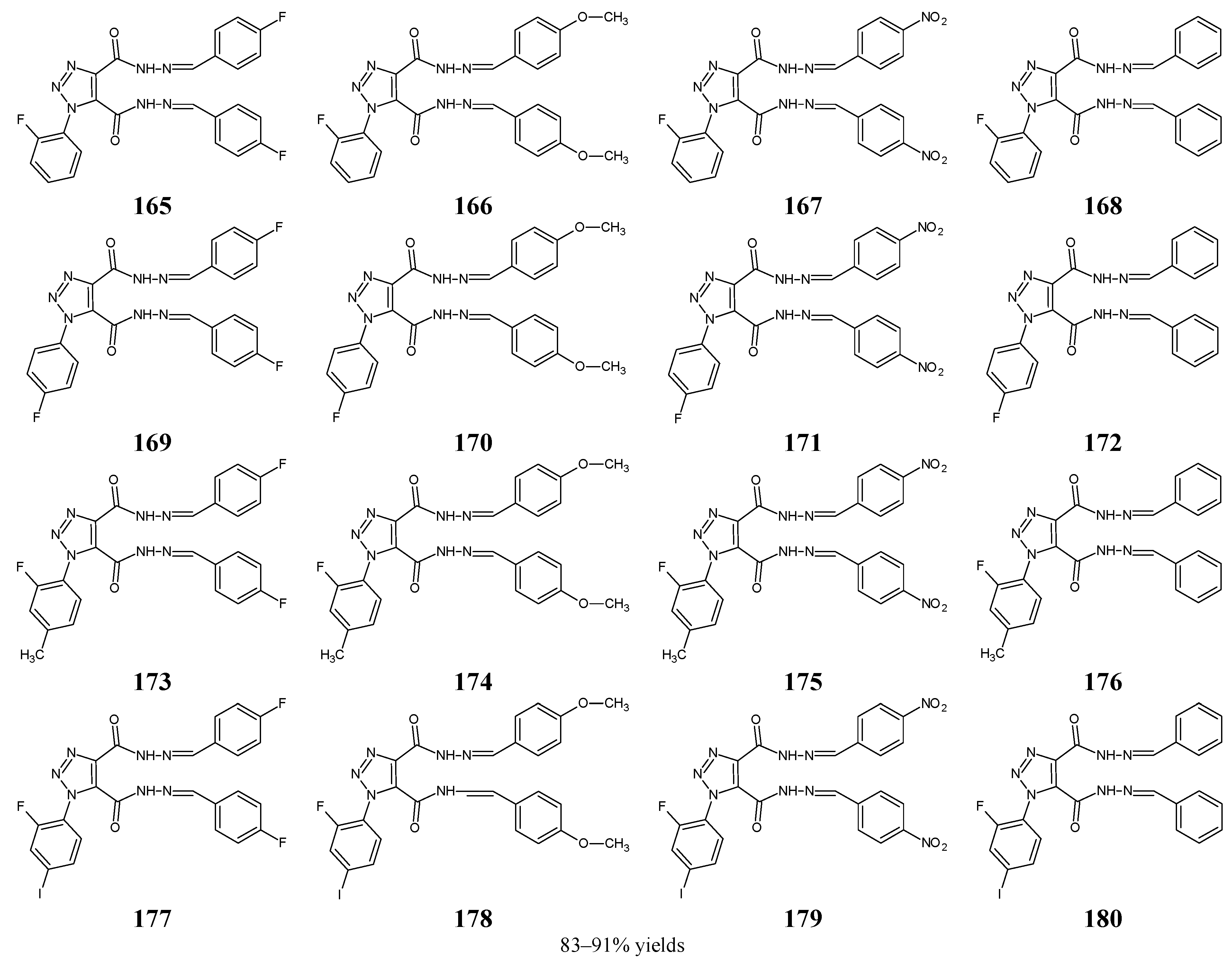
Aouad [
62] has reported the synthesis and biological evaluation of fluorinated
bis-hydrazones (
165–
180) (
Figure 43). The synthesis of these hydrazide-hydrazones was performed by refluxing 1-(R-phenyl)-1
H-1,2,3-triazole-4,5-dicarbohydrazide with benzaldehyde and its derivatives, such as 4-fluorobenzaldehyde, 4-methoxybenzaldehyde, or 4-nitrobenzaldehyde, for 2 h in ethanol with catalytic assistance of small amount of hydrochloric acid.
Standard pathogenic strains of Gram-positive (
S. aureus RCMB 010025,
S. pneumoniae RCMB 010010,
B. subtilis RCMB 010067) and Gram-negative (
P. aeruginosa RCMB 010043,
K. pneumoniae RCMB 010058,
E. coli RCMB 010052) clinical isolates have been included to assess antibacterial activities of hydrazones
165–
180 in the assay based on two-fold serial dilutions. As a positive control, a broad-spectrum fluoroquinolone ciprofloxacin was used to confirm the susceptibility of all bacteria recruited. Fluorinated
bis-hydrazone structures
165,
167,
169,
171,
175,
177–
180 have been reported as the most active molecules, revealing remarkable activities (with MIC values ranging from 6.0 to 28.6 µM) against all bacterial strains (
Table 34). Their efficacy towards
S. pneumoniae,
S. aureus and
P. aeruginosa was often higher or comparable to that of ciprofloxacin. The author proved that enhanced antibacterial potency was achieved by introducing two preferred
para-fluorophenyl or
para-nitrophenyl moieties. He suggested that an azomethine bridge as well as the 1,2,3-triazole scaffold are necessary for the antibacterial activity in this series of molecules [
62].
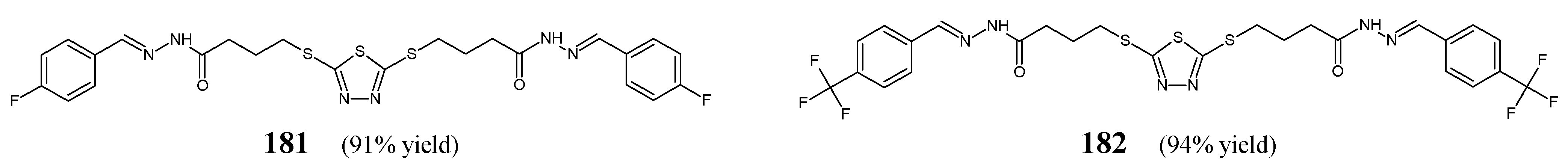
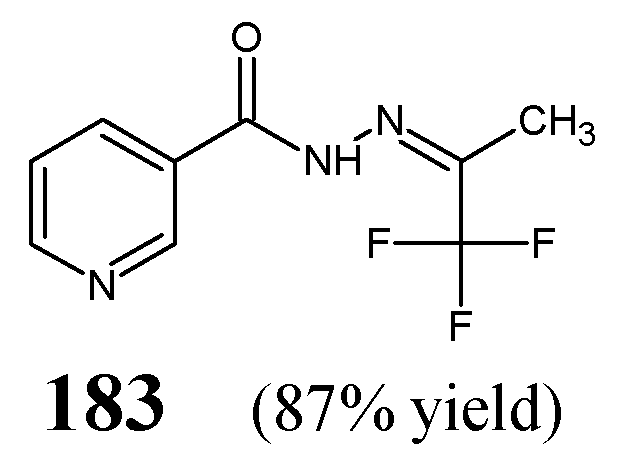
Rezki et al. [
63] have reported the synthesis, structural characterization and antibacterial evaluation of fluorinated
bis-hydrazide-hydrazones
181 and
182 (
Figure 44). The synthesis of hydrazones
181 and
182 was carried out by refluxing 4,4′-(1,3,4-thiadiazole-2,5-diyldisulfanediyl)dibutanehydrazide with 4-fluorobenzaldehyde or 4-trifluoromethylbenzaldehyde, respectively, in ethanol for 4–6 h with the use of catalytic assistance of small amount of hydrochloric acid.
Rezki and co-workers have recruited Gram-positive (
S. pneumoniae RCMB 010010,
B. subtilis RCMB 010067,
S. aureus RCMB 010025) and Gram-negative (
P. aeruginosa RCMB 010043,
K. pneumoniae RCMB 010058,
E. coli RCMB 010052) bacterial strains of clinical interest to assess antibacterial activities of fluorinated hydrazones
181 and
182 in the assay based on two-fold serial dilutions. As a positive control, a broad-spectrum fluoroquinolone ciprofloxacin was used to confirm the susceptibility of all microorganisms recruited. Both fluorinated
bis-hydrazide-hydrazones have been disclosed to possess remarkable antibacterial effects against all pathogenic microorganisms with MIC values ranging from 7.1 µM to 24.2 µM (
Table 35). In addition, it is clearly seen that three bacterial strains:
S. aureus,
E. coli and
B. subtilis are more susceptible to
bis-hydrazone
181, containing two
para-fluorophenyl moieties. Simultaneously, the activity of both fluorinated
bis-hydrazones against
S. pneumoniae and
P. aeruginosa as well as the efficacy of compound
181 towards
S. aureus was better or similar to that of ciprofloxacin [
63].
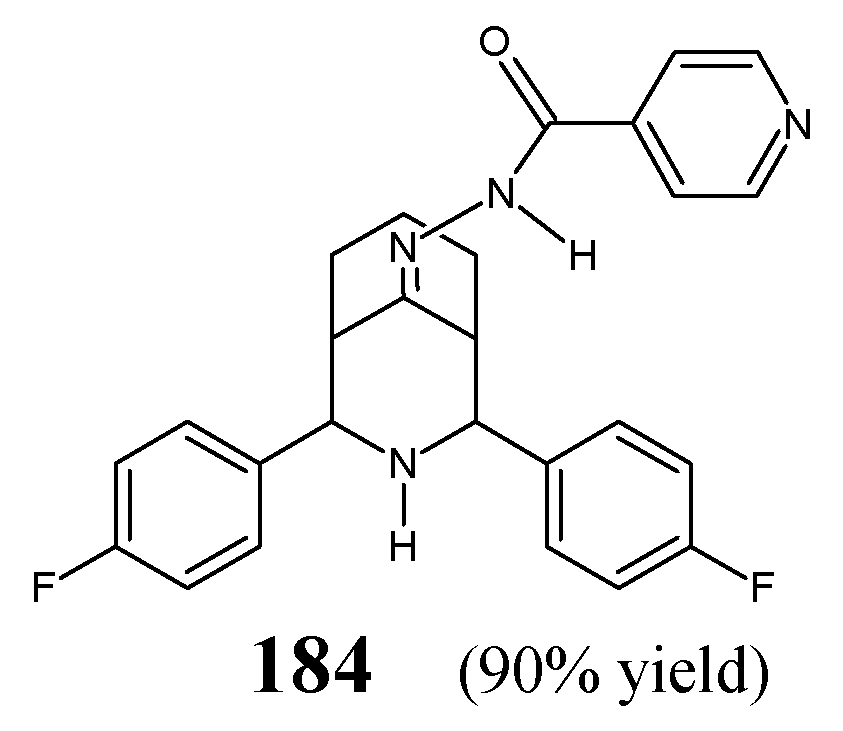
Morjan et al. [
64] have synthesized fluorinated hydrazide-hydrazone
183, i.e.,
N′-[(2
Z)-1,1,1-trifluoropropan-2-ylidene]pyridine-3-carbohydrazide (
Figure 45) by refluxing in ethanol pyridine-3-carbohydrazide with 1,1,1-trifluoropropan-2-one.
The activity of fluorinated hydrazone
183 against
P. aeruginosa,
K. pneumoniae and
S. aureus has been established in the two-fold dilution assay. The tested molecule revealed remarkable potency against
P. aeruginosa. Unfortunately, its antibacterial activity has not been compared to any known antibacterial agent (
Table 36) [
64].
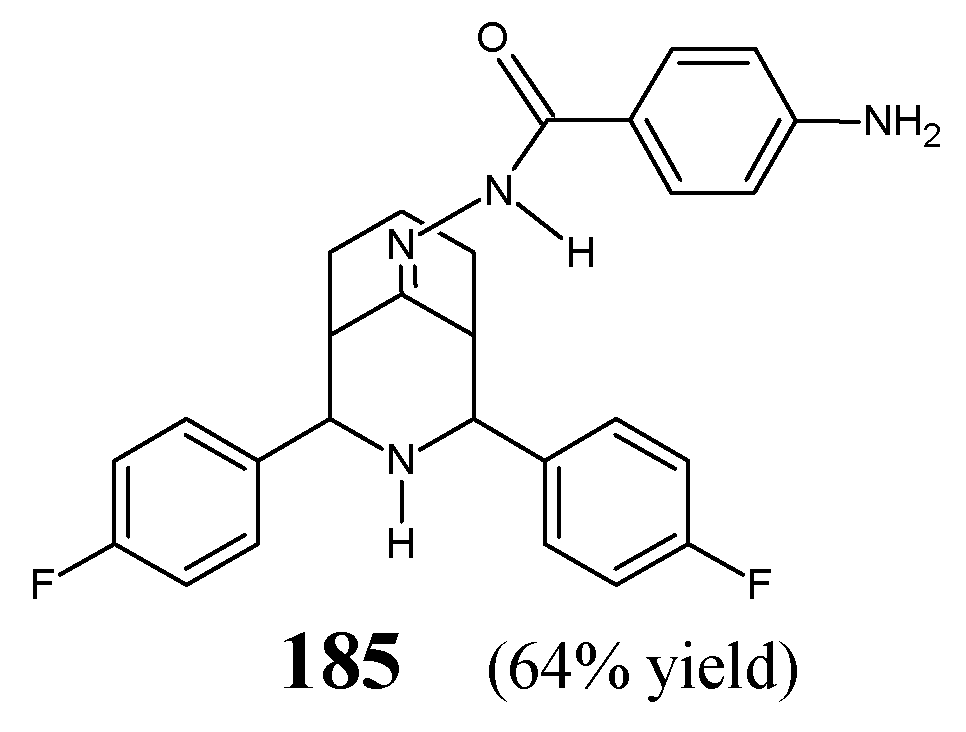
Sankar and Pandiarajan [
65] have carried out a study on fluorinated hydrazone
184 (
Figure 46) incorporating in the molecular framework the pharmacophoric isonicotinic acid hydrazide-hydrazone moiety. This moiety is also present in antimycobacterial hydrazones of aromatic aldehydes obtained from isoniazid, such as ftivazid, verazid and furilazon, which have been used in clinical practice as antitubercular agents that were less toxic than isoniazid. The synthesis of this hydrazone was accomplished successfully by reacting 2,4-
bis(4-fluorophenyl)-3-azabicyclo[3.3.1]nonan-9-one with an excess of isonicotinic acid hydrazide for 2–3 h in refluxing two-component methanol-chloroform solution (1:1), containing a small amount of acetic acid as an efficient catalyst.
Two clinically important strains of
M. tuberculosis, i.e.,
M. tuberculosis H
37Rv ATCC 27294 and resistant to isoniazid
M. tuberculosis, have been selected by the authors to evaluate antimycobacterial activities (expressed as the percentage of reduction in the Related Lights Units—RLU) of fluorinated hydrazone
184 at two concentrations (2.24 and 4.48 µM) in the luciferase reporter phage assay. In addition, two Gram-positive (
S. aureus NCIM 2492,
B. subtilis NCIM 2439) and three Gram-negative (
E. coli NCIM 2345,
P. aeruginosa NCIM 2035,
K. pneumoniae) bacteria have been recruited to study antibacterial activities of this molecule. Isoniazid, penicillin G and streptomycin were used as standard antibacterial agents. Fluorinated structure
184 was proposed as a potential antimycobacterial agent, showing at concentrations of 2.24 and 4.48 µM very good in vitro potency against
M. tuberculosis H
37Rv (76.63 and 86.12% reduction in RLU, respectively) and
M. tuberculosis strain resistant to isoniazid (67.63 and 75.08% reduction in RLU, respectively). Moreover, this hydrazone was found to be active against
B. subtilis and
S. aureus with a MIC value of 112.0 µM, and was able to completely inhibit the growth of
E. coli and
P. aeruginosa at a MIC value of 224.0 µM (
Table 37). In addition, its activity against
S. aureus was only 1.3-fold lower than that of streptomycin, and against
B. subtilis—1.5-fold lower than that of penicillin. G. Sankar and Pandiarajan have suggested that the release of the active hydrazide structure of isoniazid via hydrolysis of an azomethine bond and the presence (at both phenyl moieties) of two fluorine atoms capable of forming the strong hydrogen bond, are responsible for the promising antitubercular action of hydrazone
184 [
65].
Xaiver et al. [
66] have synthesized fluorinated hydrazide-hydrazone, i.e., 4-amino-
N′-[2
r,4
c-
bis(4-fluorophenyl)]-3-azabicyclo[3.3.1]non-9-ylidene)benzohydrazide (
185) (
Figure 47) by condensing 4-aminobenzoic acid hydrazide (in a molar excess) with 2,4-difluorophenyl-3-azabicyclo[3.3.1]nonan-9-one in methanol/chloroform (1:1
v/
v) under reflux for 2–4 h.
The fluorinated hydrazone
185 has been tested against
S. typhimurium MTCC 98,
E. coli MTCC 443,
V. cholerae,
S. typhi MTCC 531,
P. aeruginosa MTCC 741,
K. pneumoniae MTCC 2272,
B. subtilis MTCC 121 and
S. aureus MTCC 96 in the two-fold serial dilution assay. Its antibacterial activity expressed as MIC values has been compared to that of streptomycin (
Table 38). This hydrazone proved to be the most active against
B. subtilis, although its potency against this bacterial strain was found to be 2.5-fold lower than that of streptomycin. Additionally, the activity of this molecule against
V. cholerae was only 1.2-fold weaker than that of the standard drug [
66].
Kodisundaram et al. [
67] have obtained fluorinated hydrazide-hydrazone
186 (
Figure 48), by refluxing 4-methyl-1,2,3-thiadiazole-5-carboxylic acid hydrazide (in a molar excess) with 2,4-difluorophenyl-3-azabicyclo[3.3.1]nonan-9-one, in a methanol/chloroform mixture (1:1
v/
v), for 3–4 h, in the presence of catalytic amount of acetic acid.
The authors have screened the antibacterial activities of hydrazone
186 against two Gram-positive (
B. subtilis,
S. aureus) and three Gram-negative
(K. pneumoniae,
E. coli,
P. aeruginosa) strains in the two-fold serial dilution assay, and compared its activities to those of streptomycin. This fluorinated hydrazone proved to be 1.6-fold more potent towards
B. subtilis,
K. pneumoniae and
E. coli than the standard drug (
Table 39) [
67].
Kaki et al. [
68] have synthesized two fluorinated hydrazide-hydrazones, i.e., 2-(2,3-dihydro-1-benzofuran-5-yl)-
N′-[(1
E)-1-(4-fluoro-2-hydroxyphenyl)ethylidene]acetohydrazide (
187) and 2-(2,3-dihydro-1-benzofuran-5-yl)-
N′-[(1
E)-1-(4-fluorophenyl)ethylidene]acetohydrazide (
188) (
Figure 49) by refluxing 2-(2,3-dihydro-1-benzofuran-5-yl)acetohydrazide (in a molar excess) with 1-(4-fluoro-2-hydroxyphenyl)ethanone or 1-(4-fluorophenyl)ethanone, respectively, in ethanol for 8 h in the presence of glacial acetic acid as an efficient catalyst.
These fluorinated hydrazones (
187 and
188) have been screened for their activity against
E. coli MTCC 443 and
P. aeruginosa MTCC 424 (Gram-negative bacteria) as well as
S. aureus MTCC 96 and
S. pyogenes MTCC 442 (Gram-positive bacteria) in the disc-diffusion assay, using as a standard antibiotic ampicillin at a concentration of 715.5 μΜ. Both compounds—
187 at a concentration of 761.4 μΜ and
188 at a concentration of 800.4 μΜ—were capable of revealing remarkable antibacterial activity against all the recruited bacterial strains, as their zones of inhibition were comparable to those of ampicillin [
68].
Skrickus et al. [
69] have synthesized
bis-hydrazide-hydrazone
189 (
Figure 50) by condensing 3,3′-[disulfanediylbis(benzene-2,1-diylimino)]dipropanoic acid hydrazide with 4-fluorobenzaldehyde (in molar ratios 1:2.5) in boiling isopropanol for 2–3 h.
Fluorinated hydrazone
189 has been screened for its activity against
S. aureus ATCC 9144,
L. monocytogenes ATCC 35152,
E. coli ATCC 13076 and
S. enterica ATCC 8739 in the microbroth assay based on two-fold serial dilutions, using a first-generation cephalosporin—cefazolin––as a standard drug. This compound was found to be antibacterially active, revealing significant potency against
L. monocytogenes and moderate activities against the remaining recruited bacterial strains. Simultaneously, its MIC value against
L. monocytogenes proved to be slightly lower than that of a standard drug [
69].
Haj Mohammad Ebrahim Tehrani et al. [
36] have described the antibacterial evaluation of fluorinated hydrazide-hydrazones
190–
191 (
Figure 51) obtained from stoichiometric ratios of benzohydrazide or isonicotinic acid hydrazide and 5-fluoro-1
H-indole-2,3-dione, in refluxing ethanol for 4–6 h, with assistance of small amount of glacial acetic acid as an efficient catalyst. The authors have reported that microwave irradiation at 110 °C for 5 min was an alternative method of their synthesis.
Antibacterial screening of hydrazide-hydrazones
190–
191 has been performed in the assay based on two-fold serial dilutions against three Gram-positive (
S. aureus ATCC 25923, methicillin-resistant
S. aureus ATCC 43300,
E. faecalis ATCC 29212) and two Gram-negative (
P. aeruginosa ATCC 27853,
E. coli ATCC 25922) bacterial strains of clinical interest. Amikacin (an aminoglycoside antibiotic) was included as a positive control. The results of the study have confirmed that fluorinated hydrazone related to benzoic acid hydrazide (
190), revealing a broader spectrum of antibacterial activity, is distinctly more active than fluorinated hydrazone related to isonicotinic acid hydrazide (
191) (
Table 40) [
36].