Abstract
Graves’ ophthalmopathy (GO), or thyroid eye disease (TED), is the most frequent extrathyroidal manifestation of Graves’ disease (GD). Inflammation and subsequent aberrant tissue remodeling with fibrosis are important pathogenesis. There are many proposed mechanisms and molecular pathways contributing to tissue remodeling and fibrosis in GO, including adipogenesis, fibroblast proliferation and myofibroblasts differentiation, oxidative stress, endoplasmic reticulum (ER) stress, hyaluronan (HA) and glycosaminoglycans (GAGs) accumulation in the extracellular matrix (ECM) and new concepts of epigenetics modification, such as histone modification, DNA methylation, non-coding RNAs, and gut microbiome. This review summarizes the current understanding of ECM proteins and associated tissue remodeling in the pathogenesis and potential mediators for the treatment of GO.
1. Introduction
Graves’ ophthalmopathy (GO), also called thyroid eye disease (TED), is the most frequent extrathyroidal manifestation of Graves’ disease (GD) and is cosmetically disfiguring and potentially vision-threatening. Inflammation in the early phase, and subsequent aberrant tissue remodeling with fibrosis are important pathogeneses of GO [1]. Clinical manifestations, including lid retraction, proptosis, diplopia due to ocular motility, and compressive optic neuropathy restriction result from abnormal tissue remodeling of the orbital soft tissues. Severe abnormal orbital tissue remodeling and fibrosis may cause orbital deformity and vision loss (Figure 1). Current pharmacological agents are systemic steroids and immunomodulating agents, which may reduce inflammation but have limited effects on the long-term sequela [1]. Teprotumumab, a human monoclonal antibody inhibitor binding the extracellular alpha subunit of the IGF-1R, was approved by the U.S. Food and Drug Administration (FDA) in 2020 for patients with active moderate to severe GO [2]. However, the price of teprotumumab is extremely high and hearing loss is a major concern as an adverse event of teprotumumab [3]. Thyroidectomy could be considered for cases with recurrent hyperthyroidism; however, the evidence of thyroidectomy on GO is variable [4,5,6]. Thus, to identify novel targets of abnormal tissue remodeling and fibrosis in GO it is necessary to develop better therapeutics.
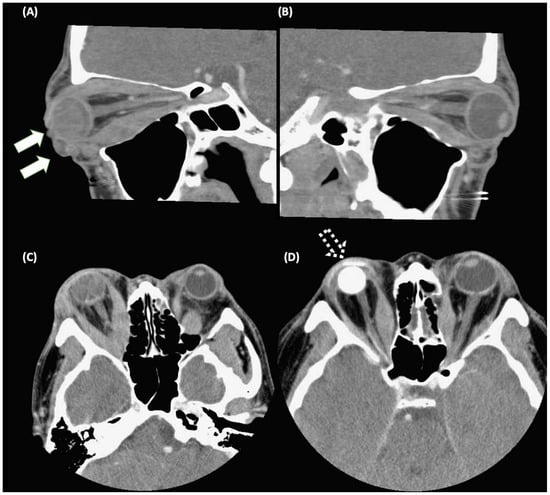
Figure 1.
Computed tomography images in a patient with Graves’ ophthalmopathy (GO). (A) Exposure keratopathy with eyeball rupture (solid arrows) due to severe extraocular muscle hypertrophy and adipogenesis in the right eye. (B) Extraocular muscle hypertrophy in the left eye. (C,D) The patient received an evisceration with an implant (as dashed arrows) of the right eye, and orbital decompression of the left eye. The pre-operative image and post-operative image were demonstrated in (C) and (D), respectively.
This review summarizes the current understanding of ECM proteins and associated tissue remodeling in the pathogenesis and potential mediators for the treatment of GO, and are summarized in Figure 2 and Table in Section 9.4, respectively.
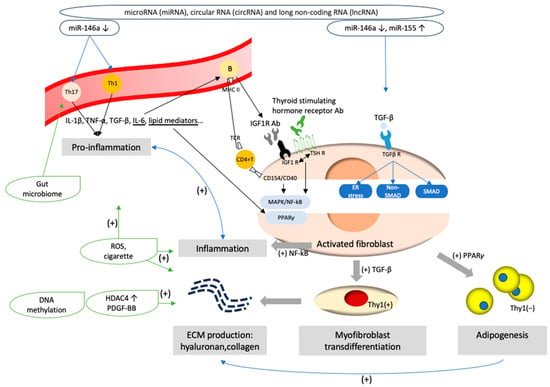
Figure 2.
Pathogenesis of Graves’ ophthalmopathy. (1) Aberrant epigenetic modifications, such as dysregulation of microRNA (miRNA)-146, miRNA 155, circular RNAs and long non-coding RNA, might trigger pro-inflammatory cascades and disturb the expression of signaling molecules critical for myofibroblast transdifferentiation and adipogenesis processes in orbital fibroblasts. (2) The interaction through CD40–CD154 ligation and T cell receptor (TCR)–major histocompatibility complex class II (MHC II) ligation activates pro-inflammation and orbital fibroblasts with the secretion of inflammatory mediators, such as IL-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and lipid mediators. Orbital fibroblasts further upregulate the release of pro-inflammatory cytokines. IL-6 modulates B cell immunoglobulin secretion. Autoantibodies of insulin-like growth factor-1 (IGF-1) and thyroid-stimulating hormone receptor antibodies (TSH Ab) interact with their receptors and activate the thyrotropin/IGF-1 receptor complex, promoting the further mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) downstream signaling pathways, which induce orbital fibroblast proliferation and inflammation. (3) The interaction of thyrotropin/IGF-1 receptor complex and lipid mediators upregulates peroxisome proliferator-activated receptor-γ (PPAR-γ) expression, and induce adipocyte differentiation from Thy1 negative orbital fibroblasts. (4) TGF-β binds to its receptors and activates Smad/non-Smad transcription factors. Thy1 positive orbital fibroblasts are activated and result in myofibroblast transdifferentiation. (5) Contents of the extracellular matrix, such as hyaluronan (HA) or collagen, are produced by activated orbital fibroblasts. Aberrant histone modification and DNA methylation, such as the stimulation of platelet-derived growth factor-BB (PDGF-BB) and histone deacetylases (HDAC) 4, would promote the mRNA expression of HA production and pro-inflammation. Factors of adipogenesis, such as prostaglandin D2 (PGD2) and PGJ2, enhance ECM production. (6) Cigarette enhances oxidative stress and upregulate TGF-β1, IL-1β, adipogenesis and the fibrosis-related gene expression in orbital fibroblasts. (7) Endoplasmic reticulum (ER) stress promotes fibrogenesis after the stimulation of TGF-β. (8) The gut microbiome may foster an imbalance in Th17 and T regulatory cells and impact the levels of TSH Ab.
2. Cell Mediators in GO
Activated GO fibroblasts are a hallmark in GO. During the active stage, the ligation of T cell receptor and major histocompatibility complex class II (MHC II) on B cells, and the interaction through CD40–CD154 (CD40 ligand) ligation between CD4+ T cells and fibroblasts, activates pro-inflammation and orbital fibroblasts [7]. CD4+ T lymphocytes in the orbit, and CD8+ T cells, macrophages, plasma cells and B cells in the extraocular muscle and adipose tissue produce lots of inflammatory mediators, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β and lipid mediators [8]. Additionally, autoantibodies against thyroid-stimulating hormone receptors (TSHR) and insulin-like growth factor-1 receptors (IGF-1 R) stimulate cAMP production and downstream mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways and phosphoinositide 3-kinase (PI3K) cascades [8,9], inducing orbital fibroblasts to secrete more inflammatory mediators, and increase the inflammatory cycle. Activated orbital fibroblasts differentiation into fat storing adipocytes through adipogenesis and myofibroblast transdifferentiation and ECM molecule secretion will be discussed in the following sections.
3. Adipogenesis and ECM of GO
Adipogenesis, the process of adipocyte (fat cell) development, results in the expansion of orbital adipose tissue and proptosis in GO. The differentiation of preadipocytes into mature adipocytes was associated with orbital fibroblasts. There are two distinct orbital fibroblasts: Thy-1(+) or Thy-1(−). Thy-1 is a classical T lymphocyte marker (CD90) expressed on fibroblasts. Fibroblasts expressing Thy-1 produce HA and inflammatory mediators, and are responsible for myofibroblast transdifferentiation. Thy-1(−) fibroblasts are capable of mature adipocyte differentiation. Lehmann et al. [10] demonstrated that the imbalance between Thy-1(+) and Thy-1(−) orbital fibroblasts facilitated adipogenesis in GO.
The PI3K pathway and lipid mediators induce the activation of peroxisome proliferator-activated receptor γ (PPARγ) and enhance adipogenesis. PPARγ is a nuclear receptor functioning as a transcription factor in lipid uptake and adipogenesis. Kumar et al. [11] demonstrated that several adipogenesis-associated mRNAs in orbital adipose tissues from GO patients, including leptin, adiponectin, PPARγ and preadipocyte factor-1, were elevated in parallel with TSH receptors. After IGF-1 stimulation, PPARγ was upregulated, and the PI3K pathway was activated [12]. Thus, the inhibitors of PPARγ may be a potential therapeutic target for GO. Notably, some type 2 diabetes medications, rosiglitazone or piogliatozone, which would activate PPARγ, may deteriorate GO [13].
4. Transforming Growth Factor-β1 (TGF-β1)-Induced Myofibroblast Transdifferentiation and ECM in GO
Multiple studies have established the involvement of TGF-β in tissue remodeling. TGF-β, along with insulin-like growth factor (IGF)-1, IL-4, and platelet-derived growth factor (PDGF), has been observed to increase the proliferation of orbital fibroblasts in GO compared to normal controls [14]. Among these mediators, TGF-β plays a crucial role in tissue remodeling. GO orbital fibroblasts show significantly higher levels of TGF-β protein and mRNA expression, and TGF-β is capable of triggering the transformation of Thy-1(CD90)-positive orbital fibroblasts into myofibroblasts [15]. There are three isoforms of TGF-β in mammals, with TGF-β1 being the primary contributor to tissue remodeling in GO [16].
TGF-β induces tissue fibrosis through either the canonical SMAD pathway or the non-canonical SMAD signaling pathway [17]. The TGF-β molecule consists of three parts: latent TGF-β binding protein (LTBP), latency-associated peptide (LAP), and activated TGF-β. Cleavage of LTBP and LAP by matrix metalloproteinases (MMP) releases activated TGF-β, which then interacts with TGF-β receptors. This interaction leads to the phosphorylation of SMAD 2/3, forming a complex with SMAD 4. The SMAD 2/3/4 complex enters the nucleus, initiating myofibroblast transdifferentiation and ECM production [18,19,20].
In the non-SMAD pathway, TGF-β activates non-Smad mediators, including the mitogen activated protein kinases (MAPK), phosphatidylinositol-3-kinase (PI3K) and Rho-like GTPases, to regulate gene expression [21]. The MAPK family consists of p38, c-Jun N-terminal kinase (JNK), and extracellular-signal-regulated kinase (ERK). In our prior study [22], the increased phosphorylation of p38 and JNK (but not ERK) after the induction of TGF-β1 was demonstrated, as well as the expression of connective tissue growth factor (CTGF), α-smooth muscle actin (α-SMA), fibronectin, tissue inhibitors of metalloproteinases 1 (TIMP-1) and TIMP-3. The levels of matrix metalloproteinases 2 (MMP-2) and MMP-9 were inhibited. Our study proved that the non-SMAD pathway of p38 and JNK contributed to the TGF-β-induced myofibroblast transdifferentiation in human GO Fibroblasts and ECM production. Inhibiting the p38 or JNK pathway may be a potential therapeutic strategy to treat fibrosis in GO.
When the activated Smad/non-Smad proteins enter the nucleus, they regulate transcription factors and increase ECM production, including collagen, CTGF, fibronectin, and alpha-smooth muscle actin (α-SMA). In our previous study, the expression of CTGF, fibronectin, and α-SMA in Graves’ orbital fibroblasts were stimulated after the induction of TGF-β1 [23,24,25]. The protein levels of fibronectin and α-SMA were increased after induction by recombinant human protein CTGF (rhCTGF) and decreased after knockdown of CTGF, which indicates CTGF is an essential downstream mediator for TGF-β1-induced myofibroblast transdifferentiation. Moreover, the elevated CTGF was correlated with clinical manifestations in GO patients [21].
Inhibiting TGF-β1 has potential as a therapeutic approach to target fibrosis; however, TGF-β is involved in not only tissue remodeling, but also cell proliferation and immune responses. The blockage of TGF-β1 might cause unexpected systemic side effects [26,27]. Thus, blockage downstream of TGF-β, such as the p38 or JNK pathway or CTGF, may bring a potential therapeutic target to treat fibrosis in GO.
5. Hyaluronan Formation by Orbital Fibroblasts
ECM consists of collagen and hyaluronan (HA). HA is the main contributor to volume expansion in GO because it occupies around 75,000 times the volume of that of an equivalent weight of collagen [28]. HA synthesis is by the actions of hyaluronan synthases (HAS). There are three isomers of HAS: HAS1, HAS2 and HAS3, and HAS2 mainly contributes to HA synthesis by orbital fibroblasts [29]. HA synthesis could be stimulated by inflammation. It has been reported that leukoregulin, IL-1, TNF-α, IFN-γ, TGF-β, IGF-1, PDGF and prostaglandins enhance HA synthesis in GO fibroblasts. Hyaluronic acids could induce MAPK and NF-κB phosphorylation; while HA-induced transcription of COX-2 was halted by the administration of MAPK or NF-κB inhibitors [30,31,32,33] and inhibited by methylprednisolone and dexamethasone. Additionally, HA synthesis could be stimulated by adipogenesis. Zhang et al. [34] showed that adipogenesis in orbital preadipocytes was associated with increased HAS2 transcripts and HA accumulation. Guo et al. [35] revealed the elevated expression of HAS mRNA in GO fibroblasts after treatment with mast cell derived prostaglandin D2 (PGD2) and PGJ2, which are both factors of adipogenesis. Although HA accumulation mainly leads to volume expansion in GO, the mechanism of HA degradation and the relationship between HA synthesis and degradation are not fully understood [34].
6. Oxidative Stress and Smoking in the ECM of GO
Oxidative stress represents an imbalance between the antioxidant defense system and reactive oxygen species’ (ROS) production, including superoxide anions, hydrogen peroxide, and hydroxyl radicals. When the production of ROS overwhelms the body’s antioxidant defenses, oxidative stress arises. Sources of ROS in GO include activated immune cells (such as infiltrating lymphocytes and macrophages) and orbital fibroblasts, which induce the expression of proinflammatory cytokines and chemokines [36]. Additionally, the GO autoantibodies could bind to orbital fibroblasts and activate NADPH oxidase, and further trigger the generation of ROS and exacerbate oxidative stress [37].
Although the exact pathogenic mechanisms of oxidative stress in GO are not fully specified, orbital fibroblasts are considered as the major effector cells. Our prior study found elevated urinary 8-hydroxy 2′-deoxyguanosine (8-OHdG) in active GO patients, which is an important biomarker of oxidative DNA damage [38]. Not only 8-OHdG, but also malondialdehyde (MDA), a product of lipid peroxidation, as well as the superoxide anions and hydrogen peroxide were increased in cultured orbital fibroblasts from patients with GO compared to controls, which indicated prominent oxidative DNA damage, lipid peroxidation, and ROS production in the pathogenesis of GO [39]. After the induction of hydrogen peroxide (H2O2), there were increased ROS contents (MDA, H2O2, and manganese-dependent superoxide dismutase) and imbalance of the ratio change between reduced (GSH) and oxidized glutathione (GSSG) in GO orbital fibroblasts [40]. Systemic glucocorticoids could decrease the level of urinary 8-OHdG in patients with active GO [35]. Similar results were obtained by Akarsu et al. [41], Abalovich et al. [42] and Bednarek et al. [43].
Cigarette smoking, an important risk factor for GO deterioration, has been established in multifactorial ways. Yuksel et al. [44] demonstrated the smoking effects on oxidative stress and mitochondrial homeostasis. Two proteins associated with proper mitochondrial function, paraoxonase (PON) and mitochondrial transcriptional factor A (MTFA), were found to be significantly decreased in GO smokers compared to GO nonsmokers or healthy subjects. Cawood et al. [45] showed cigarette induced hyaluronic acid production and adipogenesis via the synergizing effects of IL-1 and ROS. Additionally, smoking impairs endothelial function and disrupts the integrity of the vascular barrier within the orbit. Decreased superior ophthalmic venous blood flow velocity and choroidal blood circulation were found in GO [46,47]. The hypoxic environment might induce the production of hypoxia-inducible factor-1 (HIF-1) and stimulate the HIF-1-dependent adipogenesis pathway. The levels of HIF-1α were correlated with the clinical activity score (CAS) [48]. Similar results regarding smoking were demonstrated in our study. After induction by cigarette extracts, there was increased oxidative stress, fibrosis-related gene expression (apolipoprotein J, CTGF, and fibronectin), and intracellular levels of TGF-β1 and IL-1β in GO orbital fibroblasts [49].
7. Endoplasmic Reticulum (ER) Stress in the ECM of GO
The endoplasmic reticulum (ER) is an important intracellular organelle facilitating the conversion of nascent proteins to functional forms. Dysfunction of the ER leads to ER stress and triggers the unfolded protein response (UPR) of the chaperone protein through three main effector pathways, involving PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1α (IRE1α). Severe or prolonged ER stress has been proposed in multiple organs [50] with the development of fibrotic disorders, including liver, kidneys, heart, and lung, while ER stress in GO pathogenesis is in its infancy.
In orbital tissues from GO patients, Huang et al. revealed that ER stress-related gene (ATF6, PERK, and IRE1α) expression was higher than in control orbital tissues [51]. Further study showed that silencing PERK could reduce oxidative stress and adipogenesis in the GO orbital fibroblasts [52]. Our recent study (unpublished data) further demonstrates that ER protein TXNDC5 plays an important role in TGF-β1-induced myofibroblast trans-differentiation and ECM protein in GO orbital fibroblasts. We used lentivirus transfected TED orbital fibroblasts with small hairpin RNAs to knockdown TXNDC5 protein expression levels, and found that TXNDC5 knockdown attenuated TGFβ1-induced myofibroblast transdifferentiation and ECM protein production, whereas increasing TXNDC5 expression by recombinant TXNDC5 addition increased ECM protein expression.
8. Epigenetics and the Gut Microbiome in the ECM of GO
With advanced gene sequencing technology, the epigenetics in the pathogenesis of GO has been studied in recent years [53,54]. Although the research is still in its infancy, histone modification, DNA methylation and non-coding RNAs bring emerging insights and novel potential therapeutic strategies.
8.1. Histone Deacetylases (HDACs) and DNA Methylation
Histone deacetylases (HDACs) are enzymes that epigenetically control gene transcription through the modification of histone proteins. HDACs remove acetyl groups from histone lysine residues and reduce the expression of target genes. The downregulation of HDACs could induce the gene activity of proinflammation and immunity; thus, HDAC inhibitors (HDACis) may have therapeutic potential [55].
HDACs are known to be associated with malignancy, autoimmune diseases, as well as thyroid disease [53,56,57]. Limbach et al. [58] performed a genome-wide analysis in patients with Graves’ disease and showed dysregulated DNA methylation and histone modifications at T cell signaling genes. Sacristán-Gómez et al. [59] analyzed different kinds of HDAC and demonstrated that elevated HDAC9 and decreased Tip60 histone acetyltransferases (HAT) might suppress the activation of regulatory T cells and promote proinflammation.
Elevated DNA methylation in GO fibroblasts was demonstrated in recent years, after analysis of the proteomics and DNA methylation in orbital fibroblasts. Ekronarongchai et al. [60] revealed higher HDAC4 mRNA expression in GO orbital fibroblasts compared to those in normal subjects after stimulation with platelet-derived growth factor-BB (PDGF-BB). The expression of hyaluronan synthase 2 (HAS2), collagen type I alpha 1 chain (COL1A1), Ki67, α-SMA, and HA were reduced after silencing of HDAC4. Adding HDAC4i (tasquinimod) decreased the mRNA expression of HAS2 and α-SMA. Inhibitors of HDAC4 might be potential therapeutic targets for GO therapy.
8.2. MicroRNA in GO
Non-coding RNAs (ncRNA) are a class of RNA lacking protein-coding functions, and are classified into microRNA (miRNA), circular RNA (circRNA) and long non-coding RNA (lncRNA). Among these, MiR-146a is the most studied miRNA associated with GO. MiRNA is a small non-coding RNA that contains 21 to 23 nucleotides. It binds to 3′ untranslated regions of the target mRNA and post-transcriptionally regulates gene expression. MiR-146a was found to affect immune regulation, cell proliferation, differentiation, apoptosis and ECM [61,62]. Downregulated miR-146a expression would attenuate CD4+ T cell differentiation and proliferation and induce elevated IL-17 levels, which promotes orbital inflammation [63]. Jang et al. [62] demonstrated that miR-146a downregulates TGF-β-induced fibronectin, collagen Iα and α-SMA protein from GO orbital fibroblasts through Smad 4 and the tumor necrosis factor receptor-associated factor 6 (TRAF6) pathway. In addition to miR-146a, miR-155, causing the opposite effect against miR-146a, has been proposed to be associated with GO. Li et al. [64] revealed that increased miR-155 and decreased miR-146a promote orbital fibroblast proliferation. Woeller et al. [61] revealed that TSH stimulates proliferation of orbital fibroblasts through PI3K/Akt, miR-146a and miR-155. Recent findings suggest miR-155 upregulates collagen synthesis, which further induces the fibrosis process in GO [65]. With the high throughput of advanced technology, more non-coding RNA have been proposed in association with GO, such as miR-29, miR-21, MiR-27a, miR-27b, miR-130a [16,66,67,68]. Additional research is warranted to develop the crucial role of miRNA and to elucidate potential targets.
8.3. Other Non-Coding RNAs in GO
Circular RNA (CircRNA) is a closed loop RNA with joined of 3′ and 5′ ends. They acts as mRNA sponges and induce mRNA transcription [69]. Wu et al. [70] identified 163 circRNAs interacting with 607 mRNAs in GO orbital adipose, and circRNA_14936, circRNA_14940, and circRNA_12367 were associated with cytokine–cytokine interaction and ECM receptor interaction. Although the literature is limited, the co-expression of circRNA and mRNA was demonstrated in GO pathophysiology but warrants further evaluation.
Long non-coding RNAs (LncRNAs), involving more than 200 non-transcribed nucleotides, regulate gene expression in Hashimoto thyroiditis and other autoimmune diseases. Christensen et al. [71] firstly reported lncRNA (designated Heg) was negatively related to thyroid receptor autoantibodies in untreated Graves’ patients, which manifested anti-inflammatory effects. In the latest study, lncRNAs from the GO orbital tissue were identified as being associated with 52 kinds of ECM genes [72].
8.4. Gut Microbiome in GO
The relationship between gut microbiota and autoimmune diseases, including Graves’ disease (GD) or GO, is an area of growing interest and research. Literature revealed alterations in the gut microbiome that may induce GO by impacting the levels of thyroid-stimulating hormone receptor antibodies (TSH Ab) and fostering an imbalance in Th17/Treg [53,73,74]. Experimental findings revealed heterogeneity in the gut microbiome in a TSHR-immunized GD/GO mouse model and a reduction in disease severity with oral antibiotic vancomycin treatment [75].
However, fecal microbiota transplantation from GD/GO patients exacerbated the condition [73,75]. Studies revealed GD/GO patients exhibit distinct differences in microbiota composition compared to healthy controls and the composition of microbiota could be impacted by various factors, such as glucocorticoids, antithyroid drugs and immunosuppressants [75]. Significant differences in gut microbiota composition caused by various factors could explain variations in study results and clinical application. To elucidate the link between GO and the gut microbiome warrants further investigation.
In summary, histone modification, DNA methylation, and non-coding RNAs may play a crucial role in GO, but further evaluation is warranted to elucidate the applications.
9. Treatment for Tissue Remodeling in GO
9.1. Biologic Agents
Current pharmacological agents are systemic steroids and immunomodulating agents, which may reduce inflammation but have limited effects on the long-term tissue remodeling. ECM production, tissue remodeling and fibrosis of the orbital soft tissues are responsible for the clinical manifestations of proptosis, lid retraction and compressive optic neuropathy in GO. They highlight the urgency required to develop novel targets against GO related fibrogenesis.
Teprotumumab is a monoclonal antibody that targets the insulin-like growth factor-1 receptor (IGF-1R). It decreases levels of the receptors of TSH and IGF-1 on orbital fibrocytes and attenuates TSH-stimulated pro-inflammatory cytokines, including IL-6, IL-8, and TNF-α. A randomized, double-blind, placebo-controlled, multicentered, phase III trial enrolled 41 patients with active, moderate-to-severe GO [2]. Either intravenous teprotumumab or placebo infusions were prescribed every 3 weeks for a total of eight infusions. After 24 weeks, patients receiving teprotumumab had significant improvement of proptosis (83% vs. 10%, p < 0.001). This “breakthrough therapy” teprotumumab was approved by the US FDA in 2020. However, the high price is difficult to afford, and the infusion requirement limits the extensibility. The side effects of hyperglycemia and hearing impairment need to be solved [76]. Exploring other potential therapeutic targets is necessary.
Other biologic agents, including rituximab and tocilizumab, have been reported. Rituximab is a monoclonal antibody against CD20 targeting B cell lymphocytes. It works by depleting B cells, which leads to the reduction in autoantibodies and pro-inflammatory cytokines. Rituximab has been used clinically, while the results from the randomized control trials (RCT) are inconsistent. Salvi et al. [77] demonstrated better outcome of ocular motility and quality of life in cases of moderate to severe GO after use of intravenous rituximab compared to intravenous methylprednisolone. However, Stan et al. [78] showed no benefit in cases of moderate to severe GO after use of intravenous rituximab compared to placebo. Tocilizumab is a humanized antibody against IL-6 in T cell and B cell activation. Additionally, IL-6 may change the ECM remodeling by inducing the expression of the thyrotropin receptor and thyroid-stimulating immunoglobulin (TSI) in orbital fibroblasts, leading to the differentiation into myofibroblasts or adipocytes [79]. By blocking IL-6, tocilizumab decreases B cell activation, T cell recruitment and orbital fibroblast activation. However, there are no published randomized clinical trials. Biologic agents show promise in treating GO, the benefits and side effects warrant evaluation of more RCTs.
9.2. Potential Therapeutic Target—Antioxidants
Multiple potential antioxidants have been described in GO, including selenium, pentoxifylline, quercetin, enalapril, allopurinol, nicotinamide, vitamin C, N-acetylcysteine, melatonin, β-carotene, and statins [80,81,82]. A recent systematic review [80] included four clinical and ten in vitro studies from 1993 to 2018, and demonstrated that selenium was the only antioxidant based on in vitro and randomized controlled trial studies. The following paragraphs highlight the potential therapeutic effects of selenium, pentoxifylline, allopurinol, and nicotinamide, because they were reported to be effective in both clinical and experimental reports. β-carotene, retinol, vitamin E, vitamin C, melatonin, resveratrol, N-acetyl-l-cysteine, and quercetin showed potential in in vitro studies, but need more clinical data.
9.2.1. Selenium (Se)
Selenium is a trace mineral that is incorporated into several selenoproteins, such as glutathione peroxidase, thioredoxin reductase and iodothyronine deiodinases (D1, D2 or D3). Selenoproteins have antioxidative and immune-modulating effects through activating T cells and reducing the release of tumor necrosis factor α (TNFα) and cyclooxygenase 2 (COX2) [83,84]. Thus, Se as a supplementary modality, may have a role in GO treatment.
One randomized control trial (RCT) [85] founded by the European Group on Graves’ Orbitopathy (EUGOGO) compared the effect of selenium (100 μg twice daily), pentoxifylline (600 mg twice daily) or placebo in 159 patients with mild GO. The trial demonstrated noteworthy enhancements in both quality of life and ocular involvement, coupled with a reduced progression rate in patients with mild GO following a 6-month selenium supplementation. However, this recommendation was not endorsed by the American Thyroid Association (ATA) and the European Thyroid Association (ETA), since selenium deficiency is not present in the US and Europe [86,87]. Additionally, Se could be consumed by daily intake, such as egg, milk and meat. The risk between long-term Se intake and diabetes was uncertain [88,89] and the role of Se on moderate to severe GO found insufficient evidence [90,91]. More RCT was needed to confirm the efficacy and safety.
Despite the uncertainty of Se supplementation, its ability to maintain adequate Se serum levels and to avoid severe Se deficiency deserves clinical attention.
9.2.2. Pentoxifylline
Pentoxifylline is a methyl-xanthine derivative and is approved by the FDA to improve ischemic symptoms from peripheral arterial diseases. In 1993, Chang et al. [92] demonstrated that pentoxifylline inhibited the proliferation of fibroblasts from GO patients, as well as glycosaminoglycan synthesis in vitro. Further studies revealed that cytokine induced HLA-DR expression, serum levels of GAG, TNF-α, anti-TSH-receptor, anti-eye muscle, anti-thyroglobulin and anti-thyroid peroxidase antibodies were suppressed by pentoxifylline [93,94]. A prospective RCT [95], held in 2004, with a total of 18 inactive GO patients, distributed patients to two groups randomly: one group with pentoxifylline 1200 mg orally/day for 6 months, and the other group with placebo for initial 6 months sequentially pentoxifylline for another 6 months. The study demonstrated a significant change in proptosis after 3 months and 6 months of pentoxifylline use. However, inconsistent results were found in the large-sampled double blinded RCT [85] from the EUGOGO group, which revealed the insignificant effect of pentoxifylline (600 mg twice daily for 6 months) in mild GO patients. More RCT were needed to confirm the efficacy in different severity GO patients (active, inactive; mild, moderate and severe).
9.2.3. Nicotinamide and Allopurinol
Nicotinamide, an amide form of vitamin B3, is a component of the coenzymes of nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide (NADH), nicotinamide adenine dinucleotide phosphate (NADP+), and nicotinamide adenine dinucleotide phosphate (NADPH), in the oxidation–reduction assay. It was found that nicotinamide could inhibit HLA-DR expression on orbital fibroblasts from GO patients and suppressed superoxide-induced fibroblast proliferation. It also stimulated cytokine-induced activation and enhanced apoptosis through the Fas ligand on fibroblasts in vitro [96,97].
Allopurinol is an inhibitor of xanthine oxidase. Uric acid is a powerful scavenger of ROS; through reducing purine metabolism, allopurinol is regarded as an antioxidant. Experimental studies revealed allopurinol reduced the level of xanthine oxidase, malondialdehyde, glutathione and nitric oxide derivatives in hyperthyroidism in rats [98,99].
In a non-randomized prospective study [100], oral allopurinol (300 mg daily) and nicotinamide (300 mg daily) were used in 11 GO patients. After 3 months of usage, significant improvements in visual acuity (82%) and in the soft tissue inflammation (90%) were found, compared to the placebo group. However, limitations were the small numbers, newly diagnosed GO patients from mild to severe severity and two antioxidants used simultaneously. Large-numbered RCT with separate therapy is warranted to evaluate the efficacy. Additionally, allopurinol-induced subclinical hypothyroidism (odds ratio 1.51) was found in a recent large-scaled observational study [101]. Because allopurinol may induce the elevation of thyroid-stimulating hormone levels, the safety of long-term use warrants more clinical attention.
9.3. Potential Therapeutic Target—Pirfenidone
Pirfenidone is indicated for the treatment of idiopathic pulmonary fibrosis. Regarding GO, it was found that pirfenidone attenuates levels of cyclooxygenase 2, prostaglandin E2 and tissue inhibitors of metalloproteinase (TIMP)-1 in IL-1β–induced orbital fibroblasts [102,103,104]. In our previous study [105], we found pretreatment of pirfenidone decreased the levels of α-SMA, CTGF, fibronectin, and collagen type I on TGF-β1-induced orbital fibroblasts, through the p38 and c-Jun N-terminal kinase (JNK) pathways. The role of pirfenidone on ECM homeostasis and tissue remodeling provides a potential strategy.
9.4. Other Potential Targets
Factors influencing endoplasmic reticulum stress and oxidative stress, and the influence of smoking have been recognized as critical components in the disease’s development and severity. Epigenetic regulators, particularly histone deacetylases (HDACs), have emerged as key players in modulating the immune responses and fibrotic processes that drive GO. In addition, Chen et al. [106] demonstrated that targeting CD40–CD40L signaling by a specific RNA aptamers (CD40Apt) could reduce the levels of CD40, collagen I, TGF-β, and α-SMA in orbital muscle and adipose tissues of model mice. This represents a promising antagonist of CD40–CD40L signaling for TAO treatment. Further, the emergence of advanced gene sequencing technologies has illuminated the roles of microRNAs (miRNAs), circular RNAs (circRNAs), and long non-coding RNAs (lncRNAs) in the pathogenesis of GO, offering new avenues for understanding and potentially controlling the disease. We summarize the potential therapies in Table 1.

Table 1.
Current and potential treatment for Graves’ ophthalmopathy (GO).
10. Concluding Remarks
In this review, we have demonstrated a picture of the crucial roles and emerging issues related to ECM proteins in GO pathogenesis. To understand the mechanisms of ECM protein dysregulation in GO, it is important to elucidate the molecular basis of the excessive proliferation and ECM production by GO orbital fibroblasts and the involvement of certain signaling pathways, leading to tissue expansion/remodeling and fibrosis in GO.
Current pharmacological agents are systemic steroids and immunomodulating agents, which may reduce inflammation but have limited effects on the long-term sequela. While teprotumumab, a monoclonal antibody targeting the insulin-like growth factor-1 receptor (IGF-1R), has shown remarkable success in managing active proptosis in GO, its cost, adverse effects, and infusion requirements emphasize the need for additional therapeutic options. Multiple antioxidants and antifibrotic agents, such as pirfenidone, hold promise as potential therapies or prophylactic agents, while warranting long-term and large-scaled clinical trials. The identification of novel biomarkers, including antagonists in ER stress, the PPARγ pathway, small molecules of histone and non-coding RNA, provide a more comprehensive understanding of the disease pathways. The ideal scenario would involve the oral or subcutaneous delivery of these novel therapies with minimal side effects. Broadening the concepts of the ECM and tissue fibrosis will help in the development of new therapeutic targets in GO.
Funding
The work was supported by a grant (112-2314-B-075-003-MY2) from the Taiwan National Science and Technology Council and a grant (V113C-021) from the Taipei Veterans General Hospital, Taiwan.
Data Availability Statement
Not applicable.
Conflicts of Interest
No potential conflicts of interest were reported by the authors.
References
- Taylor, P.N.; Zhang, L.; Lee, R.W.J.; Muller, I.; Ezra, D.G.; Dayan, C.M.; Kahaly, G.J.; Ludgate, M. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nat. Rev. Endocrinol. 2020, 16, 104–116. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marino, M.; et al. Teprotumumab for the Treatment of Active Thyroid Eye Disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef]
- Sears, C.M.; Azad, A.D.; Amarikwa, L.; Pham, B.H.; Men, C.J.; Kaplan, D.N.; Liu, J.; Hoffman, A.R.; Swanson, A.; Alyono, J.; et al. Hearing Dysfunction After Treatment with Teprotumumab for Thyroid Eye Disease. Am. J. Ophthalmol. 2022, 240, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, P.R.K.; Sabaretnam, M.; Kumar, S.C.; Zwalitha, S.; Devi, N.V. Regression of Ophthalmopathic Exophthalmos in Graves’ Disease After Total Thyroidectomy: A Prospective Study of a Surgical Series. Indian J. Surg. 2017, 79, 521–526. [Google Scholar] [CrossRef]
- Liu, Z.W.; Masterson, L.; Fish, B.; Jani, P.; Chatterjee, K. Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy. Cochrane Database Syst. Rev. 2015, CD010576. [Google Scholar] [CrossRef]
- Ryan, H. Thyroidectomy and thyrotropic exophthalmos (exophthalmic ophthalmoplegia) a review of 1001 thyroidectomies. Br. J. Ophthalmol. 1949, 33, 769–773. [Google Scholar] [CrossRef][Green Version]
- Feldon, S.E.; Park, D.J.; O’Loughlin, C.W.; Nguyen, V.T.; Landskroner-Eiger, S.; Chang, D.; Thatcher, T.H.; Phipps, R.P. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves’ ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3913–3921. [Google Scholar] [CrossRef]
- Bahn, R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010, 362, 726–738. [Google Scholar] [CrossRef]
- Smith, T.J.; Hegedus, L.; Douglas, R.S. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best. Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.M.; Woeller, C.F.; Pollock, S.J.; O’Loughlin, C.W.; Gupta, S.; Feldon, S.E.; Phipps, R.P. Novel anti-adipogenic activity produced by human fibroblasts. Am. J. Physiol. Cell Physiol. 2010, 299, C672–C681. [Google Scholar] [CrossRef]
- Kumar, S.; Coenen, M.J.; Scherer, P.E.; Bahn, R.S. Evidence for enhanced adipogenesis in the orbits of patients with Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 2004, 89, 930–935. [Google Scholar] [CrossRef]
- Zhao, P.; Deng, Y.; Gu, P.; Wang, Y.; Zhou, H.; Hu, Y.; Chen, P.; Fan, X. Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy. Exp. Eye Res. 2013, 107, 65–73. [Google Scholar] [CrossRef]
- Lehmann, G.M.; Feldon, S.E.; Smith, T.J.; Phipps, R.P. Immune mechanisms in thyroid eye disease. Thyroid 2008, 18, 959–965. [Google Scholar] [CrossRef]
- Heufelder, A.E.; Bahn, R.S. Modulation of Graves’ orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Investig. Ophthalmol. Vis. Sci. 1994, 35, 120–127. [Google Scholar]
- Koumas, L.; Smith, T.J.; Feldon, S.; Blumberg, N.; Phipps, R.P. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am. J. Pathol. 2003, 163, 1291–1300. [Google Scholar] [CrossRef]
- Tan, J.; Tong, B.D.; Wu, Y.J.; Xiong, W. MicroRNA-29 mediates TGFbeta1-induced extracellular matrix synthesis by targeting wnt/beta-catenin pathway in human orbital fibroblasts. Int. J. Clin. Exp. Pathol. 2014, 7, 7571–7577. [Google Scholar]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Robertson, I.B.; Horiguchi, M.; Zilberberg, L.; Dabovic, B.; Hadjiolova, K.; Rifkin, D.B. Latent TGF-beta-binding proteins. Matrix Biol. 2015, 47, 44–53. [Google Scholar] [CrossRef]
- Pardo, A.; Cabrera, S.; Maldonado, M.; Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 2016, 17, 23. [Google Scholar] [CrossRef]
- Budi, E.H.; Duan, D.; Derynck, R. Transforming Growth Factor-beta Receptors and Smads: Regulatory Complexity and Functional Versatility. Trends Cell Biol. 2017, 27, 658–672. [Google Scholar] [CrossRef]
- Finnson, K.W.; Almadani, Y.; Philip, A. Non-canonical (non-SMAD2/3) TGF-beta signaling in fibrosis: Mechanisms and targets. Semin. Cell Dev. Biol. 2020, 101, 115–122. [Google Scholar] [CrossRef]
- Hou, T.Y.; Wu, S.B.; Kau, H.C.; Tsai, C.C. JNK and p38 Inhibitors Prevent Transforming Growth Factor-beta1-Induced Myofibroblast Transdifferentiation in Human Graves’ Orbital Fibroblasts. Int. J. Mol. Sci. 2021, 22, 2952. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Kau, H.C.; Wei, Y.H. Essential role of connective tissue growth factor (CTGF) in transforming growth factor-beta1 (TGF-beta1)-induced myofibroblast transdifferentiation from Graves’ orbital fibroblasts. Sci. Rep. 2018, 8, 7276. [Google Scholar] [CrossRef]
- Huang, Y.M.; Chang, P.C.; Wu, S.B.; Kau, H.C.; Tsai, C.C.; Liu, C.J.; Wei, Y.H. Expression and clinical significance of connective tissue growth factor (CTGF) in Graves’ ophthalmopathy. Br. J. Ophthalmol. 2017, 101, 676–680. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, S.B.; Chang, P.C.; Wei, Y.H. Alteration of Connective Tissue Growth Factor (CTGF) Expression in Orbital Fibroblasts from Patients with Graves’ Ophthalmopathy. PLoS ONE 2015, 10, e0143514. [Google Scholar] [CrossRef]
- Horan, G.S.; Wood, S.; Ona, V.; Li, D.J.; Lukashev, M.E.; Weinreb, P.H.; Simon, K.J.; Hahm, K.; Allaire, N.E.; Rinaldi, N.J.; et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008, 177, 56–65. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-beta Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Smith, T.J.; Bahn, R.S.; Gorman, C.A. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr. Rev. 1989, 10, 366–391. [Google Scholar] [CrossRef]
- Zhang, L.; Bowen, T.; Grennan-Jones, F.; Paddon, C.; Giles, P.; Webber, J.; Steadman, R.; Ludgate, M. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: Contributory role in hyaluronan accumulation in thyroid dysfunction. J. Biol. Chem. 2009, 284, 26447–26455. [Google Scholar] [CrossRef]
- van Steensel, L.; Paridaens, D.; van Meurs, M.; van Hagen, P.M.; van den Bosch, W.A.; Kuijpers, R.W.; Drexhage, H.A.; Hooijkaas, H.; Dik, W.A. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J. Clin. Endocrinol. Metab. 2012, 97, E400–E408. [Google Scholar] [CrossRef]
- van Steensel, L.; Hooijkaas, H.; Paridaens, D.; van den Bosch, W.A.; Kuijpers, R.W.; Drexhage, H.A.; van Hagen, P.M.; Dik, W.A. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J. Clin. Endocrinol. Metab. 2012, 97, E944–E953. [Google Scholar] [CrossRef]
- Galgoczi, E.; Katko, M.; Papp, F.R.; Csiki, R.; Csiha, S.; Erdei, A.; Bodor, M.; Ujhelyi, B.; Steiber, Z.; Gyory, F.; et al. Glucocorticoids Directly Affect Hyaluronan Production of Orbital Fibroblasts; A Potential Pleiotropic Effect in Graves’ Orbitopathy. Molecules 2022, 28, 15. [Google Scholar] [CrossRef]
- Lim, H.S.; Back, K.O.; Kim, H.J.; Choi, Y.H.; Park, Y.M.; Kook, K.H. Hyaluronic acid induces COX-2 expression via CD44 in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7441–7450. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Grennan-Jones, F.; Lane, C.; Rees, D.A.; Dayan, C.M.; Ludgate, M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2012, 97, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Baglole, C.J.; O’Loughlin, C.W.; Feldon, S.E.; Phipps, R.P. Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: Implications for thyroid eye disease. J. Biol. Chem. 2010, 285, 15794–15804. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Leo, M.; Altea, M.A. Oxidative stress in graves’ disease. Eur. Thyroid J. 2012, 1, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lanzolla, G.; Marcocci, C.; Marino, M. Oxidative Stress in Graves Disease and Graves Orbitopathy. Eur. Thyroid J. 2020, 9 (Suppl. S1), 40–50. [Google Scholar] [CrossRef]
- Tsai, C.C.; Kao, S.C.; Cheng, C.Y.; Kau, H.C.; Hsu, W.M.; Lee, C.F.; Wei, Y.H. Oxidative stress change by systemic corticosteroid treatment among patients having active graves ophthalmopathy. Arch. Ophthalmol. 2007, 125, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Wu, S.B.; Cheng, C.Y.; Kao, S.C.; Kau, H.C.; Chiou, S.H.; Hsu, W.M.; Wei, Y.H. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves’ ophthalmopathy: Evidence that oxidative stress has a role in this disorder. Eye 2010, 24, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Wu, S.B.; Cheng, C.Y.; Kao, S.C.; Kau, H.C.; Lee, S.M.; Wei, Y.H. Increased response to oxidative stress challenge in Graves’ ophthalmopathy orbital fibroblasts. Mol. Vis. 2011, 17, 2782–2788. [Google Scholar] [PubMed]
- Akarsu, E.; Buyukhatipoglu, H.; Aktaran, S.; Kurtul, N. Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin. Endocrinol. 2011, 74, 118–124. [Google Scholar] [CrossRef]
- Abalovich, M.; Llesuy, S.; Gutierrez, S.; Repetto, M. Peripheral parameters of oxidative stress in Graves’ disease: The effects of methimazole and 131 iodine treatments. Clin. Endocrinol. 2003, 59, 321–327. [Google Scholar] [CrossRef]
- Bednarek, J.; Wysocki, H.; Sowinski, J. Oxidative stress peripheral parameters in Graves’ disease: The effect of methimazole treatment in patients with and without infiltrative ophthalmopathy. Clin. Biochem. 2005, 38, 13–18. [Google Scholar] [CrossRef]
- Yuksel, N.; Yaman, D.; Tugce Pasaoglu, O.; Pasaoglu, H. The Effect of Smoking on Mitochondrial Biogenesis in Patients with Graves Ophthalmopathy. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 172–177. [Google Scholar] [CrossRef]
- Cawood, T.J.; Moriarty, P.; O’Farrelly, C.; O’Shea, D. Smoking and thyroid-associated ophthalmopathy: A novel explanation of the biological link. J. Clin. Endocrinol. Metab. 2007, 92, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.J.; Bagher Abtahi, S.M. Mini Review on the Effect of Smoking on Retrobulbar Blood Flow in Thyroid Eye Disease. J. Clin. Exp. Ophthalmol. 2018, 9, 5. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Nagahara, M.; Tomita, K. Acute effects of cigarette smoking on tissue circulation in human optic nerve head and choroid-retina. Ophthalmology 1999, 106, 564–569. [Google Scholar] [CrossRef]
- Gortz, G.E.; Horstmann, M.; Aniol, B.; Reyes, B.D.; Fandrey, J.; Eckstein, A.; Berchner-Pfannschmidt, U. Hypoxia-Dependent HIF-1 Activation Impacts on Tissue Remodeling in Graves’ Ophthalmopathy-Implications for Smoking. J. Clin. Endocrinol. Metab. 2016, 101, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Kau, H.C.; Wu, S.B.; Tsai, C.C.; Liu, C.J.; Wei, Y.H. Cigarette Smoke Extract-Induced Oxidative Stress and Fibrosis-Related Genes Expression in Orbital Fibroblasts from Patients with Graves’ Ophthalmopathy. Oxid. Med. Cell Longev. 2016, 2016, 4676289. [Google Scholar] [CrossRef]
- Hung, C.T.; Tsai, Y.W.; Wu, Y.S.; Yeh, C.F.; Yang, K.C. The novel role of ER protein TXNDC5 in the pathogenesis of organ fibrosis: Mechanistic insights and therapeutic implications. J. Biomed. Sci. 2022, 29, 63. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Liang, Y.; Hu, Y.; Xia, W.; Zhang, Y.; Zhao, C.; Wu, L. Integrative metabolic analysis of orbital adipose/connective tissue in patients with thyroid-associated ophthalmopathy. Front. Endocrinol. 2022, 13, 1001349. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, J.Y.; Kyoung Chae, M.; Jig Lee, E.; Sook Yoon, J. PERK mediates oxidative stress and adipogenesis in Graves’ orbitopathy pathogenesis. J. Mol. Endocrinol. 2021, 66, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, X.M.; Wang, X.; Sun, X.; Wang, L.J.; Li, X.Q.; Liu, X.Y.; Yu, H.S. Emerging Insights Into the Role of Epigenetics and Gut Microbiome in the Pathogenesis of Graves’ Ophthalmopathy. Front. Endocrinol. 2021, 12, 788535. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Duan, H.; You, S.; Liang, B.; Chen, Y.; Huang, H. Research progress on the pathogenesis of Graves’ ophthalmopathy: Based on immunity, noncoding RNA and exosomes. Front. Immunol. 2022, 13, 952954. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Lin, Z.J.; Li, C.C.; Lin, X.; Shan, S.K.; Guo, B.; Zheng, M.H.; Li, F.; Yuan, L.Q.; Li, Z.H. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Limbach, M.; Saare, M.; Tserel, L.; Kisand, K.; Eglit, T.; Sauer, S.; Axelsson, T.; Syvanen, A.C.; Metspalu, A.; Milani, L.; et al. Epigenetic profiling in CD4+ and CD8+ T cells from Graves’ disease patients reveals changes in genes associated with T cell receptor signaling. J. Autoimmun. 2016, 67, 46–56. [Google Scholar] [CrossRef]
- Sacristan-Gomez, P.; Serrano-Somavilla, A.; Gonzalez-Amaro, R.; Martinez-Hernandez, R.; Marazuela, M. Analysis of Expression of Different Histone Deacetylases in Autoimmune Thyroid Disease. J. Clin. Endocrinol. Metab. 2021, 106, 3213–3227. [Google Scholar] [CrossRef]
- Ekronarongchai, S.; Palaga, T.; Saonanon, P.; Pruksakorn, V.; Hirankarn, N.; van Hagen, P.M.; Dik, W.A.; Virakul, S. Histone Deacetylase 4 Controls Extracellular Matrix Production in Orbital Fibroblasts from Graves’ Ophthalmopathy Patients. Thyroid 2021, 31, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Woeller, C.F.; Roztocil, E.; Hammond, C.; Feldon, S.E. TSHR Signaling Stimulates Proliferation Through PI3K/Akt and Induction of miR-146a and miR-155 in Thyroid Eye Disease Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4336–4345. [Google Scholar] [CrossRef]
- Jang, S.Y.; Park, S.J.; Chae, M.K.; Lee, J.H.; Lee, E.J.; Yoon, J.S. Role of microRNA-146a in regulation of fibrosis in orbital fibroblasts from patients with Graves’ orbitopathy. Br. J. Ophthalmol. 2018, 102, 407–414. [Google Scholar] [CrossRef]
- Wei, H.; Guan, M.; Qin, Y.; Xie, C.; Fu, X.; Gao, F.; Xue, Y. Circulating levels of miR-146a and IL-17 are significantly correlated with the clinical activity of Graves’ ophthalmopathy. Endocr. J. 2014, 61, 1087–1092. [Google Scholar] [CrossRef]
- Li, K.; Du, Y.; Jiang, B.L.; He, J.F. Increased microRNA-155 and decreased microRNA-146a may promote ocular inflammation and proliferation in Graves’ ophthalmopathy. Med. Sci. Monit. 2014, 20, 639–643. [Google Scholar]
- Eissa, M.G.; Artlett, C.M. The MicroRNA miR-155 Is Essential in Fibrosis. Noncoding RNA 2019, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.L.; Roztocil, E.; Gonzalez, M.O.; Feldon, S.E.; Woeller, C.F. MicroRNA-130a Is Elevated in Thyroid Eye Disease and Increases Lipid Accumulation in Fibroblasts Through the Suppression of AMPK. Investig. Ophthalmol. Vis. Sci. 2021, 62, 29. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Chae, M.K.; Lee, J.H.; Lee, E.J.; Yoon, J.S. MicroRNA-27 inhibits adipogenic differentiation in orbital fibroblasts from patients with Graves’ orbitopathy. PLoS ONE 2019, 14, e0221077. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yun, M.; Paik, J.S.; Lee, S.B.; Yang, S.W. PDGF-BB Enhances the Proliferation of Cells in Human Orbital Fibroblasts by Suppressing PDCD4 Expression Via Up-Regulation of microRNA-21. Investig. Ophthalmol. Vis. Sci. 2016, 57, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, R.; Diao, J.; Chen, X.; Huang, J.; Xu, K.; Ling, L.; Xia, W.; Liang, Y.; Liu, G.; et al. Differentially expressed circular RNAs in orbital adipose/connective tissue from patients with thyroid-associated ophthalmopathy. Exp. Eye Res. 2020, 196, 108036. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.J.; Habekost, G.; Bratholm, P. A RNA transcript (Heg) in mononuclear cells is negatively correlated with CD14 mRNA and TSH receptor autoantibodies. Clin. Exp. Immunol. 2008, 154, 209–215. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Liang, Y.; Chen, X.; Mou, P.; Liu, G.; Sun, X.; Qin, B.; Zhang, S.; Zhao, C. Identification of differentially expressed long non-coding RNAs and mRNAs in orbital adipose/connective tissue of thyroid-associated ophthalmopathy. Genomics 2021, 113 Pt 2, 440–449. [Google Scholar] [CrossRef]
- Su, X.; Yin, X.; Liu, Y.; Yan, X.; Zhang, S.; Wang, X.; Lin, Z.; Zhou, X.; Gao, J.; Wang, Z.; et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020, 105, 3526–3547. [Google Scholar] [CrossRef] [PubMed]
- Masetti, G.; Moshkelgosha, S.; Kohling, H.L.; Covelli, D.; Banga, J.P.; Berchner-Pfannschmidt, U.; Horstmann, M.; Diaz-Cano, S.; Goertz, G.E.; Plummer, S.; et al. Gut microbiota in experimental murine model of Graves’ orbitopathy established in different environments may modulate clinical presentation of disease. Microbiome 2018, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Moshkelgosha, S.; Verhasselt, H.L.; Masetti, G.; Covelli, D.; Biscarini, F.; Horstmann, M.; Daser, A.; Westendorf, A.M.; Jesenek, C.; Philipp, S.; et al. Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease. Microbiome 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Douglas, R.S.; Holt, R.J.; Sile, S.; Smith, T.J. Teprotumumab for patients with active thyroid eye disease: A pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021, 9, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Vannucchi, G.; Curro, N.; Campi, I.; Covelli, D.; Dazzi, D.; Simonetta, S.; Guastella, C.; Pignataro, L.; Avignone, S.; et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: A randomized controlled study. J. Clin. Endocrinol. Metab. 2015, 100, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.N.; Garrity, J.A.; Carranza Leon, B.G.; Prabin, T.; Bradley, E.A.; Bahn, R.S. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2015, 100, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Jyonouchi, S.C.; Valyasevi, R.W.; Harteneck, D.A.; Dutton, C.M.; Bahn, R.S. Interleukin-6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves’ ophthalmopathy. Thyroid 2001, 11, 929–934. [Google Scholar] [CrossRef]
- Akbarian, S.; Chaibakhsh, S.; Kashkouli, M.B.; Karimi, N.; Abdolalizadeh, P.; Ghahvehchian, H. A Systematic Review on the Role of Antioxidants in Thyroid Eye Disease. J. Curr. Ophthalmol. 2022, 34, 16–24. [Google Scholar]
- Hou, T.Y.; Wu, S.B.; Kau, H.C.; Tsai, C.C. The Role of Oxidative Stress and Therapeutic Potential of Antioxidants in Graves’ Ophthalmopathy. Biomedicines 2021, 9, 1871. [Google Scholar] [CrossRef]
- Lanzolla, G.; Marcocci, C.; Marino, M. Antioxidant Therapy in Graves’ Orbitopathy. Front. Endocrinol. 2020, 11, 608733. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the course of mild Graves’ orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Bartalena, L.; Hegedus, L.; Leenhardt, L.; Poppe, K.; Pearce, S.H. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur. Thyroid J. 2018, 7, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Burch, H.B.; Cooper, D.S.; Greenlee, M.C.; Laurberg, P.; Maia, A.L.; Rivkees, S.A.; Samuels, M.; Sosa, J.A.; Stan, M.N.; et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016, 26, 1343–1421. [Google Scholar] [CrossRef] [PubMed]
- Karalis, D.T. The Beneficiary Role of Selenium in Type II Diabetes: A Longitudinal Study. Cureus 2019, 11, e6443. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Yang, H.B.; Li, X.X.; Lei, G.H.; Yang, T.B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuk, T.; Schomburg, L. Challenges and perspectives of selenium supplementation in Graves’ disease and orbitopathy. Hormones 2020, 19, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lanzolla, G.; Marino, M.; Marcocci, C. Selenium in the Treatment of Graves’ Hyperthyroidism and Eye Disease. Front. Endocrinol. 2020, 11, 608428. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, T.C.; Kao, S.C.; Kuo, Y.F.; Chien, L.F. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol. 1993, 129, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Balazs, C.; Kiss, H.; Farid, N.R. Inhibitory effect of pentoxifylline on HLA-DR expression and glycosaminoglycan synthesis by retrobulbar fibroblasts. Horm. Metab. Res. 1998, 30, 496–499. [Google Scholar] [CrossRef]
- Balazs, C.; Kiss, E.; Vamos, A.; Molnar, I.; Farid, N.R. Beneficial effect of pentoxifylline on thyroid associated ophthalmopathy (TAO)*: A pilot study. J. Clin. Endocrinol. Metab. 1997, 82, 1999–2002. [Google Scholar] [CrossRef]
- Finamor, F.E.; Martins, J.R.M.; Nakanami, D.; Paiva, E.R.; Manso, P.G.; Furlanetto, R.P. Pentoxifylline (PTX)—An Alternative Treatment in Graves’ Ophthalmopathy (Inactive Phase): Assessment by a Disease Specific Quality of Life Questionnaire and by Exophthalmometry in a Prospective Randomized Trial. Eur. J. Ophthalmol. 2018, 14, 277–283. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Yang, D.; Miyake, I.; Koga, M.; Kameo, J.; Sato, M.; Inoue, Y.; Nonaka, K. Nicotinamide decreases cytokine-induced activation of orbital fibroblasts from patients with thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 1998, 83, 121–124. [Google Scholar] [CrossRef]
- Burch, H.B.; Lahiri, S.; Bahn, R.S.; Barnes, S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp. Eye Res. 1997, 65, 311–316. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Makay, O.; Yenisey, C.; Icoz, G.; Genc Simsek, N.; Ozgen, G.; Akyildiz, M.; Yetkin, E. The role of allopurinol on oxidative stress in experimental hyperthyroidism. J. Endocrinol. Investig. 2009, 32, 641–646. [Google Scholar] [CrossRef]
- Bouzas, E.A.; Karadimas, P.; Mastorakos, G.; Koutras, D.A. Antioxidant agents in the treatment of Graves’ ophthalmopathy. Am. J. Ophthalmol. 2000, 129, 618–622. [Google Scholar] [CrossRef]
- Choi, W.; Yang, Y.S.; Chang, D.J.; Chung, Y.W.; Kim, H.; Ko, S.J.; Yoo, S.; Oh, J.S.; Kang, D.Y.; Yang, H.J.; et al. Association between the use of allopurinol and risk of increased thyroid-stimulating hormone level. Sci. Rep. 2021, 11, 20305. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.A.; Jeon, B.K.; Choi, Y.H.; Back, K.O.; Lee, J.B.; Kook, K.H. Pirfenidone attenuates the IL-1beta-induced hyaluronic acid increase in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2276–2283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, Y.H.; Back, K.O.; Kim, H.J.; Lee, S.Y.; Kook, K.H. Pirfenidone attenuates IL-1beta-induced COX-2 and PGE2 production in orbital fibroblasts through suppression of NF-kappaB activity. Exp. Eye Res. 2013, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, Y.H.; Park, S.J.; Lee, S.Y.; Kim, S.J.; Jou, I.; Kook, K.H. Antifibrotic effect of Pirfenidone on orbital fibroblasts of patients with thyroid-associated ophthalmopathy by decreasing TIMP-1 and collagen levels. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3061–3066. [Google Scholar] [CrossRef]
- Wu, S.B.; Hou, T.Y.; Kau, H.C.; Tsai, C.C. Effect of Pirfenidone on TGF-beta1-Induced Myofibroblast Differentiation and Extracellular Matrix Homeostasis of Human Orbital Fibroblasts in Graves’ Ophthalmopathy. Biomolecules 2021, 11, 1424. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, R.; Xiong, W.; Zhang, F.; Wang, N.; Xie, B.; Cao, J.; Chen, Z.; Ma, C. RNA aptamers with specific binding affinity to CD40 (CD40Apt) represents a promising antagonist of the CD40-CD40L signaling for thyroid-associated ophthalmopathy (TAO) treatment in mouse. J. Transl. Med. 2023, 21, 396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).