Vesicular Messages from Dental Biofilms for Neutrophils
Abstract
1. Introduction
2. Results
2.1. Dental Biofilms Are Rich in BEVs of Varying Morphologies
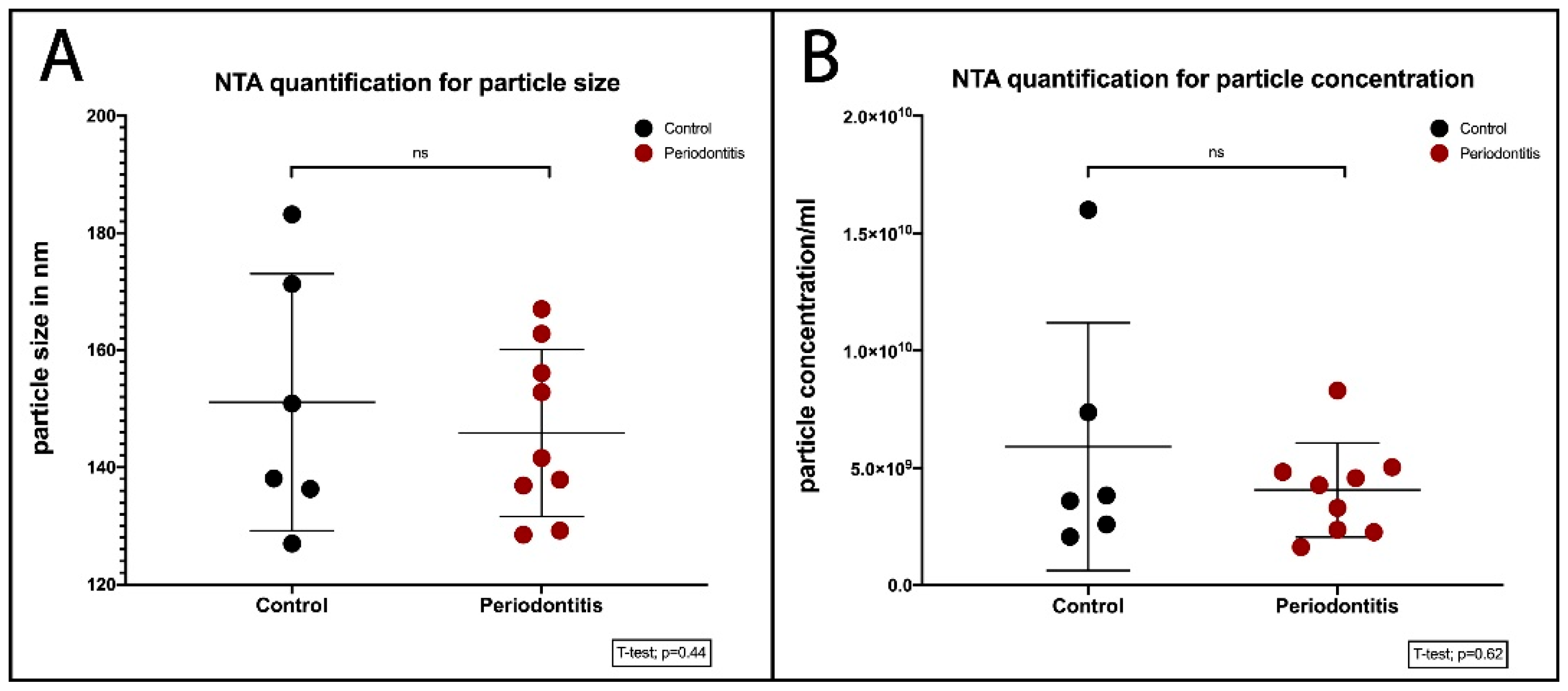
2.2. Quantification of BEVs
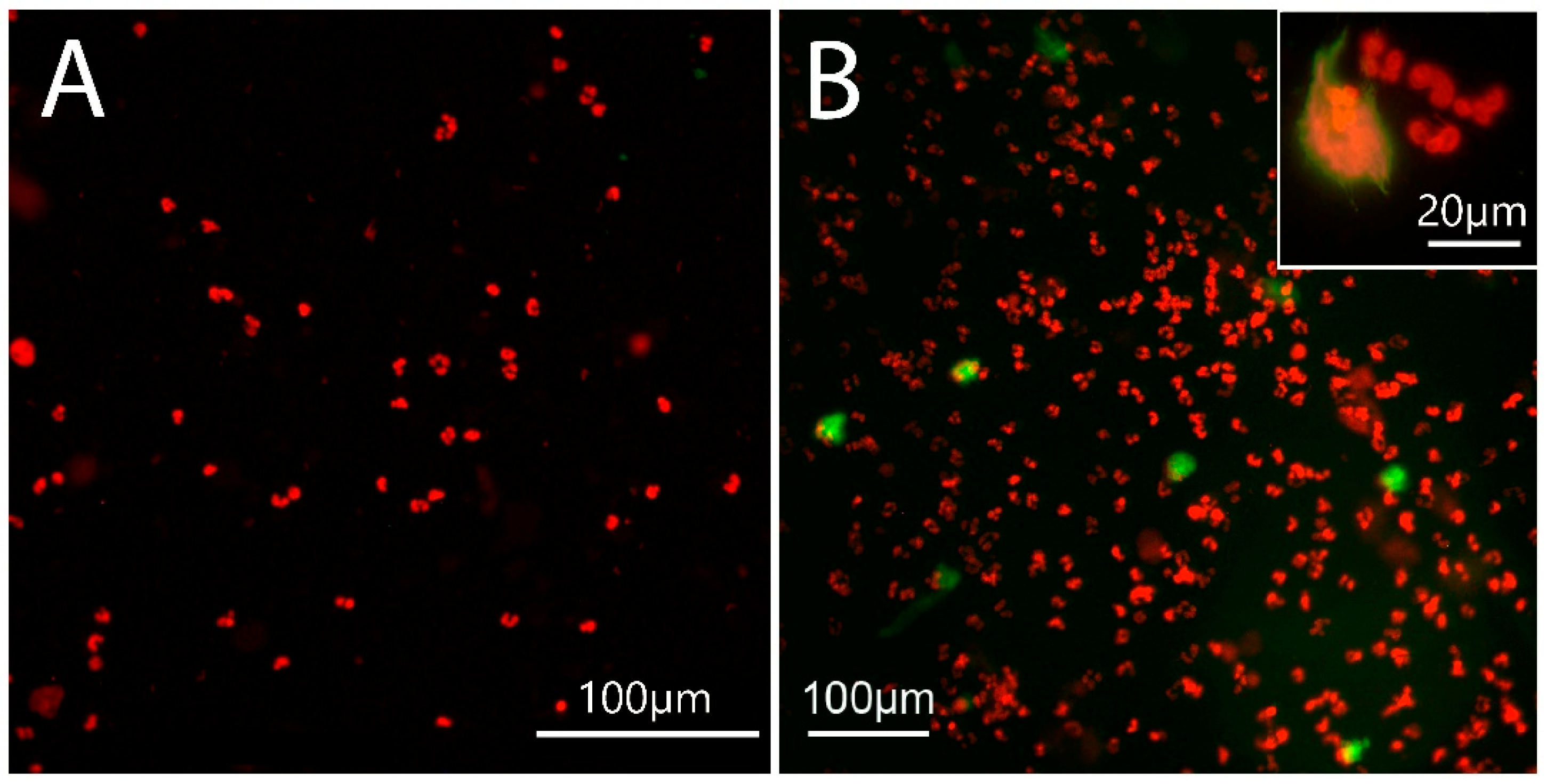
2.3. NET Morphology
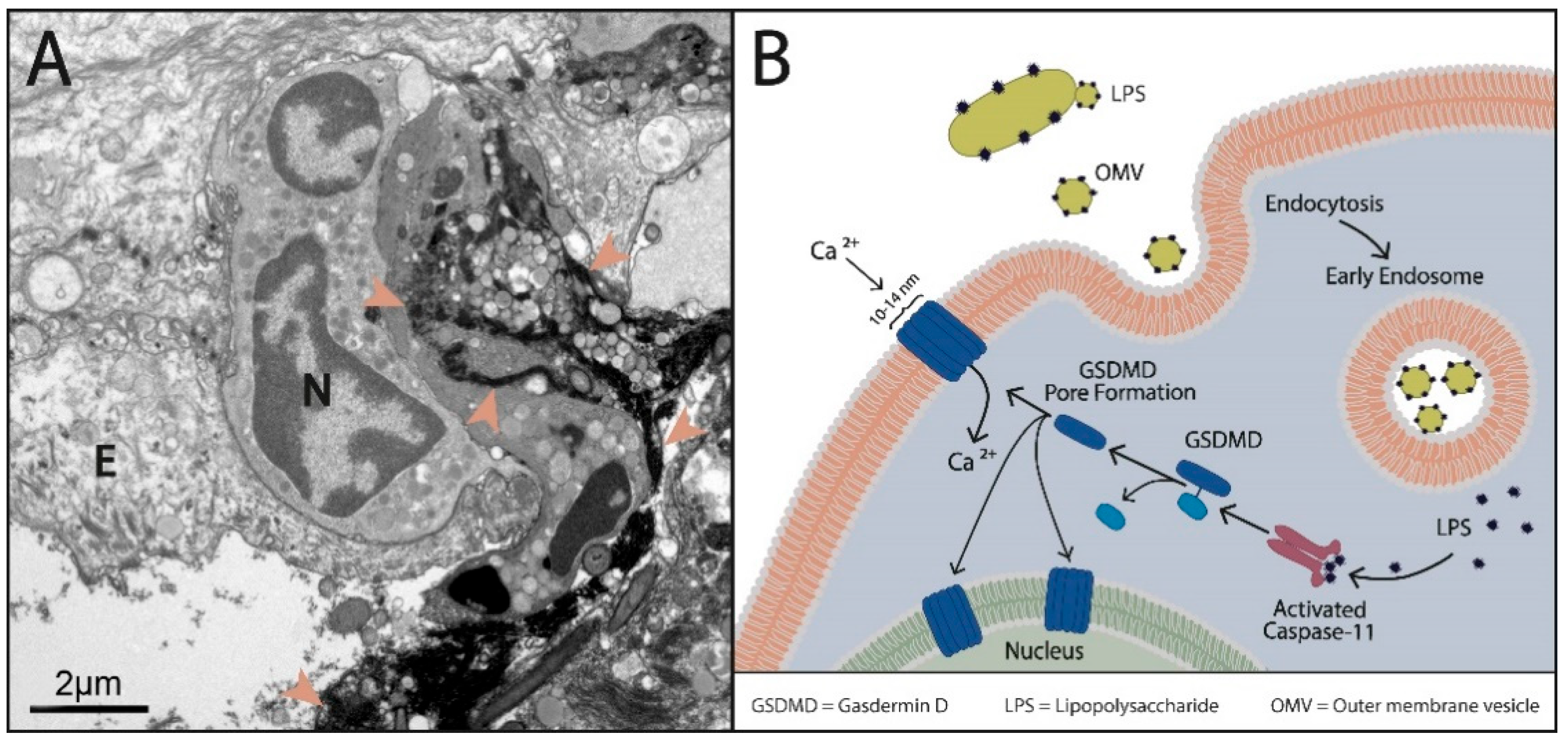
2.4. Site of NET Formation
2.5. NET Quantification in Cultured Neutrophils
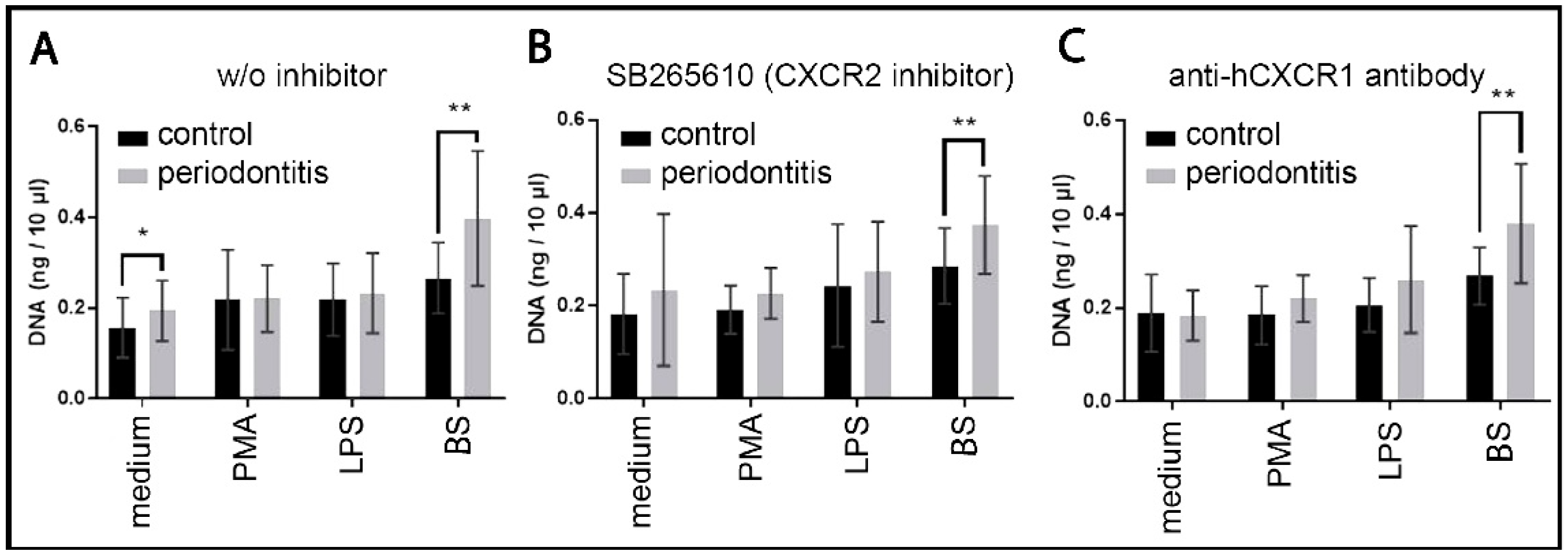
2.5.1. cfDNA Quantification in Neutrophil Culture
2.5.2. NET Dependence on Autocrine and Paracrine IL8
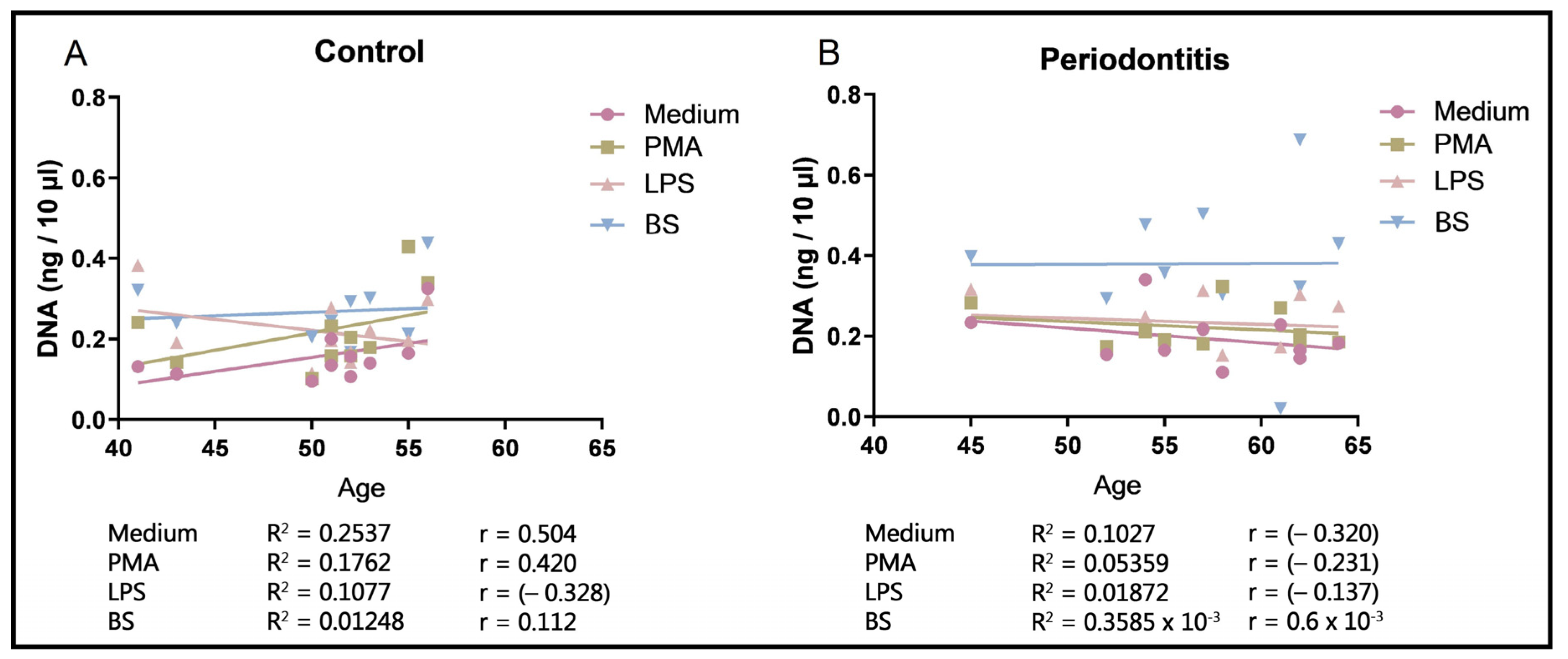
2.5.3. NET Dependence on Age
2.6. Quantification of cfDNA in Blood Plasma
3. Discussion
4. Materials and Methods
4.1. Patients’ Selection and Sample Collection
4.1.1. Gingival Biopsies
4.1.2. Blood Sample Collection
4.1.3. Dental Biofilm Collection
4.2. Electron Microscopy and Nanoparticle Tracking Analysis (NTA)
4.2.1. Scanning Electron Microscopy (SEM)
4.2.2. TEM
4.2.3. NTA
4.3. Neutrophil Isolation and Culture
4.4. Quantification of NETs from Cell Culture and Blood Plasma Cell-Free DNA
4.5. Epifluorescence Microscopy
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BEVs | bacterial extracellular vesicles |
| BS | biofilm supernatant |
| cfDNA | cell-free DNA |
| CXCR1 | interleukin 8 receptor alpha |
| CXCR2 | interleukin 8 receptor beta |
| DAMPs | damage-associated molecular pattern |
| DOAJ | directory of open access journals |
| EPS | extracellular polymeric substances |
| GCF | gingival crevicular fluid |
| GSDMD | gasdermin D |
| IL8 | interleukin-8 |
| LPS | lipopolysaccharide |
| MPO | myeloperoxidase |
| NE | neutrophil elastase |
| NETs | neutrophil extracellular traps |
| NETosis | suicidal NET formation |
| NTA | nanoparticle tracking analysis |
| OMVs | outer membrane vesicles |
| PAD4 | peptidylarginine deiminase 4 |
| PAMPs | pathogen-associated molecular patterns |
| PMA | phorbol 12-myristate 13-acetate |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| TLR | toll-like receptor |
References
- Silva, L.M.; Brenchley, L.; Moutsopoulos, N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol. Rev. 2019, 287, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Munoz, L.E.; Schoen, J.; Knopf, J.; Schauer, C.; Minnich, B.; Herrmann, M.; Hannig, M. Neutrophils Orchestrate the Periodontal Pocket. Front. Immunol. 2021, 12, 788766. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Ling-Mountford, N.; Cooper, P.R.; Chapple, I.L. Neutrophil hyper-responsiveness in periodontitis. J. Dent. Res. 2007, 86, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Vitkov, L.; Munoz, L.E.; Knopf, J.; Schauer, C.; Oberthaler, H.; Minnich, B.; Hannig, M.; Herrmann, M. Connection between Periodontitis-Induced Low-Grade Endotoxemia and Systemic Diseases: Neutrophils as Protagonists and Targets. Int. J. Mol. Sci. 2021, 22, 4647. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Divaris, K.; Chavakis, T. Maladaptive trained immunity and clonal hematopoiesis as potential mechanistic links between periodontitis and inflammatory comorbidities. Periodontol. 2000 2022, 89, 215–230. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Yu, X.; Saha, G.; Kalafati, L.; Ioannidis, C.; Mitroulis, I.; Netea, M.G.; Chavakis, T.; Hajishengallis, G. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 2022, 185, 1709–1727.e18. [Google Scholar] [CrossRef] [PubMed]
- Sigusch, B.; Klinger, G.; Holtz, H.; Süss, J. In Vitro Phagocytosis by Crevicular Phagocytes in Various Forms of Periodontitis. J. Periodontol. 1992, 63, 496–501. [Google Scholar] [CrossRef]
- Miyazaki, A.; Kobayashi, T.; Suzuki, T.; Yoshie, H.; Hara, K. Loss of Fcgamma receptor and impaired phagocytosis of polymorphonuclear leukocytes in gingival crevicular fluid. J. Periodontal Res. 1997, 32, 439–446. [Google Scholar] [CrossRef]
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Neutrophil fate in gingival crevicular fluid. Ultrastruct. Pathol. 2010, 34, 25–30. [Google Scholar] [CrossRef]
- Vitkov, L.; Minnich, B.; Knopf, J.; Schauer, C.; Hannig, M.; Herrmann, M. NETs Are Double-Edged Swords with the Potential to Aggravate or Resolve Periodontal Inflammation. Cells 2020, 9, 2614. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Tan, M.W. Host immunity and cellular responses to bacterial outer membrane vesicles. Trends Immunol. 2021, 42, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Zlatkov, N.; Nadeem, A.; Uhlin, B.E.; Wai, S.N. Eco-evolutionary feedbacks mediated by bacterial membrane vesicles. FEMS Microbiol. Rev. 2021, 45, fuaa047. [Google Scholar] [CrossRef]
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028. [Google Scholar] [CrossRef]
- Perlee, D.; de Beer, R.; Florquin, S.; van der Poll, T.; van ‘t Veer, C.; de Vos, A.F. Caspase-11 contributes to pulmonary host defense against Klebsiella pneumoniae and local activation of coagulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L105–L114. [Google Scholar] [CrossRef]
- Chen, K.W.; Demarco, B.; Broz, P. Beyond inflammasomes: Emerging function of gasdermins during apoptosis and NETosis. EMBO J. 2020, 39, e103397. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Zhu, Y.; Dashper, S.G.; Chen, Y.Y.; Crawford, S.; Slakeski, N.; Reynolds, E.C. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS ONE 2013, 8, e71727. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Tulkens, J.; De Wever, O.; Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 2020, 15, 40–67. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia Associated with Toothbrushing and Dental Extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Tomás, I.; Diz, P.; Tobías, A.; Scully, C.; Donos, N. Periodontal health status and bacteraemia from daily oral activities: Systematic review/meta-analysis. J. Clin. Periodontol. 2012, 39, 213–228. [Google Scholar] [CrossRef]
- Wang, W.; Chanda, W.; Zhong, M. The relationship between biofilm and outer membrane vesicles: A novel therapy overview. FEMS Microbiol. Lett. 2015, 362, fnv117. [Google Scholar] [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export: Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef]
- Metruccio, M.M.E.; Evans, D.J.; Gabriel, M.M.; Kadurugamuwa, J.L.; Fleiszig, S.M.J. Pseudomonas aeruginosa Outer Membrane Vesicles Triggered by Human Mucosal Fluid and Lysozyme Can Prime Host Tissue Surfaces for Bacterial Adhesion. Front. Microbiol. 2016, 7, 871. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and surface properties influence biofilm proliferation. Adv. Colloid Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Dauros-Singorenko, P.; Hong, J.; Vanholsbeeck, F.; Phillips, A.; Swift, S. The complex, bidirectional role of extracellular vesicles in infection. Biochem. Soc. Trans. 2021, 49, 881–891. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6676. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Chen, X.; He, W.-T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Lewallen, D.M.; Bicker, K.L.; Subramanian, V.; Clancy, K.W.; Slade, D.J.; Martell, J.; Dreyton, C.J.; Sokolove, J.; Weerapana, E.; Thompson, P.R. Chemical Proteomic Platform to Identify Citrullinated Proteins. ACS Chem. Biol. 2015, 10, 2520–2528. [Google Scholar] [CrossRef]
- Vitkov, L.; Klappacher, M.; Hannig, M.; Krautgartner, W.D. Extracellular neutrophil traps in periodontitis. J. Periodontal Res. 2009, 44, 664–672. [Google Scholar] [CrossRef]
- Krautgartner, W.D.; Vitkov, L. Visualization of neutrophil extracellular traps in TEM. Micron 2008, 39, 367–372. [Google Scholar] [CrossRef]
- Fredriksson, M.I.; Gustafsson, A.K.; Bergström, K.G.; Åsman, B.E. Constitutionally Hyperreactive Neutrophils in Periodontitis. J. Periodontol. 2003, 74, 219–224. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L.C. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis: Neutrophil hyperactivity and reactivity in chronic periodontitis. Clin. Exp. Immunol. 2006, 147, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.A. Periodontitis and aging: Reviewing recent evidence. J. Am. Dent. Assoc. 1994, 125, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Hamam, H.J.; Khan, M.A.; Palaniyar, N. Histone Acetylation Promotes Neutrophil Extracellular Trap Formation. Biomolecules 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Hamam, H.J.; Palaniyar, N. Histone Deacetylase Inhibitors Dose-Dependently Switch Neutrophil Death from NETosis to Apoptosis. Biomolecules 2019, 9, 184. [Google Scholar] [CrossRef]
- Yang, S.; Qi, H.; Kan, K.; Chen, J.; Xie, H.; Guo, X.; Zhang, L. Neutrophil Extracellular Traps Promote Hypercoagulability in Patients with Sepsis. Shock 2017, 47, 132–139. [Google Scholar] [CrossRef]
- Ríos-López, A.L.; González, G.M.; Hernández-Bello, R.; Sánchez-González, A. Avoiding the trap: Mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol. Res. 2021, 243, 126644. [Google Scholar] [CrossRef]
- Huang, L.; Lu, W.; Ning, Y.; Liu, J. Reverse effects of Streptococcus mutans physiological states on neutrophil extracellular traps formation as a strategy to escape neutrophil killing. Front. Cell Infect. Microbiol. 2022, 12, 1023457. [Google Scholar] [CrossRef]
- Sutterlin, H.A.; Shi, H.; May, K.L.; Miguel, A.; Khare, S.; Huang, K.C.; Silhavy, T.J. Disruption of lipid homeostasis in the Gram-negative cell envelope activates a novel cell death pathway. Proc. Natl. Acad. Sci. USA 2016, 113, E1565–E1574. [Google Scholar] [CrossRef]
- Crasta, K.; Daly, C.G.; Mitchell, D.; Curtis, B.; Stewart, D.; Heitz-Mayfield, L.J.A. Bacteraemia due to dental flossing. J. Clin. Periodontol. 2009, 36, 323–332. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Russo, A.J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S.D.; Rathinam, V.A.K. Bacterial Outer Membrane Vesicles Mediate Cytosolic Localization of LPS and Caspase-11 Activation. Cell 2016, 165, 1106–1119. [Google Scholar] [CrossRef]
- Hirschfeld, J.; White, P.C.; Milward, M.R.; Cooper, P.R.; Chapple, I.L.C. Modulation of Neutrophil Extracellular Trap and Reactive Oxygen Species Release by Periodontal Bacteria. Infect. Immun. 2017, 85, e00297-17. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zhang, Y.; Lee, C.; Li, L. Novel reprogramming of neutrophils modulates inflammation resolution during atherosclerosis. Sci. Adv. 2019, 5, eaav2309. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, J.; Zhang, Y.; Kuang, S.; Shen, Z.; Qin, W. Blocking CXCR1/2 attenuates experimental periodontitis by suppressing neutrophils recruitment. Int. Immunopharmacol. 2024, 128, 111465. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S74–S84. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitkov, L.; Krunić, J.; Dudek, J.; Bobbili, M.R.; Grillari, J.; Hausegger, B.; Mladenović, I.; Stojanović, N.; Krautgartner, W.D.; Oberthaler, H.; et al. Vesicular Messages from Dental Biofilms for Neutrophils. Int. J. Mol. Sci. 2024, 25, 3314. https://doi.org/10.3390/ijms25063314
Vitkov L, Krunić J, Dudek J, Bobbili MR, Grillari J, Hausegger B, Mladenović I, Stojanović N, Krautgartner WD, Oberthaler H, et al. Vesicular Messages from Dental Biofilms for Neutrophils. International Journal of Molecular Sciences. 2024; 25(6):3314. https://doi.org/10.3390/ijms25063314
Chicago/Turabian StyleVitkov, Ljubomir, Jelena Krunić, Johanna Dudek, Madhusudhan Reddy Bobbili, Johannes Grillari, Bernhard Hausegger, Irena Mladenović, Nikola Stojanović, Wolf Dietrich Krautgartner, Hannah Oberthaler, and et al. 2024. "Vesicular Messages from Dental Biofilms for Neutrophils" International Journal of Molecular Sciences 25, no. 6: 3314. https://doi.org/10.3390/ijms25063314
APA StyleVitkov, L., Krunić, J., Dudek, J., Bobbili, M. R., Grillari, J., Hausegger, B., Mladenović, I., Stojanović, N., Krautgartner, W. D., Oberthaler, H., Schauer, C., Herrmann, M., Singh, J., Minnich, B., & Hannig, M. (2024). Vesicular Messages from Dental Biofilms for Neutrophils. International Journal of Molecular Sciences, 25(6), 3314. https://doi.org/10.3390/ijms25063314






